ContentslistsavailableatScienceDirect
Journal of Pharmaceutical and Biomedical Analysis
jou rn al h om e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / j p b a
Characterization of a Cell Line Model for d -Serine Uptake
István Vincze, Péter P. Lakatos, Fruzsina Bagaméry, Tamás Tábi, Éva Szök ˝o
∗DepartmentofPharmacodynamics,SemmelweisUniversity,4Nagyváradtér,Budapest,H-1089,Hungary
a r t i c l e i n f o
Articlehistory:
Received31January2020
Receivedinrevisedform4May2020 Accepted6May2020
Availableonline13May2020
Keywords:
alanine-serine-cysteine-threonine transporters
capillaryelectrophoresis cellmodel
ratprimaryastrocyte d-serinetransport SH-SY5Ycellline
a b s t r a c t
d-Serineisanimportantco-agonistoftheN-methyl-d-aspartate(NMDA)receptorsinthebrainandits alteredactivitywasidentifiedinvariouspathologicalconditions.Modificationoftheextracellulard- serinelevelissuggestedtobeabletomodulatethereceptorfunction.Itstransportersmaythusserveas potentialdrugtargets.
Theaimofthisworkwastofindaneasilyavailablehumancelllinemodelappropriateforscreening moleculesaffectingd-serinetransporters.
Characteristicsofd-serinetransportintoSH-SY5Yhumanneuroblastomacelllinewerestudiedand comparedtothoseinculturedprimaryastrocytes.Uptakewasfollowedbymeasuringintracellulard- serineconcentrationbycapillaryelectrophoresiswithlaserinducedfluorescencedetectionmethod.
WefoundthatSH-SY5YcellsexpressfunctionalASCT-1andASCT-2neutralaminoacidtransporters andshowsimilard-serineuptakekineticstoculturedastrocytes.Neutralaminoacidsinhibitedd-serine uptakesimilarlyinbothcelltypes.Completeinhibitionwasachievedbyl-alanineandl-threoninealike, whilethetwo-stepinhibitioncurveoftrans-hydroxy-l-proline,aselectiveinhibitorofASCT-1supported thepresenceoffunctioningASCT-1andASCT-2transporters.Itshigheraffinitystepcorrespondingto inhibitionofASCT-1wasresponsibleforabout30%ofthetotald-serineuptake.
BasedonourresultshumanSH-SY5Ycelllineshowssimilaruptakecharacteristicstoprimaryastrocytes andthuscanserveasasuitablemodelsystemfortestingofcompoundsforinfluencingd-serineuptake intoastrocytes.
©2020TheAuthors.PublishedbyElsevierB.V.ThisisanopenaccessarticleundertheCCBYlicense (http://creativecommons.org/licenses/by/4.0/).
1. Introduction
Proper function of N-methyl-d-aspartate (NMDA) receptors of glutamate is crucial in neuronal development and plastic- ity, the molecular basis of several higher neuronal functions suchasmemoryformation,learning,etc.DysregulationofNMDA receptor activityleadsto numerouspathological conditions. Its
Abbreviations: Asc-1, alanine-serine-cysteine-1; ASCT-1, alanine-serine- cysteine-threonine-1;ASCT-2,alanine-serine-cysteine-threonine-2;CE-LIF,capil- laryelectrophoresislaserinducedfluorescence;DAAO,d-aminoacidoxidase;ECL, enhancedchemiluminescence;FBS,fetalbovineserum;HEPES,4-(2-hydroxyethyl)- 1-piperazineethanesulfonic acid; HPA--CD, 6-monodeoxy-6-mono(3-hydroxy) propylamino--CD; NBD-F, 4-fluoro-7-nitrobenzofurazan; NMDA,N-methyl-d- aspartate;RIPA,radioimmunoprecipitationassaybuffer;SR,serineracemase;TBSS, Trisbufferedsaltsolution;TBST,Tris-bufferedsalinecontaining0.1%Tween20;
t-Pro,trans-hydroxy-l-proline.
∗Correspondingauthor.
E-mailaddresses:vincze.istvan@pharma.semmelweis-univ.hu (I.Vincze),lakatos.peter@pharma.semmelweis-univ.hu (P.P.Lakatos),bagamery.fruzsina@pharma.semmelweis-univ.hu (F.Bagaméry),tabi.tamas@pharma.semmelweis-univ.hu(T.Tábi), szoko.eva@pharma.semmelweis-univ.hu(É.Szök ˝o).
hypofunction iswell establishede.g. in schizophrenia whileits over-activation resultsin excitotoxicity and consequential neu- rodegeneration[1].
NMDAreceptoractivationrequiresthebindingofaco-agonist in addition toglutamate that canbe eitherglycine or d-serine dependingonreceptorsubtypeanditscellularlocalizationinvar- iousbrainareas.D-Serineisregardedtobetheprimaryco-agonist onthesynapticNMDAreceptorsinthehippocampusandprefrontal cortex[2].Reducedd-serineconcentrationinbloodofschizophre- niapatientswasfirstreportedbyHashimotoetal.[3]andlater confirmedbyseveralresearchgroups(forreviewsee[4]).Further- more,highdosed-serinesupplementationeitheralone[5]orin adjuncttoantipsychoticdrugtherapy[6]wasshowntoimprove thesymptomsofthedisease.
Promising preclinicaland clinical datainitiated an intensive researchtobetterunderstandthesynthesis,releaseandelimina- tionofd-serineinthecentralnervoussystem.Serineracemase(SR) wasidentifiedastheuniquesourceofd-serinedenovosynthesis [7].SRexpressionwasfirstshowninastrocyteswhere d-serine was also found highly abundant,so originally it was regarded asagliotransmitter[8].However,recentlySRwasshown being
https://doi.org/10.1016/j.jpba.2020.113360
0731-7085/©2020TheAuthors.PublishedbyElsevierB.V.ThisisanopenaccessarticleundertheCCBYlicense(http://creativecommons.org/licenses/by/4.0/).
predominantlyexpressedinneurons[9]and itsneuronspecific knockdownresultedinamoreconsiderablereductionofd-serine levelcomparedtoitsgliaspecificknockdown[10].Basedonthese resultsneuronsareregardednowastheprimarysiteofd-serine synthesis.Itsneuronalreleasewasdemonstratedtobeinducedby depolarization:however,itwasfoundcalciumindependentsug- gestinganon-vesicularmechanism[11].Alanine-serine-cysteine1 (Asc-1)transporterwasidentifiedtomediated-serinereleasefrom neurons[12].Asc-1isasodiumindependentsmallneutralamino acidexchangetransporterthatshowshighaffinitytod-serine[13].
Itsexpressionisrestrictedtoneuronsinthecentralnervoussystem andisonlyabundantinthepresynapticmembrane[14].
d-Serinewasalsoidentifiedtobesubstrateofalanine-serine- cysteine-threonine-1and-2(ASCT-1,ASCT-2),sodiumdependent hetero-exchangetransportersforsmallneutralaminoacids.Their expression was demonstrated in both astrocytes and neurons [15,16].Ribeiroandco-workersshowedfirstthatd-serineuptake intoastrocytesis mediatedby ASCTtype transportersand sug- gestedtheyplayanimportantroleintheregulationofextracellular leveloftheco-agonist[17].
Degradationofd-serineiscatalyzedbytheperoxysomalflavo- protein, d-amino acid oxidase (DAAO). The distribution of the enzymeinthebodyisuneven,itsexpressioninhigherbrainareas isinsignificantresultinginconsiderabled-serinelevel[18].
ThepossibilitiesforrestorationofthealteredNMDAreceptor activationinvariouspathologicalconditionsareextensivelystud- ied.Sincefinetuningofreceptoractivityisrequiredmodulationof theco-agonistlevelisapromisingapproach.Incase ofd-serine itssynthesizingand metabolizingenzymesaswellasits trans- portersmayserve aspotentialdrugtargets.Inhibitorsof DAAO wereusedconcomitantlywithd-serinesupplementationtoreduce itsrapidelimination.However,speciesdifferenceswereobserved suggestingdifferentroleofDAAOind-serinemetabolisminvar- iousanimals[19].Regulationofd-serinesynthesis bySRis not wellcharacterizedsofar,thusthewayofitspharmacologicalmod- ulationisnotclear.Asthetransportersplayamajorroleinthe regulationofextracellulard-serineconcentrationtheyseemtobe morepromisingdrugtargets.However,forstudyingtheeffectof drugcandidatesactingonthetransporters,asimplemodelsystem wouldberequired.Previouslymainlyprimaryastrocyteandneu- ronalcultureswereappliedforcharacterizationofthetransporters [17,20].Theirextensiveusefortestinghighnumberofmoleculesis difficultandraisesethicalissues.Celllinescouldbemoreappropri- ateforscreening.PreviouslyratC6gliomacelllinewascommonly usedfor studyingtransporters ofvarious amino acids,e.g. glu- tamine[21].d-Serinetransportintothesecellswasdemonstrated bySikkaetal[22].TheyshowedthatthecellsexpressSRandASCT-2 andintheabsenceofd-aminoacidoxidaseASCT-2isanimportant playerintheregulationofd-serinehomeostasis.ItsmRNAexpres- sionwasshowntobedependentond-serineavailability.Therole ofASCT-1inthetransport wasnotaddressed.Theotherwidely usedmodelforstudyingd-serinetransportareratMüllercellline (rMC-1)andmouseprimaryMüllercells.Thismodelwaschosen becaused-serineandSRweredetectedinMüllercells ofretina andastrocytesinvertebratesandd-serineincreasesNMDAinduced excitotoxicityinthesecells[23].Itstransportwascharacterizedby Dunetal[24]andwasfoundsodium-dependentandinhibitedby thesubstratesofASCTtransporters.Theyshowedthatthecellscon- tainedmRNAofbothASCT-1andASCT-2butbecauseglutamine, regardedspecificforASCT-2transporter,inhibitedd-serinetrans- porttheyfocusedonlyonASCT-2andclaimeditbeingimportantin theregulationofNMDAreceptoractivityoftheneighboringneu- rons.TheroleofASCT-1wasnotexplored.Fosteratal[20]werethe firstdemonstratingthatbothASCT-1andASCT-2contributestod- serinetransportintorathippocampalastrocytesandHEK293cells transfectedwithhumanASCT-1orASCT-2.Theydemonstratedthat
l-glutamineisapreferentialbutnotaspecificsubstrateofASCT-2 inthetransfectedcellsraisingthepossibilityofspeciesdifference in theaffinity of thesubstrates totheASCT transporters.They alsofounddifferingaffinityofsubstratestotransportersinastro- cytesandcellstransfectedwithhumanASCTtransporters.Thiswas explainedbythepossibledifferenceintransporterdensityonthe cellsurfaceduringtransfectionwhichcanbealimitationofusing transfectedcellsasamodel.
Regarding the suggested species difference, the goal of our presentstudywastofindahumancelllineexpressingASCT-1and ASCT-2toestablishamodelsystemforrapidscreeningofcom- poundsfor influencingd-serineuptake intoastrocytes.Wealso aimedatclarifyingthecontributionof ASCT-1transportertod- serineuptake.SH-SY5Yhumanneuroblastomacelllinewasthus studiedanditsd-serinetransportcharacteristicswascomparedto thoseofculturedprimaryastrocytes.
2. Materialsandmethods 2.1. Chemicals
d-Serine, l-alanine, l-threonine, l-glutamine, t-Pro, buffer components,trypsin,trypanblue,acetonitrile,l-cysteic-acid,acry- lamidewerepurchasedfromSigma-Aldrich(St.Louis,MO,USA) and cholinechloride wasfromAlfa Aesar (Haverhill,MS, USA).
Fluorescent reagent, 4-fluoro-7-nitrobenzofurazan (NBD-F) was providedbyTCI(Tokyo,Japan).6-Monodeoxy-6-mono(3-hydroxy) propylamino--CD(HPA--CD)waspurchasedfromCyclolabLtd.
(Budapest,Hungary).UltrapurewaterfromMilliQDirect8water purification system (Merck Millipore, Billerica, MA, USA) was appliedforallexperiments.
For preparationof cellculture medium,Dulbecco’s Modified EagleMedium/NutrientMixtureF-12(DMEM/F12)andfetalbovine serum(FBS)wereprovidedbyCorning(Tewksbury,MA,USA)and Biosera(Nuaille,France), respectivelyand stableglutamine was obtainedfromPanBiotech(Aidenbach,Germany).
For westernblot analysis Novex 4-12% polyacrylamide gels (Thermo Fisher Scientific, Waltham, MA, USA) were used.
ASCT-1andASCT-2primaryantibodiesweresuppliedbySigma- Aldrich and Cell Signaling (Danvers, MA, USA), respectively.
Horseradishperoxidaseconjugatedsecondaryanti-rabbitantibody andPierceenhancedchemiluminescence(ECL)detectionreagent fromThermoFisherScientificwereused.
2.2. Cellcultures
Wistarratpupsof1-3daysoldwerepurchasedfromToxi-Coop Ltd.(Budapest,Hungary)andusedforestablishmentofprimary astrocytecultureaccordingtotheprotocolofMechaetal.[25].
Animalexperiments wereapprovedbytheInstitutionalAnimal CareandUseCommitteeofSemmelweisUniversity(approvalnum- ber:PE/EA/850-2/2016).ProcedureswereinharmonywiththeEU CouncilDirectiveonlaboratoryanimals(86/609/EEC).Astrocytes wereusedafterreachingconfluencyofP1subculture.
SH-SY5YcellswereacquiredfromTheEuropeanCollectionof CellCultures(ECACC,Salisbury,UK)andmaintainedaccordingto therecommendationoftheprovider.
2.3. Analysisofcellulard-serineuptake
2.3.1. Time-andconcentration-dependenceoftheuptakeinto SH-SY5Ycells
Onthedayoftheexperimentcellsweretrypsinizedandsus- pendedinTrisbufferedsaltsolution(TBSS)atconcentrationof1 millioncellsin0.5mL.Cellsuspensionswereincubatedwith0,25, 50and200Mofd-serinefor0,15,30,60and120minutesat
37◦C.Aftercompletionofincubationuptakewasterminatedby coolingthesamplesonicefollowedbycentrifugation(630g,4◦C, 5min)andwashedtwicewithicecoldTBSS.Pelletedcellswere resuspendedin35Lmixtureofacetonitrile:water(2:1,v/v)and theprecipitatedproteinwasremovedbycentrifugation(3000g,4
◦C,20min).Supernatantwascollectedandstoredat−80◦Cuntil analysis.
2.3.2. Timeandsodiumdependenceoftheuptake
Fortheanalysisofsodiumindependenttransportsodiumchlo- ridewas substitutedby cholinechloride. Samplescontaining 1 millioncellswereincubatedwith50 Md-serinefor 0,15,30, 60 and 120 minutes at 37 ◦C. After completion of incubation timeuptakewasterminatedandthesampleswereprocessedas describedinsection2.3.1.
2.3.3. Characterizationofd-serinetransportkinetics
Cellsuspensionswereincubatedinthepresenceofvariouscon- centrationsofd-serine(0-10000M)for15minat37◦Ctostudy thetransportkinetics.Aftercompletionofincubationtimeuptake wasterminatedandthesampleswereprocessedasdescribedin section2.3.1.
2.3.4. Inhibitionofd-serineuptakebyneutralaminoacids
Cellswereincubatedwith25Md-serineinthepresenceof variousconcentrationsofl-alanine,l-threonine,l-glutamineandt- Pro,respectivelyfor15minat37◦C.Aftercompletionofincubation timeuptakewasterminatedandthesampleswereprocessedas describedinsection2.3.1.
2.4. Determinationofintracellulard-serineconcentration
Capillaryelectrophoresiscoupledtolaserinducedfluorescence detection(CE-LIF)analysisofd-serinecontentofcellextractswas performedaccordingtoourpreviouslypublishedvalidatedmethod [26].Briefly,5Lsampleswerederivatizedbymixingwith5L ethanolicNBD-Fsolution(3mg/mL)and5Lboratebuffer(pH 8.5;20mM)containing5Ml-cysteicacidasinternalstandard andheatingfor20minat65◦C.Preparedsampleswerestoredat
−20◦CuntilCEanalysis.
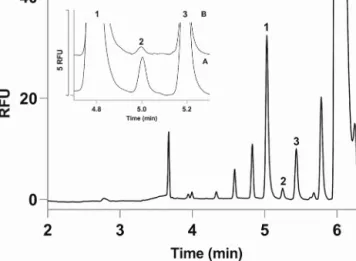
AllmeasurementswerecarriedoutonaP/ACEMDQcapillary electrophoresissystemoperatedby32Karatsoftwareversion5.0 (BeckmanCoulter,Brea,CA,USA).LIFdetectorwithArgon-ionlaser sourcewasapplied.Excitationandemissionwavelengthswere488 and520nm,respectively.Separationtookplaceinfusedsilicacapil- lariesof75midand365mod(AgilentTechnologies,SantaClara, CA,USA).Beforeusecapillarieswerecoatedwithlinearpolyacry- lamide.Representativeelectropherogramsofd-serineseparation isshowninFig.1.
2.5. Westernblotanalysis
ExpressionofASCT-1andASCT-2inSH-SY5Ycelllinewasstud- iedbywesternblotanalysis.About5millioncellswereextracted by200Lradioimmunoprecipitationassaybuffer(RIPA,Thermo FisherScientific)andthesampleswereseparatedin4-12% gra- dientgelusingTris-glycine-EDTAbufferandtransferredtoPVDF membrane.Membraneswereblockedby5%non-fatdrymilkdis- solvedinTris-bufferedsalinecontaining0.1%Tween20(TBST)for1 hourandprobedwith1g/mLanti-ASCT-1or4g/mLanti-ASCT- 2antibodiesin 5%non-fat drymilkdissolvedinTBSTovernight at4◦C.MembraneswerewashedthreetimeswithTBSTandthen incubatedwithhorseradishperoxidaseconjugatedsecondaryanti- rabbitantibodyfor1houratroomtemperature.ASCT-1andASCT-2 weredetectedonautoradiographyfilmsusingPierceECLreagent.
Fig.1.Electropherogramsdemonstratingdeterminationofintracellulard-serine contentofhumanSH-SY5Yneuroblastomacells.NumberedpeaksindicateNBD-F labelledglycine(1),d-serine(2)andl-serine(3).Intheinserttheelectropherograms ofsamples(A)afterincubationthecellswith25Md-serinefor15minand(B)after incubationthecellswith25Md-serinefor15mininthepresenceof3mMofl- alanineareshown.Separationconditions:50mMpH7HEPESbuffercontaining6 mMHPA--CD;30/40cmx75midcoatedfused-silicacapillary,−24kV.Sample injection:3474Papressurefor5sec.
2.6. Dataevaluation
GraphPadPrism8forWindows(GraphPadSoftware,LaJolla, CA,USA)wasusedfordataanalysisandcurvefitting.Exponential plateaufittingscheme,fitlogIC50fittingmethodandMichaelis- Menten curves were applied for analysis of time dependence, inhibitionandkineticsofd-serineuptake,respectively.
Forestimationofintracellulard-serineconcentration6and7
mradiusofSH-SY5Ycellsandastrocyteswereused,respectively [27].
3. ResultsandDiscussion
d-Serineuptake characteristicsofSH-SY5Ycells wereevalu- atedandcomparedtothoseofprimaryastrocytes.Firstthecell linewasincubatedinthepresenceof25,50and200Md-serine, respectivelyfor upto120minutesanditsuptake wasassessed bymeasuringitsintracellularlevel.Thetransportwasfoundcon- centrationandtimedependent.Itwassaturatedafter30minutes incubationwhen200Md-serinewasusedassubstrate,whilein caseofitslowerconcentrationsonlyatendencyofsaturationwas seenafter2hours(Fig.2).
In sodium free buffer a very limited d-serine uptake was detectedindicatingoverwhelmingdominanceofsodiumdepen- denttransportmechanismintoSH-SY5Ycellsandastrocytesalike (Fig.3AandB).Sodiumindependentportionoftheuptakewasless than10%intoeachcelltypeprobablyduetoanon-specificprocess.
ASCTtransportersareknownresponsibleforsodiumdependent uptakeofd-serineintocells andwerereportedtobeexpressed onastrocytes[16].Thesefindingssuggestthattheundifferentiated SH-SY5Ycelllinemayshowsimilard-serinetransportcharacteris- ticstothosepreviouslyreportedforculturedastrocytes[20].
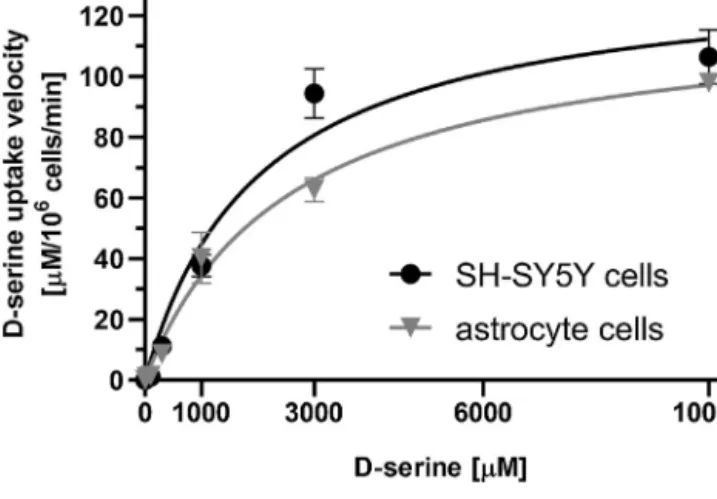
Toestablishthesimilarity, thekineticsofd-serinetransport intoSH-SY5Ycells andastrocyteswerecompared.Incubationof eachcelltypewithincreasingconcentrationsofd-serineshowed aproportionalelevationintheuptakerateaccordingtoMichaelis- Mentenkineticsandreachedplateauatsimilarconcentrationrange (Fig.4).EstimatedKMvaluesandmaximaluptakevelocity(vmax) werenotsignificantlydifferentindicatingcomparableaffinityand capacityofd-serinetransportinthesecelltypes(Table1).Wecan
Fig.2. Concentrationdependentd-serineuptakeintoSH-SY5Ycells.Intracellulard- serinecontentinSH-SY5Ycellswasdeterminedaftertheirincubationwithvarious concentrations(25,50,200M)ofd-serinefordifferentperiodsoftime(0,15,30, 60,120min).Dataareshownasmean±SEMofthreeexperiments(n=3).
Fig.3. Sodiumdependenceofd-serineuptakeintoSH-SY5Yandastrocytecells.
Uptakeofd-serineintoSH-SY5Ycells(A)andcorticalastrocytes(B)wasmeasured overaperiodof120minutesinsodiumcontainingorsodiumfreebuffers.d-Serine concentrationwas50M.Dataareshownasmean±SEMofthreeexperiments (n=3).
Fig.4. Kineticofd-serineuptakeintoSH-SY5Yandastrocytecells.Intracellulard- serineconcentrationmeasuredinSH-SY5Ycellsandratcorticalastrocytesinthe presenceofincreasingconcentrationsofd-serine.Dataareshownasmean±SEM ofthreeexperiments(n=3).Michaelis-Mentencurveswerefitted.
Table1
Kineticparametersofd-serineuptakeintoSH-SY5Yandastrocytecells.
SH-SY5Y astrocyte
mean 95%Cl mean 95%Cl p
Km(M) 2025 1081-3884 2545 1714-3847 0.5131
Vmax(M/106cells/min) 135.0 108.4-172.0 121.9 105.8-142.8 0.4339
Fig.5.WesternblotanalysisofASCT-1andASCT-2inSH-SY5Ycells.SH-SY5Ycell lysate(40gprotein)wasanalyzedbywesternblotusing4-12%gradientgeland probedbyrabbitanti-humanASCT-1andrabbitanti-humanASCT-2antibody.ASCT- 1andASCT-2weredetectedatabout52andabout66kDa,respectively.
assumethatd-serineuptakeintoSH-SY5Ycellsishighlysimilar tothatofastrocytesthusitcanserveasmodeloftheastrocytic transportofthisd-aminoacid.
TofurtherconfirmtheroleofASCTtransportersinthed-serine uptakeintoSH-SY5Ycellstheirexpressionwasstudiedbywest- ernblotanalysis.Expressionofbothtransporterswasdetectedin SH-SY5Ycells.Themostintensivebandappearedatabout52kDa usinganti-humanASCT-1antibody(Fig.5),whichiscomparable tothepredicted55kDamolecularweightreportedearlierinante- riorcingulatecortex[28].Anti-humanASCT-2antibodydetected animmunoreactivebandat66kDa(Fig.5)whichisinlinewiththe previouslyreportedmolecularweightoftheglycosylatedprotein [15].
Fig.6.Inhibitionofd-serinetransportintoSH-SY5Ycellsandratcorticalastro- cytesbyl-alanineandl-threonine.l-Alanine(A)andl-threonine(B)concentration dependentlyinhibitedthed-serineuptake.One-stepinhibitioncurvewasobserved incaseofbothcelltypes.Inallexperimentsuptakeof25Md-serinewasstud- iedfor15min.Intracellulard-serineconcentrationintheabsenceofinhibitorwas regardedas100%uptake.Dataareshownasmean±SEMofthreeexperiments(n= 3).
Asourultimateintentionisstudyingdrugcandidatesacting byinhibitionofd-serineuptakeintoastrocyteswecomparedthe inhibitorsensitivityofthetransportersonthetwocelltypes.
Natural substrates of ASCT transporters, l-alanine and l- threonine concentration dependently inhibited d-serine uptake intobothSH-SY5Ycellsandastrocytesandalmostcompleteinhi- bitionwasachieved.ComparableIC50valuesindicatetheirsimilar inhibitoraffinitytothetransportersonthetwocelltypes(Fig.6A andB,Table2).Basedonliteraturedatatheseneutralaminoacids havesimilaraffinitytoASCT-1andASCT-2[20].Inlineofthisfind- ingweobservedaone-stepsigmoidalinhibitioncurve,notallowing thedistinctionofthetwotransportersandhavinginsightifboth ASCTtransportersareinvolvedind-serineuptakeoronlyoneof themisfunctioning.
Therelativecontributionoftheindividualtransporterstothed- serineuptakewasthusassessedbyusingtheirselectivesubstrates.
t-Prowaspreviously shownhavingconsiderably higheraffinity toASCT-1[29], whilel-glutamine wasreportedbeingaprefer- entialsubstrateofASCT-2 [30].Inhibitionofd-serineuptakeby
Table2
EstimatedIC50valuesofvariousneutralaminoacidinhibitorsond-serineuptake intoSH-SY5Yandastrocytecells.
SH-SY5Y astrocyte
mean 95%Cl mean 95%Cl p
L-AlaIC50(M) 174.4 40.21-772.4 177.7 66.21-470.4 0.982 L-ThrIC50(M) 157.9 50.52-521.8 158.1 74.47-335.5 0.998 L-GlnIC50(M) 97.47 53.12-182.5 67.95 26.12-206.3 0.515 t-ProIC50(M)* 8.218 1.98-26.5 3.975 0.16-19.7 0.522
*forASCT1
Fig.7.Inhibitionofd-serinetransportintoSH-SY5Ycellsandratcorticalastrocytes byt-Proandl-glutamine.T-Proresultedintwo-stepinhibitioncurveinbothcell types(A).One-stepinhibitioncurvewasobservedinthepresenceofl-glutaminein bothSH-SY5Ycellsandculturedastrocytes(B).Intracellulard-serineconcentration intheabsenceofinhibitorwasregardedas100%uptake.Dataareshownasmean
±SEMofthreeexperiments(n=3).
t-Proshowedatwo-stepcurveindicatingcontributionofbothASCT transporters(Fig.7A).TheIC50valuesofthehigheraffinitycompo- nentcorrespondingtotheinhibitionofASCT-1werecomparable inthetwocelltypes(Table2).Becauseoftheweakinhibitionof ASCT-2byt-Procompleteinhibitionoftheuptakewasnotreached inthestudiedconcentration-rangeandtheestimationofIC50val- uesofthesecondstepsuffersfromhigherror(datanotshown).
BasedontheheightofthefirststepthecontributionofASCT-1to d-serinetransportwasabout30%inbothcelltypes.Foralongtime,
theinvolvementofASCT-1ind-serineuptakewasneglected.How- ever,usingtransfectedcellsorculturedastrocytesitwasshown tobesubstrateofbothtransporters[12,20].Ourpresentresults furtherconfirmthatASCT-1 ispartiallyresponsibleford-serine uptakeintocultured astrocytesand similarresultwasfoundin SH-SY5Ycells.Usingl-glutaminehowever,asingle-stepinhibition curvewasdetectedcontrarytothefindingsofFoster etal[20], whoobservedslightselectivitytowardsASCT-2.Thecontradiction isprobablyexplainedbytherathersmalldifferenceintheaffinity ofl-glutaminetotheASCTtransporters(Fig.7B).
4. Conclusion
OurpresentresultsdemonstratethathumanSH-SY5Yneurob- lastomacellsexpressfunctionalASCT-1andASCT-2transporters and show comparable uptake kinetics of d-serine to cultured astrocytes.Assimilarinhibitioncharacteristicsoftheestablished competitive uptake inhibitor small neutral amino acids were observedin both celltypes, theundifferentiated SH-SY5Ycells seemtobeanappropriatemodelsystemforscreeningpotential inhibitorsubstances ofd-serineuptake intoastrocytes byASCT transporters.Theadvantages ofusingthis celllinearetheeasy availability of functioning human transporters, its inexpensive maintenanceandtheavoidanceofsacrificinglaboratoryanimals.
Authorstatement
EvaSzoko:Supervisionoftheproject,dataevaluation,prepara- tionofthepublication;IstvanVincze:Planningandperformingthe experiments,CEmeasurements,dataanalysis,preparationofthe draftofthemanuscriptandthefigures;PeterP.Lakatos:Perform- ingsomeexperiments,dataanalysis,preparation ofthefigures;
FruzsinaBagamery:Maintenanceofthecells,preparationofthe primaryculture,performingsomeoftheexperiments;TamasTabi:
Evaluationofexperimentaldata,preparationofthepublication
DeclarationofCompetingInterest
Theauthorsdeclarethattheyhavenoknowncompetingfinan- cialinterestsorpersonalrelationshipsthatcouldhaveappearedto influencetheworkreportedinthispaper.
References
[1]L.Pollegioni,S.Sacchi,MetabolismoftheneuromodulatorD-serine,CellMol LifeSci67(2010)2387–2404.
[2]J.P.Mothet,M.LeBail,J.M.Billard,TimeandspaceprofilingofNMDAreceptor co-agonistfunctions,JNeurochem135(2015)210–225.
[3]K.Hashimoto,T.Fukushima,E.Shimizu,N.Komatsu,H.Watanabe,N.
Shinoda,M.Nakazato,C.Kumakiri,S.Okada,H.Hasegawa,K.Imai,M.Iyo, DecreasedserumlevelsofD-serineinpatientswithschizophrenia:evidence insupportoftheN-methyl-D-aspartatereceptorhypofunctionhypothesisof schizophrenia,ArchGenPsychiatry60(2003)572–576.
[4]S.E.Cho,K.S.Na,S.J.Cho,S.G.Kang,Lowd-serinelevelsinschizophrenia:A systematicreviewandmeta-analysis,NeurosciLett634(2016)42–51.
[5]J.T.Kantrowitz,A.K.Malhotra,B.Cornblatt,G.Silipo,A.Balla,R.F.Suckow,C.
D’Souza,J.Saksa,S.W.Woods,D.C.Javitt,HighdoseD-serineinthetreatment ofschizophrenia,SchizophrRes121(2010)125–130.
[6]G.Tsai,P.Yang,L.C.Chung,N.Lange,J.T.Coyle,D-serineaddedto antipsychoticsforthetreatmentofschizophrenia,BiolPsychiatry44(1998) 1081–1089.
[7]H.Wolosker,K.N.Sheth,M.Takahashi,J.P.Mothet,R.O.BradyJr,C.D.Ferris, S.H.Snyder,Purificationofserineracemase:biosynthesisofthe
neuromodulatorD-serine,ProcNatlAcadSciUSA96(1999)721–725.
[8]M.J.Schell,M.E.Molliver,S.H.Snyder,D-serine,anendogenoussynaptic modulator:localizationtoastrocytesandglutamate-stimulatedrelease,Proc NatlAcadSciUSA92(1995)3948–3952.
[9]K.Miya,R.Inoue,Y.Takata,M.Abe,R.Natsume,K.Sakimura,K.Hongou,T.
Miyawaki,H.Mori,Serineracemaseispredominantlylocalizedinneuronsin mousebrain,JCompNeurol510(2008)641–654.
[10]M.A.Benneyworth,Y.Li,A.C.Basu,V.Y.Bolshakov,J.T.Coyle,Cellselective conditionalnullmutationsofserineracemasedemonstrateapredominate localizationincorticalglutamatergicneurons,CellMolNeurobiol32(2012) 613–624.
[11]D.Rosenberg,E.Kartvelishvily,M.Shleper,C.M.Klinker,M.T.Bowser,H.
Wolosker,NeuronalreleaseofD-serine:aphysiologicalpathwaycontrolling extracellularD-serineconcentration,FASEBJ24(2010)2951–2961.
[12]D.Rosenberg,S.Artoul,A.C.Segal,G.Kolodney,I.Radzishevsky,E.
Dikopoltsev,V.N.Foltyn,R.Inoue,H.Mori,J.M.Billard,H.Wolosker,Neuronal D-serineandglycinereleaseviatheAsc-1transporterregulatesNMDA receptor-dependentsynapticactivity,JNeurosci33(2013)3533–3544.
[13]Y.Fukasawa,H.Segawa,J.Y.Kim,A.Chairoungdua,D.K.Kim,H.Matsuo,S.H.
Cha,H.Endou,Y.Kanai,Identificationandcharacterizationofa
Na(+)-independentneutralaminoacidtransporterthatassociateswiththe 4F2heavychainandexhibitssubstrateselectivityforsmallneutralD-and L-aminoacids,JBiolChem275(2000)9690–9698.
[14]L.Helboe,J.Egebjerg,M.Moller,C.Thomsen,Distributionandpharmacology ofalanine-serine-cysteinetransporter1(asc-1)inrodentbrain,EurJNeurosci 18(2003)2227–2238.
[15]C.M.Gliddon,Z.Shao,J.L.LeMaistre,C.M.Anderson,Cellulardistributionof theneutralaminoacidtransportersubtypeASCT2inmousebrain,J Neurochem108(2009)372–383.
[16]Z.Shao,A.Kamboj,C.M.Anderson,Functionalandimmunocytochemical characterizationofD-serinetransportersincorticalneuronandastrocyte cultures,JNeurosciRes87(2009)2520–2530.
[17]C.S.R.Ribeiro,M.Panizzutti,R.deMiranda,J.Wolosker,H.Glial,Transportof theNeuromodulatorD-Serine,BrainResearch(2002)202–209.
[18]L.Pollegioni,L.Piubelli,S.Sacchi,M.S.Pilone,G.Molla,Physiologicalfunctions ofD-aminoacidoxidases:fromyeasttohumans,CellMolLifeSci64(2007) 1373–1394.
[19]C.Rojas,J.Alt,N.A.Ator,A.G.Thomas,Y.Wu,N.Hin,K.Wozniak,D.Ferraris,R.
Rais,T.Tsukamoto,B.S.Slusher,D-Amino-AcidOxidaseInhibitionIncreases D-SerinePlasmaLevelsinMouseButnotinMonkeyorDog,
Neuropsychopharmacology41(2016)1610–1619.
[20]A.C.Foster,J.Farnsworth,G.E.Lind,Y.X.Li,J.Y.Yang,V.Dang,M.Penjwini,V.
Viswanath,U.Staubli,M.P.Kavanaugh,D-SerineIsaSubstrateforNeutral AminoAcidTransportersASCT1/SLC1A4andASCT2/SLC1A5,andIs TransportedbyBothSubtypesinRatHippocampalAstrocyteCultures,PLoS One11(2016),e0156551.
[21]M.Dolinska,etal.,GlutaminetransportinC6gliomacellsshowsASCT2 systemcharacteristics,NeurochemInt43(4-5)(2003)501–507.
[22]P.Sikka,etal.,D-SerinemetabolisminC6gliomacells:Involvementof alanine-serine-cysteinetransporter(ASCT2)andserineracemase(SRR)but notD-aminoacidoxidase(DAO),JNeurosciRes88(8)(2010)1829–1840.
[23]E.R.Stevens,etal.,D-serineandserineracemasearepresentinthevertebrate retinaandcontributetothephysiologicalactivationofNMDAreceptors,Proc NatlAcadSciUSA100(11)(2003)6789–6794.
[24]Y.Dun,etal.,FunctionalandmolecularanalysisofD-serinetransportin retinalMullercells,ExpEyeRes84(1)(2007)191–199.
[25]M.Mecha,ProtocolExchange,2011.
[26]T.Jako,E.Szabo,T.Tabi,G.Zachar,A.Csillag,E.Szoko,Chiralanalysisofamino acidneurotransmittersandneuromodulatorsinmousebrainbyCE-LIF, Electrophoresis35(2014)2870–2876.
[27]Bionumbers,in,2020.
[28]S.Weis,I.C.Llenos,J.R.Dulay,N.Verma,S.Sabunciyan,R.H.Yolken,Changes inregion-andcelltype-specificexpressionpatternsofneutralaminoacid transporter1(ASCT-1)intheanteriorcingulatecortexandhippocampusin schizophrenia,bipolardisorderandmajordepression,Journalofneural transmission(Vienna,Austria:1996)114(2007)261–271.
[29]J.Pinilla-Tenas,A.Barber,M.P.Lostao,Transportofprolineand
hydroxyprolinebytheneutralamino-acidexchangerASCT1,TheJournalof membranebiology195(2003)27–32.
[30]A.Broer,N.Brookes,V.Ganapathy,K.S.Dimmer,C.A.Wagner,F.Lang,S.Broer, TheastroglialASCT2aminoacidtransporterasamediatorofglutamineefflux, JNeurochem73(1999)2184–2194.