CHAPTER 1
Conjugation in Bacteria
JULIAN D . GROSS
I. I n t r o d u c t i o n 1 I I . C o n j u g a t i o n i n Escherichia coli K 1 2 2
A . D e t e r m i n a t i o n of M a t i n g T y p e 2 B . T h e Origin of t h e F e r t i l e C e l l s i n F+ P o p u l a t i o n s 7
C . I n t e r m e d i a t e D o n o r S t r a i n s 8 D . T h e M o d e of A t t a c h m e n t of t h e S e x F a c t o r t o t h e C h r o m o s o m e 10
I I I . O t h e r S y s t e m s of C o n j u g a t i o n 11 A. C r o s s e s b e t w e e n S t r a i n s of Escherichia coli 11
B . C r o s s e s b e t w e e n Escherichia coli a n d O t h e r E n t e r o b a c t e r i a c e a e 13
C . C r o s s e s w i t h i n O t h e r S p e c i e s of E n t e r o b a c t e r i a c e a e 14 D . C o n j u g a t i o n M e d i a t e d b y C o l i c i n o g e n y F a c t o r s 15
E . T r a n s m i s s i b l e D r u g R e s i s t a n c e 15
F . FQ-lac F a c t o r of Salmonella typhosa 16
G. B a c t e r i o p h a g e r 17 H . S t r e p t o m y c i n - M u t a b i l i t y T r a n s f e r F a c t o r 17
I . C o n j u g a t i o n i n Pseudomonas aeruginosa 17
J. C o n j u g a t i o n i n Vibrio cholerae 18 K . C o n j u g a t i o n in Serratia marcescens 19 I V . U n i o n b e t w e e n D o n o r a n d R e c i p i e n t C e l l s 19
A. Surface P r o p e r t i e s of M a l e C e l l s 20 B . M o d i f i c a t i o n of t h e A b i l i t y of M a l e Cells t o C o n j u g a t e 20
C. T h e K i n e t i c s of Cellular U n i o n 23 D . E n e r g y R e q u i r e m e n t for Cellular U n i o n 26
E . T h e M a i n t e n a n c e of Cellular U n i o n 27 F . P h y s i o l o g i c a l E f f e c t s of C e l l u l a r U n i o n 27 V. C h r o m o s o m e T r a n s f e r d u r i n g C o n j u g a t i o n 28
A . O r i e n t e d T r a n s f e r 28 B . K i n e t i c A n a l y s i s of T r a n s f e r 32
C . P h y s i c a l D e t e c t i o n of C h r o m o s o m e T r a n s f e r 33 V I . T r a n s f e r of N o n c h r o m o s o m a l M a t e r i a l d u r i n g C o n j u g a t i o n 34
A. T r a n s f e r of C o n j u g a t i o n F a c t o r s 34 B . T r a n s f e r of D e t e r m i n a n t s O t h e r T h a n C o n j u g a t i o n F a c t o r s 35
C . P h y s i c a l D e t e c t i o n of T r a n s f e r of N o n c h r o m o s o m a l M a t e r i a l 35
D . T r a n s f e r of C y t o p l a s m i c M a t e r i a l 36
V I I . G e n e t i c R e c o m b i n a t i o n 36 A . T h e Efficiency of R e c o m b i n a t i o n 37
B . T h e T i m e of R e c o m b i n a t i o n 38 C. T h e M e c h a n i s m of R e c o m b i n a t i o n 39
D . A n a l y s i s of L i n k a g e 41
R e f e r e n c e s 44 I. Introduction
Three mechanisms of genetic exchange are known in bacteria : transfor
mation, transduction, and conjugation. I n each, a portion of the genetic
1
material of one cell is introduced into another, giving rise to a partially diploid zygote.1 T h e genetic material introduced from the donor cell cannot generally be maintained as an independently multiplying entity. However, genetic recombinants, i.e., clones of cells having characteristics of both the donor and the recipient cell, m a y arise from the zygotes as a result of ge
netic exchange between homologous regions of the two parental genomes.
I n transformation the agent of transfer is purified D N A (deoxyribonu
cleic acid) extracted from the donor cell; in transduction bacteriophages act as vectors of genetic material. Both processes involve the transfer of relatively small amounts of genetic material, and their study has been of great importance in elucidating the chemical nature and fine structure of the genetic material. However, knowledge of the over-all organization of the genetic material in bacteria has been derived mainly from the study of conjugation, in which large amounts of genetic material, occasionally even a complete genome, m a y be transferred.
The outstanding studies of Lederberg and his collaborators,1' 2 followed by those of Jacob and W o l l m a n3 and of H a y e s ,4 have established t h a t the genetic determinants of the characteristics of E. coli K12 are arranged in linear fashion on a single structure, or chromosome. T h e analysis of conju
gation in other species suggests t h a t the same is probably true of all Entero
bacteriaceae. Evidence has been obtained t h a t the chromosome of E. coli K12 is a closed or circular structure, t h a t is, one having no ends.3
The ability to conjugate is conferred upon cells by the presence of dis
crete genetic determinants, which m a y exist independently of the bacterial chromosome and be transferred with high frequency during conjugation.
They are closely allied to certain other determinants such as prophages and determinants of bacteriocin production.5 T h e application of conjugation to analysis of the nature of these determinants has demonstrated t h a t some, if not all, are capable of existing in two alternative states: an "autonomous"
state, in which they are transferred independently of the bacterial chro
mosome, and an "integrated" state where they are transferred along with it.5 Such determinants, known as episomes, are the subject of a separate chapter and will be considered here only when relevant to an understanding of conjugation. Conjugation in E. coli has been the subject of a number of excellent reviews.1 , 3» 6 - 8
II. C o n j u g a t i o n in Escherichia coli K 1 2
A . DETERMINATION OF M A T I N G T Y P E
I n 1946 Lederberg and T a t u m observed the formation of prototrophic recombinants in mixtures of different multiple auxotrophic derivatives of Escherichia coli K12. T h e prototrophs arose a t frequencies of about 1 per
1. CONJUGATION I N BACTERIA 3 107 parental cells and appeared as isolated colonies on solid medium on which neither of the two parental types of auxotrophic cells could g r o w .9'1 0 Recombinant formation was later shown to require direct contact between cells of the parental strains, since supernatants or filtrates of cultures of either strain were incapable of yielding recombinants when mixed with cells of the other s t r a i n .1 0 Furthermore, no recombinants were formed when cul
tures of the two parental strains were placed in the separate arms of a U-tube divided by a sintered glass filter which prevented passage of intact cells from one arm to the other, but allowed thorough mixing of the culture fluids.11
The parental strains employed in some of the early crosses differed in characters other t h a n their auxotrophic requirements and the important observation was made t h a t these unselected characters did not assort at random among recombinants selected for prototrophy.2' 9 > 1 0 This indicated t h a t the genetic determinants (markers) controlling these characters were physically associated in some precise manner. At t h a t time genetic recom
binants in bacteria were believed to arise by a process similar to meiosis in zygotes formed by the fusion of complete parental genomes. However, data obtained in further studies were difficult to reconcile with this assumption.
T h e first evidence t h a t the parental cells do not play identical roles in conjugation was provided by Hayes, who showed t h a t the cells of one of the parents could be pretreated with streptomycin and their viability drastically reduced without markedly affecting the yield of recombinants, whereas treatment of cells of the other parent prevented recombinant for
m a t i o n .1 2 I n addition, ultraviolet irradiation of the former strain stimulated recombinant formation while irradiation of the latter led to reduction in yield parallel with loss of viability.1 3 These observations were interpreted as showing t h a t cells of one of the parents serve as donors of genetic m a t e rial, while those of the other act as recipients and give rise eventually to the recombinant clones.
The division of strains into donors and recipients was confirmed by the chance discovery of derivatives of a "donor" strain which were no longer fertile when mixed with cells of a "recipient" s t r a i n .1 4 - 1 6 Systematic study indicated t h a t combinations of donor and recipient cultures were generally about 10 times more fertile t h a n mixtures of two donor strains and t h a t crosses of two recipient strains were always sterile. Prior to this time all the combinations of strains which had been examined had evidently in
volved either mixtures of two donors strains or of a donor and a recipient strain. Cells of recipient type were found to be converted with high effi
ciency to donors by conjugation with donor c e l l s .1 4 - 1 6 T h e ability to act as donor appeared therefore to depend on the presence of a determinant, termed F,lé which was transmitted with high frequency from donor ( F + )
to recipient ( F ~ ) during conjugation, in marked contrast to the low effi
ciency of transfer of chromosomal determinants (see Table I ) .
I t was observed t h a t recombinants tended to inherit most of their un- selected markers from the F ~ p a r e n t .4'1 5- 1 6 This led to the suggestion that the donor usually transfers only a p a r t of its genome to the recipient cell, so t h a t recombinants are derived from incomplete z y g o t e s .4-1 6-1 7 An alternative explanation involving the elimination of p a r t of the donor genome after its transfer was also p r o p o s e d1 , 1 5 , 1 8 but was abandoned as a result of studies which will be considered below.
T A B L E I
P R O P E R T I E S O F H F R A N D F+ D O N O R C E L L S '
Property F+ donor Hfr donor
F r e q u e n c y of t r a n s f e r of c h r o m o s o m a l d e t e r m i n a n t s
V e r y l o w ( 1 0 -4- 1 0 ~6) for all d e t e r m i n a n t s
R a n g i n g f r o m h i g h ( 1 0_ 1) t o l o w ( 1 0 -4) for dif
ferent d e t e r m i n a n t s F r e q u e n c y of transfer of
d o n o r a b i l i t y
V e r y h i g h ( 0 . 5 - 1 0 -1) L o w (10-3-10-4)
T y p e of d o n o r a b i l i t y t r a n s f e r r e d
F + H f r
L i n k a g e of d o n o r a b i l i t y t o c h r o m o s o m a l d e t e r m i n a n t s
U n l i n k e d L i n k e d t o d e t e r m i n a n t s t r a n s f e r r e d w i t h l o w e s t f r e q u e n c y S u s c e p t i b i l i t y of d o n o r
a b i l i t y t o a c r i d i n e s
S u s c e p t i b l e N o t s u s c e p t i b l e
a S e e t e x t for r e f e r e n c e s .
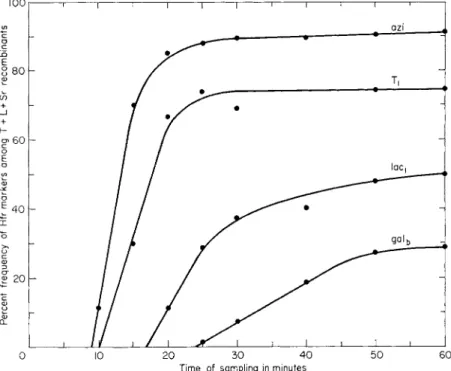
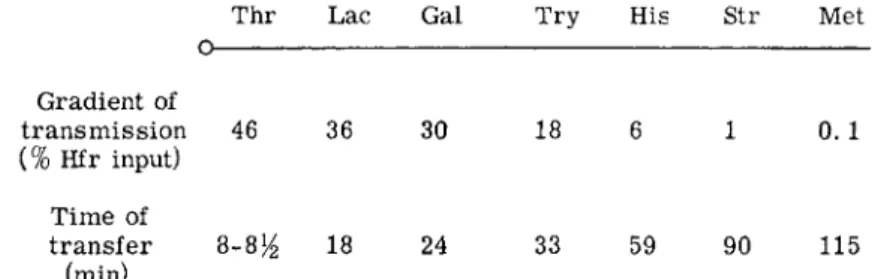
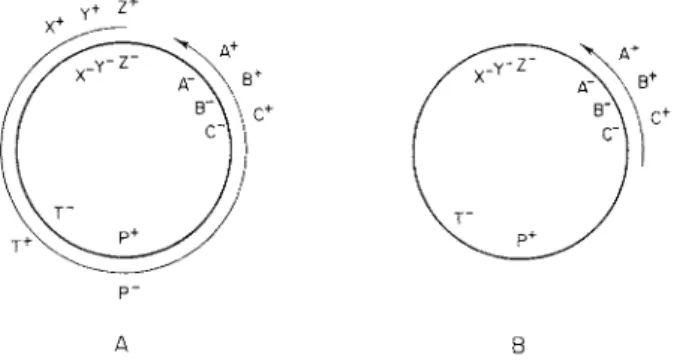
The key to an understanding of the role of the F factor was provided by crosses involving a new type of donor cell, which originated from an F + strain, and is referred to as H f r4 , 1 9 (high frequency of recombination). I n such crosses the yield of recombinants depends on the selected donor marker. With some markers the yield is up to 104 or 105 fold greater t h a n in F + crosses while with others it is not much more than in F+ crosses.4 Jacob and Wollman have shown t h a t the markers of any Hfr strain can in fact be arranged in a continuous gradient with respect to their frequency of transmission to recombinants.3- 2 0 This gradient is due to the fact t h a t transfer is a slow oriented process which starts at the same point of the chromosome or "origin," in all the cells of any one Hfr strain, and is inter
rupted by random spontaneous breakage of the chromosome as transfer progresses. As a result recipient cells receive fragments all of which start
1. CONJUGATION I N BACTERIA 5 at the same point but have variable lengths.3 , 2 0 B y contrast populations of F + cells give rise to approximately the same number of recombinants irrespective of the particular marker selected.
Comparison of the inheritance of donor ability in F + and Hfr crosses provides strong evidence t h a t the F factor is in an autonomous state in F + cells, whereas it is attached to the bacterial chromosome in Hfr cells. A summary of the relevant observations is given in Table I. F + cells t r a n s fer donor ability with high frequency and independently of the bacterial chromosome whereas Hfr cells only rarely transfer the ability to act as d o n o r1 4 , 1 5 ; only those recombinants t h a t inherit donor markers which are located at the furthest extremity of the chromosome and are thus trans
ferred with lowest frequency m a y be donors and those t h a t are donors are invariably of Hfr and not F + t y p e .4 , 2 1« 2 2 The Hfr character thus behaves
Hfr s t r a i n
Hfr H Ο T h r — Leu — T6 T r y — His — Str Met — Thi — HfrC Ο Τ6 Leu — T h r — Thi — Met — Str — His T r y — J 4 Ο Thi — Met — Str His — T r y — T6 Leu — T h r — G10 0 Met — Thi — T h r — Leu — T6 T r y — His Str —
AB-311 Ο His — T r y — T6 Leu — Thr — Thi — Met Str FIG. 1 . T h e order of transfer of various c h r o m o s o m a l markers b y different Hfr strains.
Ο stands for the origin, the c h r o m o s o m a l e x t r e m i t y which first enters t h e recipient cell during transfer. T h e m e a n i n g of the s y m b o l s is g i v e n in t h e legend for Fig. 6 .
as a chromosomal determinant linked to the terminal region of the Hfr chromosome. Further evidence for the chromosomal attachment of the F factor in Hfr cells is provided by the finding t h a t their donor ability is not affected by growth in acridine orange, which is known to act upon various types of cytoplasmic particle, whereas after similar t r e a t m e n t a large proportion of F + cells are converted to F-.2 3 T h a t the F factor is actually present in Hfr cells is indicated by the fact t h a t Hfr cells can only arise from F + strains and t h a t they can revert to the F + s t a t e .4 , 1 4 T h e transi
tion from F+ to Hfr and vice versa thus corresponds to chromosomal at
tachment and detachment of the F factor.
Jacob and Wollman have compared the orientation of chromosome transfer in a group of Hfr strains isolated from the same F + p a r e n t .2 2 Interrupted mating experiments (see Section V, A) showed t h a t all the cells of a given strain transfer their determinants in a precise sequence but t h a t the order of transfer is different for each Hfr strain. T h e order of transfer of several Hfr strains is shown in Fig. 1. I t m a y be seen t h a t
despite the difference in order of transfer the relative position of the m a r k ers does not change at a l l .2 2 This observation provides convincing evidence t h a t the determinants of E. coli K12 are located on a single chromosome.
I t is apparent t h a t although the chromosomal structure transferred by the cells of any one Hfr strain has definite ends and m a y therefore be repre
sented as a straight line the chromosome of the F + strain from which the various Hfr strains arose cannot be represented in this way. For, no matter in which linear sequence the markers are written, Hfr strains can be found which transfer the markers at opposite ends of the sequence in im
mediate succession. If, however, the F + chromosome is represented by a circle then the sequence of transfer by a given Hfr m a y be derived by
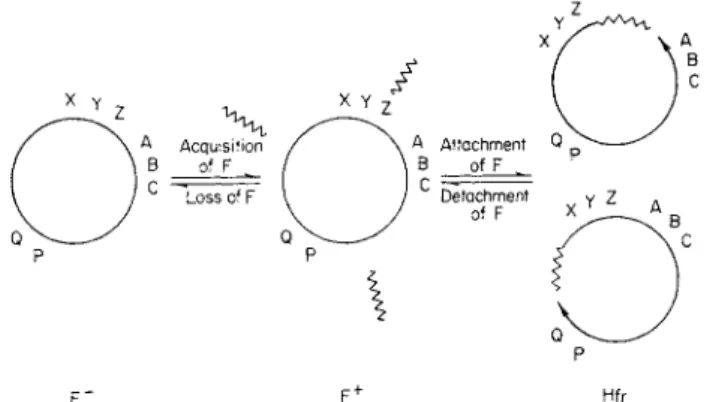
F " F+ Hfr
FIG. 2. D i a g r a m m a t i c representation of t h e sexual t y p e s in E. coli K 1 2 . T h e F fac
tor is indicated b y a short zigzag line. T h e letters represent h y p o t h e t i c a l chromosomal markers and t h e arrows the leading extremities (origins) of t w o possible Hfr t y p e s .
F r o m Jacob and W o l l m a n (ref. 3, p . 187).
opening it at the appropriate point and reading the sequence of markers in one or the other direction from t h a t point.2 2
Figure 2 contains a diagrammatic representation of the hypothesis con
cerning the determination of sexual types t h a t has been developed by Jacob and Wollman.3 , 22> 2 4 T h e F ~ cell has a closed chromosome and no sex factor, while the F + cell also has a closed chromosome b u t contains autonomous F factors which enable it to conjugate with F ~ cells. T h e F factors are transmitted with high frequency during conjugation so t h a t recombinants from F + X F ~ crosses are generally F + . T h e transition from F + to a given Hfr type is due to attachment of an F factor at some point on the F + chromosome followed by actual or potential rupture of the chromosome at the point of attachment to give a linear structure. T h e extremity to which the F factor remains attached upon rupture corresponds to the distal extremity of the Hfr chromosome and the other to the leading extremity in transfer.
1. CONJUGATION IN BACTERIA 7 When the F factor becomes integrated to the chromosome a mechanism is set up which represses the multiplication of autonomous F factors.2 5' 2 6 Consequently Hfr cells do not harbor any autonomous F factors, and re
combinants from Hfr X F ~ crosses are F ~ except for the few which inherit the terminal chromosomal extremity and are Hfr. The integrated sex factor of Hfr cells very occasionally reverts to the autonomous F+ state;
sometimes, as discussed below, it carries with it a fragment of the bacterial chromosome adjacent to its site of attachment, giving rise to an intermedi
ate donor strain in which the autonomous sex factor has a high affinity for its original site of attachment.
B . T H E ORIGIN OF THE F E R T I L E C E L L S IN F + POPULATIONS
The fact t h a t F + cells transfer the autonomous F factor efficiently but give rise to very few genetic recombinants, whereas cells in which F is integrated initiate chromosome transfer with extremely high efficiency suggests t h a t F+ cells as such, i.e., cells in which F is not associated with the chromosome, are unable to bring about chromosome transfer.6 , 2 7 The rare recombinants formed in mixtures of F+ and F~~ cells are thought to be due to the presence in F + cultures of a small number of cells in which F has become attached to the chromosome. Since the F factor can become integrated at different sites the gradients of marker transfer corresponding to each particular site of attachment tend to cancel one another out, so that, among the population as a whole, all markers are transferred at ap
proximately the same frequency.6 , 2 7
I t is not clear whether the association between F and the chromosome in the fertile cells of an F + population is invariably similar in its stability to t h a t observed in the known Hfr strains or whether the latter represents only one extreme of a wide spectrum of stability. T h e attachment in an Hfr strain must be quite stable if it is to be isolated and maintained in the laboratory and even among the known Hfr strains the frequency of re
version to the F + state does v a r y somewhat.2 8
Jacob and Wollman have observed t h a t the variation in fertility between small independent cultures of F+ cells is considerably greater t h a n between samples from the same culture.2 7 This result indicates t h a t fertility can be clonally inherited and therefore t h a t a considerable proportion of the fertile cells which arise have some degree of stability. T h e same workers were able to isolate stable Hfr cells from most of the fertile cells detected in an F + population.2 7 However, this does not necessarily mean t h a t most of the fertile cells are typical Hfr's since the technique used in detecting the fertile cells involved indirect selection by replica plating and would be biased in favor of more stable donor types.
There is, in fact, some evidence t h a t the fertile cells in F + populations m a y not be quite like typical Hfr cells. For example the majority of re-
combinants from F + X F ~ crosses are F + , and reconstruction experiments have shown t h a t they must have acquired F from the cell which contributed the chromosomal marker to the r e c o m b i n a n t .2 9 - 3 1 The fertile cells in F + populations must therefore possess and be able to transfer F, unlike estab
lished Hfr cells. However, this difference is of doubtful significance, since irrespective of their stability a sizable proportion of the fertile cells present at any one time in an F + population must be of recent origin. T h e y would therefore be expected to continue to harbor and transfer F pending its elimination as a result of repression of its multiplication. T h e existence of unstable donor cells is also indicated by experiments on the stimulation of the fertility of F + populations by ultraviolet (UV) irradiation.4 I t was found t h a t the fertility of F + cultures reached a maximum about 1 hour after irradiation and then gradually fell off, indicating t h a t most, if not all, of the increased fertility is due to unstable donor cells.
W o r k with conjugation systems other t h a n t h a t of E. coli K12 supports the idea t h a t fertility can result from unstable interactions between F and chromosome. Zinder has reported t h a t the fertile cells present in cultures of Salmonella typhimurium which have acquired the F factor by con
jugation with E. coli K12, show some clonal stability. However, he was un
able to isolate stable Hfr derivatives except after heavy UV irradiation of the donor cells.3 2 I n addition it has not proved possible to isolate any stable Hfr derivatives from P + strains of Pseudomonas which have donor
properties similar to those of F + cells3 3 (see Section I I I , I ) .
I t appears, therefore, t h a t the F factor can bring about transfer of the bacterial chromosome by becoming transiently attached to it. While there is no doubt t h a t Hfr cells contribute to the fertility of F+ populations the frequency of transfer of individual markers m a y be accounted for by
assuming t h a t the majority of fertile cells represent those in which the F factor has become transiently attached to one of an indefinite number of chromosomal sites.8 Preliminary mapping of the origins of a number of Hfr strains (Fig. 7, p. 31) suggests t h a t the number of sites of stable at
tachment is restricted.
C . INTERMEDIATE DONOR STRAINS
Adelberg and B u r n s3 4 have studied a derivative of an Hfr strain, which transfers its chromosome with high frequency and with the same orienta
tion as the parent strain, but gives rise to recombinants which are them
selves high frequency donors and transfer donor ability to cells with which they conjugate. This aberrant strain thus combines the infective proper
ties of an F + with the high frequency chromosome transfer of an Hfr, and has been referred to as an intermediate donor. I t appears to harbor a sex factor which has incorporated a segment of the bacterial chromosome near its site of attachment in the parent Hfr strain, and thus possesses a
1. CONJUGATION I N BACTERIA 9 region of homology with the chromosome a t the point where it had origi
nally been attached. As a result it frequently becomes integrated at t h a t point and brings about high frequency chromosome transfer with the same orientation as in the original Hfr p a r e n t .3 4
The chromosomal segment thought to be incorporated in this hybrid sex factor does not carry any known determinant. However, if the ex
planation of the behavior of the aberrant donor strain is correct, it should be possible to isolate cells harboring hybrid sex factors which carry known bacterial markers, provided t h a t the F factor in the Hfr strain which one uses as source is located close to a convenient marker. Factors of this kind (called F') have been isolated from several different Hfr strains.2 8' 3 5« 3 6 T h e segment of bacterial chromosome in the F' factors is variable in size but always corresponds to the distal end of the Hfr chromosome from which they originated. I n addition, these factors invariably bring about high frequency chromosome transfer with the same orientation as in the parent Hfr s t r a i n s .2 8 , 3 5 Their properties thus provide additional proof of the role of attachment of sex factor to chromosome in transfer of the latter.
A different type of intermediate donor strain has been described. I t s properties m a y be illustrated by further observations made by Adelberg and Burns. T h e y found t h a t when the hybrid sex factor was eliminated from the original intermediate donor strain by acridine orange treatment and the resulting F ~ cells infected with F the derivatives obtained were again intermediate donors, despite the fact t h a t they now only harbored and transmitted a normal F factor. This remarkable observation suggested t h a t the intermediate donor strain had originated in an event which not only gave rise to the hybrid sex factor but also left a piece of F a t the initial point of attachment. This piece of F would not by itself be able to bring about chromosome transfer but would provide a region of homology for attachment of autonomous sex factors. Consequently, cells of this t y p e harboring a normal F factor should mediate chromosome transfer with high frequency owing to the frequent association between F and the in
serted material, which is referred to as a "sex factor affinity" (sfa) locus.3 4 Richter has studied a strain which carries an sfa locus at a different loca
t i o n .3 7 I t was isolated as a m u t a n t incapable of fermenting maltose, after UV irradiation of an F + culture, and transfers its chromosome with high frequency and with constant orientation. Analysis of the inheritance of the intermediate donor property of this strain has provided direct proof of the presence of the sfa locus. Intermediate donor activity is transferred as a distal character during oriented chromosome transfer, just as is donor ability in Hfr cells. A further point of interest is t h a t the sfa locus and the mal~ mutation are inseparable, showing t h a t they must have arisen in one and the same event.
Intermediate donor strains thus fall into two categories: one possessing
an sfa locus and normal F factor, and the other a hybrid F factor and a chromosome without any sfa locus. I n each the high frequency of oriented chromosome transfer is due to homology between the sex factor and a spe
cific point on the bacterial chromosome.
Cells harboring an sfa locus and normal F can exist in alternative states corresponding in all essentials to the F + and Hfr states of normal K12 strains. Owing to frequent transition from one state to the other cultures grown from cells with F in the autonomous state contain about 1% of cells in which F is in the integrated state. Such populations therefore trans
fer chromosomal markers at moderately high frequency as well as being highly infective for F. Similarly, cultures grown from cells with F in the
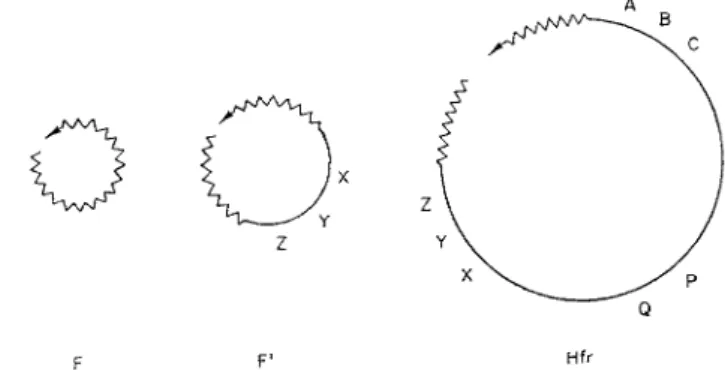
F F' Hfr
FIG. 3. H y p o t h e t i c a l structure of F and F' factors and of the Hfr c h r o m o s o m e . T h e genetic material of the F factor is represented b y a zigzag line and that of the bacterial c h r o m o s o m e b y a straight line. T h e arrows indicate the e x t r e m i t y of each structure which first penetrates the recipient cell and the letters represent h y p o t h e t i c a l chromo
s o m a l markers.
integrated state contain about 1% of cells in which the sex factor has re
verted to the autonomous s t a t e .3 7 I n intermediate donor strains of the other category the frequency of transition of the hybrid sex factor from auton
omous to integrated states, and vice versa, appears to be so great t h a t cul
tures in which one or the other state predominates cannot be obtained.3 4
D . T H E M O D E OF ATTACHMENT OF THE S E X FACTOR TO THE CHROMOSOME
A general hypothesis for the mode of attachment of the sex factor is illustrated in Fig. 3. I t is based on the idea t h a t chromosome transfer is a special case of transfer of the sex factor itself, and on the fact t h a t attach
ment of the sex factor not only determines the point at which the chromo
some opens, but also specifies the direction of its transfer. I t is assumed t h a t transfer of F is itself an oriented process, and t h a t the orientation of chro
mosome transfer is a direct result of this basic orientation. I t is possible
1. CONJUGATION I N BACTERIA 11 t h a t the F factor is a closed structure which opens a t a specified break point during conjugation. If so7 this scheme would suggest t h a t the Hfr chromo
some is also closed except during its transfer, which is in accord with r e sults obtained b y Taylor a n d Adelberg.3 7 a T h e scheme also allows for the possibility t h a t in addition to t h e distal piece of F whose transfer is neces
sary for t h e inheritance of the H f r character, there is also a piece located at the leading end of t h e c h r o m o s o m e .3 4 , 3 8
T h e major feature of the scheme in Fig. 3 is the colinearity of F and t h e chromosome in Hfr cells. I t h a s two advantages. T h e first is that, as al
ready discussed, it could account well for t h e opening of t h e F + chromo
some in t h e mutation t o t h e Hfr state and for t h e imposition of a unique direction of chromosome transfer. Secondly, a single genetic exchange be
tween a circular F or F' factor and t h e bacterial chromosome would give rise to a chromosomal structure like t h a t in Fig. 3. T h e scheme would thus explain t h e ability of Ff factors to bring about chromosome transfer. F o r further discussion the reader is referred to the stimulating review b y C a m p bell.3 9
III. O t h e r Systems o f C o n j u g a t i o n
All systems of conjugation which have been studied in detail are con
trolled by episomal elements which are similar to t h e F factor. These ele
ments will be referred to as conjugation factors. T h e only known properties conferred upon t h e cell b y some conjugation factors are those directly connected with t h e ability to conjugate. Others carry, in addition, deter
minants of properties such as resistance to antibiotics or ability to ferment sugars. T h e main features of the conjugation systems known in Eubacteria are listed in Table I I . Genetic exchange in actinomycetes is considered separately in Chapter 5.
A. CROSSES BETWEEN STRAINS OF Escherichia coli
Lederberg and his co-workers have performed crosses between an F + strain of E. coli K 1 2 and auxotrophic derivatives of a large number of other E. coli strains. Only about one in twenty of t h e combinations was fertile.1 4 0rskov and 0rskov examined 200 independent strains and de
tected recombinants in 18% of the crosses with an F + donor, and in 30%
with H f r donors.4 0 T h e somewhat higher degree of interfertility observed by the latter workers is probably due to greater homogeneity of t h e strains examined. T h e infertility of most of these crosses is presumably due either to inability to form a cellular union, or to poor homology between t h e genetic material of t h e participating strains which would interfere with genetic recombination. I n addition, other factors such as colicin production
T A B L E I I
D I S T R I B U T I O N A N D M A J O R F E A T U R E S O F C O N J U G A T I O N S Y S T E M S "
Bacterial species in which conjugation was originally
observed
Transmissible conjugation
factor
Determinants associated with
conjugation factor
Transfer of chromo
somal de
terminants
Species to which conjugation factor
has been transferred Escherichia coli
K 1 2
F N o n e
+
S. typhimurium,Shigella s p . E. coli K 1 2 F' S e g m e n t s of t h e
b a c t e r i a l c h r o m o s o m e
V. cholerae, S.
marcescens, Salmonella s p . E. coli U n n a m e d
f a c t o r s
N o n e r e p o r t e d
+
E. coli K 1 2 Salmonella typhimurium*
E. coli K 1 2 f
col I (col B)
B a c t e r i o c i n p r o d u c t i o n
V a r i o u s e n t e r o - b a c t e r i a
E. coli
Shigella sonnei Shigella flexneri
R A n t i b i o t i c r e s i s t
a n c e
+
V a r i o u s e n t e r o - b a c t e r i a , V.comma Salmonella typhosa, F°-lac L a c t o s e f e r m e n t
a t i o n
N o n e d e t e c t e d
V a r i o u s e n t e r o - b a c t e r i a , V.
cholerae E. coli M u t a t o r
t r a n s f e r f a c t o r
I n s t a b i l i t y at s t r e p t o m y c i n l o c u s
N o t e x a m i n e d
E. coli K 1 2
Pseudomonas ae
ruginosa
FP N o n e
+
N o t e x a m i n e dVibrio cholerae Ρ B a c t e r i o c i n p r o
d u c t i o n (?)
+
N o t e x a m i n e d Serratia mar ces -cens
N o n e y e t d e t e c t e d
—
+
—a R e f e r e n c e s c o n c e r n i n g t h e v a r i o u s s y s t e m s are g i v e n i n t h e a p p r o p r i a t e p a r t of t h e t e x t .
* col I w a s i n t r o d u c e d f r o m Shigella sonnei b y m i x e d c u l t u r e .6 0
t col I w a s i n t r o d u c e d f r o m Salmonella typhimurium b y m i x e d c u l t u r e .6 3
1. CONJUGATION I N BACTERIA 13 or the presence in the donor of prophages or other determinants m a y pre
vent the formation of recombinant clones.4 1
T h e F factor of E. coli K12 has been transferred to several other strains of E. coli as shown b y the recovery of cells able to transmit i t back t o t h e donor or to act as donors of genetic material themselves ; t h e fragmentary data available indicate t h a t these F + strains are generally infertile in crosses with E. coli K 1 2 .1 4 , 4 2» 4 3 The infertility is probably n o t due to in
ability of the F factor t o bring about chromosome transfer in these hosts but rather to t h e same causes as t h e infertility observed in interstrain crosses in which E. coli K12 cells are t h e donors. Bernstein h a s in fact studied a n F + strain which was infertile when crossed with recipients of strain K12 b u t fertile in combination with derivatives of its own s t r a i n .4 2 However, other strains were fertile as recipients in crosses with donors of strain K12 but could not be made fertile with K12 recipients b y infection with F .4 2 F + strains of E. coli Β can be produced and are fertile both with other derivatives of E. coli Β and with F ~ strains of K 1 2 .4 4 - 4 7
A large number of E. coli strains have been tested for ability t o yield recombinants in crosses with an auxotrophic F ~ derivative of strain K12.
About one in forty was found t o be fertile ;4 8 i t is possible t h a t a consider
ably higher proportion are fertile within themselves.4 9 Some of t h e fertile strains must harbor transmissible conjugation factors since they are able to transfer fertility to a n F ~ strain of E. coli K 1 2 .1 4>1 5 These factors are not identical to F since some of them give rise to unstable donor strains when introduced into K 1 2 ,1 4 a n d there is evidence from examination of the segregation of unselected markers t h a t the affinity of these factors for chromosomal sites is different.4 9 T h e inability of certain other fertile strains to transfer fertility t o E. coli K12 m a y be due to an even greater instability of their conjugation factor in the K12 host.
One interesting strain showed low fertility in crosses with K12 recipients and was unable to transfer fertility t o the latter. I t could be infected with F a n d t h e derivative obtained showed similar fertility to F + strains of K12 b u t reverted with high frequency t o t h e original donor t y p e .4 2 Other strains have been found which show little or no fertility in crosses with K12 recipients but can transmit t o them conjugation factors which render them fertile.4 9
B. CROSSES BETWEEN Escherichia coli AND OTHER ENTEROBACTERIACEAE
Crosses between donor strains of E. coli K12 and strains of Salmonella are generally infertile, b u t a few Salmonella strains do give rise to a low yield of recombinants.5 0"5 3 If these recombinants are used as recipients in further crosses t h e yield of recombinants is considerably increased and
analysis of such crosses has demonstrated over-all similarity in the se
quence of chromosomal determinants in the two species.5 3 , 5 4 I t has been suggested t h a t the majority of cells of Salmonella strains are unable to act as recipients in crosses with E. coli K12 and t h a t the few recombinants t h a t are produced are derived from rare fertile m u t a n t s and consequently act as efficient recipients in further crosses. Such mutants can in fact be isolated by replica plating.5 5 The mutation involved seems likely to affect the efficiency of cellular union with the donor cell rather t h a n the ac
quisition of increased genetic homology with the E. coli chromosome since mutants with a greatly improved ability to act as recipients of F' factors from E. coli have been isolated in Serratia marcescens.56
The importance of genetic homology in the formation of recombinants is indicated by the fact t h a t some Salmonella strains which do not yield any detectable genetic recombinants can act as recipients of autonomous determinants such as F' factors.5 7 Moreover, crosses between E. coli K12 donors and strains of Shigella as recipients are fertile b u t the yield of re
combinants is a hundred to a thousand times lower than when F ~ strains of E. coli K12 are used as recipients. T h e low yield is due to low recombina
tion efficiency, since the chromosomal segment carrying lambda prophage has been shown to be transferred with high efficiency.5 8
The F factor has been transferred from E. coli K12 to Salmonella typhi
murium82 and to strains of Shigella.58 The F + strains obtained are able to transfer F back to E. coli, but with considerably lower efficiency than to cells of their own species.
C . CROSSES WITHIN OTHER SPECIES OF ENTEROBACTERIACEAE
Zinder has studied the fertility of crosses involving derivatives of Sal
monella typhimurium which have received the F factor by conjugation with E. coli. T h e yield of recombinants in F + X F~~ crosses in Salmonella is similar to t h a t in E. coli, and the frequency of inheritance of unselected markers indicates t h a t the chromosomal segments transferred are also of about the same size in the two systems.3 2
Analysis of the fluctuation in yield of recombinants from independent F + cultures indicates t h a t recombinants are derived from a small minority of fertile cells which have some degree of clonal stability, as in F + cultures of E. coli K12. Attempts to isolate stable Hfr strains by indirect selection were unsuccessful although an initial increase in the proportion of fertile cells was obtained. Hfr strains could, however, be isolated after irradiating the F + cells with large doses of ultraviolet light.3 2 The Hfr character has been transferred from E. coli to Salmonella by selecting for recombinants inheriting a marker close to the distal chromosomal extremity of the Hfr strain used as donor.5 4
1. CONJUGATION IN BACTERIA 15 T h e properties of F + strains of Shigella are strikingly different from those of F + strains of Salmonella; none of them gave any recombinants in crosses either with other Shigella derivatives or with F ~ strains of E. coli K12 despite repeated attempts with a variety of selected m a r k e r s .5 8 This infertility m a y perhaps result from inability of the F factor to mobilize the Shigella chromosome for transfer due to the absence of any chromosomal site for its attachment.
D . CONJUGATION MEDIATED BY COLICINOGENY FACTORS
Fredericq and other workers have shown t h a t the production of colicines and related substances is under the control of autonomous determinants which m a y be transferred during conjugation (see Chapter 4). I t has been shown recently t h a t certain determinants of colicine production, notably col I and col B, are able to mediate their own conjugal transfer as well as transfer of other colicinogeny determinants.5 9' 6 0 Cells of established col- icinogenic strains transfer the col I or col Β determinant with only low efficiency. However, cells which have recently acquired the determinant transmit it with very high efficiency. This difference appears to be due to a difference in the efficiency with which they form contact with recipient cells.6 1
Ozeki and Howarth have shown t h a t cells of S. typhimurium which have recently acquired col I can bring about transfer of chromosomal deter
minants with very low frequency.6 2 Similar low frequency chromosome transfer has been observed in E. coli K 1 2 .6 3 If the cells of S. typhimurium harbor col El in addition to col I the frequency of chromosome transfer is increased approximately 100-fold.6 2 The mechanism of this stimulation is unknown.
Chromosome transfer is unidirectional from col+ to col~ cells and ap
pears to involve chromosome segments similar in size to those in /^-medi
ated transfer.6 3' 6 4 T h e study of coi-mediated recombination adds further support to the evidence t h a t the genetic determinants of Salmonella are arranged on a single chromosome and in the same sequence as in E. coli K 1 2 .6 4 Moreover, it is not possible to ascribe any ends to the chromosome since all adjacent pairs of markers show a high frequency of joint transfer.
I t m a y therefore be concluded t h a t the chromosome of S. typhimurium like t h a t of E. coli K12 is a closed structure.6 4
E . TRANSMISSIBLE D R U G RESISTANCE
Since 1955 numerous drug resistant strains of Shigella and E. coli have been isolated from patients with bacillary dysentery in J a p a n (for review see ref. 65). Most strains are resistant to streptomycin, chloramphenicol, tetracycline, and sulfonamide, but others are only resistant to certain com-
binations of these four drugs. The determinants of drug resistance are lo
cated on an episomal structure, termed an R factor, which is capable of bringing about its own transfer by conjugation.6 5-6 7 Multiple drug re
sistance can be transferred between Shigella and E. coli, and to most other species of E n t e r o b a c t e r i a l and to Vibrio cholerae.™ As in col /-mediated transfer, cells which have recently acquired an R factor transmit it with much higher efficiency t h a n do cells of established resistant s t r a i n s .6 5 Transfer of R factors is stimulated by ultraviolet irradiation, which in addition renders the R factor very sensitive to elimination by acridine d y e s .6 9
R factors are capable of bringing about transfer of chromosomal deter
minants at low frequency.7 0 The frequency of chromosome transfer is in
creased 100-fold by the presence of the sex factor affinity locus sfa which, as discussed above, is believed to be a piece of the F factor inserted in the bacterial chromosome. Moreover, this transfer has the same orientation as t h a t found by Richter in a strain harboring sfa and F factor.7 0 These observations point to the existence of homology between F and R factors, and also support the general validity of the idea t h a t chromosome transfer results from physical association between conjugation factor and chromo
some.
Most R factors which have been examined have the interesting property of almost completely suppressing transfer of F or of the chromosome when introduced into F + or Hfr cells of E. coli K 1 2 .6 5 This effect is associated with suppression of the characteristic surface component associated with the F factor (see Section IV,A) which is almost certainly required for con
tact formation.7 1 The mechanism of this suppression will be considered be
low.
F . F°-lac FACTOR OF Salmonella typhosa
Strain ST-2 of Salmonella typhosa, which was isolated from a hospital patient, is similar to other strains of typhoid bacteria except for its ability to ferment lactose. I t is able to transfer this ability with varying efficiency to a wide variety of E n t e r o b a c t e r i a c e a e .7 2'7 3 T h e lactose fermenters so ob
tained are in turn able to transfer this character. I t was a t first thought t h a t strain ST-2 was an Hfr strain with the exceptional property of t r a n s ferring the Hfr character to all recombinants.7 2 However, further analysis has shown t h a t the determinant of lactose fermentation is located on an autonomous structure, termed F°-lac, which is capable of mediating its own conjugal transfer as a single unit, independently of the bacterial chro
mosome.7 3 Small doses of UV stimulate F°-lac transfer 10- to 50-fold;
stimulation reaches a maximum about an hour after irradiation and then decreases. A variety of strains harboring F°-lac have been tested for ability to transfer several different chromosomal markers, with negative results.
1 . CONJUGATION IN BACTERIA 17 The lac determinant of F°-lac and the lac segment of E. coli K12 m a y be at least partially homologous since they can undergo genetic recombina
tion. In addition, the F° determinant appears to be related to the F factor since cells harboring F°-lac exhibit a weak but definite cross-reaction with antisera to the antigen of F+ cells (see below). Also F°-lac, like F or F' factors, is transferred more than a hundred times less efficiently to F + or Hfr strains of E. coli K12 t h a n to F ~ strains.
G . BACTERIOPHAGE Τ
A system of genetic transfer involving phage τ has been described briefly in a Japanese a b s t r a c t7 4 and in the review of multiple drug resistance.6 5 Phage τ is a temperate phage which can lysogenize F ~ cells of E. coli K12.
I t adsorbs to F + cells but cannot establish lysogeny or multiply in them.
It was initially thought t h a t the transfer of genetic material mediated by τ required cellular contact. However, recent evidence indicates t h a t the system involves transduction by free phage.
H . STREPTOMYCIN-MUTABILITY TRANSFER FACTOR
Strains of E. coli exhibiting a high rate of mutability at the streptomycin ocus have been isolated from hospital patients. These strains show a nor
mal rate of mutation at all other loci tested. Evidence has been obtained t h a t the instability at the streptomycin locus is due to a determinant of episomal n a t u r e .7 5 Cells harboring the mutator episome transfer it at low frequency to E. coli K12. The frequency of transfer of the mutator episome is increased if the cells are made F + by infection from K12, but not by ir
radiation of the donor cells with ultraviolet light.
I. CONJUGATION IN Pseudomonas aeruginosa
Conjugation in Pseudomonas was discovered by Hollow a y .7 6 I t is con
trolled by a transmissible factor which has been termed FP.78 Crosses be
tween F P + and F P ~ strains yield about 1 recombinant per 107 parental cells; those between two F P + strains yield somewhat fewer recombinants;
while mixtures of F P ~ strains are sterile.7 7 The production of recombinants has been shown to require cellular contact.7 6
Of four independent strains of P. aeruginosa which were studied three were found to act as donors of genetic material. Donor ability was not affected by treatment with acridines and consequently infection experi
ments could not be performed between derivatives of the same strain.
However, both the donor strains t h a t were tested were able to transfer fertility to the fourth strain. The efficiency of transfer in the two cases was
different and the fertile derivatives resulting from acquisition of the FP factor from one of these donor strains were u n s t a b l e .3 3 Both these observa
tions suggest t h a t the donor strains harbor different conjugation factors.
There is good evidence t h a t the production of recombinants results from unidirectional transfer of genetic material from F P + to F P- cells. T h u s if F P + and F P ~ cells are first incubated together for half an hour, de
struction of the F P + parent with virulent phages does not markedly reduce the number of recombinants formed, whereas destruction of the F P ~ abolishes recombinant formation. Furthermore, transfer is incomplete since the unselected markers of the recombinants tend to be derived mainly from the F P - p a r e n t .3 3
Attempts to clarify the origin of the small number of fertile cells in F P + populations gave inconclusive results although there appeared to be some variation in the fertility of independent F P + cultures. One culture gave an unusually high number of recombinants. Its fertility was found to decrease progressively on further subculturing and attempts to isolate stable Hfr strains by indirect selection either from this culture or from other F P + populations with or without prior UV irradiation were entirely unsuccess
ful. I t thus appears t h a t the capacity to initiate chromosome transfer, which m a y be supposed to result from chromosomal attachment of the FP factor, is always unstable.
The pattern of inheritance of seven markers has been examined in crosses involving various types of selection. T h e results indicate t h a t all seven are located on a single physical structure, and are consistent with a linear arrangement of the markers. This result suggests t h a t the genetic material of Pseudomonas aeruginosa, like t h a t of E. coli and S. typhi
murium, is organized as a single chromosome.3 3
J. CONJUGATION IN Vibrio cholerae
While screening pairs of strains of Vibrio cholerae for recombinant for
mation, one particular strain was observed to give rise to about 1 re
combinant per 106 cells in mixtures with various other strains and to pro
duce a lytic agent which was active on other V. cholerae s t r a i n s .7 9 Lysis was at first thought to be due to production of bacteriophage but it now seems more likely t h a t a bacteriocin is i n v o l v e d .8 0 , 8 1 The ability to produce the lytic substance could be transferred to other strains, and its transfer was invariably accompanied by acquisition of fertility. The determinant P, responsible for the synthesis of the lytic substance, thus appears to play the role of a conjugation factor. As in the conjugation systems already considered, mixtures of P + strains are less fertile than combinations of P + and P ~ , while P ~ mixtures are of course sterile.7 9 , 8 1
Transfer appears to be unidirectional from P + to P_ cells and is in
complete. Transfer of unselected markers has been detected but it is rare, suggesting t h a t the segments of chromosome transferred are smaller in size than in F-mediated conjugation. Alternatively, the genetic determinants of V. cholerae may not be organized in a single chromosome.
1. CONJUGATION I N BACTERIA 19 K. CONJUGATION IN Serratia marcescens
Belser and Bunting have detected the formation of prototrophic recom
binants in mixtures of auxotrophic derivatives of a strain of Serratia mar
cescens.82 Recombinant formation requires cellular contact, and neither D N a s e (deoxyribonuclease) nor trypsin has any effect on t h e yield of re
combinants. T h e various derivatives of the single S. marcescens strain which was examined could be divided into two groups, members of one of the groups being infertile with each other b u t fertile with members of t h e other group. This suggested t h a t cells of t h e former group can act only as recipients while those of t h e other m a y act as donors of genetic material.
This interpretation was only partially substantiated by further obser
vations. UV irradiation of cells of the group of "donor" strains leads to a very striking increase in fertility, just like similar t r e a t m e n t of F + cells of E. coli K12. However, irradiation of cells of the "recipient" strain also in
creases the yield of the cross. With the exception of one pair of markers no joint transfer of more than one marker was ever detected even when sensi
tive selective techniques were employed. These observations suggest t h a t the segments of genetic material transferred during conjugation in this system are much smaller t h a n in F-mediated chromosome transfer, and more analogous to those transferred in transduction or transformation.
Analysis of t h e inheritance of unselected markers indicated t h a t cells of both groups of strains are able to act as donors. Although in most experi
ments the recombinants tended to inherit t h e unselected markers of the recipient strain there were generally some which inherited those from the donor strain ; occasionally the proportion of these was very high.8 2
I n summary, it appears t h a t t h e genetic recombinants formed in S.
marcescens result from the transfer during conjugation of very small seg
ments of genetic material. The derivatives of the one strain which has been examined can be divided into two groups, which are in some ways very similar to F + and F ~ types, on the basis of interfertility and with respect to the effect of U V irradiation on their fertility. However, cells of both groups show some ability to act as donors.
IV. Union b e t w e e n Donor a n d Recipient Cells
The capacity to conjugate m a y be regarded as a specific differentiation of the donor cell which makes possible infective transfer of the conjuga
tion factor. From this point of view the process of conjugation is compa
rable with the infective transmission of bacteriophage particles. Transfer of phages takes place by means of free extracellular particles each possess
ing its own apparatus for attaching and injecting into a new host cell, whereas in conjugation it occurs by the formation of a temporary inter
cellular connection.
A. SURFACE PROPERTIES OF M A L E C E L L S
The first demonstration of a physiological difference between donor and recipient cells of E. coli K12 was provided by Maccaearo, who showed t h a t F + cells have a greater affinity for acidic dyes than female cells, and pre
cipitate out of suspension in less acid media. H e also observed t h a t F + cells have a greater tendency to a u t o a g g l u t i n a t e .8 3'8 4 The inference t h a t the surface of F + cell is less negatively charged t h a n t h a t of F ~ cells has recently been confirmed by electrophoresis of intact cells.8 5 F + cells have also been found to be less motile than F ~ cells,8 6 and there is evidence t h a t male and female cells can be separated by countercurrent distribution.8 7
A characteristic surface antigen, termed / + , has been detected in strains which have acquired the F factor.8 8 Antibody to the / + antigen cross-re
acts weakly with the surface of cells harboring the F°-lac factor of Sal
monella typhosa,73 indicating t h a t this factor controls the production of an analogous and related antigen. I n addition, the presence of a surface antigen which is serologically unrelated to / + has been demonstrated in cells of a wild strain of E. coli harboring a conjugation factor different from F 8 9
Phages which adsorb to F + or Hfr cells of E. coli but not to F~~ cells have been isolated by several workers. Surprisingly the genetic material of these male-specific phages is R N A (ribonucleic acid) rather t h a n D N A .9 0 - 9 3 Phages which multiply in F ~ cells but are unable to multiply in male cells have also been isolated. One of them has been shown to adsorb less efficiently to male cells, presumably because its adsorption site is cov
ered by the male-specific surface component(s) ,9 2 Another adsorbs equally well to male and female cells of S. typhimurium but cannot multiply in the m a l e .9 4
B. MODIFICATION OF THE ABILITY OF M A L E C E L L S TO CONJUGATE
The evidence t h a t the surface component (s) responsible for the specific properties of male cells play a direct role in conjugation is derived from various instances of phenotypic or genetic modification of the ability of male cells to conjugate.
1. T H E ABILITY OF M A L E C E L L S TO A C T AS R E C I P I E N T S
Extensive pairing and clumping of cells may be observed microscopi
cally in mixtures of male and female cells.9 5 , 9 6 Such interactions are vir
tually absent from cultures of male or female cells alone, indicating t h a t specific pairing can only take place between cells of opposite mating type.
The same conclusion is suggested by the low fertility of crosses between male strains observed in several conjugation systems; those recombinants which do arise in mixtures of male strains are believed to result from con-
1. CONJUGATION IN BACTERIA 21 j ligation involving as the recipient a male cell in which the specific surface component is transitorily absent.
The role of surface components in specific pairing is strongly supported by the properties of so-called aF ~ phenocopy cultures." These are cultures of cells of male genotype which have temporarily lost their ability to act as donors as a result of being grown to maximum cell density with aera
tion.1 4 Such F ~ phenocopy cultures have a markedly increased ability to act as recipients in crosses with normal donor cells.1 4 Their behavior is just what would be expected if synthesis of the male surface component were temporarily suppressed. This interpretation is supported by the demon
stration t h a t aerated F + cultures adsorb male-specific phage much less efficiently than nonaerated cultures.9 2
2. DEVIRILIZING ACTION OF PERIODATE TREATMENT
Further evidence t h a t a surface component plays a role in conjugation is provided by the demonstration t h a t brief exposure of male cells of E.
coli K12 to periodate markedly reduces their ability to pair with female cells.9 7 The treated cells regain their mating ability after 1 or 2 hours' growth in broth. The identity of the periodate reactive surface component with t h a t responsible for the adsorption of male-specific phages has been demonstrated by the observation t h a t periodate-treated cells have reduced ability to adsorb male-specific phages.9 2 Transfer of R factors has also been shown to be temporarily suppressed by periodate t r e a t m e n t ,6 5 indi
cating t h a t R factor-mediated conjugation also involves a periodate-sen- sitive surface component.
3. SUPPRESSION OF CONJUGATION IN ESTABLISHED STRAINS
Cells which have recently acquired certain types of conjugation factor conjugate with very high frequency but lose the ability to conjugate after a few generations.6 1 , 6 5 In the case of col /-mediated conjugation it has been conclusively shown t h a t loss of ability to conjugate is due to inability to form contacts with recipient cells ; this observation again points to the importance of some surface component in conjugation.6 1 Preliminary ex
periments have not revealed the presence of any specific surface antigen on cells recently infected with col /.61
4. SUPPRESSION OF P - M E D I A T E D CONJUGATION BY R FACTORS
Several groups of workers (see ref. 65) have observed t h a t transfer of F or F' factors is virtually eliminated and chromosome transfer by Hfr cells markedly reduced by the introduction of R factors into male strains of E.
coli K12. This effect is not due to elimination of the F factor since the cells regain their original properties when the R factor is lost. I t appears instead
to be due to suppression of the synthesis of the surface substance con
trolled by the F factor, since cells harboring F and R factors have been shown to lack the sites for adsorption of F-specific male p h a g e s .4 1 More
over, some R factors have been isolated which do not suppress the ability of the F + or Hfr cells to adsorb male-specific phages and they also do not suppress their fertility.4 1
W a t a n a b e and F u k a s a w a have proposed t h a t suppression is due to syn
thesis, under the control of the R factor, of a surface component which replaces or covers the corresponding component synthesized by the F factor.9 8 This explanation would imply t h a t F-mediated transfer cannot take place through intracellular connections formed as a result of specific pairing involving the R factor surface component. An alternative which avoids this implication is suggested by the fact t h a t R factor-mediated conjugation is one of the systems which is suppressed in cells in which the conjugation factor has become established. If this is due to production of a repressor which inhibits synthesis of a surface component by the R factor,8 this repressor might also act on the F factor, inhibiting its func
tional activity. This explanation gains some support from the evidence of homology between F and R factors indicated by the affinity of the latter for the sfa locus of Richter's s t r a i n .4 0 If it is correct, then F-mediated transfer should not be inhibited in cells newly infected with R factor. F u r thermore, transfer of those R factors which do not supress F-mediatcd transfer m a y perhaps itself not be suppressed in established strains.
I t m a y be noted t h a t all the instances of suppression of fertility in E.
coli K12 have been found to involve loss of the the component responsible for adsorption of the male-specific phage. I t therefore seems probable t h a t one and the same component is responsible for all the spécifie surface prop
erties of male cells. The periodate sensistivity of this component suggests t h a t it is a polysaccharide. Strains of E. coli K12 harboring either JP9 9 , 1 0 0 or R factors6 5 retain their ability to conjugate when converted to spheroplasts, indicating t h a t the male component is located in t h a t p a r t of the cell wall which is retained by the spheroplast.
Nothing is known about the nature and degree of specificity of the sur
face structure of recipient cells. No treatment has yet been found which impairs ability to act as recipient. However, the low efficiency of conjuga
tion in most interstrain or interspecies crosses is possibly due in p a r t to poor complementarity with the male component, and mutations leading to increased ability to act as recipient m a y well involve alterations in surface structure.
Various male strains which exibit a heritable alteration of fertility have been isolated ; in some cases the mutation has been unambiguously shown to be located on the F factor. Some of the mutations probably result in al-