CHAPTER 22
Enzyme Inhibition by Quinones
O. Hoffmann-Ostenhof
I. Introduction 145 II. Quinone Inhibition of Enzymes by Interaction w i t h - S H G r o u p s . . . . 146
III. Quinone Inhibition of Enzymes of Oxidoreduction 150
References 157
I. INTRODUCTION
Although quinones are closely related to certain aromatic compounds, namely, p - and o-dihydroxybenzene (catechol and hydroquinone) and their homologues, to which they can easily be transformed by reduction, their properties differ very strongly from those of aromatic substances.
Their oxidizing capacities, their unsaturated character, and also their carbonyl groups explain their great reactivity. Therefore, it is under
standable that quinones exert various actions on living cells and whole organisms.
In the literature numerous reports are to be found on more or less specific bactericidal, fungicidal, antimitotic, and cytostatic effects of quinones. Attempts to use these actions for practical purposes, e.g., by using certain quinones as fungicidal agents or in the chemotherapy of malaria and cancer have been made with some success.
On the other hand, a great variety of quinones are known to be formed by microorganisms, plants, and animals (1). Most of these quinones appear to be final metabolic products, but quinones are also formed as intermediates in some processes, e.g., in melanin formation. Quinones also seem to play an important role in the respiratory chain. Although the exact mechanism of the participation of quinoid compounds in this process is not y e t completely elucidated, it seems to be established that the naphthoquinone derivatives of the vitamin Κ series as well as the so-
145
146
called ubiquinones ("coenzyme Q"), and perhaps some other quinones, like α-tocopherylquinone and—in plants only—plastoquinone, act as carriers in the respiratory chain and may also have some function in oxidative phosphorylation. An interesting discussion of the development in this field can be found in the proceedings of a recent symposium (2).
Most of the mentioned biological effects of the quinones are probably caused by their action on certain enzymes. This reasoning has induced many workers to investigate quinone inhibition of various enzymes with the purpose of establishing correlations between these effects and the biological actions of the quinones.
In very many, but not in all, cases quinone inhibition of enzymes must be considered an attack of the quinone on sulfhydryl groups essential for the catalytic function of the enzyme. Interaction with such SH groups is apparently the only mechanism responsible for quinone inhibition of nonoxidative enzymes. Usually, the enzymes can be protected against quinone inhibition by SH compounds like cysteine, glutathione, and BAL;
by addition of these substances to an already inhibited enzyme, partial reversal of the inhibition can frequently be obtained.
Quinone interaction with SH groups of enzymes must be considered a much more complex and, on the other hand, less specific effect than the action of some of the classic reagents for sulfhydryl groups of enzymes, such as iodoacetate, iodoacetamide, arsenicals, or p-chloromercuribenzo- ate. From studies of the interaction of quinones with low molecular sulfhydryl compounds, it may be deduced that several modes of action are possible.
B y virtue of their oxidizing power, quinones can oxidize two SH groups to an S—S linkage [Eq. ( 1 ) ] .
II. QUINONE INHIBITION OF ENZYMES BY INTERACTION WITH SH GROUPS
ο
OH+
\ < SH SH
(1)
Ο OH
This action, which originally was considered the only explanation of enzyme inhibition by quinones (5), is probably predominant with qui-
nones in which all four positions of the quinone ring are substituted, and should produce an enzyme inhibition which can easily be reversed by addition of SH compounds.
A mode of action frequently encountered when studying the reaction of low molecular sulfhydryl compounds with quinones is the addition of the sulfhydryl group to the reactive double bond of the quinone. The primary step seems to be 1,4-addition. With equivalent amounts of quinone and sulfhydryl compound and in the absence of oxygen, the formation of a monosubstituted hydroquinone can be observed [see Eq. (2)] U ) .
Ο OH
(2)
Zuman and Zumanova (5) have shown that 2,3-dimercaptopropanol (BAL) and benzoquinone can react in a 1:2 ratio according to Eq. (3).
Ο OH
In most cases, however, secondary processes take place which frequently lead to unidentifiable polymerization products. Schubert (6), who studied the interaction of p-benzoquinone with thioglycolic acid, observed the formation of a tetraalkylthio derivative of the quinone and explains the formation of this compound by a sequence of reactions. The originally formed monosubstituted hydroquinone is oxidized to a quinone, and the 1,4-addition is repeated. This reaction cycle goes on until all free positions of the quinone are substituted.
Closer examination of the interactions of quinones with cysteine (7-9), which lead mainly to the formation of insoluble polymer prod
ucts, have shown that bicyclic quinone imide compounds can be isolated from the reaction mixtures and may be considered as intermediates. Using cysteine ethyl ester with p-xyloquinone and 1,4-naphthoquinone, respec-
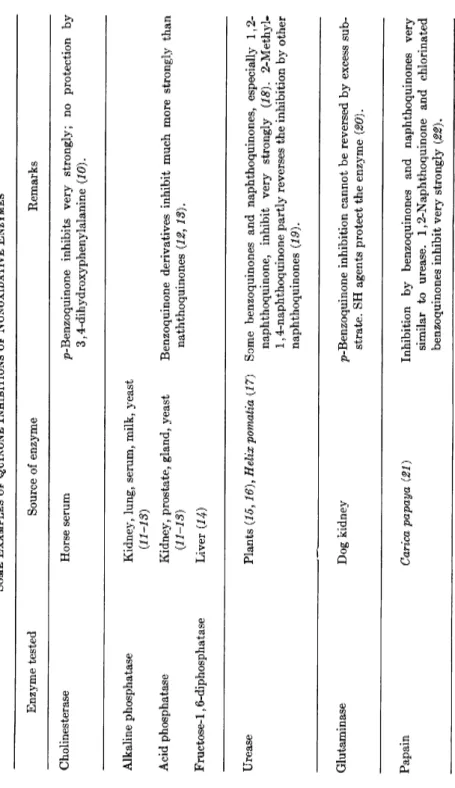
TABLE I SOME EXAMPLES OF QUINONE INHIBITIONS OF NONOXIDATIVE ENZYMES Enzyme tested Source of enzyme Remarks Cholinesterase Horse serum p-Benzoquinone inhibits very strongly; no protection by 3,4-dihydroxyphenylalanine (10). Alkaline phosphatase Acid phosphatase Fructose-1,6-diphosphatase
Kidney, lung, serum, milk, yeast {11-18) Kidney, prostate, gland, yeast (11-13) Liver (14)
Benzoquinone derivatives inhibit much more strongly than naththoquinones (12, 18). Urease Plants (15,16), Helix pomatia {17) Some benzoquinones and naphthoquinones, especially 1,2- naphthoquinone, inhibit very strongly (18). 2-Methyl- 1,4-naphthoquinone partly reverses the inhibition by other naphthoquinones (19). Glutaminase Dog kidney p-Benzoquinone inhibition cannot be reversed by excess sub- strate. SH agents protect the enzyme (20). Papain Carica papaya (21) Inhibition by benzoquinones and naphthoquinones very similar to urease. 1,2-Naphthoquinone and chlorinated benzoquinones inhibit very strongly (22).
Proteinase Yeast Very similar to papain (23). Proteinase Potato Benzoquinone inhibits this SH enzyme (24) · Deoxyribonuclease Pancreas 1,4- and 1,2-Naphthoquinones inhibit, whereas some benzo- quinones, as well as polyphenols transformable into qui- nones, show a slight activating effect (25). Hyaluronidase Bovine testicles Inhibition by p-benzoquinone and related quinones. Alkali- treated quinone solutions inhibit much more strongly than quinone itself (26), Pyruvate carboxylase Yeast (27), fungi (28) Various benzoquinones, 1,2- and 1,4-naphthoquinones, anthraquinones inhibit very strongly (27). The enzyme can be protected by SH compounds. Carbonic anhydrase Plants Quinones inhibit by attack on essential SH groups (29, 80). Xylose isomerase Pseudomonas hydrophila p-Benzoquinone inhibits this SH enzyme (81).
150
tively, Kuhn and Hammer (8) have isolated compounds to which they ascribe structures (I) and (II).
Ο Ο Finally, although no evidence for such a reaction can be derived from experiments with low molecular weight sulfhydryl compounds, the possi
bility of a reaction of the SH group with the two carbonyl groups of the quinones should not be overlooked. Considering the ease with which the carbonyl groups of aldehydes and quinones react with SH groups, it is hard to imagine why the otherwise very reactive carbonyl groups of quinones should be completely unable to undergo such an addition re
action. B y carbonyl addition mono- or dithiohemiacetals could be formed.
It is still impossible with our present knowledge to decide which of the mentioned reaction mechanisms may be predominant in the interaction of SH groups of an enzyme protein with a given protein, causing an inhibition of enzyme activity. Of course, the structure of the quinone must play an important role in determining which of the reactions will take place. Thus, quinones with no free positions in the ring, like the vitamins Kx and K2, the ubiquinones, or duroquinone, will not participate in 1,4-addition reactions. But other factors, such as the concentration of the quinone, the pH conditions, and also the steric position of the SH groups in the polypeptide chains of the enzyme, may have a major influence.
In Table I, which is not intended to be complete, some representative examples of quinone inhibition of nonoxidative enzymes are given. Al
though it has not been established in all cases listed that interactions with SH groups is the underlying mechanism, this must be considered probable, and almost all the enzymes mentioned in Table I are known to be SH enzymes.
III. QUINONE INHIBITION OF ENZYMES OF OXIDOREDUCTION
Even more complex than the mode of action of quinone inhibition of nonoxidative sulfhydryl enzymes is the mechanism of quinone action on enzymic oxidoreduction processes.
The first observations of a quinone inhibition of oxidative processes were made by workers who wanted to use n-benzoquinone as a hydrogen acceptor instead of methylene blue in experiments with the Thunberg technique (32, 33). Since then, many reports on the inhibitory actions of quinones on various oxidoreductases and enzyme systems involving oxidoreduction have been published; a list of some representative exam
ples is given in Table II.
In some cases, the enzymes acted upon are also sulfhydryl enzymes and therefore attacked by one or more of the mechanisms discussed above.
However, other modes of action must also be considered.
As already mentioned in the introduction, some quinones are believed to have an important function in the respiratory chain. Although neither the exact mechanism of their participation nor their localization in the respiratory chain has been definitely established, it must be assumed that these quinones act as hydrogen and/or electron carriers in a way which is comparable, e.g., to that of the flavins. It also has been postulated by several authors that quinones participate in the phosphorylation processes of the respiratory chain, and various attractive theories about the mecha
nism of this action have been suggested. A profound discussion of this problem is to be found in an article by Slater, Copla-Boonstra, and Links (59).
Some of the effects of quinones on oxidoreductases or oxidative enzyme systems can possibly be explained by a competition between the quinone inhibitor and the quinone endogenous to the respiratory chain. But even without such competition with a quinone carrier of the system, it could be imagined that the added quinones serve as electron carriers which bypass some essential participants of the respiratory chain. B y such a shunting action, the uncoupling of phosphorylation by quinones, a fre
quently observed phenomenon, can be explained.
The older work on quinone effects on enzymic oxidation reactions (cf. Table II) did not take into account the carrier function of quinones endogenous to the respiratory chain. Some more recent papers, however, deal especially with the action of inhibitory quinones on respiratory systems requiring ubiquinones or vitamin Κ for their function and are therefore of special interest.
We shall first discuss the studies of quinone action on ubiquinone- requiring systems. Crane (46) investigated the ability of some quinones to restore the antimycin-sensitive succinate cytochrome c reductase and succinate oxidase activities in mitochondrial systems which were deprived of ubiquinone by solvent extraction. He found that only quinones very closely related to the endogenous ubiquinone, namely, ubiquinones with
TABLE II SOME EXAMPLES OF QUINONE INHIBITION OF OXIDOREDUCTASES AND OXIDOREDUCTIVE ENZYME SYSTEMS Enzyme tested Source of enzyme Remarks Triose phosphate dehydrogenase Bacteria (34) Menadione inhibits. Glucose dehydrogenase Bovine liver (35) o- and p-Quinones inhibit. Glycollate dehydrogenase Plants (36) p-Benzoquinone inhibits while being partly reduced. Estrone reductase Yeast (37) The reduction of estrone to 17£-estradiol is inhibited by p-benzoquinone, whereas the reverse process is not affected. 3-Hydroxyanthranilate oxygenase Kidney, liver (38, 39) Menadione inhibits the transformation of 3-hydroxyanthrani- late to quinolinic acid. Pyrocatechol oxygenase Bacteria (40) o-Benzoquinone inhibits the reaction—probably through in- teraction with SH groups—and cannot be considered an intermediate of the conversion of pyrocatechol to cis,cis- muconic acid. Catalase Blood, liver (41, 42) The action is inhibited by several p-benzoquinones derivatives and naphthoquinones, and also by meriquinoid amines.
NADH-oxidase system Lupine mitochondria, (45), Tetra- SN 5949 inhibits; it appears that the NADH-cytochrome c hymena puriformis (44), animal reductase part of the system is mainly attacked (44)- See mitochondrial systems (45) also text. Succinate oxidase system Various sources (32, 46-56) Glycolytic system Ascites tumor cells (57, 58) The system is attacked by quinones, apparently at several places; the substrate reducing part (succinate dehydrogen- ase), probably by interaction with essential SH groups of the enzyme; in the later stages of electron transport the quinones may interfere with ubiquinone- or vitamin K- requiring steps (cf. also text). E39 Bayer [2, 5-dipropoxy-3,6-bis(ethylenimino)-p-benzo- quinone] and related cancerostatic substances inhibit glycolysis but not respiration (58).
154
from 3 to 10 isoprenoid units in the side chain in the 6-position ( U Q3- U Q J O )1 were able to restore the mentioned activities completely, whereas several other quinones acted as strong inhibitors. Of these, the action of the diethoxy analogue of U Q1 0 can be reversed by addition of U Q1 0; the diethoxy analogue seems to act as a competitive inhibitor of the system. The inhibiting action of other quinone inhibitors on the system, however, cannot be reversed by addition of ubiquinone. They include naphthoquinones such as S N 5949 [2-hydroxy-3-(2-methyloctyl)-l,4- naphthoquinone], the vitamins Kx and K2, lapachol, and norlapachol, whereas 2-hydroxy-l,4-naphthoquinone, 2,3-dimethyl-l,4-naphthoqui- none, 2-undecyl-l,4-naphthoquinone, α-tocopherylquinone, and p-xyloqui- none do not influence the system at all.
The influence of duroquinone and some lower homologues of the ubiquinones on the P / O ratios of the oxidation of added N A D H by a mitochondrial system has been studied by Jacobs and Crane {60). U Q0 (the ubiquinone molecule without a polyisoprenoid chain in the 6-posi
tion), UQ2, and also duroquinone showed marked uncoupling effects on the system.
According to Smith and Lester (47), who studied the influence of U Q0 and its bromo derivative (2,3-dimethoxy-5-methyl-6-bromo-p-benzoqui- none) on succinate oxidase and on pyruvate oxidase activities and the accompanying phosphorylation in beef heart mitochondria and active preparations derived from them, the observed inhibitory effects of these ubiquinone analogues are not competitive, since the inhibitions could not be reversed with any of the ubiquinone homologues. The authors explain their results by suggesting two modes of action of the inhibiting quinones:
(a) These quinones react with vital sulfhydryl groups in the electron transport and phosphorylation systems, which is indicated by the fact that cysteine and glutathione are capable of protecting the systems against the effect and even of partially reversing them when added later, and (b) the inhibiting quinones serve as electron carriers which bypass some carriers endogenous to the electron transport and phosphorylation system.
Naphthoquinones of the vitamin Κ group seem to play a role in respira
tion and phosphorylation systems similar to that of the ubiquinones.
However, all evidence existing seems to prove that these two groups of quinones are not interchangeable or, in other words, that their localiza-
1 Following the rules of the Enzyme Commission (45), the abbreviation UQ for ubiquinone is used in this chapter. The index, e.g. UQw, denotes the number of monounsaturated isoprenoid units in the side chain in the 6-position of the ubiquinone molecule.
u Η3Ο Ο γ Α χ Η3
H3C O ' ^ ^s| f ^ ^ C H2— C H = C ^ - C H2— C H2— CH = (j>^CH3
Ο CHS CH3
Ubiquinone with 10 isoprenoid units in the side chain (UQ10) Ο
2—CH =C-£cH2—CH2—CH2—CH- -CH3
Vitamin KX
Ο
SN 5949
156
tion in the respiratory chain is different. There are indications that some species contain both types of quinones as participants of the respiratory chain, whereas in others only one is present.
In the case of the action of exogenous quinones on respiration and phosphorylation systems, it is frequently very difficult to distinguish between attacks on the ubiquinone and the vitamin K-dependent parts of the systems. As the vitamins Κ have already been known for some time and the participation of these compounds in the respiratory chain was considered possible before the ubiquinones were discovered, many work
ers investigated the action of various presumable vitamin Κ antagonists on oxidative systems without knowing that another quinone carrier may be involved in the reactions studied. Therefore, the conclusions drawn from some of these earlier experiments are at least partly invalidated, and in all these cases, the question of which of the presumable vitamin Κ antagonists are in fact attacking the system at the site of vitamin Κ participation needs reassessment. We have seen previously that one of the most typical vitamin Κ antagonists, namely, the so-called S N 5949, is capable of interfering with a system requiring ubiquinone rather than vitamin K.
In order to study the specific role of vitamin Κ in a bacterial system, Brodie and Ballantine (61, 62) have devised a very ingenuous method.
In a bacterial phosphorylating NADH-oxidase system, all bound vitamin Κ can be specifically destroyed by irradiation at 360 ταμ. It is possible to restore the oxidative, but not the phosphorylating, capacities of the system by addition of riboflavin phosphate. For the restoration of phos
phorylation, however, the addition of vitamin Κ or some closely related compounds proved to be necessary. Napthoquinones which are active in this system contain a methyl group in the C-2 position and an unsatu
rated side chain of at least five carbon atoms in the C-3 position of the quinone ring. Other quinones restored only oxidation, whereas phos
phorylation was either very low or completely suppressed. The authors (62) also investigated the action of some uncoupling agents on the complete system, i.e., without previous destruction of vitamin Κ by irradiation, and found that dicoumarol and also lapachol (2-hydroxy-3- butenyl-l,4-naphthoquinone) act as competitive inhibitors of phosphory
lation, whereas oxidation is not inhibited. In this context, the interesting suggestion of Russell and Brodie (63) that only such compounds which can be transformed by reduction to β-chromans can participate in phos
phorylation, must be mentioned.
Enzymes capable of reducing quinones have been recognized for several years (64). They appear not to be highly specific with regard to the
157 quinone substrate, as several quinones are reduced at about the same rate.
Quite recently, however, Marki and Martius (65) have isolated and puri
fied a flavin protein from beef liver which catalyzes hydrogen transfer from reduced pyridine nucleotides to vitamin K2 and some other naphtho- and benzoquinones, but not to vitamin Kx or to U Q1 0. The authors con
sider it a part of the respiratory chain involving vitamin K2. The enzyme is highly sensitive to small concentrations of dicoumarol, but no experi
ments on the action of naphthoquinone analogues of vitamin Κ on its activity are reported. It would be highly desirable to know whether compounds like lapachol or S N 5949 inhibit the vitamin K2 reductase of Martius and Marki and whether this action is competitive.
In conclusion, it may be said that the study of quinone inhibitions of oxidoreductive systems has already proved to be a valuable tool for the investigation of the role of quinone carriers participating in the respira
tory chain and in the concomitant phosphorylation system. Our knowl
edge of these systems is still far from complete. Some other quinones, like the plastoquinones in photosynthesizing plants (66-68) or a-tocopheryl- quinone, may also function in respiration and phosphorylation systems, and it may be hoped that the use of quinone analogues will help to get a closer insight into the intimate mechanisms of respiratory systems. But the study of quinone inhibitions of oxidative systems may also be of use for practical purposes. It is not improbable that some specific quinone inhibitors acting on respiratory systems will prove to be of therapeutic value.
REFERENCES
1. R. H. Thomson, "Naturally Occurring Quinones." Academic Press, New York, London, 1957.
2. G. E . W. Wolstenholme and C. M. O'Connor, eds., "Quinones in Electron Transport," Ciba Foundation Symposium. Little, Brown, Boston, Massa
chusetts, 1961.
3. L. Hellerman and Μ. E. Perkins, J. Biol. Chem. 107, 241 (1934).
4. G. H. Meguerrian, J. Am. Chem. Soc. 77, 5019 (1955).
5. P. Zuman and R. Zumanova, Tetrahedron 1, 289 (1957).
6. M. Schubert, J. Am. Chem. Soc. 69, 712 (1947).
7. R. Kuhn and H. Beinert, Ber. 77, 606 (1944).
8. R. Kuhn and I. Hammer, Ber. 84, 91 (1951).
9. H. Burton and S. B. David, J. Chem. Soc. p. 2193 (1952).
10. W. K. Berry, K. P. Fellows, P. J. Fraser, J. P. Rutland, and A. Todrick, Biochem. J. 59, 1 (1955).
11. I. W. Sizer, J. Biol. Chem. 145, 405 (1942).
12. O. Hoffmann-Ostenhof and E . Putz, Monatsh. Chem. 79, 421 (1948).
13. O. Hoffmann-Ostenhof and E . Putz, Monatsh. Chem. 81, 703 (1950).
14. E. O. F. Walsh and G. Walsh, Nature 161, 976 (1948).
15. J. H. Quastel, Biochem. J. 27, 1116 (1933).
158
16. L. Hellerman, Μ. Ε. Perkins, and W. M. Clark, Proc. Natl. Acad. Sci. U.S.
19, 855 (1933).
17. R. Russ, Z. vergleich, Physiol. 38, 284 (1956).
18. O. Hoffmann-Ostenhof and W. H. Lee, Monatsh. Chem. 76, 180 (1946).
19. W. H. Schopfer and E. C. Grob, Helv. Chim. Acta 32, 829 (1949).
20. F. W. Sayre and E . Roberts, J. Biol. Chem. 233, 1128 (1958).
21. T. Bersin and W. Logemann, Z. physiol. Chem. Hoppe-Seyler* s 220, 209 (1933).
22. O. Hoffmann-Ostenhof and E. Biach, Monatsh. Chem. 78, 53 (1948).
23. O. Hoffmann-Ostenhof and H. Moser, Monatsh. Chem. 79, 570 (1948).
24. A. Niemann, Z. physiol. Chem. Hoppe-Seyler's 305, 196 (1956).
25. O. Hoffmann-Ostenhof and W. Frisch-Niggemeyer, Monatsh. Chem. 83, 1175 (1952).
26. S. Roseman and A. Dorfman, J. Biol. Chem. 199, 345 (1952).
27. R. Kuhn and H. Beinert, Ber. 80, 101 (1947).
28. M. W. Foote, J. E . Little, and T. J. Sproston, J. Biol. Chem. 181, 481 (1949).
29. K. Kondo, H. Chiba, and F. Kawai, Bull. Research Inst. Food Sci. Kyoto Univ. 13, 12 (1954).
30. K. Kondo, H. Chiba, and F. Kawai, Bull. Research Inst. Food Sci.f Kyoto Univ. 13, 23 (1954).
31. R. M. Hochster, Can. J. Microbiol. 1, 589 (1955).
32. H. Wieland and K. Frage, Ann. 477, 1 (1929).
33. H. Wieland and A. Lawson, Ann. 485, 193 (1932).
34. H. Schreiber, Med. Klin. (Munich) 48, 747 (1953).
35. M. Nakamura, J. Biochem. (Japan) 41, 67 (1954).
36. W. Franke and I. Schulz, Ann. 578, 147 (1952).
37. K. Repke, Arch, exptl. Pathol. Pharmakol., Naunyn-Schmiedeberg's 230, 178 (1957).
38. S. Auricchio, M. Piazza, and E. Quagliarello, Boll. soc. ital. biol. sper. 33, 1211 (1957).
39. S. Auricchio, M. Scotto, and M. Piazza, Boll. soc. ital. biol. sper. 33, 1223 (1957).
40. O. Hayaishi, A. A. Batchett, and B. Witkop, Ann. 608, 158 (1957).
41. O. Hoffmann-Ostenhof and E. Biach, Monatsh. Chem. 76, 319 (1946).
42. L. Horner and C. Betzel, Ann. 571, 225 (1951).
43. Τ. E. Humphreys and Ε . E. Conn, Arch. Biochem. Biophys. 60, 226 (1956).
44. H. J. Eichel, J. Biol. Chem. 222, 121 (1956).
45. "Report of the Commission on Enzymes of the International Union of Biochemistry," Pergamon Press, N e w York, 1961.
46. F. L. Crane, Arch. Biochem. Biophys. 87, 198 (1960).
47. A. L. Smith and R. L. Lester, Biochim. et Biophys. Acta 48, 547 (1961).
48. H. Bergstermann and W. Stein, Biochem. Z. 317, 217 (1944).
49. E. G. Ball, C. B. Anfinsen, and O. Cooper, J. Biol. Chem. 168, 257 (1947).
50. H. Heymann and L. F. Fieser, J. Biol. Chem. 176, 1359 (1948).
51. W. W. Ackerman and V. R. Potter, Proc. Soc. Exptl. Biol. Med. 72, 1 (1949).
52. A. E. Reif and R. Potter, J. Biol. Chem. 205, 279 (1953).
159 53. H. W. Clark, H. A. Neufeld, C. Widmer, and E. Stotz, J. Biol. Chem. 210,
851 (1954).
54. C. Widmer, H. W. Clark, H. A. Neufeld, and E. Stotz, J. Biol. Chem. 210, 861 (1954).
55. A. Herz, Biochem. Z. 325, 83 (1954).
56. K. Okunuki and J. Sekutsu, J. Biochem. (Japan) 42, 397 (1955).
57. W. Remmele and W. Rick, Z. Krebsforsch. 61, 449 (1957).
58. H. Holzer, P. Glogner, and G. Sedlmayr, Biochem. Z. 330, 59 (1958).
59. E . L. Slater, J. P. Copla-Boonstra, and J. Links, in "Quinones in Electron Transport," Ciba Foundation Symposium (G. E. W. Wolstenholme and C. M. O'Connor, eds.), p. 161. Little, Brown, Boston, Massachusetts, 1961.
60. Ε . E. Jacobs and F. L. Crane, Biochem. Biophys. Research Communs. 2, 218 (1960).
61. A. F . Brodie and J. Ballantine, J. Biol. Chem. 235, 226 (1960).
62. A. F. Brodie and J. Ballantine, J. Biol. Chem. 235, 232 ( I 9 6 0 ) .
63. P. J. Russell and A. F . Brodie, in "Quinones in Electron Transport," Ciba Foundation Symposium (G. E. W. Wolstenholme and C. M. O'Connor, eds.), p. 205. Little, Brown, Boston, Massachusetts, 1961.
64. W. D. Wosilait and A. Nason, J. Biol. Chem. 206, 255 (1954).
65. F. Marki and C. Martius, Biochem. Z. 333, 111 (1960).
66. R. L. Lester and F. L. Crane, J. Biol. Chem. 234, 2169 (1959).
67. Ν . I. Bishop, Proc. Natl. Acad. Sci U.S. 45,1696 (1959).
68. Ν. I. Bishop, in "Quinones in Electron Transport," Ciba Foundation Sym
posium (G. E . W. Wolstenholme and C. M. O'Connor, eds.), p. 385.
Little, Brown, Boston, Massachusetts.