C H A P T E R 9
Plant Diseases Caused
toy Fungi
Introduction
F u n gi are small, generally microscopic, plants lacking chlorophyll and conductive tissues. Most of the 100,000 fungus species known are strictly saprophytic, living on d e ad organic matter which they h e lp d e c o m p o s e. S o m e, about 50, species cause diseases in man, and about as many cause diseases in animals, most of them b e i ng superficial dis- eases of the skin or its a p p e n d a g e s. More than 8000 species of fungi, however, can cause diseases in plants. All plants are attacked by s o me kinds of fungi, and each of the parasitic fungi can attack one or many kinds of plants. S o me of the fungi can grow and multiply only by re- maining in association with their host plants during their entire life (obligate parasites), others require a host plant for part of their life cy- cles but can complete their cycles on artificial media, and still others can grow and multiply on d e ad organic matter as well as on living plants (nonobligate parasites).
209
Characteristics of Plant Pathogenic F u n gi Morphology
Most fungi have a vegetative body consisting of more or less elon- gated, continuous filaments which may or may not have cross walls (septa). T h e body of the fungus is called mycelium, and the individual branches or filaments of the mycelium are called hyphae (Fig. 16).
E a ch hypha or mycelium may b e uniform in thickness or may taper into thinner or broader portions. H y p h ae of s o me fungi are only 0.5 μ in diameter, while in others they may b e more than 100 μ thick. T h e length of the mycelium may b e only a few microns in some fungi, but in others it may produce mycelial strands several meters long.
In some fungi the mycelium consists of cells containing one or two nuclei per cell. In others the mycelium is coenocytic, i.e., it contains many nuclei and either the entire mycelium is one continuous, tubu- lar, branched or unbranched multinucleate cell, or it is partitioned by several septa, each segment b e i ng a multinucleate hypha. Growth of the mycelium occurs at the tips of the hyphae.
S o me lower fungi lack true mycelium and produce instead a naked, amoeboid, multinucleate p l a s m o d i um (e.g., Plasmodiophorales) or a system of strands of grossly dissimilar and continuously varying diam- eter called a rhizomycelium (e.g., Chytridiales).
Reproduction
F u n gi reproduce chiefly by means of spores (Fig. 17), which are specialized propagative or reproductive bodies, usually consisting of one or a few cells. Spores may b e formed asexually (i.e., through the separation of minute fragments of the mycelium into spores without any nuclear change), or as the result of a sexual process in which there is nuclear change.
In the lower fungi, and in the phycomycetes of the true fungi, asex- ual spores are produced inside a sac called a sporangium and are re- leased through an opening of the sporangium or upon its rupture.
S o me of these spores are motile by means of flagella and are, there- fore, called zoospores. Other fungi produce asexual spores called co- nidia by the cutting off of terminal or lateral cells from special hyphae called conidiophores. In some fungi terminal or intercalary cells of a hypha enlarge, round up, form a thick wall and separate to form chla- mydospores. In still other fungi, asexual spores (conidia) are produced inside thick-walled structures called pycnidia.
Characteristics of Plant Pathogenic Fungi
F i g. 16. A p p e a r a n ce of the vegetative b o dy (mycelium) of two fungi in culture. U p p e r:
Physalospora cydoniae. L o w e r: Phoma sp. E a ch of the two cultures of e a ch fungus was g r o w i ng on slightly different nutrient m e d i u m.
Sexual reproduction, or processes resembling it, occur in most groups of fungi. In some, sexual reproduction is brought about by the union of two cells (gametes) of equal size and of similar appearance, which unite and produce a zygote, called a zygospore. In others, the gametes are of unequal size and the zygote which they form is called an oospore. In s o me fungi no definite gametes are produced, but in-
211
Zoospor e Sporangiospoj ^ Zygospor e Sporangiu m Oospor e Zoosporangiu m Conidi Conidi a Conidi a i
novcnidiu m Nake d asc i Apotheciu m Peritheciu m Cleistotheciu m Ascu s contain - in g ascospore s Chlamydospore s Conidi a Conidi a Conidi a o
nsporodochiu m Conidi a
\
Conidi a i
nacervulu s Conidi a i
npycnidiu m Basidiospore s o
n basidium Pycniospore s i
n Aeciospores i
n Uredospores i n Teliospore s i
npycniu m aeciu m urediu m teliu m
Fig. 17. Representative spores and fruiting bodies of the main groups of fungi.PHYCOMYCETES ASCOMYCETES IMPERFECT FUNGI BASIDIOMYCETES
Characteristics of Plant Pathogenic Fungi 213
stead any cell of one mycelium may unite with any cell of another compatible mycelium. Spores p r o d u c ed from such mycelium are usually called teliospores. T h e zygote formed in the above ways may b e c o me a ne w one-celled plant or may produce mycelium of various types. In one group of fungi (Ascomycetes) the zygote is formed by the union of two unequal-sized gametes. T h e sexual spores, usually eight in number, are p r o d u c ed within the zygote cell, the ascus, and the spores are called ascospores. T h e asci may or may not b e enclosed by a fruiting b o dy called ascocarp. In another group of fungi (Basidio- mycetes), sexual spores are p r o d u c ed on the outside of the zygote cell called the b a s i d i um and the spores are called basidiospores.
For a large group of fungi (Fungi Imperfecti) no sexual reproduction is known either b e c a u se they do not have one or b e c a u se it has not yet b e e n discovered. Apparently these fungi reproduce only asexually.
T h e union of the sexual nuclei in the zygote produces a diploid (2 N) nucleus. Usually the first divisions of this nucleus are meiotic so that throughout its life the fungus contains haploid (1 N) nuclei, except immediately after the union of the gamete nuclei. In s o me groups of fungi, however, especially in the Basidiomycetes and to a lesser ex- tent in the Ascomycetes, the cells of the entire mycelium or of parts of the mycelium contain two haploid nuclei which remain separate in- side the cell. Such mycelium is called dikaryotic but behaves very much as though it were a diploid mycelium (in which the two nuclei are united).
In most fungi both male and female gametes are p r o d u c ed on the same mycelium (hermaphroditic fungi). Whe n the male gametes can fertilize the female ones of the s a me mycelium the fungus is called homothallic. In many cases, however, the male gametes can fertilize only the female gametes of another, sexually compatible mycelium, and the fungus then is called heterothallic.
Ecology and Spread
Almost all plant pathogenic fungi s p e nd part of their life on their host plants and part in the soil or on plant debris on the soil. S o me highly parasitic fungi pass all of their vegetative, active life on the host and only the spores may land on the soil where they remain inactive until they are again carried to a host on which they grow and multiply.
Other fungi (e.g., Venturia) must pass part of their lives on the host as parasites and part on d e ad tissues on the ground as saprophytes in order to complete their life cycle in nature. T h e latter group of fungi, however, remain continually associated with host tissues, whether liv- ing or dead, and, in nature, do not grow on any other kind of organic
matter. A third group of fungi grow parasitically on their hosts but they continue to live, grow, and multiply on the d e ad tissues of the host after its death, and may further move out of the host debris into the soil or other decaying plant material on which they grow and multiply as strict saprophytes. T h e d e ad plant material which they colonize n e e d not b e related at all to the host they can parasitize. This group of fungi are usually soil pathogens, have a w i de host range, and can sur- vive in the soil for many years in the a b s e n ce of their hosts. T h e y too, however, n e e d to infect a host from time to time in order to increase their populations, since protracted and continuous growth of these fungi as saprophytes in the soil results in more or less rapid reduction in their numbers.
During their parasitic p h a se fungi a s s u me various positions in rela- tion to the plant cells and tissues. S o me fungi (e.g., powdery mildews) grow outside the plant surface but s e nd their feeding organs (haustoria) into the epidermal cells of the plant. S o me (e.g., Venturia) grow only b e t w e en the cuticle and the epidermal cells. Others grow between the cells in the intercellular spaces and may or may not send haustoria into the cells. Still others grow b e t w e en and through the cells indiscriminately. Obligate parasites can grow only in association with living cells, b e i ng unable to fee d on d e ad cells. On the other hand, the mycelium of s o me nonobligate parasites never comes in contact with living plant cells, b e c a u se their enzymes macerate and kill the plant cells ahead of the mycelium. In most cases, however, regardless of the position of the mycelium in the host, the reproduc- tive bodies (spores) of the fungus are p r o d u c ed at or very near the sur- face of the host tissues to ensure their prompt and efficient dissemina- tion.
T h e survival and performance of most plant pathogenic fungi d e p e nd greatly on the prevailing conditions of temperature and mois- ture or the presence of water in their environment. F r e e mycelium survives only within a certain range of temperatures (-5° to + 4 5 ° C) and in contact with moist surfaces, inside or outside the host. Most kinds of spores, however, can withstand broader ranges of both tem- perature and moisture and carry the fungus through the low winter temperatures and the dry summer periods. Spores, however, also re- quire favorable temperatures and moisture in order to germinate. Fur- thermore, lower fungi producing zoospores require free water for the production, movement, and germination of the zoospores.
Zoospores are the only fungus structures that can m o ve by them- selves. Zoospores, however, can m o ve for only very short distances (a few millimeters or centimeters, perhaps). B e s i d e s, only some myxo- mycetes and some phycomycetes produce zoospores. T h e great major-
Characteristics of Plant Pathogenic Fungi 215
ity of the plant pathogenic fungi have very little motion of their own, and therefore they d e p e nd for their spread from plant to plant and to different parts of the same plant on chance distribution by agents such as wind, water, birds, insects, other animals, and man. F u n gi are dis- seminated primarily in the form of spores. Fragments of hyphae and hard m a s s es of mycelium known as sclerotia may also b e disseminated by the same agents although to a much lesser extent.
Spore dissemination in almost all fungi is passive, although their initial discharge in some fungi is forcible. T h e distance to which spores may b e disseminated varies with the agent of dissemination.
Wind is probably the most important disseminating agent of spores of most fungi and may carry spores over great distances. For specific fungi, other agents such as water or insects may play a much more important role than wind in the dissemination of their spores.
Classification
T h e fungi that cause diseases on plants are a diverse group, and b e c a u se of their large numbers and diversity, only a sketchy classifica- tion of the most important phytopathogenic gener a will b e presented here .
C l a s s: M Y X O M Y C E T E S - L a ck m y c e l i u m. T h e ir b o dy is a naked, a m o r p h o us Plasmo- d i u m. Z o o s p o r e s.
Order: P l a s m o d i o p h o r a l es — P l a s m o d ia p r o d u c ed within cells of roots a nd stems of plants.
G e n u s: Plasmodiophora, o ne s p e c i es c a u s i ng c l ub root of crucifers.
Spongospora, o ne s p e c i es c a u s i ng p o w d e ry scab of potato tubers.
C l a s s: P H Y C O M Y C E T AE - H a ve round or e l o n g a t ed m y c e l i um that lacks cross walls.
Order: Chytridiales — H a ve cell wall b ut lack true m y c e l i u m, at most a rhizomyce- lium. Z o o s p o r e s.
G e n u s: Olpidium, parasitic in roots of c a b b a ge a nd other plants.
Synchytrium, o ne s p e c i es c a u s i ng potato wart.
Urophlyctis, o ne s p e c i es c a u s i ng crown wart of alfalfa.
Order: S a p r o l e g n i a l e s. H a ve w e l l - d e v e l o p ed m y c e l i u m. Z o o s p o r es p r o d u c ed in z o o s p o r a n g ia attached to m y c e l i u m. O o s p o r e s.
G e n u s: Aphanomyces, c a u s i ng root rot of m a ny v e g e t a b l e s.
Order: P e r o n o s p o r a l es - Z o o s p o r a n g ia p r o d u c ed at tips of h y p h ae a nd set free. Oos- pores.
F a m i l y: P y t h i a c e ae
G e n u s: Pythium, c a u s i ng damping-off of s e e d l i n g s.
Phytophthora, one s p e c i es c a u s i ng late blight of potato, others c a u s i ng mostly root rots.
F a m i l y: A l b u g i n a c e ae
G e n u s: Albugo, o ne s p e c i es c a u s i ng white rust of crucifers.
F a m i l y: P e r o n o s p o r a c e ae
G e n u s: Plasmopara, o ne s p e c i es c a u s i ng d o w ny m i l d ew of grape.
Peronospora, one s p e c i es c a u s i ng d o w ny m i l d ew (blue mold) of tobacco.
Bremia, o ne s p e c i es c a u s i ng d o w ny m i l d ew of lettuce.
Sclerospora, one s p e c i es c a u s i ng d o w ny m i l d ew of grasses.
Pseudoperonospora, one s p e c i es c a u s i ng d o w ny m i l d ew of cucurbits.
Order: Mucorales — P r o d u ce z y g o s p o r e s. N o n m o t i le asexual spores formed in termi- nal sporangia.
F a m i l y: M u c o r a c e ae
G e n u s: Rhizopus, c a u s i ng soft rot of fruits a nd v e g e t a b l e s.
C l a s s: A S C O M Y C E T AE — P r o d u ce sexual spores, c a l l ed a s c o s p o r e s, in groups of eight within an ascus.
S u b c l a s s: P Y R E N O M Y C E T E S— A s ci in fruiting b o d i es c o m p l e t e ly c l o s ed (cleistothecia) or in fruiting b o d i es with an o p e n i ng (perithecia).
Order: E r y s i p h a l es — M y c e l i um a nd cleistothecia on surface of host plant.
F a m i l y: E r y s i p h a c e ae
G e n u s: Erysiphe, o ne s p e c i es c a u s i ng p o w d e ry m i l d ew of grasses.
Microsphaera, o ne s p e c i es c a u s i ng p o w d e ry m i l d ew of lilac.
Podosphaera, one s p e c i es c a u s i ng p o w d e ry m i l d ew of a p p l e.
Sphaerotheca, o ne s p e c i es c a u s i ng p o w d e ry m i l d ew of roses a nd p e a c h.
Uncinula, one s p e c i es c a u s i ng p o w d e ry m i l d ew of grape.
Order: S p h a e r i a l es — Perithecia with dark-colored, usually firm walls.
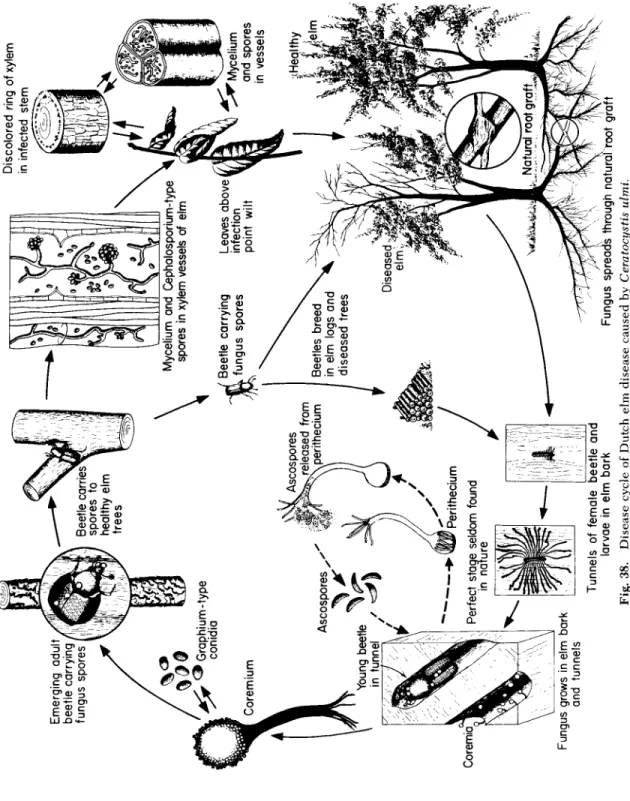
G e n u s: Ceratocystis, o ne s p e c i es c a u s i ng the D u t ch e lm d i s e a s e.
Endothia, o ne s p e c i es c a u s i ng chestnut blight.
Glomerella, one s p e c i es c a u s i ng bitter rot of a p p l e.
Roselinia, c a u s i ng root d i s e a s es of fruit trees a nd vines.
Valsa, c a u s i ng canker d i s e a s es of p e a ch a nd other trees.
Order: H y p o c r e a l es — Perithecia light-colored, or r e d or b l u e.
G e n u s: Claviceps, o ne s p e c i es c a u s i ng ergot of rye.
Nectria, c a u s i ng twig a nd stem cankers of trees.
Order: P s e u d o s p h a e r i a l es — Perithecium-like stromata with asci in separate or s i n g le large cavities.
G e n u s: Guignardia, o ne s p e c i es c a u s i ng black rot of grapes.
Mycosphaerella, c a u s i ng leaf spots of m a ny plants.
Physalospora, one s p e c i es c a u s i ng black rot of a p p l e s.
Venturia, one s p e c i es c a u s i ng a p p le scab.
S u b c l a s s: D I S C O M Y C E T E S — Asci p r o d u c ed at the surface of cup- or s a u c e r - s h a p ed apo- thecia.
Order: Pezizales — Apothecia fleshy or leathery, round in outline.
G e n u s: Coccomyces, one s p e c i es c a u s i ng cherr y leaf spot.
Pseudopeziza, one s p e c i es c a u s i ng alfalfa leaf spot.
Rhytisma, o ne s p e c i es c a u s i ng tar spot of m a p le leaves.
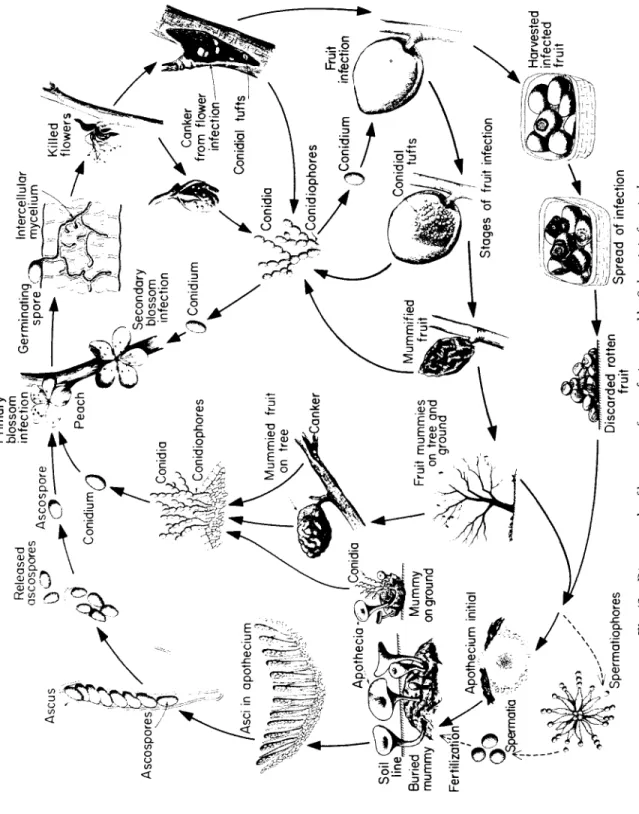
Sclerotinia, one s p e c i es c a u s i ng brown rot of stone fruits.
Order: T a p h r i n a l es — Apothecia with no definite limiting border, almost lacking.
G e n u s: Taphrina, one s p e c i es c a u s i ng p e a ch leaf curl.
C l a s s: F U N GI I M P E R F E C TI — L a ck sexual reproduction a nd structures.
Order: S p h a e r o p s i d a l es — Asexual spores p r o d u c ed in pycnidia.
G e n u s: Ascochyta, o ne s p e c i es c a u s i ng p e a blight.
Phoma, one s p e c i es c a u s i ng b l a c k l eg of crucifers.
Phomopsis, c a u s i ng blights and stem cankers of trees.
Phyllosticta, c a u s i ng leaf spots of m a ny plants.
Septoria, o ne s p e c i es c a u s i ng late blight of celery.
Order: Ì e l a n c o n i a l e s — Asexual spores p r o d u c ed in acervulus.
Characteristics of Plant Pathogenic Fungi 217
G e n u s: Colletotrichum, c a u s i ng anthracnose on m a ny field crops.
Coryneum, c a u s i ng blight on stone fruits.
Order: Moniliales —Asexual spores p r o d u c ed on or within h y p h ae freely e x p o s ed to the air.
G e n u s: Alternaria, c a u s i ng leaf spots a nd blights on m a ny plants.
Aspergillus, c a u s i ng rots of stored s e e d s.
Botrytis, c a u s i ng gray m o ld a nd blights on m a ny plants.
Cercospora, o ne s p e c i es c a u s i ng early blight of celery.
Cladosporium, o ne s p e c i es c a u s i ng leaf-mold of tomato.
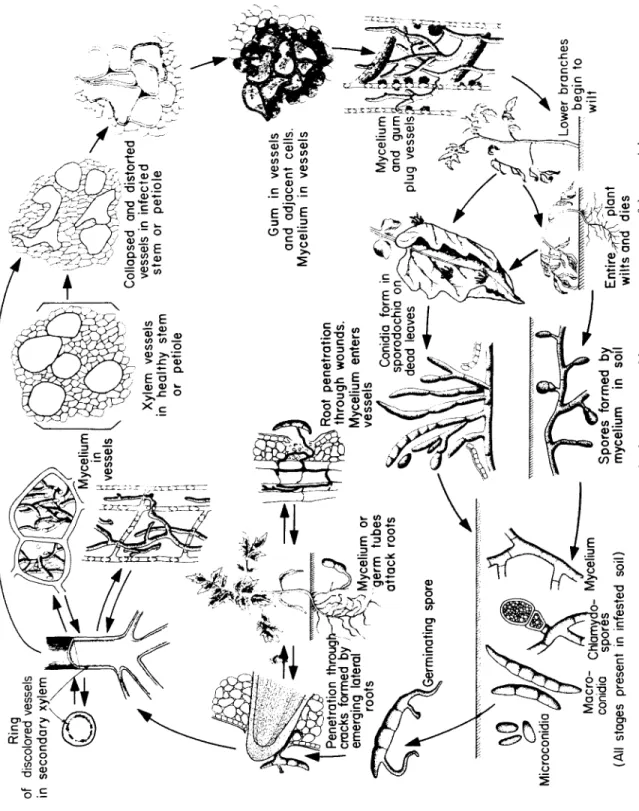
Fusarium, c a u s i ng wilt d i s e a s es of m a ny annual plants.
Helminthosporium, one s p e c i es c a u s i ng blight of oats.
Penicillium, c a u s i ng rot of fleshy organs.
Thielaviospsis, o ne s p e c i es c a u s i ng black root rot of tobacco.
Verticillium, c a u s i ng wilt of m a ny annuals a nd perennials.
Order: M y c e l ia Sterilia— No sexual or asexual spore forms known.
G e n u s: Rhizoctonia, c a u s i ng root rots a nd crown rots of annuals.
Sclerotium, c a u s i ng root a nd stem rots of m a ny plants.
C l a s s: B A S I D I O M Y C E T AE —Sexual spores, c a l l ed b a s i d i o s p o r es or sporidia, are pro- d u c e d externally on a cell c a l l ed a b a s i d i u m.
S u b c l a s s: T E L I O S P O R A E — T e l i o s p o r es single or united into crusts or c o l u m n s, remain- ing in host tissue or bursting through the e p i d e r m i s.
Order: Ustilaginales — Fertilization by m e a ns of union of c o m p a t i b le spores, h y p h a e, etc. Only teliospores are p r o d u c e d.
G e n u s: Sphacelotheca, several s p e c i es c a u s i ng loose s m ut of sorghum.
Tilletia, several s p e c i es c a u s i ng bunt, or stinking smut, of wheat.
Urocystis, o ne s p e c i es c a u s i ng smut of onion.
Ustilago, c a u s i ng s m ut of corn, wheat, barley, etc.
Order: U r e d i n a l es — S p e rm cells fertilize special r e c e p t i ve h y p h ae in p y e n ia (sperma- gonia). P r o d u ce a e c i o s p o r e s, u r e d o s p o r es (repeating spores) a nd teliospores.
G e n u s: Cronartium, o ne s p e c i es c a u s i ng white p i ne blister rust.
Gymnosporangium, one s p e c i es c a u s i ng cedar a p p le rust.
Melampsora, one s p e c i es c a u s i ng rust of flax.
Phragmidium, o ne s p e c i es c a u s i ng rust of roses.
Puccinia, c a u s i ng rust of cereals.
Uromyces, o ne s p e c i es c a u s i ng rust of b e a n s.
S u b c l a s s: H Y M E N O M Y C E T A E- B a s i d ia p r o d u c ed in a h y m e n i um b e c o m i ng e x p o s ed to the air before the spores are shot off from the sterigmata.
Order: Polyporales — H y m e n i um lining the surfaces of small pores or tubes.
G e n u s: Fomes, c a u s i ng heart rot of m a ny trees.
Thanatephorus, c a u s i ng root a nd stem rots of m a ny annual plants (Rhizoctonia).
Polyporus, c a u s i ng root a nd stem rot of m a ny trees.
Stereum, c a u s i ng silver leaf d i s e a se of trees.
Order: Agaricales — H y m e n i um on radiating gills or lamellae.
G e n u s: Armillaria, c a u s i ng root rots of fruit trees.
Identification
Since each fungus d i s e a se of plants is usually c a u s ed by only one fungus, and since there are more than 100,000 different species of
fungi, the identification of the fungus species on a d i s e a s ed plant specimen or culture of a fungus means that all but one of all the known fungus species must b e excluded.
T h e most significant characteristics of a fungus u s ed for identifica- tion are its spores and fructifications, or spore-bearing structures.
T h e se are almost always too small to b e seen with the naked eye and, therefore, they are examined under the c o m p o u nd microscope. If the specimen or culture has well-marked, mature, spore-bearing struc- tures, they are examined directly after removal from the s p e c i m en and may provide adequate information for identification. If no such struc- tures are present the s p e c i m en may b e kept moist for a few days to promote fructification development, or a pure culture of the fungus may be isolated and grown on artificial m e d ia in petri dishes, and identification is m a de on the basis of the fructifications produced on the media.
T h e shape, size, color, and manner of arrangement of spores on the sporophores or the fruiting bodies, as well as the shape, color, etc., of the sporophores or fruiting bodies are sufficient characteristics to sug- gest, to one somewhat experienced in the taxonomy of fungi, the class, order, family, or even genus to which the particular fungus belongs. In any case, these characters can b e utilized to trace the fungus through analytical keys of the fungi to the genus and, finally, to the species to which it belongs. Ther e are several p u b l i s h ed standard keys that lead to families and genera. S o me of these cover fungi in all groups, others may b e more specialized covering only the fungi in one class, one order, or even one family. Once the genus of the fungus has b e e n de- termined, specific descriptions of the species are found in mono- graphs of gener a or in specific publications in research journals.
Since there are usually lists of the pathogens affecting a particular host plant, one may use such host indexes as short cuts in quickly find- ing names of fungus species that might apply to the fungus at hand.
Host indexes, however, merely offer suggestions in determining identities, which must ultimately b e determined by referenc e to monographs and other more specific publications.
Symptoms C a u s ed by F u n gi on Plants
F u n gi cause local or general symptoms on their hosts and these may occur separately on different hosts, concurrently on the same host, or follow one another on the same host. In general, fungi cause local or general necrosis or killing of plant tissues, hypoplasia, or stunting of
Symptoms Caused by Fungi on Plants 219
plant organs or entire plants, and hyperplasia or excessive growth of plant parts or whole plants.
T h e most common necrotic symptoms are:
Root rot—Disintegration or d e c ay of part or all of the root s y s t em of a plant.
Basal stem rot — Disintegration of the lower part of the stem.
Damping-off—The rapid death a nd c o l l a p se of very y o u ng s e e d l i n gs in the s e ed b e d or field.
C a n k e r —A localized w o u nd or necrotic lesion, often s u n k en b e n e a th the surface of the s t em of a w o o dy plant.
Anthracnose —A necrotic a nd s u n k en ulcerlike lesion on the s t e m, leaf, fruit or flower of the host plant.
L e af spots — L o c a l i z ed lesions on host l e a v es consisting of d e ad a nd c o l l a p s ed cells.
S c ab — L o c a l i z ed lesions on host fruit, l e a v e s, tubers, etc., usually slightly r a i s ed or s u n k en a nd cracked, giving a s c a b by a p p e a r a n c e.
B l i g h t — G e n e r al a nd extremely rapid b r o w n i ng of leaves, b r a n c h e s, twigs, a nd floral organs resulting in their death.
Soft rots and dry rots —Maceration a nd disintegration of fruits, roots, b u l b s, tubers a nd fleshy leaves.
Almost all of the above symptoms may also cause pronounced stunt- ing of the infected plants. In addition, certain other symptoms such as leaf rust, mildews, wilts, and even certain diseases causing hyperpla- sia of some plant organs, such as clubroot, may cause stunting of the plant as a whole.
Symptoms associated with hypertrophy and distortion of plant parts include:
Clubroot — E n l a r g ed roots a p p e a r i ng like s p i n d l es or clubs.
Galls — E n l a r g ed portions of plants usually filled with fungus mycelium.
Warts — Wartlike p r o t u b e r a n c es on tubers a nd stems.
Witches'-brooms — Profuse, u p w a rd b r a n c h i ng of twigs.
L e af curls — Distortion, thickening a nd curling of leaves.
In addition to the above, three groups of symptoms may b e added:
Wilt—Usually a g e n e r a l i z ed s e c o n d a ry s y m p t om in w h i ch leaves or shoots lose their turgidity a nd droop b e c a u se of a disturbance in the vascular s y s t em of the root or of the stem.
R u st —Many, small lesions on leaves or s t e m s, usually of a rusty color.
M i l d ew —Chlorotic or necrotic areas on l e a v e s, stems, a nd fruit usually c o v e r ed with m y c e l i um and the fructifications of the fungus.
In many diseases, the pathogen grows or produces various struc- tures on the surface of the host. T h e se structures, which include my- celium, sclerotia, sporophores, fruiting bodies, spores, are called signs and are distinct from symptoms which refer only to the appearance of infected plants or plant tissues. Thus, in the mildews, for example, one sees mostly the signs consisting of a whitish, downy growth of
fungus mycelium and spores on the plant leaves, fruit, or stem, while the symptoms consist of chlorotic or necrotic lesions on leaves, fruit, and stem, r e d u c ed growth of the plant, etc.
H ow F u n gi C a u se D i s e a se in Plants
F u n gi enter plant tissues through wounds, through natural open- ings, and/or directly through the cuticle and the epidermis. Once in- side the plant, fungi remove nutrients from the plant and utilize them for their own growth and reproduction. T h e mere removal of nutri- ents, which would normally b e utilized by the plant cells for their own processes, is, sometimes, sufficient cause for development of an un- healthy condition in the host cells which leads to appearance of local- ized or generalized disease symptoms on the plant.
Most frequently, however, fungi cause diseases in plants through the direct or indirect effects of substances they secrete on the struc- tural integrity and the metabolic activities of plant cells and tissues.
Different fungi are known to produce one or more of the following groups of biologically active substances: E n z y m e s, toxins, growth regulators, polysaccharides, antibiotics. T h e se substances may injure plant cells directly or affect them indirectly, either by affecting the mechanisms controlling the metabolic processes in the cell or by in- ducing cell responses that lead to pathological manifestations. S o me of the enzymes (pectinases, cellulases, hemicellulases) break down the structural substances that make up the plant cell wall, other en- zymes (proteinases, amylases, lipases, etc.,) cause degradation of sub- stances contained in the cells, and still others affect (usually increase) the rate of respiration and decrease the respiratory efficiency in in- fected plant tissues.
Of the other substances secreted by fungi, toxins may alter the per- meability of membranes of affected cells, may cause changes in respi- ration, may act as antimetabolites by displacing structurally similar essential metabolites, or, through their chelating action, may block chemical reactions by binding metals existing as free ions or as en- zyme cofactors. F u n gi secrete many of the same growth regulators produced by host plants and may thus cause hyperplastic or hypertro- phic plant growth by direct action. F u n gi also act indirectly by secret- ing substances that affect the production, accumulation, and/or break- down by the host of growth-promoting substances and their inhibitors.
Such action may result in an increase of hormonal substances and production of overgrowths and malformations, or it may lower or alter
Life Cycles of Fungi 221
the content of hormonal substances and may cause stunting, distor- tion, leaf drop, etc. Polysaccharides are p r o d u c ed by most fungi, but they s e em to be most important when they can cause, directly or after oxidation, clogging of the vessels and wilting of the plants. Antibiotics are p r o d u c ed by several fungi and, although they are active mostly against other microorganisms, they are known to affect plant cells as well. Their m o de of action s e e ms to b e similar to that of s o me toxins, affecting mainly the permeability of cell m e m b r a n es and the respira- tion of host cells.
Life Cycles of F u n gi
Although the life cycles of the fungi of the different groups vary greatly, the great majority of them go through a series of steps that are quite similar (Fig. 18). Thus almost all fungi have a spore stage with a simple, haploid (possessing one set of chromosomes or 1 N) nucleus.
T h e spore germinates into a hypha which also contains haploid nu- clei. T h e hypha may either produce simple, haploid spores again (as is always the case in the Imperfect Fungi) or it may fuse with another hypha to produce a fertilized hypha in which the nuclei unite to form one diploid nucleus, called zygote, (containing two sets of chromo- somes, or 2 JV). In the Phycomycetes the zygote will divide to produce simple, haploid spores which close the cycle. In a brief phase of most Ascomycetes, and generally in the Basidiomycetes, the two nuclei of the fertilized hypha do not unite, but remain separate within the cell in pairs (dikaryotic or Ν + Ν) and divide simultaneously to produce more hyphal cells with pairs of nuclei. In the Ascomycetes, the dikary- otic hyphae are found only inside the fruiting body, in which they b e c o me the ascogenous hyphae, since the two nuclei of one cell of each hypha unite into a zygote (2 N) which divides meiotically to pro- duce ascospores that contain haploid nuclei.
In the Basidiomycetes haploid spores produce only short haploid hyphae. U p on fertilization, dikaryotic (Ν + N) mycelium is produced and this develops into the main b o dy of the fungus. Such dikaryotic hyphae may produce, asexually, dikaryotic spores that will grow again into a dikaryotic mycelium. Finally, however, the paired nuclei of the cells unite and form zygotes. T h e zygotes divide meiotically and pro- duce basidiospores that contain haploid nuclei.
In the Imperfect F u n g i, of course, only the asexual cycle (haploid spore —> haploid mycelium —» haploid spore) is found. E v en in the other fungi, however, a similar asexual cycle is the most common one
^ Germinating conidium Sporangiospore (or zoospore) Fertilizatio /Zygot / Sporangium Sporangiospore (or zoospore)
Conidium Fertilization
ASCOMYCETES
PHYCOMYCETES
Sporangium \
IMPERFECT FUNGI
Conidium ^ Conidioohore STERILE FUNGI
Mycelium A Basidiospores^J 3asidium ^^Basidiospore BASIDIOMYCETES Dikaryotic ^mycelium Dikaryotic spores
Fertilization Dikaryotic mycelium
/AscosporeJ^ Ascusm //Zygote* Fig. 18. Schematic presentation of the generalized life cycles of the main groups of phytopathogenic fungi.
Control of Fungus Diseases of Plants 223
by far, since it can b e repeated many times during each growth season.
T h e sexual cycle, which includes the formation of the zygote, usually occurs only once a year.
Control of F u n g us D i s e a s es of Plants
T h e endless variety and the complexity of the many fungus diseases of plants have led to the d e v e l o p m e nt of a correspondingly large number of approaches for their control. T h e particular characteristics in the life cycle of each fungus, its habitat preference s and its perform- ance under certain environmental conditions are some of the most important points to b e considered in attempting to control a plant dis- e a se c a u s ed by a fungus. Although s o me diseases can b e controlled completely by just one type of control m e a s u r e, a combination of mea- sures is usually necessary for satisfactory control of most diseases.
T h e u se of pathogen-free s e ed or propagating stock is always recom- m e n d e d and, for control of certain d i s e a s e s, it is mandatory. Destruc- tion of plant parts or refuse harboring the pathogen, destruction of volunteer plants or alternate hosts of the pathogen, use of clean tools and containers, proper drainage of fields and aeration of plants, are all very important practices in the control of most plant diseases c a u s ed by fungi. Crop rotation is helpful in controlling diseases c a u s ed by some fungi, but cannot b e u s ed for fungi that have w i de host ranges, can live saprophytically for a long time, or produce long-lived resting spores.
T h e u se of plant varieties resistant to certain pathogens has found its greatest application in controlling fungus diseases of plants. S o me of the most serious fungus diseases (e.g., rusts, Fusarium wilts) of the most important crop plants, are successfully controlled today by the u se of resistant varieties. Although the degree of control through re- sistant varieties varies with the crop and the pathogenic fungus in- volved, its s u c c e s s es as of this time and the very low overall cost of such control make this type of control the most promising for the fu- ture.
T h e most effective method, however, and, sometimes the only one available for controlling most of the fungus diseases of plants, is through application of chemical sprays or dusts on the plants, their s e e d s, or into the soil where the plants are to grow. Soil-inhabiting fungi may b e controlled in small areas by steam or electric heat, and in somewhat larger areas by volatile liquids, such as formaldehyde, chlo-
ropicrin, methyl bromide. S o me diseases c a u s ed by soil-inhabiting fungi can also be controlled, and at a much lower cost, by applying fungicides on the seeds or other propagating materials, such as tubers and corms. Such treatment will also protect the s e ed from fungus mycelium or spores carried on the seed. F u n g i c i d es u s ed for s e ed treatment include, among others, Ceresan, S e m e s a n, Panogen, chlor- anil, dichlone, captan, thiram, etc.
Most fungicides are u s ed to prevent diseases on the aboveground parts of plants and are applied on the foliage as sprays or dusts. Almost all of these are protectant, since they can only prevent fungi from causing infection, but cannot stop an infection once it has started. T h e number of such fungicides is great and includes many inorganic and organic compounds. T h e most common of these are elemental sulfur, copper compounds (Bordeaux mixture, Cuprocide, Perenox, etc.), thiocarbamates (thiram, ferbam, nabam, m a n e b, zineb, ziram, etc.), mercury compounds (Tag, Coromerc, etc.,) and several miscellaneous compounds (captan, dichlone, dodine, chloranil, Polyram, Glyodin, Botran, Folpet, etc.) In addition to these, certain antibiotics (e.g., cycloheximide) are also effective against certain fungus diseases of plants.
In some diseases (e.g., loose smuts of cereals) the fungus is carried in the s e ed and control can b e obtained only through treatment of the s e ed with hot water. In others, control of the insect vectors may b e the only available possibility. In general, great advances have b e e n m a de toward controlling fungus diseases of plants, especially through resist- ant varieties and through chemicals, and as a result, these diseases are probably much easier to control than any other group of plant dis- eases, although the losses c a u s ed by fungus diseases of plants are still very great.
Selected References
Alexopoulos, C. J. 1962. "Introductory M y c o l o g y ," 613 pp. Wiley, N e w York.
Barnett, H. L. 1955. " I l l u s t r a t ed G e n e ra of Imperfect F u n g i ," 2 1 8 pp. B u r g e s s, M i n n e- apolis, Minnesota.
B e s s e y, E. A. 1950. " M o r p h o l o gy a nd T a x o n o my of F u n g i ," 791 pp. Blackiston, Phila- delphia, Pennsylvania.
C l e m e n t s, F. E., and C. L. Shear. 1957. " T he G e n e ra of F u n g i ," 4 9 6 pp., 58 plates. Haf- ner, N e w York.
C o c h r a n e, V. W. 1958. " P h y s i o l o gy of F u n g i ," 524 pp. Wiley, N e w York.
Diehl, W. W. 1953. Identifying a pathogenic fungus. Yearbook Agr. (U.S. Dept. Agr.) pp.
31-34.
Clubroot of Crucifers 225
F e r g u s, C. L. 1960. " I l l u s t r a t ed G e n e ra of W o od D e s t r o y i ng F u n g i ," 132 pp. B u r g e s s, M i n n e a p o l i s, Minnesota.
F i n g h a n, J. R. S., a nd P. R. Day. 1963. " F u n g al G e n e t i c s ," 3 0 0 pp. F. A. D a v i s, Philadel- phia, Pennsylvania.
G a u m a n n, E. 1950. " P r i n c i p l es of Plant Infection," 5 4 3 pp. C r o s by L o c k w o o d, L o n d o n.
G o o d m a n, R. Æ., Z. Kiraly, a nd M. Zaitlin. 1967. " T he Biochemistry and Physiology of Infectious Plant D i s e a s e s ," 354 pp. Van Nostrand, Princeton, N e w J e r s e y.
Stakman, E. C , a nd J. G. Harrar. 1957. " P r i n c i p l es of Plant P a t h o l o g y ," 581 pp. R o n a ld Press, N e w York.
S t e v e n s, F. L. 1913. " T he F u n gi Which C a u se Plant D i s e a s e ," 754 pp. Macmillan, N e w York.
Clubroot of Crucifers
Occurrence and Importance
T h e clubroot d i s e a se of cruciferous plants, such as c a b b a g e, cauli- flower, mustard, radish, is widely distributed all over the world, found wherever wild or cultivated plants of the mustard family grow. It has b e e n o b s e r v ed most frequently and studied most intensively, howev- er, in Northern E u r o pe and North America, probably b e c a u se of the relatively greater importance of cruciferous plants and of the greater emphasis on plant diseases in these areas.
Clubroot is a very serious d i s e a se when susceptible varieties of any cruciferous species are grown in infested fields, and losses c a u s ed by it are sometimes very heavy. F i e l ds once infested with the clubroot pathogen remain so indefinitely and b e c o me unfit for cultivation of crucifers practically forever or until costly methods and materials are u s ed to sterilize the soil.
Symptoms
T h e first symptoms of the d i s e a se in the aboveground parts of the plant are mild and difficult to distinguish. T h e leaves are pale green to yellowish, and may show flagging and wilting in the m i d d le of hot, sunny days but may recover during the night. Affected plants show almost normal vigor at first, but then gradual or s u d d en and pro- nounced stunting sets in, which may or may not b e followed by death of the plant. Youn g plants may b e killed by the d i s e a se within a short time after infection, while older plants may remain alive but fail to produce marketable heads.
F i g. 19. Clubroot symptoms on the roots of c a b b a g e plants infected with Plasmodi- ophora brassicae. (Photo by courtesy of U.S. D e p t . Agr.)
T h e most obvious and most characteristic symptoms of the disease appear on the roots and sometimes the underground part of the stem (Fig. 19). T h e symptoms consist of small or large spindlelike, spheri- cal, knobby or club-shaped swellings on the roots and rootlets. T h e se malformations may b e isolated and cover only part of s o me roots or they may coalesce and cover the entire root system of the plant. T h e older and usually the larger c l u b b ed roots disintegrate before the e n d of the season d ue to invasion by bacteria and other weakly parasitic soil microorganisms.
The Pathogen: Plasmodiophora brassicae
It is a slime mold the body of which is a p l a s m o d i um and develops and multiplies only within the host cells (Fig. 20). T h e plasmodium gives rise to resting spores, about 4 ì in diameter, each of which upon germination releases one zoospore moving about by means of two
Clubroot of Crucifers 227
unequal-sized flagellae. Whe n the zoospore comes in contact with a root hair of a host plant, it b e c o m es amoeboid, penetrates into the host cell, and there develops into a Plasmodium —that is, a mass of proto- plasm containing many nuclei. After a few days, the Plasmodium cleaves into multinucleate portions surrounded by separate mem- branes; each portion develops into a zoosporangium. T h e zoosporan- gia are discharged outside the host through pores dissolved in the host cell wall, and each zoosporangium releases four to eight zoospores.
S o me of these zoospores fuse in pairs to produce zygotes which can cause ne w infections.
T h e Plasmodium is always intracellular. It has no wall of its own and lives in direct contact with the protoplasm of the host cell. T h e Plasmodium may move from cell to cell as an amoeba, and may divide simultaneously with dividing infected plant cells, part of the Plasmo- dium going to each host cell. It contains many nuclei, and, during sporulation, spores consisting of a nucleus, a layer of the surrounding Plasmodium, and a m e m b r a ne are formed and remain free from one another inside the host cell wall. T h e se are the resting spores of the pathogen and are released into the soil upon disintegration of the host cell walls by secondary microorganisms.
Development of Disease
T h e p l a s m o d i um resulting from the germination of the zoospores penetrates young root tissues directly; it can also penetrate secondar- ily thickened roots and underground stems through wounds. F r om these points of primary infection the p l a s m o d i um spreads to cortical cells and reaches the c a m b i um through direct penetration of host cells (Fig. 20). F r om the point of infection of the c a m b i um the plasmodium spreads in the c a m b i um in all directions, and from the infected cam- b i um the p l a s m o d i um spreads outward into cortex cells and inward toward the xylem region and into the medullary rays. Single-point infections result in spindle-shaped clubs with the greater diameter of the spindle at the point of invasion and the spindle tapering off away from it.
As the plasmodia pass through the cells, they b e c o me established in some of them and stimulate these cells to abnormal enlargement (hypertrophy) and abnormal division (hyperplasia). Infected cells may be five or more times larger than adjacent uninfected ones and their nuclei and nucleoli 5 and 30 times, respectively, larger than those of adjacent uninfected cells. T h e infected cells of a club are distributed in small groups throughout the d i s e a s ed tissue and the groups are
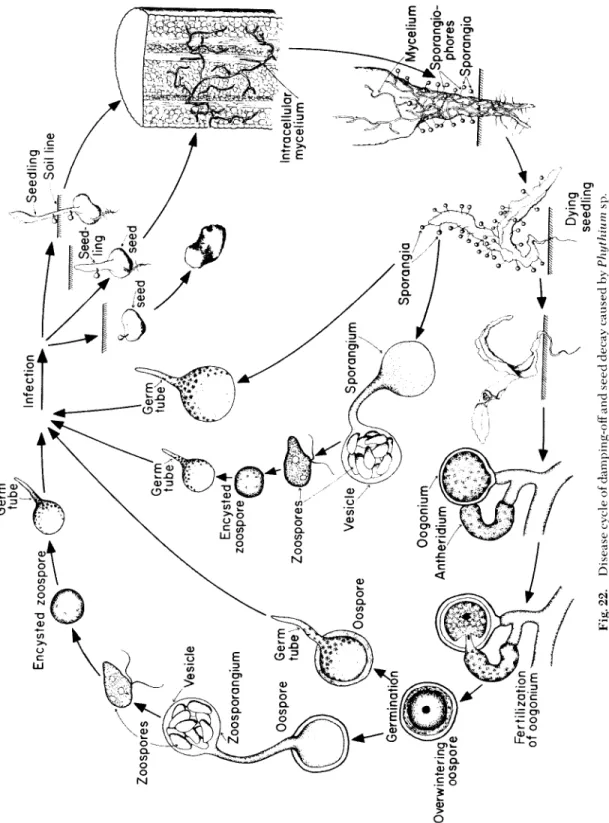
Fig. 20. Disease cycle of clubroot of crucifers caused by FlasmocLwphora brassicae.
<^Rootsof * ^infected cabbage plant ψ Development of ^ club roots
^ Infectio / of root
Zygote ^Plasmodium invades cells Hypertrophy and
Multinucleate Plasmodium
Zoospores .
Zoosporangiax Sporulation ^
Plasmodiu m sDread s
/ Plasmodium / J Infection of 7>JOOt hairs Zoospore Fungus-filled cells of cabbage root ^ ^ Germination^ Resting! spore Disintegrating cell releasing restina spores Disintegrating club rootsClubroot of Crucifers 229
usually separated by uninfected cells. T h e stimulus which is responsi- ble for the abnormal growth of the cells appears to diffuse in advance of the pathogen and acts on the noninvaded cells of d i s e a s ed tissues as well as on the infected ones. Actively growing and dividing cells, i.e., cambial cells, are more easily invaded by the pathogen and are more responsive to the stimulus than other cells.
In most cases many of the cells of infected clubs remain free from Plasmodia, but in rare instances almost all the cells of a club may b e infected. Whe n few cells are infected the plasmodia b e c o me large, whereas w h en many cells are infected, they remain relatively small.
T h u s, there s e e ms to b e a fairly constant ratio b e t w e en the volume occupied by the p l a s m o d i um and that of the d i s e a s ed tissue, the for- mer b e i ng approximately 30 % of the latter.
T h e plasmodium-infected clubs not only constitute a drag on the economy of the plant by utilizing much of the foodstuff required for the normal growth of the plant, but they also interfere with the absorp- tion and translocation of mineral nutrients and water through the root system resulting in gradual stunting and wilting of the aboveground parts of the plant. Furthermore, the rapidly growing and greatly en - larged cells of the club tissues are unable to form a cork layer at the surface and are easily ruptured and invaded by secondary, weakly parasitic microorganisms. T h e invasion of clubs by bacteria and their s u b s e q u e nt disintegration by them lead to formation of substances toxic to the plant which are partly responsible for the wilting of the tops.
Control
Growing cruciferous crops in fields known to b e infested with the clubroot pathogen should b e avoided. If that is not possible, c a b b a ge and the other susceptible cruciferous crops should b e planted in well drained fields with a pH slightly above neutral (usually about 7.2) or in fields in which the pH is adjusted to 7.2 by the addition of the proper amount of hydrated lime. T h e use of soil liming in the control of clubroot is b a s ed on the fact that spores of the clubroot organism germinate poorly or not at all in alkaline media.
Although soil fumigants capable of disinfecting fields from the club- root organism are available, the cost of materials and of application are as yet prohibitive. However, s e e d b ed areas can b e kept clubroot-free by treating the soil with chloropicrin, methyl bromide, Mylone, Va- pam, or Vorlex approximately 2 w e e ks before planting. T h e clean, clubroot-free seedlings should, upon transplanting, b e watered with a
solution containing 1 ounce of Terrachlor (or P C NB = pentachloro- nitrobenzene) per gallon of water.
T h e search for and development of varieties of cruciferous hosts re- sistant to clubroot has b e e n only partially successful. Such resistance has b e e n most highly d e v e l o p ed in varieties of rutabaga and turnip, but the extensive u se of resistant varieties in infested soil has resulted in the appearance of highly virulent ne w races of the pathogen, some- times within three years from the time of the release of the resistant varieties. Although some varieties of the most popular cruciferous hosts are resistant to certain races of the clubroot organism and can b e grown in areas free from these races, no varieties of c a b b a g e, cauli- flower, Brussels sprouts, or broccoli resistant to all the races of P. bras
sicae are presently available.
Selected References
C o l h o u n, J. 1958. Clubroot d i s e a se of crucifers c a u s ed by Plasmodiophora brassicae Woron. Commonwealth Mycol. Inst. Phytopathol. Paper 3: 108 p p.
Kunkel, L. O. 1918. T i s s ue invasion by Plasmodiophora brassicae. J. Agr. Res. 14:
5 4 3 - 5 7 2 , illus.
L a r s o n, R. H. 1934. W o u nd infection and t i s s ue invasion by Plasmodiophora brassicae.
J. Agr. Res. 49: 6 0 7 - 6 2 4.
S e a m a n, W. L., J. C. Walker, a nd R. H. L a r s o n. 1963. A n e w race of Plasmodiophora brassicae aifecting B a d g er S h i p p er c a b b a g e. Phytopathology 53: 1426-1429.
Sherf, A. F. 1964. Clubroot of c a b b a g e, cauliflower a nd broccoli. N.Y. State Agr. Expt.
Sta. (Geneva, N.Y.) Bull. 1130: 4 p p.
Williams, P. H. 1964. H o st parasite nuclear relations in clubroot of crucifers. Phytopath
ology 54: 9 1 2 (abstr.).
Williams, P. Ç. , Í . T. K e e n, J. O. Strandberg, a nd Sharon S. M c N a b o l a. 1968. Metabo- lite synthesis and degradation d u r i ng clubroot d e v e l o p m e nt in c a b b a ge hypocotyls.
Phytopathology 58: 9 2 1 - 9 2 8 .
Woronin, M. 1878. Plasmodiophora brassicae. U r h e b er der Kohlpflanzen — H e r n i e.
Jahrb. Wiss. Botan. 11: 548-574. E n g l i sh Transl. by C. C h u pp in Phytopathol. Clas
sics 4: 1934.
Pythium S e ed Rot, Damping-off, and Root Rot Occurrence and Importance
Damping-off disease of seedlings is widely distributed all over the world. It occurs in valleys and forest soils, in tropical and temperate climates, and in almost every greenhouse.
Pythium Seed Rot, Damping-off, and Root Rot
T h e d i s e a se affects s e e d s, seedlings, and older plants of almost all kinds of vegetables, flowers, cereals, and many fruit and forest trees.
In all cases, however, the greatest d a m a ge is done to the s e ed and seedling during germination and either before or after e m e r g e n c e . L o s s es from this d i s e a se vary considerably with the plant species, the particular fungus species involved, soil moisture, temperature, etc.
Quite frequently, however, seedlings in s e e d b e ds are completely de- stroyed by damping-off, or they die soon after they are transplanted. In many instances poor germination of s e e ds or poor e m e r g e n c e of seed- lings is the result of damping-off infections in the p r e e m e r g e n c e stage.
Older plants are seldom killed when infected with the damping-off pathogen, but they d e v e l op stem lesions or root rots, their growth may be retarded considerably, and their yields may b e r e d u c ed drastically.
S o me species of the damping-off fungus also attack the fleshy organs of plants, which then rot with resultant heavy losses during storage of these products.
Symptoms
T h e symptoms c a u s ed by the damping-off fungi vary with the age and stage of d e v e l o p m e nt of the plant affected. Whe n seeds of suscep- tible plants are planted in infested soils and are attacked by the damp- ing-off fungi they fail to germinate, b e c o me soft and mushy, then turn brown, shrink, and finally disintegrate. S e ed infections taking place in the soil cannot b e observed, and the only manifestations of the d i s e a se are poor stands. Poor stands, however, are also the result of infections of the seedling by the damping-off fungus after the s e ed has germi- nated but before the seedling has e m e r g e d above the soil line. T i s s u es of such young seedlings can b e attacked at any point. T h e initial infec- tion appears as a slightly darkened, water-soaked spot. T h e infected area enlarges rapidly, the invaded cells collapse, and the seedling is overrun by the fungus and dies shortly after the beginning of infec- tion. In both cases infection takes place before the seedlings e m e r g e , and this p h a se of the d i s e a se is called p r e e m e r g e n c e damping-off.
S e e d l i n gs that have already e m e r g e d are usually attacked at or be - low the soil line (Fig. 21). T h e succulent tissues of the seedling stems are easily penetrated by the fungus, which invades and kills the cells very rapidly. T h e invaded areas b e c o me water-soaked and discolored, and the cells soon collapse. At this stage of infection the basal part of the seedling stem is much thinner and softer than the above, yet unin- vaded, parts; owing to loss of firmness and supporting power, the in- v a d ed portion of the stem cannot support the part of the seedling above it, w h e r e u p on the s e e d l i ng falls over on the soil. T h e fungus
231
F i g. 2 1 . Damping-off symptoms on b e e t seedlings infected with Pythium sp. (Photo by courtesy of the D e p a r t m e n t of Plant Pathology, Cornell University.)
continues to invade the seedling after it has fallen to the ground and the seedling quickly withers and dies. This p h a se of the d i s e a se is called postemergence damping-off.
Whe n older plants are attacked by the damping-off fungus they usually show only small lesions on the stem; these, however, if suffi- ciently large or numerous, can girdle the plant and cause stunting or death. More commonly, infections on older plants are limited to root- lets, which are d a m a g ed and frequently killed by the fungus; this re- sults in stunting, wilting, and death of the aboveground part of the plant.
Soft fleshy organs of some vegetables, such as cucurbit fruits, pota-