Surface Tension Measurements
B Y
MALCOLM DOLE
Department of Chemistry, Northwestern University, Evanston, Illinois
CONTENTS
Page
1. Introduction S06 1.1. Definitions 306 1.2. Review of Methods 306 1.3. Review of Applications 306 2. Capillary Rise Method 308
2.1. Absolute Measurements 308 2.1.1. Introduction 308 2.1.2. General Theory 308 2.1.3. Experimental Precautions 310
2.1.4. Experimental Accuracy and Reproducibility 311
2.2. Relative Measurements 312 2.2.1. Double Capillary Tube Methods 312
2.2.2. Relative Methods of Jones and Ray 313 2.3. Combined Capillary Tube-Pressure Methods 314
2.3.1. Single Capillary Tubes 314 2.3.2. Double Capillary Tubes 316 3. Maximum Bubble Pressure Methods 317
3.1. Single Tube Methods 317 3.1.1. Introduction 317 3.1.2. Theory 317 3.1.3. Applications 318 3.2. Double Tube Methods 319
3.2.1. Apparatus of Sugden 319 3.2.2. The Relative Method of Warren 320
3.2.3. Method of Long and Nutting. 321 4. Drop-Weight and Pendant Drop Methods 321
4.1. Drop-Weight Method 321 4.1.1. Introduction 321 4.1.2. Theory 321 4.1.3. Methods 322 4.2. Pendant Drop Method 324
5. Ring Methods 325 5.1. Single Ring Method 325
5.1.1. Introduction 325 5.1.2. Theory of the Ring Method 325
5.1.3. Methods 327 305
5.2. Double Ring Method..
5.3. Straight Wire Method.
References
Page 328 . 330 . 331
1. I N T R O D U C T I O N
1.1. Definitions
Surface tension is defined as the force in the interface between a liquid and gas phase or between a solid and gas phase t h a t opposes a n extension of t h e surface, t h a t is measured along a line, and t h a t is expressed with the symbol y having the units of dynes per centimeter. T h e force lying in the interface between two liquid phases, or between solid and liquid phase, t h a t opposes an extension of t h e interface is defined as t h e interfacial tension and also has the. symbol y with t h e units dynes per centimeter. T h u s surface tension is a force per unit of length.
Surface energy of an area A cm.2 is defined as y · A ergs.
There are m a n y methods of surface and interfacial tension measure- ment among which m a y be mentioned t h e capillary rise, t h e ring, t h e maximum bubble pressure, the drop weight, the Wilhelmy, the p e n d a n t drop, the centrifugal, the sessile drop, t h e ripple, t h e vibrating jet methods, and modifications of these. Double capillary tubes, twin- rings, straight wires, plates, discs, parallel plates, horizontal capillary tubes, etc. have been used. I n fact t h e study of surface tension tech- niques might be described as a s t u d y of scientific inventiveness and ingenuity.
We do not have space here t o describe and review all methods a n d modifications t h a t have been used in the past; instead t h e four most important and convenient methods will be discussed. Of these, t h e m a x i m u m bubble pressure method and t h e drop weight m e t h o d have most often been used in studying the surface tension of liquid metals and alloys, a n d the m a x i m u m bubble pressure method in t h e analysis of solutions. M a n y of t h e principles of one method are at once trans- ferable to another method, so t h a t if, for example, the theory underlying t h e capillary rise method is well understood, it is not difficult t o com- prehend t h e techniques of m a n y of the other methods.
As most metals are formed in the liquid state it is clear t h a t surface tension forces m a y influence some of their properties, in particular t h e spreading of one liquid metal over another, possibly solid, metal is
1.2. Methods
1.3. Applications
affected by t h e surface forces. Thus, Chalmers (10) has investigated t h e relationship between surface tension a n d t h e tinplating of steel by the hot dipping process. Coffman a n d P a r r (11) found t h a t H C 1 gas not only lowers t h e surface tension of a Zn-Pb solder, b u t also markedly improves the spreading. T h e connection is obvious since, as t h e surface tension of t h e liquid phase is larger, t h e tendency for it t o spread is less (the work of adhesion of t h e liquid t o t h e solid a n d t h e contact angles m u s t also be considered). As t h e surface tension of molten metals is remarkably high (10 times or more greater t h a n t h a t of water in m a n y cases) with sometimes an unusual positive t e m p e r a t u r e coefficient, it is not surprising t h a t surface tension forces play a significant role in all processes where t h e spreading of a liquid metal is concerned.
T h e accurate determination of t h e surface tension of molten metals is difficult because of two factors, first t h e ease with which metals combine with gases of different types t o form surface compounds such as oxides or hydrides t h a t upset t h e measurements, and second t h e large contact angle usually exhibited between metals and glass or quartz. I n t h e drop weight method, for example, this means t h a t t h e inner radius determines t h e size of t h e drop whereas when water is used with a quartz capillary, it is t h e outer radius which m u s t be used in t h e surface tension calculation.
Grain size in alloys is u n d o u b t e d l y affected b y surface tension: see, for example, t h e remarks of Bastien on grain size in Al-Mg alloys (5).
M a n y solutions containing surface active solutes can be analyzed b y adding base, let us say, which converts t h e surface active acid to a surface inactive compound a n d causes a sharp rise in t h e surface tension until t h e end point in t h e titration is reached, at which time t h e surface tension becomes constant (see T a u b m a n n , 63, a n d Preston, 48). T h e m a x i m u m bubble pressure m e t h o d is used, t h e pressure rising rapidly t o t h e end point. Reference can also be m a d e t o t h e filter-paper strip m e t h o d of capillary analysis carried out b y Liesegang (38).
T h e flotation of solids, as in t h e separation of minerals b y flotation, is entirely dependent on surface forces; in particular the property of t h e solid, or of t h e solid plus " collector/' t h a t largely determines t h e flotation is t h e contact angle between t h e liquid and solid phase. T h e stability of the floated ore will increase with increase in t h e value of 7( 1 — cos 0) where 7 is t h e surface tension of t h e liquid phase and 0 is t h e contact angle.
T h e r a t e of penetration of liquids into solid powders and capillary tubes is a function of t h e " penetrating p r e s s u r e / ' which is given b y t h e term
2γ cos 0t
r '
again showing t h e importance of surface tension and contact angle.
As this chapter will include only a description of methods of surface tension measurement, the reader who is interested in contact angles, frothing agents, surface film pressures, a n d other surface phenomena is referred t o t h e excellent treatise of Adam (2).
2. C A P I L L A R Y R I S E M E T H O D
2.1. Absolute Measurements
2.1.1. Introduction. Since t h e capillary rise method has been in the past t h e standard surface tension method t o which all other methods
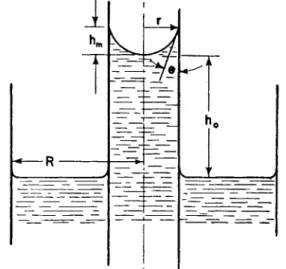
FIG. 1. Diagram for discussion of the capillary rise method.
have been referred, we shall consider it first in some detail. T h e theory underlying this method should be well understood because it is funda- mental and applicable in p a r t to all other types of surface tension measure- m e n t . I t is interesting t o note t h a t since t h e exhaustive studies of Richards and Coombs (52), Richards and Carver (51), a n d Harkins and Brown (26) there have been no important a t t e m p t s t o improve, t h e pre- cision of absolute surface tension measurements; t h e tendency has been on t h e other h a n d t o increase t h e accuracy b y relative methods which enable slight changes in surface tension t o be measured. These relative methods will be described later. T h e absolute capillary rise method is nearly as precise as we can at t h e m o m e n t interpret the data.
2.1.2. General Theory. T h e forces t h a t , at equilibrium, act on a capillary t u b e liquid column (see Fig. 1) above t h e surface of t h e liquid in t h e wide tube, are as follows: a surface tension force acting around a
periphery 2rr, with y dynes per centimeter of length a n d a vertical component proportional t o cos θ where θ is t h e so-called contact angle, or t h e angle between t h e vertical wall a n d a t a n g e n t t o t h e meniscus a t t h e point of contact; t h e b u o y a n c y force due t o air a n d vapor displaced equal t o nr2ghpv dynes where h is t h e corrected height of capillary rise, pv is t h e density of t h e vapor phase, a n d g is t h e gravitation constant;
t h e downward pull of gravity on t h e liquid supported in t h e column equal t o irr2ghpi where pi is t h e density of t h e liquid phase. E q u a t i n g these forces a t equilibrium we h a v e
β rghfo - p,)
7 2 cos θ KL)
As most p u r e liquids wet clean glass, t h e contact angle Θ is usually assumed t o be zero. Richards a n d Carver (51) in fact could find no evidence of a finite contact angle between water a n d glass using a sensitive optical method," b u t t h e y do not s t a t e how small a contact angle could have been detectable in their equipment.
T h e weight of liquid supported b y surface tension forces includes t h a t of t h e meniscus, which m u s t be t a k e n into consideration; in other words t h e t r u e height of rise is not h0 b u t h0 + hm or h. If t h e radius of t h e capillary t u b e is 0.02 cm. or below, in other words if t h e meniscus has t h e shape of a perfect hemisphere, t h e t r u e height can be calculated from t h e equation
h = ho + I (2)
which m a y be obtained for small values of r from Poisson's (46) equation
h - ho + I - £o (0.1288) (3)
or from t h a t of Hagen and Desains (23)
Α = Λ° + 3^ Τ 7 ' ( 4 ) where a is t h e capillary constant,
a* = rh (5)
T h e equation of Hagen a n d Desains is t o be preferred u p to a value of r = 0.1 cm. For still higher values of r/h u p t o r/h = 0.3 t h e equation of Porter (47) can be used
where
E q u a t i o n (6) is also based on t h e assumption t h a t t h e t u b e holding t h e bulk of t h e liquid is wide enough so t h a t t h e surface of t h e liquid is essentially flat.
A correction t o t h e observed height, A0, m u s t also be m a d e because of t h e fact t h a t t h e lower level of liquid t h a t serves as a reference point in measuring t h e height, A0, is somewhat higher because of capillary rise on t h e walls of t h e wide t u b e t h a n a free flat surface would be. T h e following equation of Lord Rayleigh (50) makes possible t h e calculation of this correction for wide t u b e s :
As tested b y Richards a n d Carver (51) this equation is valid for values of r/a greater t h a n 2.75. T h e correction factor in t h e case of smaller tubes can be obtained from t h e experimental curve given b y these authors.
For t h e most refined work a final correction needs t o be m a d e if t h e capillary t u b e is not perfectly circular in cross section. If t h e t u b e is elliptical instead of circular, with a ratio of major t o minor axis given by Qy t h e n t h e capillary rise will be greater in h u n d r e d t h s of one per cent b y t h e factor (from Richards a n d Carver),
This expression is valid only for Q values u p t o 1.10 ( 1 0 % ellipticity).
Apparently u n a w a r e of t h e work of Richards a n d Carver, Smith and Foote (58) have also discussed a n d given mathematical equations for t h e surface tension in elliptical tubes.
2.1.8. Experimental Precautions. Since t h e surface tension as measured b y t h e capillary rise m e t h o d is directly proportional to t h e height, t h e density, a n d t h e radius of cross section, it is essential t h a t all of these quantities be known with as great a precision as desired in t h e final surface tension value. T h e density of t h e pure liquid is readily determined with great accuracy, t h e height also is fairly readily measured, b u t t h e radius of cross section offers t h e greatest difficulty in its determi- nation. T h e usual procedure is t o force a small slug of mercury through t h e capillary measuring its length a t various points along t h e t u b e with an accurate comparator. H a r k i n s a n d Brown (26) point out t h a t gravitational forces m a y distort t h e meniscus when t h e t u b e is in a horizontal position a n d Harkins (25) recommends t h e adoption of Young's scheme, which eliminates t h e meniscus correction b y using a long and short section of mercury. Richards a n d Carver (51) could not observe a n y gravitational distortion in t h e case of small bore tubes, b u t applied a correction for t h e mercury meniscus b y measuring its length (8)
1900(Q - 1.00) - 4 (9)
(a distance corresponding t o hm) a n d computing t h e volume of mercury in t h e meniscus, assuming it t o be a hemisphere. One wonders why capillary tubes have not been studied in a vertical position with the mercury column supported on a column of water or some other liquid.
F r o m theoretical considerations which we shall discuss later, it would appear t h a t t h e diameter of t h e t u b e wet with a film of water is of greater significance for surface tension measurements t h a n t h a t of a d r y tube.
T h e greatest care in selecting capillary tubes of uniform t r u e right cylindrical shape should be exercised.
Capillary height measurements are m a d e with accurate cathetometers with a sharp edged black screen immersed in t h e t h e r m o s t a t t a n k just under a n d back of t h e meniscus, as recommended b y Richards and Coombs (52). T h e a p p a r a t u s should be tested for optical distortion b y measuring t h e distance between two ruled lines on a glass plate inserted in t h e b a t h a t t h e location of t h e capillarimeter and comparing this dis- tance with a similar measurement m a d e in t h e air. Richards and co-workers measured t h e height of rise with t h e capillary t u b e in four positions, front, back, inverted front, a n d inverted back, a n d took t h e average t o eliminate t h e distortion of t h e image. T h e capillarimeter, as well as t h e plates through which t h e observation is made, should be, of course, exactly vertical.
T h e final experimental precaution has t o do with cleanliness of equip- m e n t a n d p u r i t y of liquids studied. A slight a m o u n t of grease on glass surfaces will change t h e contact angle between liquid a n d glass, t h u s vitiating t h e measurement. T h e best technique for t h e removal of grease is t o wash t h e glass a p p a r a t u s with a hot mixture of nitric a n d sulfuric acids (cleaning solution should not be used since chromic oxide seems t o p e n e t r a t e slightly into t h e glass structure—as we have often noticed in making accurate density measurements in t h e Northwestern Laboratory), t o rinse with redistilled water, a n d finally t o steam out t h e a p p a r a t u s for about an hour using steam generated from redistilled water. T h e chief criticism of t h e work of Richards a n d his school lies in their use of chromic acid cleaning solution. Nevertheless, their results agreed very closely with those of Harkins a n d Brown who applied the steaming out technique t o t h e cleansing of their glass capillarimeters.
Young et al. (69) describe a useful overflow method of renewing t h e surface b y flowing t h e liquid in t h e capillary t u b e out into a t r a p . (The surface could also be renewed in t h e a p p a r a t u s of Richards a n d Coombs (52) and, indeed, was, in their habit of inverting their apparatus.)
2.1.4. Experimental Accuracy and Reproducibility. Table I gives some d a t a from which t h e degree of accuracy obtainable can be judged.
Richards a n d Carver (51) found t h a t removal of air increased the
surface tension b y t h e following a m o u n t s : water 0.02, benzene 0.14, chloroform 0.10, carbon tetrachloride 0.15, ether 0.05, and dimethyl aniline 0.10 d y n e s / c m . These values represent t h e increase in surface tensions on readmitting air t o t h e evacuated capillarimeter; one wonders
TABLE I
Surface Tension Data by Capillary Rise Method
Observer
Κ (r = 0.4, R = 20 mm.) 34.33 mm. Richards and Carver (51) Small meniscus correction .143
Large meniscus correction .013 Corrected Height 34.49 mm.
Surface tension of water at 20° 72.75 dynes/cm. Richards and Carver 72.77 Richards and Coombs (52) 72.80 Harkins and Brown (26) Surface tension of benzene at 20° 28.88 Richards and Carver
28.88 Harkins and Brown if sufficient time was allowed for t h e liquids to become resaturated with air.
2.2. Relative Measurements
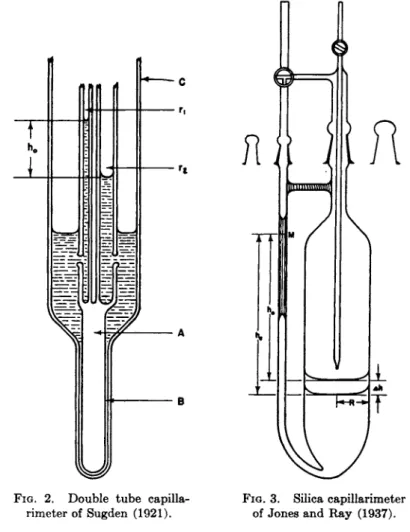
2.2.1. Double Capillary Tube Methods. Sugden (60), Mills and Robinson (43), and Bowden (8) are among those who have advocated t h e use of two small capillaries attached t o a large t u b e instead of one small tube. T h e a p p a r a t u s of Sugden is illustrated in Fig. 2 where rx and r2 represent t h e two small capillary tubes of radii ri and r2 respec
tively, A is a solid rod on which tubes η and r2 are fused and which fits into t h e socket Β of t h e wide t u b e C. T h e capillary system is t h u s easily removable from C for cleansing purposes.
If both capillary tubes η a n d r2 are so small t h a t each meniscus has the shape of a hemisphere, and if h is t h e observed difference in t h e height of rise in t h e two capillaries, then t h e surface tension is given by t h e equation (for zero contact angle) (43)
ri · r2 · g (PI - pv){3h + r2 - rQ n n.
7 = 6(n - r2) ( 1 0 )
As is obvious from equation (10) this method requires an accurate knowledge of t h e capillary radii and of t h e heights of rise.
Advantages are first, t h a t it is suitable for small quantities of liquid, second, for corrosive liquids, and third, t h a t it permits a s t u d y of changes of height of rise with time. T h u s Mills a n d Robinson (43) observed irregularly falling or rising menisci, followed b y a slow rise t o a sta
tionary m a x i m u m value in both tubes.
2.2.2. Relative Method of Jones and Ray. Although it h a d been realized b y previous workers t h a t changes in t h e height of rise could be measured b y weighing t h e q u a n t i t y of liquid in t h e wide t u b e required t o bring t h e meniscus in t h e narrow t u b e always t o t h e same point, it was
FIG. 2 . Double tube capilla- FIG. 3 . Silica capillarimeter rimeter of Sugden ( 1 9 2 1 ) . of Jones and Ray ( 1 9 3 7 ) .
Jones a n d R a y (33) w h o first brilliantly carried this method t o a high state of perfection. Using a fused quartz capillarimeter, illustrated in Fig. 3, whose wide t u b e was ground internally a n d externally t o a true right circular cylinder, t h e y were able t o measure changes in surface tension amounting t o 0.001 % or 0.0007 d y n e / c m . After water h a d been dropped, b y pipet, into t h e wide t u b e until t h e upper meniscus came t o M, t h e a p p a r a t u s was weighed t o obtain t h e weight of water, Wo, con-
tained therein. After drying, solution was admitted t o the capillarim- eter until once again the upper meniscus stood at M . Knowing t h e weight of solution Wc required to bring this about, knowing t h e densities of water and solution pi t 0 and pi,e and knowing t h e radii of t h e larger and smaller tubes R and r respectively (an exact knowledge of t h e radius of t h e large t u b e became important in concentrated solutions, b u t a p a r t from this it was unnecessary t o determine t h e radii exactly), t h e relative surface tension, γ, could be calculated from t h e equation
= 7 c = Vpi.c — P t . c l To LPi.o — Pv.o J
where h is t h e true height of rise with pure water in the capillarimeter.
T h e outstanding observation of Jones and R a y was t h a t addition of salts u p t o 0.001 Ν concentration lowered t h e surface tension, apparently, b y 0.02%. At higher concentrations, the surface tension then rose in accordance with t h e prediction of t h e Debye-Wagner-Onsager-Samaras surface tension theory of strong electrolytes (45). Langmuir (36) has explained t h e initial decrease in surface tension, called the Jones-Ray effect, as t h e result of a methodological error, arising from a vertical film of water held above t h e meniscus because of f-potential forces which diminishes in thickness in t h e presence of electrolytes, t h u s producing an apparent widening of t h e small tube and an apparent decrease of surface tension. Langmuir's theory has been further investigated by Jones and Frizzell (32), Jones and Wood (34), a n d Wood a n d Robinson (67), with nearly complete verification. Harkins (25) states t h a t t h e water film is about 25 A. thick, b u t Langmuir's calculations a n d t h e Jones-Ray effect require it t o be about 500 A. thick at t h e meniscus in t h e capillary tube.
Until this methodological uncertainty is resolved, all surface tension measurements b y t h e capillary rise method will be uncertain to t h e extent of a few t h o u s a n d t h s or hundredths of a per cent.
2.8. Combined Capillary Tube-Pressure Methods
2.8.1. Single Capillary Tubes. Single capillary tubes with t h e meniscus forced t o a predetermined point by means of air pressure have been used in b o t h t h e vertical and horizontal positions. The essential p a r t of Ferguson a n d Dowson's (17) a p p a r a t u s is shown in Fig. 4 where A is t h e tip of t h e capillary t u b e t h a t is immersed in a vertical position under t h e surface of t h e liquid whose surface tension is desired. Ε is t h e sharp end of a pointer which is sealed into t h e capillary t u b e at a con
venient height a n d serves as a reference m a r k so t h a t the height of the liquid in t h e wide dish can always be adjusted t o t h e distance h2 above
1 +
nR*h
_l
(Wo( L _ 0 U ,
Pi (Π)
t h e flat b o t t o m end of t h e capillary t u b e . This adjustment can be m a d e with great accuracy. Pressure is now applied a t Β until t h e lowest point of t h e meniscus of liquid in t h e capillary t u b e falls t o a position flush with t h e flat end. If t h e capillary t u b e is small enough so t h a t t h e meniscus is hemispherical, t h e surface tension is given b y t h e equation
- ν (*-.-£) «>
If t h e liquid in t h e m a n o m e t e r used t o measure t h e pressure is t h e same
ι I
FIG. 4 . Apparatus of Ferguson and Dowson ( 1 9 2 1 ) .
as t h e liquid under test a n d if hi is t h e difference between t h e heights in the manometer, equation (12) m a y be written
( * . - * , - { ) . (13) This m e t h o d can be carried out with great accuracy a n d has t h e a d v a n
tages t h a t (1) t h e same point on t h e capillary tube, namely its tip end, is always used, (2) t h e radius is easily measured at various orientations with a dividing engine, (3) it is unnecessary t o measure t h e height of rise in t h e capillary, only t h e relative measurements of hi in t h e manometer have t o be carried out with a cathetometer, (4) t h e capillary t u b e can be m a d e quite short a n d is t h u s more easily cleaned, and (5) t h e experiment can be quickly performed. Ferguson a n d H a k e s (18) point out t h a t b y varying A2 a n d making simultaneous measurements of hi a n d A2, t h e density of t h e u n k n o w n liquid can be determined from t h e slope of a plot of pih\ versus A2 (in this case t h e manometer liquid m u s t h a v e a known and different density).
Ferguson a n d K e n n e d y (19) eliminate density considerations entirely by measuring t h e pressure necessary t o produce a flat meniscus of liquid
at t h e open end of a horizontal capillary t u b e (contact angle of 90° at open end). N o height of rise measurement is necessary a n d no meniscus corrections are involved, t h e surface tension being given b y t h e equation
where r is t h e radius at t h e curved meniscus end of t h e liquid column.
2.8.2. Double Capillary Tubes. Double capillary tubes h a v e also been used in b o t h vertical a n d horizontal positions. S u t t o n (62) seals two tubes of radii ΤΊ a n d r2 together end t o end and measures t h e pressure required t o bring a column of liquid of length h t o a predetermined point on t h e smallest capillary with t h e system in a vertical position. T h e a p p a r a t u s is inverted and t h e pressure again recorded. If P i a n d P2 are t h e pressures in t h e two positions, we have
7 = mPi + Pi) (i5)
"
=~ w
( 6 )! - J L ( 1 7 )
k 27ΓΓΙ 2πΓ2
where r± is the radius of t h e smaller tube. T h u s from these two pressure measurements both t h e density and t h e surface tension can be determined.
If t h e a p p a r a t u s is calibrated with a liquid of known surface tension, y', we can write
I n these equations meniscus corrections have been neglected. S u t t o n obtained results accurate t o about 1 %, b u t t h e technique could no doubt be refined. Speakman (59) seals two parallel capillary tubes of radius ri a n d r2 into a wider t u b e whose vertical arm has a radius r3. H e then measures first t h e pressure required t o bring t h e liquid in rx to a pre
determined level and second t h e pressure required t o bring t h e liquid in r2 t o the same level, t h e surface tension is then given by the equation
y = ^ (19)
where AP is t h e difference in t h e two pressure measurements. For accurate calculations meniscus corrections m u s t be applied.
Achmatov (1) uses a double capillary t u b e system similar t o Sutton's described above, b u t b y laying t h e t u b e in a horizontal position, t h e surface tension can be computed from t h e following equation without a knowledge of t h e density of t h e liquid
(20)
where Ρ is t h e pressure difference a t t h e ends of t h e two capillaries just sufficient t o prevent t h e liquid from moving in either horizontal direction.
N o capillary correction is necessary for this method b u t t h e possibility of gravity distortion of t h e meniscus would have t o be investigated for precise results.
3 . M A X I M U M B U B B L E P R E S S U R E M E T H O D
3.1. Single Tube Methods
3.1.1. Introduction. One of t h e most a t t r a c t i v e methods of surface tension measurement involves t h e measurement of t h e pressure required t o form a bubble of gas in t h e liquid whose surface tension is desired.
I t is particularly applicable t o corrosive liquids like molten phosphorus ( 3 0 ) or molten metals a n d alloys ( 7 ) a n d has t h e distinct advantage of
FIG. 5. Diagram for discussion of maximum bubble pressure method.
giving results independent of t h e contact angle. B y exerting t h e neces
sary precautions great precision of technique is obtainable.
3.1.2. Theory. If we assume for t h e m o m e n t t h a t t h e radius of t h e capillary tube, Fig. 5 , is so small t h a t t h e shape of t h e bubble a t m a x i m u m pressure is hemispherical, then t h e equation for t h e total gas pressure in t h e capillary t u b e is
Ptotal = Ρ + Pi = — + Qhl(pi - Pv). (21)
r
T h e contact angle is zero a t this point; a n y lowering of t h e pressure will cause t h e bubble t o grow smaller, a n y a t t e m p t t o increase t h e pressure will produce a n increase in t h e size of t h e bubble with an increase in t h e contact angle t o a value greater t h a n 1 8 0 ° , as illustrated in Fig. 5 . T h e pressure will decrease as t h e bubble rapidly expands.
T h e pressure across t h e interface, P , is given for small values of r/L by t h e equation of Schroedinger (54)
* - ? [ - ! £ - * © " ]
where h is given b y t h e equation
Ρ = gh(j>i - P9) ( 2 3 )
(In the case of a perfect hemisphere, h of equation (23) would be identical with Ζ of Fig. 5.) For more accurate calculations t h e tables of Bashford and Adams (4) can be used. Sugden (61) has worked out a m e t h o d for t h e use of these tables in connection with t h e m a x i m u m bubble pressure method and gives additional tables which m a k e it unnecessary t o refer t o t h e work of Bashford a n d Adams. T h e height A, m u s t be measured with reference to a flat surface; i.e., if t h e outer vessel holding t h e liquid under investigation is not wide enough t o give a flat surface, a correction for capillary rise m u s t be included.
8.1.3. Applications. Harkins (25) quotes Young as recommending t h a t the maximum pressure be measured in t h e formation of a single bubble, rather t h a n measuring t h e pressure necessary t o give a stream of bubbles. Long and Nulting (40) could detect no difference between a rate of 9 and 100 seconds per bubble in their accurate relative surface tension studies. T h e capillary tip should be thin-walled.
Inasmuch as t h e pressure drops as each bubble breaks away from the tip, it is possible t o measure t h e m a x i m u m pressure even though t h e bubble cannot be watched. Thus, Hutchinson (30) has recently meas
ured t h e surface tension of white phosphorus against C 02 b y observing t h e m a x i m u m pressure required t o form bubbles of this gas in t h e molten phosphorus. His a p p a r a t u s was calibrated with known liquids before use, and a Bourdon gauge was used t o measure t h e pressure (as t h e system h a d t o be completely evacuated before use). Using a quartz capillary t u b e placed under t h e surface of fused metals, Sauerwald (53) a n d D r a t h a n d Sauerwald (13) were able t o measure t h e surface tension of a n u m b e r of fused metals a n d alloys. I n this case hydrogen was t h e gas forced through t h e capillary tip.
Hogness (29) has developed a m a x i m u m pressure m e t h o d for the measurement of t h e surface tension of mercury and fused metals in which t h e pressure necessary t o force a drop of t h e liquid metal upwards through a vertical capillary is measured. His a p p a r a t u s is illustrated in Fig. 6 where A is a t u b e into which a cylinder of t h e metal is inserted and later melted b y raising t h e t e m p e r a t u r e . Pressure is applied at Β until liquid metal drops begin t o flow out of t h e quartz capillary t u b e D . At this point t h e difference in height between t h e column of metal in C
a n d capillary tip is measured a n d t h e surface tension calculated from t h e equation
7 2 V 3 Ρ 6 Ρ2 / K }
where Ρ is t h e m a x i m u m pressure a n d ρ is t h e density of t h e liquid metal. Hogness found it necessary t o use hydrogen gas a n d t o bake out his glass a p p a r a t u s thoroughly before admitting t h e metal in order t o eliminate all traces of water. Bering a n d Pokrowsky ( 6 ) h a v e more recently carried out a similar investigation in which t h e y studied t h e surface tension of mercury and amalgams against a v a c u u m as well as hydrogen gas. Hogness's m e t h o d has t h e advantages of continually supplying a fresh surface and of being independent of t h e contact angle.
FIG. 6. Apparatus of Hogness FIG. 7. D o u b l e (1921) for the measurement of capillary tip surface the surface tension of fused tension apparatus of metals and alloys. Sugden (1924).
3.2. Double Tube Methods
3.2.1. Apparatus of Sugden. Figure 7 illustrates a convenient appa
r a t u s invented by Sugden (60) for t h e measurement of surface tension by a relative method. First the pressure necessary t o produce bubbles from t h e small tip, radius riy is determined. T h e stop cock is t h e n opened
and t h e maximum pressure measured for bubbles formed at t h e tip of t h e larger capillary tube, radius r2. If Ρ is t h e difference in pressures measured, t h e surface tension can be calculated from t h e equation,
where χ = and must be calculated from the tables given by Sugden (61). This is particularly true for t h e large capillary t u b e ; for the small tube
with sufficient accuracy. T h e recommended values of η and r2 in centimeters are
Sugden points out t h a t for approximate calculations, good to 1 part in 1000, t h e following equation is satisfactory
where A is a constant, r2 is expressed in centimeters and Ρ in d y n e s / c m2. B o t h capillary tips m u s t be a t t h e same level in t h e liquid.
Bircumshaw (7) carried out an extensive study of t h e surface tension of liquid metals and alloys using the double tip m a x i m u m bubble pressure method. Good agreement with t h e d a t a of Hogness was obtained.
Using a similar double capillary tip a p p a r a t u s Hutchinson (30) deter
mined t h e interfacial tension of a n u m b e r of liquid pairs b y measuring the pressure required t o form a liquid bubble of one liquid in another.
This is a particularly good method for interfacial tension measurements as here again t h e contact angle does not enter into t h e phenomenon.
3.2.2. The Relative Method of Warren. I n t h e double t u b e a p p a r a t u s of Warren (66) two identical tips (made b y carefully breaking a capillary t u b e at one point) are immersed in separate vessels, b o t h containing t h e same fluid a t first. B y suitable mechanical means it is possible t o adjust and measure accurately t h e d e p t h of immersion of t h e jets. One of t h e jets is moved u p or down until t h e rate of escape of bubbles from its tip is exactly equal t o t h e r a t e of bubble formation at t h e other tip.
The liquid in t h e second vessel is t h e n removed and replaced b y t h e liquid under investigation. T h e difference in surface tension between t h e two liquids is then given b y t h e equation
(26)
0.005 < r i < 0.01 0.1 < r2 < 0.2 c m .
(27)
r 1
- 7 2 = g 7y (P2^2 — Pihi) + £ gr2(pi — p2) (28)
where hi a n d h2 are t h e depths of immersion of t h e tip in t h e experiments on t h e two liquids plus t h e radius r in each ease. W a r r e n ' s m e a n error in t h e determination of t h e surface tension of sodium chloride solutions was 0.022 d y n e s / c m .
3.2.3. Method of Long and Nutting. Long and N u t t i n g (40) describe a relative m e t h o d similar t o W a r r e n ' s in which the change in t h e depth of immersion was calculated from t h e weight of liquid added a n d from t h e d e p t h of immersion of a rod of known volume. (By careful regulation of t h e depth of t h e rod t h e height of t h e liquid level in one of t h e vessels is varied until t h e bubbling r a t e is equal from t h e two jets.) W i t h pure water initially in b o t h vessels, t h e depth of immersion, ho, m u s t necessarily be equal for t h e two jets. After a small a m o u n t of salt solution is added to one of t h e halves of t h e a p p a r a t u s , a n d pure water t o balance t h e bubbling rate added t o t h e other half, t h e relative surface tension can be calculated from t h e equation
7 = - = l + f [ΛΟ(ΡΟ - P I ) + Ahopo - AhlPl] (29)
T h e reproducibility of Long and N u t t i n g ' s d a t a is somewhat better t h a n 0 . 0 1 % .
Apparently t h e Langmuir film or possibly a finite contact angle which resulted in t h e Jones-Ray effect does not exist in t h e m a x i m u m bubble pressure method—which suggests t h a t this method m a y be funda
mentally sounder for surface tension measurements t h a n others.
4. D R O P - W E I G H T A N D P E N D A N T D R O P M E T H O D S
4.1. Drop-Weight Method
4.1.1. Introduction. Another useful m e t h o d of surface a n d interfacial tension measurement is t h e drop-weight m e t h o d in which t h e surface tension can be calculated from t h e m a x i m u m weight of a drop. I t is, perhaps, most i m p o r t a n t from t h e standpoint of interfacial tension measurements difficult t o m a k e b y other methods. T h e closely analogous p e n d a n t drop m e t h o d is in practice less accurate b u t has its own a d v a n tage in being particularly suitable for studying t h e variation of surface tension as a function of time.
4.1.2. Theory. T h e theory of t h e shape of drops has been investi
gated b y Bashford a n d A d a m s (4), Lohnstein (39), F r e u d a n d Harkins (21) a n d a n u m b e r of early workers. Harkins (25) has stressed t h e principle of similitude, namely t h a t all drops h a v e t h e same shape if their values of r/Vl are equal where r is t h e radius of t h e tip from which t h e drop falls and V is t h e volume of t h e drop. Harkins a n d Brown (26)
give a table of their so-called F-faetors from which values of F can be obtained applicable t o t h e particular liquid under s t u d y a n d which when multiplied b y mg/r gives t h e surface tension in dynes per centimeter.
I n t h e case of interfacial tension measurements t h e equation for t h e tension is
7 - V(pi - P I )Q- -F (30)
where V is t h e volume of a single drop of liquid of density pi formed in a second liquid of density p2.
T h e F-factors have been determined experimentally b y first finding the surface tension of t h e liquids by t h e capillary-rise method, a n d then determining t h e drop-weights in a drop-weight a p p a r a t u s . T h e F-factors are finally calculated from t h e equation
F =
£
mg<
31>
T h e y are apparently valid for liquids having such diverse surface tensions as exhibited b y benzene on t h e one h a n d a n d mercury on t h e other, as D u n k e n (14) has recently shown. Iredale (31) a n d Michelli (42) have suggested an alternate method of surface tension calculation for t h e drop weight method based on an equation of Worthington (68). If r is the capillary tip radius and a t h e radius of t h e drop assuming it t o be spheri
cal, then Iredale points out t h a t t h e ratio r/a is roughly a linear function of r. Suppose a is determined for a liquid of unknown surface tension, the value of r/a is t h e n calculated and from t h e nearly linear curve of ro/cto as a function of ro for water, t h e value of r0 for water is found which would give a value of ro/a0 equal to r/a for t h e unknown liquid. T h e surface tension can then be calculated from t h e equation
2 2
= 4
(32)P7o r0 2
where t h e subscript zero identifies the values for water. As a m a t t e r of fact this method is essentially t h a t of Harkins and Brown inasmuch t h a t , if
7 7o
then ψϊ - and t h e F-factors are equal.
4.1.8. Methods. T h e m e t h o d of Harkins and Brown (26) is capable of a precision of 0.02 t o 0 . 0 3 % with certain liquids. T h e following precautions should be observed:
(a) A flat ground tip, circular with sharp edges and not polished on t h e b o t t o m should be used.
(b) I t s vertical sides should be polished t o prevent liquid wetting t h e side walls.
(c) T h e size of t h e tips should be between 5-7 m m . in diameter for most liquids in t h e case of surface tension measurements a n d between 9-11 m m . in diameter for interfacial tension measure- m e n t s . High density liquids call for smaller tips t h a n low density liquids.
(d) T h e a p p a r a t u s should be held in a vertical position, b u t a 3°
inclination from t h e vertical can be tolerated as Gans a n d H a r k i n s (22) have pointed out (the weight becomes less t h e greater t h e angle of inclination).
(e) As t h e weight of t h e drops increases with increasing velocity of flow, it is i m p o r t a n t t o form each drop over a 5-minute interval.
Actually t h e liquid is allowed t o flow until t h e drop approaches its m a x i m u m size, t h e n it is allowed to stand for a few minutes t o reach equilibrium (some dilute liquids of long linear organic molecules m a y t a k e half an hour to a t t a i n surface equilibrium).
Finally, t h e volume of t h e drop is allowed to increase slowly and preferably automatically until it drops. T h e drops are collected in a suitable weighing bottle and weighed.
(f) I t is not correct t o calibrate t h e instrument with a liquid of known surface tension unless t h e proper i'-factor correction is applied both t o t h e known a n d unknown surface tension calculation.
(g) I n interfacial tension measurements W a r d a n d Tordai (65) collect drops of t h e heavier liquid b y displacement of t h e lighter liquid from a special pycnometer. T h e increase in weight of t h e pycnometer gives just t h e required information needed for t h e interfacial tension calculation. T h e authors point out t h a t a weight measurement of four drops is more accurate t h a n a volume measurement of 40 drops.
Figure 8 illustrates t h e a p p a r a t u s of Harkins a n d Brown (26), suitable for t h e s t u d y of volatile liquids. T h e capillary tip is shown at t h e right;
t h e rod at t h e left which is a t t a c h e d t o a rack a n d pinion device serves t o raise gradually t h e reservoir of liquid, t h u s causing t h e drop t o grow very slowly; t h e intermediate vessel containing a small a m o u n t of liquid prevents excessive evaporation from t h e surface of t h e drop.
K u r t z (35) points out t h a t a film of plastic or other solid material forming on t h e surface of t h e drop can vitiate the surface tension measure- ments b y preventing t h e drop from taking on its normal shape. E d w a r d s
FIG. 8 . Drop-weight apparatus of Harkins ( 2 5 ) .
(16) uses an electrical counter in measurements of interfacial tension of sulfuric acid against various oils.
4.2. Pendant Drop Method
Andreas et at. (3) as well as Smith (57) h a v e applied a m e t h o d involv- ing the measurement of t h e shape of p e n d a n t drops to t h e s t u d y of surface tension. This method has t h e a d v a n t a g e of being a static one, a single drop being investigated over a considerable period of time, or until its shape becomes constant. This m e t h o d is t h e only known surface tension method which permits an accurate s t u d y of surface composition (as indicated by surface tension) as a function of time.
T h e experimental problem is chiefly an optical one, once a proper dropping tip has been obtained. B y proper choice of magnifying system a n d camera results having a probable error of ± 0 . 5 % are obtained. As with t h e drop weight method, correction factors given in tables must be used t o convert measurements of t h e diameter of t h e drop at its equator a n d at a selected plane t o surface tension. Good agreement for both surface a n d interfacial tension measurements on s t a n d a r d substances has been obtained. Andreas et al. believe t h a t this m e t h o d will prove t o be particularly valuable for t h e s t u d y of ( 1 ) viscous liquids, ( 2 ) surface- active solutions ( 3 ) small samples of rare chemicals and ( 4 ) systems in which t h e contact angle is not zero.
5 . R I N G M E T H O D S
5.1. Single Ring
5.1.1. Introduction. Because of t h e ease a n d rapidity with which t h e force necessary t o pull a ring out of t h e surface of a liquid can be measured, the ring method of surface tension measurement has h a d great popularity.
Like t h e drop weight a n d t h e m a x i m u m bubble pressure m e t h o d s this method is a dynamic one a n d requires a knowledge of t h e force necessary to r u p t u r e the liquid-air interface. T h e d a t a obtained b y t h e ring method are quite sensitive t o t h e size of wire a n d dimension of ring employed, so t h a t absolute values of surface tension cannot be obtained without applying corrections. I n fact it is rather surprising t o consider t h e large n u m b e r of ring surface tension determinations in which t h e shape of t h e surface was not taken into account. I t was not until 1 9 2 4 - 2 6 when Lenard ( 3 7 ) who used a straight wire instead of a ring and Harkins et al. ( 2 8 ) first correctly calculated surface tension values from t h e surface r u p t u r e forces t h a t the theory of the ring m e t h o d was p u t on a sound basis. W e are including t h e work of Lenard in this discussion as t h e problems involved in pulling a straight wire out of t h e surface are similar in m a n y ways t o those applicable t o work with circular rings.
5.1.2. Theory of the Ring Method. If a ring pulled a true hollow cylinder of liquid with a vertical plane of contact with the ring from the surface, t h e n at t h e m o m e n t t h e liquid surface ruptured, t h e weight of liquid supported t o t h e point of r u p t u r e above a flat level surface would be given b y t h e equation
W · g = 4T T# T (33)
where R is t h e radius of t h e ring measured to the center of the wire of which t h e ring is composed. However, the liquid surface will t a k e on t h e shape illustrated in Fig. 9 as t h e ring is raised (or t h e liquid lowered).
FIG. 10. Cenco Du Noliy tensiometer. (Courtesy of the Central Scientific Co.) Harkins and J o r d a n (27) m a d e an extensive s t u d y of t h e weight of liquid supported b y rings of various r a n d R values using liquids of known surface tension a n d found t h a t in t h e majority of cases t h e weight of liquid supported is greater t h a n t h e simple equation (33) would predict.
T h e y have published tables of F-factor corrections for t h e ring method which m a y a m o u n t to as much as 2 5 % under certain conditions. Thus, the correction factors m a y be extremely large. Knowing F, t h e surface
FIG. 9. Schematic diagram of the ring method.
tension is t o be calculated from t h e equation W · g
4Τ Γ# • F (34)
To determine F from the tables both R a n d r m u s t be known as well as V, t h e volume of liquid supported at the m o m e n t of break (V = m/p).
Freud a n d Freud (20) have carried out numerical integrations of Laplace's equation relating surface curvature to surface tension a n d t h u s succeeded in calculating t h e F-factors theoretically.
For this reason we can now regard t h e ring m e t h o d as being an absolute method.
5.1.3. Methods. For t h e most precise determi
nations using rings t h e technique of Harkins a n d J o r d a n (27) should be followed although Schwen- ker's straight wire m e t h o d (55) seems t o be some
w h a t more sensitive. T h e relative t w i n - r i n g m e t h o d of Dole a n d Swart out (12) described below has t h e greatest precision of all methods involving a r u p t u r e of t h e surface. Various commercial ten- siometers are on t h e market, t h e best known being t h e D u Noliy (15) tensiometer sold b y t h e Central Scientific Co., Fig. 10. I n t h e last instrument t h e liquid is lowered from t h e ring and t h e force at r u p t u r e measured b y a torsion wire balance. T h e torsion force is calibrated b y known weights in advance of t h e surface tension measurement.
T h e Harkins /^-factor corrections should be applied, of course.
Returning t o the techniques of Harkins and Jordan, Fig. 11 illustrates their surface tension flask in which t h e p a n holding t h e liquid is sealed into another flask in order t o allow t h e whole t o be immersed in a constant t e m p e r a t u r e bath, and which has t h e side tubes A and Β so t h a t t h e surface can be renewed b y flushing through A a n d b y sucking t h e over
flow liquid out through B . During measurement t h e liquid is held stationary a n d t h e ring slowly raised b y using a mechanical gear arrange
m e n t t o raise t h e chainomatic balance on whose left beam t h e ring is supported. T h e chief difficulty comes from slight impurities, particu
larly in t h e case of aqueous solutions, which are picked u p b y t h e ring, thereby changing t h e contact angle. T h e pull on t h e ring at r u p t u r e is a rather sensitive function of t h e contact angle (44) as it also is of t h e angle of inclination of t h e ring (27). Precautions and experimental recom
mendations can be listed as follows.
FIG. 11. Surface tension flask of Harkins and Jordan (1930).
(a) Harkins (25) recommends raising t h e ring rather t h a n lowering the solution as t h e latter might produce disturbing ripples in t h e surface. Dole and Swartout (12), however, show t h a t a precision of 0.002% is attainable on lowering t h e liquid (the precision of Harkins a n d J o r d a n was a b o u t 0.2%).
(b) T h e diameter of the pan should be such t h a t it is 4 - 5 cm. greater t h a n the diameter of the ring.
(c) T h e angle of inclination of t h e plane of the ring with the free surface should be less t h a n 0.47° for the error of measurement to be less t h a n 0.1 %. A positive angle can be detected by carefully observing the ring as it approaches t h e surface of t h e liquid. If it makes contact with the liquid simultaneously all about its circumference, then no angle of inclination exists (assuming, of course, t h a t t h e ring is plane).
(d) Harkins and J o r d a n adjusted t h e volume of liquid in the p a n so t h a t the surface would be plane at t h e m o m e n t of rupture.
(e) T h e ring should be circular and all in a plane.
(f) T h e a p p a r a t u s should be cleaned with a hot mixture of nitric and sulfuric acids, rinsed with redistilled water, steamed (the steam generated from water t h a t had previously been refluxed with K M n 04) , and rinsed again. T h e p H of t h e rinse w a t e r should be checked to insure t h e complete removal of t h e acid.
These rigorous cleaning directions are of significance chiefly in t h e measurement of t h e surface tension of aqueous solutions.
(g) T h e ring is preferably m a d e of p l a t i n u m - 1 0 % iridium a n d should be ignited in a flame shortly before use.
(h) T h e dry weight of t h e ring must be known. T h e W of equation (34) is the weight at the moment of r u p t u r e less t h e dry weight.
T h e fact t h a t drops of liquid adhere t o t h e ring after t h e r u p t u r e is of no significance except t h a t it indicates a desirable wetting of the ring b y t h e liquid.
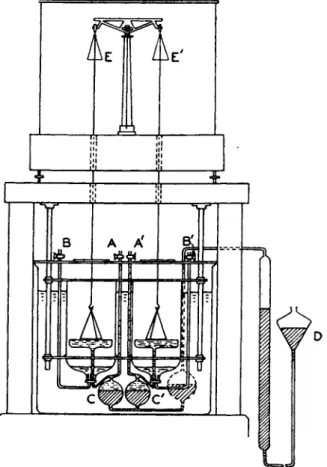
5.2. Double Ring Method
Dole and Swartout (12) have recently invented a twin-ring ten- siometer, illustrated in Fig. 12, which makes possible t h e a t t a i n m e n t of a relative precision to 0.002%. In this method it is unnecessary t o observe the m a x i m u m weight of pull or t h e weight at t h e m o m e n t of r u p t u r e ; instead notice is t a k e n merely of t h e ring which breaks from t h e surface first as t h e two liquid surfaces are lowered. T h e weaker tension on this side is then balanced b y t h e addition of weights t o this side of the balance and t h e process repeated. I t is possible to obtain a balance between the forces of tension and the weights such t h a t 0.1 mg. increase in weight
on one side will cause t h e ring on t h e other side to break first from t h e surface. As t h e total pull on either ring a m o u n t e d to 5.7 g., t h e relative uncertainty is 1/57,000 or about 0.002%. W i t h a more accurate balance an even greater sensitivity might be obtainable.
FIG. 1 2 . Twin-ring tensiometer of Dole and Swartout ( 1 9 4 0 ) .
After the surface tension forces have been balanced b y weights, t h e liquid being t h e same in b o t h pans, one of t h e liquids is removed a n d replaced b y an u n k n o w n (in Dole a n d Swartout's work b y a dilute aqueous solution). T h e change in weight required to bring about a new balance AW is a measure of t h e difference in surface tensions, or
where W is t h e total pull on t h e ring in contact with liquid of surface tension 70. I n t h e range of dilute solutions studied b y Dole and Swartout it was unnecessary t o apply a H a r k i n ' s F-factor correction.
T h e Jones-Ray effect was observed using t h e twin-ring a p p a r a t u s which indicates t h a t either the contact angle is changed slightly b y t h e addition of electrolytes or t h a t something like t h e Langmuir film enters in. This indicates t h a t t h e ring method, similar to t h e capillary rise method, does not give the true surface tension of salt solutions, particu
larly in t h e dilute range. A theoretical explanation of the Jones-Ray effect as exhibited b y the ring method is badly needed, because there will be some doubt as to t h e precise significance of all ring surface tension measurements until we understand the reason for its existence.
5.3. Straight Wire Method
Lenard a n d co-workers (37) and Schwenker (55) measure t h e force necessary t o pull a straight wire out of the surface. This wire is supported in a hori
zontal position on a stout frame and in Schwenker's work was buoyed u p b y a submerged float so t h a t t h e residual r u p t u r e force amounting only to a few milli
grams could be measured on a sensitive torsion bal
ance. Figure 13 illustrates the wire, A, in its frame Β sealed into a glass float C which has a side arm D for the admission of mercury to adjust it t o a suitable weight. On t h e hook Ε are hung weights after the wire has been pulled through the surface so as to might call the dry weight.
F I G . 1 3 . Straight wire tensi- ometer and float of Schwenker ( 1 9 3 1 ) .
obtain w h a t we Letting
W - Wo
21 ( 3 6 )
where W is t h e weight of m a x i m u m pull, Wo is t h e weight after the wire has ruptured t h e surface a n d I is t h e length of t h e wire, t h e surface tension can be calculated from the equation
( 3 7 )
I n equation (37) r is t h e diameter of cross section of the wire. Schwenker obtained results with an uncertainty of somewhat less t h a n 0 . 0 1 % .
I t is interesting t o note t h a t this straight wire or rod method was suggested b y Michelson in 1891 while a t Clark University a n d was
first carried out experimentally b y Hall (24). Hall discovered t h e m a x i m u m in t h e weight as a function of rod distance above t h e surface (see also H a r k i n s a n d J o r d a n (27)) a n d eliminated end effects b y t h e use of t w o rods of different length.
T h e r e are a n u m b e r of other m e t h o d s of measuring surface tension a m o n g which can be m e n t i o n e d centrifugal m e t h o d s (41, 56), m e t h o d of impacting jets (9, 49) ripple m e t h o d (64), etc. all of which require r a t h e r complicated a p p a r a t u s or h a v e not yet been developed t o great a c c u r a c y ; hence t h e y will not be discussed here.
REFERENCES 1. Achmatov, Α., Kolloid. Z. 66, 266 (1934).
2. Adam, Ν. K., The Physics and Chemistry of Surfaces. 3rd ed. Oxford University Press, London, 1941.
3. Andreas, J. M., Hauser, Ε. Α., and Tucker, W. B., J. Phys. Chem. 42,1001 (1938).
4. Bashford and Adams, An Attempt to Test the Theory of Capillary Action.
Cambridge, 1883.
5. Bastien, P., Chimie & Industrie 46, No. 3 bis, 27 (1941).
6. Bering, B. P., and Pokrowsky, N . L., Acta Physicochim. U.R.S.S. 4, 861 (1936).
7. Bircumshaw, L. L., Phil. Mag. [7] 3, 1286 (1927); 12, 596 (1931); 17, 181 (1934);
and others.
8. Bowden, S. T., J. Phys. Chem. 34, 1866 (1930).
9. Buchwald, E., and Konig, H., Ann. Physik. 26, 659 (1936).
10. Chalmers, B., Trans. Faraday Soc. 33, 1167 (1937).
11. Coffman, A. W., and Parr, S. W., Ind. Eng. Chem. 19, 1308 (1927).
12. Dole, M., and Swartout, J. Α., J. Am. Chem. Soc. 62, 3039 (1940).
13. Drath, G., and Sauerwald, F., Z. anorg. allgem. Chem. 162, 301 (1927).
14. Dunken, H., Ann. Physik 41, 567 (1942).
15. Du Nouy, P. Lecomte, Surface Equilibria of Biological and Organic Colloids.
Chemical Catalog Co., New York, 1926.
16. Edwards, J. C , / . Set. Instruments 6, 90 (1929).
17. Ferguson, Α., and Dowson, P. E., Trans. Faraday Soc. 17, 384 (1921).
18. Ferguson, Α., and Hakes, J. Α., Proc. Phys. Soc. London 41, 214 (1929).
19. Ferguson, Α., and Kennedy, S. J., Proc. Phys. Soc. London 44, 511 (1932).
20. Freud, Β. B., and Freud, Η. Z., Science 71, 345 (1930); J. Am. Chem. Soc. 62, 1772 (1930).
21. Freud, Β. B., and Harkins, W. D., Phys. Chem. 33, 1217 (1929).
22. Gans, D. M., and Harkins, W. D., J. Am. Chem. Soc. 52, 2287 (1930).
23. Hagen and Desains, Ann. chim. phys. [3] 61, 417 (1857).
24. Hall, T. P., Phil. Mag. 36, 390 (1893).
25. Harkins, W. D., in Physical Methods of Organic Chemistry. Edited by Arnold Weissberger, Interscience, New York, 1945, Chapter VI.
26. Harkins, W. D., and Brown, F. E., / . Am. Chem. Soc. 38, 246 (1916); 41, 499 (1919).
27. Harkins, W. D., and Jordan, H. F., J. Am. Chem. Soc. 62, 1751 (1930).
28. Harkins, W. D., Young, T. F., and Cheng, L. H., Science 64, 333 (1926).
29. Hogness, T. R., J. Am. Chem. Soc. 43, 1621 (1921).
30. Hutchinson, E., Trans. Faraday Soc. 39, 229 (1943).
31. Iredale, T., Phil. Mag. [6] 46, 1088 (1923).
32. Jones, Grinnell, and Frizzell, L. D., / . Chem. Phys. 8, 986 (1940).
33. Jones, Grinnell, and Ray, W. Α., Am. Chem. Soc. 69, 187 (1937).
34. Jones, Grinnell, and Wood, L. Α., J. Chem. Phys. 13, 106 (1945).
35. Kurtz, S. S., Jr., J. Am. Chem. Soc. 49, 1991 (1927).
36. Langmuir, I., Science 88, 430 (1938); J. Chem. Phys. 6, 894 (1938).
37. Lenard, P., v. Dallwitz-Wegener, R., and Zachmann, E., Ann. Physik. 74, 381 (1924).
38. Liesegang, Ε., Z. anal. Chem. 126, 172 (1943); 126, 334 (1944).
39. Lohnstein, T., Ann. Physik. 20, 237 (1906).
40. Long, F. Α., and Nutting, G. C , J. Am. Chem. Soc. 64, 2476 (1942).
41. Meyerstein, W., and Morgan, J. D., Phil Mag. 35, 335 (1944).
42. Michelli, L. I. Α., Phil. Mag. [7] 3, 581 (1927).
43. Mills, H., and Robinson, P. L., / . Chem. Soc. 1927, 1823.
44. Nietz, A. H., and Lambert, R. H., / . Phys. Chem. 33, 1460 (1929).
45. Onsager, L., and Samaras, Ν. Ν. T., / . Chem. Phys. 2, 528 (1934); also Wagner, C., Physik. Z. 26, 474 (1924).
46. Poisson, Nouv. Theor. d. Tact, capill. Paris, 1831.
47. Porter, A. W., Trans. Faraday Soc. 27, 205 (1931); 29, 1307 (1933).
48. Preston, J. M., / . Soc. Dyers Colourists. 61, 161 (1945).
49. Puis, H. O., Phil Mag. 22, 970 (1936).
50. Rayleigh, Lord, Proc. Roy. Soc. A92, 184 (1915).
51. Richards, T. W., and Carver, Ε. K., J. Am. Chem. Soc. 43, 827 (1921).
52. Richards, T. W., and Coombs, L. B., J. Am. Chem. Soc. 37, 1656 (1915).
53. Sauerwald, F., Z. Metallkunde 18, 137, 193 (1926).
54. Schroedinger, E., Ann. Physik. 46, 410 (1915).
55. Schwenker, G., Ann. Physik. 11, 525 (1931).
56. Searle, G. F. C , Proc. Phys. Soc. London 63, 681 (1941).
57. Smith, G. W., </. Phys. Chem. 48, 168 (1944).
58. Smith, W. O., and Foote, P. D., Ind. Eng. Chem. 21, 567 (1929).
59. Speakman, J., J. Chem. Soc. 1933, 1449.
60. Sugden, S., Chem. Soc. 119, 1483 (1921).
61. Sugden, S., Chem. Soc. 121, 858 (1922); 125, 27 (1924).
62. Sutton, T. C , Proc. Phys. Soc. London 46, 88 (1933).
63. Taubmann, Α., Ζ. physik. Chem. A161, 129 (1932).
64. Tyler, E., Phil. Mag. 31, 209 (1941).
65. Ward, A. F., and Tordai, L., Sci. Instruments 21, 143 (1944).
66. Warren, E. L., Phil. Mag. [7] 4, 358 (1927).
67. Wood, L. Α., and Robinson, L. B., J. Chem. Phys. 14, 258 (1946).
68. Worthington, Proc. Roy. Soc. London 32, 362 (1881).
69. Young, T. F., Gross, P. L. K., and Harkins, W. D. See Harkins (25).