ContentslistsavailableatScienceDirect
Journal of Pharmaceutical and Biomedical Analysis
jo u r n al ho me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / j p b a
Structural characterization of fondaparinux interaction with per-6-amino-beta-cyclodextrin: An NMR and MS study
Bianka Várnai
a, Márkó Grabarics
b,c, Zoltán Szakács
d, Kevin Pagel
b,c, Milo Malanga
e, Tamás Sohajda
e, Szabolcs Béni
a,∗aSemmelweisUniversity,DepartmentofPharmacognosy,Üll ˝oiút.26,H-1085,Budapest,Hungary
bFreieUniversitätBerlin,InstituteofChemistryandBiochemistry,Arnimallee22,14195,Berlin,Germany
cFritzHaberInstituteoftheMaxPlanckSociety,DepartmentofMolecularPhysics,Faradayweg4–6,14195,Berlin,Germany
dGedeonRichterPlc.,SpectroscopicResearchDepartment,H-1475,Budapest,P.O.B.27,Hungary
eCycloLab,CyclodextrinR&DLtd,Budapest,H-1097,Illatosút7,Hungary
a rt i c l e i nf o
Articlehistory:
Received16October2020
Receivedinrevisedform28January2021 Accepted30January2021
Availableonline3February2021
Keywords:
Heparin NMR
Electrostaticinteraction Antidote
Degradation
a b s t ra c t
Thehighlyanionicsyntheticpentasaccharidefondaparinux(FDPX)–representingtheantithrombinbind- ingsequenceofheparin–isinclinicaluseasapotentanticoagulant.Contrarytotheunfractionated heparin,FDPXlackspotentantidotecompletelyreversingitsanticoagulantactivity,thereforeitisof greatimportancetoidentifynewstructuresexhibitingstrongintermolecularinteractionstowardsFDPX.
Thepolycationicheptakis(6-amino-6-deoxy)-beta-cyclodextrin(NH2--CD)canserveasanexcellent modelcompoundtomimictheseinteractionsbetweentheoppositelychargedoligosaccharides.Herein, extensiveNMRspectroscopicandnano-electrosprayionizationmassspectrometric(nESI-MS)studies wereconductedtounderstandthemolecular-levelinteractionsintheFDPX-NH2--CDsystems.NMR experimentswereperformedatpD7.4and2.0.Job’smethodofcontinuousvariationand1HNMRtitra- tionexperimentssuggestedtheformationofFDPX·NH2--CDcomplexatpD7.4,whilethepresenceof multiplecomplexeswasassumedatpD2.0.Stabilityconstantsweredeterminedbyseparate1HNMR titrations,yieldinglogˇ11=3.65±0.02atpD7.4,whilelogˇ11≥4.9valuesuggestedahigh-affinity systematpD2.0.2DNOESYNMRstudiesindicatedspatialproximitiesbetweentheanomericresonance
␣-l-iduronicacidresidueandthecyclodextrin’smethyleneunitintheproximityofthecationicamino function.AcidicdegradationofFDPXwasinvestigatedbyNMRandMSforthefirsttimeindetailconfirm- ingthatdesulfationoccursinvolvingonetotwosulfatemoieties.ThedesulfationofFDPXwasinhibitedby thecationiccyclodextrininthecaseofequimolarratioatpD2.0.Thisisthefirstreportonthestabilizing effectofcyclodextrincomplexationonheparindegradation.
©2021TheAuthor(s).PublishedbyElsevierB.V.ThisisanopenaccessarticleundertheCC BY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Heparinisananionic,linearpolysaccharide,amemberofthe glycosaminoglycan(GAG)familythatisnaturallysynthesizedand stored in mastcells [1]. Althoughthecorestructure ofheparin consistsofrepeating1,4-linkeduronicacidandglucosaminedis- accharides,ithasamicroheterogenousandpolydispersestructure duetovariousmodificationofthemonosaccharidesubunits.The hexuronicacidiseither␣-l-iduronicacid(IdoA)or-d-glucuronic acid(GlcA),whichmaybe2-O-sulfated.Thehexosamineresidue isd-glucosamine(GlcN),whichmaybesulfatedatthe3-Oand6-
∗Correspondingauthor.
E-mailaddress:beni.szabolcs@pharma.semmelweis-univ.hu(S.Béni).
OpositionsandN-sulfated(GlcNS),N-acetylated(GlcNAc)ormay beunmodified asa primary amine[2]. Heparin is best known foritsanticoagulantactivity,exertedthroughtheinteractionwith theproteaseinhibitorantithrombin-III(AT-III).AT-IIIisthemajor plasmacoagulationinhibitor,whosemaintargetsareIIaandXa activatedcoagulationfactors[3].Heparinisawidelyusedanticoag- ulantdrugintheclinicalpracticedespiteitsnumerousundesirable sideeffects.
Nowadays,lowmolecularweightheparins(LMWHs)areapplied intheclinicalpracticeasananticoagulantagentduetotheirpre- dictableactivityprofile,betterbioavailabilityandlongerhalf-life, that contribute to their safer applicability. LMWHs are pre- pared from unfractionated heparin by chemical or enzymatic depolymerizationreactionsthatproducepolydispersemixturesof heparin-derivedoligosaccharides[4].
https://doi.org/10.1016/j.jpba.2021.113947
0731-7085/©2021TheAuthor(s).PublishedbyElsevierB.V.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/
4.0/).
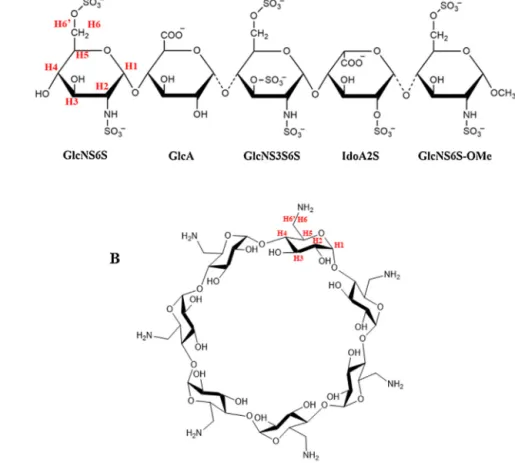
Fig.1.Chemicalstructuresandnumberingofthefondaparinux(A)andtheheptakis(6-amino-6-deoxy)-beta-cyclodextrin(B).
Another alternative in heparin-based anticoagulant therapy is the synthetic pentasaccharide fondaparinux (FDPX). It was designedtomimictheheparinpentasaccharidesequencepresent ineachheparinderivativeandrequiredtobindAT-III,thusenhanc- ingitsinhibitoryactivitytowardfactorXa[5].FDPXisamethyl glucoside analogue of the heparin pentasaccharide sequence responsibleforAT-IIIbinding(Fig.1).Toneutralizetheanticoag- ulanteffectof heparin,protaminesulfate isusually usedinthe clinicalpractice.Itis abasic,polycationicpeptideisolatedfrom salmonsperm, whichbinds tothepolyanionicheparin through electrostaticinteractions,thusforminganinactivecomplexwith- out anticoagulant effect[6].Unfortunately, protamineis unable toreversetheanticoagulantfunctionofLMWHsandisevenless effectiveforfondaparinux.Therefore,itisnecessarytofindnew antidotes,whichshouldbeeffectiveforallheparinderivatives[7].
Amongthepossiblecorestructuresasfutureantidotes,modified carbohydratesandcyclodextrinsstandoutaspromisingcandidates [8].
Cyclodextrins are cyclic oligosaccharides built of d- glucopyranose units linked via ␣-1,4-glycosidic bonds. As a result,theyhaveatruncatedconeshape withalipophilicinner cavity andahydrophilicoutersurface[9].Thisparticularstruc- ture enablesreversibleinclusion complexformation withguest moleculeswithappropriateproperties[10].Interactionsbetween the cyclodextrin host and the guest molecules are generally noncovalent: hydrogen bonds,van der Waals and hydrophobic interactionsarecommon,whileincaseofionicderivativeselec- trostaticinteractionsdominate.Themostcommonlyusednative cyclodextrinsarethe␣-,-and␥-cyclodextrincomposedofsix, sevenandeightglucoseunits,respectively.Syntheticmodification ofthehydroxylgroupsoftheglucopyranoseunitsoffersnumerous possibilitiesforthepreparationofvariouscyclodextrins,suchas cationicandanionicderivatives.Inthepresentstudythecationic heptakis(6-amino-6-deoxy)-beta-cyclodextrin (NH2--CD) was
utilized(Fig.1).Thesechemicalmodificationsareoftenaimedat providingstructure-specificinteractions,therebycontributingto anevenmorepronouncedsupramolecularassembly[11,12].
Thecurrent study aims to characterize themolecularinter- actions between the polyanionic FDPX and the polycationic cyclodextrinNH2--CDasapotentialheparinantidote.Further- more,thestabilizingeffectoftheNH2--CD(hereinafterdenoted as CD) on FDPX degradation under acidic conditions was also investigated.Toestablishamoreprofoundunderstandingofthe intermolecularinteractionaswellasthedegradationofFDPXunder acidicconditions,extensiveNMRandMSexperimentswereper- formed.
2. Materialsandmethods 2.1. Chemicalsandreagents
CDwasaproductofCycloLabLtd.(Budapest,Hungary).D2O (99.9atom%D)waspurchasedfromMerck(Darmstadt,Germany), whilefondaparinuxwasisolatedfromArixtra®2.5mg/0.5mLinjec- tions.Briefly,tenprefilledinjectionswerecombinedandsubjected todialysis(Spectra/Por®dialysismembrane–Biotech-CETubing MWCO:100−500Da)againstdeionizedwaterforthreeconsecu- tivedays.Thereafter,thedialysatewassubjectedtofreeze-drying.
Otherbasechemicalsofanalyticalgradewerepurchasedfromcom- mercialsuppliersandwereusedwithoutfurtherpurification.
2.2. NMRspectroscopy
Nuclear magnetic resonance (NMR) spectroscopy measure- ments were carried out on a 600 MHz Varian DDR NMR spectrometer(AgilentTechnologies,PaloAlto,CA,USA),equipped witha5mminverse-detectionprobeheadandgradientunit.Stan- dardpulsesequencesandprocessingroutinesavailableinVnmrJ
3.2C/Chempack 5.1 wereused. Thecomplete resonance assign- ments of the fondaparinux and the CD were established from direct1H–13C,long-range1H–13C,andscalarspin-spinconnectiv- itiesderivedfrom1D1H,2D1H-1HgCOSY,zTOCSY(mixingtime of150ms),ROESYAD(mixingtimeof400ms),NOESYand1H–13C gHSQCAD(J=140Hz–accordingtotheonebondheteronuclear couplingconstant)experiments.The1Hchemicalshiftswereref- erencedtothemethylsinglet(ı=3.31ppm)ofinternalCH3OH,a referencesubstancewithoutanypossibleinteractionwiththeCD.
AdditionalNMRstudieswereperformedonan800MHzBruker Avance III HDX800 MHz spectrometer equipped with a 5 mm
1H/13C/15NTripleResonance13CEnhancedSaltTolerantColdProbe (1H:799.7MHz,13C:201.0MHz),controlledbytheTopSpin3.5pl7 software(BrukerBiospinGmbH,Rheinstetten,Germany).AllNMR spectrawereacquiredinstandard5mmNMRtubesat25◦C.
2.2.1. 1HNMRtitrationexperiments
ThestoichiometryofFDPXcomplexationwithCDwasinvesti- gatedbytheJob’smethodofcontinuousvariation[13].Samples werepreparedin0.1MphosphatebuffersofpD2.0andpD7.4,at 25◦Ctemperature.Thetotalmolarconcentrationofthetwocom- ponents,cFDPX+cCD waskeptconstantat3mM,whilethemole fractionofFDPX,xFDPX=cFDPX/(cFDPX+cCD)wasvariedgraduallyin 0.1unitstepsfrom0to1.1HchemicalshiftsıFDPXwererecordedat 600MHzforseveralFDPXprotonsandcomplexation-induceddis- placementvalues,ıFDPX=
ıFDPX−ıFDPXwerecalculatedwith respecttoıFDPXmeasuredintheabsenceofCD.ToconstructJob’s plots,ıFDPXvaluesweremultipliedbythemolefractionofFDPX anddepictedasafunctionofxFDPX.Analogousplotsweregenerated forselectedprotonsoftheCD.SincetheexperimentaldesignunderlyingJob’splotsmaynot beoptimaltodeterminethestabilityconstantsoftheformedcom- plexes[14], separateNMRtitrations wereperformedunderthe sameexperimentalconditionsasusedfortheJob’splot.AtpD7.4, increasingportionsrangingfrom20to280Lof20.1mMCDsolu- tionwereaddedto600Lof3.4mMFDPXsolutionresidingin theNMRtube.AtpD2.0,600Lof3.18mMFDPXsolutionwas titrated with10–250Lof20.9 mMCDstocksolution.Follow- ingequilibration,1HNMRspectrawererecordedineachtitration stepat600MHzand25◦Ctemperature.Theexperimentaltitra- tion curves for well-resolved resonancesof FDPXand CD were evaluatedbytheOPIUMsoftware[15]accordingtotheprinciples summarizedherebyassumingtheformationofbothFDPX·CDand FDPX·2CDcomplexesasageneralcase.Ifcomplexationoccurswith rapidkineticsontheNMRchemicalshifttimescale,theobserved chemicalshiftıFDPX,iofanycarbon-boundprotoninFDPXbecomes amole-fractionweightedaverage[14]ofthespecies-specificval- uesintheuncomplexedFDPX(ıiFDPX)andthoseinthecomplexes (ıiFDPX·CDandıiFDPX·2CD),
ıFDPX,i=[FDPX]ıiFDPX+[FDPX·CD]ıiFDPX·CD+[FDPX·2CD]ıiFDPX·2CD cFDPX
(1) where square bracketsdenoteequilibrium concentrations andi usedhereinisarunningindex.Analogouslydefinedintrinsicchem- icalshifts(ıjCD,ıjFDPX·CD,ıjFDPX·2CD)andresonancesignalaveraging applyforanycarbon-boundprotonofthecyclodextrin:
ıCD,j=[CD]ıjCD+[FDPX·CD]ıjFDPX·CD+2 [FDPX·2CD]ıjFDPX·2CD
cCD (2)
where square bracketsdenoteequilibrium concentrations andj usedhereinisarunningindex.Themass-balanceequationsforboth constituentsread:
cFDPX=[FDPX]+[FDPX·CD]+[FDPX·2CD] (3) cCD=[CD]+[FDPX·CD]+2 [FDPX·2CD] (4)
whichcanbereformulatedintermsoftheˇassociation(i.e.binding orformation)constantsofthecomplexes,yielding:
cFDPX=[FDPX] (1+ˇ11[CD]+ˇ12[CD]2) (5) cCD=[CD] (1+ˇ11[FDPX]+2ˇ12[FDPX][CD]) (6) BasedontheinputvaluesofcFDPXandcCDforeachtitrationpoint (theratioofwhichwascheckedfromnon-overlapping1H NMR integralsofthecomponents)aswellasinitialguessesoftheˇsta- bilityconstants,theOPIUMprogramsolvedthenonlinearsystemof Eqs.(5)and(6)forthevariables[FDPX]and[CD].Thesespeciation calculationswereintegratedintoaleast-squaresfittingprocedure ofEqs.(1)or(2)tothemeasureddatasetinordertoiteratively refinethestabilityconstant(s).SincetheOPIUMprogramlacksa graphicaloutput,theresultingequilibriumconstantswereused tocomputespeciesdistributiondatacoveringthe0–2.74interval oftheccCD
FDPX ratiobytheORCHESTRAprogram[16].Thesedatasets weresubsequentlyimportedintoMicrosoftExcel2002,whereEqs.
(1)and(2)wereusedtocalculateanddepictthecontinuouscurves fittedtotheexperimental1HNMRtitrationdatasets.
2.2.2. NMRstructuralstudiesoncomplexformation
Toexplorethespatialarrangementofthehost-guestcomplexes andidentifytheinteractingmolecularsequences,nuclearOver- hausereffect(NOE) type experimentswereperformed atcCD = cFDPX=1mMinunbufferedsamplesatdifferentpDvalues(2.0–7.4) toeliminatethepossibleinterferingeffectofthephosphateions.
NOEisa manifestationof dipolarcross relaxationbetweentwo nonequivalentnuclearspinsthatarecloseenough(<5Å)through space[17].TheNOEintensitiesarescaledwithr−6,whererrepre- sentsthemeandistancebetweentheprotons.2DNOESYspectra wereacquiredonthe600MHzinstrument,withmixingtimesof 400msusing16scanson1258×512datapoints.Fortheacidic sample,2DNOESY spectrawerealsorecorded onthe800MHz instrument,collecting24scanson4096×512datapoints,applying mixingtimesof350ms,400ms,500msand650msalongwitha 250-WpresaturationontheHDOsignalforD1=2.5s.
2.2.3. NMRinvestigationsofFDPXdegradation
TodeterminethestructureoftheFDPXdecompositionproduct,
1Handseveral2D(COSY,TOCSY,HSQC,ROESY)NMRspectrawere recordedat600MHzwiththesameparametersdescribedinchap- ter2.2.Forthisexperiment,5mMFDPXwasdissolvedina0.1M phosphatebufferedD2OatpD2.0.
Duringthestabilityinvestigation,FDPX:CDsampleswith1:0, 2:1and1:1molarratiosweretested.Allsamplesweredissolvedin 0.1MphosphatebufferedD2OatpD2.0.Inthecaseofthefirstsetof experiments,1HNMRspectraofthefreshlypreparedsampleswere recorded.Afterkeepingthesesamplesat25◦Cforaweek,their
1HNMRspectrawererecordedagain.Asecondexperimentwas carriedouttoperformanaccelerateddecompositioninvestigation.
1HNMRspectraofthefreshsampleswererecordedat25◦C,then sampleswereincubatedat60◦Cfor14h.Afterwardsallsamples werecooleddownto25◦Cagainand1Hspectrawerere-recorded.
2.3. Electrosprayionizationmassspectrometry
Massspectrometricmeasurementswereperformedinnegative ionmodeonamodifiedSynaptG2-SHDMSQ-IMS-ToFhybridmass spectrometer(WatersCo.,Manchester,UK),equippedwithanano- electrosprayion(nESI)sourceandanrf-confiningdriftcell(250.5 mminlength).AnMKS647Cmultichannelcontroller(MKSInstru- ments,Andover,Massachusetts,US)providedgasflowandpressure controlinthedriftcell,whichwasfilledwithHebuffergas(99.999%
purity)atapressureof1.8torr.
For studying thecomplex formation betweenFDPXand CD, thecompoundsweredissolvedinwater:methanol(50/50,v/v)to reachaconcentrationof20MforFDPXand200MforCD.The solventmixturewasprepared usingMilli-Q® waterand LC–MS grademethanol(Merck,Darmstadt,Germany).ForMSexperiments involvingtheFDPXdegradationproducts, 5mMFDPXwasdis- solvedina0.1MphosphatebufferedD2OatpD2.0andincubated at60◦Cfor14h,identicallytothesolutionusedforNMRspectro- scopicdegradationmeasurements(seeSection2.2.3).Thisacidic solutionwasdilutedinwater:methanol(50/50)andspikedwitha 10mMaqueoussolutionofCDtoreachaconcentrationof20M forFDPXdegradationproducts(calculatedforthepre-incubation FDPXcontent)and200MforCD.
For generating negative ions using nESI, 5 L of the sam- plesolutionsdescribedabovewasloadedintoin-houseprepared borosilicateneedlescoatedwithPt/Pd(80/20),andavoltageof- 0.60to-0.85kVwasappliedtothecapillary.Typicalinstrument settings oftheSynapt G2-SHDMSinstrument wereasfollows:
capillaryvoltage-0.60to-0.85kV;sourcetemperature100◦C;sam- plingcone2.0;sourceoffset0.0;trapgasflow2.0mLmin−1;trap DCentrance4.0;trapDCbias2.0;trapDC−2.0;trapDCexit1.0;
IMSDCentrance−23.0;heliumcellDC55.0;IMSbias45.0;helium exit−40.0;IMSDCexit5.0;transferDCentrance5.0;transferDC exit15.0;trapwavevelocity250ms−1;trapwaveheight0.8V;
transferwavevelocity300ms−1;transferwaveheight4.0V.All massspectrawererecordedinresolutionmode.Forclarity,polar- itiesandunitsofthevaluesaboveareprovidedasdisplayedinthe MassLynx4.1softwarepackage(WatersCo.,Manchester,UK).As such,somevoltagesaregivenonlywithnumericalvalueswithno unitspecified.
Data processing and analysis were performed using MassL- ynx4.1(WatersCo.,Manchester,UK)andOrigin2017(OriginLab, Northampton,MA,US)softwarepackages.
3. Resultsanddiscussion
3.1. ExploringthecomplexformationequilibriabyNMRtitrations IntermolecularinteractionsbetweenFDPXandCDwereinvesti- gatedattwodifferentpDvalues(pD2.0and7.4),wherethecharge distributionoftheinteractingspeciesisdifferent.Atthephysiolog- icalpDvalue,theCDderivativebearsanaveragepositivechargeof 5.1(pKavaluesfortheCDareasfollows:9.50(1),8.89(1),8.33(1), 8.07(1),7.57(1),7.35(1),6.75(1)[18]),whileatpD2.0thehostis fullyprotonated,carrying sevenpositive charges.Inthecase of FDPXaccordingtothepublishedrelatedoligosaccharidesthepKa
valuesare2.35and3.44for-d-GlcAandIdoA2S[19],thusatpD 7.4bothhexuronicacidresiduesaredeprotonated,thereforeFDPX possesses10negativecharges.Under acidicconditions(pD2.0), themeanchargeofFDPXcorrespondsto−8.3.Complete1HNMR assignmentsofFDPXandCDatpD2.0and7.4canbefoundinTable S1ofSupportingInformation.
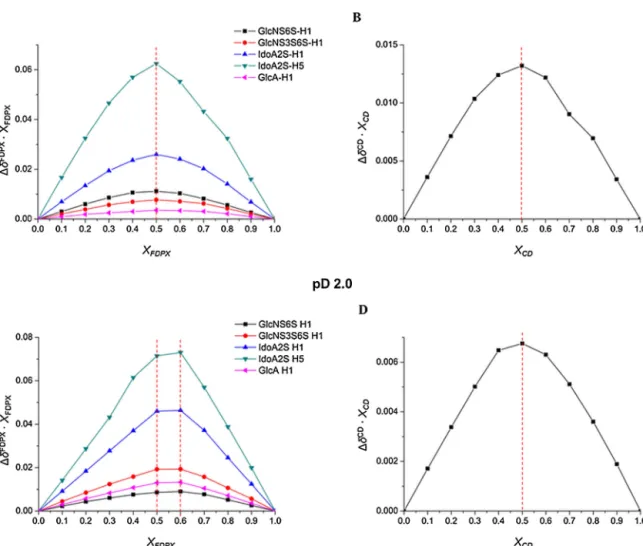
InordertoassessthecomplexationstoichiometryatpD7.4,Job’s plotswereconstructedfromwell-separatedNMRsignalsofFDPX, suchasthoseoftheanomericprotonsoftheGlcNS6S,GlcAand GlcNS3S6Sresidues,theH1andH5resonancesofIdoA2S,andthe anomericH1resonanceoftheCD.AlltheJob’splotcurvesdepicted onAand BdiagramsinFig.2showa maximumatxFDPX =0.5, suggestingthesoleformationoftheFDPX·CDcomplexwith1:1 stoichiometry.
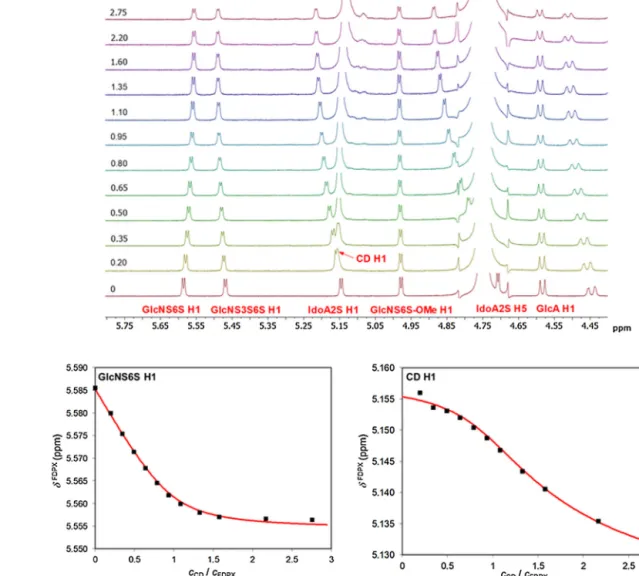
Duringthesubsequentsingle-tubeNMRtitrationofFDPXwith CDatthesamepDvalue(seethestackplotinFig.3A),thechem- icalshiftvariationofthefollowingprotonswithnon-overlapping signalsweremonitored:GlcAH1,GlcNS3S6SH1,GlcNS6S-OMeH1, GlcNS3S6SH6,IdoA2SH5andH1,GlcNS6SH1ofFDPXandH1ofthe
CDderivative.Titrationprofilesofthelattertwonucleiaredepicted inFig.3B,whilethoseoftheremainingFDPXprotonslistedabove areshowninFig.S1.Alltheeightdatasetscouldbeadequatelyfit- tedusingtheOPIUMprogrambyassumingonlythesingleFDPX·CD complex,assuggestedbytheJob’splots.Theseevaluationsyielded estimatesforthebindingconstantlogˇ11rangingfrom3.25to4.3, collectedinTableS2.Inordertoextractamorerobustandreliable, singlevalueofthisstabilityconstant[20],theeightdatasetswere subjectedto simultaneousnonlinear regression using thesame software.Thequalityoffitremainedvirtuallythesameforallthe observednucleiandthisglobalevaluationyieldedlogˇ11=3.65 withanestimatedstandarddeviationof0.02,amoderatelystrong bindingaffinityforaCDcomplex(seethespeciesdistributionplot inFig.S2).Thecomplexation-inducedchangesinchemicalshifts exceeded0.04ppmunitsfortheGlcNS3S6SH5,IdoA2SH1andH5 protonsofFDPX,theintrinsicchemicalshifts(listedinTableS3) emergingfromtheglobaldatafittingonlyslightlydifferedfrom theircounterpartsfromsingle-datasetevaluationsinTableS2.
TheJob’splotsrecordedatpD2.0revealedalessclear-cutpic- tureaboutcomplexation(seeFig.2,subplotCandD).Whilethe profileoftheanomericCDprotoninsubplotDisratherwidebut bearsamaximumatxCD=0.5,suggestingagainasimple1:1com- plexationpattern,theextremumofeachFDPXproton’sprofilein Fig.2,subplotCisconsequentlyshiftedtoca.xFDPX=0.6,indicating thepresenceofa2FDPX·CDcomplexbesidesFDPX·CD.
Toexplorethecomplexationequilibriaat pD2.0more thor- oughly,asingle-tube1HNMRtitrationofFDPXwiththeCDwas carriedout(seethestackplotinFig.S3).Titrationprofilesofthe anomericprotonsbelongingtotheGlcNS6SandGlcNS3S6Sunits aredepictedinFig.4,whilethoseforfouradditionalprotonsof FDPXaswellasfortheCDanomericprotonareshowninFig.S4.
Mosttitration profilesofFDPXprotonsconsistoftwoquasilin- earsegments,withaminimalcurvaturenearthecrossingpointat cCD=cFDPX.Thistypeoftitrationcurveischaracteristictoarather high-affinitysystem,forwhichmerelythelowerlimitofthebind- ingconstantisaccessiblebycurve-fitting[14].Theentiredataset cannotbefittedsatisfyinglyassumingtheformationof asingle FDPX·CDcomplex(seethebluecurvesinFig.4andFig.S4),the Hamilton’sRfactor,agoodness-of-fitcriterionforthesimultaneous fittingofthesevendatasets,is0.031%.Theintrinsicchemicalshifts arelistedinTableS4.Hencethetitrationcurveswereevaluatedin twosteps.
AllexperimentaldatasetscanbenicelyfitteduptoccCD
FDPX=1with theassumptionofasingleFDPX·CDcomplexonly(bluecurvesin Fig.4andFig.S4),yieldinglogˇ11=4.9±0.1,buthighervalues ofthisstabilityconstantgivevirtuallythesamegoodness-of-fit.
Additionofa2FDPX·CDspeciestotheequilibriummodel,assug- gestedbyJob’splots,didnotfurnishameaningfulvalueforˇ21, theiterationsbytheOPIUMprogramdidnotconverge.Intuitively, onecannotdescribethemonotonicchangeinFDPXchemicalshifts beyondtheequimolarcompositionbythe{FDPX·CD,2FDPX·CD} equilibriummodel.Instead,anFDPX·2CDcomplexwaspostulated besidesFDPX·CD.Aglobalfittinginvolvingthesetwospecieswas alreadyabletodescribethetitrationprofilesofFDPXandCDpro- tonsinthefullrangeofconcentrationsstudied(seethefittedred curves in Figs. 4and S4), the Hamilton’s Rfactor decreased to 0.0089%,indicatingamoreappropriateequilibriummodel.Thecal- culationsyieldedastabilityconstantoflogˇ12=8fortheFDPX·2CD complex(butthisvalueagainrepresentsmerelya lowerlimit).
TheintrinsicchemicalshiftvaluesofspeciesarecollectedinTable S5.Usingtheselowerlimitsofthestabilityconstants,thespecia- tioncurvesinFig.S5wereconstructedfortheconcentrationrange exploredintheNMRtitration.
ThediscrepancyofJob’smethodandthechemicalshifttitra- tioninestablishingthecorrectstoichiometryofcomplexationis
Fig.2. Job’splotfortheselected1HresonancesofFDPXatpD7.4(A)andatpD2.0(C)andthatoftheanomericresonanceofNH2--CDatpD7.4(B)andatpD2.0(D), respectively.
somewhat puzzling. Nevertheless, recent publications [21] [22]
emphasizethelimitationsofJob’smethodofequilibriumsystems beyondthesimplest1:1case,beinghighlysensitivee.g.totheratio ofequilibriumconstants[21].WhileJob’smethodremainsareli- abletoolinstoichiometricstudiesofinorganicmetalcomplexes, acarefulinspectionofthedistributionofresidualsofdata-fitting [20,21]ortherankingof allreasonablebindingmodelsaccord- ingtoestimateduncertaintiesandotherchemometricdescriptors [22]becametherecommendedprotocolsforthesametaskinthe fieldofsupramolecularchemistry.Sincethe{FDPX·CD,FDPX·2CD} equilibriummodelnicelyreproducedtheentiretitrationdataset, theformationofthesetwospeciescanberegardedasmostproba- bleundertheappliedexperimentalconditionsforthishigh-affinity system.
3.1.1. NMRstructuralstudies
Toobtainatomic-levelinformationaboutthe3Dstructure of theFDPX-CDcomplex,NOEexperimentswerecarriedoutforsam- pleswithoutphosphatebuffer,toeliminateanypossibleinterfering effectofthephosphateionswiththeCD.SolutionsatpD2.0–7.4 weretested.The2DNOESYspectrumforallpD valuesrevealed thattheH6protonsoftheCDgiveintermolecularcontactswiththe FDPX’sIdoA2SH1proton–asshowninFig.5forthepD2.0sam- ple.NOEexperimentswereperformedatseveralFDPX:CDmolar ratios,however,noneoftheexperimentsconfirmeddipolarcorre- lationsbetweentheinnerprotonsoftheCDandFDPX.Henceonly
outer-sphere,electrostatics-drivencomplexformationcanoccur betweentheseoligosaccharidesasalsofoundinarecentstudy[23].
3.1.2. NMRinvestigationsofFDPXdegradation
Itiswellknownfromtheliterature,thatsulfatedpolysaccha- ridescanundergodegradation(depolymerizationanddesulfation) underacidicconditions. However,thereare nodetailed studies thatproviderelevantinformationonFDPX.Recordingthe1HNMR spectraofaqueousFDPXsampleatpD2.0afteraweekofstor- ageindicatedthatnew1Hresonancesappeared,seeFig.6.Inorder toidentifythestructureofthedegradationproduct,additional2D spectrawererecorded.Thespinsystemofthemonosaccharidesub- unitsofthedegradationproductwasidentifiedbyCOSYandTOCSY experiments, respectively. 1H–13C HSQC experiment evidenced thattheanomericcarbonoftheGlcNS3S6Sunit(ı=6ppm)and theC5resonanceoftheIdoA2Smoiety(ı=2ppm)exhibited remarkableshiftscomparedtotheintactFDPX(Fig.S6).A2DROESY experiment(Fig.S7)wasperformedtoestablishtheconnectivity ofthemonosaccharides.BasedontheROESYdatathepentasac- charidechainremainedintactduringtheappliedcircumstancesof degradation.Therefore,theobservedchemicalshiftchangesupon decomposition(Fig.S8)cannotbeattributedtothedepolymeriza- tionoftheFDPXbackbone.Consequently,assupportedbyearlier studiesontheacidhydrolysisofsulfatedpolysaccharides[24],sul- fatelossof FDPXoccurredinourcase. Setoet al. followedthe chemicalshiftchanges inheparinundergoingdesulfationby1H
Fig.3.Representative1HNMRchemicalshiftchanges(subplotA)oftheFDPX1Hresonancesupontitrationof3.4mMFDPXsolutionwithincreasingportionsofa20.1mM NH2--CDsolutionatpD7.4.TitrationprofilesofFDPXGlcNS6SH1andGlcNS3S6SH1nuclei(subplotB)atpD7.4,fittedbythe1:1complexationmodelusingtheOPIUM program(redcurves).
Fig.4.TitrationprofilesoftheanomericprotonsoftheFDPXGlcNS6SandGlcNS3S6SunitsatpD2.0.Bluecurveswerefittedbythe1:1complexationmodel,whileaglobal fittingassumingtheFDPX·CDandtheFDPX·2CDspeciesareshownbyredcurves.
NMR measurements[25].Theirresultalsosupportsourdata,as desulfationleadstodownfieldshiftofparticularresonances.The largest1HNMRchemicalshiftschangeswereobservedinthecase oftheIdoA2Sandthetrisulfated(GlcNS3S6S)subunits(Fig.S8), suggestingthatthesulfatelossprimarilyaffectedtheseunits.Fur- therinvestigationswereconductedtosupportthishypothesisby massspectrometry.
ToovercometheunwanteddesulfationofFDPXinsolution,the possibleprotectivefunctionofCDwasexplored.Todemonstrate thestabilizingeffectoftheCD,1HNMRspectraofthefreshlypre- paredpD2.0sampleswith1:0,2:1and1:1(FDPX:CD)molarratios wererecorded.Allsampleswereincubatedat25◦Cforaweek, then1HNMRspectrawerere-recorded.AsshowninFig.6,almost 50%oftheFDPXdegradedin theabsenceofCD. Thedecompo- sitionprocesssloweddownsignificantlyinthepresenceofhalf
Fig.5. Partial2DROESYNMRspectraofapD2.0samplecontainingFDPXandCDat1:1molarratio,showingcross-peaksbetweentheIdoA2SH1protonandtheCDH6 resonance.
Fig.6.The1HNMRspectraofthefreshlypreparedFDPX:CDsamples(day0)andthatofthesamesamplesstoredat25◦Cforaweek(day7)atdifferentmolarratios(red 1:0,blue2:1,green1:1)underpD2.0conditions.The1Hresonancesofthedecompositionproductareindicatedby*.
equivalent CD, as only 20% degradation could be observed by
1HNMR.However,samplesofequimolarFDPXandCDhowever revealednodetectabledegradationofFDPX.Applyingevenhigher molar ratiosof CD, completeprotectionof FDPXwasobserved, therefore, a minimum ofequimolar CD isnecessary toprevent desulfation.
ToacceleratethedecompositionofFDPX,sampleswiththesame molarratioswereincubatedat60◦C for14 h.1H NMRspectra wereregisteredpriorandaftertheincubationatroomtempera- ture(Fig.S9).ThesamplewithoutCDdegradedcompletely,while thosecontainingCDexhibitedlesspronounceddesulfation.Inthe presenceof1:1molarratiominordegradationcouldbeobserved, whilemolarexcessofCD(1:2molarratio)completelyhinderedthe desulfation.
3.2. StudyingFDPXdegradationbynESI-MS
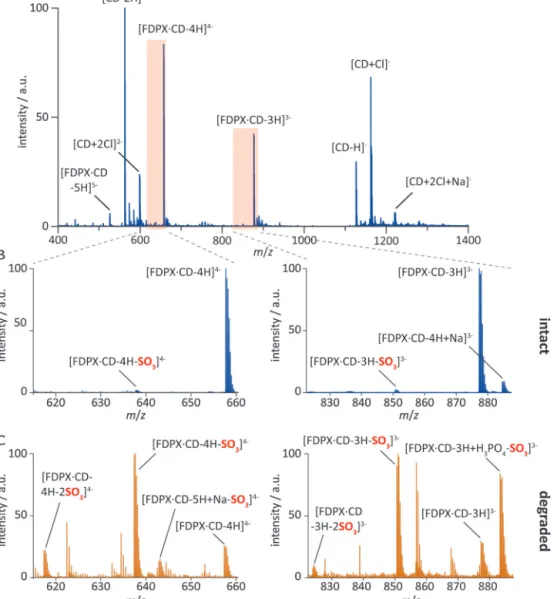
To study the complex formation between FDPX and CD by MS-based methods, the compounds were dissolved in a water:methanolmixture(50/50,v/v)ataconcentrationof20M forFDPXand200MforCD,yieldinga1:10molarratio.Arepre- sentativemassspectrumobtainedinnegativeionmodeisshown inFig.7a(non-informativepartsofthespectrumoutsidethem/z range of 400–1400 were omitted for clarity). No signal corre- spondingtounboundFDPXcouldbedetected,andthespectrumis dominatedbymasspeaksofsingly-anddoubly-chargedCDions,as wellasbytriply-andquadruply-chargedFDPX·CDcomplexesdis- playingexclusively1:1stoichiometry.Theassignmentofspecies alongwithm/zvaluesreflectingmonoisotopicmassesaregivenin
Fig.7. Complexformationoffondaparinuxanditsdegradationproductwithper-6-amino--cyclodextrinstudiedbynano-electrosprayionizationmassspectrometry.
(A)NegativeionmodenESImassspectrumofasolutioncontaining20Mfondaparinux(FDPX)and200Mper-6-amino--cyclodextrin(CD).UnboundCDionsand correspondingmasspeaks:[CD+2Cl+Na]−atm/z1220.41;[CD+Cl]−atm/z1162.45;[CD-H]−atm/z1126.47;[CD+2Cl]2-atm/z598.71and[CD-2H]2-atm/z562.73.
CD:FDPXcomplexionsandrespectivemasspeaks:[FDPX·CD-3H]3-atm/z877.14;[FDPX·CD-4H]4-atm/z657.6and[FDPX·CD-5H]5-atm/z525.88.(B)Insetshighlighting diagnosticpartsofthefullmassspectrumshownatthetop.ThelowextentofsulfatelossinthepresenceofCDisapparent:theintensityofmasspeakscorresponding to[FDPX·CD-3H-SO3]3-atm/z850.48and[FDPX·CD-4H-SO3]4-atm/z637.61areroughlytwoordersofmagnitudelowerthanthoseoftherespectiveintactspecies.(C) DiagnosticpartsofnegativeionmodenESImassspectraofasolutioncontainingfondaparinuxdegradationproducts(20M),spikedwith200MCD.Dominantspecies withonetotwosulfatelossesandtherespectivemasspeaks:[FDPX·CD-3H+H3PO4-SO3]3−atm/z883.14;[FDPX·CD-3H-SO3]3−atm/z850.48;[FDPX·CD-3H-2SO3]3−atm/z 823.83;[FDPX·CD-5H+Na-SO3]4−atm/z643.11;[CD·FDPX-4H-SO3]4−atm/z637.61and[FDPX·CD-4H-2SO3]4−atm/z617.62.Othermajorpeaksnotassignedinthefigure correspondtodeprotonatedCDionsoflowerchargestates(formingadductswithvariousnumbersofH3PO4andNa+),bearingnorelevancetothedegradationinquestion.
Allm/zvaluesgivenabovereflectmonoisotopicmasses.
thefigurecaption.ThefindingsareinagreementwithNMRspec- troscopicresults,allowingfortheconfidentdeterminationofthe FDPX·CD complex stoichiometrybasedonorthogonalanalytical methods.
In general,highly-sulfatedglycosaminoglycan(GAG)ionsare ratherfragileinthegasphase:thelossofneutralSO3 (79.96Da) uponionheatinginthesourceorduringcollisioninduceddissoci- ationisadominantdissociationchannelinMS-basedexperiments, posing a majordifficultyin theanalysisof heparin andrelated species[26–28].Interestingly,theFDPX·CDcomplexremainspre- dominantlyintactupontransferfromsolutiontothegasphase.This effectisanalogoustothepreventionofsulfateequivalentlosses bypairinghighly-sulfatedGAGswithbasicarginine-richpeptides in MS experiments [29,30]. Whenelectrosprayinga solution of FDPXinthepresenceofexcessquantitiesofCD,littlesulfatelossis
observedasshownexemplarilyinFig.7b.Thepeakscorresponding to[FDPX·CD-3H-SO3]3−and[FDPX·CD-4H-SO3]4- areweak,with intensitiesbelow5%ofthoseoftherespectiveintactcomplexes.
Theappearanceoftheseminorpeaksinthemassspectrumcanbe explainedbytwo,mutuallynon-exclusiveprocesses.Mostproba- bly,somelossofneutralSO3occursfromtheintact[FDPX·CD-3H]3−
and [FDPX·CD-4H]4- ions duringthe MSexperiment. The other possibilityis thatpartiallydegradedFDPXisalready present in solution,formingacomplexwiththecyclodextrin.
ThestabilizingeffectexertedbyCDonthesulfategroups of FDPXnotonlyfacilitatedtheanalysisoftheintactFDPX·CDcom- plex,butalsoprovedtobehighlyadvantageousforthestructural characterizationofthefondaparinuxdegradationproducts.
MSexperimentswereusedtomonitorthesulfatelossinFDPX uponincubationinacidicmedia.Forthesedegradationmeasure-
mentssampleswereincubatedasdescribedinSection2.3.Briefly, a phosphatebufferedpD 2.0solutionof 5mMFDPXwasincu- bated at60◦C for14h, dilutedinwater:methanol(50/50,v/v), thenspikedwiththecationiccyclodextrintoreachaconcentration of20MfortheFDPXdegradationproducts(calculatedforthe pre-degradationFDPXcontent)and200MforCD.Underexperi- mentalconditionsidenticaltothoseappliedtorecordthespectrum displayedin Fig.7aand b(bluetrace),markedly differentmass spectrawereobtainedforthesamplecontainingFDPXdegrada- tionproducts, ashighlightedinFig.7c(yellowtrace).Owingto potentialdifferencesintheionization/iontransmissionefficiency of thevarious species, exact quantitative information may not beextractedfromtheMSexperiments.However,thedominance of mass peakscorresponding toFDPX-related ionswithsulfate losses,suchas[FDPX·CD-3H+H3PO4-SO3]3−,[FDPX·CD-3H-SO3]3−, [FDPX·CD-3H-2SO3]3−, [FDPX·CD-5H +Na-SO3]4-,[FDPX·CD-4H- SO3]4-and[FDPX·CD-4H-2SO3]4-,isclearlyvisibleinthespectra inFig.7c.CombiningtheseresultswiththefindingthatCDcom- plexationefficientlyhindersneutrallossofSO3fromFDPXinthe gasphase,wecanconcludethatthemassspectrareflectthecom- positionofthesolutionandthedistributionofspeciestherein.That is,theintensesignalscorrespondingtotheionslistedabovearenot artefactscausedbygas-phaseSO3lossduringtheMSexperiment, butinsteadcorrespondtodegradationproductsalreadypresentin solution.
In conclusion,resultsofMSexperimentssupportNMRspec- troscopic findings,enablingtheunambiguousassignmentofthe FDPX·CD complexstoichiometry.Inaddition,utilizingthebene- ficialstabilizingeffectofCD-complexationonthesulfategroups ofFDPXinthegasphase,MSprovidesevidencethatthedegrada- tionproductsofFDPXarepentasaccharideswithanintactglycan backbone,displayingpredominantlyone,andtoalesserextenttwo sulfatelosses.
4. Conclusions
Inthepresentstudy,theintermolecularinteractionsoffonda- parinuxwithheptakis(6-amino-6-deoxy)-beta-cyclodextrinwere characterizedextensivelybyNMRspectroscopyinsolutionandby nESI-MSinthegasphase.NMRspectroscopicstudiesrevealed1:1 stoichiometryandmoderateaffinity(logˇ11=3.65±0.02)atpD 7.4,whileatpD2.0thermodynamicallymorestablespecieswere deduced:FDPX·CD(logˇ11≥4.9±0.1)andFDPX·2CD(logˇ12≥8).
ThepossibleinteractionsiteinvolvingtheIdoA2Smoietyandthe cationicpartofthecyclodextrinhasalsobeenlocalizedbasedon2D NMRstudies.Anin-depthcharacterizationoftheacidicdegrada- tionofFDPXbyNMRandMSexperimentssuggesteddesulfationof thepentasaccharidebackboneatpD2.0.Undertheseconditions, theheptacationiccyclodextrinsuccessfully preventssulfate loss bystrongelectrostaticinteractions.Thus,ourfindingscontribute to a better understanding of heparin stabilization under acidic conditions,offeringanefficientmethodtopreventtheunwanted decompositionofheparinoids.
CRediTauthorshipcontributionstatement
BiankaVárnai:Formalanalysis,Investigation,Writing-original draft,Writing - review &editing,Visualization. Márkó Grabar- ics: Investigation, Writing - original draft, Writing - review &
editing,Visualization.ZoltánSzakács:Methodology,Formalanal- ysis, Writing - review & editing, Visualization. Kevin Pagel:
Resources,Writing-review&editing,Fundingacquisition.Milo Malanga:Investigation,Writing-review&editing.TamásSoha- jda:Resources,Writing-review&editing,Fundingacquisition.
DeclarationofCompetingInterest
Theauthorsdeclarethattheyhavenoknowncompetingfinan- cialinterestsorpersonalrelationshipsthatcouldhaveappearedto influencetheworkreportedinthispaper.
Acknowledgments
S.B.thanksthefinancialsupportfromtheJánosBolyaiResearch ScholarshipoftheHungarianAcademyofSciencesandfromthe Bolyai+NewNationalExcellenceProgramoftheMinistryofHuman Capacities(ÚNKP-20-5-SE-31).M.G.andK.P.gratefullyacknowl- edgefinancialsupportfromtheFreieUniversitätBerlin,theMax PlanckSocietyandtheGermanResearchFoundationundergrant numberFOR2177/P03.M.M.andT.Saregratefulforthesupport oftheHungarianNationalResearch,DevelopmentandInnovation Office(OTKAK-125093).
ThanksareduetoProf.CsabaSzántayJr.forhissupportofthe 800MHzNMRmeasurementsatGedeonRichterPlc.
AppendixA. Supplementarydata
Supplementarymaterialrelated tothis article canbefound, in theonline version, at doi:https://doi.org/10.1016/j.jpba.2021.
113947.
References
[1]R.J.Linhardt,2003ClaudeS.Hudsonawardaddressincarbohydrate chemistry.Heparin:structureandactivity,J.Med.Chem.46(2003) 2551–2564,http://dx.doi.org/10.1021/jm030176m.
[2]C.J.Jones,S.Beni,J.F.K.Limtiaco,D.J.Langeslay,C.K.Larive,Heparin characterization:challengesandsolutions,Annu.Rev.Anal.Chem.4(2011) 439–465,http://dx.doi.org/10.1146/annurev-anchem-061010-113911.
[3]B.Mulloy,J.Hogwood,E.Gray,R.Lever,C.P.Page,Pharmacologyofheparin andrelateddrugs,Pharmacol.Rev.68(2015)76–141,http://dx.doi.org/10.
1124/pr.115.011247.
[4]E.Gray,B.Mulloy,T.W.Barrowcliffe,Heparinandlow-molecular-weight heparin,Thromb.Haemost.99(2008)807–818,http://dx.doi.org/10.1160/
TH08-01-0032.
[5]H.Naimy,N.Leymarie,M.J.Bowman,J.Zaia,Characterizationofheparin oligosaccharidesbindingspecificallytoantithrombinIIIusingmass spectrometry,Biochemistry47(2008)3155–3161,http://dx.doi.org/10.1021/
bi702043e.
[6]M.Schroeder,J.Hogwood,E.Gray,B.Mulloy,A.M.Hackett,K.B.Johansen, Protamineneutralisationoflowmolecularweightheparinsandtheir oligosaccharidecomponents,Anal.Bioanal.Chem.399(2011)763–771, http://dx.doi.org/10.1007/s00216-010-4220-8.
[7]M.A.Crowther,L.R.Berry,P.T.Monagle,A.K.C.Chan,Mechanismsresponsible forthefailureofprotaminetoinactivatelow-molecular-weightheparin,Br.J.
Haematol.116(2002)178–186,http://dx.doi.org/10.1046/j.1365-2141.2002.
03233.x.
[8]B.Kalaska,K.Kaminski,E.Sokolowska,D.Czaplicki,M.Kujdowicz,K.
Stalinska,J.Bereta,K.Szczubialka,D.Pawlak,M.Nowakowska,A.Mogielnicki, Nonclinicalevaluationofnovelcationicallymodifiedpolysaccharide antidotesforunfractionatedheparin,PLoSOne10(2015)1–21,http://dx.doi.
org/10.1371/journal.pone.0119486.
[9]L.Szente,J.Szemán,Cyclodextrinsinanalyticalchemistry:host-guesttype molecularrecognition,Anal.Chem.85(2013)8024–8030,http://dx.doi.org/
10.1021/ac400639y.
[10]L.Liu,Q.X.Guo,Thedrivingforcesintheinclusioncomplexationof cyclodextrins,J.Incl.Phenom.Macrocycl.Chem.42(2002)1–14,http://dx.
doi.org/10.1023/A:1014520830813.
[11]S.Hbaieb,R.Kalfat,Y.Chevalier,N.Amdouni,H.Parrot-Lopez,Influenceofthe substitutionof-cyclodextrinsbycationicgroupsonthecomplexationof organicanions,Mater.Sci.Eng.C28(2008)697–704,http://dx.doi.org/10.
1016/j.msec.2007.10.013.
[12]F.D’Anna,S.Riela,P.LoMeo,M.Gruttadauria,R.Noto,Thebinary pyrene/heptakis-(6-amino-6-deoxy)--cyclodextrincomplex:asuitable chiraldiscriminator.Spectrofluorimetricstudyoftheeffectofsome␣-amino acidsandestersonthestabilityofthebinarycomplex,Tetrahedron Asymmetry13(2002)1755–1760,http://dx.doi.org/10.1016/S0957- 4166(02)00418-4.
[13]P.Job,Formationandstabilityofinorganiccomplexesinsolution,Ann.Chim.
(Paris)9(1928)113–203.
[14]L.Fielding,Determinationofassociationconstants(K(a))fromsolutionNMR data,Tetrahedron56(2000)6151–6170,http://dx.doi.org/10.1016/S0040- 4020(00)00492-0.
[15]SolutionEquilibriaAnalysiswiththeOPIUMComputerProgram,Thefull versionoftheOPIUMprogramisavailable(freeofcharge)at
http://web.natur.cuni.cz/∼kyvala/opium.html(accessedon19.September, 2020).
[16]J.C.L.Meeussen,Orchestra:anobject-orientedframeworkforimplementing chemicalequilibriummodels,Environ.Sci.Technol.37(2003)1175–1182, http://dx.doi.org/10.1021/es025597s,ThefullversionoftheORCHESTRA programisavailable(freeofcharge)athttp://orchestra.meeussen.nl/
(accessedon19.September,2020).
[17]D.Neuhaus,M.P.Williamson,TheNuclearOverhauserEffectinStructuraland ConformationalAnalysis,2nded.,Wiley-VCH,2000.
[18]G.Wenz,C.Strassnig,C.Thiele,A.Engelke,B.Morgenstern,K.Hegetschweiler, Recognitionofionicguestsbyionic-cyclodextrinderivatives,Chem.-AEur.
J.14(2008)7202–7211,http://dx.doi.org/10.1002/chem.200800295.
[19]H.M.Wang,D.Loganathan,R.J.Linhardt,DeterminationofthepK(a)of glucuronicacidandthecarboxygroupsofheparinby
13C-nuclear-magnetic-resonancespectroscopy,Biochem.J.278(1991) 689–695,http://dx.doi.org/10.1042/bj2780689.
[20]W.Al-Soufi,P.R.Cabrer,A.Jover,R.M.Budal,J.V.Tato,Determinationof second-orderassociationconstantsbyglobalanalysisof1Hand13CNMR chemicalshifts,Steroids68(2003)43–53,http://dx.doi.org/10.1016/s0039- 128x(02)00114-9.
[21]F.Ulatowski,K.Dabrowa,T.Bałakier,J.Jurczak,Recognizingthelimited applicabilityofjobplotsinstudyinghost-guestinteractionsin
supramolecularchemistry,J.Org.Chem.81(2016)1746–1756,http://dx.doi.
org/10.1021/acs.joc.5b02909.
[22]D.BrynnHibbert,P.Thordarson,ThedeathoftheJobplot,transparency,open scienceandonlinetools,uncertaintyestimationmethodsandother developmentsinsupramolecularchemistrydataanalysis,Chem.Commun.
(Camb.)52(2016)12792–12805,http://dx.doi.org/10.1039/c6cc03888c.
[23]S.J.Berners-Price,A.K.Gorle,T.Haselhorst,S.J.Katner,A.VEverest-Dass,D.
James,E.J.Peterson,J.E.Koblinski,K.Takabe,M.VonItzstein,P.Nicholas, AngenwandteChemie,(n.d.).https://doi.org/10.1002/anie.202013749.
[24]A.Kalsson,S.K.Singh,Acidhydrolysisofsulphatedpolysaccharides.
Desulphationandtheeffectonmolecularmass,Carbohydr.Polym.38(1999) 7–15,http://dx.doi.org/10.1016/s0144-8617(98)00085-x.
[25]S.P.Seto,T.Miller,J.S.Temenoff,Effectofselectiveheparindesulfationon preservationofbonemorphogeneticprotein-2bioactivityafterthermal stress,Bioconjug.Chem.26(2015)286–293,http://dx.doi.org/10.1021/
bc500565x.
[26]M.J.Kailemia,L.Li,M.Ly,R.J.Linhardt,I.J.Amster,Completemassspectral characterizationofasyntheticultralow-molecular-weightheparinusing collision-induceddissociation,Anal.Chem.84(2012)5475–5478,http://dx.
doi.org/10.1021/ac3015824.
[27]R.Huang,C.Zong,A.Venot,Y.Chiu,D.Zhou,G.J.Boons,J.S.Sharp,Denovo sequencingofcomplexmixturesofheparansulfateoligosaccharides,Anal.
Chem.88(2016)5299–5307,http://dx.doi.org/10.1021/acs.analchem.
6b00519.
[28]R.L.Miller,S.E.Guimond,R.Schwörer,O.V.Zubkova,P.C.Tyler,Y.Xu,J.Liu,P.
Chopra,G.J.Boons,M.Grabarics,C.Manz,J.Hofmann,N.G.Karlsson,J.E.
Turnbull,W.B.Struwe,K.Pagel,Shotgunionmobilitymassspectrometry sequencingofheparansulfatesaccharides,Nat.Commun.11(2020)1–12, http://dx.doi.org/10.1038/s41467-020-15284-y.
[29]G.Venkataraman,Z.Shriver,R.Raman,R.Sasisekharansequencingcomplex polysaccharides,Science286(1999)537–542,http://dx.doi.org/10.1126/
science.286.5439.537.
[30]J.Zaia,Glycosaminoglycanglycomicsusingmassspectrometry,Mol.Cell Proteomics12(2013)885–892,http://dx.doi.org/10.1074/mcp.R112.026294.