Path from problem to solution in multi-stage continuous flow synthesis of pharmaceutical compounds; Bioorg. Greiner: Application of Multiple Two-Mode Centrifugal Partition Chromatography for Final Purification of a Multistep Continuous Flow Reaction;. A novel, sequential heterogeneous catalytic hydrogenation reaction was developed with a continuous flow hydrogenation reactor (H-CubeTM and H-Cube ProTM) using palladium on carbon and methanesulfonic acid catalyst in acetic acid for the synthesis of ethyl 2-(2,3-dihydro-1H-indol-2-yl)acetate (6) from ethyl 4-(2-nitrophenyl)-3-oxobutanoate (29) via ethyl 2-(1H-indol-2-yl)acetate. 2-yl)acetate (21).
A new continuous flow final product purification technique was developed by coupling a multistep flow reaction with centrifugal partition chromatography (CPC) and the target compound, 4-fluoro-2-(morpholin-4-yl)aniline (65a), was produced continuously in the purity of more than 99.9% (GC-MS).
Új tudományos eredmények
Új folyamatos áramlású végtermék-tisztítási módszert fejlesztettünk ki, többlépcsős folyamatos áramlású szintézis rendszerrel, amely centrifugális megoszlási kromatográfiával (CPC) párosul.
Új, folyamatos áramú végtermék tisztítási módszert dolgoztunk ki, többlépéses folyamatos áramú szintézis centrifugális megoszlásos kromatográfiával (CPC) kapcsolt rendszerével,
HMBC heteronuclear multiple bond correlation spectroscopy HSQC heteronuclear single quantum coherence spectroscopy LLPS liquid-liquid phase separation. data) not available NBS N-bromosuccinimide NMR nuclear magnetic resonance.
Introduction
My next major appointment was the development of a new continuous flow final product purification method using centrifugal separation chromatography (CPC). Our aim was to realize the coupling of a multi-step flow reaction system with CPC in dual multiplex mode (MDM) in order to continuously produce the high purity lead product solution.
Literature review
- Synthesis of diazepino-indole derivatives – a retrosynthetic overview
- Synthesis of 4 from 2-fluorobenzaldehyde (9)
- Synthesis of ethyl 2-(1H-indol-2-yl)acetate (21)
- Selective catalytic heterogeneous hydrogenation of 1H-indole derivatives to 2,3-dihydroindole (indoline) derivatives
- Introduction to selective hydrogenation of 1H-indole derivatives
- Selective heterogeneous catalytic hydrogenation of 1H-indole derivatives
- Summary on selective heterogeneous catalytic hydrogenation of 1H-indole derivatives
- Flow chemistry in pharma industry [3]
- Purification in multistep flow synthesis of APIs
- Continuous-flow in-line work-up
- Continuous-flow final product purification
In the next section I will discuss the possibilities of the synthesis of the key indoline intermediate (5). Therefore, in the next section I briefly summarize the challenges and difficulties of the reduction of 1H-indole derivatives. In the next section, I provide a brief overview of existing continuous flow cleaning methods and their limitations.
The circular arrow marks the simulated counterclockwise motion of the bed (this is achieved by clockwise switching the inlet and outlet ports).
Gas-Liquid phase separation 4. Use of solid phase supported
In summary, the chemical knowledge and technology for realizing multi-step flow synthesis and subsequent purification exists, but connecting individual chemical steps involves hidden pitfalls. The utility of the available continuous purification methods (Table 2.10.) can be maximized by proper design of the synthesis process; however, we hope that our proposal for a new purification method using centrifugal partition chromatography will gain wide applicability for the synthesis of complex molecules and APIs, even on a large scale, due to the advantages presented in the last chapter of the introduction.
Catch and release chromatography (with semi-batch processing)
Simulated Moving Bed (SMB) Chromatography
Crystallization or recrystallization (with semi-batch processing)
Centrifugal Partition Chromatography (CPC – this work)
- Centrifugal Partition Chromatography [183]
As a result of mixing and settling operations, extraction occurs in each of these cells and extraction channels. According to the dual-mode principle, ascending and descending modes are applied alternately with a certain frequency in such a way that the samples are injected into the feed input of the currently applied mode. It is also noted that determining the appropriate composition of mobile and stationary phases to optimize the selectivity of the separation is within the general knowledge of the person skilled in the art.
Consequently, the present discovery, which relates to the use of centrifugal partition chromatography coupled to a multi-step flow reaction for the separation or purification of the final product from the products, is conceptually novel.

Results and Discussion
- Development of the synthesis of MCHR1 antagonist’s scaffold [1]
- Description and batchwise improvements of the original discovery chemistry route of the synthesis of the scaffold (2)
- Flow chemistry techniques in the synthesis of indoline derivatives
- New continuous flow final product purification method using centrifugal partition chromatography (CPC)
In the amide group reduction (78), the amount of borane-dimethylsulfide complex from 5 eq. After the addition of the reagent, the reaction mixture was immediately analyzed by TLC, which showed full conversion. We also investigated the N -alkylation reaction of 5 with 1,2-dibromoethane to reduce the reaction time, the dilution and the high excess of the carcinogenic reagent.
We have also observed the formation of a small amount of the over-reduced product (5) when charcoal-supported palladium was used; therefore, it seemed logical to develop a consecutive catalytic hydrogenation method to prepare compound 5 from 29 without isolating the indole (21).
![Figure 3.1. Original discovery chemistry synthesis of the target compound: tert-butyl 9- 9-bromo-1H,2H,3H,4H,5H-[1,4]diazepino[1,7-a]indole-3-carboxylate (2)](https://thumb-eu.123doks.com/thumbv2/9dokorg/2497229.294214/55.892.149.743.350.943/figure-original-discovery-chemistry-synthesis-compound-diazepino-carboxylate.webp)
Experimental
- General experimental details
- Procedures for the synthesis of diazepino-indoles
- Unpublished batchwise reactions
The reaction mixture was stirred at room temperature for 1 hour, where TLC showed full conversion (DCM:MeOH:sat. To the reaction mixture was added ethanol (20 mL) and it was cooled to room temperature. First, ethanol (36 mL) was added to quench the excess reagent, then the reaction mixture was evaporated in vacuo.
The cooled reaction mixture was first basified to pH>13 with 1 M NaOH solution (ca. 46 mL), then extracted with DCM (40 mL) three times. First, ethanol (31 mL) was added to quench the excess reagent, then the reaction mixture was evaporated in vacuo. The cooled reaction mixture was first basified to pH>13 with 2 M NaOH solution (ca. 16 mL), then extracted with DCM (30 mL) twice.
After removal of the cooling bath, the reaction mixture was stirred at ambient temperature for 2 hours, when TLC showed complete transformation (DCM: Saturated. Saturated Nahc03 (28 ml) and water (28 ml) were added to extinguish the reagent surplus and then the reaction mixed. .
The reaction mixture was first cooled to 0 °C and alkalinized to pH>9 with a 2 M NaOH solution (approx. 4.5 mL). The reaction mixture was evaporated in vacuo and suspended in 2 M NaOH solution (30 ml) and filtered.
Summary
By using a biphasic sampling method to feed the CPC device, 67% of the isolated yield was achieved for the two synthetic steps and purification. The productivity of the new continuous flow final product purification with centrifugal partition chromatography (CPC) coupled to a multi-step flow synthesis can be increased by adopting a so-called single-stage sampling method for feeding the CPC device. It was shown that the target compound of 4-fluoro-2-(morpholin-4-yl)aniline (65a) could be produced with a higher productivity (by almost 60%) compared to the two-stage sample intake, where the only bottom phase of the two-phase liquid system suitable for the separation was mixed.
This system is the first continuous-flow adsorbent-free final product purification technique and should have wide application in the synthesis of APIs or their intermediates.
Yu, "Continuous Manufacturing Has a Strong Impact on Drug Quality," can be found at https://blogs.fda.gov/fdavoice/index.php/2016/04/continuous-manufacturing-has-a-strong-impact-on-drug-quality, 2016. Stanton, "Lack of talent will hinder continuous manufacturing adoption, says MIT Prof," can be found at http://www.in-pharmatechnologist.com/Processing/Lack-of -talent -will-hamper-continuous-manufacturing-uptake-MIT-Prof, 2015. Daniel, Method and Device for Separating Constituents of a Liquid Charge by Means of Liquid-Liquid Centrifuge Chromatography, 2005, WO.
Audo, a centrifugal separator, for use in chromatography, comprising a flat ring with cells divided by separators into subcells, 2006, FR2883770. Couillard, Centrifugal Liquid-Liquid Column Chromatography Device for Separating the Components of a Liquid Mixture for the Extraction and Purification of Components Comprising a Downstream Side of a Column to Maintain a Back Pressure Above a Certain Value, 2008, FR2904235. 200] Armein-Crew, “Introduction to Centrifugal Partition Chromatography CPC/CCC”, available at http://www.armen-instrument.com/downloads/Armen-Introduction-CPC-CCC.pdf, 2016.
Brennan, "http://www.in-pharmatechnologist.com/Processing/FDA-calls-on- manufacturers-to-begin-switch-from-batch-to-continuous-production," available at http://www.in-pharmatechnologist.com/Processing/FDA-calls-on-be-manufacturers-continuous-to 2015. Berndt, Carbazole and Carboline Compounds for Use in Diagnosing, Treating, Ameliorating, or Preventing Disorders Associated with Amyloid Proteins or similar to amyolide, 2015, WO2015110263. Németh, New type of extraction cell for a centrifugal separation chromatograph, as well as a centrifugal separation chromatograph containing such an extraction cell, 2016, WO2016055821 A1.
Environmentally Friendly Synthesis of Indoline Derivatives using Flow-Chemistry Techniques
We also investigated the N-alkylation reaction of 4 with 1,2-dibromoethane to reduce the reaction time, the dilution and the large excess of the carcinogenic reagent. Investigation of the continuous catalytic hydrogenation in a heterogeneous continuous-flow hydrogenator Next, we investigated the reductive cyclization reaction of 2 to ethyl (1H-indol-2-yl)acetate (3) described in the literature (shown in Table 1). During the optimization several parameters were taken into account such as the solvent, the quality and quantity of the acid catalyst, and the temperature.
Of the three solvents used (EtOH, EtOAc, and AcOH), acetic acid proved to be the best (Scheme 3, entry 2). Results of continuous flow heterogeneous N-alkylation reactions using Cs2CO3 as a solid base.[a]. Results of continuous-flow heterogeneous N-alkylation reactions using a solid-supported reagent (Silia-DMAP) as a solid base.[a].
Furthermore, bed regeneration and the use of solid-supported reagents are not common practice in industry. KGaA, Weinheim ond, reaction parameters to be optimized were used in DoE as independent variables; third, the flow-reactor system was assembled and the designed experiments were carried out; Fourth, further optimization of the reaction parameters was carried out. Our design of experiment contained 2n-1+3 cases (where is the number of factors), which included eight experiments for each of the four independent variables (corners of the cube) and three more at the midpoint (see the Supporting Information) to identify deviation of the results.
In other words, by increasing the temperature, we were able to reduce the required amount of the carcinogenic reagent. Further reduction of this reagent was not feasible if we wanted to keep the value of the reduced dependent variable above 93% (see Supporting Information). All continuous flow hydrogenation reactions were performed in an H-CubeTM continuous flow hydrogenation reactor with a reaction volume of 8 mL in Full-H2 mode at a flow rate of 0.5 mL min–1.
All continuous flow hydrogenation reactions were performed in H-CubeTM continuous flow hydrogenation reactor with 8 ml reaction volume, in Full-H2 mode with flow rate of 0.5 ml min-1.

![Figure 2.6. Main literature methods for preparation of 21. a: PPh 3 •HBr, MeCN, 7 h, ∆, 88%; [13,27–29] b: ClOCCH 2 COOEt; DCM, 3 h, rt, 71%; c: tBuOK, PhMe, 15 min, ∆, 70%;](https://thumb-eu.123doks.com/thumbv2/9dokorg/2497229.294214/21.892.163.733.297.701/figure-main-literature-methods-preparation-mecn-clocch-cooet.webp)
![Table 2.1. Reaction of indole and sodium borohydride in various carboxylic acids. [39]](https://thumb-eu.123doks.com/thumbv2/9dokorg/2497229.294214/23.892.134.759.727.984/table-reaction-indole-sodium-borohydride-various-carboxylic-acids.webp)

![Figure 2.16. Synthesis of ergot alkaloid intermediates by Carr et al. [51]](https://thumb-eu.123doks.com/thumbv2/9dokorg/2497229.294214/32.892.136.755.637.811/figure-synthesis-ergot-alkaloid-intermediates-carr-et-al.webp)
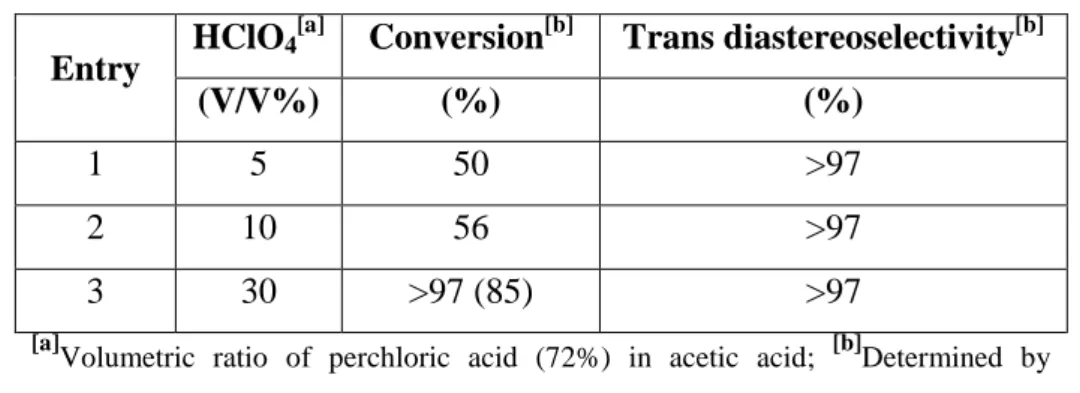
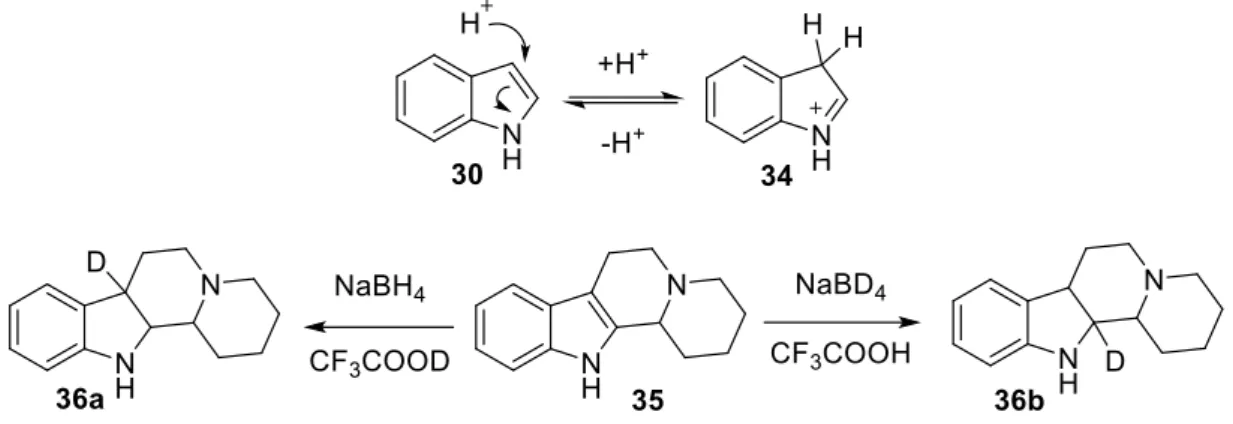
![Figure 2.21. General scheme of indole reduction by Kulkarni et. al. [41]](https://thumb-eu.123doks.com/thumbv2/9dokorg/2497229.294214/35.892.259.633.103.280/figure-general-scheme-indole-reduction-kulkarni-et-al.webp)