N a - D E P E N D E N T T R A N S P O R T O F γ - A M I N O B U T Y R I C A C I D I N S U B C E L L U L A R B R A I N P A R T I C L E S
1
S. V A R O N A N D W. W I L B R A N D T
Department of Genetics, Stanford University, Palo Alto, California, and Department of Pharmacology, University of Berne, Switzerland,
γ - A m i n o b u t y r i c acid (GABA) is of interest to the neuroscientists on a n u m b e r of grounds [ 1 1 ] . Although extensively present in lower orga- nisms and in plants, in the v e r t e b r a t e s G A B A can be found only in the central nervous system ( C N S ) . I t is formed within t h e C N S by decarboxylation of glutamic acid a n d is metabolized to succinic acid by t r a n s a m i n a t i o n (with α-ketoglutaric acid) a n d dehydrogena- tion. T h e presence of G A B A and of the three G A B A - r e l a t e d enzymes t h u s confers on t h e v e r t e b r a t e C N S t h e unique ability to b y p a s s t h e K r e b s cycle p a t h w a y for the conversion of α-ketoglutarate to suc- cinate. I t has been calculated t h a t as much as 4 0 % of t h e energetic metabolism of brain could go through this special G A B A shunt. A neurophysiological role of G A B A in t h e v e r t e b r a t e C N S is suggested by its demonstrable inhibitory effects on t h e evoked electrical a c t i v i t y of cerebral and cerebellar cortex, spinal cord, a n d other p a r t s of t h e C N S . F u r t h e r m o r e , in the crustacean peripheral nervous system, G A B A has been shown to mimic t h e stimulation of inhibitory fibers;
the finding [9] t h a t G A B A is present in such fibers, b u t n o t in motor or sensory ones, h a s raised t h e question of whether this compound acts as an inhibitory n e u r o t r a n s m i t t e r or, more generally, as an in- hibitory neuromodulator. Indeed, G A B A presents [20] a striking n u m - ber of analogies, both a t the biochemical a n d t h e functional level, with the catecholamines, t h e i m p o r t a n c e of which in t h e modulation of C N S activity is well established, even though only p a r t i a l l y under- stood.
M o r e recent findings indicate t h e existence of mechanisms for intracellular t r a n s p o r t of G A B A in brain. P a r t i c u l a t e m a t t e r sedi- mented from brain homogenates retains a sizable portion of t h e 1
This work was supported in part by Grant R-178-64 from the United Cere- bral Palsy Foundation and in part by Research Grant N B 04270-03 from the National Institute for Neurological Diseases and Blindness of the National Institutes of Health.
119
120 S. VARON AND W. WILBRANDT
t o t a l GABA content in brain, as first reported by Elliott a n d v a n Gelder [ 5 ] . Sano a n d R o b e r t s [14] showed t h a t such particles, in contrast with those from other tissues, a t 0 ° C can " b i n d " radioactive G A B A from t h e m e d i u m provided N a ions are present. Subsequent investigations, summarized in a recent symposium [22, 2 8 ] , gave evi- dence t h a t exogenous G A B A is actually t r a n s p o r t e d into brain p a r - ticles a t 0 ° C a n d a t higher t e m p e r a t u r e s , and t h a t in both cases N a ions are involved. This N a - d e p e n d e n c e of G A B A t r a n s p o r t parallels t h a t reported for other t r a n s p o r t systems both a t t h e cellular [2-4, 7, 16, 23-26, 29] and t h e subcellular [1] levels. T h e aim of t h e present p a p e r is a further analysis of t h e available d a t a on G A B A t r a n s p o r t
2
a n d a comparison with t h e other N a - d e p e n d e n t systems. I t will be shown t h a t t h e hypothesis [3, 4, 23-26] of an equilibrating carrier mechanism, coupling t h e t r a n s p o r t of s u b s t r a t e and N a - i o n s through a common carrier and supported by an energy-dependent extrusion of N a , a d e q u a t e l y fits t h e G A B A system a n d can in fact offer a suggestive basis for a relationship between the metabolic and n e u r o - physiological properties of G A B A in t h e v e r t e b r a t e C N S .
T H E GABA S Y S T E M A T 0°C [18-20, 27]
A 10% mouse b r a i n homogenate in 0.25 M sucrose yields, upon centrifugation between 1,500 and 15,000 g, a highly heterogeneous pellet which comprises mitochondria, microsomes, and pinched-off nerve endings ( s y n a p t o s o m e s ) . All three t y p e s of particles retain endogenous GABA, while no appreciable a m o u n t s of G A B A are found t o associate with t h e m e m b r a n o u s s t r a n d s a n d myelin m a t e r i a l of t h e pellet. T h e pellet also contains some free G A B A in t h e entrained sucrose s u p e r n a t a n t .
W h e n t h e pellet is resuspended in a buffered N a C l medium contain- ing a small a m o u n t of
1 4
C - G A B A , incubation a t 0 ° C results in a r a p i d accumulation of r a d i o a c t i v i t y by t h e particles (reaching a m a x i - m u m within 30 minutes) which is accompanied by a relatively smaller increase of t h e p a r t i c u l a t e G A B A . P a r t of t h e acquired r a d i o a c t i v i t y remains in equilibrium with t h e m e d i u m a n d can be diluted out by adding an excess of nonradioactive free G A B A to the system. B o t h
2
These data were obtained in Dr. E . Roberts' laboratory at the City of Hope Medical Center, California, more than two years ago. They are reported and discussed elsewhere [17-22, 27, 28]. T h e additional analysis given here, and the conclusions drawn, reflect only the personal opinions of the present authors.
T R A N S P O R T O F γ - A M I N O B U T Y R I C ACID I N B R A I N P A R T I C L E S 121
t h e r a d i o a c t i v i t y a n d t h e G A B A present in this r a p i d l y equilibrating pool are p r o m p t l y released from t h e particles after their transfer to a Na-free medium. T h e r a d i o a c t i v i t y r e m a i n i n g with t h e particles and most of their endogenous G A B A constitute a second pool, which decreases in size a n d increases in specific a c t i v i t y with prolonged incubation in N a - c o n t a i n i n g media. Such changes occur m u c h more slowly t h a n those affecting t h e first pool. T h e y become b a r e l y notice- able in Na-free media. Evidence from electron microscopy studies indicates t h a t both pools are associated with t h e same particles and are in fact present in all three t y p e s of membrane-enclosed particles occurring in t h e system. T h e following model h a s been suggested.
1. T h e m e m b r a n e s which enclose mitochondria, microsomes, a n d nerve-ending particles from brain are impermeable to free GABA.
2. These m e m b r a n e s contain sites which are capable of binding G A B A only in t h e presence of N a - i o n s . Exposure of t h e particles to a N a - c o n t a i n i n g m e d i u m t h u s results in t h e acquisition of exoge- nous G A B A a n d the formation of a r a p i d l y equilibrating pool.
3. T h e binding sites are mobile carriers a n d cross t h e m e m b r a n e (or t h e barriers within t h e m e m b r a n e ) a t a r a t e which, a t 0 ° C , is considerably slower t h a n t h a t a t which t h e y bind or exchange G A B A . Because of such carrier m o v e m e n t s , r a d i o a c t i v i t y enters t h e internal pool a n d internal G A B A moves out, with a n e t progressive loss of p a r t i c u l a t e G A B A owing to t h e higher G A B A concentration inside t h a n outside t h e barrier.
According to t h e model, an equilibrating carrier mechanism is r e - sponsible for t h e slow changes in t h e i n t e r n a l G A B A pool while t h e outer, r a p i d l y equilibrating, pool merely reflects the binding of free external G A B A by t h e N a - a c t i v a t e d carriers present a t a n y t i m e outside t h e barrier. T h e m e a s u r a b l e size of t h i s r a p i d l y equilibrating pool implies a relatively high n u m b e r of carrier molecules as compared with estimates for other carrier systems [ 6 ] .
T H E GABA S Y S T E M A T 29°C [17, 20, 22]
T h e same particles, suspended in buffered N a C l m e d i u m containing 1 4
C - G A B A a n d i n c u b a t e d a t 0 ° C for a brief period to allow t r a c e r accumulation, show a m a r k e d l y different behavior when further in- cubated a t 2 9 ° C u n d e r a c o n s t a n t air flow. T h r e e major processes t a k e p l a c e : massive release of p a r t i c u l a t e GABA, u p t a k e of free G A B A into some particles, and active metabolic d e g r a d a t i o n of G A B A within t h e same particles.
122 S. VARON AND W. WILBRANDT
1. Release of p a r t i c u l a t e G A B A occurs a t a much faster r a t e a t 29° t h a n a t 0 ° C . This can be directly d e m o n s t r a t e d for microsomes by use of pure microsomal p r e p a r a t i o n s (which, a t 0 ° C , h a v e been shown [18, 19] to behave with respect to G A B A in t h e same w a y as t h e heterogeneous suspension discussed t h u s f a r ) . I t is also clearly observable in t h e s t a n d a r d heterogeneous suspensions under such con- ditions as a nitrogen atmosphere, where u p t a k e and metabolism of G A B A are inhibited; the complete removal of p a r t i c u l a t e G A B A t h a t can be achieved with a sufficiently long incubation demonstrates t h a t this release process is not confined to the microsomal constituents of t h e suspension, b u t involves all its G A B A - c o n t a i n i n g particles.
U n d e r these inhibitory conditions, all the G A B A lost by the particles is recovered in t h e m e d i u m ; therefore, a plot of p a r t i c u l a t e GABA levels versus time yields a time curve for t h e release process. One of the most striking experimental findings was t h a t such a p a r t i c u l a t e G A B A t i m e curve is identical to those o b t a i n a b l e under air or pure oxygen, where both u p t a k e and metabolism of G A B A t a k e place, suggesting t h a t these two processes occur in such a w a y as to balance each other out. This is borne out by independent evidence indicating (see below) t h a t the G A B A newly t a k e n up is metabolized very r a p - idly and does not contribute significantly to the p a r t i c u l a t e GABA levels. I t has therefore been concluded t h a t essentially the same m a s - sive release occurs under both air a n d nitrogen and is in both cases depicted by t h e p a r t i c u l a t e G A B A time curve.
2. A t 0 ° C , t h e slow b u t progressive release of p a r t i c u l a t e G A B A results in a corresponding increase in t h e free G A B A levels of t h e medium. I n contrast with this p a t t e r n , a t 2 9 ° C t h e GABA content of the medium undergoes a considerable drop in the initial 30 to 60 m i n u t e s of incubation, a n d remains c o n s t a n t thereafter. T h i s d e - crease has been shown not to be due to metabolism of free GABA within t h e suspending fluid a n d can therefore only result from the u p t a k e of free G A B A by t h e particles. I n fact, u p t a k e of GABA by t h e particles m u s t occur a t a much greater extent and for a longer period t h a n indicated by t h e G A B A depletion in the medium since, as discussed in t h e preceding p a r a g r a p h , a considerable release of GABA is t a k i n g place throughout the incubation. T h e u p t a k e of GABA does not result in a n y observable increase of the p a r t i c u l a t e G A B A levels b u t is accompanied by an accumulation in t h e particles of G A B A metabolites. This finding, t h a t t h e newly t a k e n - u p G A B A is immediately m a d e available to metabolic degradation, d e m o n s t r a t e s
T R A N S P O R T O F γ - A M I N O B U T Y R I C ACID I N B R A I N P A R T I C L E S 123
t h a t the u p t a k e is not due to an increased binding c a p a c i t y of t h e particles and, furthermore, t h a t it t a k e s place in particles which h a v e the ability to metabolize G A B A (see below). T h e u p t a k e of free GABA by such particles does not occur in Na-free media and is in- hibited b y cardiac glycosides, dinitrophenol, lack of oxygen, and by specific inhibitors of G A B A metabolism (such as aminooxyacetic acid) ; in the last case, addition of another energy source such as p y r u v a t e restores t h e ability of the particles to t a k e u p GABA. T h u s , G A B A u p t a k e a t 2 9 ° C is a process which is both N a - d e p e n d e n t and energy-dependent and which draws n o r m a l l y t h e required energy from the metabolic degradation of GABA itself.
3. Evidence for active G A B A metabolism is provided by the rapid and progressive decrease of the t o t a l G A B A content in t h e system.
N o G A B A metabolism has been found to occur in t h e m e d i u m (after removal of the particles) or in the microsomal particles. M i t o c h o n d r i a , on the other hand, are clearly indicated as G A B A metabolizing p a r - ticles by their reported content of G A B A - t r a n s a m i n a s e [13] a n d t h e observed involvement of K r e b s cycle steps in t h e metabolic d e g r a d a - tion of GABA t a k i n g place in t h e system [ 1 7 ] . I t is possible, although less likely [ 1 3 ] , t h a t mitochondria-containing nerve-ending particles also contribute to G A B A metabolism. Inhibition of G A B A metabolism can be achieved by incubating the particles in t h e absence of oxygen (nitrogen flow) or in t h e presence of aminooxyacetic acid, a k n o w n specific inhibitor of G A B A - t r a n s a m i n a s e . I t is also observed under all conditions (see above) where u p t a k e of free G A B A is inhibited.
This, and the i m m e d i a t e metabolic d e g r a d a t i o n undergone by t h e newly t a k e n - u p G A B A (see a b o v e ) , strongly suggest t h a t most, if not all, of the G A B A degraded in the system is m a d e available to the metabolic sites by t h e u p t a k e process.
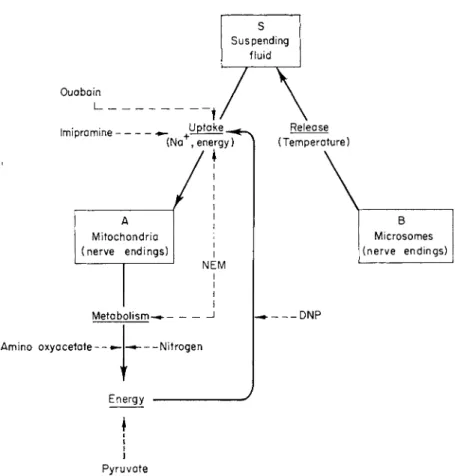
T h e m a i n features of the 29°C G A B A system are shown d i a g r a m - m a t i c a l l y in Fig. 1. T h e particles are regarded as two functionally distinct classes (A, B) linked through the suspending fluid ( S ) . Only one class (A) is the site of active G A B A metabolism and t a k e s up free G A B A from t h e medium. T h e other class (B) r a p i d l y releases its G A B A content into t h e suspending fluid a n d t h u s m a k e s it available t o u p t a k e and metabolism by t h e A particles. Owing to t h e cyclic n a - t u r e of the sequence metabolism - » energy u p t a k e -> metabolism, interference a t a n y level will bring a b o u t the interruption of both metabolism and u p t a k e .
A better u n d e r s t a n d i n g of t h e system and an exact kinetic s t u d y
124 S. VARON AND W. WILBRANDT
Ouabain
Imipramine
Amino o x y a c e t a t e - - * -
Microsomes (nerve endings)
Pyruvate
FIG. 1. Schematic representation of the interrelationships between particles A and particles B.
of t h e two t y p e s of G A B A m o v e m e n t involved will obviously have to a w a i t additional d a t a on t h e separate behavior of t h e different t y p e s of particles, as well as on sodium distribution in t h e particles.
On t h e basis of t h e available evidence, however, a n u m b e r of a d d i - tional considerations can be discussed.
The Release of GABA from the Β Particles (29°C)
T h i s process is considerably faster t h a n t h e release occurring a t 0°C. I t is, on t h e other hand, only slightly, if a t all, slowed down in Na-free media. I t is n o t affected b y t h e occurrence or absence of energy-yielding metabolism in t h e A particles. I t is also unaffected
T R A N S P O R T O F y - A M I N O B U T Y R I C ACID I N B R A I N P A R T I C L E S 125
by the concentration of free G A B A in t h e m e d i u m (which increases with time under nitrogen, decreases to a low c o n s t a n t level u n d e r a i r ) .
Based upon t h e p a r t i c u l a t e G A B A time curve, a kinetic analysis can be m a d e , as summarized in Fig. 2. I t is found t h a t a double reciprocal plot of G A B A decrement in t h e particles (p0 — p) versus time (£) yields a s t r a i g h t line, the equation for which can be written
— J — = a + β \ (1)
where a = l/p0 and the slope β characterizes t h e release a t 2 9 ° C . F r o m the previous equation, one o b t a i n s :
T h e validity of this t r e a t m e n t has been verified by i n t e g r a t i n g E q . (2) into
a n d plotting against time t h e reciprocals of t h e various experimental values for p. T h e rigorous linearity of this plot is shown in Fig. 2.
E q u a t i o n (2) states t h a t t h e r a t e a t which G A B A is released is a second-order function of t h e G A B A left in t h e particles. T h i s rules out diffusion as t h e u n d e r l y i n g mechanism for t h e release. I t is also h a r d to reconcile with t h e i n t e r p r e t a t i o n t h a t the G A B A release results from a t e m p e r a t u r e - e n h a n c e d breakdown of t h e Β particles. Possible mechanisms compatible with this second-order relationship include a carrier m e c h a n i s m operating under low s a t u r a t i o n conditions and with complexes involving two s u b s t r a t e molecules per carrier. U n d e r the same conditions, t h e above-mentioned irrelevance of t h e external G A B A concentration would be consistent with a carrier m e c h a n i s m in view of t h e large difference between the G A B A concentrations inside and outside t h e particles.
T h e r a p i d i t y with which t h e G A B A newly entering t h e particles is metabolized a n d t h e a t t r i b u t i o n to t h e releasing Β class of all G A B A - c o n t a i n i n g particles suggest t h a t u n d e r s t a n d a r d conditions
( 2 )
The Uptake of GABA into the A Particles {29°C)
126 S. VARON AND W . W I L B R A N D T
Ρ
\
I I I I I I I / 30 60 90 120 150 180 210 min
I P0-P
0 . 5 - > ^ _ L _ = + / 3 - L a
P0'P
H
t
= -kp
dt
0.1 -
I I \/t 0.01 0.03
J_
Ρ
1.5-
y ir-ir
+"
0.5- y
I I I I /
30 90 150 210 min
Fie. 2. Kinetic analysis of GABA release at 29°C from particles B.
T R A N S P O R T O F γ - A M I N O B U T Y R I C ACID I N B R A I N P A R T I C L E S 127
the free G A B A inside the A particles is brought down t o , a n d m a i n - tained at, negligible concentrations by its metabolic degradation. A G A B A gradient would t h u s obtain between external a n d internal free G A B A which could be t h e driving force for the G A B A u p t a k e into the A particles. I n fact, it w
T
as observed t h a t increasing G A B A concen- t r a t i o n s in t h e m e d i u m result in increasing r a t e s of disappearance from the medium. T h e N a - d e p e n d e n c e of t h e u p t a k e process, among other considerations, rules out a passive diffusion of G A B A along the metabolically m a i n t a i n e d G A B A gradient. I t is therefore t e m p t i n g to interpret t h e G A B A u p t a k e into A particles as a downhill t r a n s p o r t mediated by the same equilibrating carrier mechanism t h a t has been postulated in t h e 0 ° C system.
T h e issue is, however, complicated by other features of t h e process.
T h e sensitivity to ouabain, for instance, can h a r d l y be explained as a direct inhibition of metabolic processes by cardiac glycoside (for which no examples h a v e ever been c i t e d ) , and the possibility t h a t ouabain interferes with the N a - a c t i v a t e d binding ability of t h e carrier is ruled out by t h e demonstrable lack of such an effect a t 0 ° C . M o r e - over, there are experimental conditions under which t h e G A B A u p t a k e results in an accumulation of G A B A in t h e particles and, provided the p a r t i c u l a t e G A B A is in a free form, the system a p p e a r s to behave as a G A B A uphill t r a n s p o r t . This is the case, for example, where G A B A u p t a k e t a k e s place in spite of specifically blocked G A B A m e - tabolism (with the support of p y r u v a t e ) . W i t h o u t additional features, then, an equilibrating carrier system cannot account for all t h e ob- served properties of the GABA u p t a k e a t 2 9 ° C . However, the involve- m e n t of N a - i o n s and t h e sensitivity to cardiac glycosides allow for an i n t e r p r e t a t i o n of the whole process in t e r m s of a downhill equili- b r a t i n g carrier mechanism for a mixed Na-GABA carrier complex sustained by the simultanous action of a N a - p u m p . Such an inter- p r e t a t i o n is suggested by t h e comparison of t h e G A B A system with other N a - d e p e n d e n t t r a n s p o r t systems, to be discussed in the following section.
N a - D E P E N D E N T " U P H I L L " T R A N S P O R T S Y S T E M S
Since t h e 1953 report [15] t h a t cardiac glycosides inhibit sodium and potassium uphill t r a n s p o r t in red blood cells, similar observations have been m a d e in m a n y other cell t y p e s [8] ; in fact, no cell t y p e a p p e a r s to h a v e been tested t h u s far with negative results. This wide- spread inhibitory effect of cardiac glycosides on N a
+ a n d K
+
t r a n s p o r t
128 S. VARON AND W. WILBRANDT
is characterized b y two features: I t is m a i n l y affecting t h e uphill m o v e m e n t of t h e ions a n d it does n o t derive from interference w i t h t h e energy-yielding metabolism. I n recent y e a r s , a n u m b e r of other t r a n s p o r t systems concerning sugars a n d amino acids h a v e also been found to be inhibited b y cardiac glycosides; t h e suggestion occasion- ally h a s been m a d e t h a t cardiac glycosides h a v e a general action on p u m p i n g systems r a t h e r t h a n a specific one for t h e N a - K t r a n s p o r t . A v e r y strong point against such a suggestion is, however, t h a t in all the cases where cardiac glycoside inhibition was observed, t h e t r a n s - port was found to be N a - d e p e n d e n t . T h i s fact supports t h e a l t e r n a - tive interpretation, t h a t in these cases t h e glycoside inhibitory action is directly exerted on a sodium-potassium p u m p linked in some m a n - ner to t h e s u b s t r a t e t r a n s p o r t . If this i n t e r p r e t a t i o n is accepted, t h e question m a y be asked whether t h e relationship to t h e sodium t r a n s - port is by w a y of a direct coupling between sodium p u m p a n d s u b - s t r a t e p u m p or whether the role of the sodium p u m p is r a t h e r t o m a i n t a i n a sodium gradient. I n the case of iodide t r a n s p o r t , this ques- tion has been studied by Iff [7] by following t h e u p t a k e of radioactive iodide into t h y r o i d slices, continuously perfused directly u n d e r n e a t h a scintillation counter. I n an initial phase, t h e iodide u p t a k e was inhibited by using a lithium- instead of a sodium-containing medium.
L a t e r , ouabain was added and a sufficient time was allowed for t h e glycoside action to develop fully. T h u s , a condition was reached under which the sodium p u m p was certainly blocked, b u t a sodium accumu- lation h a d been p r e v e n t e d t h r o u g h t h e absence of sodium in the ex- ternal medium. T h e n , lithium was replaced by sodium without remov- ing t h e glycoside. T h e restored sodium gradient r e a c t i v a t e d t h e iodide p u m p t e m p o r a r i l y even though t h e sodium p u m p still was, and re- mained, blocked. T h e answer therefore was t h a t it is n o t necessary for iodide uphill t r a n s p o r t t h a t t h e sodium p u m p be a c t u a l l y running, b u t t h a t its functioning is required for t h e m a i n t e n a n c e of a sodium gradient.
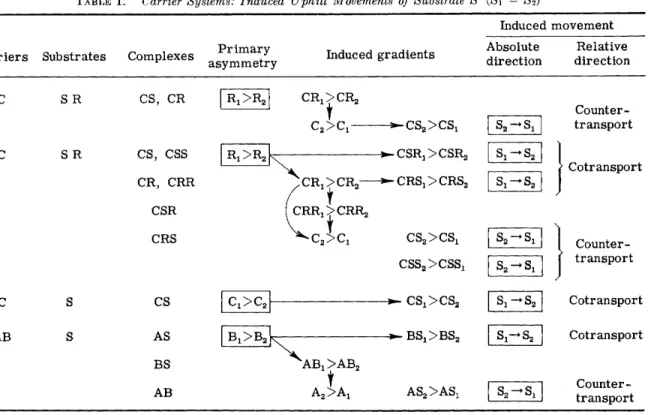
One possibility for a sodium gradient to do t r a n s p o r t work would be to h a v e the N a - t r a n s p o r t linked to a second t r a n s p o r t system by m e a n s of a common carrier. I n systems of this kind, uphill t r a n s - p o r t m a y be induced in various w a y s [ 3 0 ] . E x a m p l e s are listed in T a b l e I. Among t h e m t h e best-known case is t h a t of c o u n t e r t r a n s p o r t [ 1 2 ] , which has been observed in a n u m b e r of cell t y p e s and with different substrates. C o t r a n s p o r t in systems involving t e r n a r y com- plexes also h a s been reported, a n d recent i n t e r p r e t a t i o n s of amino
TABLE I . Carrier Systems: Induced Uphill Movements of Substrate S (Si = S 2)
Induced m o v e m e n t C a r r i e r s Substrates Complexes P r i m a r y
a s y m m e t r y Induced gradients Absolute direction
Relative direction S R
S R
C S AB S
CS, CR
CS, CSS
CR, CRR CSR CRS
CS AS BS AB
R i > R2 C R ^ C R j ,
1
J
c2> c1—
R1> R2
C i > C2 B ^ B ,
fr-CS^CS!
- C S R1> C S R2
CR^ CR2
t
CRRi > CRR^
t
C2> C1
c s2> c s1 css2>css1
^ A B ^ A B ,
t
A2> A !
C S ^ C S , B S ^ B S ,
A S2> A S1
Si~*S2 - C R S1> C R S2 Sj—S.
S 2- S , S 2 - S , Si ~*s2
S ! - S2
$2^ Si
Counter- transport
Cotransport
Counter- transport
Cotransport Cotransport
Counter- transport
TRANSPORT OF γ-AMINOBUTYRIC ACID IN BRAIN PARTICLES 129
130 S. VARON AND W. W1LBRANDT
acid t r a n s p o r t into single cells [23-26] and of glucose absorption from t h e intestine [2-4, 16] h a v e assumed mixed complexes involving one carrier molecule and different ratios of sodium ions and amino acid or sugar molecules. Of p a r t i c u l a r interest for t h e G A B A system is t h e successful analysis of glycine t r a n s p o r t into a v i a n red cells by Vidaver [ 2 3 - 2 6 ] . H e interprets t h e amino acid u p t a k e into t h e cells as a downhill m o v e m e n t of a carrier complex with 1 molecule of amino acid and 2 sodium ions. This downhill m o v e m e n t becomes in effect an uphill m o v e m e n t of the amino acid because t h e extrusion of sodium by the sodium p u m p results in the m a i n t e n a n c e of a steep gradient for t h e mixed complex. Since t h e sodium p u m p is blocked by cardiac glycosides, the uphill amino acid movement also stops in t h e presence of this inhibitor. Vidaver showed t h a t changing t h e driving force for t h e N a - m o v e m e n t either b y establishing a D o n n a n equilibrium or by reversing the sodium gradient (using t h e hemolysis method of S t r a u b ) , the m o v e m e n t of t h e amino acid could be changed a t will, and even reversed, in accordance with prediction. F r o m t h e observation t h a t a L i n e w e a v e r - B u r k plot of u p t a k e r a t e versus sodium concentration fails to give a straight line, whereas a similar plot versus the square of sodium concentration is linear, he concluded t h a t two sodium ions per molecule of amino acid are involved in t h e t r a n s p o r t complex. This was confirmed in experiments in which t h e t r a n s p o r t r a t e was v a r i e d : t r a n s p o r t increase of one molecule of glycine was accompanied by an increment of two sodium ions. A somewhat similar analysis, using short-circuit current as a measure of N a - t r a n s p o r t , was carried out in the case of glucose absorption from the intestine [ 2 , 1 6 ] .
According t o the L i n e w e a v e r - B u r k analysis, t h e effect of N a on the a p p a r e n t carrier p a r a m e t e r s appeared to be on t h e Km in Vidaver's experiments on amino acid t r a n s p o r t in pigeon red cells [ 2 3 - 2 6 ] , as well as in C r a n e ' s experiments on intestinal glucose absorption [ 3 , 4 ] . E x - periments on glucose absorption by Schultz and Zalusky [ 1 6 ] , however, indicated a n effect on Vmax. W h e t h e r , in a mixed complex system a s discussed, the effect of sodium should be expected on the a p p a r e n t Km or t h e a p p a r e n t Vmax of t h e carrier depends on t h e t y p e of binding.
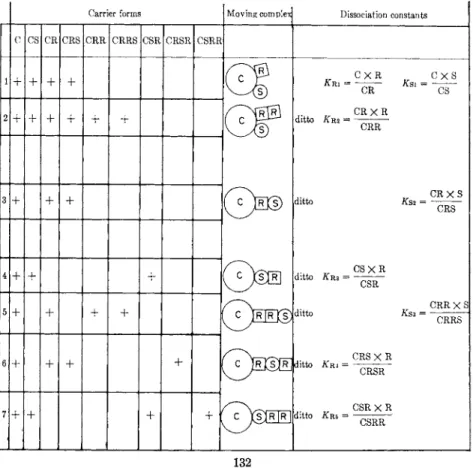
T a b l e I I shows two different possibilities for 1:1 and 2 : 1 N a - s u b s t r a t e complexes with carrier ( C ) . " I n d e p e n d e n t " binding implies binding sites for N a (R) and for s u b s t r a t e (S) without interdependence (mean- ing t h a t S reacts equally well with C and with C R or C R2) . If? then, of
T R A N S P O R T O F γ - A M I N O B U T Y R I C ACID I N B R A I N P A R T I C L E S 131
all possible complexes only C R S (case 1) or C R R S (case 2) is able to move across the m e m b r a n e , the effect of R is on t h e a p p a r e n t Vmax. On the other h a n d , a n effect on t h e a p p a r e n t Km emerges if S only, or preferentially, binds to C R (case 3) or C R R (case 5) r a t h e r t h a n to C (owing to spatial reasons or an allosteric effect of R on C ) . Theoretical possibilities also exist t h a t S has to bind before R (cases 4 a n d 7) or
in between the binding of t h e first a n d the second R (case 6 ) , yielding an effect of R both on Km and Vm&x.
I n view of t h e successful i n t e r p r e t a t i o n of these cellular s y s t e m s in t e r m s of mixed complexes, in view f u r t h e r m o r e of t h e close chemical relationship b e t w e e n G A B A a n d α - a m i n o acids, i t a p p e a r s possible t h a t a similar m e c h a n i s m is involved in t h e N a - d e p e n d e n t , o u a b a i n - sensitive u p t a k e of G A B A i n t o t h e A particles (brain m i t o c h o n d r i a ) . As i l l u s t r a t e d b y t h e scheme given in Fig. 3, t h e p a r t i c u l a r conditions o b t a i n i n g in a mixed complex s y s t e m result in t w o c h a r a c t e r i s t i c f e a t u r e s : (a) T h e net m o v e m e n t of S a n d R (in t h i s case G A B A a n d N a ) m u s t occur in a m o l a r r a t i o fixed b y t h e t y p e of complex (in V i d a v e r ' s e x p e r i m e n t s 1:2); a n d (b) t h e unidirectional fluxes are func- tions of t h e c o n c e n t r a t i o n s for b o t h S a n d R . T h e s e functions also d e p e n d on t h e t y p e of complex involved and, for different t y p e s , are identical w i t h t h e t e r m s listed u n d e r Vs in T a b l e I I . T h e difference of t h e fluxes M i _ >2 a n d M2->i (the Vs t e r m s w i t h subscript 1 a n d 2 respectively) is t h e n e t r a t e in t h e general case w h e r e S2 ^ 0.
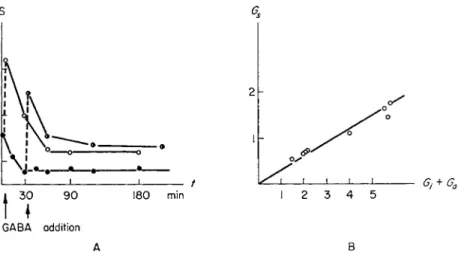
T o t e s t the mixed complex hypothesis for t h e G A B A system, t h e analysis of a somewhat puzzling observation was found suitable. D u r - ing t h e initial 30 t o 60 minutes of 2 9 ° C incubation, t h e u p t a k e of G A B A into t h e A particles exceeds the a m o u n t s of G A B A released from particles B , so t h a t t h e free G A B A levels of t h e suspending fluid decrease considerably. After this time a constant low level is m a i n t a i n e d in t h e medium, which is interpreted a s t h e a t t a i n m e n t of a s t e a d y s t a t e between Β release and A u p t a k e . I n t h e experiment depicted in Fig. 4A [21] this s t e a d y s t a t e was disturbed, or its e s t a b - lishment prevented, by an addition of G A B A to the external m e d i u m after 5 o r 35 m i n u t e s of incubation a t 2 9 ° C . T h e resulting increase in t h e external G A B A concentration is t r a n s i e n t a n d after some time a steady s t a t e is again established. I n this new s t e a d y s t a t e , however, t h e G A B A level in t h e m e d i u m is higher t h a n in t h e control experi- m e n t s (without t h e a d d i t i o n ) . T h e difference is related t o the t o t a l a m o u n t of free G A B A in t h e m a n n e r illustrated in Fig. 4 B : T h e
TABLE I I . Apparent Carrier Transport Parameters for Transport of Substrate S
Types of complexes:
A. Independent binding:
GS
Dicomplex
B. D e p e n d e n t binding:
Dicomplexes General conditions:
1. Equilibrium between s u b s t r a t e a n d carrier
2. Only fully s a t u r a t e d S-containing compound moves
Carrier forms Moving complex Dissociation constants
C CS CR CRS CRR CRRS CSR CRSR CSRR
1 + + + +
eg
O f
C J R | R ( § )
CR CS CR X R
ditto AR2 = • CRR
CR X S ditto Ks2 =
CRS
C S X R
d l t t
° *
R3 =
CSR
C R R X S 2 + + + + + +
eg
O f
C J R | R ( § )
CR CS CR X R
ditto AR2 = • CRR
CR X S ditto Ks2 =
CRS
C S X R
d l t t
° *
R3 =
CSR
C R R X S
eg
O f
C J R | R ( § )
CR CS CR X R
ditto AR2 = • CRR
CR X S ditto Ks2 =
CRS
C S X R
d l t t
° *
R3 =
CSR
C R R X S 3 + + +
eg
O f
C J R | R ( § )
CR CS CR X R
ditto AR2 = • CRR
CR X S ditto Ks2 =
CRS
C S X R
d l t t
° *
R3 =
CSR
C R R X S
eg
O f
C J R | R ( § )
CR CS CR X R
ditto AR2 = • CRR
CR X S ditto Ks2 =
CRS
C S X R
d l t t
° *
R3 =
CSR
C R R X S
4 + + +
eg
O f
C J R | R ( § )
CR CS CR X R
ditto AR2 = • CRR
CR X S ditto Ks2 =
CRS
C S X R
d l t t
° *
R3 =
CSR
C R R X S 5 + + + +
eg
O f
C J R | R ( § )
CR CS CR X R
ditto AR2 = • CRR
CR X S ditto Ks2 =
CRS
C S X R
d l t t
° *
R3 =
CSR
C R R X S 5 + + + +
eg
O f
C J R | R ( § )
d l t t
°
A S 3
" CRRS
CRS X R
d i t t
°
K
« ' = CRSR
C S R X R ditto KR& - „
NNN
CSRR
6 + + + +
eg
O f
C J R | R ( § )
d l t t
°
A S 3
" CRRS
CRS X R
d i t t
°
K
« ' = CRSR
C S R X R ditto KR& - „
NNN
CSRR
7 + + + +
eg
O f
C J R | R ( § )
d l t t
°
A S 3
" CRRS
CRS X R
d i t t
°
K
« ' = CRSR
C S R X R ditto KR& - „
NNN
CSRR
132
Exclusively in Pluricomplexes with Carrier C and Substrate R
Types o f complexes : A. Independen t binding :
Tricomplex
B.
Dependen t binding :(^C ^ (^J^^ ^ (^C ^
Tricomplexes
General conditions :
3 . S2 = R 2 = 0
S i = S R i = R
D = mobilit y o f th e movin g comple x containin g S
Relative concentration s Vs
Factors o f th e parameters
0
Fr FK
R' =
ditto R " =
Kri
R
Kr2
_S_ R' S
R' + 1 S ' + 1 CiD- R R "
CtD-
R'R" + R ' + 1 S ' + 1
y + -R ' + l R' ditto
ditto R' " =
ditto
:_S Ks2|
R"' S ' CiD — — — X -
R " ' + l R
KR3
S"' + R'R" + R ' + 1 R'R"
ditto R" " = R
Kr4
R
, , ,/
S ' CtO„ ^ X
ditto R " =R
Kr5
R " " + l 0S +„ . l/R ' + l
R ^TÏ
R
'
R
"
CtD . . . X
R' Î R' + l
I
R'R"
t
R-'R" + R ' + l | 1 + R ' |
_ ^ L Î _ L _ |
R'" + 1 J R' " +
1 1
R'R" + R ' + 1 ' " 1 S' +
i ^ R'R' * + R * + l |
R""
t
l/R ' + 1I
R " " + l | R " " - f l j
R'R
7
'
t
1I
R'R" + R ' + 1
J
R'R " + R ' + 1i
R'R" X R ' X l
° Arro w indicate s directio n o f paramete r wit h risin g R .
134 S. VARON AND W . W I L B R A N D T
GABA addition
A B
FIG. 4. A. Change of GABA concentration in the medium (S) with time.
Full circles: spontaneous time course; open and half-open circles: time course in experiments with addition of GABA after 5 and 35 minutes, respectively.
B. Steady-state level of GABA concentration in the medium (GS) as a function of the sum of initial GABA concentration in the medium, Gif and concentration increment after the addition, Ga.
FIG. 3. Schematic representation of the movement of GAB-4 and of sodium into and out of particles A.
T R A N S P O R T O F γ - A M I N O B U T Y R I C ACID I N B R A I N P A R T I C L E S 135
• Ξ
( in particles Β ) ( E x t e r n a l medium) ( In particles A )
Ξ
( O u t )
_b_
Fixed Fixed
J Uncoupled system
Fixed g,
r II Coupled
system
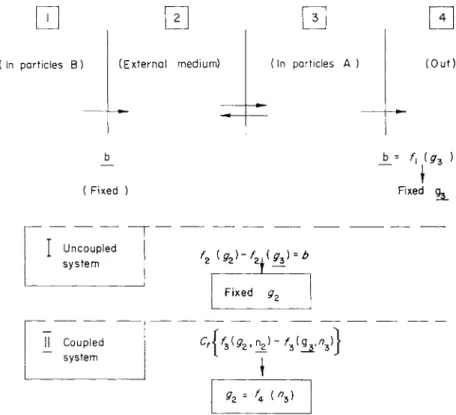
\
FIG. 5. Analysis of steady-state condition with respect to the GABA concen- tration in the medium (g2) for an uncoupled system a n d a coupled system
(GABA transport coupled to sodium transport by common carrier).
particles A a n d finally into metabolism. T h e coupled system (with mixed complexes) is compared with a n uncoupled system in which G A B A moves into particles A either b y diffusion or b y a carrier system n o t involving mixed complexes. Since t h e m o v e m e n t o u t of particles Β h a s been shown to be independent of external conditions, its r a t e a p p e a r s to control t h e s t e a d y s t a t e : both t h e m o v e m e n t into particles A a n d t h e r a t e of G A B A metabolism in t h e particles A s t e a d y - s t a t e level proved to be a linear function of t h e sum G\ -f- Ga, these symbols indicating respectively t h e external G A B A concentra- tion a t time 0 a n d its increment after t h e addition of G A B A .
Figure 5 shows a n analysis of t h e s t e a d y s t a t e with respect t o over-all G A B A movements from particles Β through t h e m e d i u m into
136 S. VARON AND W . W I L B R A N D T
m u s t equal this r a t e b. I n t h e case of t r a n s p o r t into particles A by a n y uncoupled system involving only G A B A itself (and possibly a c a r r i e r ) , regardless of whether this system follows linear kinetics or some t y p e of carrier kinetics, there is one a n d only one possible s t e a d y - s t a t e concentration of G A B A in the external medium. This is no longer so, however, if t h e t r a n s p o r t into A particles depends on both t h e G A B A a n d t h e sodium concentrations in t h e m a n n e r discussed, a n d if it is assumed t h a t the external concentration of N a is fixed due to the large external volume and t h a t t h e internal concentration of G A B A is also fixed due to t h e condition t h a t GABA metabolism equals b. I n this case, t h e postulate t h a t the r a t e of t r a n s - p o r t into particles A equals b can be m e t by a n y n u m b e r of pairs for t h e external concentration of G A B A and t h e internal concentration of sodium, and t h e external G A B A concentration in t h e s t e a d y state will increase if t h e internal N a - c o n c e n t r a t i o n rises. One possibility for such a rise would be t h e operation of t h e sodium p u m p a t m a x i - m u m r a t e , in which case the increased sodium u p t a k e into t h e particles during t h e t i m e after G A B A addition could n o t be compensated by increased removal t h r o u g h the p u m p . T h e r e are other possibilities for a sodium increase. Their discussion, however, would not be fruitful in view of the complete lack of additional d a t a .
D I S C U S S I O N
T h e experiments described here d e m o n s t r a t e the existence of so- d i u m - d e p e n d e n t and cardiac glycoside-inhibited t r a n s p o r t of GABA across m e m b r a n e s of subcellular particles. This finding in itself is of some interest since there are n o t m a n y examples in which t r a n s p o r t mechanisms k n o w n to operate across t h e cell m e m b r a n e in cellular systems were found in subcellular m e m b r a n e s as well. E v e n m o r p h o - logical c o m p a r a b i l i t y of cellular a n d subcellular m e m b r a n e s is not universally accepted, as current discussions on t h e s t r u c t u r e of t h e mitochondrial m e m b r a n e show. Subcellular sodium-dependent t r a n s - p o r t of amino acid has been d e m o n s t r a t e d across t h e m e m b r a n e of cell nuclei [ 1 ] , b u t t h e question of cardiac glycoside inhibition has not been tested in this case.
T h e relationship to observations in cellular systems is strengthened by t h e fact t h a t t h e hypothesis of mixed t r a n s p o r t complexes, which has been applied to cellular systems with considerable success, has also been helpful in t h e i n t e r p r e t a t i o n of t h e otherwise surprising a n d unexpected observation t h a t G A B A addition leads to higher
T R A N S P O R T O F γ - A M I N O B U T Y R I C ACID I N B R A I N P A R T I C L E S 137
s t e a d y - s t a t e G A B A levels in t h e m e d i u m . If t h e i n t e r p r e t a t i o n offered here is correct it also implies t h e existence of sodium p u m p s in s u b - cellular m e m b r a n e s , which to our knowledge, has not been described so far. T h u s t h e mechanisms o p e r a t i n g in cellular t r a n s p o r t of amino acids and in subcellular t r a n s p o r t of G A B A seem to be quite closely related.
A special feature of the subcellular system discussed here is t h a t the sodium dependence is a common element in t h e observations a t 0° and a t 2 9 ° C . I t therefore a p p e a r s possible t h a t the systems involved operate with the same carrier reacting both with sodium and GABA.
This results in an equilibrating t r a n s p o r t a t 0 ° C a n d in a potentially uphill system a t higher t e m p e r a t u r e . Actually such relationships would a p p e a r to be a n a t u r a l consequence of t h e concept of mixed complex downhill m o v e m e n t leading to an uphill transfer of one of t h e compo- nents. T h e additional feature introduced a t higher t e m p e r a t u r e , then, would be the energy-yielding, metabolic breakdown of G A B A and the utilization of this energy for the sodium p u m p .
As to t h e possible physiological bearing of t h e observations r e - viewed and the i n t e r p r e t a t i o n s offered here, it seems clear t h a t t h e experimental conditions differ widely from t h e biological s i t u a t i o n : I n the experiment particles originating from all p a r t s of t h e brain, including glia a n d nerve cells as well as nerve fibers, are mixed. T h e r e - fore, no direct analogy can be assumed with respect to biological conditions. Nevertheless, a few possibilities m a y be discussed briefly.
I n a general w a y t h e inter- or intracellular translocation of G A B A between sites of formation, storage, function, a n d removal m a y depend on the local concentrations of sodium ions. T o n a m e one specific a l - though speculative possibility, t h e e n t r y of sodium into a p r e s y n a p t i c nerve ending during excitation m i g h t trigger G A B A depletion by a l - lowing mitochondria to t a k e u p and metabolize G A B A . T h e p o t e n - tially uphill system in such a case would be used for accelerated r a t h e r t h a n for uphill m o v e m e n t . Likewise sodium m i g h t trigger ejec- tion of G A B A from an inhibitory nerve ending across t h e cell m e m - b r a n e in order to t r a n s l o c a t e it to a neighboring s t r u c t u r e for inhibi- t o r y action.
W h e r e a s in these cases sodium would be used to p r o m o t e t r a n s l o c a - tion of GABA, t h e coupling between G A B A a n d sodium m i g h t also be conceived to operate in the opposite sense, n a m e l y , by translocation of sodium induced by GABA. T h e general feature of inhibitory action, according to neurophysiological analysis, a p p e a r s to be a n increase
138 S. VARON AND W . W I L B R A N D T
in t h e ion conductance of t h e cell m e m b r a n e . W i t h respect to t h e sodium exchange across t h e m e m b r a n e , t h e mixed carrier complex formed in the presence of G A B A could act as a "sodium s h u n t . "
This might well be one of t h e m e a n s by which neurophysiological inhibition can be achieved.
S U M M A R Y
Sodium-dependent, cardiac glycoside-inhibited, uphill t r a n s p o r t systems a t t h e cellular level are discussed in t e r m s of recently sug- gested interpretations postulating downhill m o v e m e n t of s u b s t r a t e - sodium-carrier complexes in conjunction with t h e operation of a so- dium p u m p . A subcellular t r a n s p o r t system, d e m o n s t r a t e d in brain particles for γ - a m i n o b u t y r i c acid and having in common with these systems N a - d e p e n d e n c e and cardiac glycoside sensitivity, is reviewed and discussed in t e r m s of t h e same hypothesis. I t is shown t h a t the mixed complex mechanism is compatible with all available experimen- tal d a t a and offers interesting neurophysiological implications.
REFERENCES
1. Allfrey, V. E., Meudt, R., Hopkins, J. W., and Mirsky, A. E., Proc. Natl.
Acad. Sci. U S. 47, 907 (1961).
2. Barry, R. J. C , Dikstein, S., Matthews, J., Smyth, D . H., and Wright, E. M., J. Physiol. (London) 171, 316 (1964).
3. Crane, R. K., in Biophysics & Physiology of Biological Transport, a Sym- posium, Rome 1965. Protoplasma (1966), in press.
4. Crane, R. K., Miller, D., and Bihler, L, in "Membrane-transport and M e t a b o - lism" (A. Kleinzeller and A. Kotyk, eds.), p. 439. Academic Press, New York, 1961.
5. Elliott, K. A. C , and van Gelder, N . M., J. Neurochem. 3, 28 (1958).
6. Glynn, I. M., / . Physiol. (London) 136, 148 (1957).
7. Iff, H.-W., and Wilbrandt, W., Biochim. Biophys. Acta 70, 711 (1963).
8. Kahn, J. B., Proc. 1st Intern. Pharmacol. Meeting, Stockholm, 1961, Vol.
3, p. I l l , Macmillan (Pergamon), New York, 1962.
9. Krawitz, Ε. Α., and Potter, D. D., J. Neurochem. 12, 323 (1965).
10. Riklis, E., and Quastel, J. H., Can. J. Biochem. Physiol. 36, 347 (1958).
11. Roberts, E. (ed.), "Inhibition of the Nervous System and GABA." Sym- posium. Macmillan (Pergamon), New York, 1960.
12. Rosenberg, T., and Wilbrandt, W., J. Gen. Physiol. 41, 289 (1957).
13. Salganicoff, L., and De Robertis, E., Life Sci. 2, 85 (1963).
14. Sano, K., and Roberts, E., Biochem. Pharmacol. 12, 489 (1963).
15. Schatzmann, H.-J., Helv. Physiol. Pharmacol. Acta 11, 346 (1953).
16. Schultz, St. G., and Zalusky, R., J. Gen. Physiol. 47, 567 (1964).
17. Varon, S., Weinstein, H., Baxter, C. F., and Roberts, E., Biochem. Pharmacol.
14, 1755 (1965).
T R A N S P O R T O F γ - A M I N O B U T Y R I C ACID I N B R A I N P A R T I C L E S 139
18. Varon, S., Weinstein, H., and Roberts, E., Biochem. Pharmacol. 13, 269 (1964).
19. Varon, S., Weinstein, H., Kakefuda, T., and Roberts, E., Biochem. Pharmacol.
14, 1213 (1965).
20. Varon, S., Weinstein, H., and Roberts, E., in preparation.
21. Varon, S., Weinstein, H., and Roberts, E., Biochem. Pharmacol. (1966), in press.
22. Varon, S., Weinstein, H., and Roberts, E., in Biophysics & Physiology of Biological Transport, a Symposium, Rome, 1965. Protoplasma (1966), in press.
23. Vidaver, G. Α., Biochemistry 3, 662 (1964).
24. Vidaver, G. Α., Biochemistry 3, 795 (1964).
25. Vidaver, G. Α., Biochemistry 3, 799 (1964).
26. Vidaver, G. Α., Biochemistry 3, 803 (1964).
27. Weinstein, H., Varon, S., Muhleman, D. R., and Roberts, E., Biochem. Phar- macol. 14, 273 (1965).
28. Weinstein, H., Varon, S., and Roberts, E., in Biophysics & Physiology of Biological Transport, a Symposium, Rome, 1965. Protoplasma (1966), in press.
29. Weissbach, H., Redfield, B. J., and Titus, E., Nature 185, 99 (1960).
30. Wilbrandt, W., and Rosenberg, T., Pharmacol. Rev. 13, 109 (1961).