ContentslistsavailableatScienceDirect
Colloids and Surfaces B: Biointerfaces
jou rn a l h om ep ag e :w w w . e l s e v i e r . c o m / l o c a t e / c o l s u r f b
Aggregation of PEGylated liposomes driven by hydrophobic forces
Tamás Bozó
a,∗,1, Tamás Mészáros
b,c,1, Judith Mihály
d, Attila Bóta
d, Miklós S.Z. Kellermayer
a,e, János Szebeni
b,c, Benedek Kálmán
baDepartmentofBiophysicsandRadiationBiology,SemmelweisUniversity,1094Budapest,T ˝uzoltóStr.37-47,Hungary
bNanomedicineResearchandEducationCenter,SemmelweisUniversity,1089Budapest,NagyváradSquare4,Hungary
cSeroScienceLtd.,1089Budapest,NagyváradSquare4,Hungary
dBiologicalNanochemistryResearchGroup,InstituteofMaterialsandEnvironmentalChemistry,ResearchCentreforNaturalSciences,HungarianAcademy ofSciences,1117Budapest,Magyartudósokkörútja2,Hungary
eMTA-SEMolecularBiophysicsResearchGroup,SemmelweisUniversity,1094Budapest,T ˝uzoltóStr.37-47,Hungary
a r t i c l e i n f o
Articlehistory:
Received4November2015 Receivedinrevisedform23June2016 Accepted27June2016
Availableonline28June2016
DedicatedtothememoryofBerci(Benedek Kálmán),agreatcolleagueandfriend.
Keywords:
Liposome PEG Aggregation Fusion
Hydrophobiceffect Ammoniumsulfate Kosmotropicsalt
a b s t r a c t
Polyethyleneglycol(PEG)iswidelyusedtostericallystabilizeliposomesandimprovethepharmacoki- neticprofileofdrugs,peptidesandnanoparticles.Herewereportthatammoniumsulfate(AS)canevoke theaggregationofPEGylatedvesiclesinaconcentration-dependentmanner.Liposomeswith5mol%
PEGwerecolloidally stableatASconcentrationsupto0.7mM,abovewhichtheyprecipitatedand formedmicron-sizeaggregateswithirregularshape.Whileaggregationwasreversibleupto0.9Mof AS,above1Mfusionoccurred,whichirreversiblydistortedthesizedistribution.Zetapotentialoflipo- somesmarkedlyincreasedfrom−71±2.5mVto2±0.5mVuponraisingtheASconcentrationfrom0 to0.1M,butnoconsiderableincreasewasseenduringfurtherASaddition,showingthattheaggrega- tionisindependentofsurfacecharge.TherewasnoaggregationintheabsenceofthePEGchains,and increasingPEGmolar%shiftedtheaggregationthresholdtolowerASconcentrations.Changesinthe FTIRspectralfeaturesofPEGylatedvesiclessuggestthatASdehydratesPEGchains.Otherkosmotropic saltsalsoledtoaggregation,whilechaotropicsaltsdidnot,whichindicatesageneralkosmotropicphe- nomenon.Thedrivingforcebehindaggregationislikelytobethehydrophobiceffectduetosaltingout thepolymersimilarlytowhathappensduringproteinpurificationorHydrophobicInteractionChro- matography.Sinceliposomeaggregationandfusionmayresultindifficultiesduringformulationand adversereactionuponapplication,thephenomenadetailedinthispapermayhavebothtechnological andtherapeuticalconsequences.
©2016ElsevierB.V.Allrightsreserved.
1. Introduction
Polyethyleneglycol(PEG),alinearpolymerof[–CH2–CH2–O]
units,iscommonlyusedindrugdeliverytomodifypharmacoki- neticproperties ofactiveagents. AttachingPEGchainstosmall molecules, peptides, proteins, oligonucleotides or nanoparticles may effectively reduce their enzymatic degradation and pro- longtheircirculationtimeinblood[1].Animportantexampleis liposomePEGylation,whichprovides“stealth”characteristicsto liposomeshelpingthemtoevadephagocytosisbymacrophages,
∗Correspondingauthor.
E-mailaddresses:bozo.tamas@med.semmelweis-univ.hu(T.Bozó), tmeszaros@seroscience.com(T.Mészáros),mihaly.judith@ttk.mta.hu(J.Mihály), bota.attila@ttk.mta.hu(A.Bóta),miklos.kellermayer@med.semmelweis-univ.hu (M.S.Z.Kellermayer),jszebeni2@gmail.com(J.Szebeni).
1 Theseauthorscontributedequallytothiswork.
which extends their lifetime in the body and results in dose- independentpharmacokinetics(exceptforverylowdoses)[2,3].
Liposometherapymadeitpossibletoincreasethebioavailability ofdrugsthatarepoorlyabsorbed(likeamphotericin-B),toreduce sideeffectsofhighlycytotoxicanti-canceragents(suchasdoxoru- bicin)andtoopennewroutestogeneratesite-selectiveeffect(e.g.:
photodynamictherapyinmaculardegeneration)[4].However,new benefitsmaybeaccompaniedbynewrisks:e.g.,itturnedoutthat liposomes can inducecomplement activation related pseudoal- lergy(CARPA),anewtypeofdrug-inducedacuteimmunetoxicity [5].Itissuspectedthatoneofitscausescouldbethepresenceof liposomalaggregatesintheformulatedproduct[6].
Here we reportthat ammoniumsulfate (AS) andother kos- motropicagentsmayelicittheaggregationandeventhefusionof PEGylatedliposomes.Theaggregatesareformedbyhydrophobic interactionsduetothesolvophobiceffectofincreasingsaltconcen- tration[7].Thisphenomenonissimilartothesalting-outmethod http://dx.doi.org/10.1016/j.colsurfb.2016.06.056
0927-7765/©2016ElsevierB.V.Allrightsreserved.
Table1
Liposomecompositions.
PEGmolar% molarratio(Cholesterol:mPEG:HSPC)
0 38.4:0:61.6
2 38.7:2:59.3
5 38.4:5:56.6
10 38.4:10:51.6
regularlyusedinproteinfractionationand purification[8,9].An understandingofthemechanismofaggregateformationcouldpro- videinvaluableinformationforsuccessfuldrugformulationswhere liposomeaggregationcouldbeeitherpreventedorcontrolled.
2. Materialsandmethods 2.1. Materials
Cholesterol, mono PEGylated 1, 2-distearoyl-glycero-3- phophoethanolamine (mPEG-2000-DSPE), and hydrogenated soybeanphosphatidylcholine(HSPC)wereobtainedfromLipoid GmbH(Ludwigshafen,Germany).Ethanol,isopropanol,histidine, sucrose, ammonium sulfate (AS), sodium sulfate, magnesium sulfate,sodiumcitrate, magnesiumchloride,guanidine chloride (GdmCl) were purchased from Sigma Aldrich Kft. (Budapest, Hungary).Salsolinfusion(TEVAHungaryZrt.,Debrecen,Hungary) wasobtainedfromtheUniversityPharmacy,andpurified water wasproducedbyaMilli-QIntegral3WaterProductionUnit(Merck Millipore,Billerica,MA,USA).
2.2. Liposomepreparation
Liposomesuspensionwithalipidandbuffercompositionsim- ilartotheFDA-approvedandmarketedDoxil®waspreparedwith theextrusionmethod[10].Thelipidcompositionwascholesterol, mPEGandHSPC(seemolarratiosinTable1).Thelipidsweresolubi- lizedinethanol-isopropanolmixture(50:50),thenthesolutionwas addeddropwiseto0.25MAScontaining0.9%saline(SALSOL)solu- tion.Large,heterogeneouslipidparticleswereextrudedfourtimes through80nm WhatmanNuclepore (Track-EtchedMembranes) membranefilters(Whatman,Maidstone,UK)bymeansofaLipexTM Extruder(NorthernLipidsInc.,Burnaby,B.C.Canada)at50barand 70◦Ctoachieveuniformparticlesizedistribution.Theliposomes werethendialyzedagainst10mMhistidinebuffer(pH=7.5)con- taining10w/w%sucrosetoremoveASandorganicsolvents.The totalphospholipidconcentrationwasapproximately15.9mg/ml (cca.21.4mM).Theliposomalstocksolutionswerestoredat4◦C protectedfromlightandusedwithin2weeks.Thestocksolution wasfurtherdilutedasdictatedbytheexperiments.Thedegreeof dilutionandcorrespondinglipidconcentrationsaregiveninthe textandfigurecaptions.
2.3. MixingPEGylatedliposomeswithdifferentsalts
For turbidimetry, light scattering and zeta potential experi- ments20lofPEGylatedliposomesweremixedwith980lsalt solutionof appropriate concentration(50x dilution). For phase contrastandatomicforcemicroscopyexperimentsPEGylatedlipo- somes were diluted either 200x or 500x with salt solution of appropriate concentration. For 0M concentration physiological salinesolution(Salsol)wasusedfordilution.Theactualsaltand lipidconcentrationsaregiveninthetextandfigurecaptions.
2.4. Dilutionofprecipitatesforfusionand aggregation-reversibilitystudies
500lofPEGylatedliposomesweremixedwith500lofAS solutiontoproduceastockofprecipitatedsamplesofthedesired ASconcentration(from 0.8Mto2.0M).After15minincubation time,20lofthesestockswasmixedto980lASsolutionsof appropriateconcentrations(down to0.1M).Theactualsalt and lipidconcentrationsaregiveninthetextandfigurecaptions.
2.5. Turbidimetry
TheaggregationofPEGylatedliposomeswasfollowedbymea- suring the apparent optical density of the solution. Briefly, a 4lsampleofthewell-vortexedsolutionwaspipettedontothe pedestalofaNanoDrop2000UV–visspectrophotometer(Thermo Scientific Ltd., Wilmington, DE), and the optical density was recordedat250nm.Becausethelipidconcentrationwaskeptcon- stant,anincreaseinopticaldensitycorrespondstoanincreasein lightscattercausedbytheappearanceoflargerparticlesdueto aggregation.Forcomparability,identicallipidconcentrationswere usedinthedifferentsamples.
2.6. Dynamiclightscatteringmeasurement
Thesizedistributionofliposomesandaggregates werechar- acterizedbydynamic lightscattering(DLS)onaZetasizerNano Sinstrument (MalvernInstrumentsLtd,Malvern,UK). Fromthe intensityfluctuationsofa633-nmlaserlightscatteredathighangle fromthefreelymovingsuspendedparticlestheirdiffusionconstant wasobtained.SizedistributionwascalculatedbyusingtheStokes- Einsteinequationbythebuilt-in algorithmsoftheinstrument’s software.Light scatteringwas measuredat25±1◦C. Z-average valuesaredisplayedthroughoutthearticle,whichrepresentthe primaryandmoststableparameterproducedbyDLStechnique[11]
andrecommendedforqualitycontrolreports(ISO22412:2008).Z- averagevaluesrepresentagoodapproximationofhydrodynamic diameterofwelldispersedparticleswithmonomodalsizedistri- bution(indexofpolydispersitytypicallylowerthan0.1)andthus arewellapplicableforPEGylatedvesicles.TheZ-average,however, doesnotreflecttherealsizeofprecipitatedsamplesthatareoften heterogeneousinsizeandmaybeirregularlyshaped.Inthelatter caseZ-averagewasusedonlyforroughestimationofparticlesize, whichenabledustofollowliposomeaggregationwithoutexact determinationofaggregatedimensions.Sincedifferentbatchesof PEGylatedliposomeswereusedinthedifferentexperiments,minor variationsareseenintheaveragesizeofcontrolvesicles.
2.7. Zetapotentialmeasurements
PEGylatedliposomesweredilutedwithASsolution,and750l ofthismixturewasinjectedcarefullyintofoldedcapillarycells(PCT Kft.,Mosonmagyaróvár,Hungary)toavoidbubbleformation.Zeta potentialmeasurementswereperformedbyusingaZetasizerNano ZSequipment(MalvernInstrumentsLtd.,Worcestershire,UK)in whichparticlevelocityismeasuredaccordingtoalightscattering techniquebasedonDopplereffectevokedbyapairofmutually coherentlaserbeams (4mW,He-Nelaser at633nm).Fromthe autocorrelationfunctionofthescatteredlightintensitytheelec- trophoreticmobilityand,viatheHenryequation,thezetapotential are calculated.Measurements werecarried out in triplicates at 25◦C.
2.8. Analysisofzetapotentialdata
Bindingofionstoliposomalsurfaceandtheconcomitantchange ofsurfacepotentialcanbedescribedwellbyLangmuir-Freundlich isotherm[12].
=0+max· (K·c)n 1+(K·c)n
whereisthemeasuredzetapotential,0isthezetapotentialat zeroASconcentration,maxisthemaximalchangeofzetapoten- tial,Kisthebindingconstant,cistheligandconcentrationandn istheindexofheterogeneitydescribingthecooperativityofion binding.
2.9. Atomicforcemicroscopyandimageanalysis
Atomic forcemicroscope (AFM) images were recorded with a Cypher instrument (Asylum Research, Santa Barbara, CA) by scanningthesamplesinfluid witha gold-coatedsilicon nitride cantilever (Olympus Biolever, A lever, typical spring constant:
30pN/nm).100lsamplewasappliedona cleanedborosilicate glasscoverslipandincubatedinavaporchamberat23±1◦C.Non- contact-modeimageswererecordedatalinescanrateof0.5–1Hz.
Allmeasurementswerecarriedoutat28±1◦C.Imageswereana- lyzedbyusingthebuilt-inalgorithmsoftheAFMdrivingsoftware (IgorPro,WaveMetrics,Inc.,LakeOswego,OR).
2.10. Phasecontrastmicroscopy
MicrographswererecordedwithaNikonEclipseTi-Uinverted microscope(Auro-ScienceKft.,Budapest,Hungary)equippedwith auEyeUI1220LEdigitalcamera(IDSImagingDevelopmentSys- temsGmbH,Obersulm,Germany)usinga40xNikonSPlanfluor phasecontrastobjective.
2.11. Infraredspectroscopy
ATR-FTIRspectrawerecollectedwithaVarian2000FTIRScimi- tarSeries(VarianInc.,PaoloAlto,CA)spectrometerequippedwith a‘GoldenGate’(SpecacLtd.,London,UK)singlereflectiondiamond ATRaccessory.Themeasurementswereperformedatroomtem- perature:3lsamplewasmountedonthetopofthediamondATR crystalandacapwasusedtoavoidsampledrying;128scanswere collectedataresolutionof2cm−1.ATRcorrectionwasexecuted aftereachdatacollection.Allspectralmanipulations,includingsub- tractionsand spectraldeconvolutionswere performedbyusing theGRAMS/32softwarepackage(GalacticIndustriesIncorporation, USA).Bandpositionsforcurvefittingweredeterminedusingthe secondderivative.BandshapeswereapproximatedbyLorentzian functions.Theintensitiesandthebandwidthofeachcomponent wereallowedtovaryuntiltheminimal2parameterwasreached.
Afterthefittingprocedure,therelativecontributionofa partic- ularcomponentwascalculatedfromtheintegratedareasofthe individualcomponents.
3. Resultsanddiscussion
3.1. PEGylatedliposomescanbeprecipitatedbyammonium sulfate
TheadditionofAStoPEGylatedliposomesinfewmolarcon- centrationinitiated therapidincreaseof opacityofthesample.
Toassessthemagnitudeofliposomeprecipitation,wemeasured theturbidityofliposomalsuspensionsat0–2MASconcentration.
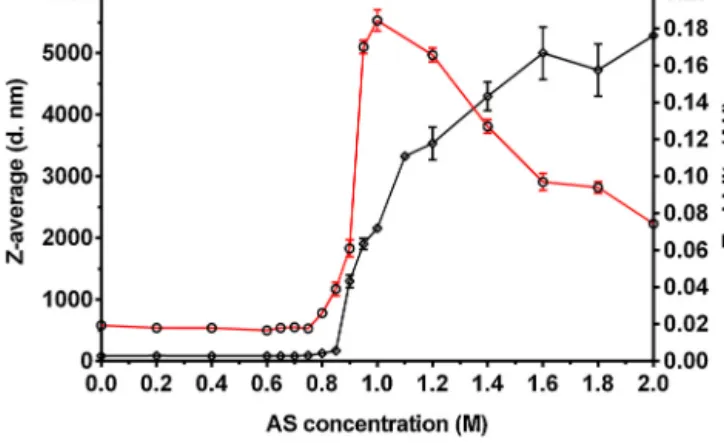
Accordingtothe turbidityvs. ammonium-sulfateconcentration
Fig.1.Turbidity(blackline)andaverageparticlediameter(redline)ofliposomal suspensionsasafunctionofammoniumsulfate(AS)concentration.Average±SD valuesofthreeindependentmeasurementsareshown.Lipidconcentrationwaskept constantthroughoutthemeasurement(50xdilution,0.318mg/ml).(Forinterpre- tationofthereferencestocolourinthisfigurelegend,thereaderisreferredtothe webversionofthisarticle.)
curve(Fig.1),noprecipitationoccursbelowanASconcentrationof 0.75M.Above0.75M,turbidityrisesabruptlythenlevelsoffabove 1Mtoavalueanorderofmagnitudegreaterthanintheabsenceof AS.Fromthiscurveweconcludedthatprecipitationbegansome- wherebetween0.75and0.8MASconcentration.Thefallofthe turbidimetrycurveabove1MASconcentrationmaybeexplained byincreasingheterogenityofthesystem,i.e.theformationofa lipid-richprecipitateandalipid-pooraqeousphase.
Wehypothesizedthattheabruptriseinturbiditywasrelatedto asizeincreaseduetotheaggregationofthevesicles.Toassessthe sizeoftheprecipitatesasafunctionofASconcentration,dynamic light scattering measurements werecarried out (Fig.1).Below a concentrationof0.8Mthemeanparticlesize variedbetween 83.2nmand91.5nmindependentlyoftheASconcentration.Upon increasingtheASconcentrationfurther,however,sizeincreased abruptlyto∼2000nmintherangeof0.8–1Mthenmoreslowly above1M.UponreachinganASconcentrationof2Mthemeanpar- ticlesizeexceeded5000nm.Notably,particlesizeisapproximated withtheZ-averagevaluewhichiscalculatedbyassumingspherical geometryandmonomodalsizedistribution.Incaseofaggregation, particleshapeislikelytodeviatefromspherical,which,together withgrowingpolydispersity indices measuredabove0.75MAS (datanotshown)meansthattheZ-averageparametermaycor- respondonlytoanapproximation,withinanorderofmagnitude, oftheaverageparticlediameter.Theresultsoftheturbidimetryand dynamiclightscatteringmeasurementsleadtosimilarconclusion:
precipitationbeginsuponreachinganASconcentrationthreshold (0.7–0.8M),thenprogressivelylargeraggregatesareformedupon incerasingtheASconcentrationfurther.Sinceprecipitationtakes placeinstantenouslyuponmixingthePEGylatedliposomeswith AS,itisratherdifficulttofollowaggregationkinetics.Sizeincreases rapidlyandconsiderablyinthefirstminuteneededtosetupaDLS measurement.Afterthislagtimeafurthercontinousincreaseof sizewasobservable(Fig.S1),butexactrateandkineticscouldnot beendetermined.
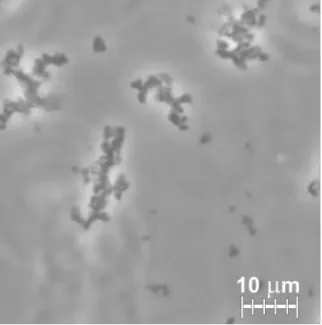
Torevealthemicroscopicdetailsoftheprecipitationprocessand assesswhethervesicleaggregationtakesplaceindeed,morpholog- icalmeasurementswerecarriedout.Phasecontrastmicroscopyof precipitatedsamplesshowedbranchingobjectsofirregularshapes apparently formedofsmallerclusters(Fig.2)which resembled electronmicrographsofliposomalaggregatesseenearlier[13].
Toresolvetheultrastructureofaggregatesandtofollowtheir formation we imaged liposomes withatomic forcemicroscopy (AFM) at various ASconcentrations (Fig.3). At0 and 0.7M AS
Fig.2.Phasecontrastmicrographofliposomalaggregatesin1MAS.Largebranching objectsandsmalleraggregatesareseen(200xdilution,0.0795mg/mllipidconcen- tration).
individual, interaction-free liposomes were observed. At an AS concentrationof0.8Mmany liposomeswereobservedinlinear assemblies,pointingattheonsetofaggregation.AtanASconcen- trationof0.9M,largeaggregates wereclearlyseen.In0.8MAS (Fig.3C),besidesthevesiclesflatpatcheswithasmoothsurfaceand atopographicalheightof5–7nmwereobserved.Weidentifythem asbilayers,althoughtheyaresomewhatthinnerthanalipidbilayer
coveredwithaPEGpolymerbrushonbothsides(approx.12nm) calculated withadifferent method[14,15]. Thebilayerpatches probablyemergebecauseasosmolalityincreases,vesiclesexhibit agreaterpropensitytoburstonthesubstrate[16,17].Patchforma- tionisageneralphenomenoncharacteristictoliposomalsamples.
Patchesofvarying sizesarefoundin almostallliposomal AFM imagesthroughoutthecorrespondingliterature[18–20]andalsoin Fig.4.Interestingly,patchformationappearstodependontheAS concentrationasevidencedbyourresultsshowninFig.3.While patchformation is only sporadicatlower ASconcentrations, it becomespronouncedabove0.8M(Fig.3C),andat0.9Mmostofthe substrateiscoveredwithaconfluentsupportedlipidbilayer(see thebackgroundofclustersandvesiclesinFig.3D).Liposomeclus- tersobservedin0.9MAS(Fig.3D–F)havediversesizesvaryingfrom fewhundrednmtofewm,whichisinanorder-of-magnitudecor- relationwiththeDLSdata(seeFig.1).Notethattheirregularvesicle shapemightbetheresultofimagingartifactsandnotexquisitely ofliposomalshapetransformations.UponraisingtheASconcen- trationabove0.8Mimagingbecamedifficult,whichismostlikely duetothepresenceoflarge,softaggregatesincompletelyimmo- bilizedonthesurface.Severalattemptshavebeenmadetoimage samplesatevenhigherASconcentrationstofindlargeraggregates, butunsuccessfully.
3.2. Aggregationmaypromotevesiclefusion
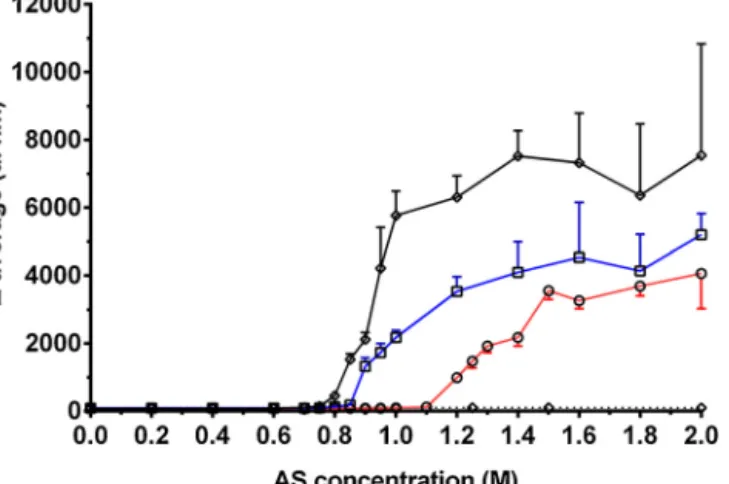
Totestwhetherliposomeaggregationisfollowedbyfusionand todeterminethethresholdconcentrationoffusion,liposomalsam- pleswereprecipitatedinvariousconcentrationsofAS,then15min laterdilutedto0.1MAS.DLSdatashowednoconsiderablechange ofaveragesizeat0.8and0.9Mprecipitatingconcentration.Aslight increasewasobservedat1.0and1.1Mfollowedbyamoresignif-
Fig.3.Amplitude-contrastAFMimagesofliposomesat(A)0M,(B)0.7M,(C)0.8M,(D)0.9MASconcentration.Sampleswerediluted1to500withtheircorresponding solution(0.0318mg/mllipidconcentration).Figure(E)showstheheight-contrastAFMimageoffigure(D)color-coded,while(F)representstheheightsectionprofiletaken alongsidethethickredlinein(E).Thewhiteandredtrianglesin(E)and(F),respectively,pointatthehighestpointoftheliposomecluster.Thecolorbarinthelowerright cornerdisplaystheheightscaleoffigure(E).(Forinterpretationofthereferencestocolourinthisfigurelegend,thereaderisreferredtothewebversionofthisarticle.)
Fig.4.(A)Sizeofliposomes(Z-average±SD)dilutedfromvariousprecipitatingASconcentrations(indicatedonxaxis)to0.1M.Reddottedlineshowsthesizeofcontrol liposomes.(B)Amplitude-contrastAFMimageoftheliposomalsuspensioninphysiologicalsalinesolution.(C)and(D)Amplitude-contrastAFMimagesofa1Mand2M AS-aggregatedsampledilutedbackto0.1MASconcentration.WhitearrowheadindicatesalargervesicleinpanelC.InpanelDfewofthelargervesiclesrupturedduring thescantoformbilayerpatchesonthesurface.Lipidconcentrationwasidenticalinthesamples(200xdilution,0.0795mg/mllipidconcentration).(Forinterpretationofthe referencestocolourinthisfigurelegend,thereaderisreferredtothewebversionofthisarticle.)
icantandmonotonousincreasefrom1.2M(Fig.4A).AFMimages ofthesesamplesrevealedthattheincreaseofaveragesizemaybe attributedtotheoccurrenceoffusedliposomes.Boththesizeand relativeamountoffusedvesiclesincreasedwithASconcentration:
at1Mliposomeslargerthan theaverageappeared sporadically (Fig.4C),whileat2Mevenlargervesicleswithanirregularshape becamedominant(Fig.4D).Thefindingsareinaccordwithsize distributionshowninFig.S2,theaveragesofwhichareshownin Fig.4A.Upto1.1Mamoderate,thenfrom1.2Mamoresignificant upwardshiftisseenduetotheappearanceoflargerobjects.Inaddi- tion,from1.5Maminorpopulationof several-micron-diameter particlesappearedwhichwasnotobservedinAFMimagespossibly duetotheirsmallnumber.
Takentogether,PEGylatedliposomesmaynotonlyaggregate butalsofuseuponASaddition.Therateoffusionisconcentration dependent,andthethresholdconcentrationis1Mattheemployed incubation time.This alsomeansthat AS-drivenaggregationof thevesiclescanbereverseddependingonASconcentrationand incubationtime.Todisruptaggregatedliposomestheyshouldbe dilutedwellbelowtheASconcentrationatwhichtheystartedto precipitate(Fig.S3).
3.3. Precipitationdoesnotdependonsurface-chargeproperties
Physicalstabilityofcolloidalvesiclesdependslargelyontheir surfacechargedensity,which isapproximatedwiththeelectric potentialdifference(orzetapotential)betweenthebulksolution andtheslippingplaneofionsassociatedtothevesicularsurface.
Itiswidelyacceptedthatazetapotentiallowerthan30mVmakes colloidaldispersionspronetoaggregation[21].Sincethesurface chargepropertiesandthusphysicalstabilityofliposomalvesicles canbelargelyaffectedbyions[22],modificationoftheliposomes’
zetapotentialbyASadditionmightbeakeyfactorinevokingaggre- gation.Toassessthecharge-modulatingeffectofASonPEGylated vesicles,zetapotentialmeasurementswerecarriedout.
Zetapotentialofthecontrolsamplewas−71.7±2.5mV,which impliesastrongnegativesurfacepotentialandcorrespondstoan extremelyhighcolloidalstability[21].AddingASledtoamassive increaseofthezetapotentialvalueatevenrelativelylowconcen- trations(Fig.5),whichmaybeexplainedbytheassociationofNH4+ ionstotheoriginallynegativevesicularsurface.Coordinationof NH4+ cationstotheetheroxygenswasproposedbyBaileyand Callard[23].Analternativeexplanationisthatstructuralmodifica- tionsofthecharge-alteringPEGchains(discussedlater)mayhave ledtotheobservedchargeincrease.
TheLangmuir-Freundlichisothermfitswelltothedatapoints suggestingmonolayerabsorptiontoaheterogeneoussurface.The adsorptionconstantof1001M−1pointsatoutstandingaffinityof NH4+ionstothePEG-coveredsurfaceand0.73asindexofhetero- genityindicatesnegativecooperativityoftheions.
Thepronouncedsurface-chargealteringeffectofASlevelsoff ataslowconcentrationasabout0.1M,whichisnearlyanorder ofmagnitudelowerthanthethresholdconcentrationforaggrega- tion.Furthermore,noconsiderablechangeofzetapotentialisseen reachingtheconcentrationregimeofaggregation(above0.75M,
Fig.5.ZetapotentialofPEGylatedliposomesasafunctionofASconcentration.Red lineshowstheLangmuir-Freundlichisotermfittedtothedata.Zetapotentialwas measuredintriplicates,errorbarscorrespondtostandarddeviation.Zetapotential at0ASconcentrationwas−71.7±2.5mV.Insetshowsthedataplottedonaloga- rithmicxaxis.Dilution:50x(0.318mg/mllipidconcentration).(Forinterpretation ofthereferencestocolourinthisfigurelegend,thereaderisreferredtotheweb versionofthisarticle.)
Fig.6. EffectofPEGconcentrationontheASconcentrationdependenceofliposomal aggregation.Dilution:50x(0.318mg/mllipidconcentration).Brokenline:0%mPEG;
redline:2%mPEG;blueline:5%mPEG;blackline:10%mPEG.(Forinterpretation ofthereferencestocolourinthisfigurelegend,thereaderisreferredtotheweb versionofthisarticle.)
seeFig.1).ThesetogethersuggestthattheASmediatedincreaseof zetapotentialisnotthemechanismbehindliposomeaggregation.
3.4. PrecipitationisPEG-related
ToelucidatetheroleofPEGchainsinliposomeaggregation,lipo- someswithdifferentamountsofPEGchainsontheirsurfacewere producedandmixedwithAS.DLSdatarevealedthatconventional liposomes(i.e.,oneswithoutPEGylation)showednosizeincrease upto2MAS(Fig.6).Thisindicatesthatnoprecipitationtakesplace intheabsenceofthePEGbrushontheliposomalsurfaceandhigh- lightsthattheaggregationevokingeffectofASismediatedviathe PEGpolymerlayer.UponincreasingthePEGcoveragefrom2to 10M%,theprecipitationcurvesshiftedtotheleft,meaningthat lowerconcentrationsofASwereenoughtoelicitaggregation.It againunderpinsthatprecipitationisPEG-related.Consideringthat modificationofsurfacechargedoesnotaffecttheaggregationof liposomes(seeabove),wehypothesizethatsomestructuraltran- sitionsofthesurfacepolymerchainsmusthaveledtothehigher propensityforaggregation.
3.5. ASdehydratesPEGchains
ConsideringthatprecipitationisrelatedtothepresenceofPEG, itisplausiblethatAS,whichisakosmotropicagent[24,25]dehy- dratedPEGpolymers,leadingtoaggregationofthePEG-covered vesiclesthroughhydrophobicinteractions.Toestimatethehydra- tion level of the PEG layer, attenuated total reflection Fourier transforminfraredspectroscopy(ATR-FTIR)combinedwithcurve fittingprocedurewasapplied.ThemethodwasproposedbyVarga etal.tocharacterizethePEG-layerofstealthliposomesbasedon theratiooftransandgaucheconformationsofC O CgroupsofPEG chain[26].ThecomplexstretchingvibrationC O Cbandaround 1100cm−1 ofthePEGchain canbedecomposed intofive band componentsduetonon-interactingvibrations.Thebandaround 1093cm−1 is related to C O C groups of PEGin trans confor- mation,whiletheonearound1113cm−1 belongs tothegauche conformationrelativetoC Cbond[27].Thetwoextremecom- ponentswithsmallerintensitiesaround1139and1029cm−1can beassignedto␦( CH2 )deformationand(C C)stretchingvibra- tions,respectively.Therelativelyintensebandcomponentaround 1068cm−1mightberelatedtothe(C OH)bands.Ahigherextent ofintramolecularH-bondingoftheC O CgroupsofthePEGmoi- etytoaneighboringethericoxygenresultsinagreaterproportion ofthe moreconstrained gaucheconformer withan appropriate increaseofrelativeintensity.Thus,theratioofthetransandgauche conformationscanbeamarkertocharacterizethePEGlayerstruc- tureand,indirectly,thehydrationstate[26].
ATR-FTIRspectraofthePEG-liposomeswith1–2wt.%concen- trationaredominatedbythestrongwaterabsorptionbands;so,asa firststepofspectralevaluationthesubtractionofwaterbackground (ASsolution)wasperformed.Sincethe(C O C)vibrationbands overlapwiththephosphatestretchingvibrations(PO2−)ofthe
Fig.7. Deconvolutionofthebandaround1100cm-1afterspectralsubtractions:A)PEGylatedliposomesinwater,B)PEGylatedliposomesin1MASsolution,C)PEGylated liposomesin2MASsolution.Emptycirclesdenotethemeasureddatapoints,solidlinesrepresentthefittedspectra,theindividualbandcomponentsandtheresiduals.
Fig.8.Thetrans/gaucheratioofthe(C O C)bandofPEGchainsforSSLsamples inwater,in1Mammoniumsulfateandin1Mguanidinechloride.Averages±SDs ofthreeindependentmeasurementsareshown.Thehighstandarddeviationfor guanidinechloridesamplesmightbecausedbythedifficultyinguanidinechloride backgroundsubtraction.
lipidcomponents,thereferencespectrumofpurehydratedHSPC (hydrogenatedsoyphosphatidylcholine,mainlipidcomponentfor PEGylatedliposomes)wascarefullysubtractedfromthespectraof PEGylatedliposomes.Typicaldeconvolutionsofthebandaround 1100cm−1afterspectralsubtractionarepresentedinFig.7.
Thehighertherelativeintensityoftransconformers,thehigher thehydration levelofthePEGlayer.By addingammoniumsul- fatesalt (1Mconcentration)therelative intensityof (C O C) ingaucheconformationincreases(Fig.8),indicatingthatthekos- motropicsaltreducesthehydrationofthePEGpolymerchains.As tothehigheramountofAS(2Mconcentration),however,anew bandcomponentat1068cm−1dominatesthespectrum.Thisband componentmightbeassignedto(C OH)groups.Interestingly,no bandcomponentbelongingto(C O C)transcomponentcould bedeconvoluted.Thismayindicateaconformationalchangemore pronouncedthanthetrans−gauchevariation.Similarphenomenon wasobservedalsoformicellesformedbypureDSPE-PEG2000lipid (∼10wt.%)inwater(unpublishedresults).
FTIRdatasupportthenotionthatthemechanismbehindaggre- gationofstealthvesiclesmightbethereductionofhydrationof PEGpolymerchainsduetokosmotropiceffectofAS.Thisisfurther underpinned by the observation that a chaotropic salt, guani- dinechloride(GdmCl)didnotaffectsignificantlythe(C O C) trans/gauche ratio and thus thehydration level of the polymer (Fig.8.,seespectrum,anddeconvolutionofthebandsinFig.S4.
andTableSI.).
3.6. Otherkosmotropicsaltsalsoleadtoprecipitationof PEGylatedliposomes
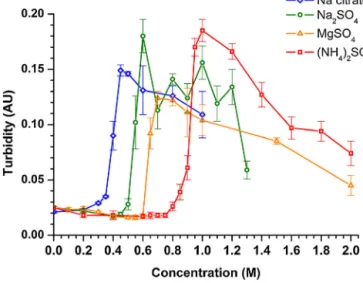
Westudiedtheeffectofvarioussaltsonstealthliposomesto testthehypothesisthatPEG-relatedprecipitationofliposomesis not specificfor ASbut ageneralkosmotropic phenomenon. All examinedkosmotropicsalts(sodiumcitrate,sodiumsulfate,mag- nesiumsulfate)ledtotheprecipitationof thevesicles (Fig.S5).
Bycontrast,chaotropicsalts(magnesiumchlorideandguanidine chloride)didnotaggregatetheliposomes(Fig.S6).Theprecipi- tatingeffectofkosmotropicsaltswasfoundtobeconcentration dependent, and theorder of theirthreshold concentrationwas thefollowing: sodiumcitrate<sodiumsulfate<magnesiumsul- fate<ammoniumsulfate(Fig.9).Thisorderisingoodaccordance withtwophaseformingcapacityofionsinPEG-salt-watersystems observedearlier[28].
Thesurfacechargemodifyingeffectofkosmotropicsaltslev- elled off at much lower concentrations than that needed for aggregation(Fig.S7),similarlytowhatwasobservedforAS(Fig.5).
Theadsorptionconstantsoftheionsdonotcorrelatewiththeir precipitatingability.Furthermorenotonlykosmotropic,butalso chaotropicsalts,whichdonotaggregatePEG-liposomes,shiftedthe
Fig.9. Turbidityofliposomalsuspensionsasafunctionofsaltconcentration.Aver- age±SDvaluesofthreeindependentmeasurementsareshown.PEGconcentration was5mol%andlipidconcentrationwaskeptconstantthroughoutthemeasurement (50xdilution,0.318mg/ml).
zetapotentialofliposomesfromstronglynegativevaluestoneutral regime(Fig.S7).Thesetogetherclearlysuggestthataggregation phenomenon isnot connected toionadsorption drivensurface chargealterationofthevesicles,buttokosmotropiceffect.
4. Conclusions
Here we demonstrated that ammonium sulfate and other kosmotropicsaltshavea precipitatingeffectonPEGylatedlipo- somes.Aggregationtakesplaceaboveathresholdconcentration (Figs.1,3,6and9)andleadstotheformationofirregular,micron- sizedaggregates(Figs.2and3D–F).AtcertainASconcentrations theprocessisreversible;aggregatescanbefullydisintegratedby dilution,buthigherASconcentrationsmayevoketheirreversible fusionofvesicles(Fig.4).Reductionofsurfacechargedoesnothave anyeffectonaggregationofvesicles(Fig.5).At0M%PEGcontentno aggregationoccurswhilethepropensityforprecipitationincreases withPEGcoverageintherangeof2–10M%(Fig.6),whichclearly showsthatAS-drivenaggregationofstealthvesiclesisrelatedto thePEGchains.ASleadstothedehydrationofPEGpolymerchains, whileGdmCldoesnotaffectit(Figs.7and8).Otherkosmotropic salts(suchasNa3citrate,Na2SO4,MgSO4,)alsoprecipitatePEGy- latedliposomes(Fig.9), whilechaotropicsalts(likeGdmCland MgCl2)do not. We propose thatkosmotropic saltsmay induce saltingout onthepolymerchainspromotingtheirhydrophobic interaction [9] and leading tothe separation of thePEG phase togetherwiththecoupledliposomes.Reversibilityofaggregation isthesimpleconsequenceofthereductionofconcentrationofthe kosmotropicagent,similarlytowhathappens incase ofprotein purificationorduringtheelutionphase ofhydrophobicinterac- tionchromatography.TheobservationthathighASconcentration resultsinrapidfusionofthevesiclesmaybeexplainedbyexces- sivestructuralalterationsofPEGchainsduetotheirhydrophobic modification.ModificationofstructureandhydrationofPEGchains mayleadtostericimbalanceofthevesiclesasitwasshownearlier [15,29].
SinceAS(andotherkosmotropicagents)maybeusedduring formulation of PEGylated nanoparticles(e.g., remote loadingof doxorubicin intoliposomes[14]), great careis neededtoavoid unwantedaggregationorfusionphenomena,whichmaytakeplace during eitherproduction or storage. Thereis a hypothesis that complementactivationrelatedpseudoallergy(CARPA),observed
intheclinicalpracticewhenPEGylatedliposomesareadministered intravenously,maybecausedbysporadicaggregationorfusionof liposomes[6].Theresultspresentedabovemayopentherouteto produceaggregatesorfusedvesiclesinacontrolledmannerand testtheirroleinpseudoallergicreactions.Reversibleaggregation mayalsobeusedtoseparatePEGylatedliposomes/nanoparticles fromtheirouteraqueousphaseduringformulation.
Acknowledgements
ZetapotentialexperimentswerecarriedoutatDepartmentof Pharmaceutics,SemmelweisUniversitywithsupportofDr.István AntalandtechnicalassistanceofDr.ViktorFülöp,forbothofwhich theauthorsaregrateful.WehighlyappreciateDr.PálGróf’shelpin analysisofzetapotentialdata.
ThisworkwassupportedbygrantsfromtheHungarian Sci- enceFoundation(OTKAK109480).Theresearchleadingtothese results has received funding from the European Union’s Sev- enthFrameworkProgram(FP7/2007-2013)undergrantagreement no HEALTH-F2-2011-278850 (INMiND)and NMP-2012-309820, (NanoAthero).Theauthorsalsoacknowledgethefinancialsupport bytheHungarian GovernmentNationalResearch, Development andInnovationFund(TÉT-13-IL-2-2014-0001).TheFTIRspectro- scopicpartwassupportedbytheJánosBolyaiResearchScholarship oftheHungarianAcademyofSciences(J.M.).
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,athttp://dx.doi.org/10.1016/j.colsurfb.2016.06.
056.
References
[1]F.M.Veronese,G.Pasut,PEGylation,successfulapproachtodrugdelivery, DrugDiscov.Today10(2005)1451–1458.
[2]D.Papahadjopoulos,T.M.Allen,A.Gabizon,E.Mayhew,K.Matthay,S.K.
Huang,K.D.Lee,M.C.Woodle,D.D.Lasic,C.Redemann,Stericallystabilized liposomes:improvementsinpharmacokineticsandantitumortherapeutic efficacy,Proc.Natl.Acad.Sci.88(1991)11460–11464.
[3]T.M.Allen,C.Hansen,Pharmacokineticsofstealthversusconventional liposomes:effectofdose,Biochim.Biophys.Acta(BBA)-Biomembr.1068 (1991)133–141.
[4]T.M.Allen,P.R.Cullis,Liposomaldrugdeliverysystems:fromconceptto clinicalapplications,Adv.DrugDeliv.Rev.65(2013)36–48.
[5]J.Szebeni,Complementactivation-relatedpseudoallergy:anewclassof drug-inducedacuteimmunetoxicity,Toxicology216(2005)106–121.
[6]J.Szebeni,P.Bedocs,Z.Rozsnyay,Z.Weiszhar,R.Urbanics,L.Rosivall,R.
Cohen,O.Garbuzenko,G.Bathori,M.Toth,R.Bunger,Y.Barenholz, Liposome-inducedcomplementactivationandrelatedcardiopulmonary distressinpigs:factorspromotingreactogenicityofDoxilandAmBisome, Nanomed.:Nanotechnol.Biol.Med.8(2012)176–184.
[7]P.Cummins,B.O’Connor,Hydrophobicinteractionchromatography,in:D.
Walls,S.T.Loughran(Eds.),ProteinChromatography,vol.681,HumanaPress, 2011,pp.431–437.
[8]Interactionsofnanoparticleswithplasmaproteins:implicationonclearance andtoxicityofdrugdeliverysystems,ExpertOpin.DrugDeliv.8(2011) 343–357.
[9]M.J.Hey,D.P.Jackson,H.Yan,Thesalting-outeffectandphaseseparationin aqueoussolutionsofelectrolytesandpoly(ethyleneglycol),Polymer46 (2005)2567–2572.
[10]R.C.MacDonald,R.I.MacDonald,B.P.Menco,K.Takeshita,N.K.Subbarao,L.R.
Hu,Small-volumeextrusionapparatusforpreparationoflarge,unilamellar vesicles,Biochim.Biophys.Acta1061(1991)297–303.
[11]Dynamiclightscattering−commontermsdefined,in,vol.2015,www.
malvern.com.
[12]X.L.R.Iraolagoitia,M.F.Martini,Ca2+adsorptiontolipidmembranesandthe effectofcholesterolintheircomposition,ColloidsSurf.B:Biointerfaces76 (2010)215–220.
[13]G.Drin,V.Morello,J.-F.Casella,P.Gounon,B.Antonny,Asymmetrictethering offlatandcurvedlipidmembranesbyagolgin,Science(NewYork,N.Y.)320 (2008)670–673.
[14]Y.Barenholz,Liposomeapplication:problemsandprospects,Curr.Opin.
ColloidInterfaceSci.6(2001)66–77.
[15]O.Tirosh,Y.Barenholz,J.Katzhendler,A.Priev,Hydrationofpolyethylene glycol-graftedliposomes,Biophys.J.74(1998)1371–1379.
[16]E.Reimhult,F.Höök,B.Kasemo,Intactvesicleadsorptionandsupported biomembraneformationfromvesiclesinsolution:influenceofsurface chemistry,vesiclesize,temperature,andosmoticpressure,Langmuir19 (2003)1681–1691.
[17]B.Seantier,B.Kasemo,Influenceofmono-anddivalentionsontheformation ofsupportedphospholipidbilayersviavesicleadsorption,Langmuir25 (2009)5767–5772.
[18]J.Jass,T.Tjarnhage,G.Puu,Fromliposomestosupported,planarbilayer structuresonhydrophilicandhydrophobicsurfaces:anatomicforce microscopystudy,Biophys.J.79(2000)3153–3163.
[19]J.Jass,T.Tjärnhage,G.Puu,Atomicforcemicroscopyimagingofliposomes,in:
D.Nejat(Ed.),MethodsinEnzymology,vol.367,AcademicPress,2003,pp.
199–213.
[20]R.P.Richter,A.R.Brisson,Followingtheformationofsupportedlipidbilayers onmica:astudycombiningAFM,QCM-D,andellipsometry,Biophys.J.88 (2005)3422–3433.
[21]T.M.Riddick,ControlofColloidStabilityThroughZetaPotential,Zeta-Meter Inc.viaLivingstonPublishingCompany,Wynnewood,1968.
[22]L.Wu,J.Zhang,W.Watanabe,Physicalandchemicalstabilityofdrug nanoparticles,Adv.DrugDeliv.Rev.63(2011)456–469.
[23]F.E.Bailey,R.W.Callard,Somepropertiesofpoly(ethyleneoxide)1inaqueous solution,J.Appl.Polym.Sci.1(1959)56–62.
[24]M.G.Cacace,E.M.Landau,J.J.Ramsden,TheHofmeisterseries:saltand solventeffectsoninterfacialphenomena,Q.Rev.Biophys.30(1997)241–277.
[25]J.A.Queiroz,C.T.Tomaz,J.M.S.Cabral,Hydrophobicinteraction chromatographyofproteins,J.Biotechnol.87(2001)143–159.
[26]Z.Varga,J.Mihály,S.Berényi,A.Bóta,Structuralcharacterizationofthe poly(ethyleneglycol)layerofstericallystabilizedliposomesbymeansofFTIR spectroscopy,Eur.Polym.J.49(2013)2415–2421.
[27]M.Rozenberg,A.Loewenschuss,Y.Marcus,IRspectraandhydrationof short-chainpolyethyleneglycols,Spectrochim.ActaA:Mol.Biomol.Spectrosc.
54(1998)1819–1826.
[28]K.P.Ananthapadmanabhan,E.D.Goddard,Aqueousbiphaseformationin polyethyleneoxide-inorganicsaltsystems,Langmuir3(1987)25–31.
[29]A.Priev,A.Samuni,O.Tirosh,Y.Barenholz,Theroleofhydrationin stabilizationofliposomes:resistancetooxidativedamageofPEG-grafted liposomes,in:G.Gregoriadis,B.McCormack(Eds.),TargetingofDrugs6,vol.
300,Springer,US,1998,pp.147–167.