The Spoilage of Fresh-Water Fish
FRITZ BRAMSTEDT A N D MARGARETHE AUERBACH
Universitäts-Krankenhaus Eppendorf, Physiologisch-Chemisches Institut, H a m b u r g , Germany
I. Introduction 3 6 1
II. Amino Acid Changes 7 6 1
III. Breakdown Products Indicative of Spoilage 622
IV. Enzymatic Activities 624
V. Rigor Mortis 6 6 2
VI. The Role of Bacteria 9 6 2
VII. Fish Silage 9 6 2
VIII. Other Substances 0 6 3
IX. Oil Oxidation 631 X. Immunity Reactions 632 XI. Concluding Remarks 633
References ^34
I. Introduction
Our present knowledge of the spoilage processes in fresh-water fish is poor compared with that of sea fish. There are, however, many similarities between the spoilage patterns of these two categories of fish.
In recent years it has been shown that not only bacteria and their metabolic products are responsible for spoilage, but that the enzymes of the fish muscle and the intestines also are involved in the spoilage.
The muscle enzymes are particularly active in the initial phases. As early as the first day after capture, biochemical changes take place which can readily be detected by variations in the amount of individual amino acids, established through paper chromatography or microbiological assays (Siebert, 1958).
In storing whole fish, as is commonly done with fresh-water fish, in
testinal enzymes may invade the muscle tissue, thus causing spoilage.
Most essential, however, is the finding that the bacterial enzymes may exert a certain influence. But not only bacteria are responsible for the spoilage of fresh-water fish. Intrinsic enzymes may participate too. The presence of certain fish muscle enzymes appears to be a prerequisite for an optimal growth of bacteria which cause spoilage.
613
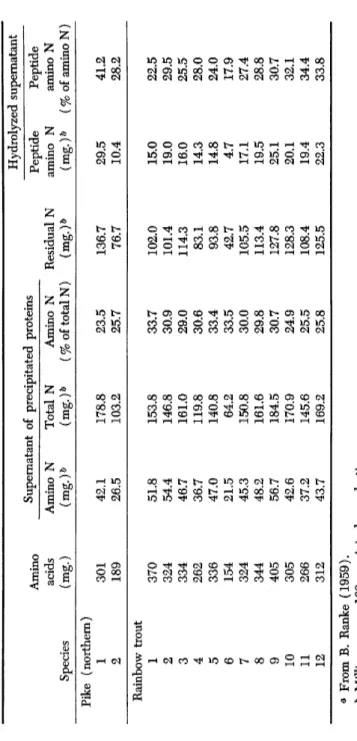
TABLE I NITROGEN COMPOSITION OF MAJOR FRESH-WATER FISH^ Hydrolyzed supernatant Amino Supernatant of precipitated proteins Peptide amino Ν Peptide amino Ν acids Amino Ν Total Ν Amino Ν Residual Ν Peptide amino Ν Peptide amino Ν Species (mg.) (mg.)*» (mg.)» (% of total N) (mg.)& (mg.)& (% of amino Ν) Pope 476 66.6 160.0 41.6 93.4 6.0 8.3 Lake perch 1 521 73.0 189.5 38.5 116.5 15.0 17.1 2 437 61.2 139.2 44.0 78.0 4.8 7.3 Pike perch 741 103.8 221.2 44.9 117.4 18.2 14.9 Carp 1 386 54.1 160.5 33.7 106.4 20.2 27.2 2 331 46.3 147.5 31.4 101.2 16.3 26.0 3 310 43.4 150.1 28.9 106.7 17.7 28.5 Tench 397 55.6 204.4 27.2 148.8 24.8 30.9 Bream 1 389 54.4 151.3 36.0 96.9 14.1 20.6 2 430 60.2 136.6 44.1 76.4 7.6 11.2 Roach 1 485 67.9 179.2 37.9 111.3 15.6 18.7 2 496 69.5 182.8 38.0 113.3 19.4 21.8 3 529 74.0 192.7 38.4 118.7 16.9 18.6 <* From B. Ranke (1959). & Milligrams per 100 g. original muscle tissue.
TABLE I (continued) Species
Amino acids (mg.)
Supernatant of precipitated proteins Amino Ν Total Ν Amino Ν (mg.)*> (mg.)*> (%oftotalN) Residual Ν > (mg.)6
Hydrolyzed supernatant Peptide Peptide amino Ν amino Ν (mg.)ö (% of amino Ν) Pike (northern) 1 301 42.1 178.8 23.5 136.7 29.5 41.2 2 189 26.5 103.2 25.7 76.7 10.4 28.2 Rainbow trout 1 370 51.8 153.8 33.7 102.0 15.0 22.5 2 324 54.4 146.8 30.9 101.4 19.0 29.5 3 334 46.7 161.0 29.0 114.3 16.0 25.5 4 262 36.7 119.8 30.6 83.1 14.3 28.0 5 336 47.0 140.8 33.4 93.8 14.8 24.0 6 154 21.5 64.2 33.5 42.7 4.7 17.9 7 324 45.3 150.8 30.0 105.5 17.1 27.4 8 344 48.2 161.6 29.8 113.4 19.5 28.8 9 405 56.7 184.5 30.7 127.8 25.1 30.7 10 305 42.6 170.9 24.9 128.3 20.1 32.1 11 266 37.2 145.6 25.5 108.4 19.4 34.4 12 312 43.7 169.2 25.8 125.5 22.3 33.8 » From B. Ranke (1959). δ Milligrams per 100 g. original muscle tissue.
TABLE II AMINO ACID CONTENT IN FRESH-WATER FISH0 Aspartic Species Cysteine Lysine Arginine Histidine ß-Alanine acid Glycine Proline Rainbow trout
+ + + ++ + + + +
Brown trout+ + + ++ + + + +
Northern pike+ + + + + + +++ + + + ++ +
Common carp+ ++ + + + ++ +
Tench+ + + ++ + + + ++ + +
Lake bream+ + ++ + + + + ++ + +
Roach+ + + ++ + + + + ++ + +
Pope++ + + + ++ + + + +++ + ++ +
Lake perch++ + + + ++ + + + +++ + +
Pike perch+++ + + + + + + + + +
<* From B. Ranke (1959).II. Amino Acid Changes
Each species has an "amino acid pool" in the intracellular system of the muscles which is specific and does not change fundamentally, even under varying environmental conditions (Florkin, 1949, 1955). This pool differs basically from a corresponding pool in the liver which is used in the synthesis of protein. In spite of this specificity of the amino acid pool in the muscle tissue, minor changes do take place in their relative con
centration under the influence of varying environmental conditions, par
ticularly in proline and glutamic acid.
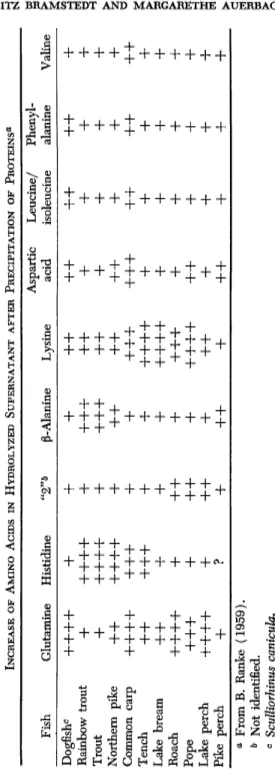
As to differences between species, the percids, such as the pope, lake perch, and pike perch, show a higher content of free cysteine compared to other species of this same family. One fraction, designated "gbF"—
later identified as carnosine—was, on the whole, not present among the percids. Nor did cyprinids, such as common carp, tench, bream, and roach, possess this fraction. They show a "normal" content of cysteine, lysine, and arginine, but lesser amounts of histidine and glycine (Tables I and I I ) .
After precipitating the proteins by a final alcohol concentration of 85%, the supernatant also contained, besides free amino acids, peptides and amines. After this supernatant was hydrolyzed with hydrochloric acid, an increase was observed in some amino acids, especially marked in common carp. Some fresh-water species, such as trout and smelt (from the Elbe River), carried peptides with a high amount of histidine. Tench, bream, and pope showed a considerable rise in lysine, after hydrolysis of the supernatant.
Under these same conditions ß-alanine increased in the supernatant from trout. Aspartic acid, leucine, isoleucine, phenylalanine, and valine rose only slightly (Tables III and I V ) (Bramstedt and Wurzbacher, 1960).
TABLE I I I
PEPTDDE-BOUND AMINO ACIDS IN DIFFERENT TISSUES OF THE RAINBOW TROUT0
Amino acid Muscle Skin Spleen Liver
Glutamic acid
+ ++++ ++++ ++++
Aspartic acid Histidine
+ ++ +
++++ ++ + +
Lysine
++ ++ ++ ++
Proline
( + )* ++ ++ ++
Valine
+ + ++ +++
* From B. Ranke (1959).
6 Traces only.
TABLE IV INCREASE OF AMINO ACIDS IN HYDROLYZED SUPERNATANT AFTER PRECIPITATION OF PROTEINS0 Aspartic Leucine/ Phenyl Fish Glutamine Histidine ß-Alanine Lysine acid isoleucine alanine Valine Dogfish0
+++ + + + + + + + + + + + + +
Rainbow trout+ +++ + + ++ + + + + + +
Trout+ +++ + + ++ + + + + + + +
Northern pike+ + +++ + + + + + + + + + + +
Common carp+++ + ++ + + ++ + ++ + + + + + + +
Tench+ + ++ + + + +++ + + + + +
Lake bream+ + + + + +++ + + + + +
Roach+++ + + + + + ++ + + + + +
Pope++ + + + + +++ + + + + + +
Lake perch+++ + + + + + + + + + + +
Pike perch+
?+ + + + + + + β From B. Ranke (1959). & Not identified. c Scylliorhinus canicula.
"2
The content of free amino acids varies not only from species to species, but also from one specimen to another. This depends not only upon environmental conditions ( B . Ranke, 1959), but also on the size of individual specimens.
Table I shows the exceptionally high amino acid content of pike perch as compared with other fresh-water species. The amount of amino nitro
gen in relation to total nitrogen is also high. As is to be expected from the figures for free amino acids in pike perch, no less than 44.9% of the total nitrogen consists of amino nitrogen (Table I ) . The lowest values were found in muscles of northern pike with 23.5 and 25.7%. It is ob
vious that the typical taste of different fish species depends to some extent upon their content of free amino acids. On the other hand, a higher amount of amino acids almost inevitably leads to more rapid spoilage.
This was demonstrated by B . Ranke (1959) in comparative investigations on fishes, mollusks, and crabs. When the fish are stored in a frozen con
dition, the breakdown of nitrogenous compounds (see Table V ) is greatly retarded ( B . Ranke, 1959).
Special investigations were made on Salmo gairdneri (trout) (Ranke et al, 1957) and on the influence of storage and spoilage on the nitrogen- containing substances in the alcoholic supernatants. The "gbF" fraction was only found in muscles, not in skin, liver, and spleen. The long-chained amino acids, such as leucine, isoleucine, phenylalanine, valine, methi
onine, and tyrosine, are more concentrated in skin, liver, and spleen than in muscle. Histidine is higher in muscles than in skin. In contrast to other tissues, skin contains hydroxyproline and α-, β-, and γ-aminobutyric acid.
Spleen has more aspartic acid than all other tissues (Table I I I ) .
Skin, liver, and spleen contain more peptide-bound glutamic acid than the muscles do. Peptide-bound proline and lysine were also found in the supernatant of liver, skin, and spleen, and peptide-bound aspartic acid in the liver and skin. Both valine and histidine were found in pep
tide-bound form, the former in the liver and spleen, the latter in the liver and skin (Tables IV, V I ) .
Elma Ranke and F . Bramstedt (1955) investigated the protein and nonprotein fractions of some fresh-water species (lake perch, bream, and common carp) by means of paper electrophoresis and studied the changes during spoilage. Three different fractions were obtained after staining with Amido Schwarz 10 Β . Only in relatively few cases was a fourth frac
tion seen. Fraction C was more strongly stained than the corresponding fraction of sea-water fish (codfish). Storage for 12 or 14 days at 4°C. did not result in the occurrence of additional fractions stainable with Amido
TABLE V EFFECT OF FREEZING*1 ON THE NITROGEN-CONTAINING FRACTIONS OF TROUT MUSCLE (IOOG. FRESH TISSUE)0 Amino Kjeldahl nitrogen Residual Amino Nitrogen nitrogen (%of nitrogen Free amino Condition (mg./100 g.) (mg./100 g.) Kjeldahl Ν) (mg./100g.) acids (%) Immediately after death 52.4 139.2 38.2 86.7 0.393 After eviscerating 52.6 149.6 35.2 97.0 0.379 After 12 hr. at 40°F. (5°C.) 41.7 148.6 28.1 106.9 0.298 After 2 months frozen storage 46.7 217.1 21.5 170.5 0.308 After 4 months frozen storage 53.4 232.7 23.7 178.2 0.389 After 10 months frozen storage 48.6 247.1 19.6 198.5 0.347 After 2 years at —25° C. 46.1 161.2 28.7 117.3 0.329 (whole fish) a Temperature of —23° and —24°C. » From B. Ranke (1959).
Schwarz 10 Β. The electrophoretic separation was not so optimal as com
pared to those from fresh-caught fish. The general pattern coincided with that for various gadids.
Staining with ninhydrin after paper electrophoresis of the supernatant resulted in a basic pattern with ten different fractions, with both sea- and fresh-water fish. The intensity and color of the fractions after staining were different. During spoilage, certain changes occur in the fractiona
tion pattern, both in color after ninhydrin staining and in the position on the paper strip. Some new fractions appear, but the fractions ani and ayi can be labeled as typical spoilage fractions.
TABLE V I
QUANTITATIVE ANALYSES OF DIFFERENT TISSUES OF THE RAINBOW TROUT (IOOG. FRESH W E I G H T )0
Component in
the supernatant Muscles Skin Liver Spleen
Amino nitrogen (mg.) 44.4 23.3 62.4 61.5
Total nitrogen (mg.) 142.6 61.1 119.3 107.2 Amino nitrogen (% of
total nitrogen) 31.1 38.1 52.3 57.4
Residual nitrogen (mg.) 98.2 37.8 56.9 45.7
Amino acids (mg.) 317 166 446 439
Peptide amino nitrogen (% of
hydrolysed supernatant) 26.6 25.1 23.8 18.6
» From Ranke et al. (1957).
As already mentioned, the basic patterns of sea- and fresh-water fish were almost identical if newly caught fish were compared. A difference was only found in the fraction Fgr, which had a pink color in fresh-water and a gray-bluish color in sea-water fish. Later on, after isolation of this fraction, it could be identified as a mixture of the following free amino acids: α-alanine, ß-alanine, glycine, and histidine. The differences in color were found to be due to different amounts of ß-alanine. The gadids had an Fgr fraction with a bluish color, the fresh-water, and some sea-water, fish snowed a reddish tinge.
Spoilage causes a change of the bluish Fgr fraction into red. The ratio of β- to α-alanine was 10:1. This demonstrates that spoilage induces a decline in the content of free ß-alanine, as well as a switch in color to red.
The first deviation in the basic electrophoretic pattern of ninhydrin- positive fractions was encountered in bream at the sixth day of storage at 4 ° C , in perch on the fifth or sixth day under the same conditions. The
new fraction which appears can be compared with ayi or anx in sea-water fish. In some species, both fractions an and ay were found; after 20 days of storage neither anx nor ayx was detectable. The R2 fraction was more intense. At 40 days spoilage, a new fraction appeared ( Β χ ) . Fraction hr showed characteristic changes during spoilage. After isolation from fishes stored for 1, 3, 16, 23? and 30 days, all samples contained lysine in a high amount, which could be proved not only by paper chromatography, but also by microbiological tests. In fishes stored for 1 to 3 days, glycine and arginine were also found in small amounts. After 16, 23, and 30 days of storage, glycine alone was present, besides lysine. Controls run with a mixture of lysine, glycine, and arginine were in the same position on the paperstrip as fraction hr. Fraction R i , in fish stored 16 and 30 days, was isolated and later identified as aminobutyric acid, together with small amounts of lysine. The ani and an2 fractions were identical with glutamic and aspartic acid. They were isolated from fishes stored for 16, 23, and 30 days, where aspartic acid caused a more bluish, and glutamic acid a more reddish, color.
Present knowledge of the regular proteins of fresh-water fishes and their amino acid composition is presented in Vol. II, Chapter 2. From this it is evident that a high content of methionine seems to be typical for protein of several fresh-water fish. In addition, they are characterized by a substantial amount of other organic sulfur compounds (Schormüller et al., 1950). Besides methionine, the proportion of cystine also is high according to analyses on Indian fresh-water fishes (Airan et al., 1950;
Airan, 1951). Although no direct comparisons are made, this is the gen
eral impression also from an analysis on eleven important Indonesian food fishes from fresh waters (Dupont, 1958).
III. Breakdown Products Indicative of Spoilage
The findings discussed above show that spoilage is related to distinct amino acids, especially glutamic acid, aspartic acid, lysine, histidine, and arginine. Other amino acids also show certain changes ( E . Ranke et al., 1957; Β. Ranke, 1959). The significance of these results may be seen in the fact that these amino acids may be precursors to certain substances creating bad odor and taste (Bramstedt, 1957). Lysine can be converted to cadaverine and putrescine which have been detected in spoiled fish, and arginine to δ-aminovaleric acid or δ-aminovaleric aldehyde. Lysine can further be transformed to piperidine and N-aminopiperidine as well as to pyridine, which substances have all been identified in fresh-water fish by Japanese scientists (Obata et al., 1950, 1951b, 1952). These au-
thors encountered some other substances responsible for bad odor in spoiling fish. Thus methylmercaptopropanol was isolated as well as crotonaldehyde, formaldehyde, furfurol (which forms complexes with cysteine through condensation), isovaleraldehyde, methyl mercaptan, iso- butyraldehyde, and condensation products of isobutyraldehyde and ethyl mercaptan derivatives. Aldehyde derivatives especially were detected as bad-smelling substances which occur during fish spoilage. Amano (1950) could prove that isovaleric acid and their derivates were formed during spoilage. Thus, the amino acids mentioned here may undoubtedly be involved in forming undesirable substances responsible for bad odor.
Furthermore, Yudaev (1950) found free histidine in carp and perch in amounts varying from 127 to 241 mg.%. This suggests that a metab
olism of histidine is probable, additional amounts becoming available through the splitting of peptides or proteins, containing histidine. Shimizu and Hibiki (1955) likewise found an increase in free histidine during storage, which confirms the suggestion of a rapid histidine metabolism.
Cadaverine has been reported from spoiling fish, possibly as a degrada
tion product of lysine (Figs. 1 and 2 ) (Saito and Sameshima, 1956).
Breakdown products such as formic acid, acetic acid, butyric acid, and isovaleric acid have been identified in spoiling fresh-water fish muscle by Suzuki (1953).
In the first 5 days of storage, i.e., before the main invasion of bacteria, a decrease in the content of free leucine, isoleucine, and valine was established. This indicates that compounds have been formed which no longer have the character of amino acids, as they cannot replace amino acids in microbiological tests. Amano (1950) identified isovaleric acid, which can be formed from leucine, in spoiling fish. Isoleucine can be transformed to α-keto-ß-methyl-n-valeric acid and α-methylbutyric acid, while valine may form α-ketovaleric acid and isobutyric acid.
Paladino (1945) found in La Plata fresh-water fish that volatile N-containing bases were suitable indicators of the degree of spoilage.
This entire problem is discussed in detail in Vol. I l l , Chapter 2. One hundred mg. of volatile basic nitrogen per 100 g. samples of fish muscle was the critical point. When exceeding this value, the fish in question were not acceptable as human food. E . Ranke (1960) and B . Ranke
(1959), however, demonstrated that the initial content of free amino nitrogen could not be linked to different stages of spoilage. Those authors also discovered that an initial drop in the amount of free amino acids was, in effect, the first sign of spoilage. Free lysine in fraction hr inves
tigated by E . Ranke and Bramstedt (1955) was encountered not only in
certain seawater fishes, but also in bream and lake perch. In addition, other amino acids, such as cysteine and arginine, show considerable changes indicating a rapid metabolism also in the initial stages of spoil
age.
R ι CH2 Η—C—NIL
COOH I
R I CH2 CH2-
+
co2
R I CH2
Η + NH,
R I CH2 COOH
Amino acid Amine Aldehyde Fatty acid FIG. 1. Pathways of bacterial attacks on amino acids.
COOH Η—C—NHI 2
CHI 2 I 2 CH2 CHI 2 CHo—NH, Lysine
CH2—NH2 CH2 CH2 ~
I CH2
XH
CH2 NH2 CH2
CHI 2 CH2 CH2—NH2 Cadaverine
A
H2C CH
H2C CH2
CT
COOH I Η—C—NHo
CH2 CH2 CH2—NHa Ornithine
CH2—NH2 CH2 CH2 CH2—NH2 Putrescine
Intermediary Piperidine product
FIG. 2. Possible pathways for the formation of malodorous compounds from lysine.
IV. Enzymatic Activities
Siebert (1958) showed that fish muscle tissue exhibits a much higher catheptic activity than mammalian tissues. No proteinase with an op
timum at pH 7.0 was encountered, however. Under optimal conditions, proteinases available in the fish tissue are able to destroy the entire protein of muscle tissue in less than 24 hours. These catheptic enzymes
differ from those of mammalian tissues especially with regard to their optimal temperature of action. Fish enzymes are retarded in their activity as soon as temperatures drop as low as 68 °F. ( 2 0 ° C ) , while the cathepsin of mammalian tissues have their temperature optimum at much higher levels, as, e.g., the stomach cathepsin, gastricsin (Richmond et al., 1958) at about 140°-158°F. ( 6 0 - 7 0 ° C ) . Furthermore, Togasawa and Katsu- mata (1955-56) described a dipeptidase belonging to the group of glycylglycinpeptidases, encountered in both the pyloric caecum and the muscles of fish. This enzyme was inhibited and stimulated by cobalt and manganese concentrations shown in the tabulation.
ΙΟ"2 mole 10"3 mole 10"4 mole
Mn++
+++ +
Co++ —
+
Siebert (1958) found no enzymes capable of deaminating glutamic or aspartic acid in fish muscle tissue. This suggests that the drop estab
lished in these amino acids by Bramstedt (1957) and E . Ranke (1960) may be due to bacterial action.
During prolonged storage of fresh-water fish, catalase from the eryth
rocytes enters the blood plasma and later diffuses into the tissues (Privol- nev, 1950). This enzyme activity goes parallel with the cell destruction.
In fish flesh this catalase activity is, however, less than in fish liver during later stages of spoilage.
The catalase activity of the gills also runs parallel to the degree of spoilage (Tomiyama et al., 1950). This was established not only on sea- water but also on fresh-water fish, especially the common carp. There is, in addition, a close relationship between the amount of volatile bases formed and catalase activity. Up to an amount of 30 mg.% volatile basic nitrogen in the flesh, the catalase activity remained almost constant.
These results are naturally noteworthy, as in organoleptic tests the gills are important indicators of spoilage.
For a biochemical characterization of spoilage, the paper of Lindahl and Svärd (1957) on the discovery of an enzyme capable of deaminating guanine and located in the skin is of particular interest. It is generally recognized that the fish skin shows signs of spoilage earlier than the muscle. This was confirmed in a study on trout (Ranke et al., 1957). The skin is rich in guanine. The deaminating action of this enzyme results in the formation of significant quantities of ammonia. There is also an in
crease in the residual nitrogen level.
This particular enzyme is only slightly affected by low temperatures.
Quite substantial activity was observed in frozen trout at 32°F. ( 0 ° C ) . This could possibly explain the constantly established small increase in the nonprotein nitrogen which is not derived from amino acids (Table V I ) .
The more rapid spoilage taking place in the skin produces many malodorous substances. Many pyridine and pyridine derivatives were identified and encountered specifically in the skin (Obata et al., 1952;
Amano and Bito (1950). Even when fish were stored frozen at —13 °F.
(—25 ° C ) , these enzymically induced changes took place, although at a very slow rate (Ranke et al., 1957).
In carp muscles the dehydrogenase activity was almost constant in the range of 50-86°F. (10-30°C.) (Novikov, 1954).
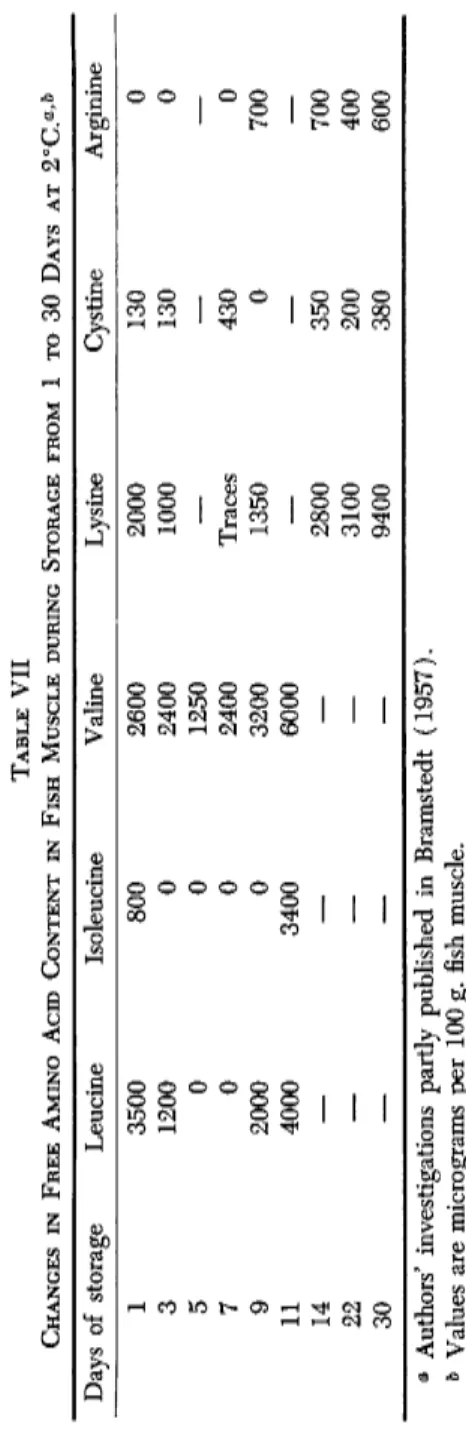
Antibiotics such as Chlortetracycline ( C T C ) , Oxytetracycline ( O T C ) , polycycline, and nisin exert an influence on enzyme systems involved in spoilage (Castell and Greenough, 1957). The enzymes studied belonged to gram-negative bacteria isolated from fish. Washed cell suspensions were used as enzyme source. Chlortetracycline and OTC did not affect the reduction of cysteine by an enzyme system belonging to Escherichia coli. None of the antibiotics was able to inhibit the formation of H2S from cysteine through these bacterial enzymes. The changes taking place in the ratio of free cystine to cysteine (Table V I I ) might be explained by these findings. This might obviously be a source of malodorous sub
stances.
One of the most characteristic biochemical changes in fish spoilage is the formation of reducing substances. Numerous investigations have been made in recent years on the parallelism between the formation of these compounds and the degree of spoilage, as measured bacteriologically, but so far without being able to formulate a reliable test in this respect. For a detailed discussion on freshness tests see Chapter 2 by Farber, in Volume III.
Moorjani et al. (1957) investigated the applicability of the tetrazolium red-test (TPTZ-test) and found it particularly useful for the estimation of spoilage in fresh-water fish. The test is, however, most reliable when the bacterial loads are high. These reducing substances are primarily interrelated with the action of bacterial enzymes.
V. Rigor Mortis
Another important factor in fish spoilage is rigor mortis. The onset and duration of this phenomenon depend upon a number of factors such as the glycogen ATP relationships. The pH, reaching acidic values after
TABLE VII CHANGES IN FREE AMINO ACID CONTENT IN FISH MUSCLE DURING STORAGE FROM 1 το 30 DAYS AT 2°C.a >h Days of storag ;e Leucine Isoleucine Valine Lysine Cystine Arginine 1 3500 800 2600 2000 130 0 3 1200 0 2400 1000 130 0 5 0 0 1250 — — — 7 0 0 2400 Traces 430 0 9 2000 0 3200 1350 0 700 11 4000 3400 6000 — — — 14 — — — 2800 350 700 22 — — — 3100 200 400 30 — — — 9400 380 600 a Authors' investigations partly published in Bramstedt (1957). & Values are micrograms per 100 g ;. fish muscle.
death, enhances the hydrolyzing activity of some enzymes and the split
ting of proteins.
Besides glycogen, a number of carbohydrate breakdown products, such as D-ribose, glucose, and hexose phosphates, are encountered in fish muscle (Tarr, 1954). Cordier and Cordier (1957) found 1.0-1.5 g. of glycogen in 100 g. of fresh muscle tissue. This would indicate a higher amount than that generally found in mammalian flesh. Anorexia caused a drop to about one-third of normal values. Earlier, the prevailing opinion was that fish muscles contained practically no glycogen.
Tarr (1954) compared the occurrence and duration of rigor mortis in marine and fresh-water fish (mackerel, red snapper, and carp). The rate of glycolysis decreased in the order: mackerel, red snapper, and carp.
The changes in the ATP content and free SH groups showed very slight differences among them. Fishes killed just after agony had a lower content of glycogen, ATP, and free SH groups, and a lower pH than the control groups.
Chapter 12 in this volume discusses the intricate relationship of rigor mortis. A few specific points relating to fresh-water fish and their spoiling will be mentioned here. The disappearance of ATP runs parallel to rigor mortis, and in carp this compound completely vanishes within 6 hours.
ATPase engineers this breakdown and, as is the case with mammals, it is tied in with the myosin fraction. The amount of free SH groups in carp muscle diminishes continuously right from the time of death, but in 15-20 hours the trend is reversed due to the process of putrefaction (Partmann, 1952-53).
In contrast to these findings are the results of Golovkin and Pershina (1957) who established an increasing amount of ATP in the flesh of fish stored for up to 10 days at 32°F. ( 0 ° C ) , 37.5°F. ( 3 ° C ) , and 43.3°F.
( 6 ° C ) . The amount of ATP was measured by analyzing readily hy- drolyzable phosphorus. This might explain the discrepancy, since com
pounds other than ATP also contain easily hydrolyzable phosphorus.
The glycogen content in the flesh of fresh-water fish decreases rapidly if they are starved. Immediately after intake of food, the glycogen con
tent rises rapidly to normal values. Any movements of the fish, whether normal or due to struggling in connection with capture, cause an ap
preciable drop in glycogen (Trautner, 1960).
The quality of fresh market fish depends upon the duration of rigor mortis and the time for its onset. The present knowledge of the bio
chemistry of rigor mortis is still scanty, so much further research is re
quired.
VI. The Role of Bacteria
Fish muscles are generally considered sterile as such in nature. But Maltschewsky and Partmann (1951) isolated a number of bacteria from the flesh of several fresh-water fishes such as lake bream, roach, tench, goldfish, river barbel, northern pike, and rainbow trout. Generally, how
ever, they did not belong to typical water bacteria. They could rather be classified as air, soil, plant, or waste bacteria. This made Maltschewsky
(1955) draw the conclusion that these bacteria were related to the feed of the respective fishes.
There is difficulty in distinguishing between the role played by bac
teria, on one hand, and intrinsic fish enzymes, on the other. Some evi
dence in this respect can be gained from Japanese studies on the part played by bacteria in fish autolyzates, this being nothing else than a controlled spoilage, sometimes with the addition of particular enzymes or bacterial strains.
In Soviet manufacturing processes the whole fish is autodigested at 126°F. (52°C.) for 7 to 9 hours. Free amino acids are then obtained.
Tomiyama and Kawazoe (1955-56) prepared a fish autolyzate under slightly acid conditions from whole fish. The final product was dehydrated into powder. It contained 4 0 % free amino acids, indicating that this amount had increased tenfold as compared to fresh-caught fish.
All these autolyzates constitute excellent substrates for bacterial growth. Soviet and Japanese investigations indicate that they are par
ticularly useful in the culturing of pathogenic bacteria.
Naturally, bacteria, besides "growing" in the fish tissue, might exert an indirect spoiling effect by the excretion of enzymes, active in various ways. The spoilage flora may obviously originate in the waters from which fish come or be due to contamination after capture in ships or ashore, particularly in handling and processing plants. Kreuzer (1954) maintains that with regard to fresh-water fish there exists a "special spoilage microflora." But the microorganisms active in this respect origi
nate largely from contamination subsequent to capture.
VII. Fish Silage
The making of fish silage, a kind of controlled fish spoilage, is based on the fermentative appearance of lactic acid due to acidifying bacteria (Kreuzer, 1954). Carbohydrates are converted into this organic acid.
The optimal temperature for the sugar degradation through Lacto
bacillus plantarum is 77°-86°F. (25-30 ° C ) . A slight increase takes place in the nitrogen content of the silage at 70 °F. ( 2 0 ° C ) . The free amino
acids increase too. The volatile bases were at the level of 94-124 mg./100 g. of silage.
Fish homogenates incubated with Bacterium vulgaris from milk at 113°F. (45°C.) and at pH 3.0 were later (after 96 hours) incubated with Aspergillus niger at 113°F. ( 4 5 ° C ) . A liquid product was obtained. This process is claimed to give much higher amino nitrogen values than a simple autolyzation (Yamazaki and Yamauchi, 1956).
In the early stages of fish spoilage, bacteria develop slowly in fish muscle but more rapidly in skin, gills, and other external parts (Horie and Sekine, 1954). A certain degree of autolytic breakdown—rendering free amino acids—presumably is a prerequisite to a more forceful bac
terial attack on fish meat (Kovaleva, 1956). Betabacterium buchneri uses glutamic acid as an energy source (V. Meyer, 1956), and is not capable of utilizing carbohydrates for this purpose. In spite of the relatively high content (from 3 % to 7 % ) of free amino acids in fish muscles ( B . Ranke, 1959), their amount does not suffice for more than the initial growth.
In marine fishes the amount of the free amino acids drops after the bacteria have invaded the tissue and initiated spoilage. As Siebert (1958) found no evidence for the presence of decarboxylating enzymes in the fish muscle, it might be possible that the invading bacteria are using amino acids in the fish muscle for their initial growth. In later stages sufficient amounts of amino acids will be released through autolysis. This in turn allows a more rapid bacterial growth. Thus, there should be an inhibition of the enzymes that would, at least in theory, control the au
tolytic process. This phenomenon might serve two purposes: ( 1 ) retard
ing the autolysis and subsequent changes in odor and taste, and ( 2 ) re
ducing the growth rate of spoiling bacteria, needing more free amino acids than are successively becoming available in the fish muscle.
Spoilage appears to be the result of a combined effect of autolysis and microbial action. Autolysis is effected by the enzymes of the tissue, and thus paves the way for microbial development. Microorganisms con
tribute to spoilage either directly by their growth or indirectly by ex
creting enzymes which exert a disintegrating action on the host tissue.
It does not seem justifiable to speak of a "special spoilage microflora,"
since this depends entirely on conditions for bacterial growth created by previous autolytic processes.
VIII. Other Substances
Besides amino acids spoiling bacteria need carbohydrates, minerals, and vitamins for optimal growth. The amount of water soluble vitamins
in red fish muscle tissue is significantly higher than in white flesh (Mori et al., 1955-56). The niacin content was further found to be higher in big
ger and more active fish. The increased enzyme activity together with a greater amount of vitamins in red muscles presumably explains the faster spoilage taking place there as compared to white muscles. The higher niacin content could be important for the synthesis of nicotinamide, con
stituting part of the enzyme systems of the respiratory chain, which are found to be involved in spoiling processes. Yanase (1956-57) estimated the vitamin B6 content of fresh-water species. The muscles contained con
siderable amounts, which were influenced only to a minor degree by age, season, and location. The highest concentrations were found in those species having a large proportion of red muscles, just as more active species, such as migrating fishes, contained higher amounts. These find
ings correspond with those for niacin and indicate that muscles normally exhibiting higher activity and an increased metabolism contain more water-soluble vitamins. All tissues with a lively intermediary metabolism, such as liver, spleen, and kidney, readily autolyze after death. This ex
plains why all tissues in a fish with a high metabolic rate are more sus
ceptible to spoilage than those with a correspondingly low rate.
Those microorganisms which cannot synthesize vitamins themselves depend for their growth on a food substrate which contains sufficient amounts of all accessory substances. Several fish bacteria belong to this category (Ingalls et al., 1950). These research workers also found high contents of all water-soluble vitamins in the fresh-water species inves
tigated, especially trout and carp. As a whole, fresh-water fishes do not differ in this respect from marine species and are good sources of both fat-soluble and water-soluble vitamins. For further information in this respect, see Chapter 13 of this volume, which gives a thorough discussion on the occurrence of vitamins in fish.
IX. Oil Oxidation
Our present knowledge of the biochemical processes of fat break
down in fresh-water fish is extremely scanty. Most investigations refer to marine species. This partly is explained by the fact that fresh-water fish are not held to the same extent as sea-water fish for any length of time.
Mostly they are consumed shortly after capture. Only trout are stored frozen. Some species, like carp and eel, may be kept smoked for some time.
Fresh-water fish fed a high-fat diet soon become unfit for human food owing to the ready development of rancidity (Mann, 1960). This ran-
cidity is most pronounced directly under the skin, where most fat is deposited. The taste of rainbow trout fed such a high-fat diet was, never
theless, superior to that of trout fed a low-fat diet, but the risk of an early off-flavor due to rancidity was far more evident in the high-fat fish.
Particularly for trout, which are stored frozen for some length of time, the use of diets with a restricted fat dosis in the feed is essential.
Lassen et al. (1951) found a rise in free fatty acids in fish during spoilage, from 1.1% after 24 hours of storage to 2.5, 7.8, or 8.1% after 120 hours of being kept at room temperature. After 6 and 24 months of storage (—13°F., —25°C.) the appearance of considerable amounts of free fatty acids and certain bad odors and off-flavors presumably derived from the degradation of fats, greatly reduces the consumer appeal of trout (Ranke et al., 1957). Frequently, these changes are so objectionable that the fish have to be discarded for human use, although the numerical changes in amounts of free amino acids and various fatty oxidation products were insignificant.
Hematin compounds are crucial in many different fish species and play a vital role in fish oil oxidation (Brown et al., 1956). These oxida
tion problems and the catalyzing action of hematin compounds are dis
cussed further in Chapter 7 of this volume.
Kelly et al. (1958) investigated the effect of the diet on the fatty acid composition of several species of fresh-water fish such as yellow bull
head, bluegill, rockbass, smallmouth buffalo, and rainbow trout. No sig
nificant changes occurred in the body fat as to fatty acids of 2-6 double bonds in the low-fat and cottonseed-oil diet. Other oils, such as that of menhaden, influenced the composition of the body oils. These results differ basically from those the author obtained on marine fish, where the body fat lost most of its naturally poly-unsaturated acids, when the fish was placed on a low-fat regimen and regained it on a menhaden-oil diet.
It is, nevertheless, evident that both marine and fresh-water fish are able to synthesize poly-unsaturated fatty acids, though not necessarily essen
tial fatty acids from nonfatty precursors. Differences between the fatty acid composition of the body fat of marine and fresh-water fish result largely from differences in their dietary fatty acids (see Chapter 7 of this volume).
X. Immunity Reactions
In studies of fish diseases the functional role of antibodies in fresh
water fish was revealed. The importance of this defense mechanism in staving off pathogenic organisms is evident and reasonably well estab-
lished. Evidently this build-up of immunity may also check the growth of the normal flora (Bisset, 1948).
The formation of agglutinins is reported from carp by Pliszka (1939), who also reviews earlier such findings with other fresh-water fishes.
Cushing (1942) established that carp produced agglutinin more pro
ficiently at 82°F. (28°C.) than at 59°F. (15°C.) Such a favorable effect of high temperature had already been found by Nybelin (1935) in eels, entirely lacking this capability at 46-48°F. ( 8 - 9 ° C ) . Similar findings are reported by Bisset (1946) on goldfish. This built-in antibacterial mech
anism is far more efficient at higher temperatures (Mackie et al., 1930).
This interesting relationship provides the fish with greater resistance when the external threat through elevated temperatures in surrounding waters is apt to be most massive. This has the surprising effect that the degree of infection shows a steady decline throughout the warm season.
The bacterial load in the opercular cavity, on the gills, or in the guts is appreciably reduced in this period. Bisset (1948) could also establish considerable seasonal fluctuations in the normal bacterial flora of powan and perch from Scottish lakes. High temperatures encourage the develop
ment of immunity. These important findings undoubtedly have a bearing on the keeping properties of fresh-water fish and might explain why such fish, even from ponds with an extremely high bacterial load, do not show any particularly rapid breakdown through spoilage.
To what degree this acquired immunity explains the Soviet finding that extracts from various body organs of fresh-water fishes (8 species studied) exhibit antibacterial activity against both gram-positive and gram-negative bacteria is difficult to establish at this stage of research (Zotov, 1958a, b ) .
This important field merits special attention in future research. It is evident that fresh-water fish are more exposed than marine fish to massive bacterial attacks in their natural environment and with a marked seasonal variation. The considerable decline in the normal bacterial flora of the fish occurring during the warm season is an important phenom
enon.
XI. Concluding Remarks
The initial steps of spoilage in the widest sense of the word are not entirely undesirable. The optimal taste and the best quality will be reached only if the biochemical processes connected with rigor mortis are resolved. Any preservation prior to rigor mortis does not give satis-
factory results. The initial steps of spoilage are characterized by the appearance of substances partly essential to an optimal taste.
In the manufacturing of fish autolyzates these spoilage processes are controlled by means of pH and partly guided through the adding of certain plant and animal enzymes. This breakdown proceeds much fur
ther than is the case in any normal kind of fish preservation. In the future it might be possible to take cognizance of the effect on taste of varying feed diets in the raising of carp and trout and to utilize such findings.
A more thorough knowledge of the degradation products of amino acids, as well as the processes governing their appearance, seems most desirable. This could be important both to an optimal taste and to a correct handling of the fresh fish. The possibility of working out, through research, entirely new taste patterns emanating from fish can be visual
ized as an intriguing future objective.
REFERENCES
Airan, J. W. (1951). Studies in Kolhapur fresh-water fishes. / . Indian Chem. Soc.
2 8 ( 3 ) , 164-166.
Airan, J. W., Ghatge, N. D., and Kalyankar, G. D. (1950). Studies in Kolhapur fresh
water fishes. Part II. Water-extractable protein and mineral contents. Indian J.
Med. Research 3 8 , 259-262.
Amano, K. (1950). The green discoloration of frozen swordfish (Xiphias gladius).
I. Isolation of iso-valine acid-like substance from green colored flesh. (In Jap
anese with English summary.) Bull. Japan. Soc. Sei. Fisheries 1 5 , 469-474.
Amano, K., and Bito, M. (1950). Changes of free amino acids generated by decom
position of fish muscle. (In Japanese with English summary.) Bull. Japan. Soc.
Set. Fisheries 1 6 ( 1 2 ) , 10-16.
Bisset, Κ. Α. (1946). The effect of temperatures on non-specific infections of fish.
/. Pathol. Bacteriol. 5 8 , 251-258.
Bisset, Κ. Α. (1948). Natural antibodies in the blood serum of freshwater fish. / . Hyg. 4 6 , 267-268.
Bramstedt, F . (1956). In "Probleme der vollwertigen Ernährung in Haushalts- und Grossverpflegung," pp. 135-140. Umschau Verlag, Frankfurt-am-Main, Germany.
Bramstedt, F . (1957). Geschmacks- und Geruchsstoffe im Fischfleisch. Arch.
Fischer eiwiss. 8 , 94-103.
Bramstedt, F . , and Wurzbacher, I. (1960). Biochemische Untersuchungen zur Frage der Aufstellung von Güteklassen bei Seefischen. Fette, Seifen, Anstrich- mittel 62, 513-517.
Brown, W., Tappel, A. J . , and Stansby, Μ. E . (1956). Oxidative deterioration in fish and fishery products. No. 1. Com. Fisheries Rev. 1 8 ( 2 ) , 10-13.
Castell, C. H., and Greenough, M. F . (1957). The effect of certain antibiotics on the production of trimethylamine and hydrogen sulphide by bacterial enzymes.
Fisheries Research Board Can. 1 4 , 771-774.
Cordier, D., and Cordier, M. (1957). Phosphorolyse du glycogene du muscle strie chez les poissons marins. Compt. rend. soc. biol. 1 5 1 , 1909-1911.
Cushing, J. E . (1942). Effect of temperature on antibody production in fish. J.
Immunol. 45, 123-127.
Dupont, A. (1958). Amino acid content of Indonesian fresh-water fish. Biochem.
Z. 330, 174-176.
Florkin, M. (1949). "Biochemical Evolution," 157 pp. Academic Press, New York.
Florkin, M. (1955). "Mosbacher Kolloquien 1954." Springer, Berlin.
Golovkin, Ν. Α., and Pershina, L. I. (1957). On the role of adenosin triphosphoric acid in cold treatment and storage of fish. (In Russian.) Rijbnoe Khozy. 33(9), 87-90.
Horie, S., and Sekine, Y. (1954). Increase of volatile basic nitrogen and growth of bacteria in the muscle of fish at the early stage of spoilage. /. Tokyo Univ.
Fisheries 41, 69-74.
Ingalls, R. L., Klocke, J. F., Rafferty, J. P., Greensmith, R. E., Li Chang, M., Tack, P. I., and Ohlson, M. A. (1950). Nutritive value of fish from Michigan waters.
Michigan Agr. Expt. Sta., Tech. Bull. No. 219, 24 pp.
Kelly, Τ. B., Reiser, R., and Hood, D. (1958). The effect of diet on the fatty acid composition of several species of fresh water fish. /. Am. Oil. Chemists' Soc. 35, 503-505.
Kovaleva, V. J. (1956). Changes in the amino acid composition as a result of the growth of coliforms on media prepared from fish autolyzates. (In Russian.) Uchenye Zapiski Dagestan. Nauch.-Issledovatel. Inst. Proizvod. Pitateln'n. Sred, No. 2, 46-47.
Kreuzer, R. (1954). Untersuchungen zur Durchführung des Fischgärungssilage.
II. Der Ablauf bakterieller und chemischer Vorgänge bei der Silierung. Arch.
Fischereiwiss. 5, 47-58.
Lassen, S., Bacon, Ε. Κ., and Dunn, Η. J. (1951). Fish reduction process. Ind.
Eng. Chem. 43, 2082-2087.
Lindahl, P. E., and Svärd, P. O. (1957). Guanine metabolism in fishes. I. The occurrence of guanine-deaminating enzyme in fish skin. Acta Chem. Scand. 11, 846-853.
Mackie, T. J . , Arkwright, J. Α., Pryce-Tannant, J. E., Mottran, J. C , Johnston, W.
D., Menzies, W. J. M., and Martin, W. (1930). Interim report of the Furun- culosis Committee. His Majesty's Stationery Office, London, 65 pp.
Maltschewsky, N. (1955). Über die in der Muskulatur von lebenden Süsswasser- fischen vorkommenden Bakerienstämme. Arch. Fischereiwiss. 6, 84-93.
Maltschewsky, N., and Partmann, W. (1951). Zur Frage der Keimfreifeit der Muskulatur lebender Süsswasserfische. Arch. Mikrobiol. 16, 252-269.
Mann, H. (1960). Personal communication.
Meyer, V. (1956). Probleme des Verderbens von Fischkonserven in Dosen. II.
Aminosäuredecarboxylase durch Organismen der Beta-bacterium buchneri- Gruppe als Ursache bombierter Marinaden. Veröffentl. Inst. Meeresforsch.
Bremerhaven 4, 1-16.
Moorjani, Μ. N., and Iyengar, J. R. (1957). Tetrazolium reduction test in relation to species of fish, pH of the media, and incubation period. Food Sei. (Mysore) 6, 203-205.
Moorjani, Μ. N., Iyengar, J. R., Bhatia, D. S., and Subrahmanyan, V. (1957). Use of 2,3,5-triphenyl tetrazolium chloride (IPTZ) for assessing the quality of fish.
Food Sei. (Mysore) 6, 275-276.
Mori, Τ., Hashimoto, Υ., and Komata, Υ. (1955-56). B-vitamins content in the muscle of fish. (In Japanese with English summary). Bull. Japan. Soc. Sei.
Fisheries 21, 1233-1235.
Novikov, S. T. (1954). Dehydrogenating ability of muscle tissue of some fish and amphibia. (In .Russian.) Doklady Akad. Nauk S.S.S.R. 95, 131-133.
Nybelin, O. (1935). Medd. Anstalt Insjöfisket, Drottningholm No. 8, 1-62.
Obata, Y. (1950). Quoted from E. Ranke (1960). Bull. Japan. Soc. Set. Fisheries 15, 551-553.
Obata, Y. (1952-53). The smell and odor of marine products. J. Japan. Soc. Food Nutrition (Eiyo to Shokuryo) 5, 115-120.
Obata, Y., and Yamanishi, T. (1950). Chemical studies on the substance of fish smell. III. Investigations of the components of mucilaginous substances of salmon. Bull. Japan. Soc. Sei. Fisheries 15, 554-556.
Obata, Y., and Yamanishi, T. (1951a). Studies on the substance of fish smell.
IV. The components of mucilaginous substances of salmons caught at sea (add, that of eels). (In Japanese with English summary.) Bull. Japan. Soc. Sei. Fish
eries 16, 361-362.
Obata, Y., and Yamanishi, T. (1951b). Flavors of soy sauce. I. Flavors developing from hydrolyzed protein and amino acids. /. Agr. Chem. Soc. Japan 24, 226-229.
Obata, Y., and Yamanishi, T. (1951c). Flavors of soy sauce. III. Synthesis of un
saturated aldehydes and ketones. /. Agr. Chem. Soc. Japan 24, 479-481.
Obata, Y., and Yamanishi, T. (1951d). Flavors of soy sauce. IV. The condition of the development of soy-sauce flavor. (In Japanese.) /. Agr. Chem. Soc. Japan 25, 8-12.
Obata, Y., and Yamanishi, T. (1952). Chemical studies in the substance of fish smell. V. Aroma of cooked fish. Bull. Japan. Soc. Sei. Fisheries 17, 326-328.
Obata, Y., Yamanishi, T., and Ileida, M. (1950). Chemical studies on the sub
stance of fish smell. II. Pyridine group compounds as the substances concerned with fishy smell. Bull. Japan. Soc. Set. Fisheries 15, 551-553.
Obata, Y., Igarashi, H., and Matano, K. (1951). Studies on the flavor of seaweeds.
I. On the component of the flavor of some green algae. (In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 17, 60-62.
Obata, Y., Yatoro, and Yamanishi, T. (1952). Studies on method of estimating freshness of fish. I. Estimation of freshness by determining the catalase activity of the gill. Bull. Japan. Soc. Set. Fisheries 17, 10.
Paladino, J. A. I. (1945). El estado de preservacion del pescado. Rev. fac. cienc.
qutm. Univ. nacl. La Flata 18, 105-117.
Partmann, W. (1952-53). Der Anteil der Autolyse am Fischverderb. Arch. Fisch- ereiwiss. 4, 40-57.
Pliszka, F . (1939). Untersuchungen über die Agglutinine bei Karpfen-Vorl. Mitt.
Zentr. Bakteriol. Parasitenk. Abt. I Orig. 143, 726-733.
Privolnev, T. (1950). Catalase of fish. (In Russian.) Dokhdy Akad. Nauk S.S.S.R.
70, 461-463.
Ranke, Β. (1959). Über die nicht-eiweissgebundenen und eiweissgebundenen Ami
nosäurenbestände von Fischen, Mollusken und Krebsen. Arch. Fischer eiwiss. 10, 117-159.
Ranke, Ε . (1960). Der Einfluss der Lagerung auf die freien Aminosäuren und Peptide beim Lengfisch Molva vulgaris und Seelachs Gadus vir ens. Arch. Fisch
ereiwiss. 11, 18-47.
Ranke, E., and Bramstedt, F . (1955). Papierelektrophoretische Untersuchungen an Fisch-, Krebs- und Muscheleiweiss. Arch. Fischereiwiss. 5, 43-46.
Ranke, E., Ranke, B., and Bramstedt, F. (1955). Über ein bei frischen Seefischen vorkommendes Peptid. Arch. Fischereiwiss. 6, 343-345.
Ranke, E., Ranke, B., and Bramstedt, F. (1957). Untersuchungen an tiefgefrorenen Forellen und Lengfisch. Arch. Fischereiwiss. 8, 53-56. I. Beiheft.
Richmond, V., Tang, J . , Wolf, S., Trucco, R. E., and Caputto, R. (1958). Chro
matographie isolation of gastriesin, the proteolytic enzyme from gastric juice with pH, optimum 3.2. Biochim. et Biophys. Acta 29, 453-454.
Saito, K., and Sameshima, M. (1956). The putrefactive products of fish tissues. II.
Isolation and identification of cadaverine. /. Agr. Chem. Soc. Japan 30, 353-357.
Schormüller, J . , Winter, H., and Baiischmieter, H. (1950). Über den Gehalt en Methionin, Cystin, Tyrosin, Typtophan, Histidin und nichteiweißartigen Begleit
substanzen in Trockeneiweiss aus Süsswasserfischen. Biochem. Ζ. 321, 180-188.
Shimizu, W., and Hibiki, S. (1955). Studies on putrefaction of aquatic Products.
XX. Consideration of difference in putrefaction for various kinds of fish. ( 3 ) — Influence of autolysis on putrefaction. Bull. Japan. Soc. Sei. Fisheries 21, 267- 270.
Siebert, G. (1958). Aktivität Eiweiss-spaltender Enzyme in Fischen. Experientia 14, 65.
Suzuki, T. (1953). Determination of volatile acids for judging the freshness of fish. Bull. Japan. Soc. Sei. Fisheries 19, 102-105.
Tarr, H. L. A. (1954). The bacterial flora of fresh fish and its technological im
plications. Proc. Symposium on Cured and Frozen Fish Technol. Sweden Inst.
Food Preserv. Research Puhl. No. 100 Paper No. 5, 7 pp. (1953).
Togasawa, Y., and Katsumata, T. (1955-56). Effects of metal ions on the glycyl- glycine dipeptidase. II. (In Japanese with English summary.) Bull. Japan. Soc.
Sei. Fisheries 21, 1070-1075.
Tomiyama, T., and Kawazoe, A. (1955-56). Biochemical studies on the liquefaction of fish body. III. On effectiveness of fish autolysate to the culture of bacteria.
Bull. Japan. Soc. Sei. Fisheries 21, 1226-1232.
Tomiyama, T., Yone, Y., and Ide, K. (1950). Studies on method of estimating freshness of fish. I. Estimation of freshness by determining the catalase activity of the gill. Bull. Japan. Soc. Set. Fisheries 16, 17-21.
Trautner, K., unpublished.
Yamazaki, T., and Yamauchi, T. (1956). Soluble feed from fish. Patent No. 60F1, July 23.
Yanase, M. (1956-57). The vitamin B6 content of fish meat. Nippon Suisangaku Kaishi 22, 51-55.
Yudaev, N. A. (1950). Control of histidine, carnosine, and anserine in muscle of some fish. Dokhdy Akad. Nauk S.S.S.R. 70, 279-282.
Zotov, A. F . (1958a). Studies on antibacterial characteristics of extracts from fish.
I. (In Russian.) Voprosy Ikthyologiji 10, 120-123.
Zotov, A. F. (1958b). Studies on antibacterial characteristics of extracts from fish II. (In Russian.) Voprosy Ikthyologiji 11, 188-191.