Chemical Control of Microbiological Deterioration
H. L. A . TARR
Technological Station, Fisheries Research Board of C a n a d a , Vancouver, British Columbia, C a n a d a
I. Earlier Investigations (prior to 1938) 639 II. The 20-Year Period 1938-1958 641
A. Methods of Application 641 B. Nitrites as Preservatives 641 C. Antibiotics as Preservatives 646 D. Miscellaneous Preservatives 665
References 670 I. Earlier Investigations (prior to 1938)
It was almost two decades ago that the early literature concerning chemical preservation of fish was very sketchily reviewed (Tarr and Bailey, 1939). This literature will now be considered more fully.
Cobb (1911) stated that salicylic and boric acids were used in mild curing of Pacific salmon for many years until their use was discontinued, owing to complaints of German importers. Later he referred to the use of boric acid as a preservative for salted cod in the United States between 1881 and 1907, when it was generally replaced by sodium benzoate (0.3-0.4%) added to the salt used for curing (Cobb, 1927).
Tower (1899) stated that washing fresh fish in 3 % boric acid solution prior to shipping them caused improvement and that salicylic acid ( 0 . 1 % ) , potassium nitrate ( 1 0 % ) , and formaldehyde ( 5 % ) were not satisfactory for various reasons. There is little doubt that both boric acid and sodium benzoate were widely used for many years on the Atlantic coast of North America in attempts to control red discoloration of salted codfish occasioned by red halophilic bacteria. It is probable that they are still used, at least in isolated areas. Indeed, most of these early ex
periments or applications of preservatives to fish were made with salted products.
One of the earliest recorded attempts to use a comparatively harm
less preservative to delay spoilage of fresh fish was that of Gibbs (1923), who reported that ice containing 0.025% of sodium hypochlorite was somewhat effective in controlling bacterial spoilage of whole, eviscerated iced fish. Not long after this, Chen and Fellers (1926) found that
639
640 Η. L. Α. TARR
washing fish in solutions containing 0.6% of available chlorine in the form of sodium hypochlorite somewhat delayed bacterial spoilage and that halibut so treated turned yellow. Lehr (1937) also indicated that chlorinated ice might have value in fish preservation. These and later experiments by several other investigators, including the writer, have indicated that chlorine, or compounds which readily liberate free chlorine, are practically ineffective in preserving fish owing to their great instability in the presence of readily oxidizable materials. Free chlorine is an excellent germicide for use in fish plants.
Killeffer (1930) found that storage of chilled fish in atmospheres containing fairly high partial pressures of carbon dioxide retarded bacterial spoilage. This finding was verified by Coyne (1933), Weedon and Notevarp (1934a), Stansby and Griffiths (1935), Notevarp (1937) and von Keller (1939), but was apparently not applied commercially.
Shewan (1949) has indicated that carbon dioxide, although it has valuable preservative properties for fresh fish, is unlikely to be used commercially because of some of the rather objectionable effects it has on the appearance of the fish so treated.
German investigators stated that the keeping quality of whole fish and of fillets could be improved if they were immersed in a 0.3%
hydrogen peroxide solution (Metzner and Oeser, 1938; Metzner et al, 1938), but Lücke (1938) disagreed with these findings on the ground that the treatment was merely surface active and gave a misleading idea of the real quality of the fish. Later studies indicated that hydrogen peroxide treatment effected no important improvement in the bacterio
logical quality of treated fish (Tarr and Sunderland, 1940a). The use of ozonized ice and water to purify shellfish and preserve fish from bacteriological spoilage has been suggested (Hurequin, 1936a, b; Salmon and L e Gall, 1936; Salmon et al, 1937), but the process does not appear to have been applied. The use of inorganic and organic acids to control bacterial spoilage of fish has also been suggested (Metzner, 1933a; Note
varp et al, 1934; Weedon and Notevarp, 1934b; Nadeau, 1939). Though organic acids, and more particularly acetic acid, are very useful in preservation of acid-cured, marinated, and possibly salted (Notevarp et al, 1934) fish products, it has been pointed out that they can have little use in successful preservation of fresh fish, since acidification of fish muscles tends to bring the proteins to their insoluble isoelectric points with a resulting serious impairment of their water-holding prop
erties (Tarr, 1940, 1942a). The application of preservatives to acid-cured fish products will be considered in greater detail in the section which
is concerned with that subject. Legislation regarding permission to use such preservatives as hexamethylenetetramine, sulfur dioxide, boric acid, benzoic acid and its esters was well reviewed by Behre and Ulex (1932) and by Metzner (1933b). The value of hexamethylenetetramine in sterilization of spices used for preparation of marinated fish products was recognized by Lehr and Kayser (1937).
II. The 20-Year Period 1938-1958
A . METHODS OF APPLICATION
It is obvious that the application of any preservative to fish should be carried out as soon as possible after capture and killing. There are three general methods of application of preservatives to fish which have been routinely employed in actual practice: ( 1 ) incorporation in ice used to ice fish; ( 2 ) immersing whole or eviscerated fish in, or spraying them with, comparatively strong concentrated solutions of the germicide and then storing them in ordinary ice; ( 3 ) addition to refrigerated sea water in which the fish are stored. Fillets cut from fish may be dipped in bacteriostatic or germicidal solutions, but if this is done it is imperative that the fish used be only of high quality. Naturally the fillet-dipping procedure is rarely used except under conditions where fillets are to be shipped unfrozen. For fish intended for freezing or canning, this procedure is usually not necessary.
During the two decades immediately under consideration, a very large number of chemical compounds have been studied for fish preservation but few have been widely investigated or actually applied.
Previous reviews of the literature include those by Dunn (1947), Soudan (1950), and by Tomiyasu and Zenitani (1957). Partmann
(1952) has reviewed the literature concerning germicidal ices. For con
venience these preservatives will be listed under separate headings.
B . NITRITES AS PRESERVATIVES
1. Experimental and Practical Applications to Fish
Though Taylor (1923) suggested the use of nitrites to cause develop
ment of a slight, and apparently desirable, pink color in muscles of treated white-fleshed fish, as far as can be ascertained the first suc
cessful use of nitrites as preservatives for fresh fish was made by Tarr and Sunderland (1939a), who showed that bacterial spoilage of fillets of halibut and flounder was very strongly retarded by dipping them in 2 % sodium chloride solutions containing from 0.02 to 0.1% of potassium
642 Η. L . Α. TARR
nitrite before storing them at 1°C. ( 3 5 ° F . ) . These investigators later showed that immersion of fillets in 2 0 % sodium chloride solutions con
taining between 0.05 and 0.2% sodium nitrite considerably extended their keeping quality after they had been lightly smoked and stored at 20° or 25°C. (Tarr and Sunderland, 1939b). In a thorough comparative study of a number of different preservatives with different fish and different test conditions, it was found that sodium and potassium nitrite salts were the most effective for retarding fish spoilage (Tarr and Sunder
land, 1940a). It was found that the efficiency of sodium nitrite as a preservative varied somewhat, and that the pH of the muscle of the fish was largely responsible for this variation (Tarr and Sunderland, 1940b). Thus, with 100 to 200 μξ./g.1 of sodium nitrite, at pH 6, sup
pression of bacterial growth was extremely marked, while at pH 6.5 it was only moderate and at pH 7 there was no retarding effect at all
(Tarr and Sunderland, 1940a).
Since it was realized that it should be more effective to apply nitrite as soon as possible after capture of fish, studies were made in which sodium nitrite was incorporated in crushed block ice used to ice evis
cerated fish (Tarr and Sunderland, 1940c). In these experiments it was shown that if the aeration technique usually used in preparing block ice was omitted, block ice containing 0.05% sodium nitrite could be pre
pared in which, though the distribution of the salt was by no means uniform, the crushed mixed ice did contain about the expected .05%
of the salt. Ice containing 0.5% sodium nitrite effected a very pro
nounced degree of preservation of treated fish. However, the flesh con
tained rather high concentrations of the salt (323 to 970 μg./g.). Ice containing 0 . 1 % sodium nitrite effected a significant improvement in keeping quality without occasioning excessive sodium nitrite concentra
tions in the flesh of the treated fish (65 to 370 μg./g.)· Addition of 0.2%
N a H2P 0 4- H20 to the water used in making ice containing 0.1% sodium nitrite did not improve the bacteriostatic activity of the ice (Tarr and Sunderland, 1940d). Halibut stored in nitrite-containing ice developed a yellow discoloration on their white ventral surfaces.
Following these initial exploratory investigations concerning the value of sodium nitrite in retarding bacterial spoilage of fish, treatment of fish fillets was practiced on a large industrial scale in the Canadian Maritime Provinces, and has now been legal for many years in Canada
1 Concentrations of preservatives have been expressed as μg./g. or ml., which is equivalent to parts per million, or on a percentage basis (1% = 10,000 parts per million).
(Anonymous, 1954a). According to these regulations ( B . 16.006.k),
"sodium nitrite [may be used] in fresh fish, preserved fish, and preserved meat only, in an amount not exceeding 200 parts per million of the finished food."
In eastern Canada sodium nitrite has been used for many years to retard bacterial spoilage of fillets of white-fleshed fish (Castell and Gunnarsson, 1956). From the results of comparative experiments in which eviscerated cod were iced with ice containing 0.1% sodium nitrite, or in which the fish were dipped for short periods in 1 or 1.5% solutions of sodium nitrite prior to icing them with ordinary ice, it was concluded by the above investigators that the latter procedure was preferable.
Unfortunately, a dipping procedure on board ship often presents an extra handling problem.
Conditions for maximum bacteriostatic effectiveness, the mode of action, and the fate of sodium nitrite in fish flesh have been the subject of considerable investigation. That acid conditions (preferably below pH 6.5) are essential for adequate bacteriostatic action in fish has al
ready been pointed out. Thus, the finding that bacterial growth is normally not seriously delayed in Atlantic cod fillets by nitrite treatment
(Castell, 1949a) is not surprising, since the pH of the flesh of these fish is often above 6.5. On the other hand, the pH of the muscle tissue of many varieties of North Pacific ocean fish is between 6.0 and 6.2 post mortem (Tarr and Ney, 1949), so that sodium nitrite has a pronounced bacteriostatic action with these fish. Neilands (cited by Castell, 1949a) observed that nitrite inhibited reduction of trimethylamineoxide to tri
methylamine by washed cells of certain fish-spoilage organisms. It was subsequently shown that in cod fillets the improvement in keeping qual
ity which results from nitrite treatment is due at least partially to the suppression of trimethylamine formation rather than to inhibition of bacterial growth (Castell, 1949b). This inhibitory effect of sodium nitrite on trimethylamine formation was also observed by Yamada and Murata (1949). This effect of sodium nitrite is similar to that of sodium benzoate which had earlier been shown to retard trimethylamine forma
tion in muscles of certain fish, even though it did not retard bacterial growth to any important extent (Tarr and Bailey, 1939).
2. Fate of Nitrites in Stored Fish
The fate of sodium nitrite in flesh of stored fish has been the subject of considerable investigation. Tarr (1944a) showed that nitrite was not reduced appreciably in lingcod or salmon flesh which was stored for
644 Η. L . Α. TARR
11 and 15 days respectively at 0.5°C, but that sodium nitrate was re
duced to nitrite, and the nitrite was further reduced in lingcod flesh stored for 3 days at 10 °C. Castell (1949b) showed that a very large proportion of the bacteria on cod fillets reduced nitrate to nitrite, and it was later shown that nitrite disappeared during storage at 3°C. (Dyer and Castell, 1949) and 5°C. (Dyer, 1949). It is of interest in this con
nection that Shewan (1950) observed a significant reduction in the nitrite content of flesh of iced codfish during storage. He was success
ful in isolating an Achromobacter species which apparently reduced nitrite to free nitrogen, only a little ammonia being detected. Dyer and Castell (1949) noted that nitrite, although it did not normally greatly inhibit bacterial growth on cod fillets, retarded formation of trimethyl
amine, tyrosine, and the normal increase in surface pH which occurs in these fish. It is also interesting to note that these investigators found that the "organoleptic borderline" during 3°C. storage tests was 5-5.5 days with untreated cod fillets, 8-8.5 days when the initial sodium nitrite concentration in the flesh was 135 μg./g., and 12-14 days when it was 270 μξ./ξ.
Since the earlier Canadian investigations, a number of studies con
cerning the effectiveness of sodium nitrite-containing ice on fish preserva
tion have been carried out in other countries. Shewan (1950) found that the freshness of iced, eviscerated cod, as evaluated by organoleptic tests, could be extended 3-4 and 7-8 days by icing with ices containing 1 and 1.5% of sodium nitrite respectively. With the 1% sodium nitrite ice the sodium nitrite content of the fish rose to 400 μg./g. and fell to zero in 23 days. It has been claimed that certain South African fish may be preserved fairly effectively by use of ice containing sodium nitrite
(Anonymous, 1951b). Recent Polish investigations (Trzesinski and Zachorowski, 1955; Borowick et ah, 1956, 1957) have been concerned with the icing of Baltic fish with ice containing 0.1^0.15% sodium nitrite. In these studies it was found that the storage life of eviscerated fish, particularly cod and haddock, was extended for about 2 days and that the nitrite content of the fish rarely exceeded the legal per
missible limit of 200 μζ./ζ. Yamada and Murata (1949) found that the preservative action of sodium nitrite ( 0 . 0 2 % ) was more marked in the presence of 5 % sodium chloride than in its absence. Partmann (1954) also found that ice containing 0.02% sodium nitrite plus 1.5% sodium chloride retarded spoilage of fresh-water fish more markedly than did ice containing 0.05% sodium nitrite alone. It was suggested that the temperature-lowering effect of the sodium chloride might have had
a favorable preservative effect. Bose et al. (1954) also found that ices containing both sodium nitrite and sodium chloride were more effective in preserving fresh-water fish than those containing the nitrite alone.
3. Action of Nitrites on Fish Spoilage Bacteria
The action of sodium nitrite on fish-spoilage and certain other bac
teria has been investigated. It was shown that, both in fish muscle and in culture media, the bacteriostatic effect of sodium nitrite was critically affected by the pH. Thus, while growth of all of 10 microorganisms isolated from fish muscle was inhibited by 200 μg./ml. of sodium nitrite at pH 5.7, at pH 7 the growth of none of them was inhibited. In general, the bacteriostatic activity of sodium nitrite was feeble or absent at pH 6.5 or above. It was found that there was no relation between the effect of sodium nitrite on the aerobic respiration at different pH values and its effect on the growth of several fish-spoilage bacteria (Tarr, 1941a, b ) . In later studies the effect of sodium nitrite on the growth of certain fish- spoilage organisms, Clostridium botulinum, Clostridium sporogenes, Staphylococcus aureus, and Eberthella typhosa, under strictly anaerobic and under semianaerobic conditions was investigated. It was found that the growth of Clostridia was inhibited by 200 \ig./g. of sodium nitrite at low pH values. Growth of the facultative anaerobes was inhibited at low pH values much more strongly in a beef extract peptone broth than in either a fish flesh digest broth or a simple ammonium lactate-inorganic salt medium (Tarr, 1942a). It was shown later that, when different heterotrophic fish spoilage bacteria were grown in nutrient broth con
taining 1% sodium nitrate at pH values maintained below 6, nitrite accumulated only slowly and the organisms gradually became nonviable.
On the other hand, when the pH was permitted to rise unchecked above pH 6, nitrite accumulated in large amounts (up to 0.695%), and viable bacteria were still present after 105 days (Tarr, 1944a).
4. Mode of Action
The mode of bacteriostatic action of nitrites is not clear, though several explanations have been offered. The possibility that nitrites can inactivate bacterial enzymes by combining with amino groups was first advanced by Quastel and Wooldridge (1927). Bernheim (1943) sug
gested that nitrous acid combines with and inactivates amino groups of certain enzymes, and later, Sciarini and Nord (1944) obtained evi
dence in support of the fact that nitrous acid combines with the amino groups of carboxylase causing inhibition, and that a diazo compound
646 Η. L. Α. TARR
is formed. Though it has been calculated that there is only 0.22 μg./ml.
of free nitrous acid present in a 200 μ^/ηιΐ. solution of sodium nitrite at pH 6 (Dyer and Castell, 1949), this concentration could conceivably combine with and inactivate amino groups of enzymes. It is of interest that comparatively high concentrations of sodium nitrite (e.g., 0 . 2 % ) are required to inhibit adenosine triphosphatase at pH 6.0-7.0, and that the inhibitory effect appears to be due to the sodium ion concentration and is of the same order as that occasioned by sodium chloride (Part- mann, 1957a). Work by Tarr and Sunderland (1940c) showed that sodium nitrite was unevenly distributed throughout ice blocks. Recently this observation has been verified and, moreover, it has been indicated that there is a 5 0 % decrease in the original sodium nitrite content of ice blocks during 3 months' storage (Borowik and Ostrowski, 1956).
In Norway, large gluts of herring have necessitated preservation of these fish before fishmeal and oils can be prepared from them. Extensive experiments have, therefore, been made in that country to determine the value of such preservatives as formaldehyde and sodium nitrite, and combinations of these, in preservation of these fish. In general, sodium nitrite has been found of greatest value (Anonymous, 1950, 1951a, 1953a, 1954b), and extensive animal trials have verified earlier work with rats and cats (Tarr and Carter, 1942) concerning the comparative nontoxicity of small amounts of sodium nitrite for various farm animals (Heen et al, 1955).
C. ANTIBIOTICS AS PRESERVATIVES
1. Canadian Investigations
Soon after Florey and his collaborators at Oxford had prepared peni
cillin and demonstrated its remarkable effectiveness in the therapy of certain human diseases (Abraham et al, 1941), investigations were commenced with a view to ascertaining the possible value of antibiotics in food preservation. At first, few antibiotics were available, and these could only be obtained for medical use. It was for this reason that the first recorded attempt to use an antibiotic to preserve a food was carried out employing fish muscle and one of the antibiotics which was only of experimental interest, namely, penicillic acid (Birkinshaw et al, 1936).
These early experiments, carried out between 1942 and 1943, showed that 100 or 1000 μg./g. of penicillic acid exerted no important inhibitory effect against bacterial spoilage of fish (Tarr, 1944b). When penicillin and streptomycin became available, further experiments were carried out
in the same laboratory, and it was found that neither of these antibiotics afforded significant protection against bacterial spoilage when incor
porated in ice used for icing fish, or directly in fish flesh (Tarr, 1946, 1948; Tarr and Deas, 1948).
Early in 1950 a number of the then newer antibiotics were tested, and it was found that aureomycin ( C T C ) , terramyein ( O T C ) , and Chloromycetin, in 10 ^g./g. concentration strongly retarded bacterial spoilage of fish flesh (Tarr et ah, 1950). A more extensive investigation in which 15 different antibiotics were used confirmed the above findings, and it was also found that CTC was more effective than OTC. Indeed, effective preservation resulted with very low concentrations of CTC;
thus, dipping lingcod steaks in an aqueous solution containing only 10 μg./ml. caused a very marked improvement in keeping quality (Tarr et ah, 1952). In these investigations the antifungal antibiotic rimocidin delayed growth of yeasts and molds which normally developed in CTC- treated flesh after a number of days storage at 0° or 3°C.
Since these initial experiments, there has been an ever increasing volume of research concerning the use of tetracycline antibiotics in fish preservation. To exert the maximum beneficial effect, it was realized that an antibiotic should be applied to fish as soon after capture as possible.
Flake ice was prepared which contained 1, 2, and 4 μg./g. of CTC, and 2 μg./g. of C T C plus 200 μg./g. of potassium acid phosphate. Ex
perimental icing of eviscerated Pacific lingcod with these ices in
dicated a very marked reduction in the rate of bacterial spoilage of the fish. In these trials the fish were well iced, and reiced frequently, in well-insulated boxes, and under these conditions all levels of CTC gave about the same amount of protection against bacterial spoilage (Boyd et ah, 1953; Tarr et ah, 1954). Subsequently, experiments were carried out during the greater part of one whole fishing season, in which flake- type ice containing approximately 1 μg./g. of C T C was compared with ordinary ice under practical fishing conditions on salmon trolling boats.
The results showed that an outstanding improvement in keeping quality of troll-caught coho salmon resulted from use of the CTC ice under these conditions (Gillespie et ah, 1954a, 1955). At present, most of the ice used in the larger fisheries on this continent is prepared as block ice by freezing large amounts of water in galvanized steel tanks in circulating brine, usually at about 10°F. The reasons for this are largely ( 1 ) com
paratively greater ease of storage of block ice than flake ice where it is essential to accumulate a large reserve of ice for gluts of fish, ( 2 ) flake- type ice requires greater storage space and sometimes has a tendency
648 Η. L. Α. TARR
to pack when stored, especially if conditions are not ideal, and ( 3 ) ex
pense of changing existing equipment would be high. For this reason, block ice containing CTC was prepared (see below) and its effective
ness determined.
The first experiment carried out was with sockeye salmon which had been packed for a short time noneviscerated in ordinary ice prior to eviscerating them for comparative storage tests in crushed block ice with and without 1 μg./g. of CTC. After 9 days' storage, the fish stored in ordinary ice had 1 96 bacteria per gram, while those stored in C T C ice had less than 0.266 bacteria per gram. After 14 days' storage, the bacterial counts were 2426 and 2 . 16 bacteria per gram, respectively.
However, the CTC ice was almost ineffective when used with salmon which were by no means fresh wThen the ice was first employed (Gilles
pie et al, 1954a, 1955). The first really large-scale tests with CTC- containing block ice were carried out on large vessels which fished for cod, flounder, and ocean perch (Sebastodes species). Ice containing 2.5 μξ./g. of C T C was used, since in this type of fishery the general technique of handling fish is poor in comparison with that followed with troll-caught salmon. Though really adequate sampling of the comparatively large amounts of fish carried on the boats (50,000-80,000 lb.) proved impossible, the results indicated a very significant quality improvement in fish landed from vessels using the CTC-containing ice
(Boyd et al, 1957a).
2. Japanese Investigations
The comparative success of Canadian experiments prompted re
search in other areas. In Japan, Tomiyama and his collaborators have made a number of original and valuable contributions to our knowledge of the preservative value of CTC in several different fisheries. They found (Tomiyama et al, 1955a) that a 1-hr. dip of round mackerel in a solution containing 10 μg./ml. of CTC plus 5 % sodium chloride at 20°C.
caused the treated fish to keep 1.7 times longer than untreated fish. A similar treatment of eviscerated mackerel effected a 2.6 times improve
ment in keeping quality. It was also shown (Tomiyama et al, 1955b) that at least a 30-min. dip in 5 % sodium chloride containing 10-20 μξ./
ml. of CTC was required for effective preservation of sardines which were subsequently stored at 18° C , but that higher concentrations were not much more effective. These same investigators found that a chemical sub
stance, neofuraskin, which had been suggested for fish preservation, was practically ineffective in 20 μξ./g. concentration. Overnight storage of
sardines in ice containing 10-20 μg./g. of CTC, followed by storing them at 4° to 8°C. without ice, also effectively prolonged their keeping quality.
Tomiyama et al. (1956a) found that storage of round herring in sea water containing 10 μg./ml. of C T C or ice containing 5 μg./g. of the antibiotic, or combinations of both techniques, greatly prolonged the keeping quality of the treated fish. In experiments with herring it was found that storage in sea water containing 10 μg./ml. or in ice containing 5 μξ./g. of the antibiotic on board ship markedly improved the keeping quality of the fish. It was concluded that the maximum improvement in storage life over that of untreated fish was 9 0 % at 20° C. and 4 0 % at —1° to 2°C. (Tomiyama et al., 1956a, b ) . Even more striking im
provements in quality were experienced in similar CTC treatments of various bottom fish (Tomiyama et al., 1956c, d ) . Thus, when yellow croaker were dipped in a 10 μg./ml. solution of CTC and iced with ice containing 5 \ig./g. of the antibiotic, the storage life of the fish on fishing boats could be improved by 8 days over that of control fish iced with ordinary ice. With a more careful icing procedure on shore this improve
ment was extended to 13 days longer than corresponding controls. When fish after landing were held at 21° to 29 °C. there was some improvement in keeping quality with fish treated with CTC.
3. United States Investigations
Färber (1954) has conducted a number of experiments to determine the comparative effectiveness of antibiotics for fish preservation. Initially he found that C T C was more effective than OTC and that neomycin gave no important protection. A brief dip in 2 μg./ml. solutions of C T C effected a marked improvement in the keeping quality of fillets stored at 2.8° to 9.4°C. as judged by three different objective tests. Later, Färber and his collaborators (Yakoubovsky-Lerke and Färber, 1956;
Lerke and Färber, 1957) studied 14 antibiotics in concentrations of be
tween 5 to 25 μ^/ιηΐ., and came to conclusions similar to those reported by earlier investigators. Their data with fillets treated in 5 % sodium chloride solutions containing the antibiotics and stored at 5.5 °C. in
dicated that CTC was the most effective of the antibiotics studied and that T C was probably no more effective than OTC, neither being as effective as CTC. Firman et al. (1956a) found that, for several species of Atlantic ocean fish iced with ice containing 5 μg./g. of CTC or treated by immersing them in a 10 μ^/πύ. solution of the antibiotic, quality, as evaluated by both organoleptic criteria and bacterial counts, was markedly improved. Kline et al. (1957) studied the effect of ice contain-
650 Η. L. Α. TARR
ing 5 μξ./g. of CTC and dips containing 25 or 100 μg./ml. of this anti
biotic on the keeping quality of eviscerated Atlantic red fish. They found that, while fish stored in ordinary ice were usually stale within 14 days of capture, those treated with either of the CTC treatments were nor
mally of acceptable quality after 21 days of storage.
4. Research in Other Countries
A Russian report (Ravich, 1956) indicated that CTC gave good results in experimental trials carried out in that country. In Greece, Kelaiditis (1956) found that ice containing about 4 μg./g. of CTC pre
served fish effectively and that it was of great value in retarding the spoilage which occurred in preparation of noneviscerated sardines, anchovies, and horse mackerel in the early stages of salt curing. Indian work (Valenkar and Kamasastri, 1958) indicated that ice containing 5 Vg./g. of CTC exerted a favorable effect on the keeping quality of several species of fish as assessed by trimethylamine and total volatile nitrogen determinations, and by bacterial counts. The improvement was most pronounced after about 7 days' storage in ice.
In Great Britain, Shewan (1956a, b ) carried out extensive tests with CTC. In his work the quality of the eviscerated cod used was very carefully evaluated by organoleptic tests, bacterial counts, and deter
minations of the amount of trimethylamine present. The results showed that eviscerated cod stored in ice containing 5 μg./g. of CTC kept very much better than those iced with ordinary ice or ice containing certain other chemical additives. Differences in quality as determined organo- leptically became apparent only after 9 days' storage, and in general it was concluded that, for long storage periods such as are so frequently experienced in distant fisheries, an improvement in keeping quality of at least 7 days resulted. He also found that fillets treated with a CTC dip had excellent keeping qualities.
It would not be possible to review all the experimental work that has been carried out concerning the use of antibiotics in fish preservation. In Germany Kreuzer (1957a) has reported favorably concerning the use of CTC-containing ice in preserving certain North Sea fish. Favorable re
sults with OTC have also been reported (Pasternack et al., 1956). Sev
eral investigators, however, have not experienced such a marked im
provement in keeping quality of CTC-treated fish as that reported by most of the above-mentioned workers (see Albertsen, 1956; Bystedt and Liljemark, 1956; Hjorth-Hansen, 1956). The reasons for this are not at present clear, but could possibly be explained by ( 1 ) inability to detect
an improvement in the quality of CTC-iced fish during a comparatively brief storage in ice before detectable bacterial spoilage occurred in con
trol fish, ( 2 ) lability and destruction of CTC in ice due to the presence of free chlorine, a high pH value of hard water used in preparing the ice, or some other undesirable CTC-destroying effect, and ( 3 ) poor dis
tribution of the CTC in the ice used.
5. Use with Refrigerated Sea Water
There have been a number of reports concerning the value of tetra
cycline antibiotics in extending the storage life of fish held at —1°C.
in sea water or weak (e.g., 3 % ) sodium chloride solutions. In general, a marked improvement in quality of eviscerated red spring salmon (Boyd et al., 1953), white spring and coho salmon (Gillespie et al., 1954a), and eviscerated and noneviscerated coho salmon, white spring salmon, chum salmon and gray cod and lemon flounders (Gillespie et al., 1955) was observed. Eviscerated young coho salmon were stored 23 and 30 days in refrigerated sea water containing between 0.7 and 1.4 μg./ml.
of CTC and remained in perfect edible condition (Steiner and Tarr, 1955). Investigations by Tomiyama et al. referred to previously have definitely shown the value of CTC in maintaining bacteriological quality of round and eviscerated fish stored in chilled sea water. Stern et al. (1957a) studied the effect of CTC and OTC in concentrations of 5, 10, and 20 μg./ml. of 3 % sodium chloride solutions on control of bacterial spoilage of noneviscerated sockeye salmon at different tem
peratures. They concluded that the bacteriostatic effectiveness of the antibiotics was most marked with fish stored at 0 ° C , and that there was no appreciable difference in the effectiveness of the three tetra
cycline antibiotics studied. With noneviscerated flounders (English sole) stored at 0-4 °C. in 3 % sodium chloride solutions, addition of 2 and 5 μg./ml. of OTC proved more effective in retarding bacterial spoilage of the fish than did similar concentrations of C T C or T C (Stern et al.,
1957b). These results were not in accord with those of Southcott et al. (1958a, b ) , who found that C T C was consistently more effective than either OTC or T C in preserving fish in refrigerated sea water or chilled brine solutions. However, these investigators observed that pre
vention of bacterial growth by C T C in chilled brine solutions was not so marked as it was in ice containing this antibiotic. The growth of red halophilic bacteria on salt fish is not inhibited by 10 μg./ml. of CTC
(Bose et al, 1954).
652 Η. L . Α. TARR
6. Use as Preservatives for Shellfish and Marine Mammals The information available on treatment of shellfish with antibiotics is limited in comparison with that for fish. Tarr et al. (1952) found that 10-20 μ&/ξ. of CTC or OTC markedly protected cooked crabmeat from bacterial spoilage but that lower concentrations were comparatively ineffective. Later, Boyd and Tarr (1956) found that, as with crabmeat, dips in low concentrations of CTC (e.g., 2 and 4 μg./ml.) were not very effective in retarding bacterial spoilage and souring of shucked oysters, but that higher concentrations (e.g., 10 and 20 μg./ml.) were fairly effective. The rather low bacterial counts recorded in this work were probably due to the fact that an incubation temperature of 37 °C.
was used in making them. Abby et al. (1957) found that treatment in 5-30 μ^/πΑ. solutions of CTC greatly retarded bacterial spoilage of freshly shucked Atlantic oysters, but that 1.0 μg./ml. of the anti
biotic was comparatively ineffective. Benarde and Littlefield (1957) studied the effect of C T C and OTC on cooked Atlantic blue crab and oyster meats, using dips containing from 5 to 120 μg./ml. Their results indicated a very significant retardation of growth of viable bacteria, but they concluded that, for various reasons such as the rapid loss of natural flavor in these products and the not very significant delay in organo- leptically detectable staleness, the antibiotic treatments were of little or no practical value. Färber (1954) found that a 2-min. dip in a 5%
sodium chloride solution containing 2 μg./ml. C T C did not appreciably improve the keeping quality of raw, whole shrimp which was subse
quently stored at 7.5 °C. Later Färber and Lerke (1957) reported that stronger CTC treatments exerted a distinctly beneficial quality on the keeping quality of shrimp. Camber et al. (1955, 1956) made a very thorough study of the effects of CTC dips and ice on the keeping quality of raw pink shrimp. They found that ice containing 10 μ^/g»
of the antibiotic gave a 4-day improvement in keeping quality, but was not so effective as a 1-5-min. dip in a 100 μg./ml. solution where a 7-day improvement in keeping quality resulted. Unfortunately, CTC ice ac
celerated formation of black spot (see below), an effect which was probably due to the presence of calcium ions in the ice used. It is in
teresting to observe that there was an approximately 6 0 % loss of anti
biotic activity during preparation of the CTC ice by the carrageen method used in these experiments, possibly because of the alkaline na
ture of the water used. In a careful assessment of several different chemi
cal treatments, Fieger et al. (1956) concluded that, although ice con
taining 10 μξ./g. of CTC delayed spoilage of raw shrimp for about
4 days, it had no value in preventing black spot (melanosis), which is occasioned by a tyrosinase-like enzyme. On the other hand, ice con
taining N a2S203 (0.1-0.15%) was only slightly effective in delaying bac
terial spoilage but strongly retarded black-spot formation. The ices used had rather alkaline reactions (pH about 9.0), and this was thought by the investigators to promote black-spot formation by possibly favor
ing tyrosinase activity. There is little doubt that this comparatively alkaline pH, and that of shrimp flesh itself, would both contribute to the comparative C T C instability and consequent rather poor activity of the ice.
It must not be forgotten that such shellfish as crab, shrimp, lobster, and oysters have very delicate flavors which are usually rapidly lost after even a few days' storage in ice. There is little doubt that these flavors will gradually disappear even if the fish are successfully preserved in a microbiological sense. Moreover, some shellfish are eaten uncooked, and it is quite apparent that if antibiotic treatments are used at the compara
tively high levels necessary to effect successful preservation, the flesh will contain much higher residues than are normally found in flesh of true fishes which are CTC-treated. In view of these facts it would cer
tainly appear that preservative treatments of shellfish should be carried out only when conditions render such treatments absolutely essential.
So far as can be ascertained, only the preservation of whales with antibiotics has been investigated, and not that of other marine mam
mals. Initial experiments in which a 55-g. charge of CTC in 10 gallons of sea water was injected into the visceral cavity of three whales shortly after death indicated that the treated animals were of improved quality as judged by bacterial counts of muscle and liver tissues and of the free fatty acid content of the oil (Crean et al., 1956). Further experi
ments carried out on a much larger scale in which 45 whales were treated as above, only this time with 100 g. of C T C dissolved in 20 gallons of sea water, verified previous findings. Bacterial counts made from muscle and liver tissues showed significant improvement as judged by comparison with similar samples taken from 49 untreated whales.
Determinations of the total volatile base and indole content of a number of the different tissues also indicated that the C T C treatment had been attended by improved quality. No significant improvement in quality was experienced when whales were treated by introducing a 50-g.
charge of either CTC or OTC, contained in a polyethylene bag beneath the black gunpowder in the bombhead of the harpoons used to slaughter the animals (Duncan et al., 1957a, b ) . Though a considerable number
654 Η. L. Α. TARR
of experiments concerning the possible industrial value of CTC and OTC for whale carcass preservation have either been completed or are in progress, few details and reliable published reports of the results of this work appear to be available. One report (Anonymous, 1958) indi
cated that a considerable quality improvement in the oil obtained from whale carcasses resulted from OTC treatment.
7. Antibiotic Residues and the Effect of Heat
A number of papers recording the amounts of tetracycline anti
biotics which are incorporated in the flesh of fish, shellfish, and whales after different treatments, and the effect of various heating and cook
ing procedures on these residues have been published (Tables I, II, I I I ) . With fish iced with CTC ice, most data indicate that significant residues are found largely or entirely in the skin or gills with very small amounts in the flesh immediately under the skin and usually none in the deep flesh itself. It is very doubtful if the edible portion of the flesh of fish treated with CTC by the usual procedures would have more than an average of 0.1 μg./g. of the antibiotic in the raw flesh. Since 80-100%
of CTC is removed by normal cooking procedures, it may be concluded that very little and usually no assayable residues of CTC as such would be consumed in cooked fish.
8. Antifungal Antibiotics
In some of the early experiments concerning antibiotics it was found that the antifungal antibiotic rimocidin retarded both yeast and mold development in fish which had been treated with CTC or OTC (Tarr et al., 1952). Since these experiments were carried out, further rather similar tests have been made with other antifungal antibiotics using them in 200 \ig./m\. concentration in dipping solutions (Southcott and Tarr, unpublished). None of these antibiotics proved more efficacious than a 1% sorbic acid solution, though several had considerable preservative effects. In this work the conditions used simulated those normally en
countered in preparation of smoked fish.
9. Public Health Aspects, Use in Practice and Miscellaneous Data It is not the purpose of this section to describe the mode of action of antibiotics and their effect on public health, since these have been ably discussed by others (Finland, 1955; Hines, 1956; Jukes, 1956).
There is no reason at all to suppose that trace antibiotic residues such as could occur in fish treated with low concentrations of tetracycline
TABLE I
TETRACYCLINE ANTIBIOTICS (EXPRESSED AS μg./g. F L E S H ) IN FISH, AND E F F E C T OF COOKING OR HEATING ON RESIDUES
A. CTC (5μ&/&) Added Directly to Fish Flesh Heated at 100°C. in a Water Bath (Bisse« and Tarr, 1952)
CTC before Heating CTC after Destruction
Fish heating time (min.) heating ( % )
Coho salmon 5.1 15 1.50 71
Coho salmon 5.1 30 0.45 91
Ling cod 4.8 15 1.80 63
Ling cod 4.8 30 0.30 94
B. CTC Content in Eviscerated Gray Cod (Steiner and Tarr, 1956) Days stored in ice containing 1 μg./g. CTC
Residue0 1 2 3 4 5 7 10
V + S 0 0 0 0 — 0.39 0.90
0 0 1.1 — 0 0.30 0.50
V — S 0 0 0.45 0 1.3 0.54 0.70
0 0 0 — — 0.30 0.30
T + S 0 0 0 0 0 0 0
0 0 0 — — 0 0.80
T — S 0 0.6 0 0 0 0 0
0 0 0 — — 0 0
Days stored in sea water containing 2.8 μg./g. CTC
1 2 4 6 7
V + S 1.5 3.0 1.5 2.16 3.0
V — s 0 1.23 ; 1.23 1.26 1.14
T + s 1.2 1.5 1.5 1.26 2.55
T — s 0 0 0 0 0
CTC content in ground gray cod flesh
After heating approximately 5 min.
Unheated 60°C. 82°C. 99°C.
6.5 1.4 1.8 < 0 . 3
1.8 1.2 < 0 . 3
16.0 2.8 2.Ό 0.98
2.8 3.0 1.20
2.8 2.4 0.92
<* V + S = Visceral cavity wall plus skin; V — S = visceral cavity wall minus skin; Τ + S = tail end of fish plus skin; Τ — S = tail end of fish minus skin.
656 Η. L. Α. TARR TABLE I (continued)
C. CTC Content in Fish under Various Conditions (Firman et al, 1956a)
Ice CTC
Fish Immersion treatment treatment0 content
Scrod steaks 10 μg./ml. CTC in tap water Ordinary 5.5 10 μg./ml. CTC in sea water Ordinary 4.5 22% freezing brine + 10 μg./ml. CTC Ordinary 3.5 Butterfish 10 μg./ml. CTC in tap water Ordinary 1.33
10 μg./ml. CTC in sea water Ordinary 0.40 22% freezing brine + 10 μg./ml. CTC Ordinary 0.82
Sea bass None CTC 0.43
5 μg./g. CTC in sea water CTC 1.55
Butterfish None CTC 0.54
5 μg./g. CTC in sea water CTC 0.98
Porgy None CTC 0.50
5 μg./g. CTC in sea water CTC 1.58
Weakfish None CTC 0.05
5 μg./g. CTC in sea water CTC 1.60
5 μg./g. CTC in tap water CTC 0.88
Croaker None CTC 0.30
5 μg./g. CTC in sea water CTC 0.30
5 μg./g. CTC in tap water CTC 0.26
Scrod None CTC 2.40
None CTC 0.91
Salmon None CTC 0.09
Halibut None CTC 0.80
a The CTC ice contained 5 μg./g. of CTC.
D. CTC Content in Eviscerated 60-cm. Cod Stored in Ice Containing 5 μg./g. CTC (Shewan, 1956a)
Skin Flesh just below skin Internal flesh 3.15-11.1 0.15-1.05 < 0 . 1 E. CTC Content in Fillets of Eviscerated Gray Cod (Boyd et al, 1957b)
Days in ice
Amnnnt CTC 3 7
in ice Rawa Baked Boiled Raw Baked Boiled
(μ&/&)
+ — + — + — + — + — + —
0 0.02 0.0 0.02 0.0 0 — 0 — 0 —
—
02.0 — 0.03 0.00 — 0 — 0.06 0 0.03 0
—
04.0 0.15 0.06 0.08 — 0 — 0.20 0 0.05 0 0.03 0
a Key: -•f- = fillets unskinned; — = fillets skinned.
TABLE I (continued)
In cooked skinned fillets stored on commercial fishing vessels in ice containing 2.5 μζ/g. CTC
CTC content after cooking
Days in ice Boiled Baked Fried
6 0 0.08 0.04
7 0 0 0.17
0 0 0.12
8 0 0
—
12 0 0.04 0
F. CTC Content in Flesh of Different Species of Fish Stored at 4°C. in Sea Water Containing 10 μg./ml. CTC (Tomiyama et al, 1957a)
Range of CTC concentration in flesh of four different species
Storage time (hr.) 0 2 6 27 50 70
CTC content 0 0-1.0 0-1.6 0-4.0 2.8 2.8
CTC content in different parts of isaki Storage time (hr.)
Part 0 2 6 27
Flesh 0 0 0 0
Viscera 0 0 0 0
Skin 0 1.0 1.0 1.0
Gills 0 1.0 1.0 1.0
CTC content in bonito flesh containing 21 μg./g. of added CTC after heating
55°C. 76°C. 93°C.
Heating CTC Destruc- CTC Destruc- CTC Destruc- time (min.) content tion (%) content tion (%) content tion (%)
0 21.0 0 21.0 0 21.0 0
5 16.0 19.0 14.0 33.3 13.0 38.0
10 15.0 28.6 10.7 49.0 8.7 58.5
30 11.0 47.5 4.0 81.0 1.6 92.5
60 10.0 52.3 0.0 100 0.0 100
G. CTC Content in Round Fish Dipped in Sea Water Containing 10 μg./ml. CTC (Tomiyama et al, 1957b)
Hours
Fish 0 2 6 27 50 70
Pilchard (7 cm.) 0 1.0 1.0 4
— —
Pilchard (13 cm.) 0 1.0 1.6 2.7 2.8 —
Common mackerel (14 cm.) 0 1.0 1.5 2.1
— —
658 Η. L . Α. TARR
TABLE I (continued)
Hours
Fish 0 2 6 27 50 70
Common mackerel (25 cm.) 0 Horse mackerel (19 cm.) 0 Horse mackerel (24 cm.) 0 Red Sea bream ( 8 cm.) 0
ο ο ο ο ο ο ο ο
ο ο ο ο
— 2.8 0 — Penetration of CTC into bonito fillets stored at 4°C. in sea water
containing 10 μg./ml. CTC
Hours
Depth of penetration 1 5
0-10 mm. from surface 10-20 mm. from surface
1.2 0
2.4 0 H. CTC Content in Skin and Flesh of Fish (Tomiyama et al, 1958a)
CTC content
Fish treated with 5 μg./g. CTC Skin Flesh
Kaidi (18 days in ice) Chidai (18 days in ice) Sea eel (18 hr. in sea water) Yellow croaker (in sea water)
1.15 2.65 3.36 1.44
0.16 0.10 0.58 0.38 Effect of heating during preparation of kamaboko on CTC content of fish flesh
Fish Initial
Destruction Final (%) Croaker flesha
Ground flesh (CTC added) Ground flesh (CTC added)
0.82 0.36 0.75
0.11 0.06 0.11
87 83 85
° In sea water containing 5 μg./ml. CTC for 18 hr.
I. CTC Content in Fish Stored under Different Conditions (Tomiyama et al, 1958b)
CTC content
Storage condition Skin Flesh
Six different species stored 22 days in ice containing 3 - 5 μg./g. CTC
Six different species stored 14 days in ice containing 3 - 5 μg./g. CTC
Five different species given a brief immersion in a solution containing 10 μg./ml. CTC and then stored
22 days in ordinary ice 14 days in ordinary ice
0.05-0.17 0.05-0.27
0.15-0.28 0.28-0.28
0-0.03 0
0.03-0.18 0.05-0.14
Uncooked Boiled Broiled Fried Five different species stored in
ice containing 5 μg./g. CTC 0.03-0.11 0-0.07 0-0.09 0-0.04 Preparation of kamaboko at
maximum temperature
76°C. 81°C.
Two different species stored in ice
containing 3.5 μg./g. CTC 0 0 Two different species dipped in a solu
tion containing 10 μ&/πι1. CTC 0.03 0 J. Decrease in the CTC Content in Fish Flesh during Preparation of Kamaboko
by Heating (Higashi et al, 1958)
r Internal temperature
CTC content of . £-
raw flesh 85°C. 93°C.
0.16 0
—
0.29 0
—
0.75 0.15-0.18 0
3.6 0.75-0.90 0.39-0.50
6.9 1.8-2.0 0.75-1.50
20.4 5.8-6.0 2.0-2.4
61.8 25.1-26.3 7.2-7.7
K. CTC Content in Skin and Flesh of Eviscerated Fish (Southcott et al, 1958b) CTC content
Fish Skin Flesh Halibut stored 19 days at —1°C. in
sea water containing 2.65 μ^/πιΐ. 0-0.74 0-0.06 CTC (at time of unloading) (avg. 0.29) (avg. 0.022)
Black cod dipped 5-60 min. in sea 0.15-0.22 0.09-0.15 water containing 50 μg./ml. CTC (avg. 0.15) (avg. 0.13)
TABLE I (continued)
Cooking procedure
660 Η. L. Α. TARR TABLE II
TETRACYCLINE ANTIBIOTICS ( EXPRESSED AS μg./g. FLESH ) IN SHELLFISH, AND E F F E C T OF COOKING OR HEATING ON RESIDUES
A. Average CTC Content in Shucked Pacific Oysters Immediately After Rinsing in Water Containing CTC (Boyd and Tarr, 1956)
CTC concentrations (μg./ml.) in water
2 4 10 20 0.55 Ö63 L 3 3ti Note: A 50-60% decrease was observed after storing 12 days at 0°C.
B. Range of CTC Concentration in Shucked Atlantic Oysters After Rinsing in Water Containing CTC (Abbey et al, 1957)
CTC concentration ^g./ml.) in water
0<* 2 3 4 5 10 20 0.1-0.55 0.53-0.62 0.08-1.20 1.6-2.1 1.75-4.4 2.6-8.3 3.0-4.2
a "False assay" due to substances affecting the procedure used.
C. CTC Content of Atlantic Blue Crab Meat Dipped 2 Min. in CTC or OTC Solutions and then Stored 15 Days at 0°C. (Benarde and Littleford, 1957;
Benarde, 1957)
Concentration ^g./ml.) in CTC or OTC solutions
15 20 30 40 CTC OTC CTC OTC CTC OTC CTC OTC
3.6 2.52 2.88 1 2.40 5.2 2.8 6.7 3.7 Shucked Atlantic oysters
dipped for 2 min. in solutions containing
Antibiotic content Shucked Atlantic oysters
dipped for 2 min. in
solutions containing Initial
After 12 days storage
After frying OTC, 5 μg./ml.
OTC, 5 μg./ml.
OTC, 15 μg./ml.
CTC, 15 μg./ml.
4.9 2.9 5.35 9.00
2.47 2.40 5.10 11.60
0.14 0.36 0.36 0.91 D. Inactivation of Added OTC and CTC in Crab Cakes of Different Thickness
Fried to Internal Temperatures of between 143° and 176° C.
(Benarde, 1957)
Initial concentration Concentration (μg./g.) after frying
CTC, 10 0.75-2.64
OTC, 10 0.37-3.19
CTC, 20 0.76-1.60
OTC, 20 0.45-1.60
TABLE I I I
TETRACYCLINE ANTIBIOTICS (EXPRESSED AS μg./g·) IN MARINE MAMMALS
(Boyd et al, 1957b)
CTC content of whale tissues taken from whale
carcasses preserved
Tissue with CTC
Muscle near backbone 0.0-0.08
Muscle near throat 0-0.08
Muscle adjacent to blubber 0-0.07
Liver 0-0.26
antibiotics could in any way impair human health. Indeed, traces of the antibiotic penicillin, to which many individuals are peculiarly sensitive, occur in about 5 % of samples of market milk in the United States, yet in no instance has a hypersensitive reaction been traced to ingestion of milk so contaminated (Welch, 1957). It is interesting to observe that trace amounts of certain antibiotics (e.g., 10-20 μg./g., and in some cases 100 μg./g.) have been included routinely in the rations of several different types of farm animals with normally an improvement in weight gain and in some cases in performance. Indeed, inclusion of antibiotics in rations of certain farm animals has been an accepted practice on the North American continent for almost a decade (Jukes, 1955). Many of the gloomy predictions regarding the hypothetical dangers which might follow use of tetracycline antibiotics in food preservation appear to have emanated from members of the medical profession. So far, these grim prognostications do not appear to have materialized, though many hundreds or thousands of tons of treated flesh foods have been con
sumed in different areas. Although the promiscuous and often uncon
trolled use of antibiotics in therapy of human disease has caused a serious problem in development of antibiotic-resistant microorganisms, the concentrations used are hundreds or thousands of times greater than those which could reach a consumer of tetraeycline-treated flesh foods.
It is interesting to speculate what might have occurred if antibiotics had been first used in food preservation and secondly in human or veterinary medicine. In this event the first claim on use of antibiotics could have been that of food preservation, a function which in many respects is as useful as that of disease therapy since in many nutritionally backward areas much disease is of nutritional origin. So far it cannot be truthfully stated that use of tetracycline antibiotics in flesh food preservation has been followed by large-scale development of disease-producing micro-
662 Η. L. Α. TARR
organisms in the foods, nor by shock reactions arising through predicted hypersensitization of consumers. In this respect, at least, this applica
tion so far has a much cleaner bill of health than has that of medical usage.
It appears to the writer that the only real danger attending the use of antibiotics in such flesh foods as fish is that which could accompany misuse. Attempts to use antibiotics to mask poor or dirty processing procedures, or to delay spoilage of fish which is already of poor quality should be most strongly condemned. Not only will such procedures fail to improve the quality of fish for the ultimate consumer but they will in all probability soon result in such unsanitary fish plant or vessel condi
tions that the antibiotic will no longer prove effective. It is important to observe that CTC has been found to inhibit development of type Ε Clostridium botulinum and of an enterotoxigenic strain of Staphylococcus aureus in fish flesh (Tarr, 1956a; Bluhm and Tarr, 1956; Boyd et al, 1957a).
Presently the use of tetracycline antibiotics for fish preservation has been sanctioned in a number of countries. In Canada the Regulations under the Pure Food and Drugs Act (Anonymous, 1954a) were amended in 1956 and again in 1959 to permit the use of C T C and OTC for fish preservation. Regulation B.21.07 states that, "No person shall sell fish that has in or upon it Chlortetracycline or Oxytetracycline unless ( a ) its presence or the presence of both, if both are used, is declared on the main panel of the label, and ( b ) the total amount, singly or in combina
tion, thereof does not exceed 5 parts per million." Japan has permitted use of C T C for certain fish intended for use in preparation of kamaboko.
On April 21, 1959 (Anonymous, 1959a), the United States Food and Drug Administration permitted the following use of CTC on certain fish and fish products. Thus the present regulation permits use of CTC as follows: "5 parts per million in or on fish (vertebrate), scallop
(shucked), shrimp (unpeeled), from application for retardation of spoilage to whole, headed or gutted fish (vertebrate), scallop (shucked), shrimp (unpeeled); each in fresh, uncooked, unfrozen form." The Chilean Ministry of Health has also permitted use of certain antibiotics as additives to ice used for icing fish (Anonymous, 1959b). It has been stated that tetracycline antibiotics are used commercially for fish preser
vation in Greece, the Philippine Islands, Brazil, Colombia, Costa Rica, Guatemala, Honduras, Iran, Mexico, Nicaragua, Panama, and Spain
(Anonymous, 1957).
In spite of the large amount of research concerning the bacteriostatic
effect of CTC on the mixed bacterial flora of spoiling fish, it has re
mained for Valenkar (1958) to determine the sensitivity of different marine organisms to this antibiotic. In his examination of 50 bacterial species isolated from marine sources, he found that 7 0 % were sensitive to 5 μ^/ιηΐ. or less of CTC.
10. Distnbution in Ice Blocks
It has been pointed out (Tarr, 1948) that germicides can normally be more readily incorporated in rapidly formed flake-type ice than in block ice, though the latter possesses certain obvious advantages as pointed out in Section II, C,l. Since most ice additives tend to migrate to form a core at the center bottom part of ice blocks, the problem of proper distribution of ice additives has often been raised. Though this has not been a problem with benzoic acid ice (Tarr and Bailey, 1939) and certain sulfa compounds (Tarr and Deas, 1948), it is certainly very true for the tetracycline antibiotics. Though sodium nitrite does tend to migrate to a core, it is occluded to an extent sufficient to ensure a fairly even distribution once the block ice is crushed and mixed (Tarr and Sunderland, 1940c).
With tetracycline antibiotics the problem has been fairly success
fully overcome. Two groups of investigators working independently (Upham et al, 1954, 1956a, b; Gillespie et al, 1954b, 1955; Boyd et al, 1955; Tarr and Gillespie, 1958) found that certain hydrophilic colloids, and more particularly carrageen and carboxymethylcellulose, were use
ful in effecting more uniform distribution of tetracycline antibiotics throughout ice blocks. Though the formulae advocated by different in
vestigators differed considerably, it has in general been found that about 0.002-0.02% of the hydrocolloid usually gives a fairly even distribution of CTC throughout ice blocks. Inorganic ions such as C a+ + and M g+ + are probably essential for adequate distribution, and the pH of the water plays an important role. N a+ has been found quite effective when carboxymethylcellulose is the colloid used (Boyd et al, 1955).
In unpublished experiments (Moyer and Tarr), other hydrocolloids such as certain alginates have been found to give good results, but one of the real difficulties appears to be that none of the hydrocolloids acts ef
fectively as a distributor at pH < 6.0, while CTC is most stable at pH values considerably below this.
Though Gillespie et al (1954a, 1955) found that C T C was com
paratively stable in melted ice, their experiments were carried out under conditions in which the C T C had been stabilized in the hard
664 Η. L . Α. TARR
water used to prepare the ice by means of citric acid, which was added in concentrations sufficient to adjust the pH to 3.5. However, work by Shewan (see Reay, 1956) has indicated a definite instability of CTC in ice in certain of his experimental work. The cause of this instability is presently uncertain. Japanese research (Tawara and Sasano, 1957) showed that free chlorine in chlorinated tap water ("sendee water") even in 1 μ^/ηιΐ. concentration, rapidly destroys the germicidal activity of 5 μ^/τηΐ. CTC solutions. They found that CTC is comparatively stable in solutions containing 0.05 μg./g. of ferric iron or 36 μg./g. of calcium.
Certain sequestering or reducing agents (citric acid, ascorbic acid, sodium nitrite, sodium bisulfite), when used in molar concentrations 5 times that of the active chlorine present, were effective in neutralizing the CTC-destroying effect of the chlorine. Sea water, which has a high content of inorganic ions and an alkaline pH, did not cause CTC de
struction, and this observation supported the earlier work of Tarr et al, who showed that CTC is rather stable in sea water and had been used successfully in refrigerated sea-water storage of fish or as a solute for CTC in experimental preservation of whale carcasses (see Section I I , C , 6 ) .
11. Concluding Remarks
It has recently been shown that tetracycline antibiotics neither retard bacterial reduction of trimethylamineoxide to trimethylamine nor the formation of hydrogen sulfide from cysteine (Castell and Greenough, 1957). The value of certain antibiotics, and especially of penicillin G, in about 2 μ^/ιχιΐ. concentration in retarding bacterial decomposition of stickwater prior to its concentration to condensed fish solubles has re
cently been demonstrated (Idler et al., 1955; Thomson et al., 1956). It has been claimed that CTC is useful in preserving whole menhaden for reduction (Firman et al., 1956b). The rewards for treatment of fish or fish products intended for reduction may be several, e.g., ( 1 ) a decrease in the objectionable odor during the reduction procedure; ( 2 ) improved yields of meal and oil, and ( 3 ) less loss of nitrogen from the products of reduction in the form of ammonia.
In conclusion it may be noted that several very useful reviews have already appeared which have covered much of the available informa
tion on use of antibiotics in fish preservation (Campbell and O'Brien, 1955; Ingram et al, 1956; Partmann, 1957b; Tarr, 1956a, b, 1957a, b ) . The review by Partmann is probably the most complete since it covers all aspects of fish preservation by use of antibiotics, including public health implications and mode of action of CTC.
D . MISCELLANEOUS PRESERVATIVES
There is abundant information in the literature published since 1938 concerning preservatives other than antibiotics and nitrites. A few of the compounds investigated have been applied industrially, particu
larly in the marinated and salt fish industries, but most of them have not been applied, and are purely of experimental interest.
1. Application to Fresh Fish and Shellfish
Carbon dioxide has been further studied and has yielded good pre
servative effects but produces products which have an unpleasant ap
pearance and texture (Shewan, 1949). Tests of a number of compounds, some of which were applied in gaseous form, showed that they were either ineffective in retarding bacterial development in fish flesh, or effected such undesirable changes as protein coagulation or fat oxida
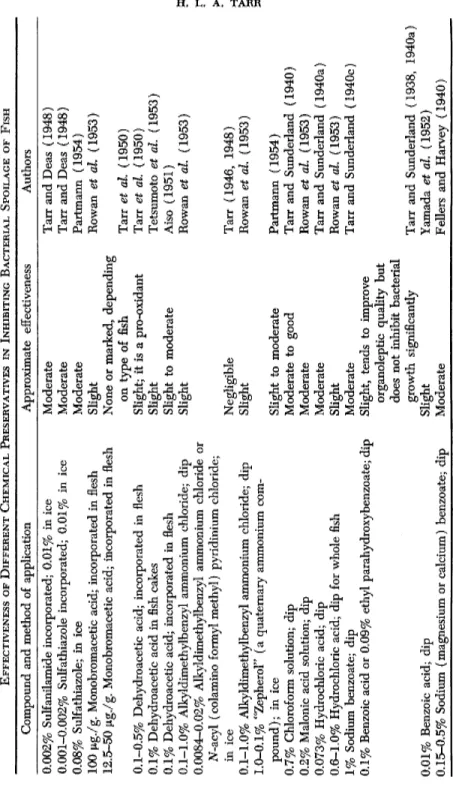
tion. Among those studied were several glycols, ethylene and propylene oxides, methyl and ethyl formates, chloramines Β and T, and ethylene dichloride (Tarr, 1944b). Several chemical substances have been studied either by incorporating them into ice used for icing fish, by dipping fillets in their solutions, or by incorporating them directly into minced fish flesh. It is impossible to compare the results obtained by different investigators in these tests very closely because the germicides, their concentrations, the type of fish, and icing conditions were, in general, very different. For the sake of brevity the results obtained by different investigators have been tabulated in Table IV. In general it may be stated that several sulfa drugs tested have given moderate preservative results, that hydroxylamine gave excellent results but is probably highly toxic, that quaternary ammonium types of compounds are comparatively ineffective, and are certainly only effective when used in high concentra
tions, and that compounds which yield active chlorine are completely inactive, though they do not adversely affect the flavor of fish (Samodel- kin, 1941; Castell, 1947; Partmann, 1955). Benzoates have given slightly successful results but are probably of value only in that they have little bacteriostatic action at the normal pH of fish flesh. Sulfurous acid, or substances which readily yield sulfur dioxide on decomposition, have been used from time to time. Thus, immersion of sole fillets in chilled 2 % sodium chloride solution containing sulfurous acid ( 0 . 1 % S 02) considerably improved keeping quality as judged by their content in tri
methylamine and bacteria but occasioned an unpleasant rancid flavor (Tarr and Sunderland, 1940a). Sodium bisulfite has been suggested as a preservative for salmon eggs intended for use in hatchery diets (Land-