Recent Findings in Fatty Acid Composition of Marine Oils
O L A V NOTEVARP
Department of Chemistry, Norwegian Institute of Technology, Trondheim, N o r w a y
I. Introduction 259 II. Structure of Polyenoic Acids 260
III. The Distribution of Fatty Acids 261 IV. Fatty Acid Composition 264
V. Differences between Marine Oils, Mammalian Depot Fats, and Seed
Fats 265 VI. Fats and Oils of Fish, Whales, and Mammals 268
VII. Fatty Acid Composition of Phosphatides and Glycerides 271
References 272
I. Introduction
In recent years much progress has been made regarding the constitu
tion of highly unsaturated fatty acids of fish oils and the distribution of such acids in marine oils. This is due chiefly to the introduction of new and better analytical methods, tests, and devices. Special mention should be made of spectrophotometric methods for determining unsaturated acids after isomerization, improvements in splitting of such unsaturated acids, chromatography, gas chromatography, and infrared spectroscopy.
The following review comprises especially the structure of polyenoic fatty acids of fish oils and the distribution of certain groups of such acids as the hexaenoic and pentaenoic in the total fatty acids of marine oils.
For the sake of comparison, some data for fats and oils from other sources are given.
It is not the intention of the writer to include all recent data on the chemistry of fish oil fatty acids. In this respect reference is made to Chapter 7 by Tsuchiya. Only new results carrying weight in elucidating the detailed composition and chemistry of fish oils will be discussed.
Several of the data presented in this review have not been published earlier, particularly those on the distribution of various polyenoic acid groups in the oils.
259
260 OLAV NOTEVARP II. Structure of Polyenoic Acids
In recent years a series of results regarding the structure of the hexaenoic, pentaenoic, tetraenoic, trienoic, and dienoic acids from oils of cod liver, herring, and South African pilchard have been published.
All of them agree on a divinylmethane arrangement of the ethylenic bonds: — C H = C H — C H2— C H = C H — . This is identical to what has been found to prevail for corresponding acids from mammalian body organs (Table I ) .
On the basis of these results, it can be concluded that practically all known polyenoic acids in fish oils, except the Ci6-tetraenoic acid, are of the linolenic type, with double bonds in positions 3, 6, 9 counted from the methyl group. As far as they have been examined, all acids show exclusively a ds-configuration. In their natural state they carry no conjugated double bonds. From Table I it will be seen that all investigators found the same configurations, and in recent reviews, Klenk (1958) and Sutton (1958) concluded that the grouping — C H = C H — C H2— C H = C H — seems to be common for fish oils. Klenk and Bongard (1952), Klenk and Lindlar (1955), and Klenk and Tomuschat (1957) found hexaenoic acids isolated from the phosphatides of different mammalian organs, especially brain phosphatides, to have the same constitution as that isolated from herring oil.
Notevarp, Höyland, Roald, and Sletnes (unpublished) isolated docosa- hexaenoic acids from cod liver oil and herring oil and found their melting points (-ξ- 41 ° C .) and spectrophotometry absorption after isomerization to be identical to those of oil isolated from herring milt. They also isolated docosapentaenoic acid and eicosatetraenoic acid from herring oil, but at the same time established that most of the pentaenoic acids in the North Atlantic fish oils and in Antarctic whale oils are eicosa (C2o) acids, and most of the tetraenoic acids are octadeca ( C i8) acids.
Klenk and Brockerhoff (1958) found the polyenoic octadeca acids of herring oil to be tetraenoic, trienoic, and dienoic (ratio, 6:1.4:2.6), and the docosa acids to contain 8 3 % hexaenoic and 9 % pentaenoic acids.
Vandenheuvel and Jangaard (1957) published figures showing Canadian herring oil to contain C i6- , C i8- , and C2 0-tetraenoic acids, C2 0- and, in small amounts, C2 2-pentaenoic acids, and C2 2-hexaenoic acid.
Summarizing, these research workers agree that all hexaenoic acids in fish oils are C2 2, the bulk of the pentaenoics is C2 0 (more than 9 0 % ), and the bulk of the tetraenoics (about 9 0 % ) is C i8.
261
III. The Distribution of Fatty Acids
In his classical work The Chemical Constitution of Natural Fats, Hil
ditch (1956) collected comprehensive data for the content of saturated fatty acids in most common fish oils and fish fats. The unsaturated acids, however, were only listed as to chain lengths and average degree of unsaturation for each chain length.
Since the method of determining unsaturated acids by means of iso
merization (conjugation) and spectrophotometric analysis was intro
duced for linoleic and linolenic acids through Mitchell et ah (1943), great efforts have gone into rendering this method serviceable also for more unsaturated polyenoic acids (Herb and Riemenschneider, 1952;
Hammond and Lundberg, 1953; Holman, 1957).
The spectrophotometric analysis gives the percentage of each group of the poly-unsaturated acids: hexaenes, pentaenes, tetraenes, trienes, and dienes. By means of the iodine values for the polyenoic acids and for the sample, the percentage of monoenoic and saturated acids may also be approximately calculated. Results of such determinations for some fish oils have been published, but they will not be listed here. Instead, results will be surveyed which have been obtained by the author and his col
laborators. Most of this material has hitherto not been published. These results refer mostly to oils from fish off the Norwegian coast, and several samples of Antarctic whale oils.
The percentages of hexaenoic, pentaenoic, and tetraenoic acids are calculated on the basis of spectrophotometric extinction coefficients, de
termined on pure acids isolated from marine oils and isomerized for 8 min. at 180°C. in 18% KOH-glycol. The trienoic and dienoic acids have been calculated on the basis of coefficients obtained through measure
ments on pure linoleic and linolenic acids after an identical isomerization procedure.
As hexaenoic acids also have absorption maxima in the pentaenoic, tetraenoic, trienoic, and dienoic bands, pentaenoic in the tetraenoic, tri
enoic, and dienoic, etc., the values are most accurate for the acids which have the highest degree of unsaturation. The values for the dienoic and trienoic acids are less accurate. For the hexaenoic and pentaenoic acids the relative errors should not exceed ± 5 % , for the dienoic ± 10 to 3 0 % , depending upon the absolute value. Values as low as 1% may obviously have larger relative errors.
TABLE I STRUCTURE OF POLYENOIC Acros FROM FISH OILS AND SOME MAMMAL ORGANS Position of double bonds From the carboxyl From the terminal Acid Isolated from group methyl group Reference Dienoic ^18 Herring oil 9,12 6,9 Brockerhoff (1957) (linoleic) Trienoic C18 Herring oil 9, 12, 15 3,6, 9 Brockerhoff (1957) C18 (linolenic) Tetraenoic ^16 S. African 6, 9,12,15 1,4, 7,10 Silk and Hahn (1954) sardine oil C18 Herring oil 6, 9,12,15 3,6, 9,12 Klenk and Brockerhoff (1957) ^18 S. African 6, 9,12,15 3,6, 9,12 Matic (1958) sardine oil C18 Herring oil 6, 9,12,15 3,6, 9,12 Notevarp and Höyland (unC18 published) C20 Ox adrenal 5, 8,11,14 6, 9,12,15 Dolby et al (1940) C20 lipids (arachidonic) Pentaenoic ^20 S. African 5, 8,11,14,17 3,6, 9,12,15 Whitcutt and Sutton (1956) sardine oil Cod liver oil 5, 8,11,14,17 3,6, 9,12,15 Klenk and Eberhagen (1957) Whale oil 5, 8,11,14,17 3,6, 9,12,15 Notevarp and Höyland (un published )
TABLE I (continued) Position of double bonds From the carboxyl From the terminal Acid Isolated from group methyl group Reference Pentaenoic (cont.) C22 Cattle liver 7,10,13,16,19 3,6, 9,12,15 Klenk and Tomuschat (1957) Herring oil 7,10,13,16,19 3,6, 9,12,15 Klenk and Brockerhoff (1958) Hexaenoic Hog brain 4, 7,10,13,16,19 3,6, 9,12,15,18 Hammond and Lundberg (tentatively) (1953) C22 Cattle brain 4, 7,10,13,16,19 3,6, 9,12,15,18 Klenk and Tomuschat (1957) c22 South African 4, 7,10,13,16,19 3,6, 9,12,15,18 Whitcutt (1957) c22 pilchard oil C22 Herring oil 4, 7,10,13,16,19 3,6, 9,12,15,18 Klenk and Brockerhoff (1958) ^22 Herring milt 4, 7,10,13,16,19 3,6, 9,12,15,18 Notevarp and Höyland (un published )
^18
264 OLAV NOTEVARP IV. Fatty Acid Composition
Table II summarizes our findings.
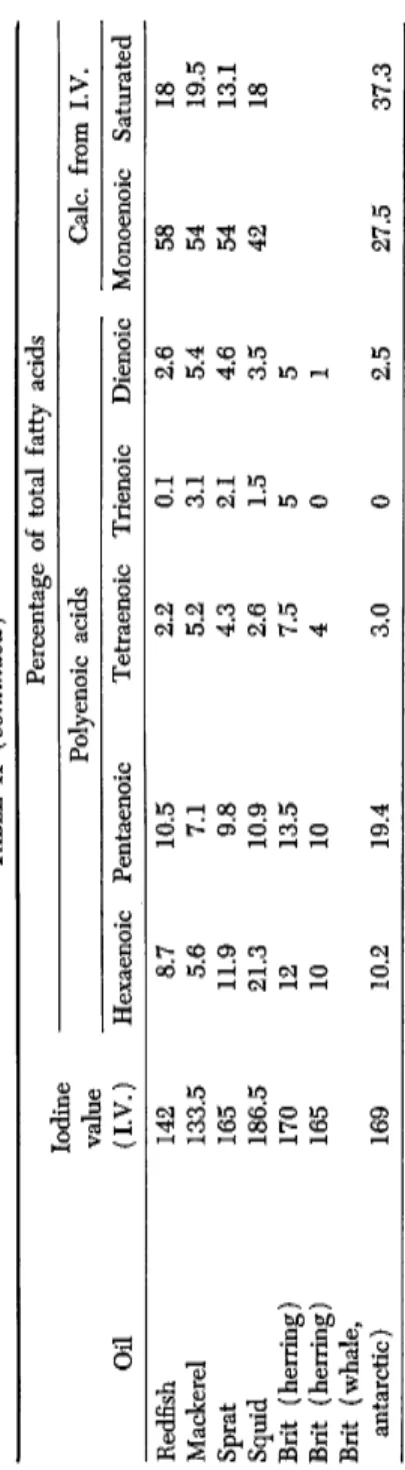
The values given for oils of cod and saithe (Gadus vir ens) livers, those of herring, capelin (Mallotus villosus), redfish, squid, and whale represent means of either a great number of samples or large quantities of oil. The figures for other oils are based on either one single sample or a few, and consequently may not be regarded as representative. The fatty acid composition may differ greatly from one individual to another, and varies, too, for the same species during the year and from different fishing grounds, depending upon feed, age of fish, spawning conditions, etc.
For a certain species, e.g., herring, the content of the most unsaturated acids, the hexaenoic and pentaenoic acids, is highest for young herring having the highest percentage of fat (summer herring) and much lower for lean herring and for old winter herring. This is in accordance with the known fact that there is an increase in unsaturation of a certain kind of fish oil when the oil content of the fish increases.
The unsaturation, and consequently the polyenoic acid content of the oils, is particularly dependent upon the diet of the fish. It was shown, e.g., by Lovern (1942) and more recently by Kelly et al. (1958a, b ) that the unsaturated fatty acid composition of the fish fat reflects to a high degree that of the feed. Kelly et al. (1959) also proved that there is a high content of hexaenoic, pentaenoic, and tetraenoic acids in the fats of a marine diatom (Nitzschia closterium), as well as in phyto-zoo- plankton, collected in the Gulf of Mexico. The fat from a fresh-water algae (Chlorella pyrenoidosa) carried no hexaenoic acid and very little pentaenoic. Altogether, its polyenoic acid distribution was close to that of the fat of mullet, a fresh-water fish.
Unfortunately, Kelly et al. only state the extinction coefficients for their oils, as they used a method of isomerization which did not lend itself to a calculation of the content of the highly unsaturated acids. So it is difficult to compare their results with those given for brit in Table II. But these figures of ours clearly illustrate that the available plankton (krill) has a high level of most unsaturated acids. These findings agree with earlier work by Lovern (1942), and it may be concluded that the algae of the sea are likely to be the producers of most of the very highly unsaturated fatty acids of marine animal life.
Table II reveals some very characteristic features as to the fatty acid composition of marine oils. They have in common a relatively high con-
8. FATTY ACID COMPOSITION OF MARINE OILS 265 tent of hexaenoic and pentaenoic acids, a much lower quantity of tetra
enoic and dienoic, and mostly a very low content of trienoic acids. The relative amount of monoenoic acids is very high, about 4 5 - 6 0 % , and fairly constant. The saturated acid content varies between 15 and 3 0 % , but shows only slight variations for oils of a certain species. The varia
tions in dienoic and trienoic acid content must be regarded as insig
nificant, because these figures are less accurate, as mentioned previously.
Altogether, the variation of the total unsaturation of a fish oil, or the iodine value, depends mainly on variations in the content of hexaenoic and pentaenoic acids, and to a less degree on the tetraenoics. A rise in the amount of the most highly unsaturated acids seems to effect a cor
responding decline in monoenoic acids and vice versa, but not to affect the content of saturated acids.
As to the composition of the saturated acids of fish oils, it seems superfluous to quote the values listed by Hilditch (1956). It should only be mentioned that the saturated acid fractions from some of the oils in Table II, especially those of cod liver, herring, and capelin, were ex
amined by distillation and gas chromatography. Their main components were found to be myristic acid ( 2 0 - 3 0 % ) , palmitic acid ( 6 0 - 7 0 % ) , and stearic acid ( 5 - 1 0 % ) .
V. Differences between Marine Oils, Mammalian Depot Fats, and Seed Fats For the sake of comparison, data as to the fatty acid composition of some mammalian depot fats and seed fats are presented in Table I I I . These figures originate from Bailey (1951), Hilditch (1956), and our own unpublished investigations.
According to Table II, marine oils do not differ greatly as regards their fatty acid composition. Fish liver oils normally have a higher con
tent of hexaenoic and pentaenoic acids than the body oils, and a lower content of saturated acids. Oil from squid is an exception. The body oils of herring and whale are not very different, except for the higher ratio of pentaenoic to hexaenoic acids in Antarctic whale oils. This is in ac
cordance with the ratio encountered in the feed of these Antarctic whales
—so-called krill. Oils from fish caught in the summer, e.g., oils from fat summer herring, mackerel, and sprat, are likely to contain more trienoic and tetraenoic acids than those collected in other seasons.
From Table III, it will be seen that depot fats from land animals and seed fats contain practically no hexaenoic, pentaenoic, and tetraenoic acids. Lard may be an exception. If the swine's feed contains fish meal,
TABLE II FATTY ACID DISTRIBUTION IN FISH AND WHALE OILS Oil
Iodine value (I.V.) Percentage of total fatty acids Oil
Iodine value (I.V.) Polyenoic acids Calc. from I.V. Oil
Iodine value (I.V.) Hexaenoic Pentaenoic Tetraenoic Trienoic Dienoic Monoenoic Saturated Cod liver, Norw. 172 15.0 11.8 2.6 1.8 4.5 45 19 Cod liver, Norw. 167 13.2 11.8 2.8 0.9 2.5 54 15 Saith liver, 1957 160 9.7 14.7 2.2 1.0 1.9 53 18.5 Saith liver, 1958 153 12.8 12.2 2.8 0.5 2.2 41 28 Saith liver, 1959 167 10.6 10.3 4.7 2.8 3.9 51 16 Herring Winter 125 6.0 8.0 3.4 1.9 3.9 52 25 Spring 123 5.4 7.4 3.3 2.2 3.8 54.5 23.5 Summer, fat 150 9.8 10.6 4.3 2.7 3.1 41.5 28 Summer, lean 125 6.4 5.0 2.9 1.6 2.4 56 25 Iceland 143 9.3 12.6 3.4 0.5 2.6 41.5 30 North Sea 143 8.9 11.0 5.6 1.5 4.1 38 30 Capelin 108 4.5 5.5 1.8 1.0 2.8 60 24 Capelin 100.5 3.9 5.7 1.3 0.7 2.2 60 26.5 Whale blubber oils Arctic fin whale 115 4.8 6.7 2.6 1.6 4.0 58 22.5 Antarctic blue and fin, 1954-55 124 5.5 10.0 1.8 0.9 3.2 53 26.5 Antarctic fin, fat 124 4.1 9.2 1.9 1.2 3.8 55.5 24 Antarctic fin, lean 132 5.2 10.4 2.9 2.2 4.0 52 23 Antarctic blue, fat 124 5.2 9.2 2.3 2.6 5.1 49 26.5 Antarctic blue, lean 140 7.5 12.8 2.4 0.7 2.5 50 25 Seal blubber 134.5 9.0 8.0 2.4 0.6 2.8 56 21
TABLE II (continued) Percentage of total fatty acids Percentage Iodine Polyenoic acids value Polyenoic acids Calc. from I.V. Oil (I.V.) Hexaenoic Pentaenoic Tetraenoic Trienoic Dienoic Monoenoic Saturated Redfish 142 8.7 10.5 2.2 0.1 2.6 58 18 Mackerel 133.5 5.6 7.1 5.2 3.1 5.4 54 19.5 Sprat 165 11.9 9.8 4.3 2.1 4.6 54 13.1 Squid 186.5 21.3 10.9 2.6 1.5 3.5 42 18 Brit (herring) 170 12 13.5 7.5 5 5 Brit (herring) 165 10 10 4 0 1 Brit (whale, antarctic) 169 10.2 19.4 3.0 0 2.5 27.5 37.3
268 OLAV NOTEVARP
hexaenoic and pentaenoic acid contents up to 0.4% have been found.
Normally, lard seems to contain 0.2% or more of tetraenoic acids.
TABLE III
FATTY Aero COMPOSITION OF SOME LAND ANIMAL DEPOT FATS AND OF SEED FATS
Percentage of total fatty acids Polyenoic
Iodine Hexa Penta Tetra Tri Di Mono Sat
Source value enoic enoic enoic enoic enoic enoic urated Depot fat from:
Hogs (Norw.) 50 0.0 0.0 0.2 1.2 7 42 35 Hogs (Norw.)
— 70 —0.4 —0.4 —0.4 1.6 —10 —50 —45
Cattle 45
— — — —
2 45 52Oils from:
Cotton seed 110
— — — —
50 23 27Corn 120
— — —
0.6 50 35 13Soybean 130
— — —
6 52 30 12Linseed 180
— — —
45 22 23 10Wheat 125
— — —
5.8 50 21 23Green pea 137
—
—- 0.1 11 53 1 35Ordinary seed oils are characterized by their high contents of dienoic and trienoic acids. Their fatty acid composition is quite different from that of fish oils, in spite of the fact that their total unsaturation as de
termined by the iodine value may be the same. The polyenoic acids of seed oils are also mainly Ci8-acids, linoleic and linolenic, of which marine oils contain only small amounts. The C2<r and C22-unsaturated acids represent most of the polyenoic acids in these oils, Ci8-tetraenoic acid being the most important exception.
VI. Fats and Oils of Fish, Whales, and Mammals
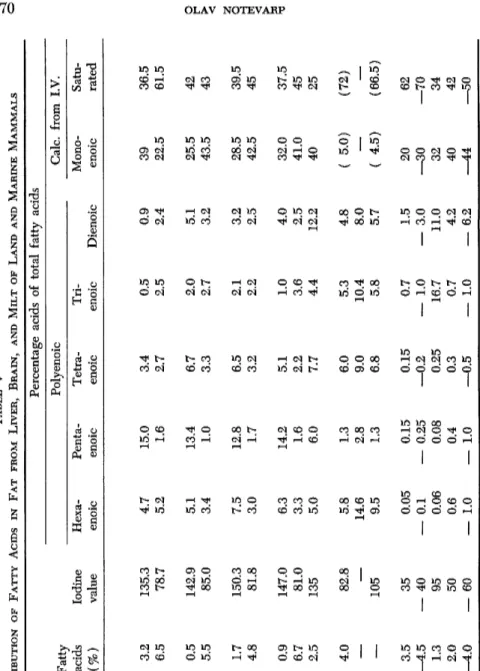
Values for the fatty acid composition of fats from flesh and different body organs of herring, cod, and salmon are brought together in Table IV. Analyses of livers and brains of whales and hogs, and of brains of a cow and a horse are given in Table V. For comparison, the average fatty acid composition of milk fats from cows, human beings, and horses is also listed.
The fatty acids from herring liver and roe contain very high per
centages of hexaenoic acid and much pentaenoic acid; the body fat is roughly identical to that of body oil (Table I I ) . The fatty acids of herring
TABLE IV ACID DISTRIBUTION OF FATTY ACIDS FROM FLESH AND ORGANS OF DIFFERENT FISH Percentage acids of total fatty acids Polyenoic Fatty Iodine Calc. from I.V. acids value HexaPentaTetraTriMonoSat Source of oil (%) (I.V.) enoic enoic enoic enoic Dienoic enoic urated Herring, winter, before spawning Flesh 14.0 127 5.3 7.2 3.0 1.5 2.7 57 23 Flesh 13.5 123.5 4.9 7.2 3.3 1.6 4.1 52 27 Liver 2.0 187 20.4 12.4 1.4 0.3 3.9 (31) (31) Liver 2.0 17.7 8.9 2.3 1.5 4.8 — — Roe (hard) 2.0 191 21.2 12.9 2.3 1.3 3.7 (24) (35) Milt (soft roe) 1.9 217 29 13.5 2.9 0.5 3.8 (10) (40) Milt (soft roe) (280) 39.5 18 2.6 1.0 6.1 Cod Flesh 0.3 236 26 18 5.5 1.5 4.1 18 27 Flesh 55 190 15 14.5 4.2 2.0 4.2 37 23 Roe 1.5 225 25.5 13 3.1 0.5 2.0 35 21 Milt (soft roe) 0.8 207 21.8 12 3.5 1.3 1.8 40.5 19 Brain 6.0 120 10.0 5.0 0.9 1.1 1.7 48 33 Salmon, Sea June 10 Flesh 4.0 165 17 8.2 2.6 1.2 2.5 38 30.5 Roe 1.8 223 23 17.5 2.4 0 1.3 35 21 Milt 1.3 196 17.8 10.8 2.3 0.8 5.4 52.5 10.5 Salmon, River August 5 Flesh 1.0 147.5 12.0 10.0 1.8 0.6 2.5 41.5 32 Roe 5.2 194 17.3 15.8 1.7 0 1.8 42 21 Liver 0.7 178 9.5 11.7 5.5 2.5 4.0 55 12 Trout, Fresh-water Liver 1.4 131 4.4 3.3 2.9 4.1 6.4 69 10
TABLE V DISTRIBUTION OF FATTY ACIDS IN FAT FROM LIVER, BRAIN, AND MILT OF LAND AND MARINE MAMMALS Percentage acids of total fatty acids Fatty Polyenoic Calc. from I.V. Source of acids Iodine HexaPentaTetraTriMonoSatu oil (%) value enoic enoic enoic enoic Dienoic enoic rated Whale Fin liver 3.2 135.3 4.7 15.0 3.4 0.5 0.9 39 36.5 brain 6.5 78.7 5.2 1.6 2.7 2.5 2.4 22.5 61.5 Fin liver 0.5 142.9 5.1 13.4 6.7 2.0 5.1 25.5 42 brain 5.5 85.0 3.4 1.0 3.3 2.7 3.2 43.5 43 Blue liver 1.7 150.3 7.5 12.8 6.5 2.1 3.2 28.5 39.5 brain 4.8 81.8 3.0 1.7 3.2 2.2 2.5 42.5 45 Blue liver 0.9 147.0 6.3 14.2 5.1 1.0 4.0 32.0 37.5 brain 6.7 81.0 3.3 1.6 2.2 3.6 2.5 41.0 45 Hog liver 2.5 135 5.0 6.0 7.7 4.4 12.2 40 25 Brain Hog 4.0 82.8 5.8 1.3 6.0 5.3 4.8 ( 5.0) (72) Cow
— —
14.6 2.8 9.0 10.4 8.0— —
Horse—
105 9.5 1.3 6.8 5.8 5.7 ( 4.5) (66.5) Milk Cow 3.5 35 0.05 0.15 0.15 0.7 1.5 20 62 —4.5 — 40 — 0.1 — 0.25 —0.2 — 1.0 — 3.0 —30 —70 Horse 1.3 95 0.06 0.08 0.25 16.7 11.0 32 34 Man 2.0 50 0.6 0.4 0.3 0.7 4.2 40 42 —4.0 — 60 — 1.0 — 1.0 —0.5 — 1.0 — 6.2 —44 —508. FATTY ACID COMPOSITION OF MARINE OILS 271 milt contain up to 39.5% hexaenoic acids. The fatty acids of cod flesh fat greatly resemble those of roe and milt. Surprisingly enough, the fats of salmon roe and milt are very similar to each other and also to that of herring roe. The content of hexaenoic acid is lower for the acids from cod brain and salmon liver, but still about 10%. The fatty acids of whale brain (Table V ) contain only 3 - 5 % hexaenoic acid, and 1-2% penta
enoic. The contents of tetraenoic, trienoic, and dienoic acids are relatively high. Hog brain fat contains still more of these last acids, but is about the same as whale brain as regards hexaenoic and pentaenoic acids. The samples analyzed of brains from the cow and the horse have a much higher content of all polyenoic acids except pentaenoic.
Whale livers contain about the same percentage of hexaenoic acids as hog livers, but much more of the pentaenoic acids. Hog livers are especially rich in dienoic acid.
The lipids of all these organs and of cod flesh consist mainly of phos
phatides and cholesterol esters. The high contents of polyenoic acids found in the fatty acids from these organs illustrate the importance of the most unsaturated acids in phosphatides and cholesterol esters, as pointed out by Hilditch (1956) and Lovern (1953).
VII. Fatty Acid Composition of Phosphatides and Glycerides
To illustrate still better the importance of the most unsaturated acids in phosphatides, values for some phosphatides and glycerides separated from lipids and extracted from different materials are listed in Table VI.
The separation of the phosphatides from the glycerides was incomplete for some materials, especially for cod and herring flesh, as some fish phosphatides are soluble in acetone, which is used for their precipitation and separation from the glycerides (Lovern and Olley, 1953). Accord
ingly, the degree of unsaturation given for the glyceride fatty acids from the materials cited tends to be too high.
It will be seen that the phosphatide fatty acids are characterized by containing much more hexaenoic acid than the glyceride acids, except for cod flesh, the lipids of which contain very little ordinary glycerides, and whose "glyceride" fraction is likely to consist mainly of soluble phosphatides. The values for the phosphatide acids of herring flesh are very close to those given in Table I I I for herring and cod roe and cod milt.
The most unsaturated fatty acids so far isolated are abundant in organs essential to metabolism, as well as in phospholipids. This is par-
272 OLAV NOTEVARP
ticularly true of hexaenoic acids, but also of pentaenoics. As such acids prevail in fish oils the conclusion seems justified that fish fats and fish oils in their natural condition are more essential to nutrition and health than hitherto realized. Recent investigations indicate, too, that the
TABLE VI
COMPOSITION OF FATTY ACIDS FROM PHOSPHATIDES AND GLYCERDDES Polyenoic acids in per cent of total fatty acids Source of Iodine Hexa Penta Tetra Tri
fatty acids value enoic enoic enoic enoic Dienoic Cod liver
Phosphatides 233 27.3 12.2 6.1 3.2 5.5
Glycerides 187 14.5 14.2 4.1 2.0 4.2
Cod flesh
Phosphatides 257 26.5 20.8 7.1 0.4 4.3
"Glycerides" 230 23.7 15.4 4.9 3.9 Herring flesh
Phosphatides 248 33.0 14.2 1.4 0.7 3.4
Glycerides 139 8.9 8.7 2.6 1.7 3.7
Egg yolk
Phosphatides 91 4.1 0.8 2.9 1.0 8.9
Glycerides 76 0 0 0 0 7.2
Hog liver
Phosphatides 174 8.4 9.1 12.6 5.6 16.1
Glycerides 96 1.5 2.7 2.8 3.2 8.2
most unsaturated fatty acids are less abundant in blood lipids from humans with atherosclerosis than in healthy persons (Antonis, 1958;
Notevarp and Cyvin, 1959a). The characteristic differences between the fats of human milk and cow's milk shown in Table IV (Notevarp and Cyvin, 1959b) may also indicate that some special importance may be attached to the hexaenoic and pentaenoic acids in foods.
REFERENCES
Antonis, A. (1958). Preliminary studies on serum fatty acids and their relationship to diet. In "Essential Fatty Acids: 4th International Conference on Biochemical Problems of Lipids" (Η. M. Sinclair, ed.), pp. 158-167. Butterworths, London.
Bailey, A. E. (1951). "Industrial Oil and Fat Products." Interscience, New York.
Brockerhoff, Η. (1957). Über die C1 8- und die C22-I>olyenfettsäuren des Hering
söls. Dissertation, Köln. De Gruyter, Berlin.
Dolby, D. E., Nunn, L. C. Α., and Smedley-Maclean, I. (1940). The constitution of arachidonic acid (preliminary communication). Biochem. J. 34, 1422-1425.
8. FATTY ACID COMPOSITION OF MARINE OILS 273 Hammond, E. G., and Lundberg, W. O. (1953). The alkali isomerization of a
methyl docosahexaenoate and the spectral properties of conjugated fatty acids.
/. Am. Oil Chemists Soc. 30, 433-438.
Herb, S. F., and Riemenschneider, R. W. (1952). Influence of alkali concen
tration and other factors on the conjugation of natural polyunsaturated acids as determined by ultraviolet absorption measurements. J. Am. Oil Chemists' Soc. 29, 456-461.
Hilditch, T. P. (1956). "The Chemical Constitution of Natural Fats." Chapman
& Hall, London.
Holman, R. T. (1957). Measurement of polyunsaturated fatty acids. Methods of Biochem. Anal. 4, 99-138.
Kelly, P. B., Reiser, R., and Hood, D. W. (1958a). The origin and metabolism of marine fatty acids. The effect of diet on the depot fats of Mugil cephalus (the common mullet). /. Am. Oil Chemists' Soc. 35, 503.
Kelly, P. B., Reiser, R., and Hood, D. W. (1958b). The effect of diet on the fatty acid composition of several species of fresh-water fish. J. Am. Oil Chemists' Soc. 35, 503-506.
Kelly, P. B., Reiser, R., and Hood, D. W. (1959). The origin of the marine poly
unsaturated fatty acids. Composition of some marine plankton. /. Am. Oil Chemists' Soc. 36, 104-106.
Klenk, E. (1958). The polyenoic acids of fish oils. In "Essential Fatty Acids:
4th International Conference on Biochemical Problems of Lipids." (Η. M.
Sinclair, ed.), pp. 57-59. Butterworths, London.
Klenk, E., and Bongard, W. (1952). Die Konstitution der ungesättigten C2 0- und C2 2-Fettsäuren der Glycerin-phosphatide des Gehirns. Z. physiol. Chem. 291, 104-118.
Klenk, E., and Brockerhoff, H. (1957). Über das Vorkommen der Δ 6 · 9 · ΐ 2 . ΐ 5 .η-
Octadecatetraensäure im Heringsöl und deren Isolierung. Z. physiol. Chem.
307, 272-277.
Klenk, E., and Brockerhoff, H. (1958). Über die C1 8- und C22-Polyensäuren des Heringsöls. Z. physiol. Chem. 310, 153-170.
Klenk, E., and Lindlar, F. (1955). Über die Dokosapolyensäuren der Glycerin- phosphatide des Gehirns. Z. physiol. Chem. 299, 74-84.
Klenk, E., and Eberhagen, D. (1957). Über das Vorkommen der Δ5·8·1 1 1 4 17- η - Eikosapentaensäure im Dorschlebertran und deren Isolierung. Z. physiol. Chem.
307, 42-48.
Klenk, E., and Tomuschat, H. J. (1957). Über die Dokosapolyensäuren der Glycerinphosphatide aus Rinderleber. Z. physiol. Chem. 308, 165-178.
Lovern, J. A. (1942). The composition of the depot fat of aquatic animals.
Rept. Food Invest. Board (London) Spec. Rept. No. 51.
Lovern, J. A. (1953). The lipids of fish. 1. Content and condition of lipids in the flesh of the haddock (Gadus aeglefinus). Biochem. J. 54, 126-128.
Lovern, J. Α., and Olley, J. (1953). The lipids of fish. 2. The acetone-soluble lipids of the flesh of the haddock. Biochem. J. 54, 128-137.
Matic, M. (1958). South African pilchard oil. 7. The isolation and structure of an octadecatetraenoic acid from South African pilchard oil. Biochem. J. 68, 692-695.
274 OLAV NOTEVARP
Mitchell, J. H., Kraybill, H. R., and Zscheile, P. F. (1943). Quantitative spectral analysis of fats. Ind. Eng. Chem. Anal. Ed. 15, 1-3.
Notevarp, O., and Cyvin, B. N. (1959a). Umettede fettsyrer i blod fra friske personer og fra pasienter med atherosclerose. Svensk Kern. Tidskr. 74, 409-410.
Notevarp, O., and Cyvin, Β. N. (1959b). Umettede fettsyrer i melk. Svensk Kern.
Tidskr. 74, 411-413.
Silk, Μ. H., and Hahn, Η. Η. (1954). South African pilchard oil. 4. The isola
tion and structure of a hexadecatetraenoic acid from South African pilchard oil. Biochem. J. 57, 582-587.
Sutton, D. A. (1958). South African pilchard oil. In "Essential Fatty Acids: 4th Conference on Biochemical Problems of Lipids" (Η. M. Sinclair, ed.), pp. 53- 56. Butterworths, London.
Vandenheuvel, F. Α., and Jangaard, P. M. (1957). Methyl esters—from marine oils. Can. Chem. Processing 41(3), 40-46.
Whitcutt, J. M. (1957). South African pilchard oil. 6. The isolation and structure of a docosahexaenoic acid from South African pilchard oil. Biochem. J. 67, 60-64.
Whitcutt, J. M., and Sutton, D. A. (1956). South African pilchard oil. 5. The isolation and structure of an eicosa-pentaenoic acid from South African pilchard oil. Biochem. J. 63, 469-475.