CHAPTER THREE
Inorganic Nutrient Nutrition of Microorganisms
D . J. D . NI C H O L A S
I. Introduction 363 II. Mineral Element Requirements 364
A. Essential and Nonessential Elements 364 B. Criteria for Essentiality of Nutrients for Growth 365
C. Pure Culture Methods 366 III. Requirement for Nitrogen and Its Compounds 372
A. Biological Fixation of Nitrogen 372
B. Ammonia 376 C. Assimilation and Dissimilation of Nitrates . 3 7 9
D. Nitrification 396 IV. Requirements for Other Nutrient Elements 398
A. Phosphorus · 398
B. Potassium and Sodium 400 C. Magnesium and Calcium 402
D. Sulfur 408 E. Iron 411 F. Copper 412 G. Zinc 415 H. Manganese 416 I. Molybdenum 418 J. Vanadium and Gallium 421
K. Cobalt 422 L. Boron 425 V. Toxicity Effects of Metals 425
VI. Conclusions 430 References 431
I. Introduction
T h e study of m i n e r a l r e q u i r e m e n t s of microorganisms has received o n l y incidental t r e a t m e n t u n t i l comparatively r e c e n t l y a n d almost all t h e observations h a v e been confined to a few genera only. P a s t e u r showed t h a t the addition of ash constituents of yeast to viable cultures of other yeast m a r k e d l y stimulated growth, a n d this w a s probably t h e first unequivocal demonstration of t h e i m p o r t a n c e of inorganic sub- stances for g r o w t h of a microorganism. Since t h a t time, m a i n l y as t h e result of p u r e culture work, t h e precise inorganic r e q u i r e m e n t s of some
363
microorganisms h a v e been determined as well as their possible role i n metabolism.
II. Mineral Element Requirements
A. E S S E N T I A L A N D N O N E S S E N T I A L E L E M E N T S
A t t h e present t i m e the m i n e r a l n u t r i e n t s found in microorganisms a r e divided into two a r b i t r a r y groups: (a) essential elements ( Ν , P , K, M g , Ca, S, F e , Cu, Z n , M n , M o , B, CI, N a , Co, V ) k n o w n to be in- dispensable for t h e g r o w t h of at least some organisms, (b) other ele- ments ( N i , T i , Se, P b , Ag, A u , Br, I, etc.) often present in t h e ash of microorganisms b u t not y e t shown to be essential for growth.
N o t all t h e elements listed u n d e r (a) a r e u n i v e r s a l l y required, b u t all of t h e m h a v e been found to be necessary for some t y p e of organism.
T h e essential elements a r e sometimes subdivided into two m a i n groups:
t h e major or macro-, nutrients a n d the micronutrients, trace elements or oligoelements; as t h e n a m e s suggest, t h e y reflect a large or a small r e q u i r e m e n t for t h e elements.
Calcium a n d sulfur, w h i c h a r e u s u a l l y required in m u c h smaller a m o u n t s b y microorganisms t h a n b y h i g h e r plants should therefore be classed as micronutrients for the former p l a n t group. Boron, w h i c h is universally required b y h i g h e r plants is also essential for some algae (Pirson 230) b u t not t h u s far for the growth of fungi or bacteria. A n - other interesting difference is t h a t cobalt, a constituent of v i t a m i n B1 2, is required b y some bacteria, e.g., Lactobacillus leichmannii, Rhizobium japonicum, Azotobacter vinelandii, a n d b y algae, e.g., Euglena gracilis
( 1 1 6 - 1 1 8 , 156, 220b, 2 2 0 c ) . Recently cobalt was shown to be essential for legumes t h a t a r e fixing nitrogen i n association w i t h the root nodule bacteria b u t not for legumes being g r o w n on combined nitrogen ( 1 , 2, 103, 2 4 4 ) . T h i s m a y be due solely to a cobalt r e q u i r e m e n t b y nodule bacteria w h e n t h e y are fixing nitrogen, a n d thus far t h e r e is no u n - equivocal evidence t h a t cobalt is essential for t h e growth of higher plants. Recently Rhizobium japonicum g r o w n on n i t r a t e outside the host p l a n t was shown to h a v e a r e q u i r e m e n t for cobalt (156, 220b, 2 2 0 c ) . A n o t h e r contrast concerns v a n a d i u m , not y e t shown to be re- quired b y higher plants, although it is essential for the growth of the green alga Scenedesmus obliquus ( 1 0 ) . T h e r e a r e n o f u r t h e r re- ports, however, t h a t v a n a d i u m is required b y other algae. Bortels ( 3 4 ) , H o r n e r et al. ( 1 1 3 ) , Bové et al. ( 3 5 ) , a n d Nicholas et al. (218) h a v e all shown t h a t v a n a d i u m can partially replace m o l y b d e n u m in the fixa- tion of nitrogen in some species of Azotobacter. T h e evidence t h a t gal- l i u m is essential for growth of Aspergillus (281) has not been sub-
3 . I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 3 6 5
stantiated in other laboratories, a n d the original claim h a s n o w been w i t h d r a w n .
E v e n in t h e m i c r o n u t r i e n t category t h e r e is a v e r y wide r a n g e of requirement, e.g., the need for iron m a y be a t h o u s a n d times t h a t for m o l y b d e n u m a n d a h u n d r e d times t h a t for cobalt or v a n a d i u m . T h e r e is some justification, therefore, for a n " u l t r a m i c r o n u t r i e n t g r o u p " of biologically i m p o r t a n t metals to include m o l y b d e n u m , v a n a d i u m , a n d possibly cobalt. Other n u t r i e n t s m a y be added to this list in due course w h e n p u r e culture methods a r e developed further, a n d r e q u i r e m e n t s for other micronutrients are established, or w h e n these metals are found to p l a y a n indispensable role in cell metabolism.
Microorganisms h a v e a regulatory m e c h a n i s m for controlling the u p t a k e of essential m i n e r a l n u t r i e n t s from t h e m e d i u m as h a v e roots of higher plants, b u t this breaks down w h e n t h e y grow u n d e r adverse conditions, e.g., high or low p H , high ionic strength, or toxic concentra- tions of nutrients. It is also clear t h a t microorganisms contain in addi- tion trace metals t h a t a r e not k n o w n to perform a n y useful function in metabolism, e.g., iodine, in m a r i n e algae.
B. C R I T E R I A F O R E S S E N T I A L I T Y O F N U T R I E N T S F O R G R O W T H
T h r e e criteria of essentiality for a n u t r i e n t w e r e proposed b y A r n o n (9) for higher plants, a n d these m a y also be applied to microorganisms.
These criteria are (a) the organism cannot complete its life cycle with- out the particular element, (b) its action m u s t be specific and cannot be replaced b y another, (c) its effect on the p l a n t m u s t be direct. As pointed out previously (208, 209, 220) concept (b) is perhaps too rigid in the light of recent work. T w o examples illustrate this point. M o l y b - d e n u m is required for nitrogen fixation b y Azotobacter, b u t in some species v a n a d i u m has a sparing action. Both trace metals w h i c h func- tion in nitrogen fixation occur in some habitats in r o u g h l y equivalent a m o u n t s so t h a t either could be utilized. I n higher plants, chloride is necessary for growth b u t bromide at higher concentrations can sub- stitute for it (cf. C h a p t e r 2 ) . According to A r n o n ' s definition neither v a n a d i u m n o r chloride can be accepted as a n essential element. To over- come this difficulty it has been proposed t h a t the concept of a "func- tional n u t r i e n t " in contrast to a n "essential n u t r i e n t " be used to include a n y m i n e r a l n u t r i e n t t h a t m a y function in some precise w a y in p l a n t metabolism irrespective of w h e t h e r or not its action is completely specific or indispensable. T h i s would also avoid problems t h a t arise w h e n a n element is required only w h e n certain substrates a r e present;
thus in Scenedesmus obliquus, the m o l y b d e n u m r e q u i r e m e n t is
abolished w h e n a m m o n i a or u r e a is substituted for n i t r a t e b u t t h e m i c r o n u t r i e n t is essential w h e n t h e alga is utilizing nitrate ( 1 1 , 119).
C . P U R E C U L T U R E M E T H O D S
As early as 1869 Raulin showed t h a t small a m o u n t s of iron a n d zinc w e r e required for growth of t h e mold Aspergillus niger ( 2 4 2 ) . T h i s w a s a r e m a r k a b l e achievement since t h e results w e r e obtained before t h e advent of p u r e culture methods w h i c h involve t h e rigorous removal of trace metals from t h e culture solutions. A t t h a t t i m e Raulin's results w e r e disputed b y others w h o assumed w r o n g l y t h a t the effects of trace metals on t h e mold w e r e akin to those of toxic substances. T h u s t h e old Arndt-Schultze hypothesis of chemical stimulation, first used to explain t h e effects of h e a v y metals on a b n o r m a l growth of a n i m a l tissues, w a s subsequently applied b y Pfeffer (229) to account for t h e effects of trace metals on p l a n t cells. T h i s controversy w a s finally resolved in favor of Raulin b y t h e careful w o r k of subsequent workers. T h u s Bertrand a n d Javillier (29) a n d later B e r t r a n d (26) showed t h a t m a n g a n e s e a n d zinc w e r e indispensable for n o r m a l growth i n Aspergillus. Steinberg, a pioneer in p u r e culture methods, demonstrated a 5 0 0 0 % increase in yields of t h e same fungus b y r e t u r n i n g iron a n d zinc to media w h i c h h a d previously been treated w i t h calcium carbonate to adsorb the two metals ( 2 8 2 - 2 8 5 ) . Other workers h a v e a m p l y confirmed a n d extended Raulin's findings (193, 202, 2 0 3 , 2 1 3 ) .
Since t h e preparation of culture solutions free from trace metals is a n i m p o r t a n t feature of a study of r e q u i r e m e n t s of microorganisms, a brief account will n o w be given of some of t h e methods used. H e w i t t
(108) (cf. Chapter 2) h a s discussed t h e methods used for growing higher plants i n cultures freed from trace metals, b u t t h e methods to be discussed i n t h e following section a p p l y p a r t i c u l a r l y to w o r k w i t h microorganisms.
1. Water Supply
Distilled w a t e r prepared from a tinned-copper still is further distilled twice from a Quickfit P y r e x glass electric distillation a p p a r a t u s w h i c h is fitted w i t h double-splash heads t h a t a r e heated w i t h a tape coil to avoid creep of t h e w a t e r condensate into t h e receiver flask ( 1 1 7 ) . D a t a relating to the removal of m e t a l at each stage of t h e distillation a r e given in T a b l e I.
W a t e r from a t i n n e d copper still is unsuitable for the demonstration of trace m e t a l deficiencies. T h e m e t a l contents of t h e w a t e r a r e m a r k - edly reduced b y distillation either once or twice from P y r e x glass stills, b u t t h e r e is no further reduction after a t h i r d distillation. T h e efficiency
3 . I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 3 6 7
Manga- Molyb- Method of distillation Copper Zinc nese Iron denum Tinned-copper still 0 . 5 0.10 0.05 0.1 0.1 Water from copper still distilled once
from Pyrex glass 0.05 0.006 0.01 0.005 0.0001 Water from copper still distilled twice
from Pyrex glass 0.025 0.002 0.005 0.001 0.00005 Water from copper still distilled thrice
from Pyrex glass 0.020 0.002 0.005 0.001 0.00005
β Values in micrograms per 50 ml.
* Reproduced from Nicholas (203).
these can adversely affect some of the slime molds, actinomycetes, a n d protozoa.
2. Culture Containers
C u l t u r e vessels m a d e of h a r d glass a r e u s u a l l y used, b u t w i t h t h e advent of toughened p o l y t h e n e (after n e u t r o n irradiation) a n d poly- v i n y l chloride t u b i n g a n d m o r e r e c e n t l y polypropylene, a n i n e r t plastic m a t e r i a l t h a t can be s t e a m sterilized a n d r e a d i l y cleaned, t h e r e is a t e n d e n c y to use these materials. A w o r d of w a r n i n g should be inter- posed h e r e since, d u r i n g t h e process of t o u g h e n i n g polythene, certain trace metals including copper, c h r o m i u m , a n d m o l y b d e n u m a r e used as catalysts. Glassware should be t h o r o u g h l y cleaned b y w a s h i n g w i t h w a r m 6 Ν nitric acid to r e m o v e surface contamination a n d t h e n several times w i t h distilled w a t e r a n d finally w i t h glass-distilled or deionized water. Glassware can be checked for t h e presence or absence of metals b y rinsing w i t h 0 . 0 0 1 % solution of diphenylthiocarbazone (dithizone) in carbon tetrachloride at p H 6.5. T h e presence of m e t a l dithizonates is shown b y a color change from the green of dithizone to red.
of t h e glass still can be checked b y p u t t i n g carrier-free radioactive cobalt-58 i n t h e flask a n d checking t h e radioactivity i n t h e receiver flask. Good grade deionized w a t e r checked w i t h a conductivity cell m a y be used instead of glass-distilled water, b u t w a t e r t h u s treated m a y con- tain a m i n e s a n d other materials, especially w h e n t h e resin m a t e r i a l has aged. T h e s e substances chelate w i t h trace metals a n d could inter- fere w i t h critical studies of the m i n e r a l m i c r o n u t r i e n t r e q u i r e m e n t s of some microorganisms. P y r o g e n s a r e also present in deionized w a t e r , a n d
T A B L E I
TRACE-METAL CONTENT" OF WATER DISTILLED BY VARIOUS METHODS6
3. Preparation of Culture Solutions Free from Trace Metals Since macrosalts of t h e culture solutions contain sufficient of t h e micronutrients as c o n t a m i n a n t s to support o p t i m u m g r o w t h of most microorganisms, it is necessary to remove t h e m from t h e basal culture solution in order to show t h a t t h e y a r e r e q u i r e d for growth. T h e m e t h - ods used should h a v e little or no effect on other constituents of the m e d i u m . T h e techniques so far developed a r e i n t h e m a i n applicable to culture solutions w h i c h contain inorganic salts w i t h either a sugar or a n organic acid as a carbon source (193, 202, 2 0 3 , 2 1 3 ) . T h e r e m o v a l of trace metals from complex m e d i a is m o r e difficult since constituents such as peptone tend to hold t h e metals even against t h e stripping action of chelating agents. Organic solvents w h i c h m a y be used to remove the m e t a l chelates also take out essential g r o w t h factors. A variety of methods used to remove trace metals from culture solutions have been reviewed elsewhere (193, 202, 2 0 3 , 2 1 3 , 2 3 4 ) . Some of t h e methods n o w in u s e are discussed u n d e r the headings a-e below.
a. Biological depletion. R a u l i n was able to reduce t h e iron a n d zinc contents of a culture solution b y growing i n it several generations of Aspergillus niger ( 2 4 2 ) . Molisch ( 1 9 0 ) , Molliard (189, a n d Ber- t r a n d a n d Javillier (29) using similar techniques confirmed Raulin's findings and, in addition, t h e y showed m a n g a n e s e to be a n addi- tional r e q u i r e m e n t for the mold. M a c L e o d a n d Snell (163) used this m e t h o d to r e m o v e m a n g a n e s e from cultures of lactic acid bacteria. T h i s m e t h o d is, however, unsatisfactory since several other constituents of the media a r e also depleted d u r i n g g r o w t h a n d these m a y become r a t e limiting for t h e subsequent growth of t h e test organism.
b. Inorganic reagents. Recrystallization of macrosalts is not satis- factory since trace metals are seldom removed completely b y this proc- ess (202, 2 0 3 , 2 7 3 ) . Boron is a n exception since it is effectively re- moved from macrosalts b y recrystallizing t h e m several times from m e t h y l alcohol since m e t h y l borate is readily soluble.
Coprecipitation methods a r e often used to remove trace metals from a solution of t h e macrosalts a n d sugar. T h u s B e r t r a n d a n d Javillier used m a g n e s i u m a m m o n i u m phosphate to adsorb m a n g a n e s e ( 2 9 ) , Bortels used active carbon ( 3 3 ) , Steinberg introduced t h e calcium car- bonate adsorption m e t h o d (280, 2 8 2 ) , a n d a combination of the last two methods was used b y Roberg ( 2 4 7 ) . A n u m b e r of investigators h a v e employed t h e copper sulfide coprecipitation m e t h o d to r e m o v e m o l y b d e n u m a n d copper from macrosalts (202, 2 0 3 , 2 1 3 , 2 8 9 ) .
c. Organic reagents. N u m e r o u s organic reagents h a v e been used -to form m e t a l complexes w h i c h a r e extracted into nonpolar solvents such
3 . I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 369
as carbon tetrachloride, chloroform, a n d e t h y l acetate. T h e reagents include dithizone, 8-hydroxyquinoline, o-phenanthroline, α,α-dipyridil a n d h y d r o x y o r g a n i c acids, a n d ethylenediaminetetraacetic acid or versene. Copper, iron, zinc, a n d m o l y b d e n u m can be removed b y shak- ing a solution of m a c r o n u t r i e n t s including a simple sugar ( p H 5.5) w i t h 5% w / v 8-hydroxyquinoline, i n redistilled chloroform (202, 203, 205, 3 1 8 ) . T h i s m e t h o d is also effective for p r e p a r i n g culture solutions free from zinc, iron, cobalt, a n d gallium. A s u m m a r y of these methods is given i n T a b l e II.
d. Ion-exchange resins. N u m e r o u s resins h a v e been used to remove metals from nonionic constituents of culture solutions, e.g., sugars.
Most cation exchange resins contain m i c r o g r a m amounts of iron, cop- per, a n d zinc, a n d these a r e removed b y percolating a 5% w / v solution of sodium chloride or m a g n e s i u m sulfate (freed of metals before use b y t h e quinoline procedure) t h r o u g h t h e columns u n t i l no m o r e trace metals a r e detected b y t h e dithizone test. T h e efficacy of ion-exchange methods in removing trace metals is shown in T a b l e III.
Chemical methods of purification a r e u s u a l l y preferable since t h e y are m o r e effective a n d specific t h a n t h e ion-exchange procedures.
Macrosalts a r e also exchanged on resin columns so t h a t culture solu- tions treated in this w a y m a y become deficient in a macronutrient. Ion- exchange methods a r e satisfactory only for treating t h e nonionic com- ponents of the media, e.g., sugars.
e. Micronutrients. O n l y m i c r o g r a m a m o u n t s of trace metals are added to culture solutions so t h a t A n a l a r , or C P . grade materials are usually satisfactory. Spectrographically checked trace metals a r e n o w readily available (Johnson & M a t t h e y , London, E n g l a n d ) for culture work. Dilute standards of t h e trace metals a r e prepared each week in graduated flasks ( h a r d glass) so t h a t risk of adsorption of metals onto glass from dilute solutions is minimized. F e r r i c chloride dissolved in 6 Ν hydrochloric acid is purified b y extracting it into isopropyl ether, a procedure t h a t leaves other metals in t h e aqueous phase (202, 203, 205).
4. Preparation of Inocula
Several serial transfers of bacteria are necessary before deficiency effects of trace metals a r e produced. T h u s in Escherichia coli, Pseudo- monas aeruginosa, Clostridium pasteurianum a n d in Azotobacter species deficiencies of copper, m o l y b d e n u m , a n d m a n g a n e s e can be obtained only b y subculturing the organisms several times in culture solutions from w h i c h t h e appropriate trace metal has been removed. T o produce a copper or a m a n g a n e s e deficiency in these bacteria it is necessary to
T A B L E I I
REMOVAL OF TRACE METALS FROM A SOLUTION OF INORGANIC MACRONUTRIENTS* AND DEXTROSE6
Trace metal
removed Method used for its removal
Residual metal after purification (μ<7/50 ml culture
solution) Copper and The solution was adjusted with 6 Ν HCl to pH 2.0, Cu 0.05
molybdenum and 5 ml 20% w / v copper sulfate solution was Mo 0.00005 added. H2S from Kipp's apparatus was passed
through for 15 min (H2S is passed through a saturated barium hydroxide solution before en- try into media to remove polysulfides). The solution was allowed to stand for 15 min then filtered through a No. 50 Whatman filter paper into a clean 2-liter Erlenmeyer flask. H2S was eliminated by boiling and aerating the solution.
A sintered glass funnel (No. 4) and Quickfit Büchner flask are convenient for filtering.
Zinc, iron The solution was adjusted to pH 5.5 with 5 Ν Fe 0.01 cobalt NaOH in a 2-liter Pyrex separating funnel. Zn 0.01 30 ml 5% w / v 8-hydroxyquinoline in chloro- Co 0.001 form was added and shaken for 1 min. The
chloroform phase was discarded. This was repeated twice more with similar amounts of quinoline. Three lots of 30 ml redistilled chloro- form were added to remove excess quinoline;
each time it was shaken for 1 min. Three lots of 30 ml redistilled diethyl ether were then added to remove excess chloroform. The ether was removed by heating the solution in a 2-liter flask on an electric hotplate at 80°C, it was aerated continuously and the flask periodically shaken.
Manganese Ten grams of C a C 03, 10 ml 20% w / v K2H P 04, Mn 0.005 10 ml 10% w / v calcium chloride were added
and then autoclaved for % hr at 15 pounds per square inch. This was cooled and filtered through a No. 42 Whatman filter paper. The pH was adjusted to 7.5.
β Macronutrients and dextrose, sufficient for 5 liter of media, dissolved in 400 ml of deionized water.
6 Reproduced from Nicholas (213).
3 . I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 3 7 1
Manga- Molyb- Resin Copper Zinc Iron nese denum
Amberlite IR-100 0 . 6 1 2 0.05 0.05
IR-100 + anion exchange IR-4B 0.05 0.5 0.05 5 0.0005
Zeocarb 215 0.05 1 2 0.01 0.05
Zeocarb 215 + anion exchange IR-4B 0.05 0.5 0.05 5 0.005
Amberlite IRC-50 0 . 8 5 3 1 0.002
IRC-50 -f* anion exchange IR-4B 1 4 2 10 0.005
Chemical method 0.05 0.1 0.01 0.01 0.00005
β Values in micrograms per 50 ml.
6 From Nicholas (203).
i m p r o v e m e n t and, i n fact, t h e drastic procedure endangers the viability of t h e spores.
5. Use of Microorganisms for Bioassay of Mineral Nutrients T h e basis of t h e m e t h o d is t h a t t h e element to be determined is re- quired specifically b y a microorganism for growth. I n its absence the yield or production of a m e a s u r a b l e metabolite is m a r k e d l y depressed.
A n increase i n a n essential n u t r i e n t from a deficiency to a sufficiency a m o u n t , w h e n all others a r e present in a d e q u a t e supply, results in a specific, a n d a quantitative increase in growth. I n this w a y a standard g r o w t h series can b e p r e p a r e d for a n y of t h e essential n u t r i e n t s . F o r bioassay a k n o w n a m o u n t of t h e test m a t e r i a l is added to a culture solution t h a t contains all t h e essential n u t r i e n t s other t h a n the one to b e tested. U n d e r these conditions t h e g r o w t h depends on the a m o u n t of the test element t h a t t h e organism derives from the material w h i c h grow t h e organisms t h r o u g h a series of about 1 0 cultures deficient i n t h e metal. I n this w a y the i n o c u l u m is depleted of the particular trace metal. T h e cells a r e washed several times w i t h t h e metal-deficient media before being used as a n inoculum. I t m a y also be necessary to collect spores from fungi w h i c h h a v e been g r o w n in c u l t u r e solutions deficient i n trace metals, b u t in Aspergillus niger a n d in Neurospora crassa t h e r e is little evidence of a substantial carry-over of trace metals in t h e spores. Some investigators h a v e leached spores w i t h dilute alkali to r e m o v e metals. I n t h e author's experience this seldom leads to a n
T A B L E I I I
TRACE METAL CONTENT OF SOLUTION OF INORGANIC MACRONUTRIENTS AND DEXTROSE AFTER PASSAGE THROUGH ION-EXCHANGE RESINS COMPARED
WITH THAT AFTER CHEMICAL TREATMENT0 6
is added. T h e growth or some related p r o p e r t y w h i c h is readily meas- u r e d a n d w h i c h is dependent u p o n it, is referred to a standard series for the element.
M a n y bacteria h a v e been used to bioassay n u t r i e n t s ; these include Streptococcus faecalis, Leuconostoc mesenteroides for potassium ( r a n g e 5 - 3 0 p p m ) , a n d m a g n e s i u m ( r a n g e 0.1-0.5 p p m ) , a n d Lactobacillus plantarum arabinosus (L. arabinosus) for m a n g a n e s e ( r a n g e 0.1-0.4 p p m ) ( 2 7 3 ) .
Several fungi h a v e been investigated in t h e determination of m i n e r a l n u t r i e n t s w h i c h a r e available in soils to crop plants. T h u s the genus Aspergillus was used b y Butkewitsch ( 4 3 , 44) a n d Kosceleckii (138) to d e t e r m i n e phosphate i n Russian soils a n d since t h e n Aspergillus niger h a s been found suitable for d e t e r m i n i n g available potassium, phos- phorus, m a g n e s i u m , copper, zinc, m a n g a n e s e , a n d m o l y b d e n u m in soils, in p l a n t extracts, a n d in e n z y m e s (69, 106, 193, 202, 203, 2 1 3 ) . T h e sensitivity of t h e m e t h o d for d e t e r m i n i n g m o l y b d e n u m is illustrated in Fig. 1.
As little as 1 X 10~4 μg m o l y b d e n u m m a y be determined b y this assay procedure. Other fungi w h i c h a r e used for the bioassay of n u t r i - ents include Cunninghamella species ( 1 7 9 ) , Rhizopus species ( 2 6 0 ) , Neurospora crassa ( 2 7 3 ) , a n d Pénicillium glaucum ( 2 0 3 ) .
III. Requirement for Nitrogen and Its Compounds
T h e utilization of nitrogen a n d its compounds ranges from t h a t b y microorganisms t h a t fix atmospheric nitrogen to utilization b y those t h a t r e q u i r e a m i n o acids or even peptides for their growth.
A. B I O L O G I C A L F I X A T I O N O F N I T R O G E N
Free-living soil bacteria of t h e genus Azotobacter a n d some of the Clostridia a n d pseudomonads a r e able to utilize atmospheric nitrogen for growth (323, 3 2 4 ) . Root nodule bacteria associated w i t h legumes a n d nonlegumes also fix atmospheric nitrogen.* T h e fixation process is also widespread in blue-green algae; according to Fogg a n d Wolfe (86) a n d W a t a n a k e (319) this occurs i n 21 species belonging to 8 genera, as shown i n T a b l e IV. T h e y are p r i m a r i l y confined to t h e M y x o p h y c e a e . M e m b e r s of t h e Chroococcales a n d Oscillatoriaceae h a v e not y e t been shown to utilize atmospheric nitrogen.
Blue-green algae w e r e first shown to fix nitrogen in 1889 w h e n F r a n k (88) found t h a t gains in combined nitrogen in soil cultures incubated in
* The problem of biological fixation of nitrogen, especially in relation to the sym- biotic nodule fixation in legumes and nonlegumes is the subject of Chapter 5 in this volume.
3. I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 373
FIG. 1. The growth of Aspergillus niger as a measure of the content of molybdenum (Mo) in a series of standard solutions. Growth of Aspergillus niger after 6 days at 25° in culture solutions containing the following amounts of Mo in micrograms
Χ Ι Ο- 4 per 50 ml of culture solution: a, 0; b, 5; c, 10; d, 50; e, 100; f, 500.
T A B L E I V
DISTRIBUTION OF THE CAPACITY TO ASSIMILATE ELEMENTARY NITROGEN I N THE MYXOPHYCEAE*
Order and family Species able to fix nitrogen Species not able to fix nitrogen Chroococcales
Chamaesiphonales Pleurocapsales Nostocales
Oscillatoriaceae
Nostocaceae
Rivulariaceae Scytonemataceae Stigonematales
Anabaena ambigua A. cylindrica A. fertilissima A. gelatinosa A. humicola A. naviculoides A. variabilis Anabaena spp.
Anabaenopsis sp.
Aulosira fertilissima
Cylindrospermum gorakhpurense C. licheniforme
C. mains
Nostoc paludosum N. punctiforme N. muscorum Nostoc spp.
Calothrix brevissima C. parietina Tolypothrix tenuis Mastigocladus laminosus
Chroococcus turgidus Chroococcus spp.
Coccochloris peniocystis (Gloeothece linearis) Diplocystis (Microcystis)
aeruginosa
Gloeocapsa membranina G. dimidiata
Synechococcus cedrorum
Lyngbya aestuarii Lyngbya spp.
Oscillatoria spp.
Phormidium foveolarum P. tenue
P. lividum Phormidium spp.
Anabaena variabilis Anabaena spp.
Aphanizomenon flos-aquae
Plectonema notatum P. nostocorum
a Reproduced from Fogg and Wolfe (86).
3. I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 375
t h e light w e r e associated w i t h t h e development of these organisms.
Subsequently Beijerinck (19) found t h a t Anabaena catenula fixed nitrogen in soil cultures w h e n incubated in t h e light. D r ewes (71) provided t h e first unequivocal evidence t h a t bacteria-free cultures of Nostoc punctiforme a n d Anabaena variabilis fixed nitrogen, a n d this has been a m p l y confirmed b y others (9, 85, 86, 8 8 ) . M o r e recent tech- niques include the use of nitrogen-15 to demonstrate fixation in Nostoc muscorum (38) a n d Calothrix parietina ( 3 8 ) .
T h e r e a r e not sufficient critical data to show w h e t h e r other algal groups a n d flagellates fix nitrogen since relatively few species h a v e been examined in detail. It is claimed t h a t some blue-green algae h a v e sym- biotic relations w i t h h i g h e r plants. T h u s Nostoc isolated from t h e root p a r e n c h y m a of Gunnera (327) a n d from t h e lichen Collema (105) fix nitrogen a n d release soluble compounds into t h e culture m e d i u m . T h e extracellular secretions of Anabaena cylindrica contain mostly poly- peptides a n d o n l y small a m o u n t s of free a m i n o acids ( 8 5 ) . T h e peptides do not a p p e a r to be utilized b y either Anabaena or Chlorella. T h e extra- cellular substances produced b y Nostoc i n lichens are readily assimi- lated b y t h e fungus w i t h w h i c h it is in association. Bond a n d Scott (32) using nitrogen-15 found t h a t two lichens a n d a liverwort containing Nostoc incorporated t h e stable isotope. N u m e r o u s authors h a v e found t h a t blue-green algae i n rice paddies fix appreciable a m o u n t s of nitro- gen d u r i n g t h e growing season (66, 2 7 1 , 3 1 9 ) .
Claims h a v e been p u t forward t h a t fungi fix nitrogen, b u t the evi- dence thus far is not convincing. E v e n i n Phoma species, often found in t h e mycorhizal flora, t h e r e is n o incorporation of t h e stable isotope of nitrogen ( 3 2 3 ) . I n Alnus glutinosa, although t h e r e is a n incorporation of atmospheric nitrogen, t h e causal organism has been variously de- scribed as a bacterium, a n actinomycete or a filamentous fungus (240) a n d m o r e r e c e n t l y as a m e m b e r of t h e Plasmodiophorales ( 1 0 4 ) .
T h e most recent development i n nitrogen fixation, w h i c h is dealt w i t h in m o r e detail in C h a p t e r 5, h a s been t h e preparation of cell-free extracts from Clostridium pasteurianum (48, 49, 2 5 5 ) , Azotobacter vinelandii (216, 217, 2 1 9 ) , blue-green algae a n d Rhodospirillum rub- rum ( 2 5 5 ) , a n d from Chromatium (12) t h a t can fix atmospheric nitro- gen. C a r n a h a n et al. (48, 49) h a v e shown t h a t the addition of sodium p y r u v a t e enhances fixation in extracts of C. pasteurianum. Nicholas a n d Fisher (216, 217) showed t h a t cell-free extracts of Azotobacter vinelandii fixed nitrogen provided t h e y w e r e disrupted in t h e m e d i u m in w h i c h t h e y w e r e grown. T h e s e results h a v e been confirmed b y the use of radioactive nitrogen-13 since cell-free extracts of Azotobacter incorporated t h e tracer after exposure for a period of 10 m i n u t e s ( 2 1 9 ) .
B . A M M O N I A
A m m o n i a is utilized b y a wide r a n g e of microorganisms, m u c h m o r e readily in fact t h a n b y higher plants. Studies w i t h bacteria h a v e shown t h a t a m m o n i a is readily assimilated provided t h e p H of t h e culture m e d i u m does not become too acid d u r i n g growth. Studies w i t h the fungus Scopulariopsis brevicaulis h a v e shown t h a t both oxygen a n d a carbon source are required before a m m o n i a is assimilated ( 5 8 ) . Its
1 1 1 ' 1
! 1
2
I Ι 1 T 1
6 pH
FIG. 2. The rate of uptake of ammonia and nitrate by Scopulariopsis brevicaulis as a function of pH. Curve Î, ammonia (two buffers) ; curve 2, nitrate. From Morton and MacMillan (191) by permission of the Oxford University Press.
utilization increased w i t h p H , a n d unlike n i t r a t e absorption it has no definite p H o p t i m u m (cf. Fig. 2) ( 1 9 1 ) .
It is claimed t h a t a m m o n i a freely enters a n d leaves the cell b y pas- sive diffusion of t h e undissociated a m m o n i a or a m m o n i u m molecule;
thus respiration h a s little influence on t h e process. T h e p r i m a r y product of a m m o n i a utilization i n most microorganisms is glutamic acid from a-ketoglutaric acid a n d t h e n other a m i n o acids are formed b y trans- a m i n a t i o n processes. Via other keto acids a m m o n i a m a y also be incor- porated into a l a n i n e a n d aspartic acid a n d into t h e amides asparagine a n d glutamine. T h e breakdown of glucose provides the carbon skeleton for these compounds. Claims in t h e literature t h a t fungi will not grow in media containing a m m o n i u m salts m u s t be treated w i t h reserve unless t h e p H effect has been excluded experimentally. T h e r e is
3. I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 377
good evidence, however, t h a t some of t h e lower phycomycetes, e.g., Blastocladiella emersonii ( 1 7 ) , Sapromyces eiongatus ( 9 5 ) , a n d Lepto- mitus lacteus (256) are u n a b l e to utilize a m m o n i u m salts as t h e sole ni- trogen source. A p a r t from these few exceptions a n d those bacteria a n d fungi t h a t r e q u i r e a m i n o acids or even peptides for their growth, a m m o - nia is u s u a l l y a suitable source of nitrogen for microorganisms provided the p H of the m e d i u m is n e a r neutrality. A m m o n i a , at high concentra- tions, is toxic. Resulting spore aberrations a r e alleviated in some fungi b y phosphate.
Recent reviews b y Syrett (293) a n d Fogg a n d Wolfe (86) give a n excellent account of the utilization of a m m o n i a a n d of n i t r a t e b y green
FIG. 3. The effect of glucose on the oxygen uptake and ammonia assimilation by Chlorella vulgaris at 25° and pH 6.1. a. Normal cells (4.75 mg dry weight per milli- liter), b. Nitrogen-starved cells (7.65 mg dry weight per milliliter). From Syrett (293).
a n d blue-green algae. I n a n adequately buffered m e d i u m autotrophic growth of Chlorella is the same w h e t h e r n i t r a t e or a m m o n i a is the nitrogen source ( 2 9 3 ) , b u t i n these experiments g r o w t h m a y h a v e been limited b y t h e r a t e of carbon assimilation. Most workers agree that, w h e n both a m m o n i u m a n d n i t r a t e ions a r e supplied together, Chlorella assimilates m u c h m o r e a m m o n i a t h a n n i t r a t e ( 2 9 3 ) .
A m m o n i a assimilation i n nitrogen-starved cells has been studied.
Syrett (293) showed t h a t cells of Chlorella vulgaris w h i c h w e r e g r o w n i n a nitrogen-free m e d i u m in t h e light for 16 hours assimilated added a m m o n i a m o r e r e a d i l y i n darkness t h a n did n o r m a l cells. T h e r a t e of assimilation of a m m o n i a b y t h e n o r m a l cells w a s increased b y adding glucose; t h a t of nitrogen-starved cells w a s n o t (Fig. 3 ) .
P r e s u m a b l y t h e nitrogen-starved cells contained sufficient carbon reserve to satisfy t h e e n z y m e systems involved i n assimilation. W h e n large a m o u n t s of a m m o n i a a r e added, assimilation continues u n t i l the carbon supply is exhausted a n d addition of m o r e glucose results i n fur- t h e r utilization of a m m o n i a .
T h e interrelation between carbon a n d nitrogen i n the n u t r i t i o n of algae m a y be s u m m a r i z e d as follows: (a) W h e n the external supply
è θ! ι I ι I »
0 100 200 Minutes
FIG. 4 . The effect of ammonia on the respiration of nitrogen-starved cells of Chlorella vulgaris at 25° and pH 6.2. Each manometer contained 1 4 . 7 mg, dry- weight, of cells. From Syrett (293).
of nitrogen or carbon is n o t r a t e limiting t h e absorption of either or both should b e conditioned b y other factors t h a t d e t e r m i n e g r o w t h a n d it is h e r e t h a t t h e effect of light or d a r k conditions m i g h t apply, (b) W h e n growth is limited b y either nitrogen or carbon supply, t h e response to either can be interpreted as interactions between t h e m .
T h e effect of adding a m m o n i u m sulfate to cells of Chlorella pyren- oidosa respiring glucose i n t h e d a r k w a s followed b y a decrease in t h e r a t e of carbon dioxide production; t h e r a t e of oxygen consumption r e -
3. I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 379
% % % % % 2,4-DNP Con- Con- Con- Con- Con-
(M) Qo2 trol Qco2 trol Qo2 trol Qco2 trol QNH8 trol 0 2.9 100 3 . 3 100 16.5 100 13.8 100 21.8 100 1 . 8 X 1 0 "4 7.2 246 7.5 228 14.7 89 13.4 97 15.2 70 2.2 X 10~4 8.3 285 8.7 264 14.5 88 13.3 96 11.9 54
β Cells were suspended in 0.0025 M MgSO* and 0.1 M phosphate. In this experi- ment and those following, respiration was measured by Warburg's "direct" method and a correction for carbon dioxide retention was applied. Each manometer contained 16.5 mg dry weight of cells. Initially, 1.5 /mioles (NHOSSO* was added and the QNHS was calculated from ammonia determinations at 15 and 30 minutes.
b Reproduced from Syrett (293).
Effects similar to those reported for t h e green algae h a v e also been found to occur in the blue-green types ( 8 6 ) . Concentrations of am- m o n i u m as low as 5 X 10"5 M suppress the formation of heterocysts b y Anabaena cylindrica.
C. A S S I M I L A T I O N A N D D I S S I M I L A T I O N O F N I T R A T E S
Nitrates m a y be utilized as t h e sole source of nitrogen b y a r a n g e of bacteria a n d fungi, although in some instances t h e r e m a y be a lag m a i n e d constant, a n d t h e respiratory quotient (R.Q.) decreased from 1.4 to 1.18 ( 2 9 3 ) . T h e change i n R.Q. w a s v e r y sharp w h e n nitrogen- deficient cells w e r e used. T h e h i g h R.Q. before the a m m o n i a w a s added showed t h a t glucose w a s being assimilated to m o r e reduced cellular materials, possibly fat. Syrett claims t h a t t h e decrease i n R.Q. after adding a m m o n i a indicates t h a t less reduced products, e.g., proteins, w e r e being formed. T h e effect of a m m o n i a on respiration of nitrogen- starved cells of Chlorella vulgaris is shown i n Fig. 4.
T h e addition of a m m o n i a to nitrogen-starved cells resulted in a respiration r a t e as high as t h a t of n o r m a l cells respiring glucose. T h e addition of a m m o n i a to these cells allowed a rapid metabolism of car- bon reserves. Syrett (293) showed t h a t 2,4-dinitrophenol reduced t h e assimilation of a m m o n i a b y nitrogen-starved cells of t h e alga Chlorella vulgaris ( T a b l e V ) . T h i s effect was interpreted as a n uncoupling of phosphorylation from respiration since the latter was stimulated.
T A B L E V
T H E EFFECT OF 2,4-DINITROPHENOL ( D N P ) ON THE RESPIRATION AND AMMONIA ASSIMILATION OF NITROGEN-STARVED CELLS OF
Chlorella vulgaris AT 25° AND pH 6.1°'6
Before ammonia addition After ammonia addition
period in growth p e n d i n g t h e induction of the nitrate-reducing enzymes.
Some bacteria r e q u i r e complex nitrogenous sources a n d will n o t grow on nitrate, e.g., Actinomycetes a n d some fungi, notably m e m b e r s of the Basidiomycetes ( 5 8 ) , Saprolegniaceae ( 2 4 3 ) , a n d Blastocladiales ( 4 7 ) , a r e also u n a b l e to grow on nitrate. I t is also found t h a t even w i t h i n a genus individual species v a r y m a r k e d l y in their use of nitrate. T h e capac- i t y to utilize n i t r a t e as a nitrogen source can be lost b y m u t a t i o n a n d thus m a y h a v e ecological significance since those t h a t c a r r y t h e m u t a - tion m a y survive only if t h e r e are other suitable nitrogenous compounds
Time, days
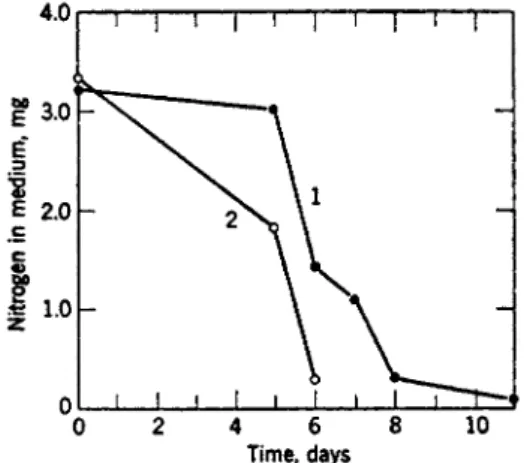
FIG. 5. The utilization of ammonium and nitrate by Scopulariopsis brevicaulis in a medium containing glucose, succinate, and ammonium nitrate. Curve / , nitrate concentration in the medium; curve 2, ammonium concentration in the medium.
From Morton and Macmillan (191).
in the habitat. It is of interest t h a t n i t r a t e cannot be utilized b y spores of Streptomyces griseus b u t a p r e g r o w n m y c e l i u m uses n i t r a t e readily
( 5 6 ) .
W h e n a m m o n i u m n i t r a t e is supplied it is u s u a l for t h e a m m o n i u m radical to be used first since t h e r e is a n initial drop in p H . I n Scopu- lariopsis brevicaulis a n d other fungi, n i t r a t e utilization is not p r o m i n e n t until t h e a m m o n i u m has been absorbed from t h e m e d i u m (Fig. 5)
( 1 9 1 ) .
This effect has also been observed i n Neurospora crassa. I n some cases a m m o n i u m salts inhibit the utilization of nitrate, b u t nitrite is not so affected. T h e effect of a m m o n i a is to depress t h e n i t r a t e reductase e n z y m e w h i c h reduces n i t r a t e to nitrite. One interesting p h e n o m e n o n is t h a t n i t r a t e reductase is induced in Neurospora g r o w n in media con-
3 . I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 3 8 1
taining a m m o n i u m n i t r a t e b u t w h e n a m m o n i u m salts a r e added aseptically to the fungus g r o w n i n a m e d i u m containing sodium n i t r a t e there is a n i m m e d i a t e reduction of t h e e n z y m e even w h e n t h e a m o u n t of a m m o n i a added is as small as Ι Ο- 8 M ( T a b l e V I ) .
M a n y microorganisms reduce n i t r a t e nitrogen to a m m o n i a w i t h sub- sequent formation of a m i n o acids a n d cell nitrogen. T h i s process is usually t e r m e d nitrate assimilation. U n d e r certain conditions some microorganisms u s e n i t r a t e or some of its reduction products as a t e r m i n a l hydrogen acceptor instead of oxygen; this process is k n o w n
T A B L E V I
T H E EFFECT OF ADDING A M M O N I U M CHLORIDE in Vivo το FUNGAL FELTS OF Neurospora crassaa GROWN WITH NITRATE AS SOLE SOURCE OF NITROGEN
ON THE NITRATE REDUCTASE ACTIVITY I N THE FELTS6
Felts harvested at times indicated after adding
ΙΟ"8 M (NH4C1) to the Nitrate reductase
medium (hours) (n^moles NO2/10 min/mg protein) 0
0.5 1 2 4
38.1 26.2 21.4 10.9 4.7
a Ammonium chloride (10~8M) was added to TV. crassa grown for 40 hours in the nitrate medium; the enzyme was determined in cell-free extracts of the felts after the further incubation times stated.
6 Walker and Nicholas (unpublished results).
as unitrate respiration" o r ctdissimilatory nitrate reduction." M a n y classifications h a v e been proposed for the various types of nitrate reduc- tion i n microorganisms. T h u s J e n s e n ( 1 2 4 ) suggested five categories according to t h e products of the reaction whereas Verhoeven ( 3 0 3 , 3 0 4 ) differentiated three types as follows: ( a ) n i t r a t e assimilation i n which n i t r a t e is reduced for subsequent formation of cell protein; ( b ) in- cidental dissimilation i n w h i c h n i t r a t e acts as a nonessential hydrogen acceptor; ( c ) t r u e dissimilatory n i t r a t e reduction i n w h i c h n i t r a t e acts u s u a l l y w h e n oxygen is limiting as t h e essential h y d r o g e n acceptor, w h i c h enables t h e organism to grow.
T h e J a p a n e s e school ( 7 2 , 7 3 , 2 5 3 , 2 9 8 ) , however, do n o t think it necessary to differentiate between Verhoeven's last two categories a n d h a v e proposed a classification of nitrate-reducing organisms based on the behavior of their cytochromes toward nitrate: ( a ) those i n which cytochrome participates i n n i t r a t e reduction, e.g., Escherichia coli and
Micrococcus denitrificans; (b) those i n w h i c h cytochromes do not participate i n n i t r a t e reduction, e.g., Neurospora crassa; (c) those organisms w h i c h reduce n i t r a t e b u t h a v e no cytochrome components, e.g., Clostridium perfringens.
It is t h e author's view t h a t subdivision into assimilation a n d dis- similation of nitrates is adequate to cover n i t r a t e utilization i n micro- organisms. Denitrification can be regarded as a special instance of n i t r a t e dissimilation i n w h i c h t h e oxides of nitrogen or nitrogen gas a r e usually produced (82, 8 4 ) .
T A B L E V I I
T H E OXIDATION-REDUCTION STATES OF SOME NITROGEN COMPOUNDS
Oxidation- reduction
state of Ν atom Formula Name
+ 7 N207
H N 04
Nitrogen peroxide Pernitric acid
+ 6 N 03
H2NO4
Nitrogen peroxide Pernitrous acid
+ 5 N206
H N 03
Nitrogen peroxide Nitric acid
4-4 N204
N 02
Nitrogen tetroxide Nitrogen dioxide
4-3 N2O3
HNO2
Nitrogen sesquioxide Nitrous acid
4-2 NO
H2NO2
Nitric oxide Hydronitrous acid
-hi
NOH N20 H2N202 N 02: N H2
N H ( O H )2
Nitroxyl Nitrous oxide Hyponitrous acid
Nitramide; imido nitric acid Dihydroxyammonia
0 N2
O H N H N H O H Nitrogen
Dihydroxylhydrazine
- 1 NH2OH Hydroxylamine
- 2 H2NNH2 Hydrazine
- 3 NH4OH Ammonium hydroxide
3 . I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 3 8 3
Nitrate Reduction
Since early intermediates a r e c o m m o n to t h e dissimilation a n d as- similation of nitrates t h e y will be considered together. T h e oxidation states of nitrogen compounds a r e given in T a b l e V I I .
G a y o n a n d Dupetit presented equations for the over-all process of denitrification b u t recognized nitrite as a n i n t e r m e d i a t e ( 9 2 ) . Beijerinck a n d M i n k m a n suggested t h a t nitrous oxide m i g h t be formed directly from either n i t r a t e or n i t r i t e a n d t h a t nitrogen was formed ex- clusively from nitrous oxide ( 2 1 ) . Others h a v e suggested t h a t nitrite, d i h y d r o x y a m m o n i a , h y d r o x y l a m i n e , a m m o n i a , a n d nitrous oxide m i g h t be intermediates ( 2 2 , 8 9 ) . K l u y v e r a n d D o n k e r presented a scheme of reduction (Eqs. 1 , 2 ) of n i t r a t e to nitrous oxide ( 1 3 4 ) .
2 H - + H20 ( 1 )
potassium nitrite /OK
N = = 0 +
potassium nitrate
2 NC „OK
+ +
2 H
2 H
N—OK
— - II + N--OK
potassium hyponitrite
2 H20 ( 2 )
T h e h y p o n i t r i t e was thought to decompose spontaneously to give nitrous oxide a n d nitrogen (Eq. 3 ) .
2 H Ν Ν III (3)
anhydride of hyponitrous acid
Since t h a t time m a n y p a t h w a y s h a v e been suggested on t h e assump- tion t h a t reduction proceeds via a n u m b e r of two-electron steps. T h e Delft school ( 1 3 5 , 1 3 6 , 3 0 3 , 3 0 4 ) has been v e r y active in this field a n d
N 03
t .
N 02-
f
NO nitroxyl (NOH) -
t
NH2OH
t +
NOH
OH I I/ O
w
I ΗOH I
N = 0 = 7
NH II
N90
• N .
(4)
h a v e suggested t h e scheme shown in sequence 4. T h e y suggest t h a t n i t r a t e is reduced to a compound of the nitroxyl t y p e ( N O H ) w h i c h in t h e assimilatory process is reduced further via h y d r o x y l a m i n e to am- monia. T h e y suggest t h a t d u r i n g denitrification nitroxyl ( N O H ) is converted b y complex molecular reorientation to nitrous oxide a n d t h e n to nitrogen gas as shown i n their scheme. T h e y consider t h a t nitrite can also be reduced to nitric oxide a n d t h a t this in t u r n is converted to N2 gas via nitrous oxide.
Quastel et al. (238) found t h a t Escherichia coli (Bacillus coli) pro- duced n i t r i t e w h e n g r o w n anaerobically; subsequently nitrite was identified as a product of nitrate reduction in bacteria, fungi, a n d higher plants. N i t r a t e reductase, t h e e n z y m e responsible for t h e reduction of n i t r a t e to nitrite, has been studied in detail in a n u m b e r of micro- organisms. N i t r a t e or nitrite is required for its induction in Neurospora crassa, Escherichia coli, a n d other microorganisms (204, 206, 2 3 1 ) . Farkas-Himsley a n d A r t m a n (78a) reported a constitutive n i t r a t e re- ductase in E. coli, b u t this is u n l i k e l y since t h e peptone used in the m e d i u m contained n i t r a t e w h i c h induced the e n z y m e .
T h e assimilatory n i t r a t e reductase from Neurospora a n d E. coli was shown to b e flavoprotein containing m o l y b d e n u m ; a n d m e c h a n i s m of e n z y m e action, w h i c h is discussed m o r e fully in Chapter 4, is shown in sequence 5.
DPNH -yHFADH2-y—Mo5 +( 2 β) γ * - NCÇ DPN — F A D - A- M o6 + — ^ NOj
It is of interest t h a t the e n z y m e is present in the fungus felts at the 4-day stage w h e n t h e y a r e growing in a n aerobic environment. W a l k e r a n d Nicholas (314) h a v e shown t h a t w h e n t h e felts w e r e submerged, n i t r a t e was dissimilated a n d t h e r e w a s a n additional iron r e q u i r e m e n t (Fig. 6 ) . W h e n the felts w e r e growing in a n aerobic environment, after 5 d a y s ' growth, only t h e assimilatory n i t r a t e reductase was present so t h a t there w a s no longer a r e q u i r e m e n t for iron. T h e dissimilatory e n z y m e is similar to the n i t r a t e reductase from Pseudomonas aeruginosa (79, 82, 8 4 ) ; the nitrate reductase functions d u r i n g denitrification as shown in sequence 6.
cytochrome —» 02
/ * oxidase
D P N H FAD -> cytochrome c (6) nitrate reductase
(Mo) -> N 03-
A cytochrome component is not required b y the assimilatory nitrate reductase system since this has a slower t u r n o v e r t h a n the m o r e active dissimilatory e n z y m e . Cytochrome c ( Ε0' + 260 millivolts p H 7)
3 . I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 3 8 5
I I I I L
10 20 30 4 0 μς iron/200ml
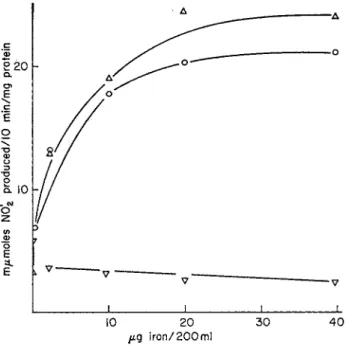
FIG. 6. Effect of the iron content of the culture medium on nitrate reductase activity in homogenates of Neurospora crassa harvested after 2, 3, and 5 days.
Neurospora was grown at 25 °C in still culture depleted of iron to which 2, 10, 20, or 40 μ% of iron per 200 ml was returned, v, Felts harvested after 5 days' growth
(314). From Walker and Nicholas (314).
cum, Clostridium welchii ( = C. perfringens), Micrococcus denitrificons, Proteus vulgaris ( 1 5 , 1 4 1 , 3 2 8 ) , a n d t h e algae Scenedesmus obliquus a n d Ankistrodesmus braunii ( 1 2 9 - 1 3 1 ) .
A p o r p h y r i n r e q u i r e m e n t for n i t r a t e reduction h a s been shown i n Haemophilus influenzae ( 9 7 ) a n d Staphylococcus aureus ( 1 4 1 ) . Cyto- chrome c is required for n i t r a t e reduction i n Pseudomonas aeruginosa a n d Micrococcus denitrificans w h e n t h e y a r e actively denitrifying
( 7 9 , 8 0 , 8 2 - 8 4 ) . T h e addition of n i t r a t e to whole cells resulted i n oxida- tion of t h e cytochromes.
is effective i n t h e dissimilatory system i n bridging t h e electrode potential g a p between flavin ( Ε0' approximately — 1 0 0 millivolts, p H 7 ) a n d n i t r a t e (E</ + 3 5 0 millivolts p H 7 ) t h u s ensuring a rapid flow of electrons from t h e substrate to nitrate, t h e t e r m i n a l acceptor. I t also facilitates a switch of t h e electrons to t h e alternative acceptor, oxygen, w h e n t h e bacteria a r e g r o w n i n a n aerobic environment.
A large n u m b e r of bacteria a n d algae use h y d r o g e n gas for n i t r a t e reduction: Aerobacter aerogenes, Azotobacter species, Bacterium formi-
I n all studies so far m a d e o n n i t r a t e reductases it is clear t h a t t h e e n z y m e is a flavoprotein containing m o l y b d e n u m of t h e t y p e described in Neurospora (204, 2 0 5 , 2 0 7 - 2 0 9 , 2 1 1 , 2 1 2 ) . T h e possible exception is t h a t of Photobacterium fischeri ( 2 5 0 ) . Recent w o r k i n this laboratory, however, has shown t h a t m o l y b d e n u m is required w h e n n i t r a t e is r e - duced b y this organism ( 2 2 0 a ) . Although cytochromes p l a y a n i m - p o r t a n t p a r t i n t h e over-all reduction sequence i n t h e dissimilatory r e - duction of nitrate, t h e y a r e u n l i k e l y to b e involved i n t h e t e r m i n a l step of electron transfer to n i t r a t e (79, 8 2 - 8 4 ) . T h e y probably act as carriers in t h e electron chain before t h e t e r m i n a l m o l y b d e n u m - c o n t a i n i n g n i t r a t e reductase.
T A B L E V I I I
T H E FORMATION OF AMMONIA AND NITRITE FROM NITRATE (INITIAL CONCENTRATION 0.02 M) BY Chlorelfo vulgaris SUSPENDED I N
0.067 M PHOSPHATE AT pH 6.0a
Mg N / 4 0 X 106 cells
In dark In light
Time Nitrite Ammonia Nitrite Ammonia (hr) Ν Ν Ν Ν
24 4 . 6 — 46 — 96 3 . 8 1.4 70 12.6 144 5 . 2 12.9 73 29.2
α From Syrett (293) after Mayer (175).
Some algae assimilate n i t r a t e i n t h e dark, b u t others do so only i n t h e light ( 8 6 , 2 9 3 ) , a n d this is in accord w i t h Burström's observations i n higher p l a n t s (40, 4 1 ) . W a r b u r g a n d Negelein found t h a t Chlorella pyrenoidosa reduced n i t r a t e to a m m o n i a u n d e r aerobic conditions b u t w h e n oxygen supply w a s decreased nitrite accumulated ( 3 1 6 ) . M a y e r (175) found t h a t C. vulgaris produced both a m m o n i a a n d nitrite from sodium n i t r a t e i n t h e light b u t i n darkness less a m m o n i a a n d nitrite w e r e formed ( T a b l e V I I I ) .
Kessler (130, 131) o n t h e other h a n d showed t h a t nitrite accumulated i n Ankistrodesmus braunii cultures w h e n g r o w n i n t h e dark w i t h nitrate. N i t r i t e did n o t accumulate i n t h e light. T h e discrepancy be- t w e e n t h e two investigations can be explained b y t h e difference i n experimental conditions. Kessler used t h i n suspensions so t h a t photo- synthesis w a s rapid w h e r e a s M a y e r used thick algal suspensions a n d photosynthesis did n o t compensate for respiration. N i t r a t e is utilized b y n e a r l y all t h e blue-green algae w h i c h h a v e been examined i n p u r e
3. I N O R G A N I C N U T R I E N T N U T R I T I O N O F M I C R O O R G A N I S M S 387
culture ( 8 6 ) . I n m a n y , however, t h e r e m a y be a lag period p e n d i n g t h e induction of t h e reductive e n z y m e s , e.g., in Anabaena cylindrica.
T h i s organism w a s found to continue fixing nitrogen after being m a i n - tained without combined nitrogen a n d t h e n subcultured into a m e d i u m containing n i t r a t e ( 8 6 ) . N i t r a t e is assimilated r a p i d l y b y Anabaena in t h e dark i n a n atmosphere of h y d r o g e n (to depress nitrogen fixation).
After about 3 - 5 hours, assimilation of n i t r a t e ceases b u t its absorption into t h e cells continues. D u r i n g assimilation t h e respiratory quotient rises from 0.9 to 1.0 to above 2. W h e r e a s i n Chlorella a similar rise in R.Q. d u r i n g n i t r a t e assimilation is caused b y a n increased output of carbon dioxide ( 8 6 ) , t h e rise i n Anabaena h a s frequently been found to be d u e to a decrease in oxygen consumption, t h e carbon dioxide out- p u t r e m a i n i n g t h e same as in t h e absence of nitrate. T h u s it appears t h a t algae can also use n i t r a t e as a n alternative h y d r o g e n acceptor to oxygen.
2. Nitrite
T h e i m m e d i a t e product of n i t r a t e reduction i n microorganisms has been identified as nitrite. A nitrite reductase e n z y m e has been charac- terized in a r a n g e of bacteria, fungi, a n d algae. I n t h e assimilatory sequence nitrite is reduced to a m m o n i a ( 2 0 0 ) , a n d w h e n dissimilating nitrate, nitric oxide, a n d nitrogen a r e products. Recently it w a s shown t h a t t h e i m m e d i a t e product of nitrite reduction in both dissimilation a n d assimilation is nitric oxide (80, 8 1 , 198, 3 1 5 ) . T h e e n z y m e from Pseudomonas aeruginosa (79) a n d Neurospora crassa (214) is a flavo- protein w h i c h requires copper a n d iron for its activity. Cells of Desul- fovibrio desulfuricans r e d u c e n i t r i t e to a m m o n i a w h e n either h y d r o g e n gas or p y r u v a t e is t h e h y d r o g e n donor, a n d ferrocytochrome c reduced nitrite n o n e n z y m a t i c a l l y ( 2 6 1 - 2 6 3 ) .
N i t r i t e serves as a nitrogen source for a n u m b e r of fungi, Fusarium niveum ( 3 2 9 ) , Coprinus species ( 9 0 ) , Phymatotrichum omnivorum
( 2 9 5 ) , Scopulariopsis brevicaulis ( 1 9 1 ) , a n d Rhizophyctis rosea ( 2 3 7 ) . Species of Aspergillus differ m a r k e d l y in their ability to use nitrite,
( 2 5 1 , 2 8 2 - 2 8 4 ) . M o s t bacteria a n d fungi fail to use n i t r i t e a n d it is often secreted into t h e m e d i u m , especially w h e n n i t r a t e is used as a n alternative h y d r o g e n acceptor to oxygen. G r o w t h in t h e presence of n i t r i t e is best i n a n alkaline m e d i u m , a result w h i c h indicates t h a t the nonionized acid is probably t h e toxic factor. N i t r i t e toxicity in Fusarium Uni results i n accumulation of p y r u v i c acid ( 2 2 2 ) , a n d it produces morphological variants in Aspergillus species (286, 2 8 7 ) . I n Neurospora crassa, nitrite secreted into m e d i a d u r i n g active dissimilation of n i t r a t e in submerged felts is reabsorbed later a n d is reduced to a m m o n i a w h e n
t h e felts break t h e surface of t h e culture fluid; oxygen is t h e n t h e alternative h y d r o g e n acceptor (314, 3 1 5 ) .
Kessler (130, 131) showed t h a t nitrite is formed from n i t r a t e in t h e alga Ankistrodesmus in darkness. T h e accumulation of nitrite is greater t h e lower t h e p H , p a r t l y because t h e r a t e of nitrite assimilation de- creases w i t h increasing acidity. N i t r i t e is assimilated b y t h e algae pro- vided t h e concentration is n o t too h i g h or t h e p H too low so t h a t it is likely to b e a n intermediate i n t h e assimilation of n i t r a t e . Nitrite is a suitable source of nitrogen for a n u m b e r of blue-green algae ( 8 6 ) . M a e r t e n s found it to be suitable for growth of Oscillatoria species, b u t n o t for Nostoc, Cylindrospermum, or Calothrix species ( 1 6 1 ) . A t concen- trations u p to 13.6 m g nitrogen p e r liter, nitrite is as effective as n i t r a t e for Microcystis aeruginosa. T h e effect of high concentrations of nitrite o n this alga w a s n o t recorded, b u t 27 m g n i t r a t e nitrogen per liter inhibits g r o w t h of Anabaena cylindrica ( 8 6 ) .
3. Nitric Oxide
G a y o n a n d D u p e t i t described t h e conversion of n i t r a t e to nitrite, nitric oxide, nitrous oxide, nitrogen a n d to a m m o n i a in a soil bacterium
( 9 2 ) . Since t h e n nitric oxide h a s been identified as a product of nitrite reduction i n denitrifying bacteria, e.g., Pseudomonas aeruginosa a n d P. stützen (62, 63, 80, 8 1 , 1 2 3 ) , Bacillus subtilis (62, 6 3 ) , Thiobacillus denitrificans ( 1 4 ) , a n d i n Micrococcus species ( 8 3 ) . N i t r i c oxide was also shown to be utilized b y Escherichia coli (Bn) as the sole nitrogen source (165, 166). Fewson a n d Nicholas (80) h a v e shown recently t h a t nitric oxide is readily utilized b y a r a n g e of bacteria, fungi, a n d algae w h e n t h e y w e r e g r o w n on n i t r a t e b u t n o t w h e n g r o w n on a m m o n i u m salts ( T a b l e I X ) . T h e y also showed t h a t n i t r i c oxide w a s utilized b y nitrogen-fixing organisms, e.g., Azotobacter vinelandii, Clostridium pasteurianum, b y t h e blue-green algae Nostoc muscorum a n d Anabaena cylindrica, a n d also b y root nodule bacteria w h e n t h e y w e r e actively fixing atmospheric nitrogen ( T a b l e X ) .
T h i s m a y m e a n t h a t nitric oxide or a compound w i t h w h i c h it equili- brates is formed d u r i n g nitrogen fixation as well as in n i t r a t e dissimila- tion a n d assimilation. N i t r i c oxide reductase from Pseudomonas aeru- ginosa w a s shown to be a flavoprotein dependent on iron o n l y ( 8 1 ) , b u t c o n t r a r y to earlier reports (63) copper is not essential for its activity.
4. Nitrous Oxide
D e h é r a i n a n d M a r q u e n n e (67) first reported t h e production of nitrous oxide b y soil microorganisms. K l u y v e r a n d Verhoeven (135) h a v e con- cluded t h a t nitrous oxide production a n d utilization is common to all