Nose-to-brain delivery of
antiglioblastoma drugs embedded into lipid nanocarrier systems: status quo and outlook
Fakhara Sabir
Q11
, Ruba Ismail and Ildiko Csoka
UniversityofSzeged,FacultyofPharmacy,InstituteofPharmaceuticalTechnologyandRegulatoryAffairs,H-6720Szeged,Eötvösu.6,Hungary
Gliobla
Q13
stoma (GBM) is one of the most devastating and deadly types of tumor. Among all the present treatment strategies, the utmost prerequisite is prolonged intervention at the malignant site. The blood–brain barrier (BBB) is the bottleneck in the delivery of ant
Q14
i-GBM drugs and invasive treatment
comes with many pitfalls. This review will discuss the potential of embedding antitumor drugs into nanocarriers for intranasal delivery. Additionally, it emphasizes the significance of applying quality by design (QbD) methodology from the early development stages to ensure the high quality, safety and efficacy of the developed carrier system.
Introduction
Malignant gliomas(MGs) arethe most lethal formsof primary centralnervoussystem(CNS)malignancy,classifiedbasedonan augmenting level of undifferentiation, anaplasia and prolifera- tion. WHO classified gliomas intofour clinical grades: grade I (astrocytoma); grade II (diffuse astrocytoma, the most distin- guished form); grade III (anaplastic variants of astrocytoma);
and grade IV (glioblastoma) [1]. Pleomorphic glioblast
Q15 oma is
calledglioblastomamultiforme(GBM)becausethesemalignant cellsshow a discrepancy in structureand morphology [2].The currenttreatmentstrategiesforGBMincludesurgery,radiothera- pyandchemotherapy.Thefocus ofresearcherstotreatGBM is challenging because surgery and radiotherapy are not good optionsbecauseofitstopographicallydiffusenature[3].Eventu- ally,understandingthepatternofspreadofindividualmalignant cellsoverlongdistancesandintopartsofthebrainisessentialfor patientsurvival.Apresentliteraturesurveyrevealsthatthereare just a few available therapies that could significantly improve survivalchances[4].Thecircumventionoftheblood–brainbarrier (BBB)throughstraightinterventionintoinsubstantialbrain tis- sues can result in severe neurotoxicity and loss of brain key functionality. Consequently, there is a need to design a more specificandrational(noninvasive)approachtotargetGBM.Itis alsonecessarytoexplorethepotentialdifferencesinpermeability
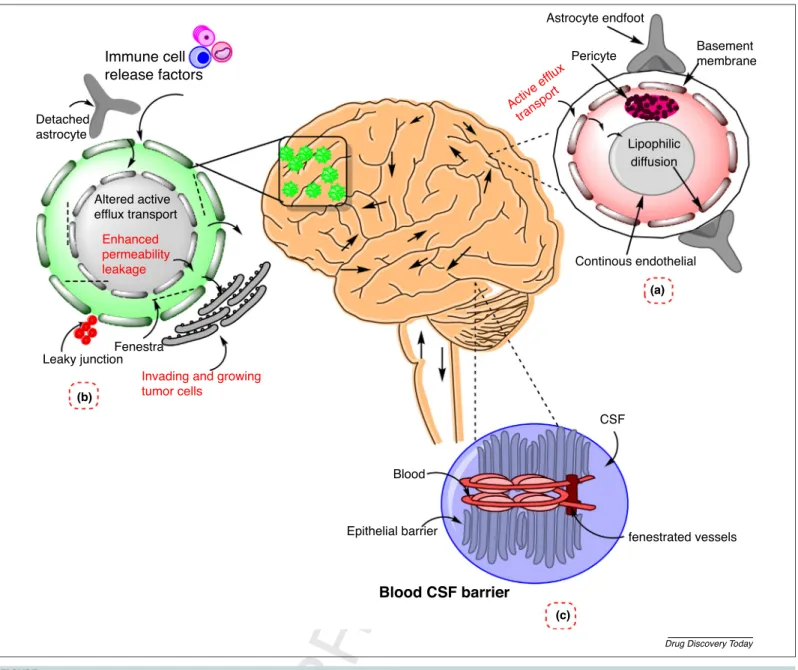
betweenthe intactand malignant brain toovercome the chal- lengesinbraintargeting[5].Figure1demonstratesthedifferences betweenbarriersintheintactbrainandinglioblastoma.
Theintranasalrouteisadirectandsimpleapproach,including many advantagesofhigher bioavailability,shorter onsetofac- tion, circumventionof systemic toxicity, noninvasivenessand clearance. Additionally, avoiding the BBB could significantly increase the concentration of theactive pharmaceutical agent inthecentralnervoussystem(CNS).Accordingtodatapresentin the literature, pharmacological active agents can be delivered throughthenasalcavityviathetrigeminalandolfactorynerves.
Drugpermeationisbasicallydependentuponthekeycharacter- istics of an active agent or carrier, like its metabolic stability, solubility,residencetimeinthemucouslayerandrateofmuco- ciliaryclearance[6].
Thesafetyandtoxicologicalevaluationofproductsdelivered intranasally is of great importance. The prolonged contact of formulationscontainingcytotoxicmaterialscancauseciliotoxi- city,tissuedamageandirritation[7].Therefore,regardlessofthe presence of carrier-free approaches for intranasal delivery, a carriersystemcouldbeusefultodeliverchemotherapeuticsvia theintranasalroute; amongalldeliverysystems, nanoparticle- basedcarriershavebeenintenselystudiedforresearchimaging, treatment anddiagnosis ofbrain tumors. Lipid-basedcolloidal systems, for example liposomes and solid lipid nanoparticles, increased drug transfer to the brain through the intranasal
ReviewsPOSTSCREEN
Correspondingauthor:Csoka,I. (csoka@pharm.u-szeged.hu)
1359-6446/ã2019PublishedbyElsevierLtd.
route[8].Forinstance,invitrohemolysisandcytotoxicitystudies ofdoxorubicin(DOX)-loadedliposomalnanoparticleswereper- formed,whichresultedinspecificityandenhancedlevelsofdrug accumulation in gliomas [9,10].Colloidal nanocarrier systems [liposomes, solid lipidnanoparticles (SLNs), lipoproteins,lipo- plexes, etc.] have shown clear amassing in gliomas but the shortening ofnoninvasive accumulationandretentionevalua- tiontoolscouldhindermonitoringtheexactdurationandloca- tionofnanoparticleswithinthebrain[11].Thisreviewwillfocus onthepotentialoflipidnanocarriersystemstodeliveranticancer drugs via the intranasal route, as well as the significance of applyingqualitybydesign(QbD)softwareforthetargeteddeliv- eryofcytotoxicmaterialsviatheintranasalroutetomaintainthe safetyandprod
Q16 uctprofile.
Strategies to circumvent the BBB: bottleneck in targeting glioblastoma
Scientists have been working to develop versatile methods to circumvent the BBB, which include the opening of the BBB, intranasaldeliveryandpenetrationviatheBBBbycellularinter- nalization.Theoverexpressionofreceptors(likelow-densitylipo- protein, nicotinic acetylene choline, insulin-like growth factor (IGF),transferrinreceptors,diphtheriatoxin,leptinandscavenger receptortypeB)hasbeenreportedontheBBB.Thespecificligand functionalizationandattachmentcaninterveneindrugtransport viatheBBB.Thispreciseandsensitivetypeofinteractionbetween ligands and receptors governs receptor-mediated transport [12–14].However,therearelimitationsin implementationofa functionalizedorspecificligandattachedmoiety.First,itcanlose Immune cell
release factors
Blood
Epithelial barrier fenestrated vessels
CSF
Continous endothelial Astrocyte endfoot
Pericyte Basement
membrane
Lipophilic diffusion
Fenestra Leaky junction
Invading and growing tumor cells
Enhanced permeability leakage Altered active efflux transport Detached
astrocyte
Active ef flux
transport
(a)
(b)
(c)
Blood CSF barrier
Drug Discovery Today
FIGURE1 Challengesinbloo
Q1 dtobraindeliveryinabraintumor.Thefigureillustratesthecomparisonbetweenbarriersinnormalbrainandinglioblastomamultiforme (GBM).(a)Normalblood–brainbarrier(BBB)composedof:astrocyte(roleinmorphology),pericyte,endothelialcells(roleintightjunctionstructureand vasoregulation).(b)Blood–tumorbarrier(deatchedastrocyte,fenestra,leakyjuctions–bloodvesselsthatsupplythetumorareleakyandincompletelyformed butthehealthybraincomponentsarestillpresentinthemainregionofGBM).(c)Blood–cerebrospinal-fluid(CSF)barrier(composedofachoroidplexushaving epithelialcellsandtightjunctions,increasedlevelofalbuminintheCSFinGBMwhichmightcausedisturbanceoftheBBBorreleasefromtumor).
ReviewsPOSTSCREEN
itstherapeuticactivity;second,allpresentstrategiesareinvasive andaccumulationofdrugcargosintheliverandotheroff-target sitesgover
Q17 nsitstherapeuticefficacy[7].Therefore,thereisaneed fornoninvasivedeliveryapproachestoachievethebesttherapeu- ticgoals.
An alternative route of administration
An alternative route of administration to CNSdrug delivery is intranasaladministration.Theintranasaldelivery(IND)routeisa noninvasive, direct and more effective route of administration thanintravenous(i.v.) deliveryandcanavoidtheBBB together with systemic side effects. The IND pathways (trigeminal and olfactory pathways) in the nasal cavity are reasons for direct deliverytothebrainandresultingoodpharmacokinetic/pharma- codynamic(PK/PD)profilesforCNSdrugs.Drugdeliveryfromthe noseviathetrigeminalpathwayfollowseitheraxonalorendocy- totictransport,whereastheolfactorypathwayisfurtherdivided intointraneuronaland extraneuronal pathways.The intraneur- onalpathwayfollowsaxonaltransportandittakeshoursordays fortheAP
Q18 Itoreachthetargetsite,whereastheextraneuronalpath followstheperineuralrouteanditjusttakesafewminutestoreach thetargetsite[15,16].Furthermore,thisdeliveryroute isanew approachforthedeliveryofpotentactiveagentsandforantineo- plastic agentsthat can beloadedinto nanocarriersto ensurea bettersafetyprofile.Byusingthisroute,nanoparticlescancarry drugseasilytothetargetsite,andcanbypassthemainbarriers:the BBBandtheblood–cerebrospinal-fluidbarrier(BCSFB).Thereare manystudiesrevealingbettertargetdeliveryofCNSdrugsviathe intranasalrouteincontrasttoi.v. administration.Schio
Q19 thetal.
reported that IGF-1, when givenintranasally, had greater CNS efficacywhencomparedwithi.v.intervention.Manyotherstudies alsoshowedthattheintranasaldeliveryoftheAPIledtobetter cure rates of CNS diseases, such as depression, autism, eating disorders, Parkinson’s disease (PD) and Huntington’s disease (HD),aswellasvariousotherdiseases yettobetreated.Besides theseadvantages,thereisalonglistoffactorsthatcanlimitthe permeabilityofdrugcarriersviatheintranasalroute.Therefore, whiledesigningintranasalformulations,thefactorsregardingthe anatomyandthephysiologyofthenasalcavityshouldbeconsid- ered.Thevibrissaeofthenasalvestibuleandthetransepithelial regionoftheatrium(narrowestregion)arethepartsthatarethe leastpermeable.Bycontrast,otherpartslikethesuperior,middle andinferiorturbinateoftherespiratoryregionaremoreperme- able,whereasthespecializedciliatedolfactorynervecellsofthe olfactoryregionhavedirect accessto theCSF. Table1explains how the structures of the nasal cavity affect permeability via the intranasal route [16,17]. By considering the crucial factors
affecting drug delivery via the nasal cavity, a formulation can bedesignedfornose-to-braindeliverywithincreasedpermeation, low clearance and high mucoadhesion by the application of theQbD[18].
Nanocarrier systems for nose-to-brain delivery
Thesafetydataforintranasalformulationsareofgreatimportance.
Nanoparticleshavethepotentialtoimprovenose-to-braindeliv- ery because they have can avoid enzymatic degradation and transportfromP-glycoprotein(P-gp)effluxproteins.Thesecarrier systemscanenhancetherapeuticbraindeliveryby usingbioad- hesivematerialsandalsobyopeningtheclosedjunctionsofthe nasal epithelial membrane. The transport of nanoparticles through the intranasal route takes place via olfactory neurons by endocytoticorneuronalpathways.Theconfocalmicroscopy study of polystyrene nanoparticles reported that nanocarriers within the range 20–200nm can follow clathrin-coated pits;
however, nanoparticles in the size range 200–1000nm can be transportedviacaveolae-mediatedendocytosis[7,15].
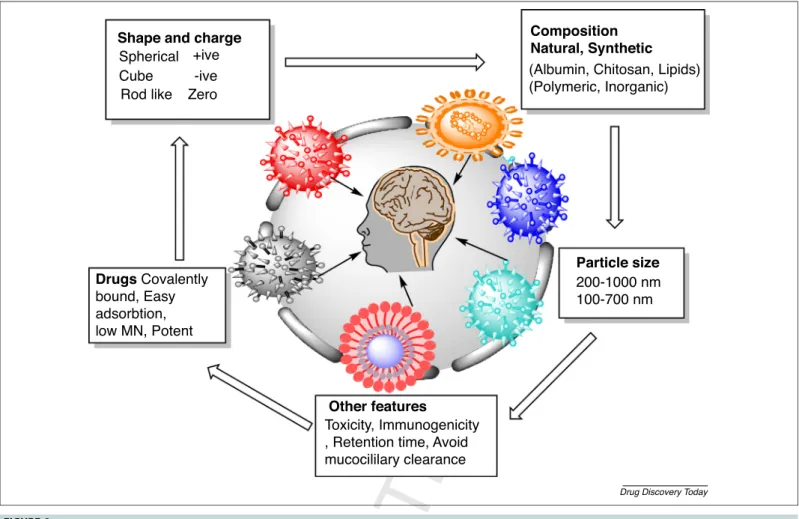
Nanoparticlescanalsofollowthetransport fromendothelial cells to olfactory neurons via endocytosis or pinocytosis and move along the axon. For this transport pathway, thesize of nanoparticlesshouldbewithinthediameteroftheaxon,whichis upto 100–700nm. Therefore, theintranasal deliveryof nano- particlescouldbeapromisingchoicefortargetinglife-threaten- ingdiseaseslikeglioblastoma.Regardingthecarriersystems,theQ20 essentialclassesofnanoparticlesthatareatthecenteroffocusfor braintargetingincludepolymericnanoparticlessuchasmicelles, ironoxidenanoparticles,goldnanoparticles,quantumdotsand lipid-based nanoparticles likenanolipid carriers (NLCs),SLNs, liposomes,lipoplexesandlipoproteins[19].Ingeneral,nanopar-Q21 ticlescaninducetoxicitydependingupontheirinternalization siteandcomposition. Itisalsoreportedthatnanoparticlescan induceinflammation,DNAdamageandoxidativestress.TheIND of metal nanoparticles into brain is relatively wellknown for harmful neurological effects. The extensive exposure of metal nanoparticles can cause serious damage and even can lead to diseasessuchasAlzheimer’sdisease(AD)andparkinsonism.The present study is relevant to lipid nanoparticles that are most biocompatible andleasttoxicin nature.Clearance ofnanopar- ticles from the brain occurs via the mononuclear phagocytes system (MPS). The aspects of toxicity and clearance via macrophages are not accurate and might be caused by variations in nanoparticles properties (like charge, shape, size, coating)anduseofdifferentquantificationmethods[20].Figure2 explains the nanoparticle attributes influencing drug delivery viatheintranasalroute.
TABLE1
Impactofvariousstruct
Q6 uralcharacteristicsofnasalcavityonpermeation
Structuralparts/regionofthenasalcavity Effectonpermeation Refs
Nasalhairs(vibrissae)ofthenasalvestibule(sebaceousglands) Leastpermeableowingtothepresenceofkeratinizedcells [17]
Transepithelialregionoftheatrium(narrowestregion) Lesspermeablebecauseithasasmallsurfaceareaandstratifiedcellsare presentanteriorly
[35]
Superior,middleandinferiorturbinateoftherespiratoryregion Mostpermeableregionduetogreatersurfaceareaandincreasedblood supply
[36]
Specializedciliatedolfactorynervecellsoftheolfactoryregion Directpathwaytocerebrospinalfluid(CSF) [35]
Ciliatedcellsandsquamousepithelialcellsofthenasopharynx Nasalcavitydrainagereceiver [17]
ReviewsPOSTSCREEN
Lipid nanocarriers: a promising approach for intranasal delivery
For significant nose-to-brain delivery, a colloidal drug delivery system is the most suitablesystem that refersto increased bio- availabilityandsustainedreleaseandhighstabilityoftheAPI.The enhancedbiocompatibility,highscalability,safetyandefficacyof lipidnanoparticlesmadethemsuperiorcarriersfornose-to-brain delivery.Incontrasttolipidnanopar
Q22 ticles,polymericnanoparti-
cleshavelowscalability,highcytotoxicity(e.g.,100%mortality was reported when cells were treated with polyester polymer nanoparticles)andpoortolerability.Mostlipidnanoparticleshave aparticlesizewithintherange50–1000nm.Asdescribedinthe previoussection,aparticlesizebetween50and700nmisthemost favorable for intranasal delivery via neuronal transport [15].
Amongtheselipidcarriers,liposomesandSLNsdiscussedinthe following section have shown greater therapeutic efficacy in GBM. The lipidnanoformulations of anticancer agentsprovide enhanceddrugstability,PK,drugdistributionandefficacycom- paredwithotherapproaches[7].
Therapeutic efficacy of lipid nanoparticles in targeting glioblastoma
Liposomes, SLNs, lipoproteins, lipoplexes and nanostructured lipid carriershave been widely usedfortargeting glioblastoma.
Liposomesarebilayersphericalbiocompatiblecarriersandthese lipid-basednanostructuresareactuallythepioneersoflipid-based particlesemployedforparenteral deliverywith diameter ranges from10to 1000nm and areprecursorsofNLCsandSLNs[21].Q23 Table2summarizestheuseofliposomesinthemanagementof MGsviadifferentdeliveryroutes.Theresultsofallthesestudies revealedanincreaseinsurvivaltimealongwiththeinhibitionof proliferation[22].Itshouldbeconsideredthatvariousliposomal factorslikethesize,particlediameteranduptakebytheMPScan becrucialfortargeting[23].
Lipid nanoparticles including SLNs and NLCs are the most suitableamongallotherlipidnanoparticles,withoutbeingcon- strainedbytheirlimitations.Thestealthabilityoflipidnanopar- ticlesishigherthanotherpolymericnanoparticlesagainsttheMPS becausetheyarefabricatedfrombiocompatiblesubstancessuchas abrewofnaturallipids[24].Table3demonstratesthetherapeutic efficacyofSLNsandNLCsintargetingglioblastoma.Theresultsof all previousstudiesrevealed the decrease intumor growth,the increaseinthelifespanoftheanimalsusedandtheinhibitionof cellproliferation.
Theprovenefficacy(fromstudiesmentionedinTables2and3) oflipidnanoparticlesintargetingglioblastomahasshowntheir potentialtoencapsulateanticancerdrugs.Furthermore,theselipid nanoparticleshaveagoodabilitytoprotecttheloadedAPIand, Shape and charge
Drugs Covalently bound, Easy adsorbtion, low MN, Potent
Other features
Particle size Composition Natural, Synthetic Spherical +ive
Cube Rod like
-ive Zero
Toxicity, Immunogenicity , Retention time, Avoid mucocililary clearance
200-1000 nm 100-700 nm (Albumin, Chitosan, Lipids) (Polymeric, Inorganic)
Drug Discovery Today
FIGURE2 Nanoparticleattrib
Q2 utesinfluencingthedeliveryofAPIviatheintranasalroute.Thefigureillustratesthesignificantfeaturesofnanoparticlesforintranasal deliverylikepysiochemicalproperties(size,charge,composition)andothersignificantfeaturessuchaslowimmunogenicity,lowtoxicity,avoidingmucociliary clearanceand
Q3 increasedretentiontime(highmucoadhesivity).
ReviewsPOSTSCREEN
because of their occlusive nature, they can also increase nasal retentiontime.Thesefeaturesmakelipidnanoparticlesasignifi- cantcarrierfornose-to-braindeliverybecausetheycanavoidthe cytotoxicityissuesofantineoplasticdrugs[7,25].
Novel lipid nanoparticle formulation for targeting glioblastoma via IND
Newdeliveryapproachesare requiredin researchto efficiently target brain tumors. Curcumin-loaded NLCs (CUR-NLC) for TABLE2
Applicationsofliposomesinthemanagementofglioblastomamultiforme(GBM)
Encapsulatedsubstance Modelofstudy Typeofliposomes Centralnervoussystem(CNS)action/effects onGBM
Refs
Doxorubicin Clinicalstudy PEG-liposomes Enhancedefficacy
Inhibitionoftumorgrowth Enhancedsurvivaltime
[37]
Interferon(IFN)-b
Plasmid
Clinicalstudy Cationicliposomes Antiproliferative Reductionoftumorsize
[38]
Recombinentherpes simplex
virusthymidineskinase (adenoviralcarrier)
Gliomamodelin mouse
Cationicliposomes Reducedimmunogenicity Antiproliferative
[39]
Antisensegrowthfactor Human
malignantglioma celllines
Cationicliposomes Inhibitionoftumorgrowth [40]
Lomustine Rabitglioma
model
Temperature-sensitive liposomes(TSL)
Thermotargetingwithinhibitionoftumorgrowth [41]
EPIplu
Q7 scelecoxib Mice PTDpeptideattachedliposomes Destructionofgliomavasculogenicmimicrychannels [42]
Q8PTX Mice Dualtargeting,cellpenetrating
peptidattachedliposomes
Selectivetargeting Inhibitionoftumorgrowth
[43]
siRNA Mice Ligand-targetedliposomes(CTX) Enhancetheefficacyandinternalizationintoglioma cells
[40]
DNRandquinacrine Mice Ligand-targetedliposomes
(WGAandTAM)
Killingofglioblastomacellsanddimishingbrain gliomas
[44]
DOXandironoxid
Q9 e Mice Ligand-targetedliposomes(RGD) Enhancetargetingabilityandsite-specificdelivery [45]
Irinotecan Rats Ligand-targetedliposomes Inhibittumorgrowthincreaselifespanofrats [46]
DOX Rats Lactoferrinliposomes Destructionoftumorcellsandsignificantenhanced
survivalrateintumor-bearingrats
[47]
TABLE3
SummaryofapplicationsofSLNs/NLCsintargetingglioblastomamultiforme(GBM)
Encapsulatedsubstance Modelofstudy TypeofSLN/NLCs Centralnervoussystem(CNS)action/
effectsonGBM
Refs
Carmustine U87cellline(Invitrohumanbrainmodel) Cationicsolidlipidnanoparticles (CASLNs)
Antiproliferativeeffect
Decreaseexpressionoftumornecrosis factor(TNF)-a
[48]
Doxorubicinandetoposide U87celllines,HBMEC,humanastrocytes CASLNs Significantreductionintumorgrowth [49]
Edelfosine(EDF) GliomacelllineC6invivoC6glioma xenografttumor
SLNs(composedofcompritolor) Theantimalignanteffect,inhibitionof tumorgrowth
[50]
Cytarabine(CRB) EL-4celllines NLCs Cytotoxiceffectontumorcellline [51]
siRNAs U87MGcelllinesandtumorxenograftforin vivostudy
Low-densitylipoprtoein(LDL) andpolyethyleneglycol(PEG) SLNs
Thedecreaseintumorcellproliferation [52]
Camptothecin(CPT) BCECPorcinebraincapillaryendothelial cellscomparedwith(RAW264.7)
CA-SLNs Highercytotoxicityinbraincells enhanceantitumorefficacy
[53]
Etoposide K562cellline,MTTassayandflow cytometry
NLCSwithtransferrin Enhancedcellularuptakeand antiproliferativeeffect
[54]
Lockednucleicacid(LNA) (antioncogenicmiR-21)
U87MG(malignantgliomacellline) Lipidnanocapsule(LNCs)with L-1peptide
ReductionofmiR-21expressionand antiproliferative
[55]
Curcumin U251MGcellline,ratsbearingC6gliomas CA-LNCs Thedecreaseintumorsizeand malignancy
[56]
Resveratrol(RVR) U87cellline FunctionalizedSLNs Enhancecytotoxicity [57]
Doxorubicin(DOX) BBBmodel(hcmec/D3cell) CA-SLNs Increasedtoxicityforglioblastomacells [58]
Polo-likekinase1(PLK1) siRNAs(siPLK1)
Ratsbearingorthotopicxenograftmodel HA-LNPs(hyaluronicacid) Increasedcelldeath(byreducing expressionofPLK1)
[59]
Temozolomide(TMZ) U87MGinvitrocellslines NLCs Verymuchenhancedantitumoractivity [60]
Vincristine(VCR)andTMZ U87MGinvitrocelllineandmiceinduced withmalignantgliomamodel
SLNsandNLCs NLCSshowbetterantitumoractivity thanSLNs
[61]
ReviewsPOSTSCREEN
intranasaladministrationweredevelopedwithaparticlediame- terof146nm,encapsulationefficiency(EE)of90%,achargeof 21mVandpolydispersityindex(PDI)ratingof0.18.Theresults ofthefollowinginvestigationrevealtheincreasedcytotoxicityof CUR-NLCcomparedwiththatoffreeCURinthegliomacellline U373MG. The biodistribution study for the same formulation showedanincreased drugconcentrationinthebrain after the intranasalapplicationof NLCs.Theresultsof thisstudyled to the conclusion that CUR-NLC is an efficient delivery system for targeting glioblastoma [23]. Temozolomide-loaded NLCs (TMZ-NLC) were prepared to ensure brain targeting via the intranasal route.Theoptimizedformulationshoweda particle sizewithinthenanorange,zetapotentialof15mV,entrapment efficiencyof81%andPDIof0.2.Theresultsoftheinvivostudies indicated the significant enhanced brain concentration of TMZ-NLCincomparisonwithTMZdispersion(i.v.,intranasal).
ThehighestconcentrationofTMZ-NLCinthebrainprovedthe efficacy of this direct intranasal administration of NLCs. The following studydescribed thattheintranasaladministrationof NLCs increasedresidencetime andresultedin higherbioavail- abilityinthebrainatlowerdoses,denotingthisdeliveryroute themostsuitablefortargetingglioma[26].
Novel farnesyl thio salicylic acid (FTA)-loaded lipid cationic hybrid nanoparticles(HNPs)wereformulatedandevaluatedfor antitumoractivityviatheintranasalroute.Glioma2(RG2)cells wereplacedintoWistarrats.Thetumor-bearingratsweretreated with FTA-encapsulated HNPsby intranasal and i.v. administra- tion.TheevaluationoftumorsizeswithFTA-encapsulatedHNPs resulted in a clear decrease (55%) in tumor size. This study proved that the intranasal intervention ofFTA-loaded HNPs is an equally effective approach in glioblastoma targeting. The resultsofallstudiessupporttheuseoftheINDroutefortargeting glioblastomabyusinglipidcarriers[27].
Mechanism of nanoparticle drug delivery via the intranasal route
Theintranasallyadministered formulationwilldepositonthe pseudostratifiedcolumnarepithelium(arespiratorytractinthe nasalcavity). Thesiteofdepositionoftheintranasalformula- tion administered in the form of solution, spray or gel (via applicator) is the front region of the nasal cavity. However, thereareafewdevicesthatcansettlethedrugformulationina higherregionofthenasalcavity.Thenanoparticlesintervene viaintranasalpassagedepositedat thesiteofthenasal cavity dependingonitsproperties likesize,charge andlipophilicity.
Therearefouroptionsforthedrug,eithertoenterviathenasal epithelial tissue and arrive at the circulation or be unloaded alongthegastrointestinaltract(GIT)throughthenasopharynx bytheciliaryclearancenetwork.Thesystemismadeupofcilia, whicharemotileandbeatinasynchronizedmanner,thereby propelling theviscoussuperiorpartdorsallyagainstthenaso- pharynxquickly(5mm/min).Inaddition,enzymaticactivityis also higher in the nasal cavity deep in the olfactory region (enzymes likecytochromeP450dependent peptidases, mono- oxygenase andproteasesare involvedinthatprocedure)[28].
Thenose-to-braindeliveryofthenanoparticleswillfollowthe transport from endothelial cells tothe olfactory neurons via
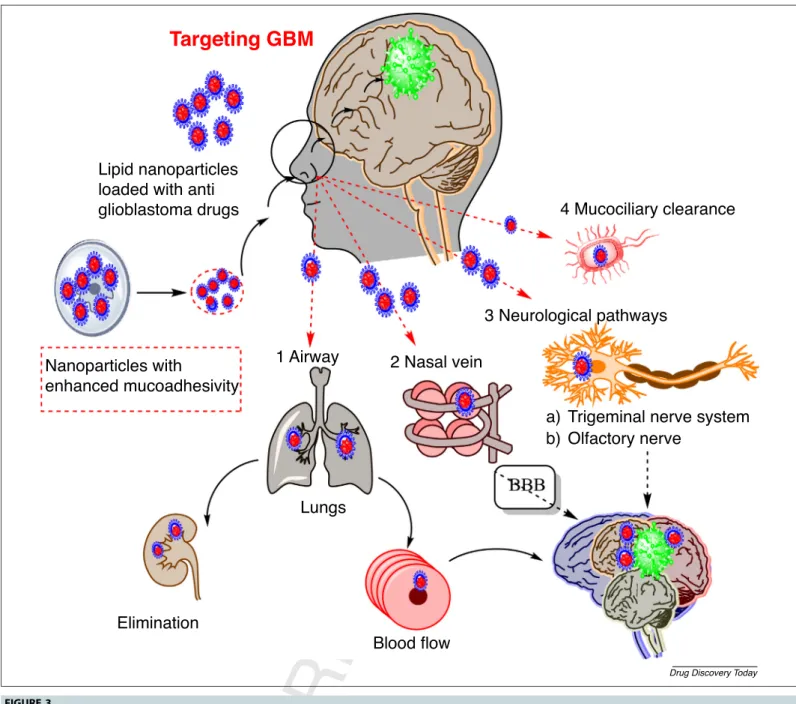
endocytosisorpinocytosisandmovealongtheaxonoritwill followthetrigeminalnerve pathway.Forthistransport path- way,thesizeofnanoparticlesshouldbewithinthediameterof theaxon: 100–700nm [29].Figure3 explains allthe possible mechanisms and pathwaysinvolved inthe transport of lipid nanoparticles.
In vitro / in vivo / ex vivo models for testing nose-to-brain delivery
Foranexplorationofthemechanismbehindthetransportof drugsviatheintranasalroute,differenttypesofinvitro,invivo andexvivomodelsareused.Thesediversetypesofmodelsare appliedfordifferentstudies:invitromodelsforpermeationand diffusion studies; in vivo models for the determination of absorptionand PK profile of theAPI; and ex vivo models for perfusionstudiesinthe nasalcavity. Theselection of invivo models should be adequatefor studyingthe anatomy ofthe nasalcavity.The firstanimalmodelusedforintranasalstudy was the rat and, later, with the development in absorption data, other animal models like sheep, monkey, mouse and rabbit were also used. For adequate PK studies rabbit, dog, sheepandmonkeymodelsarecommonlysuggested,whereas mouse and rat models are used for preliminary absorption studies[30].
Besides the significance of in vivo models, the transport mechanism of drug absorption from the nasal route had to beexplored.Invitromodelswerefabricated toreplace thein vivoandexvivomodels.Furthermore,itisdifficulttoextrapo- latethedataof theabsorption andkineticsstudiesobtained fromtheanimalmodelstohumans(owingtothedifferenceof species).Itis necessary toselectadequate celllines thatcan reproduceresultsatsignificantlylowcosts.Thereareanumber ofinvitrocellculturemodelslikeNAS2BL(originatingfromrat nasalsquamouscarcinoma),BT(originatingfrombovinetur- binates), CaCo-2 cell lines (from human colon carcinoma), Calu-3(originatingfromhumanlungadenocarcinoma),RPMI 2650 (from human nasal epithelial tissues) and 16HBE14o- (fromhumannormalbronchialepitheliumofmaleheartlung transplant patient) [30]. Among these models, CaCo-2 and RPMI2650 areusedtoevaluatepermeability andabsorption viathenasalroute.However,therearesomedisadvantagesof cell lines, for example RPMI 2650 are undifferentiated cells thatencounterthe limited expression of ciliated and goblet cells. Theabsence of a developedmonolayer makesthis cell lineimpracticaltouseforatransportstudy.Bycontrast,Calu- 3cellscandevelopmonolayersandaresuitablefortransport studybuttheoriginofthiscelllineisnotthenormalepithelial cellsofthenasalcavity.The16HBE14o-celllinepossesseshigh transepithelialelectricalresistance (TEER)makingit suitable for transportation study but this cell line originates from normalbronchialepitheliumofamaleheart andlungtrans- plantpatient.Forthedeterminationofdrugdeliveryandthe development of formulations via the intranasal route, it is important touse reliable ex vivo models. The excised tissue isusuallyfromthenasalmucosaofslaughteredorexperimen- tal animals (rats, rabbits, dogs, monkeys, sheep) or from humansaswell.Theexvivostudyisveryimportanttoobtain
ReviewsPOSTSCREEN
alltheinformationregardingthetoxicity,efflux,metabolism and permeation of the developed formulation. Besides the manyadvantagesofexvivomodels,thereareafewlimitations, suchasthelackofinterstitialflowratedeterminationandthe thicknessofnasalepithelialtissuesoftheexcisedmucosa.The Ussing chamber is the ex vivo nasal model for permeability studies[31].
Significance of QbD in the early development of a lipid carrier system for targeting glioblastoma
Dependingonup-to-dateknowledgeaboutbarrierslimitingthe INDofanticancerdrugstargetingbraintumors,lipid-basednano- carriersystemscouldofferapromisingstrategy.However,because
manyformulationparameters and regulatoryaspectsshouldbe considered, the QbD concept or the GMP of the 21st century should be followed. The main elements of QbD methodology aredescribedintherelevantguidelinesoftheInternationalCoun- cilonHarmonization(ICH),specificallyICHQ8(R2),Q9andQ10;
andtheseinclude:(i)definingthequalitytargetproductprofile (QTPP);(ii)selectingthecriticalqualityattributes(CQAs)ofthe targetedproduct;(iii)selectingtheproductionmethodanddefin- ingthecriticalprocessparameters(CPPs)thatcanhighlyaffectthe CQAs;and(iv)analysisoftheinitialriskassessment(RA),whichis followedbyoptimizingtheleveloftheriskyfactorsbyapplyinga suitabledesign ofexperiment (DOE) [32]. Thevery firststepof QbDistocollectallthedatafrompreviousstudiesthatcouldaffect
4 Mucociliary clearance
3 Neurological pathways
Trigeminal nerve system Olfactory nerve
Lungs
Elimination
Blood flow 1 Airway
Nanoparticles with
enhanced mucoadhesivity Lipid nanoparticles loaded with anti glioblastoma drugs
2 Nasal vein
Targeting GBM
a) b)
Drug Discovery Today
FIGURE3 Possiblemecha
Q4 nismsoflipidnanoparticlesacrossthenasalmembrane.Thefiguredemonstratesthatlipidnanoparticleswithenhancedmucoadhesivitywill followfourpossiblepathways:(i)airway;(ii)nasalvein;(iii)neurologicalpathway;and(iv)mucociliaryclearance.Thenanoparticleswillfollowthetransportfrom endothelialcellstoolfactoryneuronsviaendocytosisorpinocytosisandmovealongtheaxonorfollowthetrigeminalnervepathway.
ReviewsPOSTSCREEN
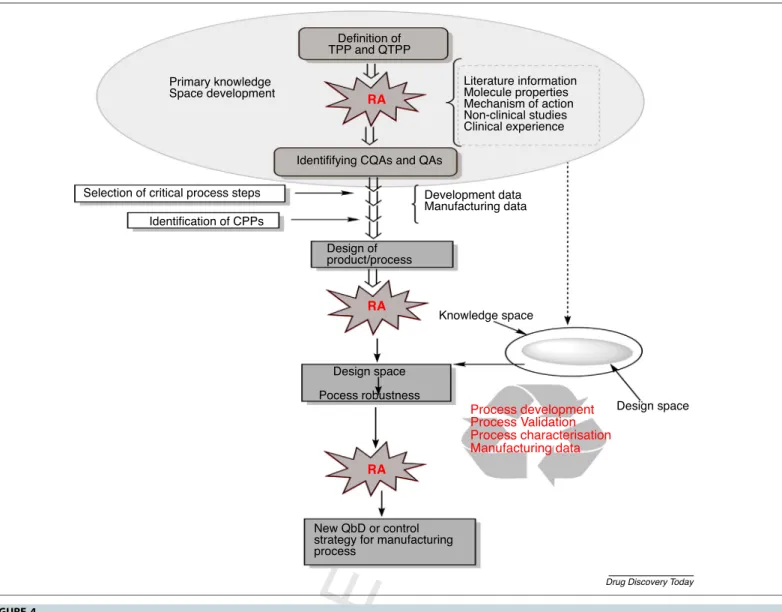
thetargetproductprofile.Afterearlyknowledgeofspacedesign and evaluation of QTPPS,CQAs and CPPs, the RA application revealedtheattributeshavingthehighestimpactonthefinallipid nanoformulation quality for IND. Figure 4 demonstrated the descriptionofQbDmethodologyinearlydevelopmentofalipid carriersystemfornose-to-braindeliverybasedontherelevantICH guidelines[33,34].
Concluding remarks and future directions
This review summarizes theimportance of nose-to-brainde- liveryintargetingGBM.Nanoformulationsare consideredas one of the most important targeting carriers. Besides many advantages,nanoformulationsloadedwithcytotoxicmaterial canstillaccumulateinotherpartsandtissuesofthebody,for instanceintheliver,spleenandkidney.Therefore,itisneces- sarytodesign andfabricateamethodthatcanovercomethe
shortcomingsofpreviouslyusedcarriersanddeliveryroutes.
The alternative route and the lipid nanocarrier provide chances to deliver anticancerdrugs (with potential efficacy) againstGBM,andthiswillbeanewandexpedientapproachto GBMtreatmentstrategies.Furthermore,forsuccessfulINDof anticancerdrugs, risk assessmentis the main componentof QbDwhichshouldbeappliedusingspecialsoftwaretocalcu- latetheriskseverityofCQAsandCPPsregardingtheencapsu- lationofpotentialAPI intolipid-basedcarriersystems.Thus, theapplicationoftheQbDconceptcansavetimeandeffortby directingthefocustowardstructuringthequalityineachstep offormulationdesign.
Conflicts of interest
Theauthorsdeclarenoconflictsofinterest,financialorotherwise.Q24 Definition of
TPP and QTPP
Identififying CQAs and QAs
Design of product/process
Design space Pocess robustness
New QbD or control strategy for manufacturing process
Primary knowledge Space development
Selection of critical process steps Identification of CPPs
Development data Manufacturing data
Knowledge space
Design space Literature information
Molecule properties Mechanism of action Non-clinical studies Clinical experience
Process development Manufacturing data Process characterisation Process Validation RA
RA RA
Drug Discovery Today
FIGURE4 Stepsandelemen
Q5 tsofqualitybydesign(QbD)methodologythatwillhelptodesignandvalidatetheprocessfordevelopmentofintranasalformulation.Step 1:identificationoftargetproductprofile(TPP)andqualitytargetproductprofile(QTPP),whichcomprisestherapeuticandotherqualityrequirements.Step2:
identificationofcriticalqualityattributes(CQAs),whichareassociatedwithin-processmaterials,andcriticalprocessparameters(CPPs)havinganeffecton CQAs.Step3:riskassessment(RA)isaprocessofcollectinginformationtosupportriskdecisionanditisalsoamainactivityofQbDmethodologythatcanbe performedatinitialandfinalphasesofdevelopment.
ReviewsPOSTSCREEN
Acknowledgments
F.S.madesubstantialcontributionstowritingthemanuscriptand designingthe figures and tables. R.I. contributedto evaluating themanuscript,writingtheQbDandgeneralopinionsections,in
addition to making critical revision of the whole manuscript.
I.C. contributed to the conception of the manuscript, planningandsupervisingtheworkateachstepandgivingthe finalapproval.
References
1Hombach-Klonisch,S.etal.(2018)Glioblastomaandchemoresistancetoalkylating agents:involvementofapoptosis,autophagy,andunfoldedproteinresponse.
Pharmacol.Ther.184,13–41
2vanSchaijik,B.etal.(2019)Circulatingtumorstemcellsandglioblastoma:areview.
J.Clin.Neurosci.61,5–9
3Zhang,Q.etal.(2018)Currentstatusandpotentialchallengesofmesenchymal stemcell-basedtherapyformalignantgliomas.StemCellRes.Ther.9,1–9 4Mughal,A.A.etal.(2018)Patternsofinvasivegrowthinmalignantgliomas–
thehippocampusemergesasaninvasion-sparedbrainregion.Neoplasia20, 643–656
5Sekerdag,E.etal.(2017)Nose-to-braindeliveryoffarnesylthiosalicylicacid loadedhybridnanoparticlesinthetreatmentofglioblastoma.J.Neurol.Sci.381, 171–172
6Tucker,C.etal.(2018)Theintranasalrouteasanalternativemethodofmedication administration.Crit.CareNurse38,26–31
7Battaglia,L.etal.(2018)Lipidnanoparticlesforintranasaladministration:
applicationtonose-to-braindelivery.ExpertOpin.DrugDeliv.15,369–378 8Shankar,R.etal.(2018)Lipidnanoparticles:anovelapproachforbraintargeting.
Pharm.Nanotechnol.6,81–93
9Lakkadwala,S.andSingh,J.(2019)Co-deliveryofdoxorubicinanderlotinib throughliposomalnanoparticlesforglioblastomatumorregressionusinganinvitro braintumormodel.ColloidsSurf.BBiointerfaces173,27–35
10Shah,M.K.etal.(2019)Lipidnanocarriers:preparation,characterizationand absorptionmechanismandapplicationstoimproveoralbioavailabilityofpoorly water-solubledrugs.Biomed.Appl.Nanoparticles2019,117–147
11Glaser,T.etal.(2017)Targetednanotechnologyinglioblastomamultiforme.Front.
Pharmacol.8,166
12He,Q.etal.(2018)Towardsimprovementsforpenetratingtheblood–brain barrier—recentprogressfromamaterialandpharmaceuticalperspective.
Cells7,24
13Pires,P.C.andSantos,A.O.(2018)Nanosystemsinnose-to-braindrug delivery:areviewofnon-clinicalbraintargetingstudies.J.Control.Release 270,89–100
14Md,S.etal.(2018)Nano-carrierenableddrugdeliverysystemsfornosetobrain targetingforthetreatmentofneurodegenerativedisorders.J.DrugDeliv.Sci.
Technol.43,295–310
15Selvaraj,K.etal.(2018)Nosetobraintransportpathwaysanoverview:potentialof nanostructuredlipidcarriersinnosetobraintargeting.Artif.CellsNanomed.
Biotechnol.46,2088–2095
16Mistry,A.etal.(2009)Effectofphysicochemicalpropertiesonintranasal nanoparticle transitinto murine olfactory epithelium. J. Drug Target17, 543–552
17Schwarz,B.andMerkel,O.M.(2019)Nose-to-braindeliveryofbiolog
Q25 ics.FutureSci..
http://dx.doi.org/10.4155/tde-2019-0013
18Pallagi,E.etal.(2018)Initialriskassessmentaspartofthequalitybydesignin peptidedrugcontainingformulationdevelopment.Eur.J.Pharm.Sci.122, 160–169
19Qindeel,M.etal.(2019)DevelopmentofnovelpH-sensitivenanoparticlesloaded hydrogelfortransdermaldrugdelivery.DrugDev.Ind.Pharm.45,1–29 20Rizvi,S.A.andSaleh,A.M.(2018)Applicationsofnanoparticlesystemsindrug
deliverytechnology.SaudiPharm.J.26,64–70
21Liao, W.et al.(2018) Recent advances on glioblastoma multiforme and nano-drug carriers:
areview.Curr.Med.Chem..http://dx.doi.org/10.2174/0929867325666180514113136 22Riaz,M.etal.(2018)Surfacefunctionalizationandtargetingstrategiesofliposomes
insolidtumortherapy:areview.Int.J.Mol.Sci.19,195
23Madane,R.G.andMahajan,H.S.(2016)Curcumin-loadednanostructuredlipid carriers(NLCs)fornasaladministration:design,characterization,andinvivostudy.
DrugDeliv.23,1326–1334
24Anand,A.etal.(2019)Braintargeteddeliveryofanticancerdrugs:prospective approachusingsolidlipidnanoparticles.IETNanobiotechnol.13,
353–362
25Sandoval,M.A.etal.(2012)EGFR-targetedstearoylgemcitabinenanoparticlesshow enhancedanti-tumoractivity.J.Control.Release157,287–296
26Khan,A.etal.(2016)Braintargetingoftemozolomideviatheintranasalroute usinglipid-basednanoparticles:brainpharmacokineticandscintigraphic analyses.Mol.Pharm.13,3773–3782
27Sekerdag, E. et al. (2017) A potential non-invasive glioblastoma treatment:
nose-to-braindeliveryoffarnesylthiosalicylicacidincorporatedhybrid nanoparticles.J.Control.Release261,187–198
28Crowe,T.P.etal.(2018)Mechanismofintranasaldrugdeliverydirectlytothebrain.
LifeSci.195,44–52
29Samaridou,E.andAlonso,M.J.(2018)Nose-to-brainpeptidedelivery–thepotential ofnanotechnology.Bioorg.Med.Chem.26,2888–2905
30Sousa,F.andCastro,P.(2016)Cell-basedinvitromodelsfornasalpermeability studies.InConceptsandModelsforDrugPermeabilityStudies(Sarmento,B.,ed.),pp.
83–100,Elsevier
31ErdÅ,F.etal.(2018)Evaluationofintranasaldeliveryrouteofdrugadministration forbraintargeting.BrainRes.Bull.143,155–170
32Nayak,A.K.etal.(2019)Applicationofqualitybydesignforthedevelopmentof biopharmaceuticals.InPharmaceuticalQualitybyDesign.pp.399–411,Elsevier Q26 33Cso´ka,I.etal.(2018)Extensionofquality-by-designconcepttotheearly
developmentphaseofpharmaceuticalR&Dprocesses.DrugDiscov.Today23, 1340–1343
34Pallagi,E.etal.(2018)Initialriskassessmentaspartofthequalitybydesignin Q27 peptidedrugcontainingformulationdevelopment.Eur.J.Pharm.Sci.122,160–169 35Saudagar,R.andKulkarni,M.M.(2017)Reviewonin-situnasalgeldrugdelivery
system.Res.J.Pharm.Technol.10,1870
36Palhal,A.P.etal.(2017)In-situnasalgel:modernisticadvancementindrugdelivery..Q28 2017.http://dx.doi.org/10.20959/wjpr201711-9512
37Beier,C.P.etal.(2009)RNOP-09:pegylatedliposomaldoxorubicineandprolonged temozolomideinadditiontoradiotherapyinnewlydiagnosedglioblastoma–a PhaseIIstudy.BMCCancer9,308
38Yoshida,J.etal.(2004)Humangenetherapyformalignantgliomas(glioblastoma multiformeandanaplasticastrocytoma)byinvivotransductionwithhuman interferonbgeneusingcationicliposomes.HumanGeneTher.15,77–86 39Jahangiri,A.etal.(2017)Convection-enhanceddeliveryinglioblastoma:areviewof
preclinicalandclinicalstudies.J.Neurosurg.126,191–200
40Aigner,A.andKo¨gel,D.(2018)Nanoparticle/siRNA-basedtherapystrategiesin glioma:whichnanoparticles,whichsiRNAs?Nanomedicine13,89–103 41Song,J.etal.(2018)Effectivenessoflomustineandbevacizumabinprogressive
glioblastoma:ameta-analysis.OncoTarget.Ther.11,3435
42Ju,R.-J.etal.(2016)Destructionofvasculogenicmimicrychannelsbytargeting epirubicinpluscelecoxibliposomesintreatmentofbrainglioma.Int.J.Nanomed.
11,1131
43Liu,Y.etal.(2016)Dualreceptorrecognizingcellpenetratingpeptide modifiedliposomesforanti-gliomatherapy.Nanomed.Nanotechnol.Biol.Med.
2,500
44Li,X.-T.etal.(2014)Multifunctionaltargetingdaunorubicinplusquinacrine liposomes,modifiedbywheatgermagglutininandtamoxifen,fortreatingbrain gliomaandgliomastemcells.Oncotarget5,6497
45Pacheco-Torres,J.etal.(2015)ImageguideddrugreleasefrompH-sensitiveion channel-functionalizedstealthliposomesintoaninvivoglioblastomamodel.
Nanomed.Nanotechnol.Biol.Med.11,1345–1354
46Chen,P.-Y.etal.(2012)Comparingroutesofdeliveryfornanoliposomalirinotecan showssuperioranti-tumoractivityoflocaladministrationintreatingintracranial glioblastomaxenografts.NeuroOncol.15,189–197
47Chen,H.etal.(2011)Lactoferrinmodifieddoxorubicin-loadedprocationic liposomesforthetreatmentofgliomas.Eur.J.Pharm.Sci.44,164–173 48Kuo,Y.-C.andLiang,C.-T.(2011)Inhibitionofhumanbrainmalignant
glioblastomacellsusingcarmustine-loadedcatanionicsolidlipid
nanoparticleswithsurfaceanti-epithelialgrowthfactorreceptor.Biomaterials 32,3340–3350
49Kuo,Y.-C.andLee,C.-H.(2015)Inhibitionagainstgrowthofglioblastoma multiformeinvitrousingetoposide-loadedsolidlipidnanoparticleswith r-aminophenyl-a-D-manno-pyranosideandfolicacid.J.Pharm.Sci.104, 1804–1814
ReviewsPOSTSCREEN
50Kuo,Y.-C.andWang,C.-C.(2015)Carmustine-loadedcatanionicsolidlipid nanoparticleswithserotonergic1Breceptorsubtypeantagonistforinvitrotargeted deliverytoinhibitbraincancergrowth.J.TaiwanInst.Chem.Eng.46,1–14 51Sharma,P.etal.(2011)Developmentandevaluationofnanostructuredlipidcarriers
ofcytarabinefortreatmentofmeningealleukemia.J.Nanosci.Nanotechnol.11, 6676–6682
52Jin,J.etal.(2011)Invivospecificdeliveryofc-MetsiRNAtoglioblastomausing cationicsolidlipidnanoparticles.Bioconj.Chem.22,2568–2572
53Martins,S.M.etal.(2013)Braintargetingeffectofcamptothecin-loadedsolidlipid nanoparticlesinratafterintravenousadministration.Eur.J.Pharm.Biopharm.85, 488–502
54Khajavinia,A.etal.(2012)Targetingetoposidetoacutemyelogenousleukaemia cellsusingnanostructuredlipidcarrierscoatedwithtransferrin.Nanotechnology23, 405101
55Griveau,A.etal.(2013)SilencingofmiR-21bylockednucleicacid–lipid nanocapsulecomplexessensitizehumanglioblastomacellstoradiation-induced celldeath.Int.J.Pharm.454,765–774
56Chen,Y.etal.(2016)Nanostructuredlipidcarriersenhancethebioavailabilityand braincancerinhibitoryefficacyofcurcuminbothinvitroandinvivo.DrugDeliv.23, 1383–1392
57Jose,S.etal.(2014)Invivopharmacokineticsandbiodistributionofresveratrol- loadedsolidlipidnanoparticlesforbraindelivery.Int.J.Pharm.474,6–13 58Chirio,D.etal.(2014)Positive-chargedsolidlipidnanoparticlesaspaclitaxel
drugdeliverysysteminglioblastomatreatment.Eur.J.Pharm.Biopharm.88, 746–758
59Cohen,Z.R.etal.(2015)LocalizedRNAitherapeuticsofchemoresistantgrade IVgliomausinghyaluronan-graftedlipid-basednanoparticles.ACSNano9, 1581–1591
60Song,S.etal.(2016)NovelRGDcontaining,temozolomide-loading nanostructuredlipidcarriersforglioblastomamultiformechemotherapy.Drug Deliv.23,1404–1408
61Wu,M.etal.(2016)Vincristineand temozolomidecombinedchemotherapy forthetreatmentofglioma:acomparisonofsolidlipidnanoparticlesand nanostructuredlipidcarriersfordualdrugsdelivery.DrugDeliv.23,2720–2725
ReviewsPOSTSCREEN