and cystoperitoneal shunting in six dogs
L ASZL O LEHNER
1*, K ALM AN CZEIBERT
2and G ABOR NAGY
31FeliCaVet Veterinary Referrals Clinic and Hospital, Retk€oz utca 16, Budapest, H-1118, Hungary
2Department of Ethology, Institute of Biology,‘E€otv€os Lorand’University, Budapest, Hungary
3National Institute of Clinical Neurosciences, Budapest, Hungary
Received: October 23, 2019 • Accepted: February 14, 2020 Published online: May 8, 2020
ABSTRACT
In this study we described two different indications of ventriculo- and cystoperitoneal shunting (VPS, CPS) procedures in six dogs, including their clinical data and magnetic resonance imaging (MRI) examinations. One dog had moderate and two dogs had severe congenital hydrocephalus, one was presented with intracranial pressure elevation due to meningoencephalitis of unknown origin (MUO) associated with congenital hydrocephalus, and two with quadrigeminal cysts (QC).
VPS procedures were done in four and CPS in two dogs, using low-pressure valve systems. The follow-up period ranged from 1 to 6 months and control MRI scans were also made. Significant improvement was detected in five cases during the short-term follow-up period (1 month) and in four cases in the medium-term follow-up (2–6 months). Major complications were found in two cases: one dog with acute-hypertensive hydrocephalus died one week after surgery, and in another case development of a chronic subdural haematoma and hygroma caused death 3 months after the surgery. Minor complications (e.g. subdural hygroma) were found in two cases. In cases of severe hydrocephalus or intracranial cysts, higher-pressure valve systems are recommended in order to prevent subdural hygroma. Transient postoperative clinical signs usually resolve within one week after the surgery.
KEYWORDS
ventriculoperitoneal shunt, cystoperitoneal shunt, brain, surgery, dogs, complications, follow-up
INTRODUCTION
Internal hydrocephalus is a common malformation of the central nervous system (CNS) in dogs, characterised by the excessive accumulation of cerebrospinal fluid (CSF) in the cerebral ventricles and enlargement of the ventricular system at the expense of the cerebral paren- chyma (W€unschmann and Oglesbee, 2001; Kitagawa et al., 2008; de Stefani et al., 2011;
Gradner et al., 2019; Schmidt et al., 2019). Hydrocephalus may result in compression atrophy of the surrounding nervous tissue parenchyma and the generation of small diverticula in the periventricular white matter of the brain (W€unschmann and Oglesbee, 2001). Marked dilation of the lateral ventricles was associated with loss of the septum pellucidum as well as marked atrophy of the corpus callosum and the periventricular white and grey matter in one study where 20 juvenile dogs underwent postmortem examination (W€unschmann and Oglesbee, 2001). Obstruction within the ventricular system or at the outflow through the lateral apertures of the fourth ventricle prevents communication between the ventricular system and the subarachnoid space, and is classified as noncommunicating hydrocephalus (Kitagawa et al., 2008; de Stefani et al., 2011; Salg€uero and Plessas, 2017; Gradner et al., 2019;
Schmidt et al., 2019). Communicating hydrocephalus develops when impaired circulation, impaired absorption of CSF in the subarachnoid space or both occur. Other terms used in the classification of hydrocephalus are hypertensive and normotensive to classify cases of
Acta Veterinaria Hungarica
68 (2020) 1, 95-104 DOI:
10.1556/004.2020.00010
© 2020 Akademiai Kiado, Budapest
ORIGINAL ARTICLE
*Corresponding author.
E-mail:dr.lehner.laszlo@gmail.com
hydrocephalus in which the intraventricular pressure (IVP) is increased or normal (Kitagawa et al., 2008; de Stefani et al., 2011; Salg€uero and Plessas, 2017; Gradner et al., 2019;
Schmidt et al., 2019). Hydrocephalus can occur secondary to tumours, brain trauma, haemorrhage, inflammation, or in association with CNS malformations (e.g. Chiari-like mal- formation, Dandy-Walker malformation) but can also be present without any obvious cause (primary hydrocephalus) (Kitagawa et al., 2008; de Stefani et al., 2011; Salg€uero and Plessas, 2017; Gradner et al., 2019; Schmidt et al., 2019). The occurrence rate of biventricular, triventricular and tetra- ventricular hydrocephalus was 35.5%, 15.5% and 26.6%, respectively, in one study (Schmidt et al., 2019). Very small (toy) and brachycephalic breeds are overrepresented with this condition, which is usually congenital and affects the ventricles bilaterally (Salg€uero and Plessas, 2017). Congen- ital hydrocephalus typically presents within the first few months of life. An enlarged, dome-shaped head with persistent fontanelles and open cranial sutures is a common finding. Patients are typically smaller than average. Ventral or ventrolateral strabismus is common in addition to a range of other visual deficits including blindness. Gait abnormal- ities and seizure activity have also been reported (Giacinti, 2016). Multiple medical and surgical options have been described for the treatment of congenital hydrocephalus in dogs. Surgical treatment typically consists of ventriculoper- itoneal shunt (VPS) placement (Kitagawa et al., 2008; Gia- cinti, 2016; Gillespie et al., 2019). The goal of medical management for this condition is to decrease the production or increase the absorption of CSF. A variety of medications including glucocorticoids, omeprazole and acetazolamide have been suggested for decreasing CSF production (Gia- cinti, 2016; Gillespie et al., 2019). Medical treatment usually does not provide long-term resolution of the clinical signs, whereas surgical treatment is an effective way of shunting the excessive amount of CSF from the cerebral ventricles into a body cavity (the peritoneum, the pleural space or the right atrium of the heart) (Kitagawa et al., 2008; de Stefani et al., 2011; Giacinti, 2016; Gillespie et al., 2019). Failure to respond to medical therapy is reported as an indication for the surgical treatment of hydrocephalus (Giacinti, 2016).
Shunt catheter obstruction is the most common cause for shunt malfunction. Obstructions may occur within all parts of the shunting system (Gradner et al., 2019). Obstruction of the proximal catheter is presumably caused by the protein content of the CSF, but it occurs with a lower incidence in dogs and cats than in humans (Gradner et al., 2019). Oc- clusion of the shunt catheters may be due to a variety of causes including debris, blood, intraparenchymal placement, placing against the choroid plexus, coaptation of the ven- tricular walls, gliosis, or infection (de Stefani et al., 2011;
Giacinti, 2016). Infection is the second most common cause of malfunction with a reported rate of 8–15% among pa- tients undergoing VPS placement (Gradner et al., 2019).
Shunt infection usually occurs within a few months of shunt surgery and is associated with a substantial risk of morbidity, including an increased risk of seizure disorders and decreased cognitive performance (Kulkarni et al., 2001).
Shunt disconnection, and coiling of the VPS shunt system are causes for undershunting. Disconnection may occur between the ventricular catheter and the anchor, between the anchor and the proximal catheter, between the proximal catheter and the valve system, or between the valve and the distal catheter (Gradner et al., 2019). Overshunting has serious consequences as it can lead to the collapse of the expanded ventricles and the subsequent tearing of vessels, resulting in subdural haematomas or hygromas (Giacinti, 2016). Bridging veins may tear and bleed into the subdural space. By contrast, haemorrhage into the epidural space is a much less common complication of ventricular decom- pression (Kalia et al., 1993).
Arachnoid cysts (AC) comprise 1% of all intracranial space-occupying lesions in dogs (Ciricillo et al., 1991; Dewey et al., 2007; Platt et al., 2016; Mustansir et al., 2018). The signs of AC vary according to their size and location. Small cysts are usually asymptomatic, requiring only observation and follow-up. However, larger cysts can have a mass effect on neurovascular structures, leading to neurological signs. Major treatment approaches for symptomatic intraarachnoideal cysts (IACs) include microsurgical resection of the AC wall, craniotomy for microsurgical fenestration, combined crani- otomy for microsurgical fenestration, and communication between cysts and a ventricle or a neighbouring cistern (cystoventriculostomy or cystocisternostomy), endoscopic fenestration, combined endoscopic fenestration and cys- toventriculostomy or cystocisternostomy, cystoperitoneal (CP) shunt, and stereotactic aspiration (Ciricillo et al., 1991;
Dewey et al., 2009; Gangemi et al., 2011; Shihab et al., 2011;
Kimiwada et al., 2015; Taroni et al., 2015; Platt et al., 2016;
Dong et al., 2018; Mustansir et al., 2018). IACs represent 1%
of all intracranial masses in humans, and they have been sporadically reported in dogs, where they occur most commonly in the quadrigeminal cistern (Taroni et al., 2015).
Diagnosis in humans and dogs is typically based on the demonstration of a large, fluid-filled structure between the caudal cerebrum and rostral cerebellum by computed to- mography (CT) or magnetic resonance imaging (MRI) (Dewey et al., 2007). The typical appearance of an IAC on MRI is a well-demarcated, cystic-appearing structure that is hypointense on T1-weighted images, hyperintense on T2- weighted images, non-contrast enhancing with intravenous gadolinium administration and suppressed onfluid attenua- tion inversion recovery (FLAIR) images (Dewey et al., 2009).
In one study the diagnosis of quadrigeminal cistern arachnoid cyst was based on the following MRIfindings: (1)fluid in the cyst similar to the CSF, (2) cyst not enhanced by contrast medium, (3) no hypoplasia or aplasia of the cerebellum, (4) cyst located at the dorsal-median area of the cerebellum, (5) no communication with the fourth ventricle, (6) no rotation of the vermis, (7) compression of the cerebellum to the ventrocaudal direction (Dewey et al., 2009). The differential diagnoses of quadrigeminal cistern arachnoid cysts include Dandy-Walker malformation, mega cisterna magna, retro- cerebellar AC and retrocerebellar arachnoid pouch; however, the MRI findings in these disorders have quite distinctive features which differ from those of quadrigeminal cisternal
cysts (Miyamori et al., 1999; Kitagawa et al., 2003; Platt et al., 2016). Quadrigeminal cysts (QC) may be classified into three types. Type I are cysts with supratentorial and infratentorial extension, Type II comprise cysts with infratentorial exten- sion (supracerebellar or supra-retrocerebellar), and Type III include cysts with a lateral extension toward the temporal lobe (Mustansir et al., 2018). As quadrigeminal arachnoid cysts compress or distort the cerebral aqueduct at an early stage (Mustansir et al., 2018), they are usually associated with hydrocephalus when symptomatic. Matiasek et al. (2007) provide guidelines for the objective assessment of brain compression by QC and give information about the degree and form of compression which is likely to be associated with clinical signs and should, therefore, help to distinguish clin- ically relevant QC from incidental findings. Occipital lobe compression greater than 14% was associated with seizure activity (Matiasek et al., 2007). In another veterinary study a parieto-occipitally located AC, as a special type of QC, was fenestrated by endoscopy towards the right lateral ventricle in a 16-month-old Hungarian Greyhound (Lehner et al., 2019).
Despite the initially observed good signs, the dog died 12 hours after the surgery. Necropsy and histopathological ex- amination showed epidural haematoma leading to trans- tentorial herniation and a collapsed, empty AC. CP shunt placement is a widely performed surgical method to treat temporal AC in human medicine (Fang et al., 2010).
Shunting had a higher success rate than fenestration (Ciricillo et al., 1991; Satyarthee et al., 2019). Another type of intra- cranial cysts has been described in the veterinary literature. A brainstem AC causing unilateral facial paresis was found in an 8-year-old Maltese dog, and it was treated conservatively (Kim et al., 2011). There is no fundamental medical treat- ment which could reduce the size of an IAC. Instead, medical therapy showed some clinical improvement through reducing increased ICP and brain oedema, but the responses were variable.
MATERIALS AND METHODS
Surgery
Fentanyl (5
m
g/kg), dormicum (0.05 mg/kg) and ketamine injections were used for premedication. The induction drug was propofol (5.5 mg/kg) and anaesthesia was maintained with a mixture of isoflurane and oxygen gas (1.5 v/v %).During the surgery Fentanyl-Ketamine infusion was maintained with an infusion pump (1 mL Fentanylþ0.06 mL Ketamine/100 mL of infusion, the infusion rate was 100 mL/10 kg/h). Ceftriaxone (30 mg/kg) was administered at the beginning of the surgery and in every 60 min. For this surgery, the dogs were positioned in sternal recumbency.
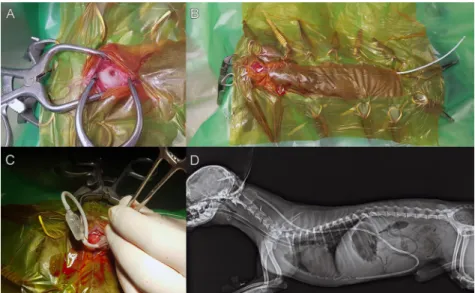
The dorsal surface of the cranial cervical area, the cranium and the face were shaved and aseptically prepared for sur- gery. The right/left lateral abdomen just caudal to the last rib was also shaved and prepared depending on the side of the shunting. A small rostrotentorial approach was used to reach the cranium and a hole in the mid-caudal parietal bone just lateral to the external sagittal crest (in the case of VPS), or where the AC is positioned, was created with a burr in a pneumatic drill depending on the diameter of the ventricular part of the catheter (Fig. 1A). The ventral aspect of the burr hole was opened with a small curette to identify the dura mater. The diameter of the hole was large enough to allow comfortable advancement of the ventricular cath- eter. From that point, a pocket was created under the skin on the caudal part of the neck with dull scissors. This space served as the location of the valve. From this space a long subcutaneous tunnel was created by a special equipment to the level of the last caudal rib. The distal catheter was placed in this tunnel and fixed to the caudal part of the valve (Fig. 1B). Ventricular catheter insertion was facilitated us- ing a 21G needle to perforate the meninges. Using a bipolar electrocauter we circularly coagulated the dura in order to
Figure 1.A: Drilled hole on the parietal region of the skull (intraoperative image), B: Abdominal part of the shunt is under the skin (intraoperative image), C: The valve and the connected ventricular and abdominal shunt (intraoperative image), D: Postoperative X-ray
from the whole body of Case 1
prevent epidural bleeding. The ventricular catheter was introduced into the lateral ventricle by aiming at the middle of the apex of the nose and directed slightly ventrally or as the cyst was located. The precise angle of insertion and depth were assessed before surgery on the basis of the dogs’
MR images and 3-dimensional reconstructions which were created to show the correct placement and relationships of the ventricular system, the cyst, the brain and the outer contour of the head. Based on the 3-dimensional recon- struction we defined the placement of the burr hole, i.e. how far it was located from the external sagittal crest and the external occipital protuberance (thus these two distances defined the point). Once the catheter was positioned in the ventricle or cyst, the stylet was removed and CSF flow confirmed. A haemostat was used temporarily to clamp the ventricular catheter to minimise CSF loss while the valve was connected (Fig. 1C). The external abdominal oblique, internal abdominal oblique and transverse abdominal muscles were cut according to theirfibre direction to open the abdominal wall in its middle third next to the costal arch. The transverse fascia and the peritoneum were then incised, and the peritoneal catheter was inserted in the peritoneal cavity and sutured to the abdominal wall. A suture was placed around the abdominal wall incision, us- ing a monofilament absorbable suture material (Surgicryl Monofilament, SMI, Belgium). The proximal catheter was secured to the cranium using the plastic holder and this was sutured to the periosteum or fascia (Surgicryl Mono- filament, SMI, Belgium). The fascia of the temporalis muscle was reapposed at the sagittal crest. After the inter- vention latero-lateral and dorso-ventral X-ray was per- formed (Fig. 1D). In our cases a low- (1–50 mmH2O) or a medium- (50–110 mmH2O) pressure valve system was used
(Medtronic CSF-Flow Control system, Medtronic Hungaria Ltd., Budapest, Hungary).
Case 1
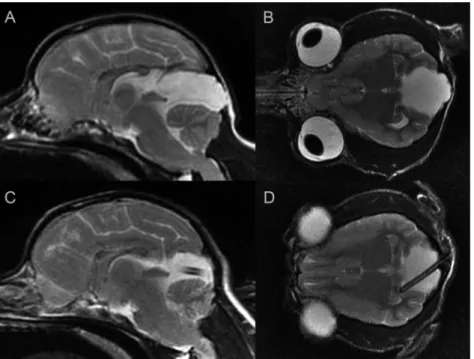
A 2-year-old male Yorkshire Terrier was referred for evalu- ation due to epileptic seizures twice per month and an intermittent body tremor. Levetiracetam (20 mg/kg) BID and carbamazepine (2 mg/kg BID) were started 7 months before referral. Neurological examination did not show any abnor- malities. The findings of abdominal and cardiac ultrasound examination findings were unremarkable. Complete blood count, chemistry panel and urinalysis were performed and showed minimal increases in alanine aminotransferase (ALT, 59 IU/L), urea (UREA, 13.9 mmol/L), glucose (GLU, 7.0 mmol/L), glutamate dehydrogenase (GLDH, 13 U/L), alka- line phosphatase (ALP, 222 U/L) and creatine kinase (CK, 144 U/L). On the basis of the clinical signs MRI examinations were recommended. MRI (1.5 T, Siemens Magnetom Avanto, Siemens, Erlangen, Germany) showed an enlarged QC which was hyperintense on T2-weighted and hypo- intense on T1-weighted and FLAIR sequences and caused a moderate cerebellar compression (Fig. 2A–B). CPS technique was performed with a low-pressure valve (1–50 mmH2O).
Awakening and the first day after the surgery were un- eventful. On the subsequent day the dog was sent home and daily online diaryfilling was recommended for follow-up. At home the previously started antiepileptic treatment was continued, and meloxicam (0.2 mg/kg QD) was started for one week. In thefirst week the dog was dull and rested and slept a lot. The appetite was reduced in that week. One week after the surgery the dog became active and happy, and the appetite was normal. One month later a control MRI was
Figure 2.Case 1, 2-year-old male Yorkshire Terrier. A: Preoperative sagittal T2-weighted image, B: Preoperative dorsal T2-weighted image, C: Postoperative sagittal T2-weighted image, D: Postoperative dorsal T2-weighted image
performed, which showed that the size of the QC had decreased and cerebellar compression was significantly reduced (Fig. 2C–D). In the 8-month follow-up period the seizures and the tremor were absent.
Case 2
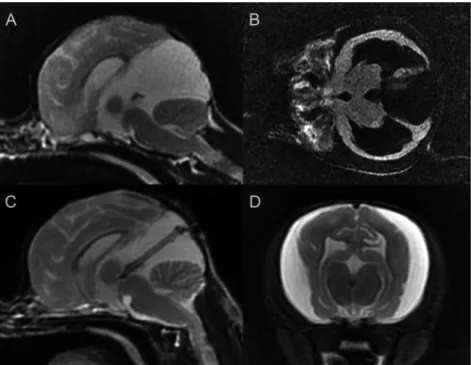
A 9-month-old male Chihuahua was referred for neuro- logical examination because of dull, periodic oral autom- atism signs and aggression on palpation of the head. Prior to referral, dexamethasone (2 mg/kg), mannitol (1 g/kg) and furosemide (2–4 mg/kg) were given occasionally by the local veterinarian. Results of the blood count and urinalysis were within the physiological range. Neurological exami- nation did not show any abnormalities except bilateral ventrolateral strabismus. MRI revealed a quadrigeminal cyst 23324326 mm in size, which was hyperintense on T2-weighted and hypointense on T1-weighted, FLAIR se- quences and compressed the cerebellum, causing cerebellar herniation and severe triventricular hydrocephalus (Fig. 3A–B). CPS insertion was performed. During the surgery the cyst and the aqueduct were explored with a 2.7- mm 308 endoscope. The large diverticula communicated with the third ventricle, therefore the ventricular part of the shunt was inserted only into the quadrigeminal diverticula and was connected to the low-pressure valve (1–50 mmH2O). Surgery and the subsequent day were unevent- ful. The dog was released home and meloxicam (0.2 mg/kg) was given for one week. One week after the intervention the previous clinical signs (oral automatism and aggression) were absent. The control MRI performed one month later revealed a quadrigeminal cyst decreased in size and absent cerebellar herniation, but enlarged ventricles and subdural
fluid were seen (Fig. 3C–D). Three months after the surgery the dog was energetic and showed no sign of the prior disturbances.
Case 3
A 4.5-year-old female Pomeranian was referred for evalua- tion because of moderate neurological signs. According to the owner, dizziness, dullness and uncertain walking were observed before presentation. The local veterinarian gave prednisolone (1.5 mg/kg BID) and acetazolamide (10 mg/kg BID) to reduce these signs. Neurological examination was normal with the exception of a stiff gait in all four limbs and mild depression. Blood and urine analyses revealed a mild decrease in red blood cell count (RBC, 5.43 T/L), mildly elevated white blood cell count (WBC, 17.9 G/L), alanine aminotransferase (ALT, 240 U/L), and glucose (GLU, 6.1 mmol/L), severely elevated alkaline phosphatase (ALKP, 2173 U/L) and gamma glutamyltransferase (GGT, 68 U/L).
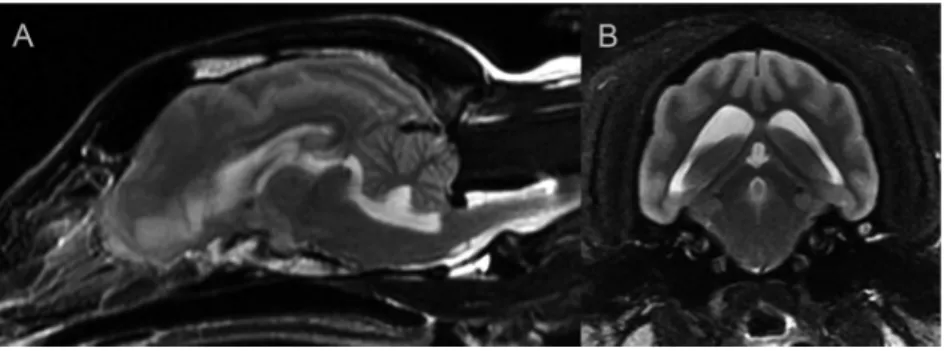
Based on the moderate neurological signs MRI was recom- mended. MRI revealed a moderate quadriventricular hy- drocephalus and mild syringohydromyelia in the C2–4, Th3–L1 and L2–L3 segments of the spinal cord (Fig. 4A–B).
VPS technique was performed with low-pressure valve sys- tem (1–50 mmH2O). One day after the surgery the dog was sent home and the previously started prednisolone and acetazolamide were continued. Ten days after the interven- tion all signs were reduced and two weeks later they were completely absent. During that 14-day period prednisolone and acetazolamide were tapered and discontinued. The one- month control MRI revealed mild hydrocephalus and mild syringohydromyelia in the C1–Th2 spinal cord segment
Figure 3.Case 2, 9-month-old male Chihuahua. A: Preoperative sagittal T2-weighted image, B: Preoperative dorsal T2-FLAIR image, C:
Postoperative sagittal T2-weighted image, D: Postoperative transverse T2-weighted image
(Fig. 4C–D). Six months after the surgery the dog was free of clinical signs without medications.
Case 4
A 7-year-old male mixed-breed dog was referred because of impaired consciousness and obtunded mental state. Four days before referral neck pain and blindness were noted. The results of ophthalmological examination were normal. Based on the changed mental status, neck pain and blindness, MRI was recommended by the local veterinarian. MRI revealed a generalised congenital hydrocephalus with periventricular oedema, increased intracranial pressure signs, Chiari-like malformation and cervical syringohydromyelia (Fig. 5A–B).
The local veterinarian prescribed methylprednisolone (2 mg/
kg), mannitol (1 g/kg) and omeprazole (1 mg/kg) to reduce the intracranial pressure. Within two days the dog became depressed and lost his consciousness. Neurological exami- nation showed semicomatose state (motor function: 4, re- flexes: 1, state of mind: 3, in all: 8 points according to the Modified Glasgow scale,Platt et al., 2001), and anisocoria.
All limb reflexes were normal. Blood pressure was 195/113 mmHg (Vet-HDO monitor MD PRO, Babenhausen, Ger- many). VPS technique was performed with low-pressure valve (1–50 mmH2O), and CSF was collected for cytological examination. One hour after the intervention the average blood pressure was 146/85 mmHg. One day after the sur- gical intervention the neurological examination showed depressed mental state, semicomatose state, deep pain sensation was normal in all limbs, while proprioception was
absent. On the second day the dog could stand and walk a short distance, the pupillary reflexes were normal but the menace response was absent. The average blood pressure was 141/82 mmHg. In the subsequent five days the dog started to show the previous signs and started to be depressed and semicomatose again. During these days methylprednisolone (2 mg/kg TID), mannitol (1 g/kg TID), omeprazole (1 mg/kg, QD), furosemide (0.7 mg/kg, TID 20 min prior to the mannitol), tramadol (2 mg/kg BID) and ceftriaxone (50 mg/kg BID) were given. CSF cytology showed mononuclear pleocytosis which suggested the following causes: granulomatous meningoencephalitis, necrotising meningo- or leukoencephalitis, viral infections, or lymphoma. One week after surgery the dog died. Histo- pathological examination of the brain revealed necrotising meningoencephalitis (NE).
Case 5
A 7-month-old male mixed-breed dog was referred for depression and loss of vision. Ophthalmological examina- tion did not show any abnormalities. The local veterinarian recommended an MRI and started to give prednisolone (1.5 mg/kg, BID). During steroid therapy the clinical signs were reduced. MRI examination revealed severe triventricular hydrocephalus with aqueductal stenosis and secondary cerebellar herniation into the foramen magnum (Fig. 6A–B).
The results of abdominal and cardiac ultrasound were negative. Blood tests and urinalysis did not show any ab- normalities. VPS surgery was performed with a low-pressure Figure 4.Case 3, 4.5-year-old female Pomeranian. A: Preoperative transverse T2-weighted image, B: Preoperative dorsal T2-FLAIR image,
C: Postoperative transverse T2-weighted image, D: Postoperative dorsal T2-FLAIR image
valve shunt system. The surgery and the subsequent day were uneventful and the dog was released home one day after the intervention. In the first 14 days after surgery the dog was slightly sleepy and slow. These signs were absent 14 days after the surgery. One month later the control MRI revealed absent cerebellar herniation and ventricles of reduced size. Around the brain, subdural hygroma was seen (Fig. 6C–D). Three months after the intervention the dog did not show any of the previous clinical signs. Prednisolone was continuously reduced and then stopped one month after the surgery.
Case 6
A 2-month-old Yorkshire Terrier was referred for evaluation because of severe depression and loss of vision. Neurological
examination revealed absent menace response and ventro- lateral strabismus on both eyes. Two big (approximately 4 cm long and 1 cm high) bone defects were observed on the frontotemporal part of skull on both sides. The fontanelle was not closed. Cardiac and abdominal ultrasound findings were normal. Blood tests and urine analysis showed mildly increased alkaline phosphatase (ALKP, 517 U/L) activity, glucose (GLU, 5.6 mmol/L) and urea (UREA, 8.2 mmol/L) levels. Based on the neurological status, MRI was performed which revealed a severe, generalised congenital hydroceph- alus, cerebellar compression and herniation (Fig. 7A–B).
VPS technique was performed with a low-pressure valve.
Immediately after the surgery amoxicillin-clavulanic acid (20 mg/kg BID) and tramadol (2 mg/kg TID) were started. At midnight the dog started to be depressed and went into a semicomatose state. Horizontal nystagmus was observed and Figure 5.Case 4, 7-year-old male mixed-breed dog. A: Preoperative sagittal T2-weighted image, B: Preoperative transverse T2-weighted
image
Figure 6.Case 5, 7-month-old male mixed-breed dog. A: Preoperative sagittal T2-weighted image, B: Preoperative transverse FLAIR image, C: Postoperative sagittal T2-weighted image, D: Postoperative transverse T1-weighted image
the skin above both of the open fontanelles was tense due to the underlying pressure. During a short anaesthesia (Pro- pofol injection, 5 mg/kg) 10 ml blood-contaminated fluid was aspirated from both sides. The cytological examination showed chronic haemorrhage and signs of acute haemo- contamination. After this intervention methylprednisolone (2 mg/kg TID) and furosemide (0.7 mg/kg, TID) were given for 5 days. On the subsequent day, neurological control examination did not show any abnormalities except bilateral horizontal nystagmus. The dog started to eat and move, and started to be energetic and inquisitive. Five days later the dog was sent home and amoxicillin-clavulanic acid (20 mg/kg BID) was continued at home for one week. Three weeks after the surgery control CT was performed to check the position of the shunt and give a basis for the subsequent controls regarding the changes in the size of the fontanelles (Fig. 7C and F). CT revealed bilateral subarachnoid signs of fluid accumulation, and the ventricular part of the shunt was in correct position. During these three weeks the dog was normal, played with other dogs and its appetite was normal.
Neurological examination did not show any sign of abnor- malities except mild horizontal nystagmus. Two months after the intervention control MRI was performed and it showed the absence of the previous hydrocephalus and developing brain tissues. Around the cerebral cortex, bilat- eral hygroma and subdural haematoma were observed which caused a moderate compression of the brain (Fig. 7D–E).
Before the intervention the brain tissue diameter was 2.4 mm around the dilated ventricles. After the surgery, the measured diameter of the brain tissues between the bilateral hygroma was 21.4 mm. The dog did not shown any sign of neurological abnormalities. Because of the bilateral hygroma, the low-pressure valve was changed to a medium- pressure valve to reduce CSF outflow which supports the dilation of ventricles and expansion of the brain tissues. This mechanism reduces the space around the brain and prevents
the accumulation of hygroma. The hygroma and the chronic subdural haematoma were drained off. During the surgery the same anaesthetic protocol was performed as at thefirst intervention. One day after the surgery the dog was alert and the neurological examination findings were normal. Five days later the dog started to be obtunded, and did not eat or drink. The neurological examination revealed decreased mental state, absent menace response, reduced palpebral reflexes, and narrow, non-responsive pupils. Through the skull defects ultrasound examination was performed, which showed massive subarachnoid fluid accumulation. Under general anaesthesia (Propofol injection, 5 mg/kg), 18 mL and 20 mL reddish-brown, viscousfluid was drained with an 18G needle and syringe from the left and the right side of the brain, respectively. After this intervention methylpredniso- lone (2 mg/kg BID) and furosemide (0.7 mg/kg, TID) were started for 5 days. After draining off thefluid the dog started to get better and it could walk, eat and responded to stimuli.
Two days later obtundation and narrow pupils were observed again, and 13 and 15 mLfluid was drained off from the brain with an ultrasound-aided technique. Because of the refilling of the subdural space and the worsening clinical signs the owner requested euthanasia. Postmortem exami- nation revealed a bilateral compartmental space with brownishfluid which compressed the brain parenchyma and caused transtentorial herniation of the occipital lobe.
Normal-sized ventricles with ventricular shunt and devel- oping brain tissues were observed. Histopathological exam- ination confirmed the suspected diagnosis which was subdural haematoma and hygroma.
DISCUSSION
To date, no exact threshold level of ventricular volume that discriminates hydrocephalus from ventriculomegaly has Figure 7.Case 6, 2-month-old male Yorkshire Terrier. A: Preoperative transverse T2-weighted image, B: Preoperative dorsal FLAIR image, C: Postoperative lateral viewed CT reconstruction, D: Postoperative transverse T2-weighted image, E: Postoperative dorsal FLAIR image, F:
Postoperative dorsal viewed CT reconstruction
been described in the veterinary literature. One study described MRI signs of elevated intracranial pressure in clinically relevant hydrocephalus and ventriculomegaly (Laubner et al., 2015). In fact, large ventricles are a common incidental finding in brachycephalic dog breeds and have been referred to as ‘ventriculomegaly’ to differentiate this finding from relevant internal hydrocephalus, and the ventricle/brain (V/B) index provides information for com- parison. The V/B index was defined as the maximum continuous distance between the internal borders of the ventricles divided by the maximum width of the brain pa- renchyma in the same image. A V/B index greater than 0.6 together with an elevated corpus callosum and the presence of a deformed intermediate mass, periventricular oedema, dilation of the olfactory recess, thinning of the sulci and/or the subarachnoid space or disruption of the internal capsule adjacent to the caudate nucleus are highly suspicious for clinically relevant dilation of the lateral cerebral ventricles due to increased IVP and cause clinical relevant hydro- cephalus. It is widely accepted that if this increase in ven- tricular volume is not associated with clinical signs, VPS is not indicated (Schmidt et al., 2017). In that study Cavalier King Charles spaniels (CKCS) were chosen to compare the association with white matter loss as occurs in internal hy- drocephalus. Based on these changes, it must be considered that canine ventriculomegaly is not a physiological variant of ventricular dimensions as previously reported but may be a preliminary or arrested form of internal hydrocephalus. In our case study hydrocephalus was observed in four cases (Cases 2, 4, 5 and 6) without any sign of ICP elevation like periventricular oedema on FLAIR sequences and/or cere- bellar herniation. In Case 3 MRI did not show any exact sign of ICP elevation but after the VPS procedure all clinical signs were absent. In Case 4 the clinical signs were caused by encephalitis, and ventriculomegaly was a congenital conformation. Inflammation led to ICP elevation which did not respond to conservative therapy. Therefore, shunt placement was used to reduce ventricular size and conse- quently the elevated ICP. After this intervention the clinical signs were improved for a short term but the inflammation did not respond for further conservative therapy. Shunting procedures are well-known techniques for decreasing the pressure of the dilated ventricles and cysts (Kitagawa et al., 2008; Lowrie et al., 2009; Reed et al., 2009; Schmidt et al., 2019). One case report has been published about the use of quadrigeminal VPS technique in a cat (Lowrie et al., 2009).
In that study, one Persian cat had a QC and 4 months after the intervention the size of the cyst was reduced on the control MRI. The main clinical signs were absent.
Complications of VPS in dogs include infection, under- shunting caused by dislocation, kinking of the catheter, leakage, valve breakage, skin necrosis, craniostenosis, microencephaly, aqueduct stenosis or obstruction, head- aches or overshunting resulting in ventricular collapse and subdural haematoma. Overshunting may lead to the devel- opment of subdural haematomas and hygromas (de Stefani et al., 2011; Shihab et al., 2011). Overshunting results in a rapid decrease in intracranial pressure caused by
overdrainage of CSF, and a resultant injury to the veins in the subdural space. It also leads to clinical signs when the haematoma is large or acute. Subdural haematomas have been reported in people and dogs after VPS placement (de Stefani et al., 2011; Shihab et al., 2011). In one study shunt catheter obstruction was by far the most common cause for shunt malfunction (Gradner et al., 2019). Obstructions may occur within every part of the shunting system. Obstruction of the proximal catheter occurred in 9.6% of the patients and kinking of the distal catheter in 2.7%. According Gradner et al. (2019), infection is the second most common cause of malfunction with a reported rate of approximately 8–15%
among patients undergoing VPS placement. In three dogs, hygroma was observed after VPS placement on the control MRI. One of these three dogs had a large subdural haema- toma, too. One dog had a hygroma before the surgery and two dogs had a severe hydrocephalus with a very thin sur- rounding brain tissue. According to the authors, in this situation a higher-pressure valve is recommended to avoid the rapid collapse of the severely dilated ventricles and the development of subdural haematoma and hygroma. If a hygroma is formed and it causes any clinical sign, a change of valve is recommended from a lower- to a higher-pressure valve. If subdural haematoma also develops besides the hygroma during the valve-changing procedure, the haema- toma material has to beflushed out using two small crani- otomy holes. If hygroma or subdural haematoma develops and it does not cause any clinical signs, clinical observation and MRI control examinations are recommended at 6 months and 1 year after the intervention. In case of quad- rigeminal cysts a low-pressure valve is used because the cyst is an altered area inside the skull and its complete reduction is recommended. In our cases where hydrocephalus was diagnosed the main complication was overshunting by low- pressure valve (in three out of the six cases, in Cases 2, 5 and 6). In conclusion, we can say that the decision as to whether a surgical intervention should be performed relies on both the clinical signs present and the results of the structural imaging methods.
ACKNOWLEDGEMENTS
The authors would like to thank Judit Benczik and Zoltan Kerekes for their assistance with the MRI ex- aminations.
REFERENCES
Ciricillo, S. F., Cogen, P. H., Harsh, G. R. and Edwards, M. S.
(1991): Intracranial arachnoid cysts in children. A comparison of the effects of fenestration and shunting. J. Neurosurg. 74, 230–235.
de Stefani, A., de Risio, L., Platt, S. R., Matiasek, L., Lujan-Feliu- Pascual, A. and Garosi, L. S. (2011): Surgical technique, post- operative complications and outcome in 14 dogs treated for
hydrocephalus by ventriculoperitoneal shunting. Vet. Surg.40, 183–191.
Dewey, C. W., Krotscheck, U., Bailey, K. S. and Marino, D. J. (2007):
Craniotomy with cystoperitoneal shunting for treatment of intracranial arachnoid cysts in dogs. Vet. Surg.36, 416–422.
Dewey, C. W., Scrivani, P. V., Krotscheck, U., Cerda-Gonzalez, S., Smith Bailey, K. and Marino, D. J. (2009): Intracranial arach- noid cysts in dogs. Compend. Contin. Educ. Vet.31, 160–167.
Quiz 168.
Dong, F., Wang, Z., Li, Y., Chen, Z., Zhang, S. and Wan, F.
(2018): Shunt dependency syndrome after cyst-peritoneal shunt resolved by keyhole microsurgical cyst resection: Two case reports and literature review. Neuropediatrics49, 310–
313.
Fang, T., Xu, J., Wang, S., Ma, Z. and Xing, J. (2010): Analysis of therapeutic choices for slit ventricle syndrome after cyst-peri- toneal shunting for temporal arachnoid cysts in children. J.
Neurosurg. Pediatr.6, 474–480.
Gangemi, M., Seneca, V., Colella, G., Cioffi, V., Imperato, A. and Maiuri, F. (2011): Endoscopy versus microsurgical cyst excision and shunting for treating intracranial arachnoid cysts. J. Neu- rosurg. Pediatr.8, 158–164.
Giacinti, J. A. (2016): Ventriculoperitoneal shunt for treatment of hy- drocephalus in a French bulldog puppy. Can. Vet. J.57, 309–312.
Gillespie, S., Gilbert, Z. and De Decker, S. (2019): Results of oral prednisolone administration or ventriculoperitoneal shunt placement in dogs with congenital hydrocephalus: 40 cases (2005–2016). J. Am. Vet. Med. Assoc.254, 835–842.
Gradner, G., Kaefinger, R. and Dupre, G. (2019): Complications associated with ventriculoperitoneal shunts in dogs and cats with idiopathic hydrocephalus: A systematic review. J. Vet.
Intern. Med.33, 403–412.
Kalia, K. K., Swift, D. M. and Pang, D. (1993): Multiple epidural hematomas following ventriculoperitoneal shunt. Pediatr.
Neurosurg.19, 78–80.
Kim, J.-W., Jung, D.-I., Kang, B.-T., Kang, M.-H. and Park, H.-M.
(2011): Unilateral facial paresis secondary to a suspected brainstem arachnoid cyst in a maltese dog. J. Vet. Med. Sci.73, 459–462.
Kimiwada, T., Hayashi, T., Narisawa, A., Shirane, R. and Tominaga, T. (2015): Shunt placement after cyst fenestration for middle cranial fossa arachnoid cysts in children. J. Neurosurg. Pediatr.
16, 533–539.
Kitagawa, M., Kanayama, K. and Sakai, T. (2003): Quadrigeminal cisterna arachnoid cyst diagnosed by MRI infive dogs. Aust.
Vet. J.81, 340–343.
Kitagawa, M., Ueno, H., Watanabe, S., Igarashi, O., Uzuka, Y., Kanayama, K. and Sakai, T. (2008): Clinical improvement in two dogs with hydrocephalus and syringohydromyelia after ventriculoperitoneal shunting. Aust. Vet. J.86, 36–42.
Kulkarni, A. V., Drake, J. M. and Lamberti-Pasculli, M. (2001):
Cerebrospinalfluid shunt infection: A prospective study of risk factors. J. Neurosurg.94, 195–201.
Laubner, S., Ondreka, N., Failing, K., Kramer, M. and Schmidt, M.
J. (2015): Magnetic resonance imaging signs of high intraven- tricular pressure – comparison of findings in dogs with
clinically relevant internal hydrocephalus and asymptomatic dogs with ventriculomegaly. BMC Vet. J.11, 181.
Lehner, L., Nagy, G., Czeibert, K. and Jakab, C. (2019): Ven- triculoscopy in a dog–fenestration of a parieto-occipital cyst into the lateral ventricle with an endoscope [in Hungarian, with English abstract]. Magy. Allatorv.141, 145–156.
Lowrie, M., Wessmann, A., Gunn-Moore, D. and Penderis, J.
(2009): Quadrigeminal cyst management by cystoperitoneal shunt in a 4-year-old Persian cat. J. Feline Med. Surg.11, 711–
713.
Matiasek, L. A., Platt, S. R., Shaw, S. and Dennis, R. (2007):
Clinical and magnetic resonance imaging characteristics of quadrigeminal cysts in dogs. J. Vet. Intern. Med. 21, 1021–
1026.
Miyamori, T., Okabe, T., Hasegawa, T., Takinami, K. and Matsu- moto, T. (1999): Dandy-Walker syndrome successfully treated with cystoperitoneal shunting–case report. Neurol. Med. Chir.
(Tokyo)39, 766–768.
Mustansir, F., Bashir, S. and Darbar, A. (2018): Management of arachnoid cysts: A comprehensive review. Cureus10, e2458.
Platt, S., Hicks, J. and Matiasek, L. (2016): Intracranial intra- arachnoid diverticula and cyst-like abnormalities of the brain.
Vet. Clin. North Am. Small Anim. Pract.46, 253–263.
Platt, S., Radaelli, S. and McDonnell, J. (2001): The prognostic value of the modified Glasgow Coma Scale in head trauma in dogs. J.
Vet. Intern. Med.15, 581–584.
Reed, S., Cho, D. Y. and Paulsen, D. (2009): Quadrigeminal arachnoid cysts in a kitten and a dog. J. Vet. Diagn. Invest.21, 707–710.
Salg€uero, R. and Plessas, I. N. (2017): Clinical presentation and magnetic resonance imaging findings in a juvenile dog with unilateral hydrocephalus and presumed periventricular encephalitis. Vlaams Diergeneeskd. Tijdschr.86, 297–302.
Satyarthee, G. D., Moscote-Salazar, L. R. and Agrawal, A. (2019):
Cystoperitoneal shunt surgery during infancy in porencephalic cyst located in frontal region led to regaining of developmental milestone. Rom. Neurosurg.XXXIII, 67–70.
Schmidt, M. J., Hartmann, A., Farke, D., Failling, K. and Kolecka, M. (2019): Association between improvement of clinical signs and decrease of ventricular volume after ventriculoperitoneal shunting in dogs with internal hydrocephalus. J. Vet. Intern.
Med.33, 1368–1375.
Schmidt, M. J., Kolecka, M., Kirberger, R. and Hartmann, A. (2017):
Dynamic susceptibility contrast perfusion magnetic resonance imaging demonstrates reduced periventricular cerebral blood flow in dogs with ventriculomegaly. Front. Vet. Sci.4, 137.
Shihab, N., Davies, E., Kenny, P. J., Loderstedt, S. and Volk, H. A.
(2011): Treatment of hydrocephalus with ventriculoperitoneal shunting in twelve dogs. Vet. Surg.40, 477–484.
Taroni, M., Seurin, M.-J., Carozzo, C. and Escriou, C. (2015):
Supratentorial arachnoid cyst management by cystoperitoneal shunt in a 1-year-old European cat. JFMS Open Rep. 1, 2055116915593970.
W€unschmann, A. and Oglesbee, M. (2001): Periventricular changes associated with spontaneous canine hydrocephalus. Vet. Pathol.
38, 67–73.