Standard Solutions (Microtitration Techniques)
In general, standard solutions for microchemical work are prepared and standardized in the same manner as those for macroanalytical work. Methods for the latter are found in many works on analytical c h e m i s t r y .
7 2-
1 5 5'
2 1 5Where macromethods use normal and tenth normal solutions, microanalysis employs one or two hundredth normal s o l u t i o n s .
4 2-
7 2'
8 3'
8 4'
1 4 4'
1 4 5'
1 4 7'
1 6 1'
1 6 8-
1 7 1-
1 9 4The reader is referred to the above-mentioned works if there exist any doubts regarding the preparation of standard solutions required, other than those discussed below.
Fortunately, practically all of the standard solutions are commercially
1available and have proved satisfactory in the author's laboratory. Where the standards are prepared in the laboratory, freshly boiled, distilled water is generally used.
Standard solutions of hydrochloric acid, sodium hydroxide, potassium biiodate, iodine, sodium thiosulfate, potassium sulfate and barium chloride are the most frequently used. Potassium biiodate (also called biniodate) may be used either as an acid in alkalimetric and acidimétrie or in iodometric titra
t i o n s
5 3-
1 4 5 , 1 9 4as shown by the following equations:
( 1 ) Alkalimetric—Acidimétrie
K H ( I 03)2 + N a O H - > N a I 03 - f K I 03 + H20
( 2 ) Iodometric
K H ( I 03)2 + 10KI + 11HC1 = 11KC1 + 6 H20 + 6 I2 6 I2 + 1 2 N a2S203 = 12NaI + 6 N a2S406
The normality of a solution of potassium biiodate differs depending on whether it is to be used as an acid or in iodometric titrations, the same solution being twelve times as strong for the latter as for the former, as seen from the equations.
Dissolved carbon dioxide must be removed before proper end points can be obtained in acid-base titrations. Consequently, the solutions should be boiled for about 30 seconds before beginning the titration and then again for a few seconds toward the end. (The one exception to this is the titration of the
102
103 Reagents
dissolved ammonia in boric acid, obtained in the Kjeldahl determination of nitrogen—see Chapter 8.)
Reagents
STARCH SOLUTION*
Starch solution may be prepared by any of the methods generally used in macroanalysis (see above-noted references), or by that suggested by E l e k
5 3'
1 4 5-
1 9 4which is as follows:
An approximate 1 % solution is made by triturating one gram of water- soluble starch with a few milliliters of cold distilled water and adding this mixture to about 95 ml. of boiling 2 0 % aqueous solution of sodium chloride.
The resulting mixture is boiled for 5 minutes, cooled and filtered. The solution should be kept in a refrigerator.
An alternate reagent is prepared according to the following which is recommended by the Association of Official Agricultural Chemists
1 2:
Two grams of finely powdered potato starch is mixed with a little cold distilled water to form a thin paste. About 2 0 0 ml. of boiling distilled water is added with constant stirring and the resulting mixture is immediately allowed to cool. One milliliter of mercury is added as a preservative.
THYMOLPHTHALEIN^
A 0 . 1 % solution in 9 5 % ethanol is prepared.
SODIUM ALIZARIN SULFONATE127 ldd
A 0 . 0 3 5 % solution in water is prepared and stored in a glass-stoppered flask or bottle. The material is also known as Alizarin Red S.
BROMOCRESOL GREEN-METHYL R E D9 4'1 0 9'1 2 8-1 9 4'2 2 6
Five parts of 0 . 2 % bromocresol green and 1 part of 0 . 2 % methyl red, both in 9 5 % ethanol, are mixed and stored in a dropping bottle.
METHYL RED-METHYLENE B L U E3 6'1 4 9 , 1 9 0'1 9 4'2 2 5'2 2 6
Two parts of 0 . 2 % methyl red and 1 part of 0 . 2 % methylene blue, both in 9 5 % ethanol are mixed and stored in a dropping bottle.
METHYL R E D1 4 4 1 4 5 1 9 4
In a flask are placed 0.15 gram of methyl red powder and 4 0 ml. of 0 . 0 I N sodium hydroxide solution. The mixture is shaken and then filtered and the filtrate stored in a glass-stoppered dropping bottle.
* A 0 . 2 % aqueous solution of amylose,1 8 8 was found to be an excellent indicator by Aluise, Hall, Staats and Becker.8
PHENOLPHTHALEIN1**1*5194
A 1% solution of phenolphthalein in 9 5 % ethanol is prepared.
TETRAHYDROXYQUINONE
( ^ Η Ο ;
4-
2 5-
8 7 1 5 0·
1 9 4-
1 9 8 2 1 0·
2 1 2This is kept in the solid state and used as such. It is used together with the glass filter listed below.
Apparatus
BURETTES
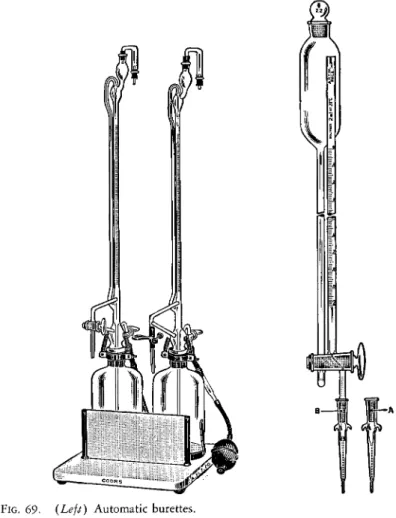
Automatic filling microburettes of the type shown in Figs. 69 and 70, are preferred for storing and titrating standard solutions, although storage in ordinary bottles or volumetric flasks and titrating from the hand-filled type of burette is permissible. The automatic b u r e t t e
8 3'
8 4'
1 4 4-
1 4 5'
1 6 8-
1 7 1'
1 9 4shown in Fig. 69 has a storage reservoir of 0.5-1.0 liter. The solution is forced up into the top of the burette when the pressure in the reservoir is increased by pump
ing the hand-operated bulb. The level of the solution is automatically adjusted to coincide with the 0.00-ml. mark since the delivery tube is at this mark and the tube is so constructed that it acts as a siphon to remove the excess. The flow of solution from the burette is controlled by means of a glass bead inside the rubber tube, a pinch clamp, or a ground glass stopcock. The burettes are usually 10-ml. capacity, graduated to 0.05-ml. The solution in the burette and in the reservoir is protected from carbon dioxide of the air by the Ascarite tubes at the top and between the reservoir and bulb, respectively. The stop
cock between the reservoir and the bulb should be closed at all times except when the solution is being pumped up into the burette. Solution which has remained in the burette for more than an hour or two should be discarded.
Obviously, the reservoir should be shaken each day to mix any condensate that occurs on standing and which alters the normality of the solution, unless mixed in.
The t y p e
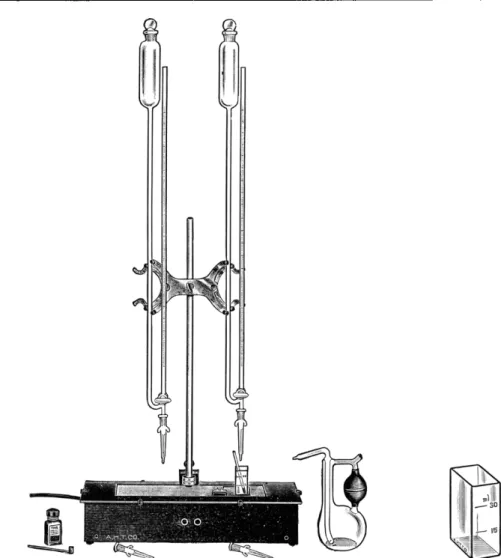
1 0 6'
1 9 4of burette shown in Fig. 70 is available in sizes of 1, 2, 5, and 10 ml. This type is graduated in 0.01 or 0.05 ml. and has its reservoir at the top. Manipulation of the three-way stopcock allows the solution to flow from the reservoir into the burette. The capacities of the reservoirs are usually approximately 100 ml.
ILLUMINATED TITRATION STAND ASSEMBLY78150 1 9 4·1 9 8
The titration stand shown in Fig. 71 is used for standardizing B a C l
2solution
using tetrahydroxyquinone ( T H Q ) indicator together with the orange-brown
glass filter plate described below. The stand is illuminated from below by
means of fluorescent lighting. The base is a frosted glass plate. This should be
105 Apparatus
covered with the masking plate or with black paper which has an oblong section cut from it, just large enough for the filter plate (see below) and cuvette (Fig. 7 2 ) to set in, side by side. T h e cuvette which is prepared from
FIG. 69. (Left) Automatic burettes.
FIG. 70. (Right) Automatic burette with stoppered reservoir and two interchangeable
ground tips; for calibration ( A ) and for delivery of finest drops in tests ( B ) .
glass has the dimensions, 20 χ 4 0 χ 60 mm. high, and has graduation marks indicating the 15- and 30-ml. capacities.
ORANGE-BROWN GLASS FILTER P L A T E4 6-1 5 0-1 9 4'1 9 8-2 1 2
Glass color filter, approximately 2.5 X 26 χ 45 mm. having a spectral trans
mission, uncorrected for reflectance, of 3 7 % at 5 5 0 ιππίμ ± 2 ιηηιμ is required.
FIG. 7 1 . (Left) Illuminated titration stand assembly, showing cuvette and orange- brown filter plate in place.
FIG. 72. (Far right) Cuvette.
pH METER
Zeromatic pH meter, No. 9 6 0 0
1 6or comparable instrument is used in the adjustment of the pH of solutions, such as in the standardization of thorium nitrate.
PHOTOELECTRIC FILTER PHOTOMETER1271*21371™
This instrument is of particular value in the titration of fluoride with thorium
nitrate using sodium alizarin sulfonate as the indicator, although it undoubtedly
107 Apparatus
can be found to be useful in other titrations in which the color change at the end point is gradual. It also makes possible the handling of larger volumes of solutions of low concentrations. The instrument has a small lamp in the center with a Meyer Trioplan lens ( 3 inches, f 2.8, provided with adjustable iris diaphragm) and green filter (maximum transmittance, 520 ιημ) on each side.
The light from the lamp, on each side, passes through the lens and filter, then through a titrating cell containing solution, and finally impinges onto a
FIG. 73. Photoelectric filter photometer.
photocell (Weston photronic photocell, model 594, Weston Instruments Divi
sion of Daystrom Inc., Newark, New Jersey). The two photocells are con
nected in opposition, a mirror galvanometer being mounted in parallel. The latter is shunted (by Ayrton shunt, Fisher Scientific Company, New York, and Pittsburgh) which permits stepwise variation of the sensitivity over a range.
Provision is made for magnetic stirring and titrating. For direct titration,
a blank solution and the indicator are placed in the left side titrating cell
while the solution to be titrated and the indicator are placed in the right side
titrating cell or vice versa. The various stages of operation of the instrument
are described below in the standardization of thorium nitrate. Figure 73 shows
a commercially available instrument, the type used in the author's laboratories (photoelectric filter photometer, model PFP-1, Marley Products Co., Hyde Park, New York) .
1 3 2It is completely encased. The switch on the extreme left controls the galvanometer light, light source and magnetic stirrers. The next control, the second from the left, controls the speed of the magnetic stirrers.
The third switch from the left shorts out the galvanometer and photocells when not in use. The switch on the extreme right is a three-stage shunt for varying the sensitivity. At the top are the galvanometer knob for zero adjust
ing and two diaphragm adjusting wheels which are used to balance the light source to the photocells. Two clear plastic dishes (ordinary refrigerator dishes) are used as titrating cells.
Procedures
It should be remembered that the detecting of an end point is a personal matter, which is more exaggerated when working with one hundredth normal solutions. Therefore, the analyst who is going to perform the particular volumetric procedure should also standardize the solutions, or at least make certain that he sees the same end point as the one who does the standardization.
SODIUM THIOSULFATE, 0.07 Ν
A liter of distilled water is brought to a boil to remove carbon dioxide and cooled while loosely covered. Sodium thiosulfate, N a
2S
20
3· 5 H
20 (2.48 grams) is dissolved in the water and the solution made up to 1000 ml. It should be transferred to a brown bottle equipped with a rubber stopper. One ml. of chloroform is added and the contents of the bottle shaken for a few minutes.
The chloroform acts as a preservative.
9 3 , 1 9 4The use of a rubber stopper over a ground glass one is preferred as the former absorbs chloroform, increasing the efficiency of the preservative. This solution is quite stable as long as a pool of chloroform is present, but should be standardized at intervals of about one month. In the absence of chloroform, standardization should be done every few days.
7 4Instead of the rubber-stoppered brown bottle, an automatic burette of the type shown in Fig. 69 may be used. I f so, it is preferable to use a brown bottle, but if clear glass is used, the solution should be standardized more frequently. Obviously, chloroform must be present at all times, regardless of the setup, or standardization must be done daily.
Standardization:
About 2 - 3 mg. of solid potassium biiodate is accurately weighed (using a
porcelain boat or charging tube—see Chapter 3 ) , transferred to a 125-ml. glass-
stoppered Erlenmeyer flask, and dissolved in 5 ml. distilled water. T o this is
109 Procedures
added 1.5 ml. concentrated hydrochloric acid, followed by 1 ml. of freshly
prepared 4 % solution of potassium iodide, and the flask stoppered. After 2minutes, the solution is diluted with water to 20 ml. and the liberated iodine is titrated with the sodium thiosulfate solution. When the iodine color has almost disappeared (yellow) several drops of the starch solution is added.
Thiosulfate is added until the blue color has been converted to a faint pink at most (end point). I f it is easier for the analyst to detect the end point by titrating to a colorless solution, such action is permissible, but being con
sistent is an absolute necessity.
Calculation:
From the equations shown at the beginning of the chapter,
1 K H ( I 03)2 12 1 _ 1 2 N a2S203
M . W . 389.928 " M w - 1522.92 — M . W. 1897.368 389.928 1897.368 ' · mg. K H ( I 03)2 mg. N a2S203
Now 1 ml. of Ν N a2S203 contains 158.114 mg. of N a2S2Os
mg. N a2S203 ml. N a2S203 X 158.114 1897.368 X mg. K H ( I 03)2
* * 389.928 X ml. N a2S203 X 158.114 i NN a2s2o3 mg. Κ Η ( Ι 03)2 X 0.03077
. ' . ml. N a2S203 = NN a2s2o3
IODINE, 0.01Ν
Approximately 1.27 grams of resublimed iodine crystals are dissolved in a solution of 4 grams of potassium iodide in 4 ml. of water. (Note: Iodine dissolves rather rapidly in a concentrated solution of potassium iodide, but very slowly in a dilute solution.) After about 2 0 - 3 0 minutes (first making certain that solution is complete) the concentrated solution is transferred to a 1-liter volumetric flask and diluted to the mark, with freshly boiled distilled water.
S tan da rdizatio η :
The resulting solution is standardized by titration against the 0 . 0
I N thiosulfate (prepared above) using starch indicator.
This solution is not stable and should be standardized daily.
POTASSIUM SULFATE, 0.0 7 Ν
Exactly 0.8713 gram of reagent grade potassium sulfate is placed in a 1-liter volumetric flask, dissolved in a little distilled water and the resulting solution diluted to the mark, with distilled water.
BARIUM CHLORIDE, 0.07 Ν
Approximately 1.04 grams of barium chloride is dissolved in water and made up to 1 liter. The solution is standardized against the potassium sulfate, prepared above, using the illuminated titration stand, orange-brown filter plate, cuvette (see above), and tetrahydroxyquinone indicator as follows: Three to
5* 1 5 0 , 1 9 4 , 1 9 8 m
\
0f standard potassium sulfate, 0.01N, is placed in the cuvette,
one or two drops of phenolphthalein indicator solution added, and enough 0.1N sodium hydroxide to render the solution alkaline after which it is back- titrated with 0.01N hydrochloric acid just to expel the color. (The titration using T H Q indicator must be done in a fairly neutral solution. Although the potassium sulfate solution is already neutral, the above procedure of making alkaline and back-titrating is recommended since this technique is used in the actual titration of sulfate in the determination of sulfur—see Chapter 10—and therefore the B a C l
2solution is standardized under the identical conditions as when used.) Enough water is added to bring the volume to 15 ml. and this is followed by adding 15 ml. of 9 5 % ethanol. One-half scoop (provided with the indicator) of dry T H Q indicator is added and dissolved by stirring with a glass rod protected on the end by a rubber sleeve (or policeman) to prevent scratching of the cuvette. T h e container is then placed on the illuminated titration stand alongside the orange-brown filter plate so that these are il
luminated from below and the rest of the stand is blacked out to avoid inter
ference with the visual comparison of the solution with the filter plate. The barium chloride solution is added to the yellow contents of the cuvette, with stirring, until the color gradually shifts towards the red and matches that of the filter plate when viewed from the top, looking through the entire depth of solution.f This is taken as the end point and is very sharp, an additional drop changing the color markedly more toward the red. I f the end point has been overstepped the solution cannot be back-titrated. The same solution will have a different normality factor for each filter plate used since the titration would be to a slightly different end point for each. However, this is of no consequence as long as the factor for the particular filter is determined.
* The amount is regulated so that the appearance of solution plus B a S 04 at the end point is about the same as the filter glass. Considerably more or less B a S 04 present does not give a mixture comparable in appearance to the filter.
f Note: Barium salt of indicator is purple-red.1 8 9
I l l Procedures
SODIUM HYDROXIDE, 0.01Ν
This solution may be obtained, commercially,
1and as such is quite satisfactory.
Otherwise, it is prepared from carbonate-free sodium hydroxide as follows:
A 5 0 % solution (about 2 0 N )
9 0>
2 1 9of reagent grade of sodium hydroxide is prepared and stored in a stoppered paraffin-lined bottle until the carbonate has settled out, which usually takes some weeks. This is then decanted and diluted with freshly boiled distilled water to about 0 . 0 I N and standardized as fol
lows.
7 2About 12 to 14 mg. of reference-standard potassium acid phthalate, K H C
8H
40
4, is weighed into a 125-ml. Pyrex Erlenmeyer flask. This is dis
solved in about 5 ml. of distilled water, a few drops of phenolphthalein in
dicator added, and the mixture boiled for about 30 seconds to drive out dissolved carbon dioxide. The hot solution is titrated with the above-prepared sodium hydroxide to a faint pink end point.
K H C8H404 + N a O H -* K N a C8H404 + H20 Calculation:
l N a O H 1 K H C8H404
M . W . 39.999 " M . W . 204.228 mg, NaOH mê- K H C8H404
' " 39.999 204.228
mg. K H C8H404 X 39.999 mg. N a O H =
204.228
Now 1 ml. of Ν N a O H contains 39.999 mg. of N a O H mg. N a O H
- - = Normality of N a O H
ml. N a O H X 39-999
mg. K H C8H404 X 39.999
204.228 X ml. of N a O H X 39.999 " Na0H mg. K H C8H404
204.228 X ml. of N a O H Na0H
Similarly, potassium b i i o d a t e
1 4 5 , 1 9 4or benzoic a c i d
7 2'
1 9 4may be used. For the
latter, neutral 9 5 % ethanol (see Chapter 1 5 ) is employed as the solvent. For
both of these acids, one molecule of acid is equivalent to one molecule of
sodium hydroxide:
l N a O H 1 K H ( I 03)2 M . W . 39.999 M . W . 389.928
l N a O H M . W . 39.999
l C6H5C O O H M . W . 122.125 mg. K H ( I 03)2
389.928 X ml. of NaOH NaOH
and
mg. C6H5C O O H
122.125 X ml. of NaOH NaOH HYDROCHLORIC ACID, 0.0 7 Ν
This solution may be obtained commercially
1or it may be prepared from more concentrated solution, diluting with freshly boiled distilled water and standard
ized against standard, 0 . 0 I N sodium hydroxide solution, using phenolphthalein as the indicator. Just before the end point is reached, the mixture is boiled for at least 30 seconds to expel carbon dioxide. (Note: If the acid is titrated with the alkali, at the end point, boiling increases the color, so that the end point should be approached only with a hot, well-boiled mixture.)
SODIUM FLUORIDE, 0.07 N1 2 7 1 3 7 1 9 9
Exactly 0.4200 gram of reagent grade sodium fluoride is placed in a one-liter volumetric flask, dissolved in a small amount of distilled water and the re
sulting solution diluted to the mark, with distilled water.
THORIUM NITRATE, 0.07 N1 2 7 1 3 7 1 9 9
Approximately 1.38 grams of thorium nitrate tetrahydrate, T h ( N 0
3)
4- 4 H
20 , are dissolved in water and the resulting solution diluted to one liter. This is standardized against the sodium fluoride, prepared above, using sodium alizarin sulfonate as the indicator with the aid of the photoelectric filter photometer described above. (Before performing actual titrations, the proper sensitivity for the instrument must be selected. This is accomplished by doing a blank determination and determining which sensitivity setting gives a deflection of 25 units upon the addition of 0.05 ml. of the thorium nitrate when the iris openings are the same as during the actual determination, preferably as large as p o s s i b l e . )
1 3 2One-, 2-, 3-, 4-, 5-, 6-, 7-, 8-, and 10-ml. portions of 0.01N sodium fluoride
are titrated with the thorium nitrate so that a plot may be made of ml. of
thorium nitrate vs. mg. of fluorine. Each of the above-mentioned portions of
sodium fluoride are treated in the following manner: The fluoride is transferred
to the clear plastic titrating cell and diluted with distilled water to a volume
113 Additional Information for Chapter 5
of 4 5 0 ml. This solution is adjusted to pH 3 . 0 ± 0 . 0 5 (using O.lN hydrochloric acid), and 2 ml. of 0 . 0 3 5 % sodium alizarin sulfonate indicator is added. A duplicate clear plastic titrating cell containing 4 5 0 ml. of distilled water ad
justed to pH 3 . 0 ± 0 . 0 5 and 2 ml. of 0 . 0 3 5 % sodium alizarin sulfonate in
dicator is placed in one compartment of the photoelectric filter photometer and the cell containing the fluoride solution in the other. (Note: Since sodium alizarin sulfonate is also an acid-base indicator, all pH adjustments are most
critical.) With the third switch from the left in the position that both thegalvanometer and photocells are shorted out (short) and the lights and stirrers
on, the galvanometer is adjusted to the zero mark by rotating the galvanometeradjusting knob at the top of the instrument. (Note: The lights should be turned on in advance of the time that the instrument is to be used.) The third
switch from the left is then turned to the "on" position to connect the galvanometer and photocells. The switch on the extreme right should be turned to the position of predetermined sensitivity (maximum preferred). The gal
vanometer is again adjusted to the zero mark this time by means of the
diaphragm adjusting wheels (usually only one—that on the side of the fluoridesolution). The fluoride is then titrated with the thorium nitrate until a de
flection of 25 units
127'137'199on the galvanometer is obtained. (Note: The color change is from a yellow to a pink.) After a number of different size portions of sodium fluoride have been titrated, a curve is plotted showing the relation
ship between milliliters of thorium nitrate and milligrams of fluorine titrated.
This is preferable to labeling the fluoride as "so-much" normal, since a straight- line function does not exist except possibly over a small range of amount of fluorine and then only under certain limited conditions.
ADDITIONAL INFORMATION FOR CHAPTER 5
Although the volumetric procedures described in this chapter as well as through
out this entire book call for the use of burettes of the types shown in Figs.
69, and 70, the analyst encounters problems and procedures elsewhere calling
for the use of other volumetric glassware. For the sake of completeness, pieces
are shown here for which recommended specifications have been published
by the Committee on Microchemical Apparatus of the Division of Analytical
Chemistry of the American Chemical S o c i e t y .
1 9 5-
1 9 7These include micro-
volumetric flasks (Fig. 7 4 ) , pipettes (Fig. 7 5 ) to be used with the flasks,
and a centrifuge tube (Fig. 6 1 , Chapter 4 ) .
T O P V I E W O F F O O T
N O T T O E X C E E D 1 0 0 M M .
F O R A N Y . . S I Z E , H J
Q
J 8 S T O P P E R„ . r
5 - I 5 M M .
2 5 - 3 0 M M .
C f )
. 8 M M . M A X .
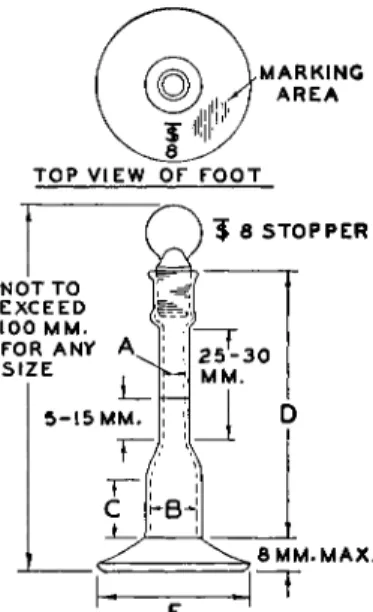
FIG. 74. Microvolumetric flask—details of construction.
A Β
Inside Inside C D Ε
Capacity diameter diameter (Approx.) (Maximum) (Maximum) Tolerance
(ml.) (mm.) (mm.) (mm.) (mm.) ( mm.) (ml.)
1 4.2-4.6 8.0-8.5 10 70 37 ± 0 . 0 1 0
2 5.0-5.4 10.5-11.0 13 70 39 ± 0 . 0 1 5
3 5.0-5.4 13.25-13.75 14 72 39 ± 0 . 0 1 5
4 6.2-6.6 13.75-14.25 18 75 39 ± 0 . 0 2 0
5 6.2-6.6 15.5-16.0 18 75 39 ± 0 . 0 2 0
To be marked " T . C . (capacity) 20° C."
1-ml. size to weigh less than 19 grams empty (stopper included).
Shape of bases may be either round or hexagonal. Dimensions given in column Ε are maximum permitted for distance between parallel sides of hexagonal bases and are maximum diameters of round bases.
115 Additional Information for Chapter 5
ALL TOPS GLAZED
II5±
I 5 M M . h-6.5±|
0.75 M M .
0 . 0 . I
II5±
I5MM.
h-6.5±l
0.75 MM' 0.0
Κ 0.75 MM
0 . 0 . I
115*
I 5 M M .
-3.0 ±0.5 MM.O.D.
-0.55-0.70 MM. ID.
1 2 5 ± I 5 M M .
ALL PIPETTES HAVE SIMILAR TIPS-WITH ENDS GLAZED 0 . 2 M L . 0 . 5 M L . I M L . 2 M L . 3 M L .
OC 5
\~7.7S±
0.75 M M O.D.
125 + I5MM
$7
AT LEAST 80 M M .
— 8 . 5 + 0.75 M M
O.D 3 0 0 M M .
M A X .
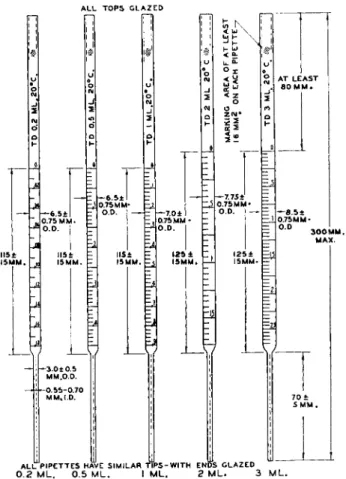
FIG. 7 5 . Micropipette with cylindrical tip—details of construction.
Interval Lining Number at
Capacity Subdivision graduated Ring at l/2 ring at 0 and each Tolerance
(ml.) (ml.) (ml.) each ml each ml. ml. (ml.)
0 . 2 0 . 0 1 0 to 0 . 1 8 0 . 0 2 0 . 0 1 0 . 0 2 ± 0 . 0 0 5
0 . 5 0 . 0 1 0 to 0 . 4 5 0 . 0 5 0 . 0 1 0 . 1 ± 0 . 0 1
1 0 . 0 2 0 to 0 . 9 0 0 . 1 0 . 0 2 0 . 1 ± 0 . 0 2
2 0 . 0 5 0 to 1 . 7 5 0 . 2 5 0 . 0 5 0 . 5 ± 0 . 0 4
3 0 . 0 5 0 to 2 . 7 0 0 . 2 5 0 . 0 5 0 . 5 ± 0 . 0 6
No graduations to appear in tapered portion.
Tip may be tapered at junction with body, but outside diameter at this point may not exceed 4 . 5 mm.
Tip outlet to be glazed, with least possible constriction.
Calibrated to deliver at 2 0 ° C. touching off last drop.
The volumetric flasks
1 9 5are of a new design which combines conveniency in use with accuracy. The wide base affords greater stability against upset (Fig.
7 4 ) . In the case of the one-ml. size, the diameter of the base is small enough to permit placement on the microchemical balance pan and the weight is restricted to a maximum of 19 grams when empty so that when full of a solution of spécifie gravity of one or less, the capacity of the balance is not exceeded.
In order to increase the usefulness of the above flasks, special measuring pipettes (Fig. 7 5 ) were designed.
1 9 5The long narrow delivery stems of the pipettes with cylindrical tip (0.2-, 0.5-, 1-, 2-, and 3-ml. sizes, Fig. 7 5 ) reach to the bottom of the flasks, permitting almost complete withdrawal of the contents. Actually, all but a few hundredths of a milliliter can be withdrawn.
The microliter pipettes
1 9 6shown in Figs. 76, and 77 also have dimensions such that they too may be used with the microvolumetric flasks. These types, sizes, and dimensions were recommended after a study was made of the returns from a questionnaire sent out to interested parties. Returns were received from 56 individuals, 48 of whom were users of this type of pipette, and a number of whom have since tested the finished items and found them to be quite satisfactory. The following procedure is recommended for calibration of these microliter pipettes
1 9 6:
"Standard procedure for micropipette calibration consists of filling the pipette with mercury, discharging the mercury into a porcelain dish, weighing the mercury, and making the appropriate weight-temperature-volume calcula
tion. This method has been compared with that of weighing the pipette both empty and mercury filled, and has been found less difficult and equally precise."
(Note: For calibration purposes where mercury is used, the edge of the mercury
meniscus should coincide with the top of the line.)
Micro washout pipettes
1 9 7of the types shown in Figs. 78 and 79, re
spectively, are used extensively. They are designated as micropipettes instead of as microliter pipettes because of their particular applications and not because of their range. With both, adhering material is transferred by means of wash liquid, being sucked up through the tip in the case of the Folin-type
6 5and added to the cuplike top of the other.
8 4The density-type pipettes
2 , 1 9 7described in Chapter 22, Fig. 217, will also prove useful, particularly because they have ground tips and caps.
Self-filling, self-adjusting polyethylene type of measuring units developed by Sanz are commercially available.
1 7As an aid to pipetting, hypodermic syringes are attached to microliter pipettes by means of plastic tubing.' This affords a means of better control.
The burette
9shown in Fig. 80 may be used as either a micro- or ultra-
microburette. It has a 3.5-ml. reservoir through which passes a power driven
MIN WALL , 0.5 2-3
OV/ΡΟΔΙ 1 LENGTH -J 20-35 ^ AT Lt .AST 5 25-40 * 1 Ξ£^Γ - -""~^T " GLAZED , SAFETY BULB χ 5-6 /O.D. NOT MORE) \ THAN 7 MM / GRADUATION MARK (RING) PERPENDICULAR TO LONG AXIS OF PI PET
2-5 DELIVERY TIP 1.7-2.5
MIN WALL t 0.5 (TO 0.7^ THAN 7 MM (DIMENSIONS IN MILLIMETERS) 1 TO 4 MICROLITER SIZES TIP MUST BE GROUND FLAT NORMAL TO AXIS AND SLIGHTLY BEVELED ,
I
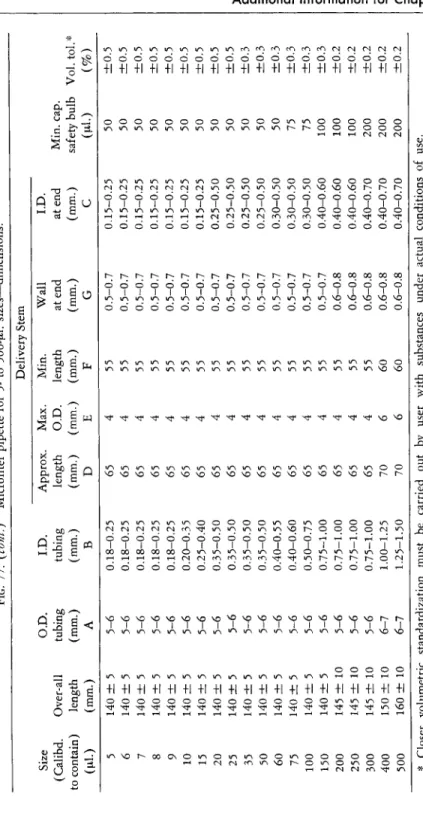
GLAZINGOPTIONAL] AFTER GRINDING - NO CONSTRICTION OF BORE J FIG. 76. Microliter pipette for 1- to 4-μ1. sizes—details of construction. ^ Size (Calibd. to contain) (μ1)
Over-all length (mm.) I.D. tubing (mm.) Β I.D. at end (mm.) C
Min. cap. safety bulb (μΐ.) Vol. toi.* (%) 1 140 ± 5 0.12-0.16 0.10-0.20 50 ±1 2 140 ± 5 0.16-0.25 0.15-0.25 50 ±1 3 140 ± 5 0.20-0.28 0.15-0.25 50 ±1 4 140 ± 5 0.24-0.32 0.15-0.25 50 ±1 * Closer volumetric standardization must be carried out by user with substances under actual conditions of use.
117 Additional Information for Chapter 5
MIN WALL 0.5 2-3 I. D.. I MIN. GLAZED
OVERALL LENGTH 20-35 T AT LE; ,/ , \ST 5 N \ . MAX DELIVERY TIP
t
1MAX DELIVERY TIP
^ 7 -
L .. - λ 15E NOTESAFETY BULB Θ D APPROX GRADUATION MARK (RING) ID1CULAP
2Z5
i4
2.0-3.0\
MAX O.D. OF BULBS I MM MORE THAN MAX A (DIMENSIONS IN MILLIMETERS) 5 TO 500 MICROLITER SIZESPERPENDICULAR TO LONG AXIS OF PIPET
TIP MUST BE GROUND FLAT NORMAL TO AXIS AND SLIGHTLY BEVELED GLAZING OPTIONAL AFTER GRINDING -NO CONSTRICTION OF BORE FIG. 77. Microliter pipette for 5- to 500-μ1. sizes—details of construction. For dimensions see next page.
FIG. 77. (cont.) Microliter pipette for 5- to 500-μ1. sizes—dimensions. Delivery Stem Size O.D. I.D. Approx. Max. Min. Wall I.D. (Calibd. Over-all tubing tubing length O.D. length at end at end Min. cap. to contain) length (mm.) (mm.) (mm.) (mm.) (mm.) (mm.) (mm.) safety bulb Vol. toi (μΐ.) (mm.) A Β D Ε F G C (μΐ.) (%) 5 140 ± 5 5-6 0.18-0.25 65 4 55 0.5-0.7 0.15-0.25 50 ±0.5 6 140 ± 5 5-6 0.18-0.25 65 4 55 0.5-0.7 0.15-0.25 50 ±0.5 7 140 ± 5 5-6 0.18-0.25 65 4 55 0.5-0.7 0.15-0.25 50 ±0.5 8 140 ± 5 5-6 0.18-0.25 65 4 55 0.5-0.7 0.15-0.25 50 ±0.5 9 140 ± 5 5-6 0.18-0.25 65 4 55 0.5-0.7 0.15-0.25 50 ±0.5 10 140 ± 5 5-6 0.20-0.35 65 4 55 0.5-0.7 0.15-0.25 50 ±0.5 15 140 ± 5 5-6 0.25-0.40 65 4 55 0.5-0.7 0.15-0.25 50 ±0.5 20 140 ± 5 5-6 0.35-0.50 65 4 55 0.5-0.7 0.25-0.50 50 ±0.5 25 140 ± 5 5-6 0.35-0.50 65 4 55 0.5-0.7 0.25-0.50 50 ±0.5 35 140 ± 5 5-6 0.35-0.50 65 4 55 0.5-0.7 0.25-0.50 50 ±0.3 50 140 ± 5 5-6 0.35-0.50 65 4 55 0.5-0.7 0.25-0.50 50 ±0.3 60 140 ± 5 5-6 0.40-0.55 65 4 55 0.5-0.7 0.30-0.50 50 ±0.3 75 140 ± 5 5-6 0.40-0.60 65 4 55 0.5-0.7 0.30-0.50 75 ±0.3 100 140 ± 5 5-6 0.50-0.75 65 4 55 0.5-0.7 0.30-0.50 75 ±0.3 150 140 ± 5 5-6 0.75-1.00 65 4 55 0.5-0.7 0.40-0.60 100 ±0.3 200 145 ± 10 5-6 0.75-1.00 65 4 55 0.6-0.8 0.40-0.60 100 ±0.2 250 145 ± 10 5-6 0.75-1.00 65 4 55 0.6-0.8 0.40-0.60 100 ±0.2 300 145 ± 10 5-6 0.75-1.00 65 4 55 0.6-0.8 0.40-0.70 200 ±0.2 400 150 ± 10 6-7 1.00-1.25 70 6 60 0.6-0.8 0.40-0.70 200 ±0.2 500 160 ± 10 6-7 1.25-1.50 70 6 60 0.6-0.8 0.40-0.70 200 ±0.2 * Closer volumetric standardization must be carried out by user with substances under actual conditions of use.
119 Additional Information for Chapter 5
SIZE ML.
A
O.D. MM. VOLUMETRIC TOLERANCE Ul 01 6 ±0.5 ± 1 0.2 6Î0.5 + 1 0.5 8+0.5 + 1 1 10+05 + 1 2001 10 MM. I0-20MM.i
L_ 5-6 MM. O.D. ^3.45-0.60MM. ID. " GRADUATION RING AT LEAST 5 MM. FROM EITHER END OF CAPILLARYHi i - A
WALL I MM. APPROX. ,'!(-3±0.5MM.0.D. SLIGHTLY BEVELED—4 —
0.55- 0.70 MM I.D. FIG. 78. Micro washout pipettes—details of construction.20+ 2 MM >5.75 MM. MAX. APPROX. RANGE 90-125 m
APPROX. RANGE 90-125 Ml 155 - Ι.βΟ MM. 1.0. SIZE ML.
VOLUMETRIC TOLERANCE μ* 0.1 i ι 0.2
t
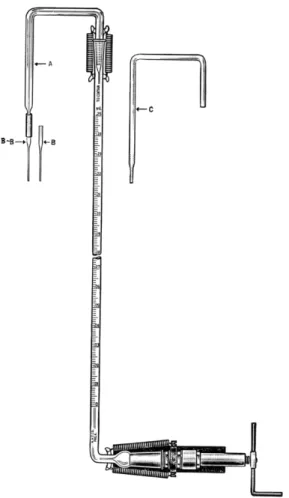
2 TIPS GROUND AND BEVELED "\J, FIG. 79. Micropipettes, Folin-type—details of construction.FIG. 8 0 . Aminco automatic power-driven burette.
121 Additional Information for Chapter 5
Vycor* plunger. A revolution counter is so calibrated that it registers to the nearest 0 . 0 0 1 ml. and a graduated drum mounted on the input shaft to the counter registers 0 . 0 0 0 1 ml. per division. Meniscus reading has been eliminated.
The delivery type is immersed in the solution to be titrated.
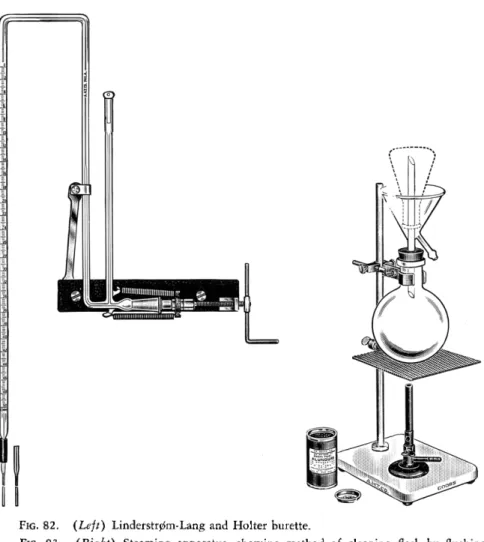
FIG. 8 1 . Rehberg burette. ( A ) Separate delivery tube with ground joint to facilitate cleaning. ( B ) Removable glass tip. ( C ) Gas bubbling tube for insertion in solution being titrated to provide stirring action.
Besides the types of burettes described and shown in the preceding pages, many others have been described, particularly for measuring smaller quantities of solutions. Figures 8 1 and 8 2 show two of these capillary b u r e t t e s
1 2 0'
1 6 3* Silica glass ( 9 6 % ) No. 7 9 0 .4 6
123 Additional Information for Chapter 5
which require micrometer screw type of manipulation. For descriptions of these, the reader is referred to the book by K i r k .
9 8A steaming apparatus of the type shown in Fig. 83 is useful for cleaning flasks in connection with volumetric p r o c e d u r e s .
1 4 5 - 1 4 7For complete information regarding titration curves, equilibrium for acid and for base dissociation, pH, buffer action, the theory and choice of in
dicators, electrometric titrations, etc., the reader is referred to the treatise by Clark.
4 4FIG. 82. (Left) Linderstr0m-Lang and Holter burette.
FIG. 83. (Right) Steaming apparatus, showing method of cleaning flask by flushing with steam.
T A B L E 15
ADDITIONAL INFORMATION ON R E F E R E N C E S * RELATED TO C H A P T E R 5
For other information the author wishes to call to the attention of the reader, a number of references are given in this table. (See author's statement regarding such information at top of Table 4 of Chapter 1.)
Books Automatic titration
Belcher and Godbert, 22, 23 Cotlove, Trantham, and Bowman, 47 Clark, E. P., 42 Simon, 185
Clark, S. J . , 43 Simon, Kovâks, Chopard-dit-Jean, Clark, W . M., 44 and Heilbronner, 186
Grant, 83, 84
K irk 9 8 Conductometric titration
Knights, McDonald, and Ploompuu, 105 S t o c k> 2° 3 Malissa and Benedetti-Pichler, 130
Milton and Waters, 140, 141 Natelson, 142
Niederl and Niederl, 144, 145 Niederl and Sozzi, 147 Pregl, 161
Roth, 168-171 Schneider, 174 Steyermark, 194
EDTA titration
Baker, 13Belcher, Close, and West, 20, 21 Flaschka, 5 6 - 5 9
Flaschka and Abdine, 60 Flaschka and Amin, 61
Flaschka, Barnard, and Broad, 62, 63 Flaschka, ter Haar, and Bazen, 64 Kinnunen and Wennerstrand, 95 Lott and Cheng, 124
Sporek, 192
Potentiometric titrations
Alimarin and Petrikova, 5 Cunningham, Kirk, and Brooks, 48 Frediani and Warren, 67 Furman, 7 0 - 7 2 Goddu and Hume, 77 Ingold, 91
Kirk, 98
Kirk and Bentley, 99
Kirsten, Berggren, and Nilsson, 102 Levy, 119
Lindner and Kirk, 122 Lykken and Rolf son, 126 Schwarz, 180
Simon, 184 Stock, 200-202 Tompkins and Kirk, 214 Wade, 221
Cerimetry
Amperometric titrations
Bradbury and Hambly, 35 Mader and Frediani, 129 Nikelly and Cooke, 148 Parks, 151High-frequency titration
Bien, 26Takahashi, Kimoto, and Sakurai, 211 Reilley and McCurdy, 164
Blaedel and Malmstadt, 28 Hara and West, 88 Lane, 113 Stock, 204
Nonaqueous titrations Heterometric titration
Bobtelsky and Welwart, 30-33
Ballard, Bamford, and Weymouth, 14 Belcher, Berger, and West, 18, 19
* The numbers which appear after each entry in this table refer to the literature citations in the reference list at the end of the chapter.
125 Table of References
T A B L E 15 Nonaqueous titrations (Conf.)
Fritz, 68
Maurmeyer, Margosis, and Ma, 136 Pifer and Wollish, 156, 157
Pifer, Wollish, and Schmall, 158-160 Riddick, 166
Wollish, Colarusso, Pifer, and Schmall, 230
Wollish, Pifer, and Schmall, 231 Spectrometry, colorimetric, etc.,
equipment Ellis and Brandt, 54 Kinsey, 96 Mason, 134 Microtitrators
Allen, 7
Burettes, ultramicrotitrations, etc.
Belcher, Berger, and West, 18, 19 Blake, 29
Daimler, 49 Gilmont, 75, 76 Wingo and Johnson, 227 Wise, 229
Burettes, pipettes, general Alber, 2
Allan, 6 Anderson, 10, 11 Barth and Sze, 15
Beckman Instruments, Inc., 16, 17 Benedetti-Pichler, 24
Birket-Smith, 27 Boguth, 34
British Standards Institution, 37 Buck, Keister, and Zelle, 38 Chinoy, 39
ClafT, 40, 41
Dern, Pullman, and Williams, 50 Dusing, 52
Feuer, 55 Flaschka, 56 Folin, 65
Foster and Broeker, 66 Gilmont, 75
Gorbach, 7 9 - 8 1 Gorbach and Haack, 82
(Continued)
Burettes, pipettes, general (Conf.) Grunbaum and Kirk, 85
Hallett, 86
Harman and Webster, 89 Kirk, 97, 98
Kirk and Bentley, 99 Kirsten, 100, 101 Knights, McDonald, and
Ploompuu, 104, 105 Korenman and Rostokin, 107 Koros and Remport-Horvath, 108 Lacourt, 110
Lacourt, Stoffyn, and Timmermans, 111 Lacourt and Timmermans, 112 Langer and Pantages, 114
Lascalzo and Benedetti-Pichler, 115 Lazarow, 116, 117
Levvy, 118
Linderstro'm-Lang and Holter, 120 Llacer and Sozzi, 123
Lundbak, 125 Marsh, 133
Mattenheimer and Borner, 135 Natelson and Zuckerman, 143 Rehberg, 163
Rieman, 167 Saunders, 173 Scholander, 175
Scholander, Edwards, and Irving, 176 Schôniger, 177
Schreiner, 178
Shaffer, Farrington, and Niemann, 182 Sômiya and Kamada, 191
Stock and Fill, 2 0 5 - 2 0 9 Thompson, 213 Upson, 217 Willits, 224 Winteringham, 228 Wise, 229
Wyatt, 232 Stirring devices
Claff, 40, 41 Stock and Fill, 205 Calibration
Alber, 2
Buck, Keister, and Zelle, 38
T A B L E 15 (Continued) Calibration (Conf.)
Pecar, 154 Thompson, 213 Upson, 218 Flasks
Thompson, 213 Indicators
Bradbury and Hambly, 35 Cooper, 45
Dorf, 51 Jerie, 92
Kinnunen and Wennerstrand, 95 Manohin, Kakabadse, and Crowder, 131 Preisler and Berger, 162
Schulze, 179
Sendroy and Hastings, 181 Sher, 183
Steinitz, 193 Standard solutions
Underwood, 216 Primary standards
Williams, 223
General, applications, etc.
Albrink, 3 Garschagen, 73
General, applications, etc. (Conf.) Knights, 103
Lazarow, 116, 117 Pecar, 153, 154 Richter, 165
Simon, Morikofer, and Heilbronner, 187 Titration of sulfate
Fritz and Yamamura, 69 Milner, 139
Smith-New York, 189
Standardization of sodium thiosulfate Kassner and Kassner, 93
Niederl and Niederl, 144, 145 Peacocke, 152
Pharmacopeia, 155 Walker and Allen, 222
Standardization of hydrochloric acid Lindner, 121
Niederl and Niederl, 144, 145 Fluoride titration
Ma and Gwirtsman, 127
Mavrodineanu and Gwirtsman, 137 Megregian, 138
Rowley, 172
Venkateswarlu, Ramanthan, and Narayana Rao, 220
127
ReferencesREFERENCES
1. Acculate, Anachemia Chemicals, Ltd., Montreal, Quebec and Champlain, New York.
2. Alber, H. K., Ind. Eng. Chem., Anal. Ed., 12, 764 ( 1 9 4 0 ) . 3. Albrink, M. J . , / . Lipid Research, 1, 53 ( 1 9 5 9 ) .
4. Alicino, J . F., Anal. Chem., 20, 85 ( 1 9 4 8 ) .
5. Alimarin, I. P., and Petrikova, M. N., / . Anal. Chem., U.S.S.R., {English Trans
lation), 9, 137 ( 1 9 5 4 ) .
6. Allan, J . C , S. African J . Med. Sci., 11, 157 ( 1 9 4 6 ) . 7. Allen, Κ. Α., Anal. Chem., 28, 277 ( 1 9 5 6 ) .
8. Aluise, V . Α., Hall, R. T., Staats, F. C , and Becker, W . W., Anal. Chem., 19, 347 ( 1 9 4 7 ) .
9. American Instrument Co., Silver Springs, Maryland.
10. Anderson, H. H., Anal. Chem., 20, 1241 ( 1 9 4 8 ) . 11. Anderson, H. H , Anal. Chem., 24, 579 ( 1 9 5 2 ) .
12. Association of Officiai Agricultural Chemists, "Official Methods of Analysis,"
8th ed., Association of Official Agricultural Chemists, Washington, D . C , 1955.
13. Baker, J . T., Chemical Co., "The E D T A Titration. Nature and Methods of End Point Detection."
14. Ballard, D.G.H., Bamford, C. H., and Weymouth, F. J . , Analyst, 81, 305 ( 1 9 5 6 ) . 15. Barth, L. G., and Sze, L. C , Rev. Sci. Instr., 22, 978 ( 1 9 5 1 ) .
16. Beckman Instruments, Inc., Fullerton, California.
17. Beckman Instruments, Inc., Spinco Division, Palo Alto, California.
18. Belcher, R., Berger, J . , and West, T . S., / . Chem. Soc. (London), p. 2877 ( 1 9 5 9 ) . 19. Belcher, R., Berger, J . , and West, T. S., / . Chem. Soc. (London), p. 2882 ( 1 9 5 9 ) . 20. Belcher, R., Close, R. Α., and West, T. S., Chemist Analyst, 46, 86 ( 1 9 5 7 ) . 21. Belcher, R., Close, R. Α., and West, T . S., Chemist Analyst, 47, 2 ( 1 9 5 8 ) .
22. Belcher, R., and Godbert, A. L., "Semi-Micro Quantitative Organic Analysis," Long
mans, Green, London, and New York, 1945.
23. Belcher, R., and Godbert, A. L., "Semi-micro Quantitative Organic Analysis," 2nd ed., Longmans, Green, London, 1954.
24. Benedetti-Pichler, Α. Α., "Introduction to the Microtechnique of Inorganic Analy
sis," p. 234, Wiley, New York, 1942.
25. Betz, W . H., & L. D., Philadelphia, Pennsylvania.
26. Bien, G. S., Anal. Chem., 26, 909 ( 1 9 5 4 ) .
27. Birket-Smith, E., Scand. J . Clin. & Lab. Invest., 3, 234 ( 1 9 5 1 ) . 28. Blaedel, W . J . , and Malmstadt, H. V., Anal. Chem., 22, 734 ( 1 9 5 0 ) . 29. Blake, G. G., Metallurgia, 41, 413, 415 ( 1 9 5 0 ) .
30. Bobtelsky, M., and Welwart, Y . , Anal. Chim. Acta, 9, 281 ( 1 9 5 3 ) . 31. Bobtelsky, M., and Welwart, Y . , Anal. Chim. Acta, 9, 374 ( 1 9 5 3 ) . 32. Bobtelsky, M., and Welwart, Y . , Anal. Chim. Acta, 10, 151 ( 1 9 5 4 ) . 33. Bobtelsky, M., and Welwart, Y . , Anal. Chim. Acta, 10, 464 ( 1 9 5 4 ) . 34. Boguth, W . , Z. physiol. Chem. Hoppe-Seyler's, 285, 93 ( 1 9 5 0 ) .
35. Bradbury, J . H., and Hambly, A. N., Australian J . Sci. Research, 5A, 541 ( 1 9 5 2 ) . 36. Brecher, C , Wien. klin. Wochschr., 49, 1228 ( 1 9 3 6 ) .
37. British Standards Institution, Brit. Standards 1428, Pt. D l ( 1 9 5 2 ) , Pt. D2 ( 1 9 5 0 ) , Pt. D 4 ( 1 9 5 4 ) , Pt. D 5 , and Pt. D 6 ( 1 9 5 5 ) .
38. Buck, J . B . , Keister, M. L., and Zelle, M. R., Anal. Chim. Acta, 4, 130 ( 1 9 5 0 ) . 39. Chinoy, J . J . , Current Sci. (India), 14, 102 ( 1 9 4 5 ) .
40. Clafï, C. L., Science, 105, 103 ( 1 9 4 7 ) .
4 1 . Claff, C. L., Science, 108, 67 ( 1 9 4 8 ) .
42. Clark, E. P., "Semimicro Quantitative Organic Analysis," Academic Press, New York, 1943.
43. Clark, S. J . , "Quantitative Methods of Organic Microanalysis," Butterworths, Lon
don, 1956.
44. Clark, W . M., "The Determination of Hydrogen Ions," 2nd ed., Williams & Wil- kins, Baltimore, Maryland, 1927.
45. Cooper, S. S., Ind. Eng. Chem., Anal. Ed., 13, 466 ( 1 9 4 1 ) . 46. Corning Glass Works, Corning, New York.
47. Cotlove, E , Trantham, H. V., and Bowman, R. L., / . Lab. Clin. Med., 51, 461 ( 1 9 5 8 ) .
48. Cunningham, B . , Kirk, P. L., and Brooks, S. C , / . Biol. Chem., 139, 11 ( 1 9 4 l ) . 49. Daimler, Β . H., Chem. Ingr. Tech., 22, 104 ( 1 9 5 0 ) .
50. Dern, R. J . , Pullman, T. N., and Williams, H. R., / . Lab. Clin. Med., 36, 494 ( 1 9 5 0 ) .
51. Dorf, H., Anal. Chem., 25, 1000 ( 1 9 5 3 ) . 52. Dusing, W., Chem. Fabrik, p. 313 ( 1 9 3 4 ) .
53. Elek, Α., and Harte, R. Α., Ind. Eng. Chem., Anal. Ed., 8, 267 ( 1 9 3 6 ) . 54. Ellis, G. H., and Brandt, C. S., Anal. Chem., 21, 1546 ( 1 9 4 9 ) . 55. Feuer, L, Nucleonics, 13, 68 ( 1 9 5 5 ) .
56. Flaschka, H., Mikrochemie ver. Mikrochim. Acta, 36/37, 269 ( 1 9 5 1 ) . 57. Flaschka, H., Mikrochemie ver. Mikrochim. Acta, 40, 21 ( 1 9 5 3 ) . 58. Flaschka, H., Mikrochemie ver. Mikrochim. Acta, 40, 42 ( 1 9 5 3 ) . 59. Flaschka, H., Mikrochim. Acta, p. 55 ( 1 9 5 5 ) .
60. Flaschka, H., and Abdine, H., Mikrochim. Acta, p. 3 7 ( 1 9 5 5 ) .
61. Flaschka, H., and Amin, A. M., Mikrochim. Acta, p. 410 ( 1 9 5 3 ) ; Chem. Abstr.
48, 1198 ( 1 9 5 4 ) .
62. Flaschka, H., Barnard, A. J . , and Broad, W . C , Chemist Analyst, 46, 112 ( 1 9 5 7 ) . 63. Flaschka, H., Barnard, A. J , and Broad, W . C , Chemist Analyst, 47, 22 ( 1 9 5 8 ) . 64. Flaschka, H., Haar, K. ter, and Bazen, J . , Mikrochim. Acta, p. 345 ( 1 9 5 3 ) ; Chem.
Abstr., 48, 1198 ( 1 9 5 4 ) .
65. Folin, O., / . Biol. Chem., 77, 421 ( 1 9 2 8 ) .
66. Foster, R. H. K., and Broeker, A. G., / . Lab. Clin. Med., 32, 918 ( 1 9 4 7 ) . 67. Frediani, Η. Α., and Warren, W . B . , Ind. Eng. Chem., Anal. Ed., 13, 646 ( 1 9 4 1 ) . 68. Fritz, J . S., "Acid-Base Titrations in Nonaqueous Solvents," G. F. Smith Chemical
Co., Columbus, Ohio, 1952.
69. Fritz, J . S., and Yamamura, S. S., Anal. Chem., 27, 1461 ( 1 9 5 5 ) . 70. Furman, Ν . H., Anal. Chem., 26, 84 ( 1 9 5 4 ) .
71. Furman, Ν . H., Ind. Eng. Chem., Anal. Ed., 14, 367 ( 1 9 4 2 ) .
72. Furman, Ν . H., ed., "Scott's Standard Methods of Chemical Analysis," 5th ed., Vol. II, Van Nostrand, New York, 1939.
73. Garschagen, H., Z. anal. Chem., 169, 49 ( 1 9 5 9 ) .
74. Geilmann, W., and Hôltje, R., Z. anorg. Chem., 152, 69 ( 1 9 2 6 ) . 75. Gilmont, R., Anal. Chem., 20, 1109 ( 1 9 4 8 ) .
76. Gilmont, R., Anal. Chem., 25, 1135 ( 1 9 5 3 ) .
77. Goddu, R. F., and Hume, D . N., Anal. Chem., 26, 1740 ( 1 9 5 4 ) .
78. Godfrey, P. R., and Shrewsbury, C. L., / . Assoc. Offic. Agr. Chemists, 28, 336 ( 1 9 4 5 ) .
79. Gorbach, G., Chem. Fabrik. 14, 390 ( 1 9 4 1 ) .
129
References80. Gorbach, G., Mikrochemie ver. Mikrochim. Acta, 31, 109 ( 1 9 4 4 ) . 81. Gorbach, G., Mikrochemie ver. Mikrochim. Acta, 34, 183 ( 1 9 4 9 ) . 82. Gorbach, G., and Haack, Α., Mikrochim. Acta, p. 1751 ( 1 9 5 6 ) .
83. Grant, J . , "Quantitative Organic Microanalysis, Based on the Methods of Fritz Pregl," 4th ed., Blakiston, Philadelphia, Pennsylvania, 1946.
84. Grant, J . , "Quantitative Organic Microanalysis," 5th ed., Blakiston, Philadelphia, Pennsylvania, 1951.
85. Grunbaum, B . W., and Kirk, P. L., Anal. Chem., 27, 333 ( 1 9 5 5 ) . 86. Hallett, L. T., Ind. Eng. Chem., Anal. Ed., 14, 956 ( 1 9 4 2 ) .
87. Hallett, L. T., and Kuipers, J . W., Ind. Eng. Chem., Anal. Ed., 12, 360 ( 1 9 4 0 ) . 88. Hara, R., and West, P. W., Anal. Chim. Acta, 15, 193 ( 1 9 5 6 ) .
89. Harman, J . W., and Webster, J . H., Am. J . Clin. Pathol., 18, 750 ( 1 9 4 8 ) .
90. Hodgman, C. D., and Lange, Ν. Α., "Handbook of Chemistry and Physics," 9th ed., p. 395, Chemical Rubber, Cleveland, Ohio, 1922.
91. Ingold, W., Helv. Chim. Acta, 29, 1929 ( 1 9 4 6 ) . 92. Jerie, H., Mikrochim. Acta, 40, 189 ( 1 9 5 3 ) .
93. Kassner, J . L., and Kassner, Ε. E., Ind. Eng. Chem., Anal. Ed., 12, 655 ( 1 9 4 0 ) . 94. Kaye, I. Α., and Weiner, N., Ind. Eng. Chem., Anal. Ed., 17, 397 ( 1 9 4 5 ) . 95. Kinnunen, J . , and Wennerstrand, B . , Chemist Analyst, 46, 92 ( 1 9 5 7 ) . 96. Kinsey, V . E., Anal. Chem., 22, 362 ( 1 9 5 0 ) .
97. Kirk, P. L., Mikrochemie, 14, 1 ( 1 9 3 3 ) .
98. Kirk, P. L., "Quantitative Ultramicroanalysis," Wiley, New York, and Chapman
& Hall, London, 1950.
99. Kirk, P. L., and Bentley, G. T., Mikrochemie, 21, 250 ( 1 9 3 7 ) . 100. Kirsten, W . J , Anal. Chem., 25, 1137 ( 1 9 5 3 ) .
101. Kirsten, W . J . , Anal. Chem., 29, 460 ( 1 9 5 7 ) .
102. Kirsten, W . J . , Berggren, Α., and Nilsson, K., Anal. Chem., 30, 237 ( 1 9 5 8 ) . 103. Knights, Ε. M., Jr., / . Am. Med. Assoc., 166, 1175 ( 1 9 5 8 ) .
104. Knights, Ε. M., Jr., McDonald, R. P., and Ploompuu, J . , Am. J . Clin. Pathol, 30, 91 ( 1 9 5 8 ) .
105. Knights, Ε. M., Jr., McDonald, R. P., and Ploompuu, J . , "Ultramicro Methods for Clinical Laboratories," Grune & Stratton, New York, and London, 1957.
106. Koch, F. C , / . Lab. Clin. Med., 11, 774 ( 1 9 2 6 ) ; 14, 747 ( 1 9 2 9 ) . 107. Korenman, I. M., and Rostokin, A. P., Zavodskaya Lab., 14, 1391 ( 1 9 4 8 ) . 108. Koros, E., and Remport-Horvath, 2 . , Chemist Analyst, 46, 91 ( 1 9 5 7 ) .
109. Kuck, J . Α., Kingsley, Α., Kinsey, D., Sheehan, F., and Swigert, G. F., Anal.
Chem., 22, 604 ( 1 9 5 0 ) .
110. Lacourt, Α., Metallurgia, 38, 355 ( 1 9 4 8 ) .
111. Lacourt, Α., Stofïyn, P., and Timmermans, A. M., Mikrochemie ver. Mikrochim.
Acta, 33, 217 ( 1 9 4 8 ) .
112. Lacourt, Α., and Timmermans, A. M., Bull, classe sci. Acad. roy. Belg., 32, 52 ( 1 9 4 6 ) .
113. Lane, E. S., Analyst, 82, 406 ( 1 9 5 7 ) .
114. Langer, S. H., and Pantages, P., Anal. Chem., 30, 1889 ( 1 9 5 8 ) .
115. Lascalzo, A. G., and Benedetti-Pichler, Α. Α., Ind. Eng. Chem., Anal. Ed., 17, 187 ( 1 9 4 5 ) .
116. Lazarow, Α., / . Lab. Clin. Med., 32, 213 ( 1 9 4 7 ) . 117. Lazarow, Α., / . Lab. Clin. Med., 35, 810 ( 1 9 5 0 ) . 118. Levvy, G. Α., Chem. & Ind. (London), p. 4 ( 1 9 4 5 ) .
119. Levy, R., Bull. soc. chim. France, p. 497 ( 1 9 5 6 ) .
120. Linderstr0m-Lang, K., and Holter, H., Compt. rend. trav. lab. Carlsberg, 19, 1 ( 1 9 3 3 ) .
121. Lindner, J . , Z. anal. Chem., 91, 105 ( 1 9 3 3 ) ; Mikrochemie, Molisch Festschrift, p. 301 ( 1 9 3 6 ) .
122. Lindner, R., and Kirk, P. L., Mikrochemie, 23, 269 ( 1 9 3 8 ) .
123. Llacer, A. J . , and Sozzi, J . Α., Anales farm, y bioquim. (Buenos Aires), 16, 82 ( 1 9 4 5 ) .
124. Lott, P. F., and Cheng, K. L., Chemist Analyst, 47, 8 ( 1 9 5 8 ) . 125. Lundbak, Α., Kern. Maanedsblad, 24, 138 ( 1 9 4 3 ) .
126. Lykken, L., and Rolfson, R. B . , Ind. Eng. Chem., Anal. Ed., 13, 653 ( 1 9 4 1 ) . 127. Ma, T . S., and Gwirtsman, J . , Anal. Chem., 29, 140 ( 1 9 5 7 ) .
128. Ma, T. S , and Zuazaga, G., Ind. Eng. Chem., Anal. Ed., 14, 280 ( 1 9 4 2 ) .
129. Mader, W . J . , and Frediani, Η. Α., / . Am. Pharm. Assoc. Sci. Ed., 40, 24 ( 1 9 5 1 ) . 130. Malissa, H., and Benedetti-Pichler, Α. Α., "Anorganische qualitative Mikroanalyse,"
Springer, Wien, 1958.
131. Manohin, B . , Kakabadse, G. J , and Crowder, M. M., Analyst, 81, 730 ( 1 9 5 6 ) . 132. Marley Products Co., New Hyde Park, New York.
133. Marsh, Ο., A.M.A. Arch. Ind. Health, 12, 688 ( 1 9 5 5 ) . 134. Mason, A. C , Analyst, 76, 172 ( 1 9 5 1 ) .
135. Mattenheimer, H., and Borner, Κ., Mikrochim. Acta, p. 916 ( 1 9 5 9 ) .
136. Maurmeyer, R. K., Margosis, M., and Ma, T . S., Mikrochim. Acta, p. 177 ( 1 9 5 9 ) . 137. Mavrodineanu, R., and Gwirtsman, J . , Contribs. Boyce Thompson Inst., 18, 181
( 1 9 5 5 ) .
138. Megregian, S., Anal. Chem., 26, 1161 ( 1 9 5 4 ) . 139. Milner, Ο. I., Anal. Chem., 24, 1247 ( 1 9 5 2 ) .
140. Milton, R. F., and Waters, W . Α., "Methods of Quantitative Microanalysis,"
Longmans, Green, New York, and Arnold, London, 1949.
141. Milton, R. F., and Waters, W . Α., "Methods of Quantitative Microanalysis," 2nd ed., Arnold, London, 1955.
142. Natelson, S., "Microtechniques of Clinical Chemistry for the Routine Laboratory,"
Thomas, Springfield, Illinois, 1957.
143. Natelson, S., and Zuckerman, J . L., / . Biol. Chem., 170, 305 ( 1 9 4 7 ) .
144. Niederl, J . B . , and Niederl, V., "Micromethods of Quantitative Organic Elemen
tary Analysis," Wiley, New York, 1938.
145. Niederl, J . B . , and Niederl, V., "Micromethods of Quantitative Organic Analysis,"
2nd ed, Wiley, New York, 1942.
146. Niederl, J . B , Niederl, V , and Eitingon, M , Mikrochemie ver. Mikrochim. Acta, 25, 143 ( 1 9 3 8 ) .
147. Niederl, J . B . , and Sozzi, J . A , "Microanalisis Elemental Organico," Calle Arcos, Buenos Aires, 1958.
148. Nikelly, J . B , and Cooke, W . D , Anal. Chem., 28, 243 ( 1 9 5 6 ) .
149. Ogg, C. L , Brand, R. W , and Willits, C. O., / . Assoc. Offic. Agr. Chemists, 31, 663 ( 1 9 4 8 ) .
150. Ogg, C. L , Willits, C. O , and Cooper, F. J , Anal. Chem., 20, 83 ( 1 9 4 8 ) . 151. Parks, T . D , Anal. Chim. Acta, 6, 553 ( 1 9 5 2 ) .
152. Peacocke, T . A. H., Chem. & Ind. (London), p. 1245 ( 1 9 5 2 ) . 153. Pecar, M , Microchem. ] . , 3, 557 ( 1 9 5 9 ) .
154. Pecar, M., Microchem. J . , 4, 73 ( I 9 6 0 ) .