C H A P T E R 13
Plant Diseases Caused by
Nematodes
Introduction
N e m a t o d es are the only plant parasites b e l o n g i ng to the animal kingdom that are studied in plant pathology. N e m a t o d e s, sometimes called eelworms, are wormlike in appearance but quite distinct taxo- nomically from the true worms. Most of the several thousand species of nematodes live in great numbers freely in fresh or salt waters or in the soil feeding on microscopic plants and animals. Numerous species of nematodes attack and parasitize man and animals, on which they cause various diseases. Several h u n d r ed species, however, are known to fee d on living plants as parasites and to cause a variety of plant dis- eases.
Characteristics of Plant Pathogenic N e m a t o d es Morphology
Plant parasitic nematodes are small, 3 0 0 - 1 0 0 0 μ with s o me up to 4 m m long by 15-35 μ w i de (Fig. 114). Their small diameter makes
499
Salivary gland ducts Lumens of esophagus
Egg
Spermatheca
Cross section of nematode
at the nerve ring region Spicule^
Male and female adults of a typical plant-parasitic nematode
-Anus
^Phasmid Bursa
F i g. 114. Morphology and anatomical characteristics of typical male and female plant parasitic nematodes.
í Lip Λ region
rStylet yCuticle
Stylet knobs \
Head Lateral
View / h ( _ips
•Mou Stylet tipf-
^Stylet
Nerve rin
-Salivary glands
^s-Basal bulb of
\ \ esophagus Median bulbs
\ o f esophagus
^ ^ x c r e t o r y pore
- Lumen ot esophagus
r Intestine
Ovary
Testis Cuticle
O ^ H y p o d e r m a l Nerve ring
-Cuticular annulations
Sperm
sr-Uterus
%^-Vulva
Characteristics of Plant Pathogenic Nematodes 501
them invisible to the naked eye, but they can b e easily observed un- der the microscope. N e m a t o d es are, in general, eel-shaped, round in cross section, with smooth, u n s e g m e n t ed b o d i es without legs or other a p p e n d a g e s. T h e females of s o me s p e c i e s, however, b e c o me swollen at maturity and have p e a r s h a p ed or spheroid b o d i es (Fig. 115).
Anatomy
T h e nematode b o dy is more or less transparent (Fig. 114). It is cov- ere d by a colorless cuticle which is usually marked by striations or may show bristles, punctuations, warts, or other markings. T h e cuticle molts w h en nematodes go through their successive larval stages. T h e cuticle is p r o d u c ed by the hypodermis, which consists of living cells and extends into the b o dy cavity as four chords separating four b a n ds of longitudinal muscles. T h e se muscles enable the nematode to move.
Additional specialized muscles exist at the mouth and along the diges- tive tract and the reproductive structures.
T h e b o dy cavity is rudimentary in its d e v e l o p m e nt and contains a fluid through which circulation and respiration take place. T h e diges- tive system is a hollow tube extending from the mouth through the buccal cavity, esophagus, intestine, rectum, and anus. L i p s, usually six in number, surround the mouth. All plant parasitic nematodes have a protrusible, hollow stylet or spear which originates in the buccal cavity and is u s ed to puncture plant cells. T h e excretory system is not well d e v e l o p ed in nematodes. On the contrary, the nervous system is well d e v e l o p ed and consists of many nerves, ganglia, and sensory structures.
T h e reproductive systems are well developed. F e m a le nematodes have one or two ovaries followed by an oviduct and uterus terminating in a slitlike vulva. T h e male's reproductive structures are similar to the female's but has a testis, seminal vesicle, and ejaculatory duct and terminates in a common cloaca with the intestine. A pair of protrusi- ble, copulatory spicules are also present in the male. Reproduction in nematodes is through eggs and may b e sexual, hermaphroditic, or par- thenogenetic. Many species lack males.
Life Cycles
T h e life histories of most plant parasitic nematodes are, in general, quite similar. E g gs hatch into larvae, w h o se appearance and structure are usually similar to those of the adult nematodes. Larvae grow in size and each larval stage is terminated by a molt. All nematodes have
0 250éé 500á 750u. lOOOu. i2507TH500Tl I750u 2000u. 2250á 25O0u 2750á 3000u
yi
£l 01
6
8
L 9
17
Æ
Helicotylenchus ^ Dolichodoru s Belonolaimu s Anguin a
^Xiphinemaoplolaimu s
' Rotylenchus =» Hemicycliophor a ^ Ditylenchu s Aphelenchoide s ^ Tylenchorhynchu s Trichodoru s
> Radopholus
=» Pratylenchus
> Criconemoides Paratylenchu s
> Longidoru
s IS
202 1 > 2 Ρ
1Meloidogyn e \ Heteroder a V
Tylenchulu s .
>iconema c
Characteristics of Plant Pathogenic Nematodes 503
four larval stages, with the first molt usually occurring in the egg. After the final molt the nematodes differentiate into adult males and fe- males. T h e female can then produce fertile eggs either after mating with a male or, in the a b s e n ce of males, parthenogenetically, or it can produce sperm herself.
A life cycle from e g g to e g g may b e c o m p l e t ed within 3 or 4 weeks under optimum environmental, especially temperature, conditions, but will take longer in cooler temperatures. In s o me species of nema- todes the first or second larval stages cannot infect plants and d e p e n d for their metabolic functions on the energ y stored in the egg. Whe n the infective stages are produced, however, they must fee d on a sus- ceptible host or starve to death. A b s e n ce of suitable hosts may result in death of all individuals of certain nematode species within a few months, but in other species e g gs may remain dormant in the soil for years.
Ecology and Spread
Almost all plant pathogenic nematodes live part of their lives in the soil. Many of these live freely in the soil, feeding superficially on roots and underground stems, and although they may cause injury to plants they are not strictly parasitic. E v en in the most highly specialized sedentary parasites, the eggs, the preparasitic larval stages, and the males are found in the soil for all or part of their lives. Soil tempera- ture, structure (porosity), moisture and aeration affect survival and m o v e m e nt of nematodes in the soil. N e m a t o d es occur in greatest a b u n d a n ce in a layer of soil from 0 to 15 c m d e e p, although species vary in this respect and their preference is influenced by location and by the host plant. Distribution of nematodes in cultivated soil is irreg- ular and is greatest in or around roots of susceptible plants, which they follow sometimes to considerable depths (30-150 c m or more). T h e greater concentration of nematodes in the region of host plant roots is d ue primarily to their more rapid reproduction on the food supply available and also to attraction of nematodes by substances released into the rhizophere. T o these must b e a d d ed the so-called hatching factor effect of substances originating from the root which diffuse or are carried into the surrounding soil and markedly stimulate the hatching of e g gs of certain species, although most nematode eggs hatch freely in water in the a b s e n ce of any special stimulus.
F i g. 115. M o r p h o l o gy a nd relative size of the m o st important plant parasitic nema- todes.
Nematodes spread through the soil very slowly under their own power. T h e overall distance traveled by a nematode probably does not e x c e e d a few feet per season. T h e s p e ed of m o v e m e nt in the soil s e e ms to b e related to pore diameter, particle size, and water content of the soil, and diameter and relative activity of the nematode. Nema- todes move faster in the soil when the pores are lined with a thin (a few microns) film of water than w h en the soil is waterlogged. In addi- tion to their own movement, however, nematodes can b e easily spread by anything that moves and can carry particles of soil. F a rm equip- ment, irrigation, flood or drainage water, animal feet, and dust storms spread nematodes in local areas, while over long distances nematodes are spread primarily with farm produce and nursery plants. A few nematodes that attack aboveground parts of plants spread through the soil as described above and are s p l a s h ed to the plants by falling rain, overhead watering, or ascend wet plant stem or leaf surfaces on their own power. Further spread takes place upon contact of infected plant parts with adjacent healthy plants.
Classification
All plant-parasitic nematodes b e l o ng to the phylum Nemathel- minthes, class Nematoda. Most of the important parasitic gener a b e - long to the subclass Secernentea, order Tylenchida:
Superfamily: T y l e n c h o i d ea F a m i l y:
T y l e n c h i d ae
G e n u s: Anguina, w h e at or seed-gall n e m a t o de Ditylenchus, stem or b u lb n e m a t o de Tylenchorhynchus, stunt n e m a t o de H e t e r o d e r i d ae
G e n u s: Heterodera, cyst n e m a t o de Meloidogyne, root-knot n e m a t o de H o p l o l a i m i d ae
G e n u s: Helicotylenchus, spiral n e m a t o de Rotylenchus, spiral n e m a t o de Hoplolaimus, lance n e m a t o de
Pratylenchus, lesion or m e a d ow n e m a t o de Radopholus, b u r r o w i ng n e m a t o de
Rotylenchulus, reniform n e m a t o de Belonolaimus, sting n e m a t o de Dolichodorus, awl n e m a t o de T y l e n c h u l i d ae
G e n u s: Tylenchulus, citrus n e m a t o de C r i c o n e m a t i d ae
Symptoms Caused by Nematodes 505
G e n u s: Criconema, ring n e m a t o de Criconemoides, ring n e m a t o de Paratylenchus, pin n e m a t o de Hemicycliophora, sheath n e m a t o de Superfamily: A p h e l e n c h o i d ea
F a m i l y:
A p h e l e n c h o i d i d ae
G e n u s: Aphelenchoides, leaf n e m a t o de or c h r y s a n t h e m um ee l w o rm
T h r e e important gener a of nematodes b e l o ng to the subclass Adeno- phorea, order Dorylaimida:
F a m i l y:
T y l e n c h o l a i m i d ae
G e n u s: Longidorus, n e e d le n e m a t o de Xiphinema, d a g g er n e m a t o de T r i c h o d o r i d ae
G e n u s: Trichodorus, stubby root n e m a t o de
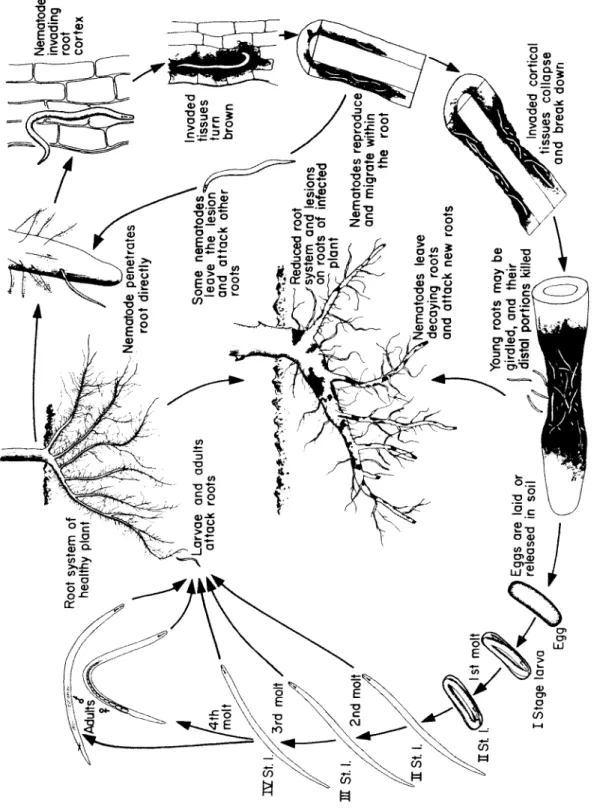
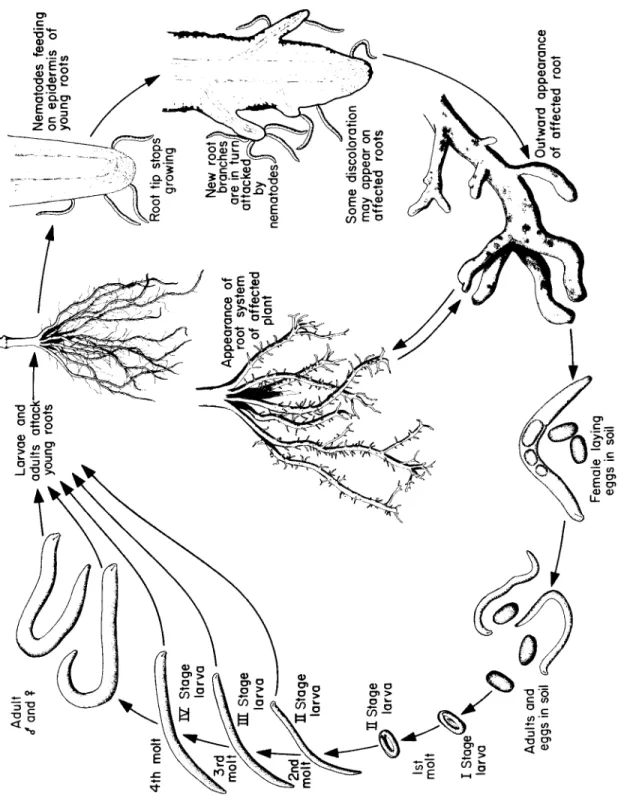
In terms of habitat, pathogenic nematodes are either ectoparasites (species that do not normally enter root tissue but fee d only on the cells near the root surface) or endoparasites (species that enter the host and fee d from within), and both of these can b e either migratory (species that live freely in the soil and fee d on plants without b e c o m- ing attached), or sedentary (species that, once within a root, do not m o ve about). T h e ectoparasitic nematodes include the ring nematode (sedentary) and the dagger, stubby root, and sting nematodes (all migratory). T h e endoparasitic nematodes include the root knot, cyst, and citrus nematodes (all sedentary), and the lesion, stem and bulb, burrowing, leaf, stunt, lance, and spiral nematodes (all somewhat migratory). Of these, the cyst, lance, and spiral nematodes may b e somewhat ectoparasitic, at least during part of their lives.
Symptoms Caused by Nematodes
N e m a t o de infections of plants result in the appearance of symptoms on the roots as well as on the aboveground parts of plants. Root symp- toms may appear as hypertrophy, necrosis, or abnormal growth, and include:
Root knots or root galls, which are enlargements of the root c a u s ed by feeding of nematodes that may or may not b e e n c l o s ed within them. T h e swellings may vary in size from 1 m m to more than 2 cm.
Root lesions, discolored and often collapsed portions of the root, consisting of cells on which nematodes have fed. T h e y vary in size
from almost invisible to the naked eye, to lesions girdling the whole root.
Excessive root branching, resulting from the formation of numerous short lateral roots in the vicinity of nematode injury.
Injured root tips, when the nematodes fee d on or near root tips and cause them to stop growing, enlarge or disintegrate.
Root rots, when nematode infections are accompanied by plant pathogenic or saprophytic bacteria and fungi.
T h e se root symptoms are usually accompanied by noncharacteristic symptoms in the aboveground parts of plants appearing primarily as r e d u c ed growth, symptoms of nutrient deficiencies such as yellowing of foliage, excessive wilting in hot or dry weather, r e d u c ed yields, and poor quality of products.
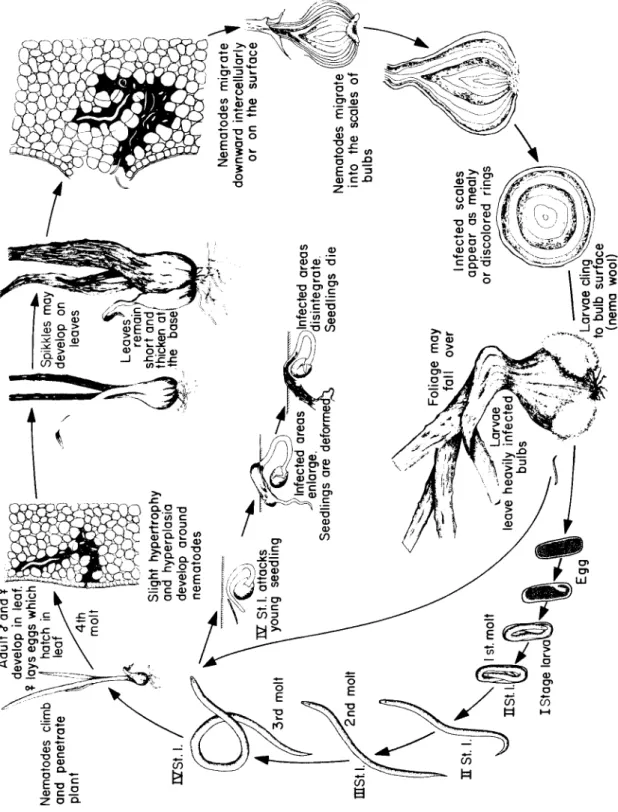
Certain species of nematodes invade the aboveground portions of plants rather than the roots, and on these they cause galls, necrotic le- sions and rots, twisting or distortion of leaves and stems, and abnormal development of the floral parts. Certain nematodes attack grains or grasses forming galls full of nematodes in place of seed.
H ow Nematodes Affect Plants
Nematodes d a m a ge plants only slightly by direct mechanical injury inflicted upon the plants during feeding. On the contrary, most of the d a m a ge s e e ms to b e c a u s ed by a secretion injected into the plants while the nematodes are feeding. This secretion, called saliva, is pro- d u c ed in three glands from which it flows forward into the esophagus and is ejected through the stylet. S o me nematode species are rapid feeders. The y puncture a cell wall, inject saliva into the cell, suck part of the cell contents and m o ve on within a few seconds. Others fee d much more slowly and may remain at the s a me puncture for several hours or days. T h e s e, as well as the females of species which b e c o me permanently established in or on roots, inject saliva intermittently as long as they are feeding.
T h e saliva of plant nematodes s e e ms to aid the parasite in the pene - tration of cell walls and, possibly, in liquefying the cell contents, mak- ing them easier to ingest and assimilate. T h e feeding process causes the affected plant cells to react resulting in d e ad or devitalized root tips and b u d s, lesion formation and tissue break down, swellings and galls of various kinds, and crinkled and distorted stems and foliage.
S o me of these manifestations are c a u s ed by dissolution of m i d d le lamellae and cell walls of infected tissues by nematode enzymes, which, with or without the help of toxic metabolites, cause tissue dis-
Interrelationships between Nematodes and Other Plant Pathogens 507
integration and death of cells. Others are c a u s ed by abnormal cell en - largement (hypertrophy), as in the case of giant cell formation by root- knot nematodes; by suppression of cell division, caused, for example, by the stubby root nematode on roots which it has fed on and in which the apical meristem stops dividing and the root stops growing; or by stimulation of cell division p r o c e e d i ng in a controlled manner and resulting in the formation of galls or of large numbers of reticulate lat- eral roots at or near the points of infection.
Plant d i s e a se syndromes c a u s ed by nematodes are complex. Root- feeding species probably decrease the ability of plants to take up wa- ter and nutrients from soil and thus cause symptoms of water and nu- trient deficiencies in the aboveground parts of plants. However, it is the plant-nematode biochemical interactions which impair the overall physiology of plants and the role they play in providing courts for en- try of other pathogens that are primarily responsible for plant injury, while the mechanical d a m a ge or withdrawal of food from plants by nematodes is generally less significant.
Interrelationships b e t w e en N e m a t o d es and Other Plant Pathogens Although nematodes can cause diseases to plants by themselves, most of them live and operate in the soil where they are constantly surrounded by fungi, bacteria, and viruses, many of which can also cause plant diseases. In many cases an association develops b e t w e en nematodes and certain of the other pathogens in which nematodes b e c o me a part of an etiological complex resulting in a c o m b i n ed path- ogenic potential far greater than the s um of the d a m a ge either of the pathogens can produce individually.
Several nematode-fungus d i s e a se complexes are known. Fusarium wilt of several plants increases in incidence and severity when the plants are also infected by the root knot, sting, reniform, burrowing, or stunt nematodes. Similar effects have also b e e n noted in d i s e a se com- plexes involving nematodes and Verticillium wilt, Pythium d a m p i ng off, Rhizoctonia and Phytophthora root rots, and in some other in- stances, although in none of these cases is the fungus transmitted by the nematode. Plant varieties susceptible to the respective fungi are d a m a g ed even more when the plants are infected with the nematodes, the c o m b i n ed d a m a ge b e i ng considerably greater than the sum of the d a m a ge c a u s ed by each pathogen acting alone, and varieties, ordinar- ily resistant to the fungi, b e c o me infected by them after previous infection by nematodes. T h e importance of n e m a t o d es in these com- plexes is indicated by the fact that soil fumigation a i m ed at eliminat-
ing the nematode, but not the fungus, greatly reduces the incidence and the d a m a ge c a u s ed by the fungus-induced d i s e a s e.
Although it s e e ms quite probable that the mechanical wounding c a u s ed to plants by nematodes is an important factor in providing avenues of entry for the fungus, the continuation of the effect that nematodes have on host susceptibility in later stages of plant develop- men t suggests that the nematodes may also cause some host response that lowers natural resistance to the fungus. It should also b e noted that, in at least some such complexes, there is a greater mass of myce- lium present in nematode infected than in nematode free tissues of the same plant and also that higher populations of nematodes are pres- ent in fungus infected than in fungus free tissues of a d i s e a s ed plant.
Relatively few cases of nematode-bacterial d i s e a se complexes are known. In most of these the nematode role s e e ms to b e that of provid- ing the bacteria with an infection court and to assist bacterial infection by wounding the host.
In one such plant d i s e a se complex, however, involving strawberry infection by the leaf nematode and the bacterium Cory neb acterium fascians, a more involved interaction b e t w e en the two pathogens
s e e ms to exist. Strawberry plants inoculated with bacteria only de- velop a leafy gall, while plants infected only with nematodes d e v e l op a rosette of rudimentary leaves surrounding the apical meristem.
Plants inoculated with both pathogens, however, produce the so- called cauliflower symptom, quite distinct from either of the symp- toms produced by each pathogen alone.
Much more important, and more common, are the interrelationships b e t w e en nematodes and viruses. Several plant viruses such as grape- vine fanleaf virus, arabis mosaic virus, tomato ringspot virus, tobacco black ring virus, raspberry ringspot virus, tobacco rattle virus, and others, are transmitted through the soil by means of nematode vectors.
All these viruses, however, are transmitted by only one or more of the three gener a of dagger, n e e d l e, and stubby-root nematodes: Xiphine- ma, Longidorus, and Trichodorus. T h e se nematodes can transmit some of the viruses after feeding on virus infected plants for one day or more. Once they have acquired virus from an infected plant the nematodes remain infective for periods of 2-4 months and sometimes even longer. All stages, larval and adult nematodes, can transmit vi- ruses, but it is not known whether the virus is carried from one larval stage to another and to adults through molts or whether the virus pas- ses from adults, through eggs, to larvae. In general, there is no infor- mation as to how virus is acquired from the plants by nematodes, what
Control of Nematodes 509
the path of the virus is in the nematode, whether there are any patho- logical effects of the virus on the nematode vectors and how nematodes transmit the virus from infected to healthy plants. That nematodes do carry viruses, however, can b e shown in many ways and by the fact that virus transmission can b e obtained by rubbing healthy leaves of host plants with suspensions of cut s p e c i m e ns of infective nematodes.
T h e role of nematodes in nematode-virus complexes s e e ms to b e that of vector of the virus. T h e fact, however, that only three taxonomi- cally closely related gener a of nematodes can transmit viruses sug- gests that this interrelationship may d e p e n d on certain physiological or anatomical characteristics of these nematodes absent or different from those in the other genera.
Control of N e m a t o d es
Several methods of effectively controlling nematodes are available, although certain factors such as e x p e n se and types of crops, limit their applicability in s o me cases. Four general types of control methods are employed: Control through cultural practices, biological control through resistant varieties and certain other means, control by means of physical agents, e.g., heat, and control through chemicals.
Cultural Practices
T h e y result in partial or complete control of nematodes and include:
C R OP ROTATION TO N O N H O ST P L A N TS
Several nematode species can infect only a few crops. Since all plant-pathogenic nematodes are obligate parasites, the absence of susceptible hosts from the soil for 2 to 3 years will result in elimina- tion of the nematodes from that area through starvation and inability to reproduce. This method, of course, requires k n o w l e d ge of the kinds of nematodes present in the soil and of what plants are resistant or sus- ceptible to them. It is limited by the fact that many nematodes can at- tack a w i de variety of plants, thus limiting the choice of nonhost plants; it is impractical for permanent plantings, such as orchards, and when the nonhost crops are drastically different from the one previ- ously grown and require specialized production know-how and equipment.
SANITARY PRACTICES
T h e y include cleaning of all machinery thoroughly before moving into an uncontaminated area; taking care not to bring nematodes into a field by means of contaminated nursery stock, s e e d, containers, etc.;
maintaining soil fallow free from host plants which deprive nema- todes of roots on which to feed.
F L O O D I NG
S o me species of nematodes have b e c o me adapted to living in the normal soil under ordinary moisture content and d e p e nd on a certain amount of aeration present in it. F l o o d i ng of the land for a period of several months results in the death of these nematodes and thus frees the land from these pathogens. However, in only very few fields is it possible or practical to flood the land for long periods and, therefore, the applicability of this control method is quite limited.
Resistant Varieties
Several crop varieties resistant to nematodes are available and oth- ers are under development. Whe n resistant varieties with the desired horticultural qualities are available their cultivation, instead of the susceptible ones, provides the most convenient and least expensive way of combatting nematodes. Experimental control of nematodes has also b e e n obtained by interplanting with nematode-trapping plants which attract the nematodes away from the susceptible crop plants;
and adding organic matter or mulching to increase the populations of certain fungi and predatory nematodes which fee d on plant parasitic nematodes.
Heat Treatment
T wo types of heat treatment are effective in controlling nematodes.
Raising the temperature of the soil to about 50°C for 30 minutes by means of steam or hot water is sufficient to kill most nematodes and nematode eggs. T h e commonly practiced soil "sterilization" at 82°C for 30 minutes will eradicate all nematodes along with practically all other soil organisms. Heat treatment is the most effective and most commonly u s ed method for treating soil to b e u s ed for a container or greenhouse benc h crop. It is sometimes u s ed for treatment of ground b e ds and small outdoor areas but is too expensive and impractical for
Control of Nematodes
use in large acreages. Its use, even in the greenhouse, is limited to preplanting applications.
Hot water dip treatments are u s ed to a limited extent to eradicate nematodes from within roots, bulbs, etc., and also those clinging to the surfaces of roots or other propagative materials of vegetatively propa- gated nursery stock before they are planted in nematode-free soil.
Temperatures around 50°C for periods of time varying from a few minutes to 30 minutes are usually e m p l o y ed for this purpose. T h e ability to withstand such temperatures varies from plant to plant and with the stage of growth of the plant, dormant plants or plant organs b e i ng more tolerant to high temperatures than actively growing ones.
Frequently the margin of safety b e t w e en the temperature lethal to nematodes and injurious to plants is very narrow, and extreme precau- tions must b e taken to avoid d a m a g i ng the plants treated.
Chemical Control
T h e most promising method of controlling nematodes in the field has b e e n through the use of chemicals called nematocides. S o me of these, including chloropicrin, methyl bromide, My lone, Vapam, Vor- lex, give off gases after application to the soil and are general purpose preplant fumigants; they are effective against a wide range of soil mi- croorganisms including, in addition to nematodes, many fungi, in- sects, and w e e d s. Other nematocides, e.g., N e m a g o n, Zinophos, are of low volatility, are effective against nematodes and insects, and can b e applied before and after planting of many crops which are tolerant to these chemicals.
Nematocides u s ed as soil fumigants are available as liquids, emulsi- fiable concentrates, or granules. Application of nematocides in the soil is m a de either by applying the chemical evenly over the entire field (broadcast) or by applying it only to the rows to b e planted with the crop (row treatment). In both cases the fumigant is a p p l i ed through delivery tubes attached at the back of tractor-mounted chisel-tooth injection shanks or disks s p a c ed at variable widths and usually reach- ing 6 inches b e l ow the soil surface. T h e nematocide is covered in- stantly by a smoothing and firming drag or can b e mixed into the soil with disk harrows or rototillers. Highly volatile nematocides should be immediately covered with polyethylene sheeting, and this should b e left in place for at least 48 hours. Whe n small areas are to b e fumi- gated, the most convenient method is through injection of the chemi- cal with a hand applicator or by placement of small amounts of gran- ules in holes 6 inches d e e p, 6-12 inches apart, and immediately
511
TABLE I
N a m es a nd Properties of the M o st C o m m on N e m a t o c i d es
C h e m i c al a nd trade n a m es Control Volatility F o r ma J. Applied only before planting
Chloropicrin ( L a r v a c i de 100, Picfume, etc.)
Methyl b r o m i de (usually with a small a m o u nt of chloropicrin a d d e d ), ( D o w f u me M C - 2, D o w- fume M C - 3 3, B r o z o n e, etc.) S o d i um methyldithiocarbamate,
or S M DC (Vapam, V P M, C h e m - V a p e)
Dimethyltetrahydrothiadiazinethione, or D M T T (Mylone)
Methyl isothiocyanate-dichloropro- p e n e mixture, or M I TC
(Vorlex)
E t h y l e ne d i b r o m i de or E D B ( D o w f u me W-85)
D i c h l o r o p r o p e n e- D i c h l o r o p r o p a ne ( D - D, V i d d en D, N e m a f u m e, T e l o n e, etc.)
E D B a nd T e l o ne (Dorlone) D -D a nd Chloropicrin ( N e m e x)
N e m a t o d e s, soil fungi, Ver y high L or G soil insects, w e e d
s e e ds
N e m a t o d e s, soil insects, Ver y high L or G soil fungi, w e e d
s e e ds
N e m a t o d e s, s o me soil H i gh fungi, germinating
w e e d s e e d s, soil insects
N e m a t o d e s, soil fungi, H i gh w e e d s e e d s, soil
insects
N e m a t o d e s, soil fungi, H i gh w e e d s e e d s, soil
insects
N e m a t o d e s, soil insects M o d e r a te N e m a t o d e s, soil insects M o d e r a te
//. Applied before and after planting D i b r o m o c h l o r o p r o p a n e, or D B CP
( N e m a g o n, F u m a z o n e)
Diethylpyrazinyl phosphorothioate (Zinophos, C y n e m)
D i c h l o r o p h e n yl diethyl phosphorothioate (VC-13 N e m a c i d e)
N e m a t o d es
N e m a t o d e s, soil fungi soil insects, w e e d s e e ds
N e m a t o d e s, Pythium fungi (damping-off) N e m a t o d e s, soil
insects
N e m a t o d e s, soil insects
M o d e r a te H i gh
W P or GR
L L
L ow to L or GR m o d e r a te
L ow L or GR L ow L
aL = liquid; G = gas; W P = w e t t a b le p o w d e r; GR = granules.
L
L
L L
Control of Nematodes 513
covering the holes with soil. In all cases of preplant soil fumigation with phytotoxic nematocides, at least 2 w e e ks must e l a p se from the time of treatment before s e e d i ng or planting in the field to avoid plant injury.
In the above types of nematocide application, only a small portion of the soil and its microorganisms c o me in contact with the chemical immediately. T h e effectiveness of the fumigants, however, is b a s ed on the diffusion of the nematocides in a gaseous state through the pores of the soil throughout the area in which nematode control is desired.
T h e distance of m o v e m e nt of the vapors is influenced by the size and continuity of soil pores, soil temperature (best range b e t w e en 10° and 20°C), soil moisture (best at about 8 0 % of field capacity), and by the type of soil (more material is required for soils rich in colloidal or or- ganic matter). Nematocides with low volatility, such as N e m a g on and Zinophos, do not diffuse through the soil to any great extent and must b e mixed with the soil mechanically or by irrigation water or rainfall.
Most nematocides, with the exception of the highly volatile ones, can b e a p p l i ed in irrigation water as soaks or drenches, but only low-vola- tility nematocides can b e a p p l i ed through overhead sprinkler systems.
T h e most common nematocides and s o me of their properties are listed in T a b le I.
S e l e c t ed References
A n o n y m o u s. 1962. Plant n e m a t o d e s. U.S. Dept. Agr., Agr. Res. Serv. Spec. Rept. 2 2 - 8 3 : 24 p p.
Christie, J. R. 1959. Plant n e m a t o d e s, their b i o n o m i cs a nd control. Fla. Univ. Agr. Expt.
Sta. (Gainesville) 2 5 6 p p.
Christie, J. R., a nd A. L. Taylor. 1965. Controlling n e m a t o d es in the h o me garden. U.S.
Dept. Agr. Farmers' Bull. 2 0 4 8 : 10 p p.
C o l e, H., Jr., a nd J. R. B l o o m. 1962. Plant parasitic n e m a t o d es a nd their control. Venn.
State Univ. Circ. 503 : 14 p p.
G o o d, J. M., a nd A. L. Taylor. 1965. C h e m i c al control of plant parasitic n e m a t o d e s. U.S.
Dept. Agr., Agr. Handbook 286: 2 8 p p.
Harrison, B. D. 1964. T h e transmission of plant viruses in soil. In " P l a nt V i r o l o g y" ( M.
K. Corbett a nd H. D. Sisler, eds.), p p. 118-147. Univ. of F l o r i da Press, G a i n e s v i l l e, Florida.
J e n k i n s, W. R., a nd D. P. Taylor. 1967. " P l a nt N e u r o l o g y ," 2 7 0 p p. R e i n h o l d, N e w York.
Krusberg, L. R. 1963. H o st r e s p o n se to n e m a t o de infection. Ann. Rev. Phytopathol. 1:
2 1 9 - 2 4 0 .
Pitcher, R. A. 1963. R o le of plant-parasitic n e m a t o d es in bacterial d i s e a s e s. Phytopath- ology 53 : 3 5 - 3 9 .
Powell, Í . T. 1963. T h e role of plant-parasitic n e m a t o d es in fungus d i s e a s e s. Phyto- pathology 5 3 : 2 8 - 3 5 .
Raski, D. J., a nd W. B. Hewitt. 1963. Plant-parasitic n e m a t o d es as vectors of plant vi- ruses. Phytopathology 53: 3 9 - 4 7 .
R o h d e, R. A. 1965. T h e nature of r e s i s t a n ce in plants to n e m a t o d e s. Phytopathology 55 : 1159-1162.
Southey, J. F. (ed). 1959. Plant nematology. Ministry of Agr., Fisheries Food. Tech. Bull.
7.
T h o r n e, G. 1961. " P r i n c i p l es of N e m a t o l o g y ," 553 p p. M c G r a w - H i l l, N e w York.
Root-Knot N e m a t o d es
Occurrence and Importance
Root-knot nematodes occur in most parts of the world. O ne species has b e e n reported from as far north as C a n a da and Alaska, but most are found more frequently and in greater numbers in areas with warm or hot climates and short or mild winters. Root-knot nematodes are also found in greenhouses everywhere w h en nonsterilized soil is used.
Certain root-knot nematode species are more or less localized in large geographical areas, but others are much more widely distributed.
T h e y attack more than 2000 species of plants including almost all cul- tivated plants. S o me plants are attacked by only one or two species of the nematode, but others are attacked by most of its known species although they may b e more susceptible to s o me than to others.
Root-knot nematodes d a m a ge plants by devitalizing root tips and stopping their growth and by causing formation of swellings of the roots which not only deprive plants of nutrients but also cause disrup- tion of the vascular system of the plant and interfere with translocation of water and minerals from the soil. Swellings also disfigure and re- duce the market value of many root crops. L o s s es c a u s ed by root-knot nematodes vary with the host plant attacked, the nematode species and the cultural conditions. Whe n susceptible plants are infected at the seedling stage, losses are heavy and may result in complete de- struction of the crop. Infections of older plants may have only slight effects on yield or they may reduce yields considerably.
Symptoms
Plants infected with root-knot nematodes exhibit aboveground symptoms that are similar to those c a u s ed by many other root diseases
Root-Knot Nematodes 515
or environmental factors that result in r e d u c ed amounts of water avail- able to the plant. T h e se symptoms consist of r e d u c ed growth of the plant and fewer, small, pale-green or yellowish leaves that tend to wilt in warm weather. Blossoms and fruits are either wholly lacking or are dwarfed and of poor quality. Affected plants usually linger through the growing season and are seldom killed prematurely. T h e stems, pet- ioles, and leaves of a few plant species are attacked directly by root- knot nematodes and d e v e l op galls similar to those produced on roots.
T h e most characteristic symptoms of the d i s e a se are those appear- ing on the underground parts of the plants. Infected roots swell at the point of invasion and d e v e l op into the typical root-knot galls which may b e two or three times as large in diameter as the healthy portions of the root (Fig. 116). Several infections may take place along the s a me root and the d e v e l o p i ng galls give the root a rough, c l u b b ed appear- ance. Roots infected by certain species of this nematode d e v e l o p, in addition to galls, several short root branches which rise from the up- per part of the gall and result in a d e n s e, b u s hy root system. Usually, however, infected roots are greatly retarded in growth and may show various stages of necrosis. Considerable rotting of the roots frequently develops particularly late in the season. Whe n tubers, edible root or other fleshy underground organs are attacked they produce small swellings or p i m p l i ng over their surface which b e c o m es quite promi- nen t at times and may cause distortion of the organs or cracking of their skin.
The Pathogen: Meloidogyne sp.
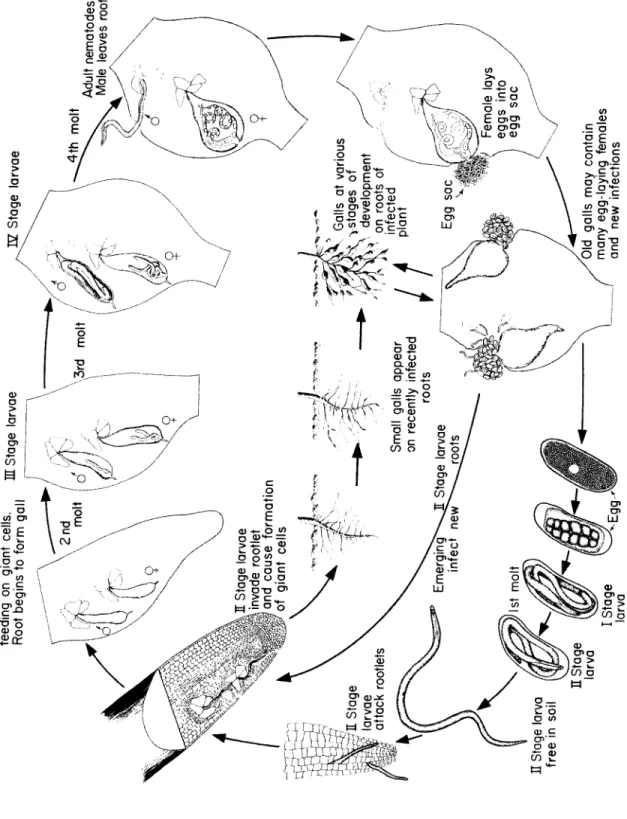
T h e adult m a le and female root-knot nematodes are easily distin- guishable morphologically (Fig. 118). T h e males are wormlike and about 1.2-1.5 m m long by 30-36 μ in diameter. T h e females are pear- s h a p ed and about 0.40-1.30 m m long by 0.27-0.75 m m wide. E a ch female lays approximately 500 eggs in a gelatinous substance pro- d u c ed by the rectal glands of the nematode. In favorable weather one first stage larva d e v e l o ps inside each e g g and after undergoing the first molt within the e g g it b e c o m es second-stage larva. T h e latter e m e r g e s from the e g g into the soil, where it moves until it finds a susceptible root. T h e second-stage larva is wormlike and is the only infective stage of this nematode. If a susceptible host is present in its vicinity, the larva enters the root, usually near the tip, b e c o m es sedentary, and grows in thickness, a s s u m i ng a sausage-shaped form. T h e nematode feeds on the cells around its h e ad by inserting its stylet and secreting saliva into these cells. T h e saliva stimulates cell enlargement and also liquefies part of the contents of the cells, which are then s u c k ed by the
F i g. 116. Galls on the roots of tomato plant c a u s ed by the root-knot n e m a t o d e. (Photo by courtesy of U.S. D e p t. Agr.)
nematode through its stylet. T h e nematode undergoes a second molt and gives rise to the third-stage larva, which is similar to, but stouter than, the second-stage larva. T h e third-stage larva goes through the third molt and gives rise to the fourth-stage larva, which can b e distin- g u i s h ed as either male or female. A male fourth-stage larva b e c o m es wormlike and is coiled within the third cuticle. It undergoes the fourth and final molt and emerge s from the root as the wormlike adult
Root-Knot Nematodes 517
male which b e c o m es free-living in the soil. T h e fourth-stage female larva continues to grow in thickness and somewhat in length, under- goes the fourth and final molt and b e c o m es an adult female which appears pear-shaped. T h e adult female continues to swell and, with or without fertilization by a male, produces e g gs which are laid in a ge- latinous substance that serves as a protective coat. T h e eggs may b e laid wholly or partly inside or outside the root tissues d e p e n d i ng on the position of the female. E g gs may hatch immediately or they may overwinter and hatch in the spring. A life cycle is completed in 25 days at 27°C, but it takes longer at lower or higher temperatures.
Whe n the eggs hatch, the infective second-stage larvae may migrate from within galls to adjacent parts of the root and cause ne w infections in the s a me root, or they may e m e r g e from the root and infect other roots of the s a me plants or roots of other plants. T h e greatest numbers of root-knot nematodes are usually in the root zone from 5 to 2 5 cm b e l ow the surface, but galls have b e e n found on peach and other roots 2-2.5 meters d e e p. T h e ability of root-knot nematodes to move on their own power is limited, but they can b e spread by water or by soil clinging to farm e q u i p m e nt or otherwise transported into uninfested areas.
Development of Disease
Infective second-stage larvae usually enter roots first b e h i nd the root tip, p u sh their way b e t w e en cells or break their way through cells until they reach positions b e h i nd the growing point, where they b e - c o me permanently established with their h e ad in the plerome (Fig.
118). In older roots the h e ad is usually in the pericycle. S o me cell d a m a ge occurs along the path of the larva and, if several larvae have entered, the cells near the root tip c e a se to divide and growth of the root stops. On the other hand, cortical cells near the point of entry b e - gin to enlarge as sometimes do cells of the pericycle and endodermis near the path of the larvae. T wo or three days after the larva has b e - come established s o me of the cells around its h e ad fail to differentiate and b e g in to enlarge. Their nuclei divide but no cell walls are laid down. T h e existing walls b e t w e en s o me of the cells break down and disappear and the protoplasmic contents of several cells coalesce, giv- ing rise to giant cells (Figs. 117 and 118). E n l a r g e m e nt and coalescing of cells continues for 2-3 w e e k s, and the giant cells invade the sur- rounding tissues irregularly. E a ch gall usually contains 3-6 giant cells, which may form in the cortex as well as in the stele. T h e en- largement of the cells s e e ms to b e brought about by the substances
a nd giant cells in the stele. (B) Section of a tomato root s h o w i ng a root-knot nema- tode feeding on the giant cells surrounding its head. (Photos by courtesy of R. A.
Rohde.)
518
Fig. 118. Disease cycle of root-knot caused by nematodes of the genus Meloidogyne.
i
Stag e larv a
1
Stag e larv a
H Stag e larv a fre e i n soi l
JMst mol
t
Stag e larva e ^-^root s Emerging'^ ^ infec t ne w
11
Stag e \
1larva e attac k rootlet s P
IT Stag
e larva e invad e rootle t an d caus e formatio n of gian t cell s
VSmal l gall s appea r on recentl y infecte d
rootsGall s a t variou s >stage s o f
\ development on root s o f infecte d / ~ plan t / Eg g sa c Femal e lay s egg s int o eg g sa c /Ol d gall s ma y contai n man y egg-layin g female s an d ne w infection s
Lat e I E stag e larva e feedin g o n gian t cells . Roo t begin s t o for m gal l HI Stag e larva e
\3rdmol t /
^E Z Stag e larva e ^4t h mol t Adul t nematodes . Mal e leave s roo t
contained in the saliva secreted by the nematode in the giant cells during feeding. After male nematodes c e a se to fee d and when female nematodes die or are killed experimentally, the giant cells degener- ate. Whe n giant cells form in the stele, irregular xylem elements may develop around them or d e v e l o p m e nt of xylem vessels may b e inter- rupted. X y l em elements already present may b e crushed or disrupted by the mechanical pressure exerted by the enlarging cells. In the early stages of gall development the cortical cells enlarge in size but, during the later stages, they also divide rapidly. S w e l l i ng of the root results also from hypertrophy and hyperplasia of the vascular parenchyma, pericycle, and endodermis cells surrounding the giant cells and from enlargement of the nematode. As the females enlarge and e g g sacs are formed, they p u sh outward, may split the cortex and may b e c o me ex- p o s ed on the surface of the root or may remain completely covered, d e p e n d i ng on the position of the nematode in relation to the root sur- face.
In addition to the disturbance c a u s ed to plants by the nematode galls themselves, frequently d a m a ge to infected plants is increased by certain parasitic fungi, which can easily attack the w e a k e n ed root tis- sues and the hypertrophied, undifferentiated cells of the galls. More- over some of these fungi grow and reproduce much faster in the galls than in other areas of the root, thus inducing an earlier breakdown of the root tissues than would have b e e n possible otherwise.
Control
Root knot can b e effectively controlled in the greenhouse with steam sterilization of the soil or soil fumigation with nematocides. In the field the best control of root knot is obtained by fumigating the soil with chemicals such as D D at 25 gallons per acre or E D B at 15 to 20 gallons per acre. E a ch treatment usually gives satisfactory control of root knot for one season. For certain crops and for certain geographical areas and locations, good control of root knot has b e e n obtained by flooding the soil for several weeks or months, by dry summer fallow and dry tillage of the soil, or by changing the time of planting so that crops grow during the period of least activity of the nematodes. Con- trol of root knot by crop rotation although possible is usually impracti- cal b e c a u se of the large number of crop plants that can b e attacked by root-knot nematodes. Varieties resistant to root-knot nematodes are available in relatively few crops, but resistance to root knot has b e e n found in many crops, and increasing emphasis is b e i ng placed on ef-
Soybean Cyst Nematode
S e l e c t ed References
Bird, A. F. 1961. T h e ultrastructure a nd histochemistry of a n e m a t o d e - i n d u c ed giant c e l l . /. Biophys. Biochem. Cytol. 1 1 : 7 0 1 - 7 1 5 .
Brodie, Â. B., a nd W. E . Cooper. 1964. Relation of parasitic n e m a t o d es to post-emer- g e n c e damping-off of cotton. Phytopathology 54: 1 0 2 3 - 1 0 2 7 .
Christie, J. R. 1936. T h e d e v e l o p m e nt of root-knot n e m a t o de galls. Phytopathology 26:
1-22.
Christie, J. R. 1946. Host-parasite relationships of the root-knot n e m a t o d e, Heterodera marioni. II. S o me effects of the host on the parasite. Phytopathology 36: 3 4 0 - 3 5 2 . D r o p k i n, V. H., a nd P. E. N e l s o n. 1960. T h e histopathology of root-knot n e m a t o de
infections in s o y b e a n s. Phytopathology 50: 4 4 2 - 4 4 7 .
H o d g e s, C. F., a nd D. P. Taylor. 1966. Host-parasite interactions of a root-knot n e m a- tode a nd c r e e p i ng b e n t g r a s s, Agrostis palustris. Phytopathology 56: 8 8 - 9 1 .
Linford, Ì . B. 1937. T h e f e e d i ng of the root-knot n e m a t o de in root-tissue a nd nutrient solution. Phytopathology 27: 8 2 4 - 8 3 5 .
Linford, Ì . B. 1941. Parasitism of the root-knot n e m a t o de in leaves a nd stems. Phyto- pathology 3 1 : 6 3 4 - 6 4 8 .
Sasser, J. N. 1954. Identification a nd host-parasite relationships of certain root-knot n e m a t o d es (Meloidogyne sp.). Maryland Agr. Expt. Sta. Bull. A-77: 30 p p.
Smith, J. J., a nd W. F. Mai. 1965. Host-parasite relationships of Allium cepa a nd Meloi- dogyne hapla. Phytopathology 5 5 : 6 9 3 - 6 9 7 .
Tyler, J o c e l y n. 1933. T h e root-knot n e m a t o d e. Calif. Agr. Expt. Sta. Circ. 330: 34 p p.
Soybean Cyst N e m a t o de Occurrence and Importance
T h e soybean cyst nematode has b e e n found in northeastern Asia, Japan, and the states North Carolina, Virginia, T e n n e s s e e, Missouri, Illinois, Arkansas, Kentucky, Mississippi, Florida, Georgia, and Ala- bama. It continues to spread slowly to n e w areas in spite of the strict quarantine measures i m p o s ed on the presently infested areas. T h e most severely affected host is soybean, but several other l e g u m e s, such as common bean, vetch, l e s p e d e z a, lupine, and a few nonlegumi- nous plants are also attacked by this nematode. D e p e n d i ng on the d e g r ee of infestation, it can cause losses varying from slight to com- forts to incorporate this resistance into horticulturally acceptable vari- eties.
521
plete destruction of the crop. Usually, however, in heavily infested fields yield is r e d u c ed from 30 to 75 %.
Symptoms
Aboveground parts of infected soybean plants appear stunted and have an unthrifty appearance. T h e foliage turns yellow prematurely and falls off early. T h e plants bear only a few flowers and a few small seeds. If infected plants are growing on sandy soil they may die. In- fected plants growing on fertile soils with plenty of moisture may show only slight chlorosis of the older leaves, little or no stunting, and may produce a nearly normal yield for a year or two. In s u b s e q u e nt years, however, d ue to the tremendous build-up of nematodes in the soil, plants in these areas also b e c o me severely chlorotic and dwarfed.
T h e root system of infected plants appears smaller than that of healthy plants, but no macroscopic lesions, galls, or other type of ab- normalities are evident on infected roots. Roots of infected plants usually have considerably fewer bacterial nodules than those of healthy plants (Fig. 119). T h e most characteristic symptom of this dis- ease is the presence of female nematodes in various stages of develop- men t and of cysts attached on the soybean roots (Fig. 120). Youn g fe- males are small, white and partly buried in the root with only part of them protruding on the surface. Older females are larger, almost completely on the surface of the root, and appear yellowish or brown d e p e n d i ng on maturity. D e a d, brown cysts are also present on the roots.
The Pathogen: Heterodera glycines
T h e soybean cyst nematode overwinters as a brown cyst in the up- per 90-100 cm of soil. T h e cysts are the carcasses of the females and are filled with eggs. T h e eggs contain fully d e v e l o p ed second-stage larvae about 440 μ long (Fig. 121). Whe n temperature and moisture b e c o me favorable in the spring, the larvae e m e r ge from the cysts and infect roots of host plants.
At 4-6 days after penetrating the roots, the larvae molt and produce the third-stage larvae. T h e male third-stage larva is slightly more ro- bust than the female, and both are much stouter than the second-stage larvae. About 9-12 days after infection, fourth-stage larvae b e g in to appear. T h e male larva resembles in size and shape the third-stage male larva. T h e female fourth-stage larva, however, loses its some- what slender appearance and develops the typical flask shape, mea-
Soybean Cyst Nematode 523
F i g. 119. (A) S o y b e an roots infected with the s o y b e an cyst n e m a t o d e. N o te a b s e n ce of nitrogen-fixing nodules. (B) H e a l t hy s o y b e an roots b e a r i ng n u m e r o us beneficial n o d u l e s. (Photo by courtesy of U . S. D e p t. Agr.)
suring approximately 0.40 m m in length by 0.12-0.17 m m in width. By day 12 to 15, adult males and females appear.
T h e adult male is slender and wormlike, measuring about 1.3 m m long by 30-40 μ in diameter. T h e males remain in the root for a few days, during which they may or may not fertilize the females, then m o ve into the soil and soon die.
T h e adult females w h en fully d e v e l o p ed are lemon-shaped, mea- suring 0.6-0.8 m m in length and 0.3-0.5 m m in diameter. T h e y are white to pale-yellow at first, b e c o m i ng yellowish brown as they ma- ture. T h e body cavity of the female is almost completely filled by the ovaries, and as the ova gradually d e v e l op into fully formed eggs the
F i g. 120. L e m o n - s h a p ed e n c y s t ed f e m a le n e m a t o d es attached to s o y b e an roots. (Photo by courtesy of U.S. D e p t. Agr.)
body cavity of the female b e c o m es completely filled with eggs. As the female body distends during e g g production it crushes cortical cells, splits the root surface, and protrudes until it is almost entirely e x p o s ed through the root surface. A gelatinous mass, usually mixed with dirt and debris, surrounds the posterior e n d of the females and the nema- todes deposit some of their eggs in it. E g gs deposited in this matrix contain already fully d e v e l o p ed second-stage larvae, the first molt tak- ing place inside the e g g and while the e g g is still inside the female.
E a ch female produces 300-600 eggs, most of which remain inside her body when the female dies. E g gs in the gelatinous matrix may hatch immediately and the emerging second-stage larvae may cause ne w infections. E g gs remaining in the body of the female pass through the embryonic stages and reach the second larval stage, which either emerge s or lies quiescent in the e g g inside the d e ad female. Finally the carcass, darkening to brown, b e c o m es the cyst. Approximately 21-24 days are required for the completion of a life cycle of this nema- tode. T h e size of the brown cyst is determined by the extent of the
Fig. 121. Disease cycle of the soybean cyst nematode Heterodera glycines.
Syncyti a begi n t o degenerat e Femal e May s egg s
in gelatinou s mas s
' Root surfac e
I Stage " larvae^ * emerg e fro m
ιegg s
Femal e cys t fille d wit h egg s stil l attache d to roo t
Ε Stag e larva e
irVegg s insid e brow n cys t overwinterin g
JQ^in soi l '$βϊ* *
TL
Stag e larva e emerg e fro m cys t ΠΓ Stag e larv a fre e i n soi l I Stag e larva e
Iattac k root s
E Stag e larva e attac k youn g root s
H Stag e larv a invade s roo t an d cause s formatio n o f syncyti o
ÌStag e mal e an d femal e larva e feedin g o n syncyti a
"ΠΣStag e larva e ^Syncyti a o f mal e pegin s t o degenerat e Adul t nematode s Mal e
Ñleave s Femal e begin s to produc e egg s _
Female s a t variou s
>stage s o f developmen t attache d t o roo t
^2nd
^mol t
3rd \mol t
growth of the female before death occurs. T h e cyst consists of the female cuticle transformed through the secretions of the nematode into a tough, brown sac that persists in the soil for many years and pro- tects the eggs which have b e e n formed within the body. T h e surface of the cyst shows markings appearing as short, zigzag lines without order.
Development of Disease
T h e infective second stage larvae enter young primary roots or api- cal meristems of secondary roots by direct penetration of the epider- mis (Fig. 121). T h e advance into the cortex is mostly intracellular and results in distortion and death of invaded cells. T h e larvae orient themselves in various directions in relation to the axis of the roots. In many cases they pass through the cortex and pierce their stylets into cells of the endodermis or the pericycle. Cells adjacent to invaded ones are frequently stimulated by n e m a t o de secretions, elongate toward the penetrated region, and fill the s p a ce m a de by the invading larva. Within 2 days from penetration larvae come to rest and fee d on cells of the cortex and stele tissues causing the enlargement of these cells. Nuclei of cells surrounding those penetrated by nematode sty- lets migrate through dissolved walls of the enlarged cells and clump.
Such groups of enlarged cells, called syncytia, are surrounded by a single layer of small, hyperplastic cells which, however, disappear gradually as the cells associated with the feeding site of the nematode enlarge and their cell walls undergo further dissolution and allow en- largement of the syncytia. E n l a r g ed cortical cells are connected through perforated walls to tissues of the endodermis, pericycle, and to vascular elements. During the d e v e l o p m e nt of the third-larval stage cortical cells surrounding the nematode are crushed by the expanding nematode body. Greater distortion of cortical tissue is c a u s ed by de- veloping females than by comparable males. Whe n the nematode feeds on the pericycle adjacent to protophloem tissue, syncytial devel- opment is restricted largely to pericyclic tissue. However, when the nematode feeds near or opposite protoxylem poles, syncytial develop- men t is relatively unrestricted and occurs in tissues of the d e e p er peri- cycle, phloem, and secondary cambium. It has b e e n observed that larvae feeding on cortex, endodermis or pericycle tissues which are opposite protophloem or crushed protophloem d e v e l op into males, while larvae feeding on syncytia which extend into the stele at or near the protoxylem poles and incorporated pericyclic, phloem, and sec- ondary cambial tissue, d e v e l op into females. Syncytia in contact with