Bacterial Luminescence
W . D . M C E L R O Y
I. Introduction 479 II. Chemistry of Bacterial Luminescence 480
A. The General Problem of Light Emission 480 B. Purification and Identification of the Compounds Required for Light
Emission 482 III. Physiology of Luminous Bacteria 490
A. The Metabolism of Luminous Bacteria 490 B. Oxygen Requirement for Luminescence—Anaerobic Flash 495
C. Relationship of the Light Reaction to Other Electron Transport Proc-
esses 496 D. Use of Luminous Bacteria for Studying Drug Action 497
E. Mutations Affecting Growth and Light Emission 499
IV. Taxonomy and Evolution 504 A. Distribution, Isolation, and Classification of Luminous Bacteria 504
B. Evolutionary Significance of the Light-Emitting Reaction 506
References 507
I. Introduction
T h e first suggestion t h a t the "phosphorescence" of dead fish and flesh might be due t o living things was made b y Baker in 1742.1 Aristotle knew of t h e curious phenomenon of light emission b y dead fish, b u t it was not until later in the history of the development of science t h a t one finds ex- perimental work in this area. Robert Boyle in 1668 was the first to demon- strate the importance of air for light emission b y organisms, and M a r t i n in 1761 actually discovered the necessity of a high salt environment for marine luminous bacteria, although he was not aware t h a t he was studying micro- organisms at the time. I t was not until the late eighteenth and early nine- teenth centuries t h a t specific experimental work was done to demonstrate the cause of this light.
Earlier investigators thought t h a t light emission was connected with the decay and decomposition process of the tissues, b u t it was Heller in 1853 who definitely named an organism, Sarcina lutea, as t h e cause of light emis- sion b y meat. Heller demonstrated t h a t new flesh could be inoculated with t h e luminous material. Several years later, in 1875, Pfluger demonstrated t h a t the bacteria from fish was "filterable" and would grow on complex cul- ture media; in 1878 Cohn proposed the name Micrococcus phoreus for this organism. Following the work of Pfluger there were numerous isolations of new forms of luminous bacteria and each isolation was apparently given a new name. The most comprehensive early study of the various species of
479
480 W . D . M C E L R O Y
luminous bacteria was made by Fisher, a ship's medical officer, and an early worker in the field of marine bacteriology. H e discovered Bacterium phos- phorescens from the West Indies and another species from the Baltic Sea.
H e studied in some detail the cultural characteristics as well as the general properties of these various forms of luminous bacteria. I t is clear from the studies b y Fisher, as well as those of workers following him, t h a t m a n y of the bacteria previously described were the same or very similar organisms (see Section IV).
T h e brilliance and intensity of the bacterial light emission has been noted by m a n y investigators. Numerous a t t e m p t s have been made to meas
ure the light intensity of a single bacterium; the reader is referred to H a r vey's book for a review of these observations. Eymers and Van Schouwen- burg have made direct measurements in absolute units of the amount of light energy emitted by a suspension of Photobacterium phosphorium (2 mg.
dry weight). They observed t h a t at 22° C. 7.18 ergs per second were emitted and Harvey has indicated in his studies on luminous bacteria t h a t a single organism has an intensity of approximately 2 X 10~1 4 foot-candle. T h e colonies of luminous bacteria are so bright t h a t t h e suggestion was made during the last war t h a t they might be used during blackouts.
Although there were early speculations t h a t the luminescence of the sea is due to luminous bacteria, modern investigations have clearly shown t h a t bacteria emit light only after they have developed in great numbers on dead fish and other organisms. T h e brilliant luminescence of the sea is usually due to large numbers of dinoflagellates, jellyfish, and other luminous salt water forms.
T h e emission spectra of luminous bacteria have been studied b y a n u m ber of workers. T h e most recent studies by Spruit Van der Berg and b y Eymers and Van Schouwenburg indicate t h a t the peak of light emission b y luminous bacteria varies only slightly, ranging from approximately 465 to about 495 πΐμ. Recent observations have also shown t h a t the light emit
ted in cell-free extracts, as well as in protoplasts, of luminous bacteria, are essentially identical to those obtained in the intact organism.2 Thus, the broad spectral distribution observed in luminous bacteria is not due to scattering effects of the cell wall or other materials. Although the peak in
tensity for light emission in the bacteria is in the blue or blue-green region, light is emitted over the range from 400 to 650 ιημ. Since, however, the major emission is around 480-490 ηΐμ the light is blue or blue-green in a p pearance.
II. Chemistry of Bacterial Luminescence
A. T H E G E N E R A L P R O B L E M O F L I G H T E M I S S I O N
Molecules can be made to emit light in a number of ways. T h e one familiar to most workers is fluorescence, the emission of light from substances during
the time they are exposed to radiations of various kinds such as ultraviolet light or X-rays. If the light emission persists after the exciting radiation is cut off, it is called phosphorescence. There are m a n y other examples of lu
minescence such as electroluminescence, thermoluminescence, and sonolu- minescence. Chemiluminescence, light emission accompanying a chemical reaction, is the process of immediate interest to the students of biolumines- cence. T h e chemical reactions in luminous organisms which lead to the excitation of a molecule are oxidative and are catalyzed b y an enzyme called luciferase. T h e immediate electron acceptor is always molecular oxygen.
T h e oxidizable substrate is called luciferin. T h e n a t u r e of t h e latter com
pound is different for the various luminous forms. Students of biolumi- nescence would like to know t h e exact chemical composition of the compo
nents participating in the enzymic reaction leading to light emission. I n addition, what is the relationship of this oxidative process t o the general electron transport mechanisms t h a t occur in t h e cell and are necessary for its viability? T h e fundamental question of the mechanism of excitation is a physical-quantum chemical question difficult for biologists to answer a t the present time, b u t certainly a basic one relating to the general problem of energy transfer. I n general, modern chemical theory postulates t h a t a molecule acquires excess energy with the displacement of an electron from a lower to a higher energy level, namely, the excited state of the molecule.
I n the case of bioluminescence the energy for this displacement comes from a chemical reaction. When the electron in the excited state returns to ground level a q u a n t u m of light is emitted. T h e color of the light depends upon the amount of energy t h a t is liberated in the transition from the excited state to the ground state. I n contrast to t h e very broad spectra of incandescence or high temperature radiation the spectra of light emitted b y organisms are usually very narrow bands of light which give them a definite color, as in the case of luminous bacteria (blue-green), the firefly (yellow-green), and t h e South American railroad worm (red).
One of the important thermodynamic problems t h a t biochemists face in explaining luminescence is the large a m o u n t of energy required for t h e excitation process. For luminous bacteria which have a peak emission spec
t r u m around 490 πΐμ one can calculate from the general equation, Ε = hy, t h a t the energy requirement is approximately 60 kcal. per mole. Consider
ing the possibility t h a t the initial excitation process m a y lose approximately 10 kcal. calories before the electron returns to the ground state, it seems likely t h a t an excess of 70 kcal. might be required for the excitation process.
T h e oxidation-reduction reactions in organisms are coupled in such a way t h a t usually no more t h a n 10 kcal. are liberated in any single step. There
fore, one of the major problems in t h e bioluminescent reaction is to discover new processes which will liberate large amounts of energy in one single step in order t h a t the basic molecule m a y be excited to luminescence. Peroxida-
482 W . D . M C E L R O Y
tion of organic moleules often leads to light emission and this has been sug- gested as a possible explanation for bioluminescent reactions.
B . P U R I F I C A T I O N A N D I D E N T I F I C A T I O N O F T H E C O M P O U N D S R E Q U I R E D F O R L I G H T E M I S S I O N
1. H I S T O R I C A L
DuBois in 1885 reported the first definitive experiments regarding the nature of the chemical components necessary for light production by or- ganisms.1 H e found t h a t the luminous organs of a beetle would cease to emit light if immersed in hot water. H e also noted, however, t h a t a cold water extract which had ceased to luminesce could be stimulated to emit light by adding the hot water extract. On the basis of this type of experi- ment DuBois proposed the theory t h a t there was in the hot water extract a substance stable to heat which was destroyed during its luminescent oxida- tion through the action of a catalyst present in the cold water extract. H e named the heat-stable substance luciferin and the heat-labile substance luciferase. Numerous studies since t h a t time have confirmed and extended DuBois' observations to include other luminous forms.
T h e earlier a t t e m p t s to separate the light-emitting process from the luminous bacterial cells were negative. These observations lead Beijerinck3 to conclude t h a t luminescence was bound u p with living protoplasm. T h e indispensability of peptones for these bacteria was described by him to be due to the fact t h a t they were converted into a special form of matter, the photoplasm which was the necessary material for light emission.
Judged by present-day knowledge concerning the mechanism of cell free bacterial luminescence, there are two very important historical observations which should have greatly influenced and facilitated the study of light emis- sion in bacteria. T h e first of these concerns the observations of Gerretsen.4 Gerretsen reported in 1920 t h a t he was able to obtain a weak luciferin- luciferase reaction from extracts of Photobacterium javanense. Gerretsen also observed an increase in luminescence after a short ultraviolet t r e a t m e n t ; these observations have been repeated by Harvey and other investigators.
As will be discussed later, observations by McElroy and associates have demonstrated t h a t ultraviolet radiation liberates a long chain aldehyde which is one of the essential components for light emission.
Doudoroff5 in earlier studies found t h a t added riboflavin was essential for maximum luminescence of dim strains during growth. Interestingly, the respiration of the bright and dim strains was the same in the presence and absence of this riboflavin. From these observations Doudoroff concluded, correctly, as we now know, t h a t a flavin was connected with one of the en- zymes involved in light production b y the bacteria.
I n 1951 Shoup and Strehler6 noted t h a t acetonized powders from
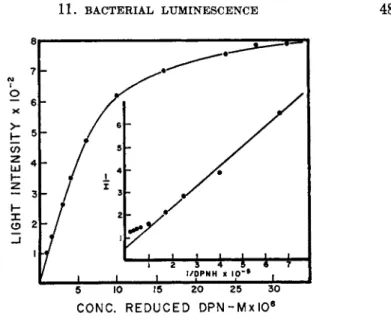
5 10 15 2 0 2 5 3 0
C O N C. R E D U C ED D P N - M x l Oe
FIG. 1. The relationship between D P N H and light intensity (McElroy and Green1 1).
Achromobacter fischeri would luminesce brightly for 15 minutes after being suspended in water. Following these observations Strehler and Cormier7 un- dertook an examination of the luminescence of t h e acetonized Achromobacter fischeri powders. T h e y observed t h a t the duration of luminescence depended
upon the concentration of the extracts and t h a t a luciferin-luciferase reac- tion was obtainable if sufficiently concentrated materials were employed.
T h e factor which first became limiting for luminescence in the crude extract was shown to be reduced diphosphopyridine nucleotide ( D P N H ) . T h e re- lationship between D P N H and light intensity is shown in Fig. 1.
2. R E Q U I R E M E N T S O F F L A V I N M O N O N U C L E O T I D E A N D A L D E H Y D E
Initial a t t e m p t s to demonstrate other requirements for the luminescence of the crude extracts were inconclusive in t h a t prolonged dialysis of the acetone powder gave preparations which were still capable of responding to added D P N H . B y ammonium sulfate fractionation of bacterial extracts which had been lysed with distilled water, McElroy and associates8 were able to show the requirement of flavin mononucleotide ( F M N ) as an essen- tial factor for light emission. These preparations would not emit light in the absence of added F M N , t h u s indicating t h a t a luminescent p a t h w a y consisted of a DPN-flavin electron transport system.
McElroy and associates9 also observed a requirement for an additional factor which they termed bacterial luciferin. This factor was in certain re- spects analogous to the component in firefly luminescence which has been called firefly luciferin and is used during the course of light emission. This material, which would restore luminescence in crude extracts, was rapidly
484 W. D. MCELROY
I I I ι ± - J
0.2 0.4 I 2
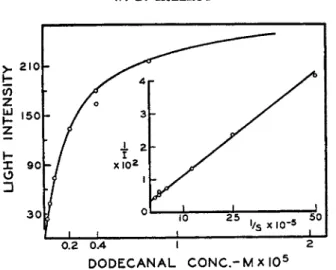
D O D E C A N AL C O N C . - M X I O5
FIG. 2. The relationship between aldehyde concentration and light intensity (McElroy and Green1 1).
liberated from bound form b y irradiating a number of tissues with ultra
violet light. Subsequent work b y Cormier and Strehler1 0 indicated t h a t this component occurred in high concentrations in hog kidney cortex and they were able to isolate this material and identify it as the long-chain aldehyde, palmital. Subsequent work has indicated t h a t a number of t h e long-chain aliphatic aldehydes, from Ce to Cis, will support light emission. T h e rela
tionship of dodecanal concentration to light intensity is shown in Fig. 2 while in Fig. 3 total light production is related to aldehyde. F r o m these
ML D0DECYL ALDEHYDE
FIG. 3. The relationship between total light production and aldehyde (McElroy and Green1 1).
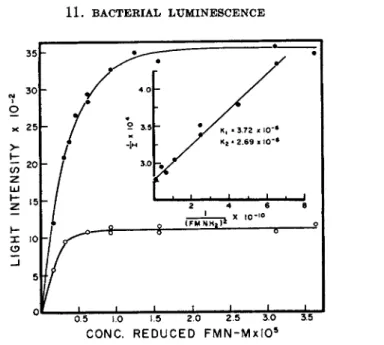
W 0 5 1.0 ϊ*5 2Ό 23 3Ό 3.5 CONC. R E D U C ED F M N - M x I O5
FIG. 4. The relationship between light intensity and reduced FMN concentration (McElroy and Green1 1).
relationships it is clear t h a t a limited amount of light is emitted for a given a m o u n t of aldehyde; t h u s McElroy and Green1 1 concluded t h a t t h e alde
hyde was used along with F M N H2 during the process of light emission.
Subsequent work b y Cormier et ai.,1 2 as well as b y McElroy and Green,1 1 has indicated t h a t one enzyme is involved in the light-emitting process and t h a t the main components for light emission are reduced F M N , long-chain aldehyde, molecular oxygen, and the bacterial luciferase.
Kinetic analysis of the light emission with varying concentrations of re
duced F M N is shown in Fig. 4. T h e reciprocal plot indicates t h a t two re
duced flavins are required for light emission; Κχ and Κ2 are the dissociation constants for the reduced FMN-luciferase complex. Similar observations with regard to the aldehyde indicates t h a t one molecule of the latter sub
stance is used. T o t t e r and Cormier1 3 have also studied the binding of the flavin to t h e luciferase molecule and have concluded t h a t there is one tightly bound flavin and one which freely dissociates. F r o m these observations McElroy and Green1 1 concluded t h a t during luminescence one molecule of aldehyde combined with one molecule of the reduced flavin to form an F M N - a l d e h y d e compound. Normally this compound would be oxidized directly b y molecular oxygen through a catalytic reaction in which peroxide and t h e corresponding acid are eventually formed. This oxidation would liberate considerable energy b u t would be approximately 40 kcal. short of t h a t required for light emission. T h e y proposed therefore t h a t the second reduced F M N molecule could react with oxygen to form an unstable organic peroxide similar t o t h a t suggested b y D r e w1 4 for aminophthalic hydrazide
486 W . D . M C E L R O Y
and t h a t this peroxide would then act as an oxidant for the aldehyde-FMN compound to give a highly excited molecule which would emit light. This proposal therefore is in keeping with the idea t h a t two reduced flavin mole
cules are necessary for light emission, one for combination with the alde
hyde and a second for forming an organic peroxide which acts as the oxidant.
T h e idea t h a t aldehyde can combine with the reduced intermediate prior to oxidation or peroxidation is not fundamentally different from the idea sug
gested by Racker for triosephosphate dehydrogenase.
T h e reasons for suggesting t h a t modified flavin is bacterial luciferin are several fold. Flavin fluorescence is a yellow-green in contrast to the blue light of the bacteria which has a peak around 480 πΐμ. I t is possible t h a t modification of the flavin structure by the addition of a long-chain aldehyde would give a conjugated system whose excited state would emit light in the blue region of the spectrum. I n addition the energy release b y the peroxida
tion of a long-chain aldehyde to form acid would be adequate to satisfy the requirements for excitation.
3. F A C T O R S A F F E C T I N G L I G H T E M I S S I O N I N E X T R A C T S
Earlier a t t e m p t s to remove or purify luciferase t o the point where it failed to respond to reduced pyridine nucleotides were unsuccessful. Pro
longed dialysis against metal-free phosphate buffer and cyanide in the pres
ence and absence of glutathione were ineffective in the further purification of bacterial luciferase. Cormier et al.12 were able by dialysis at low p H to inactivate the sytem which stimulated the utilization of reduced D P N for light production. Recently Green and McElroy have been able to separate by calcium phosphate gel column the D P N H - F M N oxidase system from the bacterial luciferase system. Under these circumstances the purified lu
ciferase does not respond to reduced pyridine nucleotide. Cormier and Tot
ter demonstrated t h a t their partially inactivated preparation would emit light with D P N H if they added a preparation from Escherichia coli which was capable of reducing F M N with reduced D P N . T h e results indicate, therefore, t h a t the partially purified luciferase contains an enzyme which transfers the electrons from reduced pyridine nucleotides to flavin and a second enzyme, legitimately called bacterial luciferase, which catalyzes light emission.
A number of agents which will reduce F M N will support luminescence.
Strehler et al.ls reported earlier t h a t reduced riboflavin would support lu
minescence in crude extracts. McElroy and Green1 1 were able to show with their purified enzyme, which is essentially free of F M N , t h a t no light is ob
tained with reduced riboflavin. However, the addition of F M N t o these preparations allowed the utilization of a number of reducing agents for light production. Various reduced dyes, such as safarine, indigo trisulfonate,
and rosindulin 2 G, support luminescence provided F M N is added. Re
duced rosindulin 2 G was approximately 50 % as effective as reduced F M N in supporting light emission, while reduced riboflavin gave only 1 0 % of maximal flash. From these studies it was apparent t h a t reduced dyes whose oxidation reduction potential were more positive t h a n the indigo trisulfo- nate (—0.081, p H 7.0) would not initiate light emission.
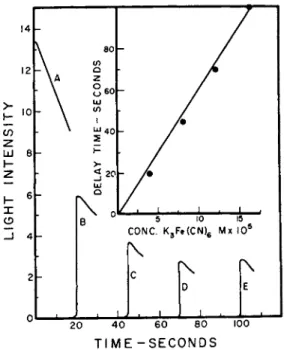
T h e partially purified bacterial luciferase which contains the D P N H - flavin oxidase rapidly oxidizes D P N H without light production provided F M N is added. I n addition, the enzyme will catalyze the rapid reduction of methylene blue, various quinones, and ferricyanide, but not inorganic iron. If ferricyanide is added to a normal reaction mixture no light is emitted until all of the ferricyanide is reduced. T h e relationship between ferricyanide concentration and delayed light emission is shown in Fig. 5. T h e fact t h a t no light is emitted until the ferricyanide is reduced suggests a competition between the latter and F M N for the electrons from D P N H . T h e oxidation of D P N H b y ferricyanide proceeds normally even in the presence of 10~3 molar K C N , suggesting t h a t the electron transport process does not require metal as a necessary cofactor. These observations would offer a plausible
ο Ε
0I ι I ι ι • ι I l 1 • ' ' 1 20 4 0 6 0 80 100
T I ME - S E C O N DS
FIG. 5. The inhibition of light by ferricyanide. Various amounts of ferricyanide were added to the reaction mixture and D P N H was added at zero time. Light emis
sion occurred only after the ferricyanide was completely reduced (McElroy and Green1 1).
488 W . D . MCELROY
explanation for the fact t h a t various reduced dyes will support light emis- sion in the presence of F M N . Cormier and T o t t e r1 6 have obtained a prep- aration from luminous bacteria which will catalyze the reduction of cyto- chrome c by reduced pyridine nucleotide. Their preparation also contains bacterial luciferase and it is presumed t h a t this system is operating in the reduction of the cytochrome system. T h e crude bacterial luciferase will also reduce various naphthoquinones. T h e results indicate t h a t the inhibition of light emission by quinones is accomplished by removing reduced pyridine nucleotides. Spruit and Schuiling1 7 have made similar observations on whole cells and have concluded t h a t quinone inhibits light emission in the intact bacteria b y competing with the light system in the electron transport process. T h e relationship of the light-emitting oxidative reactions to elec- tron transport in the intact bacteria will be considered in a later section.
I n the presence of F M N and aldehyde both D P N H and T P N H will sup- port light emission when a partially purified luciferase is used. T h e T P N H concentration required for maximum light output is about three times the D P N H concentration. Also t h e maximum light intensity obtainable with T P N H is only 8 0 % of t h a t observed with D P N H . T h e fact t h a t both re- duced pyridine nucleotides will function in light emission is of considerable interest with regard to alternate pathways for light emission. Friedman1 8 had earlier demonstrated t h a t both pathways of carbohydrate metabolism exist in the luminous bacteria; one leading to t h e reduction of triphosphopy- ridine nucleotide via glucose-6-phosphate dehydrogenase, and the usual glycolytic pathway leading to the reduction of D P N . T h e existence of these two pathways for the formation of reduced pyridine nucleotides is of interest when one a t t e m p t s to explain earlier observations on the inhibition of light emission in intact bacteria.
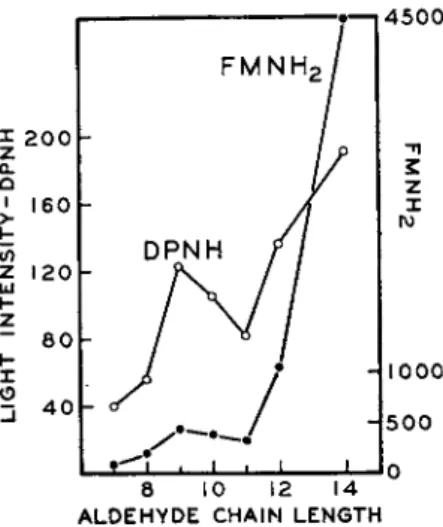
I n crude enzyme preparations a large amount of the aldehyde is bound to the protein and is slowly made available for luminescence through some unknown reaction. Ultraviolet radiation in some way releases the aldehyde and makes it more available for light production.9 This seems to explain why ultraviolet radiation stimulates light emission in crude extracts. I n the presence of a continuous supply of reduced D P N , the crude enzyme prep- arations will continue to emit a weak light for hours. W i t h the purified enzyme, however, very little bound aldehyde is present, and under these circumstances light emission is completely dependent upon the addition of aldehyde. A large amount of evidence has been presented which indicates t h a t the aldehyde is used during the luminescent reaction and t h a t in all probability the corresponding acid is produced. T h e effect of aldehyde chain length on maximum cell-free luminescence, using either D P N H or reduced F M N , is shown in Fig. 6. From experiments b y Rogers and M c E l r o y1 9 , 2 0 on the effect of aldehyde concentration on light emission, it is clear t h a t the
4 5 0 0
Η Ι 0 0 0
H500
β 10 12 14 A L D E H Y D E C H A I N L E N G T H
FIG. 6. The effect of aldehyde chain length on cell-free luminescence using either D P N H or F M N H2 (Rogers and McElroy2 0).
affinity of the luciferase for these substances is greater as the carbon chain length increases. T h e stimulation of light production b y undecanal and nonanal appears to be consistently out of line with respect to t h a t observed by the other aldehydes in t h e series. There is no apparent explanation for this anomalous behavior. T h e peculiarity of the series is apparently due to some specificity of the enzyme and probably not to impurities. I t m a y be t h a t t h e optimal chain length for luminescence for the odd-number alde
hydes is approximately C9 whereas the effectiveness of t h e even-numbered aldehydes continues t o increase even beyond tetradecanal. Unfortunately other aldehydes have not been available for testing.
T e r p s t r a2 0 a has studied an enzyme preparation from a dark strain of Photobacterium splendidum which appears t o be different from other lucif
erase preparations in t h a t aldehyde and F M N H2 appear t o compete for t h e same sites on t h e enzyme.
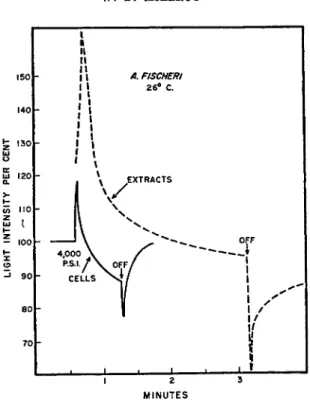
Strehler and Johnson2 1 have studied the effect of pressure on light emis
sion in extracts and in living cells; the results of one such study are shown in Fig. 7. T h e general relationships observed in the intact bacterium are essentially the same as those observed for t h e extracts. These observations are of considerable interest when they are compared to the earlier extensive observations of Johnson2 2 and Johnson et al.2Z on the effect of temperature and pressure on light emission in t h e whole cell. Cormier and Strehler2 4 have studied the extracts of a number of strains of luminous bacteria and have demonstrated t h a t t h e temperature optima in the cell-free extracts agree remarkably well with those observed for the intact bacterium, although
490 w. D . M C E L R O Y
140
£ ISO]
ω υ
n o t
A FISCHERI
26° C.
ι ι I I
2 r
MINUTES
FIG. 7. Effect of pressure on light emission in extracts and in cells. Pressure ap
plied at 30 seconds (Strehler and Johnson2 1).
there was some variation in the activation energies for the different reac
tions.
I t is not possible at the present time to describe in physicochemical terms the exact mechanism of light emission in the bacterial extract. How
ever, apparently the only components required are those discussed in the last section. The best preparations of bacterial luciferase which have been made at the present time apparently contain no other cofactors, and it must be presumed t h a t one of the four components mentioned, or some complex of them, becomes excited during the luminescent reaction.
III. Physiology of Luminous Bacteria
A . T H E M E T A B O L I S M O F L U M I N O U S B A C T E R I A
1. N U T R I T I O N
All luminous bacteria which have been studied will grow on ordinary nutrient agar with 0 . 3 % glycerol. For the salt water bacteria 3 % sodium chloride must be added. Although there was much work on the nutrition of luminous bacteria by earlier investigators, it was not until Doudoroff2 5 -2 6
made an extensive study on the nutritional requirements of several species t h a t a minimum medium was devised. This has been slightly modified by the work of Farghaly and M c E l r o y2 7 to ensure greater growth. T h e minimal medium (as published by Farghaly2 8) to which is added 0.1 % peptone gives vigorous growth and will yield in shake cultures at 23° C. approximately 6 g. wet weight of Achromobacter fischeri per liter in an 18-hour period. Studies on the growth requirements of luminous bacteria have been too numerous to report in detail. I t is of interest to note t h a t it was Beijerinck in 1889 who introduced the auxanographic method to determine the effects of various substances on growth and light production. He grew the bacteria on a solid medium with insufficient nutrients so t h a t growth and lumines- cence soon ceased. After this a few drops of various substances were added in order to test their ability to support growth and light production. Bei- jerinck classified his substances as "light n u t r i e n t s ., ,
There is a great deal of variation in the carbon sources as well as the nitrogen sources which will support growth and light emission in different strains of luminous bacteria. T h e one strain of Achromobacter fischeri stud- ied b y Farghaly is capable of using only glycerol or glucose as the sole car- bon source whereas other strains have been reported to grow on lactose, maltose, and a variety of other sugars. Needless to say all of these com- pounds are adequate in the support of both growth and luminescence.
J o h n s o n2 9 as well as H a r v e y have studied in great detail the carbon sources which will support luminescence in resting cell preparations and they have found a n u m b e r of compounds which will support light emission b u t which do not necessarily support the growth of the organism. Although Achromo- bacter fischeri appears to be capable of using fructose oxidatively it is not an adequate carbon source for maximum growth unless a number of amino acids are added to the culture medium. F r o m the work of Friedman1 8 it would appear t h a t some inhibitory products of fructose metabolism are formed and t h a t amino acids are necessary to prevent the inhibition of growth and luminescence. Anderson3 0 has also studied the variation in nu- tritional requirements at different temperatures. H e found when the lumi- nous bacteria failed to grow at a temperature above 26-27° C. on Doudoroff minimal medium the addition of hydrolyzed casein permitted normal growth and luminescence a t much higher temperatures. T h e results suggested t h a t the temperature sensitivity m a y be related to the synthesis of a particular amino acid. Anderson found t h a t a combination of methionine, glutamic acid, and serine was particularly effective in allowing growth and lumines- cence at the higher temperatures. T h e development of luminescence during growth at the higher temperature, 29° C , was critically dependent upon the concentration of these .amino acids. I t was possible to vary the con- centration of glutamic acid to such an extent t h a t the light intensity was almost completely eliminated without greatly affecting growth.
4 9 2 W . D . M C E L R O Y
Ο O.I 0.2 0.3 0.4 0.5
GLYCEROL (PER CENT)
FIG. 8. Effect of glycerol concentration on growth and luminescence of Achromo
bacter fischeri (Farghaly2 8).
2 . E N V I R O N M E N T A L F A C T O R S A F F E C T I N G G R O W T H A N D L U M I N E S C E N C E
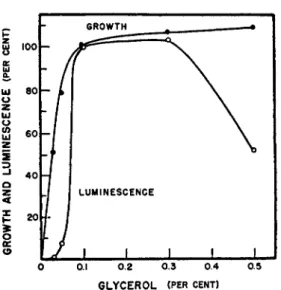
Farghaly has made a particularly careful analysis of various environ
mental factors in relation to growth and luminescence. T h e relationship between growth and luminescence as a function of glycerol concentration for Achromobacter fischeri is shown in Fig. 8 . T h e results indicate t h a t 0 . 3 % glycerol is adequate for maximal growth and luminescence and t h a t t h e usual 1 % concentration which has been used b y other workers for growing these organisms led to considerable inhibition of light emission. I t should be noted t h a t the development of luminescence always lags behind growth and t h a t light was not emitted in measurable amounts until 3 0 to 4 0 % of maximum growth had taken place. However, following this lag in the lu
minescence there was a rapid development in light emission. Doudoroff in his nutritional studies of various species of luminous bacteria m a d e t h e ob
servation t h a t methionine was an essential nutrient. Farghaly has also found for Achromobacter fischeri t h a t although methionine is not required it does tend to eliminate the lag in the development of light emission when the organism is grown on the minimal medium. T h e addition of histidine to the methionine medium greatly stimulated the development of light emission.
I t has become a routine procedure among those who work with luminous bacteria to add small amounts of calcium carbonate ( 0 . 1 to 1 % ) to t h e medium in order to maintain luminescence for long periods of time. T h e effect has been assumed to be due to the neutralization of acids produced
by the organism. However, a careful study b y Farghaly on t h e effect of cal- cium carbonate demonstrated t h a t this hypothesis does not provide a complete explanation. I n t h e first place, addition of calcium carbonate t o a growing culture not only prolonged the duration of luminescence b u t in- creased its intensity over 7 5 % . I n t h e second place, determination of p H changes during growth with and without calcium carbonate showed t h a t although there was indeed a difference in p H between the two media it was not great enough to account for the increase in luminescence in the presence of C a C 03.
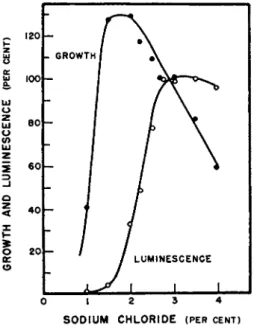
I t has been noted by m a n y of the earlier workers t h a t the salt concentra- tion of the medium on which marine bacteria are grown is very important.
Following these observations most workers have maintained the correct osmotic environment by the addition of sodium chloride. T h e work of Johnson and H a r v e y8 1 indicates t h a t the replacement of sodium chloride with other salts has no ill effect on the growth of these organisms provided the correct osmotic pressure is maintained. On the other hand, luminescence appears to be greatly affected b y the types as well as the concentration of t h e salt used. Farghaly has m a d e a careful study of t h e relationship of the concentration of sodium chloride to the growth and luminescence of Ach- rombacter fischeri. Figure 9 shows t h e relationship between growth and luminescence as a function of t h e sodium chloride concentration. Below
0 1 2 3 4
SODIUM CHLORIDE (PER CENT)
FIG. 9. Effect of sodium chloride concentration on growth and luminescence of Achromobacter fischeri (Farghaly1 8).
494 W . D. MCELROY
0 . 5 % very little growth, if any, occurs. W i t h increasing concentrations growth increased very rapidly until the maximum was reached at around 2 . 3 % after which growth gradually decreased, reaching zero at 5 % N a C l . At the concentration of sodium chloride which supported maximum growth, luminescence was only 30 to 4 0 % of maximum and at the concentration where growth declined, light continued to develop reaching its maximum intensity at approximately 3 to 3.3 % sodium chloride. Recent observations on a t t e m p t s concerned with the isolation of the light-emitting system from bacterial growth at various sodium chloride concentrations indicate t h a t it is the luciferase t h a t fails to be synthesized at the low salt concentration.
When cells are grown in 1 % sodium chloride very little luciferase is made.
If these cells, however, are immediately transferred to a solution containing 3 % sodium chloride, luciferase synthesis occurs rapidly and t h e light in- tensity increases. T h u s far only those conditions which foster cell division bring about an increase in the light intensity. I t is evident t h a t cells grown under these conditions should be excellent objects for studying protein synthesis, for it is only necessary to place the cells in front of a photocell to note the increase in the luciferase concentration which is directly meas- ured by the light emitted. I t is unnecessary to break open the cells in order to analyze for the protein which has been synthesized. Luminous bacteria can be grown on 1 % sodium chloride with adequate luciferase synthesis providing other salts are added to raise the osmotic environment. T h e ad- dition of 17 % sucrose to such a medium allows the normal development of the luciferase system. Other salts have been tried; these include KC1, K N 03, K2SO4, N a N 03, and N a2S 04. All were able to maintain the correct osmotic environment for luciferase synthesis. These observations are of considerable interest because the proteins essential for duplication are made at a normal rate in contrast to the bacterial luciferase. Two explanations appear possible. One, luciferase m a y be synthesized but is unstable in t h e low salt environment. This seems most unlikely since the partially purified luciferase is perfectly stable in a solution of low ionic environment. T h e salt itself could affect a protein-synthesizing particle in the luminous bacteria which is concerned primarily with the synthesis of luciferase.
As noted by earlier workers, when luminous bacteria are suspended in an aqueous solution light emission disappears. I t is now known t h a t in such hypotonic solutions the cells rapidly lyse, liberating their cellu- lar contents into the medium. This method has been used extensively by McElroy and Green1 1 for obtaining protein extracts from such or- ganisms. I n one species of luminous bacteria which has been isolated from a dead flounder it was found t h a t although 3 % sodium chloride was ade- quate for normal growth and light development these cells would not lyse when suspended in water. A 15-minute incubation period in 6 % sodium
chloride so conditioned the cells t h a t rapid lysis occurred when they were resuspended in distilled water. Protein analysis of the lysate as well as the debris has indicated t h a t over 90 % of the cellular protein is extracted by this procedure. I t is likely t h a t this same general technique could be used extensively for the lysis of other forms of bacteria.
I n connection with his investigations on the penetration of ammonium salts, Hill3 2 made the observation t h a t the lysis of marine luminous bac- teria led to a decrease in light emission and he has used this technique to study the penetration of a variety of compounds. Johnson and H a r v e y have made an extensive study of the respiration and luminescence of rest- ing cells of luminous bacteria in different osmotic environments. I t would appear from these studies t h a t the luminescence is more sensitive to hypo- tonic solutions t h a n is the respiration. T h e reader is referred to H a r v e y for various other findings of the effect of salts on growth and light emission.
B . O X Y G E N R E Q U I R E M E N T F O R L U M I N E S C E N C E — A N A E R O B I C F L A S H
T h e requirement of oxygen for light emission in luminous bacteria is a well-established fact and was first observed b y Boyle in 1667 for light emis- sion associated with luminous fish. Later, as the constituents of air became known, it was acknowledged b y all workers t h a t it was oxygen which was the indispensable factor required for light emission. Beijerinck first pointed out t h a t several species of luminous bacteria were facultative anaerobes and would grow without oxygen if adequate nutrients were present. T h e bac- teria failed to emit light, however, when grown under these conditions. On the admission of oxygen to such cultures, light emission occurs. T h e results indicate t h a t the components necessary for the synthesis of the light-emit- ting system could be made in the absence of oxygen. Beijerinck was also the first to utilize luminous bacteria as a test for oxygen. In his early paper in 1902 he described the detection of oxygen formed by photosynthesis in an extract of crushed clover leaves to which 3 % salt and luminous bacteria had been added. H a r v e y1 and collaborators have m a d e m a n y quantitative studies regarding the minimum amount of oxygen required for light emis- sion and in addition observed the relationship of the oxygen pressure to light intensity. Shapiro3 3 observed for Vibrio phosphorescens t h a t the light intensity is independent of oxygen pressure until the latter is reduced to about 0.14% (1.06 m m . mercury). Oxygen concentrations lower t h a n this reduced the light intensity rapidly and no further response was observed at 0.01 %. I n more recent studies b y Hastings,3 4 however, much lower con- centrations of oxygen were required to eliminate light emission completely.
When resting cells of luminous bacteria have been placed under anaerobic conditions for a few moments, the addition of oxygen or air gives rise to a very brilliant flash of light which rapidly returns to a normal base-line level.
495 W . D . M C E L R O Y
Harvey and associates were the first to measure carefully this flash re- sponse. T h e total light emitted in the flash appears to be independent of the duration of anoxia, provided these conditions are not prolonged too long and provided adequate time is given for the build-up of a substance which was earlier called luciferin. From the cell-free studies we now know t h a t the component accumulating is undoubtedly reduced F M N . Chance et aZ.3 5 made very rapid recordings of the flash reaction and concluded t h a t the half-time for the development of the maximum light intensity was approxi- mately 0.08 second. T h e flash has also been extensively studied by Johnson et aZ.3 6 by using well-washed resting cells of luminous bacteria. These work- ers have been able to study in some detail the effect of various substrates on the build-up of luciferin under anaerobic conditions. Needless to say those compounds which were found to act as excellent hydrogen donors in the respiration of the bacteria were also found to be able to lead to the ac- cumulation of bacterial luciferin. T h e effect of various inhibitors on this flash process has also been studied and all the results are in keeping with the general observations t h a t light emission following anaerobic conditions depends upon an electron donor. All of these observations, which are dis- cussed in detail by Harvey, are of considerable interest particularly with regard to our present knowledge of the cell-free bacterial light-emitting sys- tem.
I t would appear t h a t the maximum flash obtained after anaerobic condi- tions is a direct measure of the concentration of F M N H2 associated with the bacterial enzyme system and t h a t the light intensity observed under aerobic conditions is a measure of the concentration of reduced F M N in the steady state condition. T h e earlier observations on the stimulation of luminescence b y cyanide m a y now be interpreted as due to the inhibition of electron transport over the cytochrome system, which undoubtedly would lead to an increased concentration of the steady state level of reduced F M N .
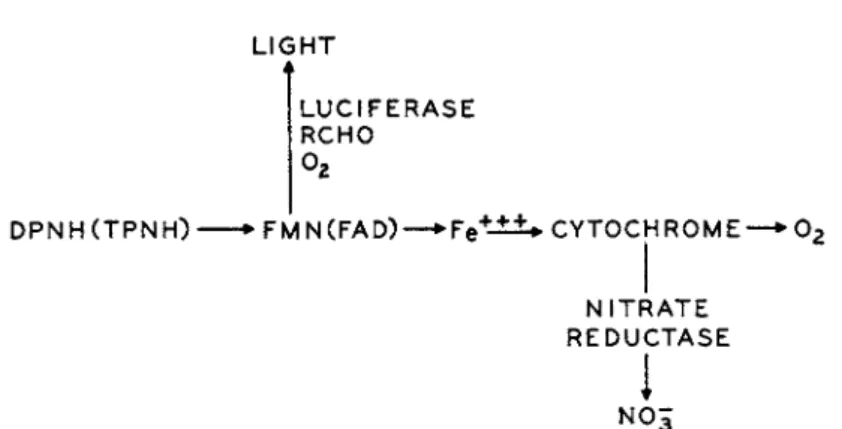
C. R E L A T I O N S H I P O F T H E L I G H T R E A C T I O N T O O T H E R E L E C T R O N T R A N S P O R T P R O C E S S E S
T h e relationship between cell respiration and luminescence has been dis- cussed in great detail b y a number of workers and Harvey has summarized most of this information. T h e earlier investigators considered luminescence simply as a consequence of respiration; this opinion was expressed as early as 1865 b y Sachs. However, Beijerinck was quite clear in pointing out the relationship between respiration an(jl light emission, and since t h a t time Harvey and collaborators have done much to clarify this relationship. I n a very extensive study involving inhibitors and oxygen tension Van Schou- wenburg3 7 * 3 8 concluded t h a t the light-emitting system is one which com-
L I G HT
L U C I F E R A SE R C HO
D P NH ( T P N H) • F M N ( F A D ) — • Fe +-± ±* C Y T O C H R O M E —* 0 2
N I T R A TE R E D U C T A SE
N 0 5
ι
FIG. 10. Relationship of the light reaction to the electron transport process (mod
ified from Sadana and McElroy3 9).
petes with the cytochrome for electrons. In addition, Van Schouwenburg clearly demonstrated t h a t there was electron transport through the light system without actual light emission. T h e scheme which he presents in his original publication in 1938 is certainly in keeping with the modern knowl
edge on the nature of bacterial luciferin and luciferase. Friedman has dem
onstrated the existence of the Embden-Meyerhof p a t h w a y as well as the oxidative p a t h w a y for carbohydrate metabolism in luminous bacteria.
Doudoroff's studies on the nature of the products produced by the anaerobic breakdown of carbohydrates has implicated a number of enzyme systems similar to those found in the Enterobacteriaceae. Sadana and M c E l r o y3 9 have isolated a nitrate reductase from luminous bacteria and have demon
strated the importance of a bacterial cytochrome in this reaction. T h e re
lationship of t h e electron transport process in luminous bacteria t o t h e oxygen-consuming reactions and the light-emitting process is illustrated in Fig. 10. W i t h this known relationship it is possible to explain all of t h e earlier observations on the action of various inhibitors on light emission including the effect of oxygen tension and cyanide. Under low oxygen tensions where the luminescent system would, in effect, be competing with the cytochrome system for oxygen, it is possible to understand now why cyanide should stimulate light emission and why added nitrate might be expected to reverse this stimulation.
D . U S E O F L U M I N O U S B A C T E R I A F O R S T U D Y I N G D R U G A C T I O N
Luminous bacteria have been a favorite organism for use in the study of the mechanism of action of drugs and H a r v e y has reviewed the earlier work from his laboratory.1 Using dimming time as a simple measure of respiration of luminous bacteria and also b y measuring the intensity of light emitted, T a y l o r4 0 was able to study quantitatively the effects of a
498 w . d . m c e l r o y
ATM. PRESi REACTION (2)
I7OOO
large number of hormones and narcotics on b o t h respiration and light emis
sion. In general, light emission is very sensitive to low concentrations of all narcotics whereas respiration is relatively unimpaired. From such investi
gations Taylor and others have concluded t h a t two processes, one involving light emission and a second, respiration, are independent of each other.
From recent studies it would appear t h a t there are essentially the two pathways of electron transport which can support light emission. One path
way makes use of the triphosphopyridine nucleotide linked glucoses-phos
phate dehydrogenase system and the other the diphosphopyridine nucleo
tide requiring triose phosphate dehydrogenase. Apparently, the narcotics affect the glycolytic system and therefore the DPN-linked electron trans
port processes before affecting the electron transport process involved in the T P N system.
Johnson2 2 and Johnson et al.2Z have used the luminous bacteria in order to study quantitatively the effect of various inhibitors, temperature, and pressure on the light-emitting process. T h e y have observed t h a t an in
creased pressure decreases the light intensity if applied to a suspension maintained at a temperature below the optimum. On the other hand, pres
sure increases the light intensity if it is applied to a suspension maintained above the optimum temperature. T h e results were explained on the as
sumption t h a t there were two reactions which proceed with an increase in volume; one was concerned directly with the light-emitting step and the second was concerned with the reversible inactivation of the enzyme b y heat. At low temperatures pressure reduced the luminescent intensity b y
2.4
>
t 2.2
ζ
I d
g 2.0
ζ ω Η 1.6
Ld
1
w_J Ο 1.2
3
1.0
.0032 .0033 .0034 .0035 .0036 ' . 0 0 3 7 I
ABSOLUTE TEMPERATURE
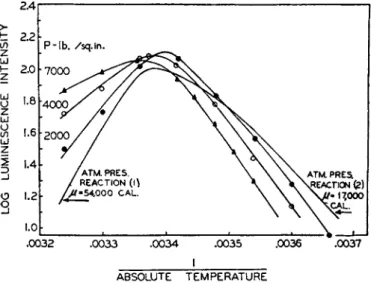
FIG. 11. Effect of pressure and temperature on light emission by Photobacterium phosphoreum [ F . H. Brown, D . Johnson and D . Marsland, Science 96, 200, (1942)].
100
4 0 .
i 7/ )-L •—^
r2"8— I
^—•-
//) i i
1 L _
<
•
* • "
> - , ο —
ι .
CHLOROFORM 0 . 0 6 m ETHYL CARBAMATE 0.78m
ETHYL ALCOHOL 0 . 5 m
PHENYL CARBAMATE 0 . 0 0 4 m
NOVOCAINE 0 . 0 0 6 4 m
p-AMINOBENZOIC ACID 0.01m
SULFANILAMIDE 0 . 0 0 3 8 m
Να-BARBITAL ETHYL ETHER 0.14m CHLORAL HYDRATE 0.016m
1000 2 0 0 0 3 0 0 0 4 0 0 0 5 0 0 0 6 0 0 0 7 0 0 0 8 0 0 0 PRESSURE IN POUNDS PER SQ. IN.
FIG. 12. Effect of pressure on inhibitors of luminescence (Johnson et al.*1) slowing the dominant light-emitting reaction whereas at elevated tempera
tures t h e dominant reaction is t h e reversible denaturation of the bacterial luciferase and pressure would tend to increase light emission b y protecting the unfolding of the enzyme. T h e results of such a pressure experiment are shown in Fig. 11. In extending these observations to the action of drugs, Johnson et al.Al were able to demonstrate t h a t certain effects of the narcotics could be reversed b y high pressure, leading to the suggestion t h a t the reversible denaturation of luciferase was not only caused by temperature b u t was also brought about b y various narcotic agents (see Fig. 12). Other workers have extended these observations to include a variety of com
pounds. T h e action of narcotics on the oxidation of glucose b y Achromobac
ter fischeri has been studied b y McElroy.4 2 I n agreement with other studies, t h e narcotics prevented the assimilation of carbohydrates. T h e effective concentrations were those which inhibited light emission approximately 5 0 % , while greatly stimulating respiration.
E . M U T A T I O N S A F F E C T I N G G R O W T H A N D L I G H T E M I S S I O N
Nonluminous strains or m u t a n t s of luminous bacteria have been known for m a n y years. However, it was Beijerinck who first realized t h e signifi
cance of this fact. H e wrote in a paper entitled "On Different Forms of Heredity Variation of Microbes" about changes in luminous bacteria which
9 0 |
LUMINESCENCE INTENSITY
IN PER CENT 7C
OF UNINHIBITED
CONTROL 6 0 AT
NORMAL
500 W . D . MCELROY
he had observed as early as 1889 and indicated how they were very simi- lar to the observations made by deVries on Oenothera. H e agreed with deVries t h a t such mutations were responsible for the origin of species.
Later, in 1912, Beijerinck4 3 pointed out t h a t the most acceptable theory of heredity is the concept t h a t the living part of the protoplasm is built u p of a great number of factors or "bearers" which determine the charac- ters of the organism. At this early date he wrote t h a t " t h e fundamental conception here to be proposed is t h a t every hereditary character of an organism corresponds to one or more 'enzymes' which exert an influence on specific substrates." Beijerinck held t h a t light emission of luminous bacteria was probably the most suitable character for studying the m u t a - tion process.
Since Beijerinck's time m a n y workers have noted dim and dark m u t a n t s of luminous bacteria. T h e important observations of Doudoroff on the stimulation of luminescence of dim strains of bacteria b y riboflavin have been mentioned previously. Giese4 4 has also observed brilliant variants in old cultures of luminous bacteria. This m u t a n t developed a particularly yellow pigment which diffused into the medium and would fluoresce in the ultraviolet light, suggesting the possibility t h a t it was riboflavin. McElroy and Farghaly2 7 made an extensive effort to induce mutations in luminous bacteria in order to dissociate growth and light emission. I n a strain t h a t requires arginine for growth, the luminescence fails to develop in a grow- ing culture unless the concentration of arginine is high enough to give approximately 30 % of the normal growth. W i t h higher concentrations of arginine the luminescent system develops rapidly and finally reaches the wild type level of intensity. I n an aspartic acid m u t a n t , luminescence de- velops only when the concentration of the amino acid is increased to a level where maximum growth is observed. I n some cases it is possible to eliminate the lag in the development of the luminescent system b y sup- plementing t h e medium with other amino acids. T h e relationship between growth, luminescence, and aspartic acid concentrations is shown in Fig.
13. T h e effect of an amino acid supplement on the development of the luminescent system is shown in Fig. 14. All of the studies on these nutri- tional m u t a n t s have merely served to emphasize the fact t h a t m a n y physi- ological functions m a y not be fully restored with concentrations of growth factors sufficient to give maximum growth. I t has been pointed out previ- ously t h a t in the rapid growth of luminous bacteria light emission often lags behind cell division. Recent studies have indicated t h a t this is a fail- ure in the synthesis of the luciferase. A specific effect of aspartic acid on luciferase synthesis has been observed in a m u t a n t requiring arginine, pro- line, histidine, lysine, tyrosine, and methionine for growth (see Fig. 15).
Miller et aZ.46 have studied extensively the conditions necessary for t h e
induction of mutation in Achromobacter fischeri. Although a number of m u t a n t s have been isolated, there is no evidence to indicate t h a t X-ray, ultraviolet, or nitrogen mustard increased the mutation rate. I t was shown during these studies, however, t h a t under nutritional conditions just suffi- cient to support t h e normal wild type strain and a t temperatures at which metabolic activity is low, there is a selective advantage favoring strains which have more exacting growth requirements. Such selections under FIG. 1 3 . Relationship between growth, luminescence, and aspartic acid concen- tration in a mutant of Achromobacter fischeri (McElroy and Farghaly2 7).
FIG. 1 4 . Stimulation of synthesis of the luminescent system by amino acids. As- partic acid mutant described in Fig. 13 was used. See text (McElroy and Farghaly2 7).
502 W . D . MCELROY
TIME (HOURS)
FIG. 15. Effect of aspartic acid on the synthesis of the luminescent system in a multiple mutant of Achromobacter fischeri. Open circles refer to light intensity. Graph on the right represents growth and luminescence in the absence of aspartic acid while the graph on the left represents the effect of supplementation with aspartic acid
(Friedman1 8).
these conditions were apparently due to a greater loss of essential nutrients from the wild t y p e cells, resulting ultimately in their death. W i t h the cold incubation technique it was possible to increase t h e apparent m u t a t i o n rate in luminous bacteria over 20 times. Gene recombination in m u t a n t s of luminous bacteria has been described b y McElroy and F r i e d m a n .4 6 U n - fortunately, strains of these particular m u t a n t s are not available for fur- ther investigation and these results have not been confirmed with other strains of luminous bacteria.
Only recently have m u t a n t s been obtained which directly affect t h e light-emitting system without influencing the growth of the bacteria. Rogers and M c E l r o y1 9 2 0*4 7 have described a m u t a n t which requires the long- chain aldehyde for light emission. T h e y found t h a t the addition of minute amounts of dodecyl aldehyde t o colonies of this dark m u t a n t immediately restored the light. An analysis of the cells indicated t h a t the m u t a n t con- tained bacterial luciferase as well as t h e other components necessary for light emission. Presumably an aldehyde-forming or aldehyde-releasing en- zyme is lacking in this particular strain. Other dark strains studied by Rogers and McElroy indicated t h a t the luciferase was lacking. T h e lumi- nous response of the dark cells to dodecanal is shown in Fig. 16. A log plot
of this d a t a demonstrates t h a t the initial response is first order. When the aldehyde concentration in the cell reaches a level where the enzyme is saturated, it can be shown t h a t the maximum light intensity becomes a measure of the amount of bacteria luciferase plus the steady state concen- tration of FMNH2 in the cell. However, initially the light intensity is a measure of the aldehyde concentration inside the cell. Therefore, the initial slope of the luminous response in light units per second is a measure of the rate of penetration of the aldehyde into the cell. Additional studies have indicated t h a t the properties of the bacterial light-emitting reaction in vitro are identical to those in the cell. Therefore, this system provides a unique one for studying rapidly and quantitatively the penetration of sub- stances which affect light emission. T h e advantage of this system over others is due to the great accuracy and sensitivity in detecting enzyme ac- tivity without disturbing cellular structure. T h e dark m u t a n t of luminous bacteria is uniquely suited for measuring t h e penetration of various alde- hydes since the latter are required to restore normal light emission. I t should be possible to analyze the penetration of other substances, however, provided they affect the light-emitting system. Direct and indirect effects can be determined by studying the response of the isolated enzyme to such agents. T h e values of maximum light intensity obtained with variation in aldehyde chain length employing intact cells show a close correspondence to those obtained with isolated luciferase when D P N H is used to initiate the reaction. This would support other evidence which indicates t h a t re- duced pyridine nucleotides are the most likely source of reducing power for luminescence in the cells.
FIG. 1 6 . Luminous response of dark mutant cells to aldehyde (Rogers and Mc- Elroy2 0).
504 W . D . M C E L R O Y
T I M E - S E C O N D S N O . O F C A R B O N S
FIG. 17. Relationship between aldehyde chain length and penetration into dark mutant cells (Rogers and McElroy2 0).
Before t h e bacterial luciferase in t h e cell is saturated with aldehyde t h e rate of increase in light intensity with time shows t h a t t h e penetration of the aldehyde is probably a first-order diffusion process. A comparison of the rates of penetration of t h e various aldehydes used can be obtained b y simply recording t h e time it takes to reach the maximum light intensity.
The relationship between aldehyde chain length and the rate of the pene- tration is shown in Fig. 17. From these d a t a it is clear t h a t t h e rate of pene- tration of these compounds through t h e membrane decreases with increas- ing number of carbons in the aliphatic chain. T h e study of t h e effect of temperature on the penetration of the aldehyde demonstrated t h a t these compounds in all probability enter t h e cell b y free diffusion and t h e re- sults clearly indicate t h a t molecular size and steric problems related to t h e structure of the cell membrane are of prime importance in t h e permeability process, and t h a t lipoid solubility is relatively unimportant. These results agree with the earlier work on Beggiatoa mirabilis in which it was demon- strated t h a t t h e penetration of nonelectrolytes depended largely upon molecular size.
T h e rapid penetration of solute molecules into cells will lead to lyses;
both Hill3 2 and Collander4 8 have used this technique with luminous bac- teria for permeability studies.
IV. Taxonomy and Evolution
A . D I S T R I B U T I O N , I S O L A T I O N , A N D C L A S S I F I C A T I O N O F L U M I N O U S B A C T E R I A
Luminous bacteria m a y be classified as parasitic, causing infection of various living animals, such as insects, fresh and salt water shrimp, amphi-
pods, etc; saprophytic, living on such dead m a t t e r as fish or m e a t ; or sym- biotic, those found in the luminous organs of fish or squid. T h e salt water luminous bacteria have been those most frequently studied and are rela- tively easy to isolate. Most will grow on ordinary nutrient agar with 3 % sodium chloride and a carbon source such as glucose or glycerol. There have been, however, a few fresh water forms isolated, and these have been reported to grow on nutrient agar with 0.9 % sodium chloride or none at all. Among the best sources of salt water luminous bacteria are dead fish or squid which have not been washed with fresh water. If such animals are placed in a 15 to 20° C. incubator overnight, one usually observes small luminous colonies developing on the surface of the organism. If one removes a small amount of this material to an agar plate, little difficulty is en- countered in obtaining a pure culture of these forms. F r o m such isolations one can obtain luminous bacteria of long or short rods, cocci or vibrios;
they m a y be quite motile or nonmotile. Probably the two forms which have received the greatest attention are those which are the most confused in classification.
Achromobacter fischeri has been used extensively in t h e laboratory during the past 25 years. I t is a motile rod approximately 0.9 b y 1.8 microns. I t is Gram-negative and requires approximately 2 . 8 % sodium chloride for optimum growth. I t is a nitrate reducer and its polar flagella and biochemi- cal characteristics classify it as a Pseudomonas. T h e temperature for op- timal luminescence is 25° C. T h e organism can be grown only aerobically unlike other closely related species. I t has been isolated from a n u m b e r of places b u t the original was obtained from a dead herring from the sea water at Kiel. T h e official name for Achromobacter fischeri now listed in
"Bergey's M a n u a l of Determinative Bacteriology" is Bacterium phos- phor escens indigenus (Eisenberg). However, most workers in the field have used the earlier and more familiar name.
T h e second species of luminous bacteria which has been studied in great detail in the laboratory is Photobacterium phosphoreum. I t will grow either aerobically or anaerobically. However, luminescence occurs only in the presence of oxygen. T h e temperature optimum for light emission is a p - proximately 15° C. I t is readily isolated from dead fish and meat and from time to time has been given the following names: Micrococcus phosphoreus (Cohn), Bacterium phosphorescens (Fisher), Photobacterium phosphorescens (Beijerinck), Streptococcus phosphoreus (Trevisan), and Bacillus phos- phoreus (Mace).
I t is evident t h a t much confusion exists in the literature on the naming of these various forms. Some of the confusion in classification has been due in part to the fact t h a t a luminous bacterium isolated from different sources has invariably been given new names. For example, luminous bacteria have been isolated from diseased insect larvae and have been given t h e
506 W . D . M C E L R O Y
name Bacterium hemophosphoreum (Pfeiffer and Stammer), from midges—
Bacterium chironomi (Issatschenko), from marine crustaceans—Bacterium giardi (Kruse), from fresh water fish—Bacterium hippanici (Issatschenko), from luminous clams such as Pholas dactylis—Bacterium pholas (DuBois), and from deep sea fish—Coccobacillus collorhynchus. I n addition, there are those interesting luminous bacteria which inhabit special glands in the deep sea fish Physiculus japonicus; these have been named Micrococcus physiculus.
Two fresh water species have been studied extensively, Vibrio albensis and Vibrio phosphorescens. T h e optimum sodium chloride concentration for growth and luminescence is approximately 0.9 %. Both species are Gram- negative and motile. Morphologically they look very much alike, how- ever, W a r r e n ' s4 9 studies on the antigenic properties of these two forms indicate a definite difference. There are other reasonably well-defined species of luminous bacteria and the reader is referred to "Bergey's M a n u a l of Determinative Bacteriology" for this information. However, it is apparent t h a t despite extensive investigations of the cultural characteristics of these various forms the separation of luminous bacterial species is in a rather unsatisfactory state. I t is certain t h a t too much attention has been paid to light emission as a unique and distinguishing characteristic. T h e earlier belief t h a t all luminous bacteria must be closely related taxonomically can no longer be accepted.
B . E V O L U T I O N A R Y S I G N I F I C A N C E O F T H E L I G H T - E M I T T I N G R E A C T I O N
As H a r v e y1 has often emphasized, a glance a t the evolutionary tree will reveal luminous species scattered in about half of the phyla with no a p - parent rhyme or reason. I n the course of evolution apparently light pro- duction has appeared again and again, and the origin of this light-emitting process has fascinated a number of workers. T h e ability to produce light does not confer a great survival value on the organisms endowed with it since there are m a n y more nonluminous than luminous forms. Secondarily, however, this ability m a y be adapted to uses which do confer a selective advantage on the luminous organism. I n the case of the firefly the light emission has been restricted to particular organs and the yellow flashing is used for the identification of t h e species to ensure sexual reproduction.
One would hardly question the long-range survival advantage of this unique ability. Luminous bacteria, on the other hand, probably do not obtain any selective advantage under most conditions from their ability to luminesce. R a t h e r their light emission has been regarded as an acci- dental mutation in which the energy liberated b y a terminal flavin oxidase is channeled into an excited state of a molecule which subsequently emits light. Certainly the ability to emit light in a number of organic oxidations