The Histamine Problem
M A S A O KIMATA
Department of Fisheries, Kyoto University, Maizuru, Kyoto, Japan
I. Introduction 329 II. Freshness and the Appearance of Histamine 331
A. Produced through Autolysis 331 B. Produced through Microbial Activity 332
C. Histidine as Precursor 336 D. White- and Dark-Meat Fish—Basic Differences 338
E. Mollusks 338 F. "Saurine" and Poisoning 340
III. Responsible Bacteria 341 A. Occurrence of Histamine-Formers 342
B. Proteus morganii 342 C. Other Bacteria 346 IV. Analytical Methods 346
References 347
I. Introduction
It has long been known that histamine may appear in spoiling fish meat. Furthermore, it is believed that this substance is produced by the action of bacteria causing spoilage. Many cases of fish poisoning have been attributed to histamine, although the causal relationships have been far from clearly understood.
Suzuki et al. (1912) reported that extracts of tuna did contain hista
mine. Igarashi (1938) affirmed that the bitter or pungent taste of less fresh fish meat was related primarily to the presence of histamine. The pungency increased with the advancing spoilage of the fish. Igarashi also determined the histamine content of numerous fish varieties and fish products collected from commercial fish markets and could confirm the presence of this substance in all the examined samples. Table I illustrates part of his experimental results (Igarashi, 1949). Recently, several investigators (Simidu and Hibiki, 1954a; Aiso et al.9 1955a;
Kawabata et al, 1955a; Miyaki et al., 1956) detected considerable amounts of histamine in several fish and fish products. The concomitant food poisoning has frequently been ascribed to this compound. These
329
TABLE I
T H E HISTAMINE CONTENT OF FISH PRODUCTS ON THE JAPANESE MARKET0
Amount of histamine
mg./1000 g. mg./1000 g.
Kind of product product dry matter Canned fish
Oil sardine 57.9 113.1
Seasoned sardine 13.1 48.0
81.6 203.1
Seasoned mackerel 1.8 6.3
49.3 148.6
Seasoned tuna 63.8 162.1
99.8 275.0
Seasoned bonito 59.9 156.3
156.9 489.5
Seasoned whale 11.3 34.9
Pink salmon 2.3 6.6
Dried fish
Sardine 124.3 150.9
Herring 300.9 366.2
Sand eel 39.5 48.5
Squid 26.0 34.5
Shark fin 1.8 2.0
Salted fish
Herring 98.3 200.7
Trout 22.5 48.8
Herring roe 5.1 11.7
Salted dried fish
Mackerel pike 297.8 522.8
Gutted cod 0.7 1.0
Sardine 15.0 23.3
Seasoned dried fish
Pressed squid 10.2 11.7
Globefish 2.4 3.6
Broiled sardine 398.9 471.8
Fish boiled in soy sauce
Sand eel 1.8 2.5
Goby 5.3 7.2
Smoked fish
Herring 345.2 520.2
Salmon 16.6 24.9
Salted fish gut
Bonito 24.7 59.8
Sea cucumber 107.2 292.2
Fish cake
Kamaboko 0.4 1.6
Kamaboko 0.7 2.3
a Analysis by Igarashi (1949).
public health aspects are further discussed in the chapter on food poison
ing through fish (see Volume II, Chapter 1 1 ) . In wastes from certain fish, histamine is so readily produced that such sources are utilized for commercial manufacture of this compound (Igarashi, 1949; Geiger, 1954).
There have been a great number of publications through the years making the assumption of a specific toxin being produced in the spoilage of fish without being able to trace the effect to any particular chemical compound (e.g., Markoff, 1939; Nowitzki, 1941). Markoff (1939) re
viewed this literature extensively and also attached the name "pelo- metoxin" to this nonprotein, heat-stable compound. Presumably he was on the track of histamine, defining this toxin as anamine, possibly diamine.
II. Freshness and the Appearance of Histamine
Since the amount of histamine in fish meat increases with the ad
vance of spoilage, it has been assumed that most of the histamine is produced by the microorganisms developing in the fish. A minor part may result from breakdown of the flesh through autolysis.
A . PRODUCED THROUGH AUTOLYSIS
Igarashi (1939, 1949) observed that comparatively large amounts of histamine were produced in the muscle tissue of the chub mackerel
(Scomber japonicus) even when these fish were treated with each of the following antiseptic solutions: 0.4% free chlorine, 0.5% a-naphthol, and 0.5% ß-naphthol. The treatment was continued for two days at 24-25°. He concluded that histamine was produced through autolysis.
In order to obtain further and more definite information on this particu
lar point, Geiger et al. (1945) and Kimata and Kawai (1953a, e) studied the production of histamine during the autolytic breakdown of several varieties of fish. They studied muscle suspensions under aseptic condi
tions (obtained by the addition of a mixture of toluene and chloroform) and confirmed that in dark-meat fish (such as mackerel, bonito, yellow- tail flounder, etc.) and in a few other kinds of fish, such as sardines and carangoids, very little histamine was formed by the action of enzymes inherent in the muscle tissue. Kimata and Kawai (1953d) found that the production of histamine was influenced by the environmental tempera
ture and pH. The optimum pH range for histamine production at 35°C.
was between 3.5 and 4.5, the maximum amount of histamine attaining 6-9 mg. per 100 g. of meat. The corresponding figure at pH 7 was about
3 mg. The optimum temperature for histamine appearance ranged be
tween 40 and 45 ° C , depending on the kind of fish examined. The maxi
mum amount of histamine produced during autolysis hardly exceeded 10-15 mg. per 100 g. of meat, even under optimum conditions with re
gard to pH and temperature. These amounts are negligible compared to those which may be produced by bacterial action. This result is in con
trast to those of previous investigators, who reported that only the histi- dine decarboxylases of bacterial origin were active in the acid range, while enzymes of animal origin operated on the alkaline side. According to Simidu et al. (1953a), the optimum pH value for the 1-histidine de
carboxylase of fish muscle tissue was 7.5, and the optimum temperature 45 °C. In their experiments, the amount of C 02 produced in reaction mixtures, consisting of 1 ml. enzyme preparation, 1 ml. phosphate buffer, and 1.5 ml. of the solution of 1-histidine, was determined, using the Warburg respiration mechanism. Since the enzyme preparations em
ployed were extracted merely with an equal quantity of phosphate buffer, there is no doubt that they contained large amounts of various other substances, originally present in the fish tissue. Hence, the optimum pH value indicated above is questionable.
In several cases, fish used for these experiments had been subjected to quite some breakdown subsequent to death, and prior to the start of investigation. It is most likely that histamine had already been pro
duced through bacterial action.
Igarashi (1939) reported that histamine was not produced at tem
peratures higher than 30°C. and that the optimum temperature for its appearance was 27-28 °C. This result is in accordance with the produc
tion of histamine expected in terms of bacterial action, as described below. It can be safely concluded that enzymes inherent in the fish muscle tissue are not responsible for the histamine appearing during the spoilage of certain fishes. In autolysis of white-meat fish, production of histamine does not take place or is very insignificant compared to what might occur in dark-meat fish under comparable conditions. In the shark, no histamine is formed through autolysis. Although the octopus tissue differs greatly from those of fish in many respects, it resembles white-meat fish with regard to the formation of histamine.
B . PRODUCED THROUGH MICROBIAL ACTIVITY
As early as 1914, Guggenheim reported that histamine was readily formed from the histidine of proteins by putrefactive microorganisms.
Van Veen and Latuasan (1950) isolated from bonito two strains of bac-
teria capable of producing histamine from the histidine of fish muscles under aerobic as well as anaerobic conditions. Geiger (1948) and Geiger et al (1945) showed that fresh fish contained very little hista
mine, but its amount increased postmortem. Small fish show higher histamine values than large specimens (Wilhams, 1954). This may be due to differences in tissue softness, since in small-sized fish minced flesh produces histamine at the same rate as intact fish whereas in large specimens this is not so (Simidu and Hibiki, 1954c). Histamine as such appears to have a slight antibacterial activity against Escherichia coli, Micrococcus pyogenes v. aureus and hemolytic streptococci (Peria and Salamone, 1954). Whether this has any practical importance is still difficult to say.
According to Kimata and Kawai (1953a) and Kimata and Tanaka (1954a), dark-meat fishes distinguish themselves from white-meat fishes with regard to the formation of histamine during bacterial spoilage. Fish belonging to the former group produce far greater quantities than those in the latter group. The quantity produced is, however, markedly in
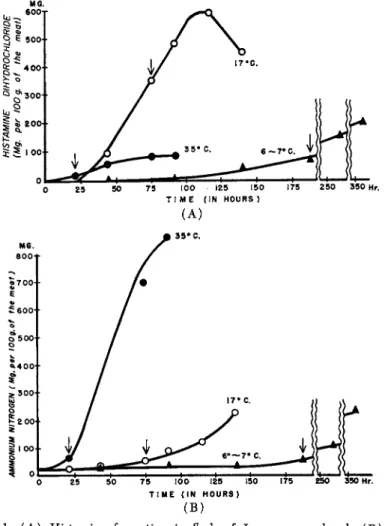
fluenced by the environmental temperature. The maximum amount and rate of histamine production are attained at about 20°C., while only a little histamine is produced at 35 °C. in the same meat. Figure 1 shows the increases of histamine and ammonia obtained when fillets of chub mackerel were kept at different temperatures and attacked by bacteria.
The arrows indicate the points at which the fillets were judged un
suitable as food. In Table II are given the time intervals up to this point and the amounts of histamine, ammonia, amino nitrogen, and histidine at this critical point, as well as the corresponding pH values. The amount of histamine in the meat was 14 mg.% at 35°C., 354 mg.% at 17°C., 50-70 mg.% at 6-7°C., at the stage when spoilage became evident.
This general trend prevails for all fish when producing histamine. The amount may vary with conditions such as the kind of fish, storage tem
perature, etc. Simidu et al (1953b) also established that intermediary temperatures produced more histamine. At very low temperatures close to 32°F. (0°C.) all histamine formation was suppressed.
It is not precarious, from the point of view of food sanitation, to judge the freshness of fish by determining the content of ammonia present as
long as the fish are kept at temperatures as high as 35°C. At this level the production of histamine is practically negligible as compared to that of ammonia. An assessment of freshness on the basis of ammonia values is, however, most unreliable when the fish are kept at room
temperature, 70 °F. ( 2 0 ° C . ) . This is particularly true in the early
stages of spoilage, within the lapse of about 70 hr. when the formation of histamine dominates. In this case, there is even a danger that the fish may be judged as "good" with regard to freshness. Even when fresh fish are kept at temperatures as low as 6°-7°C., the ammonia method may
Μ 6.
T I M E ( I N H O U R S )
( A )
T I M E ( I N H O U R S )
( B )
FIG. 1. ( A ) Histamine formation in flesh of Japanese mackerel. ( B ) Increase in ammonia in flesh of Japanese mackerel.
not be very reliable for the estimation of their freshness. As the data given above indicate, the histamine production in certain fishes pre
dominates over the ammonia production in the entire range of tempera
ture from 6° to 20° C.
Poisoning is known to occur most frequently in spring and fall. In these seasons, fish may be easily misjudged as good and thus offered for sale as food, despite the fact that in reality they are in a spoiled condi
tion, containing a comparatively large amount of histamine.
TABLE I I
SPOILAGE DATA FOR FILLETS OF Scomber japonicusa His Ammonium Amino
Temperature Time tamine Ν Ν Histidine (C.) (hours) (mg.%) (mg.%) (mg.%) (mg.%) pH
35° 21 14 59 216 875 6.4
17° 75 354 56 106 382 6.8
6°-7° 150-190 50-70 60-90 100-140 900-880 6.4
a Source: Kimata et al. (1953a).
Recent investigations once again established the close correlation between bacterial counts and histamine formation in the flesh of Japa
nese mackerel (Tsuda and Tomiyama, 1959).
Heat treatment at 60 °C. appears to kill the histamine-producing bacteria and thus prevent the formation of this compound (Hibiki and Simidu, 1959).
There is a considerable difference in the histamine production in meat extractives prepared from different kinds of dark-meat fish, even when the amount of total nitrogen and that of histidine are the same throughout the samples. For example, mackerel produces more histamine than does yellowtail flounder, although it contains more histidine (Simidu and Hibiki, 1954e). This difference may be attributed to the presence of unknown substances which may accelerate or retard the
activity of decarboxylating histidine, because in these extracts no sig
nificant difference is detected as to the growth of bacteria (Kimata and Akamatsu, 1956). Simidu and Hibiki (1954b, c, d, 1955c) attribute these differences to the coarse flesh texture of yellowtail as compared to mack
erel, causing a slower proteolytic release of histidine. This amino acid becomes available for microbial attack at a slower rate. Histidine is contained in larger amounts in the inner parts of cells than in the inter
cellular spaces, as manifested in the content of the drip as compared to the flesh (Simidu and Hibiki, 1955c). Another interesting observation was that in salted fish far more histidine was retained in the flesh, as compared to fresh fish.
An important result was reported by Simidu and Hibiki (1955a).
Experimenting with the flesh of horse mackerel, shark, and squid, they
discovered that by adding 1% of the following compounds, TMAO, urea, and glycine, the histamine formation could be effectively retarded and, in case of the first-mentioned compound, almost suppressed for approxi
mately five days. Later, a similar, still more pronounced effect through the addition of certain carbohydrates, such as sucrose, glucose, and glycogen, was described by Hibiki and Simidu (1959). Starch was a less effective inhibitor. These experiments were made by inoculating heat-sterilized meat with histamine-forming bacteria after adding the above-mentioned carbohydrates.
Indirect evidence of the vital role played by microorganisms is pro
vided by observations on histamine formation in thawed fish muscle.
It was found that the appearance of histamine was delayed and de
pressed in the thawed muscle, whereas no appreciable difference be
tween the thawed and fresh muscles was established as to the increase of ammonia and other decomposition products. Inoculation of the spoiled fish muscle immediately resulted in an acceleration of histamine forma
tion in the thawed muscle to the level observed on the fresh muscle. The depression effect undoubtedly is attributed to the destruction of hista- mine-producing bacteria in freezing and thawing (Ota and Kaneko, 1958).
There is no direct and incontrovertible relationship between degree of freshness (or spoilage) and amount of histamine. Kawabata et al.
(1955b) found no apparent differences as to pH contents of volatile basic nitrogen or TMA in samples showing widely varying amounts of histamine. Similar observations are reported by Simidu and Hibiki
(1954a).
C. HlSTIDINE AS PRECURSOR
Histidine as a free amino acid appears to be a prerequisite for the formation of histamine. There is a close relationship between these two. In general, no clear distinction is made between free histidine and histidine built into protein (Geiger, 1954). Fish with a higher content of this amino acid in the free, immediately accessible form should more readily show an accumulation of histamine. This was already demon
strated in 1909 by Suzuki and Yoshimura. The availability and amount of histamine constitute the basis for the differences between dark- and white-fleshed varieties, the former generally being more hazardous with respect to histamine. A complicating factor in the observation by Van Veen and Latuasan (1950) was that the blood of the fish they studied had a high content of histidine. The muscle is, consequently, not the
only source of this free amino acid. Simidu and Hibiki (1955a) found that a drop in the amount of histidine follows closely the mounting histamine values. Dark-fleshed varieties such as mackerel and horse mackerel show definitely much higher values (726-210 mg.%) than the white-fleshed gurnard with only traces (0.5 mg.%). The decline in the amount of histidine was less than the increase of histamine in inter
mediary temperature surveys. This was attributed to an independent bacterial action (Simidu et al., 1953b). According to more recent analyses, red-muscle fish frequently contain histidine at a level of 1%
(Hibiki and Simidu, 1959). Geiger (1954) hints at another distinction between roundfish and flatfish, the latter being short of histidine in accessible form. This is in accordance with the findings of Simidu and Hibiki (1954a) on yellowtail flounder as compared to bonito, sardine, and mackerel. As these differences disappeared in minced meat, these workers are inclined to ascribe the slower histamine formation in yellowtail flounder to flesh texture (Simidu and Hibiki, 1954c). This was refuted by a subsequent more thorough study. In spite of various softening treatments, although yellowtail contained more histidine, it produced less histamine (Simidu and Hibiki, 1954d).
The presence of a histidine decarboxylase naturally explains this rela
tionship. Numerous studies verify the existence of such a bacterial enzyme and its direct splitting activity (Simidu et al., 1953b). Whether the powerful histidine-decarboxylating activity of the entrails of some fish reported by Simidu et al. (1953a) can be attributed to micro
organisms or body enzymes is not yet established.
Nevertheless, histidine does not seem to be required for the growth of the incriminated bacterial species. In spite of carrying this specific enzyme equipment, they are still capable of growth, even when de
prived of this particular amino acid.
A seasonal variation was observed by Simidu and Hibiki (1954b), wintertime showing less readiness to form histamine. Whether this is due to differences in flesh composition or types of bacterial surface flora was not established. These workers also found in inoculation experiments that the more hours have elapsed after capture, the more histamine is formed.
It was early established by Geiger et al. (1945) that histamine is not produced by proteolysis only, liberated by a splitting of peptide linkages or through a breakdown of cells. It must exist in a preformed stage. This once again accentuates the need of distinguishing between free and protein-bound histidine.
D. W H I T E - AND D A R K - M E A T F I S H — B A S I C DIFFERENCES
Dyer and Mounsey (1945), working with cod, showed that significant amounts of higher amines, like histamine, were not formed until the fish reached a stage of decomposition considerably more advanced than the stage at which the fish would be classified organoleptically as undesir
able—in fact, a stage so advanced that food poisoning was possible.
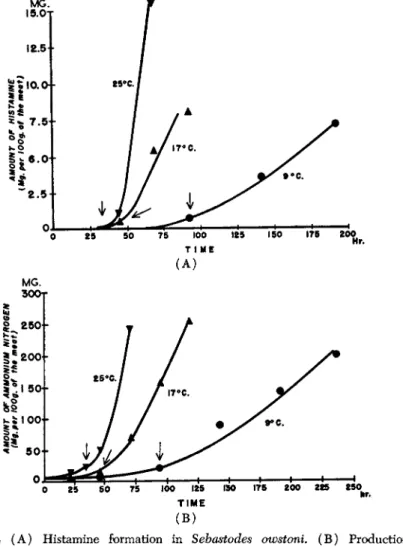
For the spoilage of white-meat fish, Kimata et al. (1954a) largely could confirm this. Histamine was produced in all cases examined as spoilage proceeded, but its production started only after the amount of both ammonia and amino nitrogen had become appreciable as is evident in Fig. 2. Since histidine in the free state was either not found at all or present only in very minor amounts, the histamine produced in spoilage must have been derived from the histidine liberated by the bacterial decomposition of muscle proteins. The amount of histamine produced in cod was related to the degree of spoilage, measured in terms of the amount of ammonia (Dyer and Mounsey, 1945). In other words, more histamine is present in fish flesh containing more ammonia and amino nitrogen. The amount of histamine produced in the flesh of white- meat fish is, however, insignificant and negligible in comparison with that appearing in red-meat fish (Simidu et al, 1954a). In white-meat fish, the amount of histamine in the meat is about 5.0 mg.% when its spoilage is clearly recognized through organoleptic tests (at this time the amount of ammonia was about 700 mg.%, and its amount was less than 1.0 mg.%
at the borderline between fresh state and beginning spoilage.
In comparing sardine, hake, and prawns, the histamine concentrations attained were far in excess with sardines, as compared with the others
(Torres-Acero, 1956). This is attributed to differences in protein com
position (see also Section C ) . Geiger (1944) found histamine in par
ticular in sardines, mackerel, tuna, and salmon.
Although investigations on shark meat are still meager, histamine does not seem to be produced by a bacterial decomposition in this type of meat. This is presumably explained by the fact that histidine is not found in shark meat when hydrolyzed by hydrochloric acid (Amano and Bito, 1951).
E . MOLLUSKS
When the meat of mollusks, such as squid and octopus, is spoiled through bacterial action, the production of histamine begins, after am
monia and amino nitrogen have reached a certain level (Kimata et al, 1954b). This phenomenon is similar to what is the case with white-meat
fish. Since the histamine produced in the flesh as the spoilage advances may be derived from histidine liberated by the bacterial decomposition of muscle protein, it is considered that a large amount of ammonia al-
M G . 15.0
T I M E
(B)
FIG. 2 ( A ) Histamine formation in Sehastodes owstoni. ( B ) Production of ammonia in the flesh of Sehastodes owstoni.
ways exists in meats containing a certain amount of histamine. But it is not considered likely that a large amount of histamine can occur in meats containing small amounts of ammonia. In the case of mollusks, the amount of histamine produced is so small as to be almost negligible,
even if histamine is formed at a rapid rate and reaches maximal quan
tities. For example, in the case of squid meat the amount of histamine per 100 g. of the meat was about 9.0 mg. (Kimata et al, 1954b), even when the spoilage went so far as to show ammonia figures attaining 1,000 mg. Also, in the case of octopus the amount of histamine was about 4.0 mg. when the amount of ammonia was about 500 mg., and 25.0 mg. when 800 mg. of ammonia was encountered. Similar values have been published by Simidu and Hibiki (1955b).
It has frequently been reported that histamine poisoning takes place due to the eating of octopus or squid, although the scientific evidence is most scanty. If such meat is erroneously judged to be still fresh, in spite of its being in a stage of advanced spoilage, the poisoning could possibly be due to histamine. But it is far more likely that other causes are to be sought for its deleterious effects on human beings.
If the stage of freshness in squid and octopus is judged by the amount of ammonia present, this seems safe from the sanitary point of view, as large amounts of ammonia generally are produced before any toxic amounts of histamine accumulate.
On the other hand, Motohiro and Tanikawa (1952) reported that poisonings due to the eating of certain mollusks resemble those caused by histamine. Octopus contains 5-hydroxytryptamine (serotonin, enter- amine) which is known as a histamine releaser (Asano, 1957). So it seems reasonable to conclude that in the octopus hazardous concentra
tions of histamine may occur under certain circumstances but this phe
nomenon is largely unknown as to its specific nature.
Crustaceans form minute quantities of histamine even when spoilage becomes very advanced. This has been studied in crab and shrimp
(Simidu and Hibiki, 1954b; Simidu et al, 1955).
F . "SAURINE" AND POISONING
Although it is evident that under certain circumstances, and par
ticularly with red-meat fish, histamine is formed and even in significant quantities, there still prevails some uncertainty as to the identity be
tween the "poison" and histamine. Pergola (1956) maintains they are identical. Kawabata et al (1955a) assume that, besides histamine, re
lated substances are formed which either are more definitely toxic or act synergistically and activate physiologically the effect of histamine.
These related substances—having a similar biological activity in stimulat
ing the vagus nerve—have even been given a separate designation—
"saurine" (Kawabata, 1955; Kawabata et al, 1955a, b ) . The name refers to the fish called in English "saury," the dried form of which causes so many of these poisoning cases. Whether such an additional vagus- stimulant is needed to enhance the toxicity of histamine or in fact con
stitutes the real culprit, this could explain Geiger's (1955) finding that spoiled fish containing as much as 190-210 mg. of histamine per kilogram did not affect cats, rats, or mice. On the other hand, shock effects were obtained when histamine could enter through the oral mucosa. These additional compounds were studied in canned mackerel and dried, seasoned saury, "samma sakuraboshi" (Kawabata et al., 1956a). In addi
tion, it was found that TMA and its oxide increased by approximately 10% the uterus reaction of experimental guinea pigs in the case of both histamine and "saurine" (Kawabata et al., 1955c). Similar results as to TMA and TMAO were obtained by Miyaki and Hayashi (1954), Hayashi (1954, 1955), and Kawabata et al. (1955d). Occasionally they have erroneously been termed synergists, although it is obvious from the findings that they merely act as stimulants.
The critical concentration for poisoning through histamine in spoiled fish seems to be about 100 mg. per 100 g. of muscle (Simidu and Hibiki, 1955d).
III. Responsible Bacteria
It is evident that bacteria active in spoilage are responsible for the appearance of histamine. Several investigators (Markoff, 1939; Geiger et al, 1945; Legroux and Levaditi, 1947; Legroux et al, 1946) reported that in sardines, mackerel, tuna, etc., histamine was produced during the spoilage process by the normal bacterial flora, i.e., not by the recognized food-poisoning pathogens. Pergola (1937) made the assumption that a special toxicogenic bacterial agent was responsible for the produc
tion of a specific fish poison, called "ichthyovenin," appearing when tuna fish and sardines spoil. Later, this toxic substance was identified as histamine and histidine was established as its precursor (Pergola, 1956).
van Veen and Latuasan (1950) isolated from bonito strains of bacteria capable of producing histamine from the histidine of fish muscle under aerobic and anaerobic conditions. Kimata and Kawai (1953c) and Kimata and Tanaka (1954a) found that most fish-spoiling bacteria failed to form any large amount of histamine. Most of the histamine appearing in the fish tissue during spoilage was primarily due to the activity of one single organism which in turn is able to produce quite appreciable amounts.
A. OCCURRENCE OF HISTAMINE-FORMERS
Since histamine is produced so commonly in the spoiling of dark-meat fish, it is presumed that so-called histamine-formers belong to the regular surface microflora of fresh fish.
Kimata and Tanaka (1954b) ascertained that histamine-formers were always present on the surface of fresh fish, irrespective of the kind of fish, although their number could be minor. The results obtained by them were as follows. About 0.1% of the total number of bacterial cells on the surface were histamine-formers in the case of market fish at the sales point. This same fish, however, carried such histamine-producing types to the extent of 1% immediately after being landed from a fishing boat, showing a difference of about ten times between these two groups.
This seems to be due to the fact that histamine-forming species did not increase, while others did so during distribution and sale. In the case of living fish in the sea, about 1% of the total number of bacterial cells seem to be histamine-formers.
From the foregoing, it may be concluded that histamine-formers are omnipresent on all fish even when fresh, and in numbers amounting to 0.1 to 1% of the total number of bacterial cells. Any fish, even when sea- fresh, appear to be carrying In^tamine-formers presumably emanating from fishing gear, nets, boxes, etc. These in turn become infected from fish carrying these bacteria in live condition. This is a likely possibility, as some living fish lack this type of bacteria.
This author in a series of studies—see list of references—identified one bacterial species, Proteus morganii, as particularly incriminated.
Several cases of this histamine-poisoning agent have been reported by the public health authorities of Japan—see Volume II, Chapter 11 by Kawabata; see also Okazaki and Harada (1959), Kimata and Akamutsu (1956), and Aiso et al. (1955c, 1956). Kawabata claims to have estab
lished appreciable amounts of both histamine and saurine. He also proved that P. morganii, isolated from poisonous tuna-fish flesh, could produce these two compounds in raw, fresh flesh of tuna species.
B. Proteus morganii 1. General Characteristics
A detailed description of the particular histamine-forming bacteria species was presented by Kimata and Kawai (1953b). In order to make the characteristics of this species more easily accessible, this description is reproduced below:
Morphology: Rods: 0.5 by 1.0-1.4 microns, with rounded ends, occur-
ring singly and in short chains. Motile, with several peritrichous flagella.
It does not produce endospores. Gram-negative.
Cultural characteristics: Agar colonies: Circular with entire margin, smooth, convex, glistening, finely granular, translucent, grayish-white.
Agar slant: Filiform, grayish-white, glistening, smooth, raised, buty- rous.
Gelatin colonies: Punctiform, pulvinate, smooth, entire, grayish-white.
Gelatin stab: Echinulate growth along the fine of puncture. No liquefaction.
Potato: Very slight growth with pale yellow.
Broth: Moderate turbid with compact and grayish-white sediment.
No surface growth.
Litmus milk: Unchanged or slightly alkaline. No coagulation or peptonization.
Physiology: Indole is formed in minor quantities. Hydrogen sulfide appears in minor quantities. Nitrates are reduced to nitrites. Starch is not hydrolyzed. Acids and gas are formed in glucose, but not in lactose, sucrose, and glycerol. Ammonia is produced from urea, but not from amino acids. Histamine is formed abundantly in the presence of histidine.
Aerobic, facultative. Optimum temperature: 20-25°C.
This organism resembled Proteus morganii in many respects as to its general characteristics. But according to Kimata and Kawai (1953b), it was named Achromobacter histamineum as a new species. The reasons for this move were the following: it was not recognized from papers pub
lished so far that Proteus morganii possesses the power to decompose histidine and thus form histamine. The genus Proteus belongs to the Enterobacteriaceae and grows very well at 37°C. The bacteria capable of producing large quantities of histamine are widely distributed, as a normal constituent of the surface flora of living fish in sea water. They grow very well at 20-25°C. At temperatures higher than 30°C., they do not grow but gradually die away. From these facts, this organism appears to be of marine origin. Later, Kimata and Akamatsu (1955a) encountered a strain which grew well at 30-35°C. This was distin
guished as Type II. Aiso et al. (1956; Aiso and Yanagisawa, 1955) re
ported that Proteus morganii isolated from fish products and causing poisoning had a strong decarboxylating action upon histidine.
Recently, Kimata et al. (1958), after renewed investigations of Achro
mobacter histamineum and comparing it with numerous strains of Proteus morganii, concluded that these two species were identical, al
though the former develops in a lower temperature range than the latter.
2. Physiological and Biochemical Features
Proteus morganii has been studied from the physiological and bio
chemical point of view by many investigators. Its characteristics con
sequently are known in considerable detail. They will not be reviewed here.
Poorly studied, however is its special capability of inducing the formation of histamine. Outside of the studies by Kimata and associates
(Kimata and Kawai, 1953b, 1958, 1959; Kimata and Akamatsu, 1955b, 1956; Kimata et al, 1958), few investigations are available (Okazaki and Harada, 1959).
a. GROWTH R A T E AND HISTAMINE PRODUCTION
The optimum temperature range for Type I is 20-25°C. and for Type II about 30°C. Actually, the former type cannot grow even at 30°C.
while the latter develops even at 35°C.
In both cases, the effect of temperature follows the general pattern.
Growth increases with temperature up to the optimum, as long as pH is suitable. Histamine production closely follows the temperature in both the descending and ascending phase. In the case of Type II, tempera
tures around 35 °C. show a deviating behavior, in that histamine is formed at a very slow rate and in extremely minute quantities, although the organism grows readily. This allows the conclusion that histamine is formed chiefly in the temperature range of 20 to 30°C. and hardly at all at higher levels.
The optimum pH value for growth of both Types I and II is in the range of 6 to 7 with no distinct difference between them. But with respect to the formation of histamine there are obvious differences. In Type I, the largest quantities and the maximum rate are found at the lowest pH value allowing a growth of these bacteria. In spite of better development at a pH shifting toward the alkaline side, hardly any histamine appears at a pH of 7. The optimum pH for Type II, on the other hand, is around 6.0, and the histamine production is reduced when pH shifts toward either the acid or alkaline side. On both sides of the optimum, histamine may, however, be produced in significant amounts even at a pH of 7. So, when fresh fish is held under weak acid condi
tions, viz., about pH 5-6, an even larger amount of histamine may be formed.
Later, Aiso et al (1955b, 1956) reported that Proteus morganii could produce a large amount of histamine in a medium containing a small amount of glucose. According to them, this may be due to the fact that
the pH value of the medium was not changed into the alkaline range, but remained slightly acid, presumably through the formation of acids. Re
cently, Kimata and Kawai (1958) found that in a culture medium which may be deficient in energy sources, the addition of glucose to it pro
motes histamine production, and the degree of promotion becomes greater with the increased concentration.
The presence of 1-histidine decarboxylase in P. morganii was firmly established by Kimata et al (1958), Kimata and Kawai (1958, 1959), and Kawabata and Suzuki (1959). No less than 13 strains were in
vestigated by Kawabata and Suzuki (1959) in this respect also as to the pH effect. The range was wide: pH 4-8. The rate of histamine production differed markedly between strains. A very slight activity of 1-histidine decarboxylase was detected in strains of P. mirabilis, P.
ret gen, or P. vulgaris by Kawabata and Suzuki (1959), but not by Kimata et al (1958). The rate of histamine production of Proteus morganii was largely stimulated in the presence of cystine, cysteine, methionine, and some other redox potential-reducers, but not in the presence of vitamin Β complex, including pyridoxal and pyridoxamine
(Kimata and Kawai, 1958).
The bacterial growth and the production of histamine are influenced by the presence of sodium chloride in the medium. The incriminated bacteria were stimulated in their development with increasing concen
trations of sodium chloride and reached their peak at a certain definite concentration—for growth, the optimum concentration was around 1%, and for formation of histamine between 2 and 3 % . No differences prevail in this respect between Types I and II. From these facts, it may be de
duced that larger amounts of histamine may be obtained in weakly salted fish than in fresh ones.
b. HISTAMINE THROUGH RESTING CELLS
When histidine decarboxylation tests were carried out with resting cells, negative results were obtained in some cases. Histamine appeared only when using cell suspensions and when cultivated under certain specified conditions (Kimata et al, 1958).
Grown on agar, they were not active in decarboxylating histidine, but when cultivated in liquid media (e.g., trypsin-digested casein) they became capable of forming histamine, although not in large quantities.
Resting cells are not suitable for this kind of test, giving inconsistent results. This was also recognized by Aiso et al (1955b).
Later, Kimata and Kawai (1959) confirmed that the histidine de-
carboxylase in the cells of Proteus morganii was easily destroyed or inactivated in the presence of oxygen and was comparatively stable under anaerobic conditions. This characteristic is markedly different from that of the histidine decarboxylases of other bacteria.
C. OTHER BACTERIA
Earlier bacteriological investigations discovered that a number of common bacteria, particularly of enteric origin, have the ability to pro
duce histamine, whether through conversion of histidine or otherwise.
Reference is made to some such studies (Ackermann, 1910; Hanke and Koessler, 1922; Hirai, 1933; Eggerth et al, 1939; Eggerth, 1939).
Notable among the species with this property are several SalmoneUa, (Koessler et al, 1928), Shigella dysenteriae (Ogasawara et al, 1953), and Clostridium (Welchia) perfnngens (Kendall and Schmitt, 1926; Ken
dall and Gebaur, 1930). This latter species seems to be able to form histamine, even when no free histidine is present (Eggerth, 1939).
Of particular interest is the fact that certain strains of Escherichia coli are clearly incriminated (Koessler and Hanke, 1919; see Eggerth et al, 1939, for further references). They might form histamine by hydrolyzing carnosine or decarboxylating histidine (Nash, 1952). Manganese inhibits this histamine formation.
Aerobacter aerogenes also is capable of producing histamine (Eg
gerth, 1939) and is particularly hazardous, in contrast to most other species uninfluenced by pH. Most of these bacteria are said to dis
tinguish themselves from the species encountered so far on fish by being favorably affected by the addition of glucose. Complicated, stimulating and retarding effects on histamine formation are induced by various amino acids (Eggerth, 1939). Escherichia coli is an exception in this particular respect, reacting, like Proteus morganii, negatively to glucose.
The fact that so many key bacteria may be responsible for producing histamine brings up the urgent need for a high degree of sanitation in fish establishments. Otherwise, the present rather restricted list of real culprits in histamine poisoning could easily be expanded.
IV. Analytical Methods
A considerable amount of work has gone into developing reliable methods of detecting and assessing histamine in fish by the United States Association of Official Agricultural Chemists. The Sager-Horwitz method
(1957) has been adopted for chemical analysis. A reliable biological method was worked out by Williams (1954, 1956).
Results obtained with these methods on a large number of samples of canned tuna fish indicated that fish classified organoleptically in classes 0, 1, and 2 yielded little or no histamine, but that fish in classes 3 and 4 tended to have considerable, but widely varying, amounts of histamine (0-736 mg./100 g. of fish) (Hillig, 1956). Also, in the "little tuna" the amount of histamine reveals the degree of spoilage prior to canning (Hillig, 1954).
Discrepancies between the biologic and chemical methods for meas
uring histamine could be eliminated by improvements in the bioassay method rendering through neutralization a more uniform sample; thus, an alkaline stimulation of the guinea pig ileum was avoided, and results checked well with those obtained by the chemical method. Collabora
tive results clearly prove that this new procedure is reproducible (Wil
liams, 1957, 1959).
An ion-exchange method using trichloroacetic acid extracts was pub
lished by Tsuda and Tomiyama (1959) and also seems to render good reproducible results. Using the same extracts, a diazo reagent was em
ployed, making it possible to develop a colorimetric method (Ota, 1958).
REFERENCES
Ackermann, D. (1910). Über den bakteriellen Abbau des Histidins. Ζ. physiol.
Chem. 65, 504-510.
Aiso, K., and Yanagisawa, F. (1955). The bacteria causing the "samma sakura- boshi" poisoning. (In Japanese.) Nihon Iji Shinpo (Japan. Med. News) No.
1625, 31-33.
Aiso, K., Yanagisawa, F., Toyoura, H., and Takeuchi, T. (1955a). Investigation on the poisoned "samma sakuraboshi" by putrefactive bacteria. (In Japanese.) Medicine and Biol. (Japan) 35, 96-99.
Aiso, K., Toyoura, H., and Fujita, H. (1955b). The specificity of histidine de
carboxylation in Proteus morganii (I). Japan. J. Bacteriol. 10, 1015-1019.
Aiso, K., Toyoura, H., and Nakano, K. (1956). The specificity of histidine de
carboxylation in Proteus morganii (II). Japan. J. Bacteriol. 11, 517-520.
Amano, K., and Bito, M. (1951). Comsequence of free amino acids generated from decomposing fish muscle. (In Japanese with English summary.) Bull.
Japan. Soc. Sei. Fisheries 16(12), 10-16.
Asano, M. (1957). Biochemistry of octopus. (In Japanese.) Kagaku-no-Ryoiki (J. Japan. Chem.) 11, 47-54.
Dyer, W. J., and Mounsey, Y. A. (1945). Amines in fish muscle. II. Development of trimethylamine and other amines. /. Fisheries Research Board Can. 6, 359-367.
Eggerth, A. E . (1939). The production of histamine in bacterial cultures.
/. Bacteriol. 37, 205-222.
Eggerth, A. H., Littwin, R. J . , and Deutsch, J. V. (1939). The determination of histamine in bacterial cultures. J. Bacteriol. 37, 187-203.
Geiger, Ε . (1944). Histamine content of unprocessed and canned fish. A tentative method for quantitative determination of spoilage. Food Research 9, 293-297.
Geiger, Ε . (1948). On the mechanism of histamine formation. Arch. Biochem.
17, 391-395.
Geiger, Ε . (1954). On the role of histamine in poisoning with spoiled fish. Proc.
Symposium on Cured and Frozen Fish Technol. Swed. Inst. Food Preserv.
Research (Göteborg) 1953 Puhl. No. 100, 5 pp.
Geiger, Ε . (1955). Röle of histamine in poisoning with spoiled fish. Science 121, 865-866.
Geiger, Ε., Courtney, G., and Schnakenberg, G. (1945). The content and forma
tion of histamine in fish muscle. Arch. Biochem. 3, 311-318.
Guggenheim, M. (1951). "Die biogenen Amine," 4th ed., S. Karger, Basel.
Hanke, Μ. T., and Koessler, Κ. K. (1922). Studies on proteinogenous amines.
XII. The production of histamine and other imidazoles from histidine by the action of microorganisms. /. Biol. Chem. 50, 131-191.
Hayashi, M. (1954). Food poisoning caused by ordinary putrefaction. IV.
Synergism between histamine and several biogenetic amines. (In Japanese with English summary.) /. Pharm. Soc. Japan 74, 1148-1151.
Hayashi, M. (1955). Food poisoning caused by ordinary putrefaotion. V. Detection of histamine in deteriorated mackerel pike. (In Japanese.) J. Pharm. Soc.
Japan 75, 1-4.
Hibiki, S., and Simidu, W. (1959). Studies on putrefaction of aquatic products—
26. Spoilage of fish in the presence of carbohydrates. (In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 24(11), 913-915.
Hillig, F. (1954). Individual volatile acids, succinic acid, and histamine as indices of decomposition in Atlantic "little tuna" (Euthynnus alleteratus). J. Assoc.
Offic. Agr. Chemists 37, 927-929.
Hillig, F. (1956). Volatile acids, succinic acid, and histamine as indices of decomposition in tuna. J. Assoc. Offic. Agr. Chemists 3 9 ( 3 ) , 773-800.
Hirai, K. (1933). Über die Bildung von Histamin aus 1-Histidin durch Bakterien.
Biochem. Ζ. 267, 1-5.
Igarashi, Η. (1938). The pungent principles of fishes produced by decrease in freshness, Part I. /. Chem. Soc. Japan 59, 1258-1259.
Igarashi, H. (1939). The pungent principles of fishes produced by decrease in freshness, Part II. Bull. Japan. Soc. Set. Fisheries 8, 158-160.
Igarashi, H. (1949). On the ptomaine of fish. Pamphlet, Municipal Office Hakodate City, 33 pp.
Kawabata, S. (1955). Poisoning by putrefaction. Allergy-like food poisoning.
Kagaku (Tokyo) 25, 336-341.
Kawabata, T., and Suzuki, S. (1959). Studies on the food poisoning associated with putrefaction of marine products. VIII. Distribution of l-( — ) histidine decarboxylase among Proteus organisms and the specificity of decarboxylating activity with washed cell suspension. (In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 25(6), 473-480.
Kawabata, T., Ishizaka, K., and Miura, T. (1955a). Studies on the food poisoning associated with putrefaction of marine products. I. Outbreaks of allergy-like food poisoning caused by dried seasoned saury and canned seasoned mackerel. (In Japanese with English summary.) Bull. Japan. Soc. Sei. Fisheries 21, 335-340.
Kawabata, T., Ishizaka, K., and Miura, T. (1955b). Studies on the food poisoning associated with putrefaction of marine products. II. Causative toxic substance and some of its chemical properties. (In Japanese with English summary.) Bull.
Japan. Soc. Set. Fisheries 21(5), 341-348.
Kawabata, T., Ishizaka, K., and Miura, T. (1955c). Studies on the food poisoning associated with putrefaction of marine products. III. Physiological and pharma
cological properties of newly isolated vagus-stimulant, named "saurine." (In Japanese with English summary.) Bull. Japan. Soc. Sei. Fisheries 2 1 ( 5 ) , 347- 351.
Kawabata, T., Ishizaka, K., and Miura, T. (1955d). Studies on the food poisoning associated with putrefaction of marine products. IV. Epidemology and the causa
tive agents of the outbreaks of allergy-like food poisoning caused by cooked frigate-mackerel meat at Kawasaki and by "samma sakuraboshi" at Hamamatsu.
(In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 21(11), 1167-1170.
Kawabata, T., Ishizaka, K., and Miura, T. (1955e). Studies on the food poisoning associated with putrefaction of marine products. V. Influence of trimethylamine and trimethylamine oxide added to histamine or "saurine" upon their own action tested on the contractility of intestine and uterus of guinea pigs. (In Japanese with English summary.) Bull. Japan. Soc. Sei. Fisheries 21(11), 1172-1176.
Kawabata, T., Ishizaka, K., Miura, T., and Sasaki, T. (1956a). An outbreak of allergy-like food poisoning caused by "sashirni" (sliced raw flesh) and the isolation of responsible bacterium, Proteus morganii. Bull. Japan. Soc. Sei.
Fisheries 21, 1100.
Kawabata, T., Ishizaka, I., Miura, T., and Sasaki, T. (1956b). Studies on the food poisoning associated with putrefaction of marine products. VII. An out
break of allergy-like food poisoning caused by "sashimi" of Parathunnus mehachi and the isolation of causative bacteria. (In Japanese with English summary.) Bull. Japan. Soc. Sei. Fisheries 22(1), 41-47.
Kendall, A. I., and Gebaur, E . (1930). The production of histamine by certain strains of the gas bacillus. Studies on bacterial metabolism. /. Infectious Diseases 47, 261-266.
Kendall, A. I., and Schmitt, F. V. (1926). Physiologic action of certain cultures of the gas bacillus. /. Infectious Diseases 39, 250-259.
Kimata, M., and Akamatsu, M. (1955a). Two different types of Achromohacter histamineum producing a large amount of histamine. Mem. Research Inst. Food Sei. Kyoto Univ. 9, 1-3.
Kimata, M., and Akamatsu, M. (1955b). Biochemistry of Achromohacter hista
mineum, II. Mem. Research Inst. Food Sei. Kyoto Univ. 9, 4-17.
Kimata, M., and Akamatsu, M. (1956). Biochemistry of Achromohacter hista
mineum, III. Mem. Research Inst. Food Sei. Kyoto Univ. 10, 1-18.
Kimata, M., and Kawai, A. (1953a). The freshness of fish and the amount of histamine present in the meat. I. Mem. Research Inst. Food Set. Kyoto Univ.
6, 3-11.
Kimata, M., and Kawai, A. (1953b). A new species of bacterium which produces large amounts of histamine on fish meats, found in spoiled fresh fish. Mem.
Research Inst. Food Set. Kyoto Univ. 6, 1-2.
Kimata, M., and Kawai, A. (1953c). The production of histamine by the action of bacteria' causing the spoilage of fresh fish. I. Bull. Research Inst. Food Set.
Kyoto Univ. 12, 29-33.
Kimata, M., and Kawai, A. (1953d). Biochemistry of Achromobacter histamineum, I. Mem. Research Inst. Food Set. Kyoto Univ. 6, 3-11.
Kimata, M., and Kawai, A. (1953e). The freshness of fish and the amount of histamine present in the meat, II. Mem. Research Inst. Food Sei. Kyoto Univ.
6, 12-22.
Kimata, M., and Kawai, A. (1958). Studies on the histamine formation of Proteus morganii. Mem. College Agr. Kyoto Univ., Fisheries Ser. Special Issue, pp. 92-99.
Kimata, M., and Kawai, A. (1959). Studies on the histamine formation of Proteus morganii (cont.). Mem. Research Inst. Food Set. Kyoto Univ. 18, 1-7.
Kimata, M., and Tanaka, M. (1954a). On the bacteria causing spoilage of fresh fish, especially on their activity which can produce histamine. Mem. Research Inst. Food Set. Kyoto Univ. 7, 12-17.
Kimata, M., and Tanaka, M. (1954b). A study whether the bacteria having an activity which can produce a large amount of histamine, so-called histamine- former, are present or not, on the surface of fresh fish. Mem. Research Inst.
Food Set. Kyoto Univ. 8, 7-16.
Kimata, M., Kawai, Α., and Tanaka, M. (1954a). The freshness of fish and the amount of histamine present in the meats, III. Mem. Research Inst. Food Sei.
Kyoto Univ. 7, 6-11.
Kimata, M., Kawai, Α., and Tanaka, M. (1954b). The freshness of fish and the amount of histamine present in the meats, IV. Mem. Research Inst. Food Sei.
Kyoto Univ. 8, 1-6.
Kimata, M., Kawai, Α., and Akamatsu, M. (1958). Classification and identification of the bacteria having an activity which can produce a large amount of histamine. Mem. Research Inst. Food Set. Kyoto Univ. 14.
Koessler, Κ. K., and Hanke, Μ. T. (1919). Studies on proteinogenous amines.
IV. The production of histamine from histidine by Bacillus colt communis.
J. Biol. Chem. 39, 539-556.
Koessler, Κ. K., Hanke, Μ. T., and Sheppard, M. S. (1928). Production of histamine, tyramine, bronchospastic, and arteriospastic substances in blood broth by pure cultures of microorganisms. /. Infectious Diseases 43, 363-377.
Legroux, R., and Levaditi, J . C. (1947). Origine de Thistamine presente dans la chair de thons responsables d'intoxications collectives. Compt. rend. soc. biol.
141, 998-1000.
Legroux, R., Levaditi, J . C , Boudin, G., and Bovat, D. (1946). Intoxications histaminiques collectives ä Tingestion de thon frais. Presse med. 39, 545-546.
Markoff, W. Ν. (1939). Zum Problem der Seefisch-Fäulnis. Zentr. Bakteriol.
Parasitenk. Abt. II 101, 151-171.
Miyaki, K., and Hayashi, M. (1954). Food poisoning caused by ordinary putrefac
tion. III. Detection of histamine and its synergistic factor in deteriorated dried mackerel pike. (In Japanese with English summary.) /. Pharm. Soc. Japan 74, 1145-1148.
Miyaki, T., Hayashi, M., and Ando, S. (1956). Putrefactive amines. (In Japanese.) Rept. Inst. Food Microbiol. Chiba Univ. 9, 135-144.
Motohiro, T., and Tanikawa, E . (1952). Studies on food poisoning of mollusks, especially of squid and octopus meat. II. Prosperity and decline of amines during putrefaction of squid meat. (In Japanese.) Bull. Fac. Fisheries Hok
kaido Univ. 3 ( 2 ) , 154-174.
Nash, J. B. (1952). Formation of histamine from carnosine and histidine by Escherichia colt. Texas Repts. Biol, and Med. 10, 639-646.
Nowitzki, Ε . von. (1941). Zum Problem der Toxinbildung durch bakterielle Zersetsung des Fischfleisches. Zentr. Bakteriol. Parasitenk. Abt. I Orig. 148(1), 364-376.
Ogasawara, K., Abe, S., Ito, I., Yoneda, M., Asano, N., Takatori, T., and Watanabe, S. (1953). Antibiological studies. II. Production of toxic amines by strains of Shigella dysenteriae. (In Japanese.) Igaku 40, 99-103.
Okazaki, H., and Harada, S. (1959). Studies on an outbreak of histamine poison
ing caused by fresh fish in Kagawa prefecture. I. General view of the outbreak and histamine-producing ability of the isolated bacteria. (In Japanese with English summary.) /. Japan. Vet. Med. Assoc. 12(6), 259-262.
Ota, F. (1958). On the formation of amine in fish muscle. III. Simple method for the detection of histamine in fish muscle. Bull. Japan. Soc. Sei. Fisheries 24(1), 37-40.
Ota, F., and Kaneko, K. (1958). On the formation of amine in fish muscle. VII.
Effect of freezing on the histamine formation in the thawed fish muscle. (In Japanese with English summary.) Bull. Japan. Soc. Sei. Fisheries 2 4 ( 2 ) , 140-143.
Pergola, M. (1937). Bacteries toxigenes chez le poisson frais avec ou sans la presence de richthyovenin. Boll. sez. ital. soc. intern, microbiol. 9 ( 5 ) , 105-108.
Pergola, M. (1956). I/ictioveleno e istamina. Rend. inst. super, sanita 19, 419-432.
Peria, G., and Salamone, F. P. (1954). Antibiotic activity of histamine in vitro.
Med. sper. 23, 177-80.
Sager, O. S., and Horwitz, N. (1957). A chemical method for the determination of histamine in canned tuna fish. /. Assoc. Offic. Agr. Chemists 4 0 ( 3 ) , 892-904.
Simidu, W., and Hibiki, S. (1954a). Studies on putrefaction of aquatic products.
XIII. Comparison on putrefaction of different kinds of fish ( 1 ) . Bull. Japan.
Soc. Sei. Fisheries 2 0 ( 4 ) , 298-301.
Simidu, W., and Hibiki, S. (1954b). Studies on putrefaction of aquatic products.
XIV. Comparison on putrefaction of different kinds of fish ( 2 ) . (In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 20(4), 302-304.
Simidu, W., and Hibiki, S. (1954c). Studies on putrefaction of aquatic products.
XV. Comparison of putrefaction for round, fillet, minced and denatured fishes.
(In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 2 0 ( 5 ) , 388-391.
Simidu, W., and Hibiki, S. (1954d). Studies on putrefaction of aquatic products.
XVI. Consideration on difference in putrefaction for various kinds of fish ( 1 ) . (In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 2 0 ( 5 ) , 392-395.
Simidu, W., and Hibiki, S. (1954e). Studies on putrefaction of aquatic products.
XVII. Consideration of difference in putrefaction for various kinds of fish ( 2 ) . Influence upon tenderness of flesh. (In Japanese with English summary.) Bull.
Japan. Soc. Set. Fisheries 2 0 ( 8 ) , 717-719.
Simidu, W., and Hibiki, S. (1955a). Studies on putrefaction of aquatic products.
XIX. Influence of certain substances upon histamine formation. (In Japanese with English summary.) Bull. Japan. Soc. Sei. Fisheries 2 0 ( 9 ) , 808-810.
Simidu, W., and Hibiki, S. (1955b). Studies on the putrefaction of aquatic products. XX. Considerations of difference in putrefaction for various kinds of fish ( 3 ) . Influence of autolysis on putrefaction. (In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 21(4), 267-279.
Simidu, W., and Hibiki, S. (1955c). Studies on putrefaction of aquatic products.
XXI. Consideration of difference in putrefaction for various kinds of fish. ( 4 ) . On difference of concentration of histidine between the interior and the ex
terior of cells. (In Japanese with English summary.) Bull. Japan. Soc. Set.
Fisheries 2 1 ( 5 ) , 357-360.
Simidu, W., and Hibiki, S. (1955d). Studies on putrefaction of aquatic products.
XXIII. On the critical concentration of poisoning for histamine. (In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 21(5), 365-367.
Simidu, W., Ikeda, S., and Kurokawa, Y. (1953a). Studies on muscle of aquatic animals, 18. On L-histidine decarboxylase. (In Japanese with English sum
mary. ) Bull. Research Inst. Food Set. Kyoto Univ. 12, 49-56.
Simidu, W., Kurokawa, Y., and Ikeda, S. (1953b). Muscle of aquatic animals.
XVII. Imidazole compounds in fish muscles, with special references to the taste of red-muscle fishes. (In Japanese with English summary.) Bull. Research Inst. Food Set. Kyoto Univ. 12, 40-48.
Simidu, W., Hibiki, S., and Nagasaki, S. (1955). Studies on putrefaction of aquatic products. XVIII. On putrefaction of some white-muscle fishes, mollusks and shrimp. (In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 2 0 ( 9 ) , 804-807.
Str0m, Α., and Lindberg, W. (1954). Forgiftning fremkalt vid nytelse av mak- rellstr0ge. Nord. Med. 26, 903-906.
Suzuki, U., and Yoshimura, K. (1909). Über die Extraktivstoffe des Fisch
fleisches. Ζ. physiol. Chem. 62, 1-35.
Suzuki, U., Yoneyama, C , and Odake, S. (1912). Composition of 'bonito' salt paste. (In Japanese.) /. Coll. Agr. Tokyo Imp. Univ. 5, 33-41.
Torres-Acero, J. M. (1956). Contenido de histamina de algunos alimentos. Anales hromatol (Madrid) 8 ( 3 ) , 345-348.
Tsuda, Α., and Tomiyama, T. (1959). Studies on the method for testing the spoilage of food. XI. A new method for determination of histamine in tissues.
(In Japanese with English summary.) Bull. Japan. Soc. Sei. Fisheries 2 5 ( 6 ) , 451-456.
van Veen, A. G., and Latuasan, Η. E . (1950). Fish poisoning caused by histamine in Indonesia. Documenta Need, et Indones. Morhis Trop. 2, 18-20.
Williams, D. W. (1954). Report on chemical indices of decomposition in fish (histamine). /. Assoc. Offic. Agr. Chemists 3 7 ( 3 ) , 567-572.
Williams, D. W. (1956). Report on chemical indices of decomposition in fish (histamine). /. Assoc. Offic. Agr. Chemists 39, 609-612.
Williams, D. W. (1957). Report on decomposition of fish (histamine). /. Assoc.
Offic. Agr. Chemists 4 0 ( 2 ) , 420-421.
Wilhams, D. W. (1959). Report on chemical indices of decomposition in fish (histamine). /. Assoc. Offic. Agr. Chemists 42(2), 287-289.