LYSOZYME
Lata S. Nerurkar
INTRODUCTION
Lysozyme (EC 3.2.1.17, mucopeptide iV-acetylmuramoyl hydro- lase or muramidase) causes lysis of many gram-positive bacteria by its action on their cell walls. It catalyzes hydrolysis of a ß(l-*4)-glycosidic linkages of polysaccharide component of the peptidoglycan (mucopolymer of the bacterial cell wall), which
is composed of alternating N-acetylmuramic acid and iV-acetyl- glucosamine residues with alternating 3(1^-4) and ß(l-*-6) link- ages, cross-linked with peptide chains. The hydrolytic action of lysozyme gives rise to disaccharide units attached to the peptide chains called muropeptides.
This enzyme is of particular interest in the study of phagocytic cells as one of the main functions of these cells is to localize the microorganisms in the body of the host, then degrade and eliminate them. Lysozyme activity of the phagocy- tic cells appears to be of lysosomal origin, it is constantly synthesized by the mononuclear phagocytes (1-4), but its secre-
METHODS FOR STUDYING Copyright © 1981 by Academic Press, Inc.
MONONUCLEAR PHAGOCYTES 6 6 7 All rights of reproduction in any form reserved.
ISBN 0-12-044220-5
tion appears to be independent of that of other hydrolases at least in case of peritoneal macrophages (1,4). Recent evidence indicates presence of distinct granules containing lysozyme in alveolar macrophages of normal and BCG-vaccinated rabbits (5).
More interesting, lysozyme has been shown to have a modulating effect on the response of neutrophils (6,7), particularly sup- pressing their inflammatory action, and may function as an im- portant mediator of tumoricidal function of macrophages (8).
Activity of lysozyme can be quantitated by a variety of methods, which are essentially based on bacteriolytic proper- ties of this enzyme. Bacterial turbidity decreases when the cell walls are acted upon by lysozyme. The most commonly used method involves the measurement of the initial rate of lysis of Micrococcus luteus in suspension that can be followed at a suitable wavelength in the visible spectrum, i.e., 450-640 nm (method I). The other methods involve the measurement of the diameter of the lytic zone after radial diffusion (method II) (9) or electrophoresis (10) of the lysozyme into agarose gels embedded with Micrococcus luteus. Recently, a radioimmunoassay procedure has also been described (11), which revealed good correlation with other previously described techniques. An immunocytochemical technique has been used for cytochemical localization of lysozyme in various cells and tissues (12,13).
All these techniques are applicable or can be adapted to the study of lysozyme activity in mononuclear phagocytes of any origin.
II. METHOD I: SPECTROPHOTOMETRIC ASSAY
A. Introduction
The spectrophotometric assay is performed according to the method of Litwack (14) with minor modifications.
B. Reagents
1. 0.067 M Sodium Potassium Phosphate Buffer pH 6.25 Stock A: 0.2 M KH2PO4, anhydrous monopotassium dihy- drogen phosphate (FW 136) 27.2 gm/liter
Stock B: 0.2 M Na^HPO. anhydrous disodium hydrogen phosphate (FW 142) 28.4 gm/liter
Mix 80 ml of stock A + 20 ml of stock B; adjust pH to 6.25 and dilute the mixture 1:3 with distilled water.
2. Buffered Substrate
Freeze-dried Micrococcus luteus (No. M0128, Sigma Chem.
Co., St. Louis, Missouri) is suspended (10 mg/100 ml) in 0.067 M phosphate buffer, pH 6.25. The absorbance of this suspension
at 450 nm should be between 0.6 and 0.7. Discard after use.
3. Enzyme Standard
Egg white lysozyme standard (Sigma, No. L6876) is prepared as a stock solution of concentration 2 mg/100 ml (20 yg/ml) in 0.067 M phosphate buffer, pH 6.25 and refrigerated.
Various working standards are prepared as shown in Table I.
C. Procedure
(1). The assay is conducted at room temperature.
(2). Set the full-scale absorbance of 0.1 on a given re- cording spectrophotometer. Set the wavelength to 450 nm. For multiple recording, use the auxiliary offset control and zero the different cuvette positions at different heights on the recording chart paper.
(3). Transfer 2.5 ml of buffered substrate to a cuvette and add 0.1 ml of sample or standard at the desired dilutions.
Mix and immediately read the change in absorbance for 1-3 min.
A macrophage equivalent of 0.5-1 x 10 cells/0.1 ml/2.5 ml sub- strate is found to be a suitable sample.
(4). Given a 10-in. recording graph paper with 0.1 in. sub- divisions, each subdivision represents 0.001 absorbance when the full scale is adjusted to 0.1A. Note down change in absor- bance (AA)/min (slope of the regression line) for each standard or sample specimen.
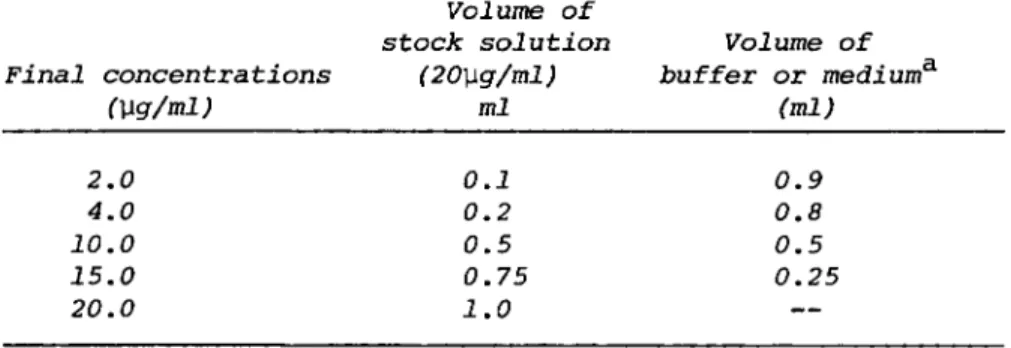
TABLE I. Preparation of Working Lysozyme Standards
Volume of
stock solution Volume of Final concentrations (20\xg/ml) buffer or medium3'
(\lg/ml) ml (ml) 2.0 0.1 0.9 4.0 0.2 0.8 10.0 0.5 0.5 15.0 0.75 0.25 20.0 1.0
aIf culture supernatants are assayed, use respective medium as the dilutent.
D. Calculation of Data
Lysozyme activity can be expressed as absorbance change (Section II. D. 1) or can be converted to the equivalent of reference lysozyme standard as follows (Fig. 1; Section II. D.
2).
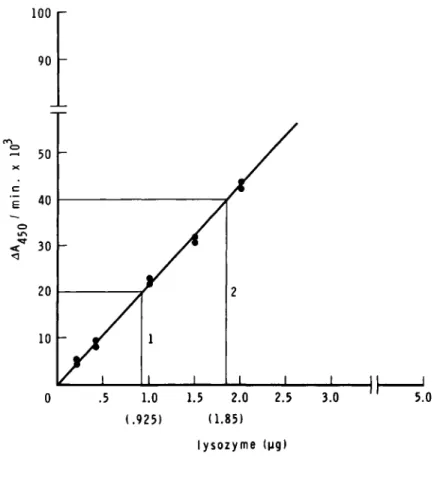
(1). One unit of activity is defined as that amount of enzyme that causes ΔΑ4 5 0 of 0.001 in a Micrococcus luteus sus- pension in 1 min at pH 6.25 in 2.6 ml reaction mixture and light path of 1.0 cm. To convert the absorbance change into units of lysozyme, simply multiply ΔΑ by 1000.
(2). Prepare standard curve by plotting ΔΑ4 5 0 readings from the initial rates against microgram of lysozyme standard
(Fig. 1). Using this curve, obtain lysozyme concentrations in the sample preparations. As given in Fig. 1, 50- and 100-yl aliquots of a macrophage extract result in ΔΑ of 0.02, and
100 r 90
Ξ 50
X
i 40 c o
<* 30
<1
20
10 Γ
/
X
//
/ 1 1
1 1 2
1 1 1 II |
T7 71 Π II Γ
.5 1.0 (.925)
2.5 1.5 2.0
(1.85) lysozyme (μς)
3.0 5.0
Fig. 1. Spectrophotometric method: Linear plot of egg white lysozyme standard (\ig/assay) and &Aäc;n/min.
0.04, respectively. Therefore, these aliquots contain activity equivalent to 0.925 and 1.85 yg egg white lysozyme, respective- ly, or 1 ml of this macrophage extract contains activity equiv- alent to 18.5 yg of egg white lysozyme. This activity can be further expressed as units/10^ cells, units/mg DNA, or units/
mg protein.
E. Critical Comments 1. Standard Lysozyme
Since crystalline hen egg white lysozyme is used as refer- ence standard by many investigators, it is important to select a preparation with at least 25,000 U activity/mg dry weight.
In fact, it is essential to specify the activity units of the reference standard used so that the literature values can be compared. There is some question as to the comparability of lysozyme assays expressed in absolute units (i.e., equivalent of microgram of egg white lysozyme) because the reference en- zyme standards used by various investigators have ranged from 8000-50,000 U/mg. In addition, rat and human lysozymes are 2- to 3.3-fold more active, respectively, than hen egg white lysozyme (1). It is thus advisable to use reference standards from more closely related biological species (1). To overcome this problem of expression of activity units, it may be even more appropriate to express the data as absorbance change units that may be more acceptable to the system(s) recommended by the International Enzyme Commission. It is, however, essential to include several concentrations of reference standards or known serum samples into each assay to ensure within - and between - day test precision.
2. pH
A pH range of 6.0-6.5 is acceptable for most of the assays.
Rabbit alveolar macrophage lysozyme has a pH optimum at 6.5 (15). The lysozyme from hen egg white and human milk have pH optima between 6.0 and 6.5, and 6.2, respectively (16). In contrast, goose lysozyme has maximal activity at acidic pH values (3.8-5.3), whereas the human plasma and leukocyte en- zymes show optimal activity at pH 7.5; human urine enzyme
(from acute monocytic and monomyelogenous leukemia) or chronic myelogenous leukemia leukocytes show high activity at more al- kaline pH values (8.5-8.6) (16). Studies with leukemic cells have to take these properties into consideration.
3. Ionic Strength
Ionic strength of the assay medium appears to be a critical factor in the determination of lysozyme (15,17). Maximal acti- vity for rabbit alveolar macrophage enzyme is seen at ionic strength of 0.04 and more than 50% inhibition of activity is observed at ionic strengths above 0.15 and below 0.017 (15).
It is thus necessary to prepare the test samples and reference standards in buffer or medium of identical ionic strength.
4. Inhibitors
Heparin interferes with the lysozyme assay (18) and should be used with caution especially when mononuclear cells are pre- pared from heparinized blood. Use of EDTA is sometimes recom- mended (19) .
5. Wavelength
Wavelengths of 450 to 640 run yield comparable results, though the assay is slightly more sensitive at the low wave- lengths .
6. Activity Range
The method yields satisfactory results only in a narrow range of activity that may be considered as the drawback of the method. The linear increases in A A4 5 Q values are observed when lysozyme concentrations in assay are varied between 0.2-
2.0 yg of egg white standard. Hence/ it is necessary to dilute or concentrate properly the test samples so that the lysozyme activity can be assayed in the sensitive range.
7. Preparation of Test Samples
Dilution of samples in a buffer or medium containing 5%
fetal calf serum may prevent loss of low levels of lysozyme activity. Ultrafiltration procedures may be used for concen- tration of culture supernatants containing low levels of lyso- zyme, however, preliminary trials should be performed to con- firm that lysozyme activity is not lost irreversibly onto the filters due to strong electrostatic binding. Concentration by dehydration against 20,000 molecular weight polyethylene glycol
(Acquacide III, calbiochem) (15) or by freeze drying (3) has been reported.
8. Advantages
The assay in our hand is fairly sensitive and reproducible with an acceptable range of variability as shown in Table II.
TABLE II. Study of Precision in Lysozyme Assay by Spectro- photometric Method
Activity
Lysozyme (U/mg) Manufacturer's value ^25,000
Laboratory value b 23,785+606^
Coefficient of variation (CV) (%) 8.5
Mean jf S.E.M. of 11 determinations.
}C7 = 100 x S£ . Mean
It has an advantage of providing results on the day of testing and it can be employed in the automated procedures.
III. METHOD II: LYSOPLATE ASSAY A. Introduction
The lysoplate assay is performed according to the method of Osserman and Lawlor (9) with minor modifications.
B. Reagents and Materials
(1). Petri dishes: 100 x 15 mm in diameter are obtained from Falcon, Oxnard, California (No. 1001)
(2) . Eppendorf micropipette: 10 \ll micropipette (No. P5062-10, Scientific products, McGaw Park, Illinois)
(3). Agarose: Agarose (electrophoresis grade) (No. 100267, ICN Pharmaceuticals, Inc., Cleveland, Ohio)
(4). Gel punchers : Thin-walled gel punching tubes with 2-mm diameter can be obtained from Bio-Rad Labs., Richmond, California (#170-4026) and used with proper template.
(5). Magnifying viewer: Calibrating viewer can be obtained from Kallestad, Inc., Chaska, Minnesota
(6). Micrococcus luteus: See Section II. B.2.
(7). 0.067 M phosphate buffer pH 6.25: See Section II.B.l.
(8). Standard lysozyme: Stock solution: 1.0 mg/ml of egg white lysozyme is prepared in 0.067 M phosphate buffer, pH 6.25. The working standards of concentrations 5, 25, 50, 100, and 500 yg/ml are prepared by 1:200, 1:40, 1:20, 1:10, 1:2, dilutions, respectively, of the stock solution with phosphate buffer.
C. Procedure
Uniform suspension of Micrococcus luteus is prepared in a small volume of phosphate buffer and is added to molten (60°- 70°C) 1% agar in 0.067 M phosphate buffer, pH 6.25 to a final concentration of 50 mg/100 ml. About 10-12 ml aliquots of this agar are then poured into petri dishes (to give a depth of 3- 4 mm). The petri dishes are covered, the agar is allowed to solidify, and kept in humid chambers for 15-20 min. The sample wells of 2-mm diameter are then cut using gel punchers. Six- teen sample wells (four rows of four wells) 20-mm apart can be cut in a petri dish of the given size (100 mm). However, for samples with high lysozyme levels, nine sample wells (three
rows of three wells) are preferred so that overlapping of the cleared zones does not occur. The gel plates can be stored for 2 weeks at 4°C; in that case, they have to be inverted, kept in plastic bags to prevent evaporation, and sample wells cut just prior to use.
Aliquots (10 yl) of the reference lysozyme standards or the test samples are filled in the respective sample wells using Eppendorf micropipette. The plates are left at room temperature for 12-18 hr. Prolonged incubations (up to 20-24 hr) may be necessary in case of samples with low lysozyme levels. As the enzyme diffuses into the gels, bacterial lysis takes place surrounding the sample wells resulting in clear zones. The diameters of the clear zones are then measured directly with an enlarged viewer or after being photographed.
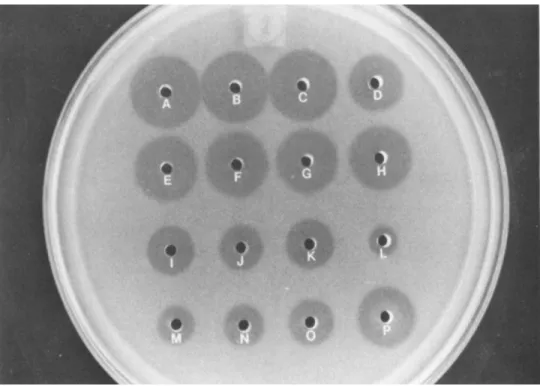
The former is slightly time-taking but inexpensive compared to the latter. It is important that sample application and the measurement of diameter of cleared zones be made as quickly as possible. A typical lysoplate is shown in Fig. 2.
D. Calculation of Data
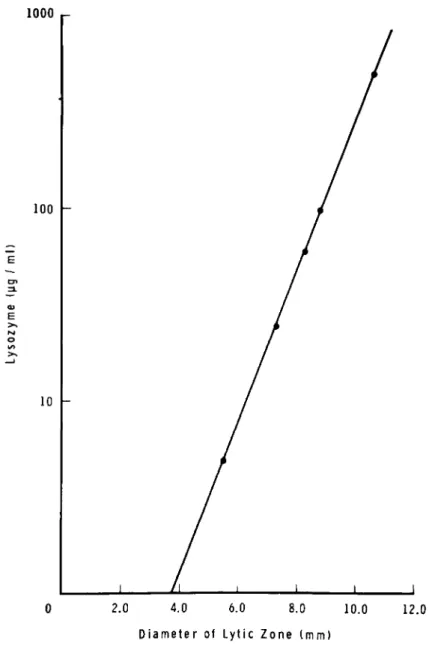
The diameters of the cleared zone are proportional to the log concentration of lysozyme (yg/ml). A semilogarithmic plot is made of diameters (mm) obtained from several reference stan- dards against their concentrations as shown in Fig. 3 and the concentrations in the test samples are interpolated from the plot.
E. Critical Comments
The lysoplate method has been carefully studied and criti- cally evaluated (20) and it appears to correlate well with the long-used spectrophotometrie method (9,21) and the recently developed radioimmunoassay (11). However, multiple factors seem to affect the lysoplate assay.
2. Enzyme standard
As described above, the activity differences in the refer- ence enzyme standards obtained from different biological spe- cies can be seen in this assay. Human urine enzyme has been shown to be more active than the hen egg white enzyme. The ratio of activities, however, changes slightly as a function of time (20). The usage of a standard from biologically re- lated or similar species may at least partially resolve this problem.
Fig. 2. Lysoplate determination of lysozyme: concentrated (50x) human lung lavage supernatant (A-D) , rabbit alveolar
macrophages homogenates (E-H), human neutrophils homogenates (I-K) , egg white lysozyme standards L = 5 \ig/ml, M = 25 Vg/ml, N = 50 ]\g/ml, 0 = 100 \ïg/ml, P = 500 \ig/ml; 10 \il samples were used in each well.
2. pH
Unlike the spectrophotometric method, where a pH optimum between 6-6.5 is observed for this enzyme in most instances, the lysoplate method shows an increase in diameters of cleared zones when pH of the system is increased from 5 to 7.5 (20).
3. Ionic Strength
Ionic strength of the gel medium influences the diameters of cleared zones in the similar manner as in the spectrophoto- metric method. In the case of both human and egg enzymes, maximum diameters were obtained when 0.1 M NaCl was added to
1000 r-
2.0 4.0 6.0 8.0 10.0 12.0 Diameter of Lytic Z o n e t m m )
Fig. 3. Lysoplate method: Semilogarithmic plot of egg white lysozyme standard (\ig/ml) and diameters in mm of the cleared zones.
0.066 M phosphate buffer, pH 6.6. The human enzyme was more sensitive than the hen enzyme to the further increased NaCl concentration up to 0.4 M (20).
4. Temperature
Unlike in the spectrophotometrie method where the lysozyme activity is far depressed at low temperatures, the lysoplate method is relatively less affected by temperature variations
(20); the effects on the latter are related to the changes in the diffusion rate of the enzyme.
5. Activity range
The lysoplate method is applicable to a wide range of lysozyme concentrations. A straight line semilogarithmic plot is obtained when concentration is varied between 5-500 yg/ml as seen in Fig. 3. Adjustment in the concentration of enzyme in test samples may not be thus required. However, the method is relatively less accurate for evaluating very low levels of lysozyme compared to the spectrophotometric method.
6. Composition of Test Sample
Salt or protein content of the test sample affects the diffusion rate of the lysozyme. Bovine serum albumin seems to have a profound effect on diameters of clearing zone, more at low than at high concentration of this enzyme (20). This ef- fect is probably related to the binding of lysozyme to the proteins (22). This may be an important factor when culture supernatants containing serum (5-20%) are assayed for lysozyme activity. The preparation of a standard curve in a medium identical to the test sample may alleviate this problem.
7. Advantages
The lysoplate method is relatively simple and does not re- quire expensive instrumentation. It can be employed where ex- tremely accurate quantitative measures are not required. It is widely used in clinical laboratory settings.
III. SIGNIFICANCE
Lysozyme is widely distributed throughout the body and is particularly high in concentration in body fluids like tears
(23,24), saliva (23), and breast milk (24-26). Using immuno- histochemical staining technique, this enzyme is detected in a variety of tissues, e.g., paneth cells (12,13,27), lymph nodes, renal tubules, respiratory tissue, stellate cells in follicle centers, and medulla of the thymus (13), serous salivary acinar cells, and lactating mammary tissue (27). The significance of
such wide occurrence of this enzyme is not well understood ex- cept that is is generally agreed that antibacterial action of this enzyme may complement the secretory immune system at the mucosal surfaces (25,26).
High concentration of lysozyme is found in phagocytic cells, particularly in neutrophils (28,29), monocytes (1,30), and tissue macrophages, e.g., alveolar macrophages (2,3,31-34), peritoneal macrophages (1,3,4,31), skin macrophages (3), and histiocytes (27). The neutrophils acquire their stores of lysozyme early in the cell development in bone marrow and do not synthesize it once the cells are mature. In contrast, as measured in in vitro cultures (1-4), mononuclear phagocytes have the capacity to synthesize actively this enzyme, which can be blocked by the inhibitors of protein synthesis (2,3).
The turnover of neutrophils and monocyte-macrophages appears to contribute to the serum lysozyme activity. This is further supported by the fact that serum activity is increased in mye- locytic and monocytic leukemias, (9,36,40), in infections (21), in certain granulomatous diseases, e.g., sarcoidosis, tubercu- losis and Crohn's granulomata (9,27,35,47,48), and is decreased in lymphocytic leukemias (36). The cells of lymphoid or eosino- philic series show little or no lysozyme activity (3,38-40).
The lysozyme content of different leukocytes is given in Table III.
Lysozyme is considered to be one of the constitutive en- zymes of macrophages (1,4). It is the major secretory product and forms about 25% of the extracellularly secreted protein of macrophages. The secretion of this enzyme by monocytes and macrophages from various sources proceeds in a continuous fash- ion (1,2,4,44) and is unaffected by a variety of stimuli such as thioglycollate, steroids, lymphokines, and endocytosis, all of which influence the secretion of other macrophage products, e.g., plasminogen activator (4). Lysozyme secretion in culture is characterized by a large net increase in total lysozyme
(1,2,4,44), 85-90% of which is excreted to the extracellular medium (1). Using -C-labeled amino acid precursors, it has been shown that lysozyme is the major labeled protein secreted into the medium (1,3) and represents up to 2.5% of the total protein (1). The ability to secrete lysozyme appears to be one of the specific features of macrophages and is often lost after hybridization of macrophages with other somatic cells (4).
Lysozyme is a cationic polypeptide whose molecular weight is about 14,000. The primary and secondary structures of some lysozomes are well characterized. Antigenically different lysozomes are detectable as seen from the lack of cross reac- tivity between the rat lysozyme and antiserum to human urinary lysozyme (45). It is interesting to note that different tis- sues show different specificities in immunohistochemical stain- ing procedures indicating possibility of isozymes (13). The
TABLE III. Lysozyme Concentration of Different Leukocytes
Cell type
Microgram lysozymec /10s cells
Method of assay
Reference
Monocytes human human Macrophages
Mouse peritoneal Mouse peritoneal
(unstimulated)
Mouse peritoneal (thioglycollate
stimulated)
Rabbit peritoneal
Rabbit alveolar-newborn Rabbit alveolar-adult Rabbit alveolar
Rabbit alveolar Rabbit alveolar (BCG) Rabbit alveolar (BCG) Human alveolar-
nonsmokers Human alveolar-
smokers Neutrophils
human human human
human (smokers and nonsmokers) Lymphocytes
0.12 1
8.3 0.4 0.35{ 0.25'
540.0d 4.0e 2.2e 3.2 3400.0ά 9.2 20.85
1.2 2.5
7.2 7.9 2.87 2.2 0.4
r
I I
II I I I II
1 38 33 1
I I I I I I I I
32 41 41 33 32 33 15 42 42
38 29 43 42 38
Hen egg white lysozyme equivalent
®Human lysozyme standard used
cRat lysozyme standard used
y9 eÇ9 white lysozyme equivalent/ml of packed cells
eAbsorbance units given in ref. (41) are converted to equivalent egg white lysozyme (\ig) units (1 \xg egg white lyso- zyme ^ 24 absorbance units)
Spectrophotometric method
^Lysoplate method
wide occurence of lysozyme in body tissues leads one to specu- late that, in addition to its antibacterial (46) and anti-in- flammatory (6) properties, this enzyme may have more basic function to perform through its binding to cell membranes, changing the surface charge density and subsequently modulating the cell function.
Acknowledgment
This work, in part, was supported by NIH Grant 5T32HD07185- 02. The author gratefully acknowledges the supply of human lung lavage materials provided by Dr. Henry Yeager.
REFERENCES
1. S. Gordon, J. Todd, and Z. A. Cohn. In vitro secretion of lysozyme by mononuclear phagocytes. J. Exp. Med. 139:
1228-1248, 1974.
2. E. R. Heise and Q. N. Myrvik. Secretion of lysozyme by
rabbit alveolar macrophages in vitro. J. Reticuloendothel.
Soc. 4:510-533, 1967.
3. D. B. L. McClelland and R. van Furth. In vitro synthesis of lysozyme by human and mouse tissues and leukocytes.
Immunology 28:1099-1114, 1975.
4. S. Gordon. Regulation of enzyme secretion by mononuclear phagocytes: Studies with macrophage plasminogen activator and lysozyme. Fed. Proc. 37:2754-2758, 1978.
5. D. B. Lowrie, P. W. Andrew, and T. J. Peters. Analytical subcellular fractionation of alveolar macrophages from normal and BCG-vaccinated rabbits with particular reference to heterogeneity of hydrolase containing granules. Bio- chem. J. 178:161-167, 1979.
6. L. I. Gordon, S. D. Douglas, N. E. Kay, O. Yamada, E. F.
Osserman, and H. S. Jacob. Modulation of neutrophil function by lysozyme, potential negative feedback system of inflammation. J. Clin. Invest. 64:226-232, 1979.
7. M. Klockars and P. Roberts. Stimulation of phagocytosis by human lysozyme. Acta Haematol. 55:289-295, 1976.
8. E. F. Osserman, M. Klockars, J. Halper, and R. E. Fischel.
Effects of lysozyme on normal and transformed mammalian cells. Nature 243:331-335, 1973.
9. E. F. Osserman and D. P. Lawlor. Serum and urinary lyso- zome (muramidase) in monocytic and monomelocytic leukemia.
J. Exp. Med. 124:921-951, 1966.
10. G. Virella. Electrophoresis of lysozyme into
Micrococcus-containing agarose gel: Quantitative and analytical applications. Clin. Chem. Acta 75:107-115, 1977.
11. T. L. Peeters, Y. R. Depraetere, and G. R. Vantrappin.
Radioimmunoassay for urinary lysozyme in human serum from leukemic patients. Clin. Chem. 24:2155-2157, 1978.
12. M. Klockars* M. C. Adinolfi, and E. F. Osserman.
Ontogeny of lysozyme in the rat. Proc. Soc. Exp. Biol.
Med. 145:604-609, 1974.
13. S. S. Spicer, R. Frayser, G. Virella, and B. J. Hall.
Immunocytochemical localization of lysozymes in respira- tory and other tissues. Lab. Invest. 36:282-295, 1977.
14. G. Litwack. Photometric determination of lysozyme acti- vity. Proc. Soc. Exp. Biol. Med. 89:401-403, 1955.
15. S. F. Carroll and R. J. Martinez. Purification and pro- perties of rabbit alveolar macrophage lysozyme. Infect.
Immun. 24:460-467, 1979.
16. P. Jolies, I. Bernier, J. Berthou, D. Charlemagne, A. Faure, J. Hermann, J. Jolies, J. P. Perin, and J.
Saint-Blancard. From lysozymes to chitinases: Struc- tural kinetic and crystallographic studies. In "Lyso- zyme" (E. F. Osserman, R. E. Canfield, and S. Beychock, eds.), pp. 31-54. Academic Press, New York, 1974.
17. R. C. Davies, A. Neuberger, and B. M. Wilson. The de- pendence of lysozyme activity on pH and ionic strength.
Biochem. Biophys. Acta 178:294-305, 1969.
18. E. Kaiser. Inhibition and activation of lysozyme.
Nature 171:607-608, 1953.
19. R. M. Bennett and T. Kokocinski. Lactoferrin content of peripheral blood cells. Br. J. Haematol. 39:509-521, 1978.
20. T. L. Peeters and G. R. Vantrappen. Factors influencing lysozyme determinations by the lysoplate method. Clin.
Chem. Acta. 74:217-225, 1977.
21. S. Zucker, D. J. Hanes, W. R. Vogler, and R. Z. Eanes.
Plasma muramidase: A study of methods and clinical applications. J. Lab. Clin. Med. 75:83-92, 1970.
22. G. Virella. The electrophoretic mobility of serum lysozyme. Experientia 31:1465-1467, 1975.
23. A. Fleming and V. D. Allison. Observations on a bacterio- lytic substance ("lysozyme") found in secretions and tis- sues. Br. J. Exp. Pathol. 3:252-260, 1922.
24. J. Jolies and P. Jolies. Human tear and milk lysozymes.
Biochemistry 6:411-417, 1967.
25. J. K. Welsh and J. T. May. Antiinfective properties of breast milk. J. Pediat. 94:1-9, 1979.
26. R. A. Lawrence. "Host-Resistence Factors and immunolo- gical Significance of human milk," pp. 73-91. The C. V.
Mosby Co., St. Louis, Missouri, 1980.
27. D. Y. Mason and C. R. Taylor. The distribution of
muramidase (lysozyme) in human tissues. J. Clin. Pathol.
25:124-132, 1975.
28. J. K. Spitznagel. Advances in the study of cytoplasmic granules of human neutrophilic polymorphonuclear leuko- cytes. In "The Phagocytic cell in Host Resistence"
(J. A. Bellanti and D. H. Dayton, eds.), pp. 77-85.
Raven Press, New York, 1974.
29. L. S. Nerurkar, L. Jacob, B. J. Zeligs, J. Walser, H.
Yeager, and J. A. Bellanti. A study of chronic granu- lomatous disease in adults. Submitted for publication.
30. F. Schmalzl and H. Braunsteiner. The cytochemistry of monocytes and macrophages. Ser. Haematol. Ill:93-131, 1970.
31. E. S. Leake and Q. N. Myrvik. Changes in morphology and in lysozyme content of free alveolar cells after the intravenous injection of killed BCG in oil. J. Reticulo- endothel. Soc. 5:33-53, 1968.
32. Q. N. Myrvik, E. S. Leake, and B. Fariss. Lysozyme con- tent of alveolar and peritoneal macrophages from the rabbit. J. Immunol. 06:133-136, 1961.
33. Z. A. Cohn and E. Wiener. The particulate hydrolases of macrophages I. Comparative enzymology, isolation, and properties. J. Exp. Med. 118:991-1008, 1963.
34. M. E. Carson and A. M. Dannenberg, Jr. Hydrolytic en- zymes of rabbit mononuclear exudate cells II. Lysozyme.
Properties and quantitative assay in tuberculous and control inbred rabbits. J. Immunol. 94:99-104, 1965.
35. S. C. Finch, J. P. Lamphere, and S. Jablon. The relation- ship of serum lysozyme to leukocytes and other constitu- tional factors. Yale J. Biol. Med. 36:350-360, 1964.
36. P. Jolies, M. Sternberg, and G. Mathe. The relationship between serum lysozyme levels and the blood leukocytes.
Israel J. Med. Sei. 1:445-447, 1965.
37. R. S. Briggs, P. E. Perillie, and S. C. Finch. Lysozyme in bone marrow and peripheral blood cells. J. Histochem.
Cytochem. 14:167-170, 1966.
38. H. J. Senn, B. Chu, J. O'Malley, and J. F. Holland.
Experimental and clinical studies on muramidase (lyso- zyme) . Acta. Haematol. 44:65-77, 1970.
39. J. L. Greenberger, A. Campos-Neto, R. Perkman, W. C.
Moloney, A. Karpas, S. F. Schlossman, and D. S. Rosenthai.
Immunological detection of intracellular and cell-surface lysozyme with human and experimental leukemic leukocytes.
Clin. Immunol. Immunopathol. 5:318-334, 1977.
40. A. T. Skarin, Y. Matsuo, and W. C. Moloney. Muramidase in myeloproliferative disorders terminating in acute leukemia. Cancer 29:1336-1342, 1972.
41. L. S. Nerurkar, B. J. Zeligs, and J. A. Bellanti.
Maturation of the rabbit alveolar macrophage during
animal development II. Biochemical and enzymatic studies.
Pediat. Res. 11:1202-1201, 1977.
42. J. 0. Harris, G. N. Olsen, J. R. Castle, and A. S.
Maloney. Comparison of proteolytic enzyme activity in pulmonary alveolar macrophages and blood leukocytes in
smokers and nonsmokers. Am. Rev. Resp. Dis. 111:519-586, 1975.
43. D. G. Wright, D. A. Bralove, and J. I. Gallin. The dif- ferential mobilization of human neutrophil granules:
Effects of phrobol myristate acetate and ionophore A23187.
Am. J. Pathol. 87:213-284, 1977.
44. L. P. Einstein, E. E. Schneeberger, and H. E. Colten.
Synthesis of the second component of complement by long- term primary cultures of human monocytes. J. Exp. Med.
143:114-126, 1976.
45. A. C. Wilson and E. M. Prager. Antigenic comparison of animal lysozymes. In "Lysozyme" (E. F. Osserman, R. E.
Canfield, and S. Beychock, eds.), pp. 127-141. Academic Press, New York, 1974.
46. A. A. Glynn. Lysozyme: Antigen, enzyme, and antibac- terial agent. Sei. Basis Med. Ann. Rev. 31:31-52,. 1968.
47. P. T. Bodel, P. T. Major, and J. B. L. Gee. Increased production of endogenous pyrogen and lysozyme by blood
monocytes in sarcoidosis. Yale J. Biol. Med. 52:241-256, 1979.
48. R. S. Pascual, J. B. L. Gee, and S. C. Finch. Usefulness of serum lysozyme measurement in diagnosis and evaluation of sarcoidosis. N. Engl. J. Med. 289:1014-1016, 1973.