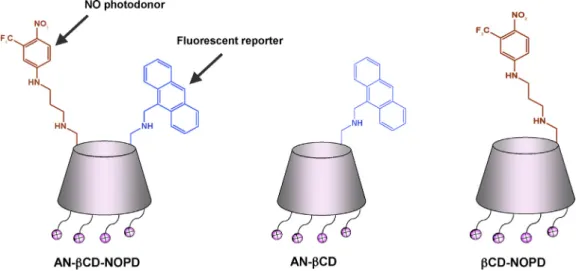
A multifunctional b -cyclodextrin-conjugate photodelivering nitric oxide with fl uorescence reporting
Gábor Benkovics
b,c,1, Marta Perez-Lloret
a,1, Damien Afonso
a, András Darcsi
d, Szabolcs Béni
d, Éva Fenyvesi
b, Milo Malanga
b,*, Salvatore Sortino
a,*
aLaboratoryofPhotochemistry,DepartmentofDrugSciences,UniversityofCatania,I-95125,Italy
bCycloLab,CyclodextrinR&DLtd,Illatosút7,H-1097Budapest,Hungary
cDepartmentofOrganicChemistry,FacultyofScience,CharlesUniversityinPrague,Hlavová8,12843Prague2,CzechRepublic
dDepartmentofPharmacognosy,SemmelweisUniversity,ÜllÅiút26,H-1085,Hungary
ARTICLE INFO
Articlehistory:
Received25March2017
Receivedinrevisedform9May2017 Accepted10May2017
Availableonline11May2017
Keywords:
Cyclodextrin Nitricoxide Singletoxygen Light Fluorescence
ABSTRACT
Thiscontributionreportsthedesign,synthesisandphotochemicalpropertiesofanovelcationic,water soluble,b-cyclodextrin(bCD)conjugateintegratingananthracenemoietyandanitroanilinederivative withintheprimarysideofthebCDscaffold.Photoinducedenergytransferbetweentheanthraceneand thenitroanilinechromophoreseffectivelysuppressesthefluorescenceoftheanthraceneunit.Excitation with visible light triggers the release of nitric oxide (NO) from the nitroaniline chromophore, accompaniedtotheconcomitantrevivaloftheanthracenefluorescence,whichactsasanopticalreporter fordetectingtheamountoftheNOreleased.Furthermore,theanthracenemoietyphotogeneratessinglet oxygen(1O2)sequentiallytoNOrelease.Theconjugateisalsoabletoaccommodatehydrophobicguests withinthebCDcavity,asprovenbyusingnaphthaleneasamodelcompound.InviewofthekeyroleNO and1O2playasanticancerandantibacterialspecies,thepresentbCDderivativerepresentsanintriguing candidate forfurther studies in biopharmaceutical research addressed to multimodal therapeutic applications.
©2017ElsevierB.V.Allrightsreserved.
1.Introduction
Cyclodextrins(CDs)havebeengreatlydevelopedduringthelast thirty years as carriers of “conventional” drugs (Davis and Brewster, 2004; Loftsson and Douchene, 2007). However, the use of CDs as suitable vehicles for photoactivable therapeutic compoundshasbeenonlyrecentlyobjectofattention(Mazzaglia etal.,2012).Duetotheirhydrophobiccavity,naturalCDscanhosta varietyof photosensitiveagents bysupramolecular interactions (BortolusandMonti,1996;MontiandSortino,2002).Nevertheless, inmostcases,thelowbindingconstantsbetweenunmodifiedCDs andguestmoleculesrepresentamajordrawbackofthesesystems asbio-carriers,makingthemodificationoftheCDstructurestrictly necessaryinviewofactualapplications(Mazzagliaetal.,2006).
ModificationoftheCDsmolecularscaffoldthroughfunctionaliza- tion of the primary and/or secondary hydroxyl groups with
suitable photoresponsiveunits allowstoobtainmultifunctional nanocarrierswithintriguingpropertieswhilst,atthesametime, maintainingthe macrocycle's capacity for guests encapsulation (Mourtzisetal.,2007,2008;Yannakopoulouetal.,2011).
Nitric oxide (NO) is a pleiotropic bioregulator of important physiological and pathophysiological processes encompassing neurotransmission,vasodilatationandhormonesecretioninliving bodies (Ignarro, 2010). Besides, NO is an excellent sacrificial antioxidant, anticancer and antibacterial agent (Ignarro, 2009).
Thisscenariohasopened aferventresearchactivity devotedto developing compounds able to release NO under physiological conditions as potential pharmaceuticals to fight a variety of diseases(Wangetal.,2002,2005;RiccioandSchoenfisch,2012;
SeabraandDurán,2010).However,thebiologicaleffectsofNOare strictlydependingonitsconcentration,locationanddose(Jiaetal., 2002).Thishasmadethelight-activatedNOdonorsveryappealing due to thesuperb spatiotemporalaccuracy the light triggering offers(Ford,2008;Sortino,2010;FryandMascharak,2011;Ford, 2013).SeveralNOphotodonors(NOPD)havebeensupramolecu- larlycombinedwithCDsderivatives(Fraixetal.,2016).Incontrast,
*Correspondingauthors.
E-mailaddresses:malanga@cyclolab.hu(M.Malanga),ssortino@unict.it (S.Sortino).
1Theseauthorscontributedequallytothiswork.
http://dx.doi.org/10.1016/j.ijpharm.2017.05.023 0378-5173/©2017ElsevierB.V.Allrightsreserved.
ContentslistsavailableatScienceDirect
International Journal of Pharmaceutics
j o u r n al h o m ep a g e: w w w . el s e v i e r . c o m / l o c at e / i j p h a r m
onlylimitedexamplesofNOphotodonorcovalentconjugateswith CDsarereportedtodate(Pirasetal.,2013).
Quantification of the NO delivery in real time is another importantissuetobefaced,especiallywhenthemaininterestisto reachacriticalmoleculeconcentrationtoinduceaspecificeffect.A suitablewaytoaddressthisquantificationtaskisbasedontheuse of a fluorescent reporter. This elegant strategy relies on the simultaneous photorelease of thedesired bioactive species, i.e.
NO,and afluorescentcomponent (thereporter) fromthesame nonfluorescentprecursor(Veldhuyzenetal.,2003;Pelloisetal., 2004;Weinstainetal.,2010).Thereleaseprocesscanbethuseasily quantifiedbymonitoringthefluorescenceemissionofthereporter, whichactsexactlyasanopticalcounterofthebioactivespecies.
Recently, we have reportedinteresting propertiesof anad-hoc designed molecular conjugate integrating two chromogenic centers within the same covalent skeleton (Vittorino et al., 2011;Kirejevetal.,2014),ananthracenemoietyandanitroaniline derivative,thislatterasasuitableNOPD(Carusoetal.,2007)Inthis compound,thetypicalemissionoftheanthracenefluorophoreis completelysuppressedbyaphotoinducedenergytransfertothe nitroanilinemoiety.WedemonstratedthatthephotoreleaseofNO leadstotherevivalofthefluorescenceofanthracenefluorophore whichactsasafluorescentreporterfortheNOdeliveryinlivingcells (Vittorinoetal.,2011;Kirejevetal.,2014).
Based on the above scenario we considered it of value to translatethisNOphotoreleasewithfluorescentreportingconcept inanovelconjugate,which covalentlyintegratestheNOphoto- releaserand theanthracene moietywithin thesame positively charged
b
CD scaffold. This contribution reports the synthesis, characterizationandphotochemicalpropertiesofthecationicb
CD conjugateAN-b
CD-NOPDandthesuitablemodelcompoundsAN-b
CDandb
CD-NOPD(Fig.1).2.Experimental 2.1.Chemicals
6-Monodeoxy-6-monoazido-
b
CD and 6-monotosyl-b
CD are finechemicalproductsofCycloLab,1,3-diaminopropanol(99%), 4-nitro-3-(trifluoromethyl)aniline(98%),glycidyltrimethylammo- nium chloride (technical grade, 90%), sodium azide (ReagentPlus1,99.5%),hydrazinemonohydrate(reagentgrade, 98%), sodium borohydride (98.0%), anthracene-9-carboxaldehyde (97.0%), p-toluenesulfonyl chloride (ReagentPlus1,99%),trimethylamine(99%),1,3-propanedithiol (99%), palladium on carbon (5%), weresourced from Sigma– Aldrich.
Dimethyl sulfoxide, formic acid, methanol, pyridine were obtainedfromMolarChemicals.
2.2.Instrumentation
1H,13CNMRspectraandDEPT-ed-HSQCspectrawererecorded in DMSO-d6 or D2O (10mg dissolved in 0.8mL of deuterated solvent)onaVarianDDR-600spectrometerat600MHzat298K.
MassspectrawereobtainedonBrukerESQUIRE3000ES-ion trap instrument with electrospray ionization (ESI) in negative mode.Allsamplesweredissolvedinwater.
Preparativechromatographicseparationswereperformedona Büchi preparative chromatographysystem using SiliCycle Silica Cartridge (4cm15cm) packed with Lichroprep (120g) RP-18 Phase (40–63
m
m) as a stationary phase and water-methanol gradientelution.Thechromatographicstationwasequippedwith BüchiUVPhotometerC-635asadetector(detectionwavelength setat390nmforNOPDappendedderivativesandat245nmforthe tosylatedintermediate).UV–visabsorptionandfluorescencespectrawererecordedwith aJascoV650spectrophotometerandaFluorolog-2(Model,F111) spectrofluorimeter, respectively. Fluorescence lifetimes were recorded with the same spectrofluorimeter equipped with a TCSPCTripleIlluminator.Thesampleswereirradiatedbyapulsed diode excitation source Nanoled at 370nm. The kinetic was monitoredat420nmandeachsolutionitselfwasusedtoregister the prompt at 370nm. The system allowed measurement of fluorescencelifetimesfrom200ps.Themultiexponentialfitofthe fluorescencedecaywasobtainedusingthefollowingequations:I (t)=A1exp(t/t1)+A2exp(t/t2)(for thebiexponentialfit) and I (t)=A1exp(t/t1)+A2exp(t/t2)+A3exp(t/t3) (for the triexpo- nentialfit).Multiexponentialfittingwereusedbecausetheygave muchbetterchi-squarevaluesthanmonoexponentialfitting.
1O2emissionwasregisteredwiththesamespectrofluorimeter as above equipped with a NIR-sensitive liquid nitrogen cooled photomultiplier, exciting the air-equilibrated samples in D2O solutionat380nmwiththefluorimeterlamp.
Photolysis of the samples in solution was performed in a thermostatedquartzcell(1cmpathlength,3mLcapacity)under
Fig.1.SchematicofthemultifunctionalbCDconjugateAN-bCD-NOPDandthemodelcompoundsAN-bCDandbCD-NOPD.
gentlestirring,byusingacontinuumlaserwith
l
exc=405nm(ca.100mW)havingabeamdiameterofca.1.5mm.
NOreleasewasmeasuredwithaWorldPrecisionInstrument, ISO-NOmeter,equippedwithadataacquisitionsystem,andbased ondirectamperometricdetectionofNOwithshortresponsetime (<5s)andsensitivityrange1nM–20
m
M.Theanalogsignalwas digitalizedwithafour-channelrecordingsystemandtransferred toaPC.ThesensorwasaccuratelycalibratedbymixingstandardsolutionsofNaNO2with0.1MH2SO4and0.1MKIaccordingtothe reaction:
4H++2I+2NO2!2H2O+2NO+I2
Irradiationwasperformedinathermostatedquartzcell(1cm pathlength,3mLcapacity)undergentlestirringbyusingthesame 405nmlaser sources described above. NO measurementswere carriedoutwiththeelectrodepositionedoutsidethelightpathin Scheme1.ReactionschemeforthesynthesisofAN-bCD.
Scheme2. ReactionschemeforthesynthesisofbCD-NOPD.
ordertoavoidfalseNOsignalduetophotoelectricinterferenceon theISO-NOelectrode.
3.Resultsanddiscussion 3.1.Syntheses
Themodelcompound AN-
b
CDwas synthesizedaccordingto theprocedureillustratedinScheme1andbrieflydescribedinthe following (the full details are reported in the Supporting Information).6-Monotosyl-
b
CD was promptly converted to the amino- analog1 byexhaustivereduction of the intermediate 6-mono- deoxy-6-monoazidob
CD,accordingtoliterature(Jicsinszkyand Iványi,2001).Theaminogroup,locatedontheprimaryrimofCD scaffold,isasuitableanchoringpointfortheintroductionofthe anthracenechromophore.Ascompound1isobtainedasfreebase, thecouplingcouldbeachievedbyanucleophilicsubstitutionin DMF with the commercially available 9-(chloromethyl)anthra- cene.Thisreactionsettingwasexplored,buttheconversionrate was not satisfactory (30% formation of compound 2) and N- dialkylated product (CD scaffold containing two anthracene moieties)wasconsiderablyformedunderthetestedexperimental conditions(10%).Althoughchromatographicpurificationofthe crude canbe attempted, theprocedure is time-consuming,noteffectiveandresultedinlow-yield.Asefficientalternativeforthe preparationofcompound(2)atwo-stepsone-potreactionbased onimine-reduction,allowinggoodconversionrateandavoiding chromatographicpurification,wasapplied.Theadditionofsilica geltothereactionmixtureduringthefirststepwasafundamental requirement for achieving quantitativeconversion totheimine intermediate 1a. The nano-porous material was effective in removingthewateraccumulatedduringthecondensationprocess thus makingconvenientthecouplingreaction.The secondstep basedonthemildreducingagentsodiumborohydrideproceeded quantitatively within expectations. The introduction of the permanent charges onthe CD scaffoldswas achievedby using the alkylating agent, glycidyltrimethylammonium chloride, as reactionsolvent.Thisapproachhastheadvantagesofminimizing theby-products formationand reducing thework-uptosimple fractionalcrystallizationcycles.
Themodelcompound
b
CD-NOPDwassynthesizedaccording totheprocedureillustratedinScheme2andbrieflydescribedin the following (the full details are reported in the Supporting Information).Theflexiblelinker,1,3-diaminopropane,waseffectivelyintro- ducedontheCDscaffoldbynucleophilicsubstitutiononthe6- monotosyl-
b
CD.Inordertoreducetheformationofby-products suchasCDdimers(twoormoreCDunitsconnectedthroughthe linker)and3,6-anhydroderivatives,1,3-diaminopropanewasused Scheme3.ReactionschemeforthesynthesisofAN-bCD-NOPD.inlargeexcess(reactionsolvent)and6-monotosyl-
b
CDwasadded portion-wise to the warm solvent solution. These precautions ensured that 6-monotosyl-b
CD was always acting as limiting reagent and promptly converted to 6-monodeoxy-6-mono-(3- aminopropylamino)b
CD.Thelargeexcessof1,3-diaminopropane in combination with an adequate work-up (evaporation under reduced pressure and precipitation with methanol-diisopropyl ethermixture)alsomaintainedcompound3infreebaseform.This isa strictrequirementtosuccessfully accomplishthefollowing reactionstepasnoextrabasecanbeaddedtothereactionmixture duetothehighreactivityof4-chloro-1-nitro-2-(trifluoromethyl) benzenetoward these species. It is worth tomentionthat the decisionofattachingtheNOPDmoietytotheCDscaffoldthroughalinker,wastheresultofseveralunsuccessfulattemptstodirectly introducethechromophoreontheCDrim.Noadequatereaction conditions werefound toreact thereagent 4-chloro-1-nitro-2- (trifluoromethyl)benzenewiththefreebaseof6-monoamino-6- monodeoxy-
b
CD.Themostprobablereasonisthatthenitroben- zenederivativeformedastronginclusioncomplexwiththeb
CD cavitylocatingthechlorineatomonthesecondaryside(Uenoand Breslow,1982).Theestablishmentofthissupramolecularinterac- tionprecludesprimary-sidesubstitution.Compound4was obtainedinmoderateyieldbyreactingthe freebaseofcompound3with4-chloro-1-nitro-2-(trifluoromethyl) benzene.Asnoextrabasecouldbeaddedtothereactionmixture without consuming thechromophore,the product was formed only at around 40% conversion rate. Reversed-phase chro- matographic purification wasneeded toseparate theunreacted compound 3 from the target product. The solubility profile of compound 4was improvedbymodifyingsomeofthehydroxyl groupswithpermanentlychargedside-chains.
ThemultifunctionalconjugateAN-
b
CD-NOPDwassynthesized according totheprocedure illustrated in Scheme3 and briefly described in thefollowing (the fulldetails are reported in the SupportingInformation).Inordertointroduceboththechromo- phores ontheprimary sideof theCD scaffold, tosylation of6- monoazido-6-monodeoxy-b
CD was performed inpyridine. This approachisselectiveforthemodificationoftheprimaryrimofthe macrocycle(Matsuietal.,1976)andgeneratesamixtureofthree pairs of pseudoenantiomers (6A-monodeoxy-6A-monoazido-6X- monotosyl-b
-CD, compound 5). The mixture of primary-side hetero-disubstitutedb
CDwas isolatedbypreparativereversed- phasechromatography.Thestrategydevelopedfortheintroduc- tionoftheNOPDchromophoreinmodelcompoundb
CD-NOPDwasappliedatthisstagealmostwithoutmodifications.Theazido moiety remained unaltered both in harsh alkaline conditions (preparationofcompound 6)andduringtheprolongedheating requiredfor the nitrobenzeneinstallation (preparationof com- pound7).Themostchallengingpartofthesyntheticworkwasthe introduction of the anthracene fluorophore on the NOPD- appended CD. A three-step one-pot reaction was carefully designed to fulfill all the necessary requirements. First, a mild reductionmethodwaschosenfortheselectivereductionofazido groupinthepresenceofthearomaticnitrogroup.Thisapproach wasbasedontheuseof1,3-propanedithiolandtrimethylamineas reductionmixturetoobtaintheamino-intermediate7a.Addition of anthracene-9-carboxaldehyde and heating of the reaction mixtureresultedinalmostcompleteconversion(90%conversion rate based on TLC estimation) to anthracene-appended imine- intermediate7b.Portion-wiseadditionofNaBH4andprolonged stirring at room temperature afforded anthracene-appended amine-intermediate, compound 8, in quantitative conversion.
Preparativereversed-phasechromatographicpurificationallowed theremovaloftheunreacted compound7aandtheisolationof targeted compound 8. As the CD scaffold is modified simulta- neously with two aromatic units, its solubility in aqueous environmentislimited.Thisdrawbackwasovercomebyintroduc- ing quaternary ammonium bearing moieties in the system, obtainingthedesiredcompoundAN-
b
CD-NOPD.3.2.Spectroscopicpropertiesandphotochemicalbehavior
All compounds synthesized are well soluble in aqueous mediumduetothepresenceofthecationicterminationmainly locatedatthesecondaryrimofthe
b
CDscaffold.Theabsorption spectrumofAN-b
CD-NOPD(ainFig.1A)exhibitsthedistinctive spectralfeatures oftheanthracenechromophorebelow 400nm andanintenseabsorptionatlongerwavelengthsextendingbeyond 450nm,duetothecontributionof thenitroanilinemoiety.This Fig.2.(A)Absorptionspectraof40mMaqueoussolutionAN-bCD-NOPD(a)andthemodelcompoundsAN-bCD(b)andbCD-NOPD(c).(B)Fluorescenceemission spectra(lexc=370nm)ofthemodelcompoundAN-bCD(d)andAN-bCD-NOPD (e).
Fig.3.NOreleaseprofileobservedforopticallymatchedaqueoussolutionsofAN- bCD-NOPD(a)andbCD-NOPD(b)uponirradiationwithlexc=405nmat25C.
spectrumreflectsquitewellthesumabsorptionspectrumofthe independentmodelcompoundAN-
b
CDandb
CD-NOPD(bandc in Fig. 2A), ruling out any significant interaction between the anthraceneandnitroanilinechromophoresinthegroundstate.On the other hand, the intense fluorescence emission of the anthracene fluorophore observed in the case of AN-b
CD (d in Fig.2)issignificantlyquenchedinthebichromophoricconjugateAN-
b
CD-NOPD(einFig.2B),suggestingaremarkableinteraction betweenthetwochromophoresintheexcitedstate.Accordingto what already demonstrated in our previous studies (Vittorino etal.,2011;Kirejevetal.,2014),thisdrasticfluorescencequenching canbeexplainedonthebasisofaphotoinducedenergytransfer betweentheanthracenefluorophoreandthenitroanilinecompo- nentsintheconjugate,whichisencouragedbythecloseproximity ofthedonorandacceptor,bothlinkedatthesamerim.The capability of AN-
b
CD-NOPD to deliver NO under lightstimuliwasinvestigatedbythedirectandreal-timemonitoringof thistransientspeciesusinganultrasensitiveNOelectrodewhich directly reveals NO with nM concentration sensitivity by an amperometric technique (Coneski and Schoenfisch, 2012). The absorptionspectralfeaturesofAN-
b
CD-NOPDallowtheselectiveexcitationoftheNOphotoreleasercomponentoftheconjugateat
l
exc>400nm.TheresultsillustratedinFig.3provideunambiguous evidencethatthebichromophoricCDconjugateisverystablein thedarkbut suppliesNO uponilluminationwithl
exc=405nm.Notethat,therateofphotoreleaseisverysimilartothatobserved foranopticallymatchedsolutionofthemodelcompound
b
CD-NOPD.
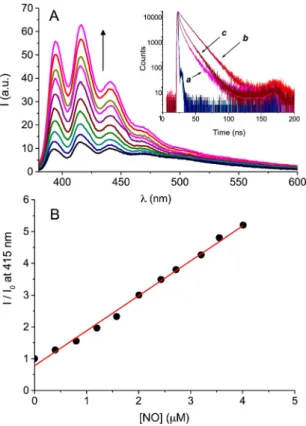
NO photorelease from AN-
b
CD-NOPD is accompanied by dramatic revival ofthe characteristicemission arisingfromthe anthracenefluorophore.Fig.4Ashowsthefluorescenceemission spectraobservedatregularirradiationtimeswithvisiblelightup to45min.Thisfluorescencerestoringisduetotheformationofa reactionproductformedafterthelossofNOthat,incontrasttothe nitroanilinederivative,isnotagoodenergyacceptorandthusis notabletoquenchthefluorescenceoftheanthracenefluorophore.This behavior also reflects in the dynamic of the emission. As shownintheinsetinFig.4A,thefluorescencedecayofAN-
b
CD-NOPD(tracea)wasshorterthanthatofthemodelcompoundAN-
b
CD(traceb).However,after45minirradiationoftheAN-b
CD-NOPDsolution,thefluorescencedynamicbecomeslower(tracec), accordingtotherevival of thefluorescence. Insucha way,the anthraceneunitrepresentsanexcellentopticalreporterfortheNO release.Infact,asillustratedinFig.4B,wefoundanexcellentlinear correlationbetweentheconcentrationofNOphotogeneratedand theincreaseofthefluorescenceintensityoftheopticalreporter.
Notethat,thesuppressionofthequenchingoftheexcitedstate ofanthraceneafterNOreleasereflectsalsointhecapabilityofthis component to photosensitize the formation of singlet oxygen (1O2), the key species in photodynamic therapy of cancer and bacterial diseases (Hasan et al., 2000). This was directly demonstrated by direct detection through monitoring of the Fig.4.(A)Fluorescence(lexc=370nm)spectralchangesobserveduponirradiation
ofanaqueoussolutionofAN-bCD-NOPD(40mM)atregularirradiationtimesof 5min(frombottomtotop)withvisiblelight(lexc=405nm).Theinsetshowsthe fluorescencedecayandtherelatedmultiexponentialfittingforsolutionsofAN- bCD-NOPD(a),themodelcompoundAN-bCD(b)andAN-bCD-NOPDafter45min irradiationwithvisiblelight(c);lexc=370nm;lem=420nm.(B)Correlationofthe fluorescenceincreaseobservedinFig.4AandtheconcentrationofNOreleasedfrom thesamesolution(IandI0representthefluorescenceintensitiesat420nmafter eachirradiationtimeandthatofthenon-irradiatedsolution,respectively).
Fig.5.Luminescencespectra detected upon370nmlight excitationofa D2O solutionsofAN-bCD-NOPD(40mM)before(&)andafter(*)1hirradiationat lexc=405nm.
Fig.6.AbsorptionspectraofanaqueoussolutionofAN-bCD-NOPD(30mM)(a) anditscomplexwithnaphthalene(b)(leftY-axis).Fluorescenceemissionspectrum (c)observeduponexcitationatlexc=280nmofthesampleb(rightY-axis).
typicalphosphorescenceof 1O2 in thenear-IRspectral window (Wilkinsonetal.,1993).Fig.5showsthatexcitationofAN-
b
CD-NOPDat
l
exc=370nmdoesnotleadtoanydetectableformationof1O2,accordingtoeffectivequenchingoccurringoftheanthracene bythenitroanilinecomponent.Ontheotherhand,excitationatthe samewavelengthafterirradiatingtheAN-
b
CD-NOPDsolutionfor1h at 405nm, leads to the appearance of the characteristic luminescencesignalsof1O2withmaximumatca.1270(Fig.5).
Despitethecovalentlinkingwiththetwochromophores,the CD cavity of AN-
b
CD-NOPD is still able to accommodate hydrophobicguests.Thiswasprovenbyusingnaphthaleneasa modelcompound,whichisinsolubleinwatermedium.Tothisend, amethanolsolutionofnaphthalenewasprepared.Afterdryingthe solvent, the resulting dried sample was added of an aqueous solutionofAN-b
CD-NOPDandthesamplewasstirredfor1hatroomtemperature.Themixturewasthenfilteredandanalyzedby UV-Visabsorption.Fig.6 showsthe spectroscopicpropertiesof AN-
b
CD-NOPDbeforetheadditiontothefilmsample(a)andafter thefiltrationprocedure(b).Thepresenceofthetypicalstructured absorptionbandsof thenaphthaleneatca.275 and285nm(b) provides unambiguous evidence for the encapsulation of the guests withintheCD cavity. Besides,thecharacteristic fluores- cenceemissionofnaphthalene(cinFig.6)furtherconfirmsthe effectiveformationofthehost-guestsupramolecularcomplex.4.Conclusions
WehavedevisedandsynthesizedanovelmultifunctionalCD conjugate,bearinganitroanilineandananthracenechromophoric unit,abletoincorporatea numberof functionalitieswithinthe samemolecularskeleton.AN-
b
CD-NOPDiswellsolubleinwaterduetothecationicappendagespresentmainlyatthelowerrimof theCDscaffoldandisabletodeliverNOundertheexclusivecontrol ofvisiblelightstimuli.NOphotoreleaserestoresthefluorescence oftheanthracenefluorophore,whichactsasanopticalreporterof NOconcentration.Tothebestofourknowledge,thisrepresentsthe first exampleof NOphotodispenser withfluorescencereporting integratedinaCDscaffold.Furthermore,wehavedemonstrated thattheanthracenecomponentisalsocapabletophotogenerate
1O2sequentiallytotheNOrelease.TheAN-
b
CD-NOPDconjugateisalsoabletoaccommodatehydrophobicguestswithinthe
b
CD cavity,asprovenbyusingnaphthaleneasamodelcompound.In viewofthekeyroleNOand1O2playsinphotodynamictherapy, thisb
CD derivative represents an intriguing candidate for biopharmaceuticalresearch.Studiesinthisdirectionarecurrently underwayinourlaboratories.Acknowledgements
We thank the Marie Curie Program (FP7-PEOPLE-ITN-2013, CYCLON-HIT608407)forfinancialsupporttoMCfellowsGB,MPL andDA,andfundingoftheresearch. SBthanksforthefinancial supportfromNKFIH109373and fromÚNKP-16-4NewNational ExcellenceProgramoftheMinistryofHumanCapacities.
AppendixA.Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in the online version, at http://dx.doi.org/10.1016/j.
ijpharm.2017.05.023.
References
Bortolus,P.,Monti,S.,1996.Photochemistryincyclodextrincavities.Adv.
Photochem.21,1–133.
Caruso,E.B.,Petralia,S.,Conoci,S.,Giuffrida,S.,Sortino,S.,2007.Photodeliveryof nitricoxidefromwater-solubleplatinumnanoparticles.J.Am.Chem.Soc.129, 480–481.
Coneski,P.N.,Schoenfisch,M.H.,2012.Nitricoxiderelease:partIII.Measurement andreporting.Chem.Soc.Rev.41,3753–3758.
Davis,M.E.,Brewster,M.E.,2004.Cyclodextrin-basedpharmaceutics:past,present andfuture.Nat.Rev.DrugDiscov.3,1023–1035.
Ford,P.C.,2008.Polychromophoricmetalcomplexesforgeneratingthe bioregulatoryagentnitricoxidebysingle-andtwo-photonexcitation.Acc.
Chem.Res.41,190–200.
Ford,P.C.,2013.Photochemicaldeliveryofnitricoxide.NitricOxide34,56–64.
Fraix,A.,Marino,N.,Sortino,S.,2016.Phototherapeuticreleaseofnitricoxidewith engineerednanoconstructs.Top.Curr.Chem.370,225–257.
Fry,N.L.,Mascharak,P.K.,2011.PhotoactiverutheniumnitrosylsasNOdonors:how tosensitizethemtowardvisiblelight.Acc.Chem.Res.44,289–298.
Hasan,T.,Moor,A.C.E.,Ortel,B.,2000.CancerMedicine,5thed.DeckerBCInc., Hamilton,Ontario,Canada.
Ignarro,L.J.,2009.Specialjournalissueonnitricoxidechemistryandbiology.In:
Ignarro,L.J.(Ed.),Arch.PharmacalRes.,.
Ignarro,L.J.,2010.NitricOxide:BiologyandPathobiology.ElsevierInc..
Jia,Q.,Janczuk,A.J.,Cai,T.,Xian,M.,Wen,Z.,Wang,P.G.,2002.NOdonorswith anticanceractivity.ExpertOpin.Ther.Patents12,819–826.
Jicsinszky,L.,Iványi,R.,2001.Catalytictransferhydrogenationofsugarderivatives.
Carbohydr.Polym.45,139–145.
Kirejev,V.,Kandoth,N.,Gref,R.,Ericson,M.B.,Sortino,S.,2014.Apolymer-based nanodeviceforthephotoregulatedreleaseofNOwithtwo-photonfluorescence reportinginskincarcinomacells.J.Mater.Chem.B2,1190–1195.
Loftsson,T.,Douchene,D.,2007.Cyclodextrinsandtheirpharmaceutical applications.Int.J.Pharm.329,1–11.
Matsui,Y.,Yokoi,T.,Mochida,K.,1976.CatalyticpropertiesofCu(II)complexwitha modifiedcyclodextrin.Chem.Lett.5,1037–1040.
Mazzaglia,A.,Micali,Monsù,L.,Scolaro,L.,2006.CyclodextrinMaterials Photochemistry.PhotophysicsandPhotobiology.Elsevier,pp.203–222andref therein.
Mazzaglia,A.,Sciortino,M.T.,Kandoth,N.,Sortino,S.,2012.Cyclodextrin-based nanoconstructsforphotoactivatedtherapies.J.DrugDeliv.Sci.Technol.22,235–
242.
Monti,S.,Sortino,S.,2002.Photoprocessesofphotosensitizingdrugswithin cyclodextrincavities.Chem.Soc.Rev.31,287–300.
Mourtzis,N.,Eliadou,K.,Aggelidou,C.,Sophianopoulou,V.,Mavridis,I.M., Yannakopoulou,K.,2007.Per(6-guanidino-6-deoxy)cyclodextrins:synthesis, characterisationandbindingbehaviourtowardselectedsmallmoleculesand DNA.Org.Biomol.Chem.5,125–131.
Mourtzis,N.,Paravatou,M.,Mavridis,I.M.,Roberts,M.L.,Yannakopoulou,K.,2008.
Synthesis,characterization,andremarkablebiologicalpropertiesof cyclodextrinsbearingguanidinoalkylaminoandaminoalkylaminogroupson theirprimaryside.Chem.Eur.J.14,4188–4200.
Pellois,J.-P.,Hahn,M.E.,Muir,T.W.,2004.Simultaneoustriggeringofproteinactivity andfluorescence.J.Am.Chem.Soc.126,7170–7171.
Piras,L.,Theodossiou,T.A.,Manouilidou,M.D.,Lazarou,Y.G.,Sortino,S., Yannakopoulou,K.,2013.S-Nitroso-b-cyclodextrinsasnewbimodalcarriers:
preparation,detailedcharacterization,nitric-oxiderelease,andmolecular encapsulation.Chem.AsianJ.8,2768–2778.
Riccio,D.A.,Schoenfisch,M.H.,2012.Nitricoxiderelease:partI.Macromolecular scaffolds.Chem.Soc.Rev.41,3731–3741.
Seabra,A.B.,Durán,N.,2010.Nitricoxide-releasingvehiclesforbiomedical applications.J.Mater.Chem.20,1624–1637.
Sortino,S.,2010.Light-controllednitricoxidedeliveringmolecularassemblies.
Chem.Soc.Rev.39,2903–2913.
Ueno,A.,Breslow,R.,1982.Selectivesulfonationofasecondaryhydroxylgroupof b-cyclodextrin.TetrahedronLett.23,3451–3454.
Veldhuyzen,W.F.,Nguyen,Q.,McMaster,G.,Lawrence,D.S.,2003.Alight-activated probeofintracellularproteinkinaseactivity.J.Am.Chem.Soc.125,13358–
13359.
Vittorino,E.,Sciortino,M.T.,Siracusano,G.,Sortino,S.,2011.Light-activatedrelease ofnitricoxidewithfluorescencereportinginlivingcells.ChemMedChem6, 1551–1554.
Wang,P.G.,Xian,M.,Tang,X.,Wu,X.,Wen,Z.,Cai,T.,Janczuk,A.J.,2002.Nitricoxide donors:chemicalactivitiesandbiologicalapplications.Chem.Rev.102,1091–
1134.
Wang,P.G.,Cai,T.B.,Taniguchi,N.,2005.NitricOxideDonorsforPharmaceuticaland BiologicalApplications.Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim, Germany.
Weinstain,R.,Segal,E.,Fainaro,R.S.,Shabat,D.,2010.Real-timemonitoringofdrug release.Chem.Commun.46,553–555.
Wilkinson,F.,Helman,W.P.,Ross,A.B., 1993.Quantumyieldsforthephotosensitized formationofthelowestelectronicallyexcitedsingletstateofmolecularoxygen insolution.J.Phys.Chem.Ref.Data22,113–262.
Yannakopoulou,K.,Jicsinszky,L.,Aggelidou,C.,Mourtzis,N.,Robinson,T.M., Yohannes,A.,Nestorovich,E.M.,Bezrukov,S.,Karginov,V.A.,2011.Symmetry requirementsforeffectiveblockingofporeformingtoxins:comparativestudy witha-,b-,andg-cyclodextrinderivatives.Antimicrob.AgentsChemother.55, 3594–3597.