Biodegradable polyesters for medical applications Judit Telegdi1,2, László Trif
1
1Institute of Materials and Environmental Chemistry, Research Centre for Natural Sciences, Hungarian Academy of Sciences, Budapest, Hungary
2 Óbuda University, Faculty of Light Industry and Environmental Engineering, Budapest, Hungary
Abstract
The book chapter gives an overview on the natural and synthetic polyesters that are used in different medical fields for bonding (suturing, fixation), as closures (covering, occlusion), for separation, scaffolds (cellular proliferation), capsulation, as drug deliverer (with great potential for controlled drug release), and could be used as prostheses. The first requirement for applications is that all monomers released from the polymers during the degradation/de-polymerization should be biocompatible. Other important polyester characteristics are the degradation rate and the mechanical stability pre-determined by the conditions of the synthesis. The chapter summarizes the historical background of the polyesters, explains the meaning of the biodegradation, biocompatibility; shortly characterizes the most interesting polyesters from the point of view of the synthesis and their medical application possibilities. Additional information demonstrates how could be the disadvantageous properties (short-time hydrolysis, mechanical instability) improved with application of co-monomers or by parallel use of different polymers. An important part of the chapter is the description of the synthetic roots with demonstration of their advantages and disadvantages.
1. Polyesters: a short historical overview
The term polyester refers to compounds containing many ester groups in each molecule.
In practice, they are polymeric materials containing an ester molecular part as major structural components in the main macromolecular chains. The ester groups are involved in the main chain either directly, as in the cellulose triacetate, poly(vinyl acetate) and
poly(methyl acrylate), or through short side-chains [1]. Thus, polyesters consist of repeated units of ester; their general formula is shown below:
where the R means aliphatic and/or aromatic groups, which determines the properties of the final polyesters, n refers to the number of the units.
The ester linkage is well-known in natural polymers. For example the shellac, this naturally occurring aliphatic polyester that has been already used in the ancient time, is biodegradable. Its application was already mentioned in the Indian epic Mahabharatha, written around 3000 BC. This biodegradable resin, which is a mixture of aliphatic polyhydroxy acids, has been used in protective coatings [3]. The success of shellac was an inspiring example that led Bakeland [3] to develop phenolic resins as substituent and, from that time - at the turn of the last century - started the development of the synthetic polymer industry. The most well-known and often used man made polyester is the polyethylene terephtalate (PET), which is a thermoplastic polymer. In its structure, the R group consists of a benzene ring (-C6H4-) and an ethylene unit (-CH2-CH2-). Because of the aromatic ring in the main-chain, its biodegradtion is not easy [2]. In contrast to PET, aliphatic polyesters, the mostly used and investigated polymers applied mainly in medical field, are biodegradable. The synthetic polymers with ester groups and an appropriate structure are mostly environmentally degradable.
It is noteworthy to mention that at the beginning of the third millennium the scientists and industrial experts turned to examples of the nature and developed bio- and environmentally accepteble polymers [4]. The first aliphatic polyesters had low molecular weight and poor mechanical properties. Some other examples are the dihydroxy-terminated poly(alkylene alkanoates), that found applications in the production of polyurethane or, as plasticizers. In the 1960s started the research of the poly(L-lactide), homo- and copolymers, as they are biocompatible, biodegradable, and bioresorbable materials [5].
Polyesters play an importnat role among the degradable polymers due to the potentially hydrolysable ester bonds. They represent a large group of the environmentally acceptable materials [6]. The lack of good mechanical and physical properties could be improved by application of co-monomers that enhance the favorable properties (biodegradation, application possibilities etc.).
2. Biodegradable polymers
The term "biodegradable polymer" can be defined on different ways [7]. It can refer to polymers, which degrade in biological environment, including any environment where biological or biochemical processes occur, regardless of the degradation mechanism. In other explanation only those polymers are involved into this group that can degrade in biochemical reactions, especially in reactions catalyzed by enzymes. By the German standard test method existed since the 1998 biodegradability is entitled “test of the compostability of plastics”. Generally the classification for the biodegradability is that more than 60% of the organic carbon must be converted in laboratory experiments within six months; additionally, under real‐life conditions in compost more than 90% of the plastic has to be degraded by fragmentation in the size of maximum 2 mm.
A biomaterial can be defined as a material that interfaces with biological systems to evaluate, treat, augment or replace any tissue, organ or function of the body.
The minimal requirements of biomaterials are that they should be: 1. non-toxic, non- pyrogenic, non-hemolytic, chronically non-inflammative, non-allergenic, non- carcinogenic and non-teratogenic; 2.effective (functionality, performance, durability); 3.
sterilizable (by ethylene oxide, irradiation, electron beams, autoclave, dry heating); 4.
biocompatible (interfacially, mechanically, biologically).
Biopolymers are the main type of biomaterials. Vast examples are for the application of biodegradable polymers. According to their degradation properties, biopolymers can be
further classified into biodegradable and non-biodegradable biopolymers. Many implants, such as bone substitute-, bone fixing- and dental materials that should have long term stability in the body. In the recent years, developments in tissue engineering, regenerative medicine, gene therapy, and controlled drug delivery have inspired researchers for development of biodegradable biomaterials with new properties. Biologically derived and synthetic biodegradable biopolymers have attracted a considerable attention [9, 10].
In the last two decades of the twentieth century, a paradigm shift took place from biostable biomaterials to biodegradable (hydrolytically and/or enzymatically degradable) biomaterials for medical and related applications [10]. The biodegradability of a polymer depends mainly on its backbone structure. The most important requirement is the presence of hydrolysable and/or oxidizable linkages in the backbone. The rate of biodegradation of polyesters mainly depends on the type of repetitive units, on the compositions, sequence length, molecular geometry, molecular weight, morphology (e.g., crystallinity, size of spherules, orientation), on the hydrophilicity, surface area, and additives [3].
3. Classification of biodegradable polyesters: natural and synthetic
Considering the raw materials, the polymers are divided into two groups: 1.
biodegradable polymers from renewable resources (polymers of microbiological origin, synthetic polymers from renewable monomers), 2. biodegradable polymers of non- renewable resources [8].
Considering the application of biodegradable polymers they are classified into three groups: ecological, medical and dual application. The other factor of classification is the origin; according to that they belong to two groups: natural and synthetic polymers.
Natural polyesters can be found in nature, in numerous animals and also in plants. In the case of bacteria, three types of polymers have been identified:
1. poly(3-hydroxybutyrate) (PHB) and its co-polymer with related repetitive units;
2. poly(β-malate) poly(L-3-carboxy-3-hydroxypropionate); they have the same carbon skeleton as PHB but they are not in the storage reservoir system;
3. shellac: secreted by the lac insect and exuded it onto different trees.
Biopolymers that originate from biological sources, could be divided into four types according to the components in the polymers: 1) poly(hydroxyalkanoate), 2) protein and poly(amino acid), 3) polysaccharide and 4) nucleotide.
From the point of view of their utilization, the degradability is one of the most important characteristic [11].
Biopolymers of natural origin have been investigated for the preparation and application of biomaterials in a range of applications.
In the modern medical industry the use of polymers as biomaterials is important. The biomaterials of either natural or synthetic origin should be biocompatible.
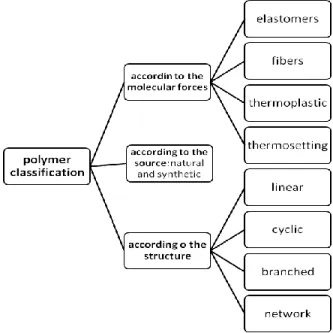
Before the discussion of the biodegradable biopolymers, a summary of the classification of the polymers is presented in Figure 1.
Figure 3.1. Classification of polymers
The classification is based on the structure, the molecular forces and material source. The first group indicates the structure of the molecules, while the second group summarizes the forces that define the physical, mechanical and thermal character of the polymers. In the third group two main clusters are involved, the natural and synthetic polymers. This study is dedicated to the biodegradable polyesters that are/could be used for medical applications.
Naturally occurring polymers have been utilized for long time; however, their application has been restricted because of their limited thermoplastic processability.
The drawback of natural polymers is that even though they are available in ample quantity, there are some limitations for their use such as immunogenecity, difficulty in processing, a potential risk of transmitting pathogens, and batch-to-batch variability [12, 98, 99].
One of the natural polyesters is the poly(β-hydroxybutyrate), which was chemically identified in 1920 and it can be found in some microorganisms as a granular component.
In some cases, they are copolymers with different alkyl groups in β-position. The natural, biodegradable, and biocompatible plastics became of industrial interest because of the wide range of applications, such as surgical sutures or packaging containers [13].
By the modification of the natural polyesters (e. g. controlled incorporation of repeating units with different chain length) tailor-made, well-defined copolymers are prepared with a range of material properties. The physical and chemical characteristics are defined by the composition.
The synthetic polymers should be produced in a reproducible manner with good quality control.
4. The most common synthetic polyesters
The polyesters are the most extensively investigated class of biodegradable polymers.
They are important, because of the synthetic versatility, as well as of the numerous monomers that build the polyesters.
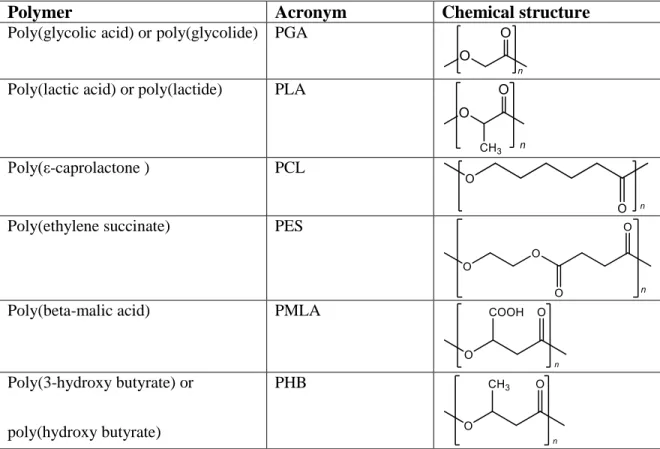
In Table 4.1. some important aliphatic polyesters, their acronyms, and their chemical structures are presented.
Several representatives of these polymers are in human applications, as pharmaceutical complementary, prosthetics, drug deliveries, and in imaging systems. These polymers are recognized by the biological environment in the human body due to the similarity with natural polymers. These materials may also avoid the stimulation of chronic
Some of the biodegradable polyesters are given in details below.
Table 4.1. Polyesters, their acronyms, and chemical structures
Polymer Acronym Chemical structure
Poly(glycolicacid) orpoly(glycolide) PGA
Poly(lactic acid) or poly(lactide) PLA
Poly(ε-caprolactone ) PCL
Poly(ethylene succinate) PES
Poly(beta-malic acid) PMLA
Poly(3-hydroxy butyrate) or
poly(hydroxy butyrate)
PHB
4.1. Poly(α-hydroxy esters)
4.1.1. Poly(Glycolic acid) (PGA)
This was the first biodegradable synthetic polymer used for biomedical application. It is a highly crystalline, hydrophobic, linear polyester, which has a high melting point and relatively low solubility in organic solvents. Its degradation goes through bulk erosion with random hydrolysis of ester bonds. The amorphous phase hydrolyzes first, which is followed by the degradation of the crystallized part and the complete hydrolysis needs
about 4-12 weeks. Its excellent fibre forming and mechanical properties are due to the high crystallinity [14-17].
4.1.2. Poly(Lactic Acid) (PLA)
The poly(lactic acid) is also a linear polyester. The presence of an additional methyl group in its molecule, compared to the PGA, results in high a hydrophobicity. Because it is highly amorphous, it dissolves easily in many organic solvents. As the molecule has a stereo center, it exits in L, D and DL form, but the most common is the L one. It has good tensile strength; low extension and high modulus (ideal biomaterial for load bearing application) and it can also form high strength fibres. Its hydrolysis results in lactic acid.
It degrades homogeneously by hydrolytic erosion as well as random scission of the ester bonds, and its degradation rate is lower than of the poly(glycolic acid). The degradation time depends on the molecular weight, crystallinity and porosity. The lactic acid, which is released in the citric acid cycle during the degradation, can induce inflammatory reaction [18, 19].
4.1.3. Poly(Lactic-co-Glycolic Acid) (PLGA)
The mechanical and physical properties of the PLGA are controlled by the co-monomers.
Both the L- and the DL-lactides were used for co-polymerization. Different ratio of monomers results in polyesters of tailor-made properties, the polymer with composition of 90% glycolic acid and 10% of lactic acid could be used for suture. The degradation rate could be decreased by increasing the ratio of lactic acid. The PLGA undergo bulk erosion through the hydrolysis of the ester bonds.
There are several other co-polymers fabricated for monofilament sutures, that contain hard and soft segments along the polymer backbone for property modulation, like poly(glycolic-co-trimethylene carbonate and poly(glycolide-co-caprolactone).
There are some other co-polymers where the components synergistically improve the basic characteristics, e.g. when the poly(lactic-glycolic acid) and poly(ethyleneglycol) are used together [25]. In this way tailor-made products are prepared that meet specific requirements.
4.1.4. Poly(Malic acid) (PMA)
Due to its low toxicity, non-immunogenicity, and biodegradability, PMA is a promising polymeric drug carrier [20, 21]. The PMA-based nanoconjugates contain multiple active sites and are ready for further engineering and modification.
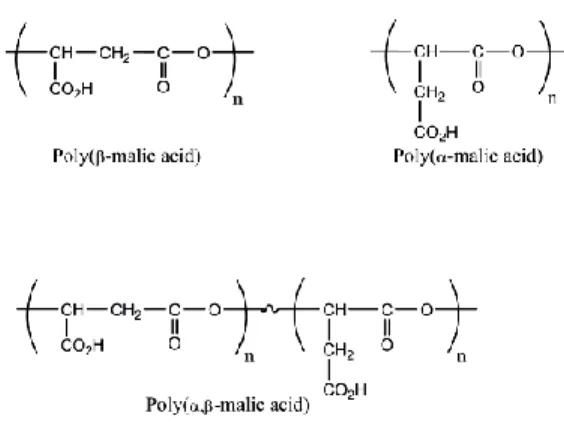
The poly(malic acid) exists in three different forms:
Figure 4.1. Polymeric forms of malic acid
The scheme shows (Figure 4.1.), that the polymerization of malic acid could result in poly(β-malic acid) or poly(α-malic acid) as well as in poly(α,β-malic acid). There is no significant difference between the structure of poly(β-malic acid) and poly(α-malic acid) molecules as they form chains and are more hydrophobic than the poly(α,β-malic acid).
The latter form is not only more hydrophilic than the two previous forms but can exist in chain-like as well as in hyper-branching structure.
Though there is a possibility to gain poly(malic acid) from natural sources or by fermentation [22], it is mainly produced by different synthetic methods, which results in significantly different molecular weights of PMAs. The factors that influence the polymer characteristics (yield, molecular weight, structure) are the synthesis conditions, the number of reaction steps, the cost of chemicals and the purification.
The PMA is a rigid polymer. In order to soften it, several co-monomers could be built in the polymers. One of the best is the β-cyclodextrin. It was experimentally demonstrated that with increasing β-cyclodextrin ratio in the polymer the polyester characteristics changes significantly. The other advantage of this co-monomer is that the inherent
complex formation ability of the β-cyclodextrin allows hosting drug molecules inside its toroid structure. Further co-monomers involved in the co-polymers (like resorcinol and salicylic acid) enables the polyesters for controlled release of monomers having anti- inflammatory or antifungal activity [23, 24].
4.1.5. Polydioxanone
Polydioxanone facilitates the formation of monofilament sutures. Its degradation goes by non-specific scission of the ester back bone. Due to the high crystallinity and hydrophobicity it is a moderately degrading polymer.
4.1.6. Polycaprolactone (PCL)
PCL is interesting polyester as the monomer is cheap; the polymer is synthesized by ring opening polymerization. It is highly processible polyester; it dissolves in a wide range of organic solvents and has the ability to form miscible blends with wide range of polymers.
Its degradation is due to the presence of a hydrolytically labile aliphatic ester linkage, but the degradation rate is slow. PCL has slow tensile strength and could be used for long- term drug delivery. Its excellent biocompatibility allows to use is as scaffold for tissue engineering. There are several co-polymeric systems, where the PCL component improves the properties of the original polymer (the degradation rate of the copolymers of caprolactone and DL-lactide is much higher. The copolymers of caprolactone and glycolic acid give less stiff fibres).
4.1.7. Poly (trimethylene carbonate) (PTMC)
The synthesis of this elastomeric aliphatic polyester goes through ring opening polymerization of trimethylene carbonate. It could be used for soft tissue regeneration.
When the molecular weight is low, it is capable to deliver drugs during its biodegradation. In vivo, the degradation goes much quicker than in vitro due to enzymes.
As a homopolymer it has low mechanical performance, this is the reason that this undesired property should be improved by co-polymerization.
4.1.8. Poly(butylene succinate) (PBS) and its copolymers
These materials are white crystalline thermoplastics, belonging to the poly(alkene- dicarboxylate) family, that are obtained by poly-condensation reactions of glycols, such as ethylene glycol and 1,4-butanediol, with aliphatic dicarboxylic acids, such as succinic and adipic acid.
5. Synthetic routes to polyesters
Polyesters are the most extensively investigated class of biodegradable polymers. The uniqueness of this class of polymers lies in the immense diversity of monomers and in the synthetic versatility. Both homo- and co-polymers of polyesters are potential materials for a variety of biomedical applications. To improve the properties and develop novel biodegradable materials, one can tailor the polymer architecture of biodegradable and biocompatible polyesters and their synthetic analogues.
There are several classifications for the polyester syntheses. One of them is the ring- opening polymerization, the other goes through condensation and there is a possibility to synthesize by catalyzed reaction. Another possible grouping is the ring-opening polymerization initiated by metal complexes and the step-by-step polymerization (that uses mild chemo/enzymatic catalysis [26].
5.1. Ring-opening polymerization
The ring-opening polymerization of cyclic esters produces biodegradable, bioassimilable and renewable polyesters. The problem is that the yield is not high because of the numerous reaction steps [27].
The ring opening polymerization goes through lactone-polymerization [28, 29]. This method results in chain-like polymers with high molecular weight [30-33].
The major steps in the polyester production are the follows:
a) synthesis of the ring monomer (generally benzyl ester of benzyl-malolactonate [28]
b) polymerization through ring opening, which often follows an anionic mechanism when initiators are applied, like tetraalkyl- ammonium benzoate [29] or potassium alkanoate in the presence of crown ethers [30, 31]. The catalyst could be stannous octoate [34] or enzyme [35].
c) The last step is the opening of the ester bond and the removal of the masking groups (in case of benzyl ester it goes through catalyzed hydrogenolysis) [30, 31].
The end-product is a polymer chain with free carboxylic groups.
Other polymers could be synthesized via modification of protecting groups or by sequential polymerization [30, 31]. The advantage of this method is the high optical purity of the product, but the disadvantage is that the end-product synthesized via many steps results in very low yield.
There are significant differences in the molecular weights of polyesters depending on the synthesis techniques
5.2. Direct poly-condensation
In order to disseminate the application of polyesters in the biomedical field, extensive research was done on their synthesis by poly-condensation, which is realized in solid state. The starting materials, with or without catalysts, are heated and the condensation takes place with the formation of the poly-condensates and water as side-product.
Usually, the reaction time is long and the by-product water is removed continuously.
In contrast to the ring opening synthesis, the poly-condensation is a simple, one-step/one- pot reaction resulting in small-, medium- and high molecular weight oligomers and polymers; they properties are influenced by the reaction conditions (catalyst, temperature, pressure, reaction time, constant removal of the water). This reaction always produces α,β-derivatives with hyper-branched structure [35-37].
In the poly-condensation hydroxyl-mono- and/or diacids react with diols or by lactone- type heterocycles [38]. The disadvantage of this method is the catalyst, which is generally toxic metal oxide (mostly tin-derivatives), but there are already some examples, when non-toxic metal oxide was used as catalyst [39].
The direct poly-condensation could run in organic solvent too, e.g. in diphenylether [32], or in dimethyl formamide [40].
This type of synthesis is mainly used for production of poly(malic acid) – one of the very important polyesters - from natural chemicals, but these polyesters could also be synthesized by fermentation [22].
5.3. Biotechnological synthesis
The biotechnological approach uses microorganisms (Penicillum, Cyclopium, Physarium Polycephalium, Aureobasidium) [41, 42, 43, 44]. In the fermentative way, when different microorganisms convert the monomer molecule into their polymers, the synthesis starts either from malic acid or from aspartic acid. This method always results in poly(malic acid). The disadvantage of the fermentative method is that the product is a mixture of polyesters and some un-reacted monomers; the separation, purification process is complicated, as well as the control of the molecular weight is difficult.
6. Degradation of natural and synthetic polyesters
The biodegradability is important in the field of biomedical application because of the biocompatibility in association with the long-term, non-degradable implants. These polymers are mainly used as drug delivery devices, scaffolds for tissue regeneration, stents, artificial skin, orthopedic implants etc.
The polymer properties in these classes vary to a high degree; the individual polymers could be selected according to the individual requirements. Many natural and synthetic polymers fulfill most of the properties, required for biomaterials. However, at biomedical applications different requirements should be fulfilled (mechanical strength, degradation time, surface properties, physicochemical parameters, degree of cross-linking, presence of functional group for modification and tagging, etc.). Some of the applications (such as bone grafting and bone repair), requires mechanical stability, in addition to the biocompatibility; these polymers are suitable for applications when they withstand to
load and have long degradation time. The use of polyesters as surgical dressings, sutures, etc. requires varying strength and degradation time, their application depends on the type of tissue and the type of injury. In tissue engineering application growth factors are considered vital for rapid healing of the tissue. The strategy is to mimic matrix and provide the necessary information or signaling for cell attachment, proliferation, and differentiation.
This particular factor is more important, when these materials are used as carriers for bioactives, where reproducible delivery is required from the carrier. Usually, polymers having higher hydrophobicity sustain the release of bioactive molecules for longer time, but sometimes instant release are required upon triggering by the external stimuli, which may be present at the target site. If the polymer is the only governing factor for the release of bioactive molecules, then a predictable release is considerable important.
Generally the system releases the active moiety in a zero-order pattern at a predetermined rate [45]. Surface-eroding polymers are considered to follow zero-order release kinetics and the release rate depends on the type of polymer/monomer.
Polyesters are widely used as basic materials for the preparation of nano-sized drug carriers. They can sustain the release for a longer time, and moreover they have very well established safety and disposition profiles from the clinical point of view [46-48].
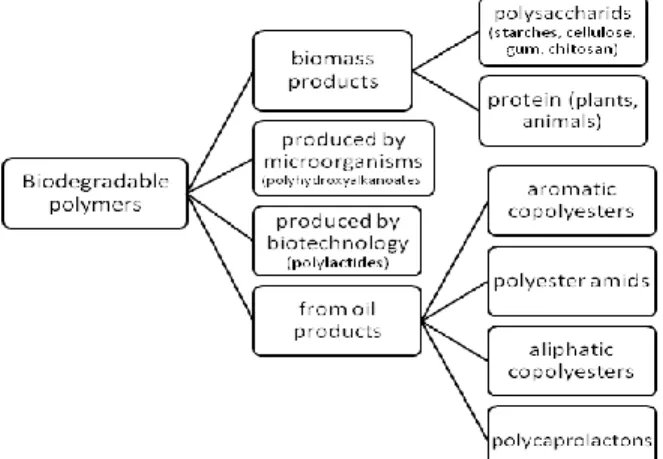
Polyesters mainly undergo bulk erosion, i.e., the polymeric matrices degrade all over their cross section and have erosion kinetics that is non-linear and usually characterized by a discontinuity. The biodegradable polymers are classified as the Figure 6.1. shows.
Figure 6.1. Classification of biodegradable polymers
The biodegradable polymers can be classified according to their structure and occurrence. Among the agro-polymers are the polysaccharides, like starch (they consist of glycosidic bonds), animal and plant based proteins (built of amino acids).
Based on the properties, the biodegradable polymers have numerous applications when important factors determine their application, stability, durability, and degradability. The biodegradable polymers have strong carbon backbones (minimal chain branching
occurs); this is the reason that their degradation starts at the end groups. The high surface area helps in the degradation by chemicals, enzymes, light etc. at higher rate as they have easier access to bounds to cleave. The crystallinity and the hydrophobicity/hydrophilicity can also interfere with the degradation process.
6.1. Routes of polymer degradation
The degradation can be monitored by measuring the change in the molecular weight, (which arises from the bond cleavage) or by following the weight loss (which is due to depletion of low molecular weight materials). The hydrolytic degradation results not only in structural but morphological and topological changes that can be visualized by scanning electron microscopy (SEM), but in formation of degraded products (determinable by LC-MS, GC-MS), and in changes in mechanical properties. Special chromatography (headspace GC-MS, LC, etc.) has been developed for the detection and identification of low molecular weight compounds in degraded polymer matrix. By these techniques, as Karlsson and Albertsson described [49], the degradation products in PHA, PLA, poly(LA-coglycolide) can be well identified.
The polymer degradation takes place mostly through scission of the main chains or side- chains of polyesters, which is induced by thermal activation, photolysis, radiolysis, hydrolysis or oxidation [51]. It takes place via water uptake when oligomers and finally monomers are formed. The first is when the water attacks the water-labile bonds by infiltrating into the polymer matrix and then the bond is hydrolyzed [52]. This is a so- called neutral nucleophilic attack, but the hydrolysis could be catalyzed by acid, base or enzymes.
Generally, two different mechanisms are discussed in literature for the degradation of polyesters: 1) bulk degradation and 2) surface erosion. In the bulk degradation process, the water molecules diffuse faster into the polymer matrix than the polymer starts to degrade. The result is that the hydrolysable bonds in the whole polymer matrix are homogeneously cleaved. Therefore, the average molecular weight of the polymer decreases homogeneously. In the case of surface erosion, the diffusion rate of water into the polymer matrix is slower than the degradation rate of the macromolecules. The degradation takes only place in the thin surface layer while the molecular weight of the polymer in the bulk remains unchanged. The surface erosion is a heterogeneous process when the rate strongly depends on the shape and the surface area of the test sample [50].
The biodegradable natural and synthetic polymers can break down and result in natural by-products such as gases (CO2, N2), inorganic salts and biomass. They can have not only ester functional groups but amide and ether, too.
6.1.1. Alkali-catalyzed polyester hydrolysis
In this hydrolytic process the hydroxide anion reversible attacks the carbonyl carbon atom of the ester group and a tetrahedral intermediate is formed; the ether is involved into a tetrahedral intermediate product that can hydrolyze, and alcohol and carboxylic acid are formed. The preference of the tetrahedral intermediate toward hydrolysis versus ester regeneration is determined by the ability of the leaving alcohol (ROH) that stabilizes the negative charge [53]. As a result, one hydroxyl and one carboxyl end groups are generated (Figure 6.2.) [54].
Figure 6.2. Alkaline-catalyzed hydrolysis of polyesters [53]
6.1.2. Acid-catalyzed polyester hydrolysis
When acid is present, the degradation of a polyester starts with protonation of the carbonyl oxygen of the ester group by the formation of a hydronium ion; thus the carbonyl carbon becomes more electrophilic due to the positive charge. Then a water molecule attacks the carbonyl carbon, a tetrahedral intermediate is formed, which decomposes into carboxylic acid and alcohol (Figure 6.3.).
Figure 6.3. Acid-catalyzed hydrolysis of polyesters [53]
6.1.3. Enzymatic degradation of polyesters
In biological environment (river, sea, soil, human and animal body fluid), and when microorganisms are present, the polyesters can also hydrolyze [55]. This happens in the case of water insoluble polymers, where the degradation rate depends on the molecular weights. At high molecular weight the microbes cannot incorporate the polymers but by excretion of extracellular enzymes the polymers will be de-polymerized [56]. On the other hand, the smaller polymers could be incorporated into the cell and will be metabolized by the microorganism. During the de-polymerization water, methane, carbon dioxide and biomass are produced [57].
7. Medical application of biodegradable polyesters
The application of various natural or artificial polymers in the modern medicine is very important, which started several decades ago [58]. Some of them stay in the body forever
(implants), others only for shorter periods, thus they should be removed or excreted from the body. The biodegradable polyesters break down in the body.
The application of biodegradable polyester is widespread. They could be grouped by the functions: bonding (suturing, fixation adhesion); by the closure (covering, occlusion); by the separation (contact inhibition, isolation); on the basis of the scaffold (cellular proliferation) and the capsulation (controlled drug delivery).
New materials are continuously developed as they should possess desired properties for highly specific purposes. Important points at the special chemicals are the physical, biochemical and the degradation properties. It is not enough to fulfill one of the requirements but almost all at the same time. The processing techniques are also important as the procedures, the biopolyesters are formed, could improve one or more of the required characteristics.
There are a lot of advantages in the application of biodegradable polyesters, like easier physiological and less invasive repair or the possibility of tissue growth. On the other hand, the degradation products could be toxic, they can induce inflammation. The sterilization could also be difficult.
The advantage of biopolymers in the medical application is that the polyesters are tailor- maid from the point of view of chemical, physical and surface properties. These properties improve the in vivo cell adhesion and proliferation as well as allow keeping the original characteristics for long time. Additionally their degradation will not take harmful effects in the body.
As it was earlier mentioned, these polyesters have great potential for controlled drug delivery, play important role in wound management (suture, and surgical meshes) and are applicable for orthopedic devices (screws, pins, and rods) and in tissue engineering. It is important is to apply a series of degradable polymers with desired physical, chemical, biological, biomechanical and degradation properties with desired physical, chemical, biological, biomechanical and degradation properties for surgical sutures, implants etc..
In the last years, the highlight is on discovery of new tissue engineers, drug deliverers.
The poly(glycolic acid) could be used in short-term tissue engineering scaffolds and, as a filler, it can be coupled with other degradable polyester, because of the relatively rapid
degradation and insolubility in many organic solvents. It is often used in mesh networks applied in scaffold for bone, cartilage, tendon, in regeneration of intestinal, lymphatic system, and spinal cord. The rapid degradation results in loss of mechanical strength, local production of glycolic acid; though it is bioresorbable, but could cause inflammation.
The poly(lactic acid), which degrades slowly is seldom applid for drug delivery. To reduce the degradation time, this polyester was modified or co-polymerized. One possibility for the modification is the irradiation by beta or gamma rays, when carbon radicals are induced which lead to branching and cross-linking; this process results in decreased crystallinity. In this form, the polyester could be used for short-term controlled delivery application as well as bone fixator. The PLA is extensively utilized in tissue engineering as scaffolds for bone, cartilage, neural, and vascular regeneration. Its composites e.g. when the PLLA is combined with the raceme PDLLA, the new polyester poly(L-lactide-co-PDLLA) is much more applicable because of the amorphous structure.
The random polymerization of PLA and PGA forms poly(lactide-co-glycolide) [59].
The different PLGAs are applied for meshes, suture reinforcement, skin replacement.
This is an ideal biomaterial for temporary medical applications (controlled drug delivery, tissue engineering). It demonstrates good adhesion and proliferation in tissue engineering application. Nanospheres and nanofibers have been developed for controlled drug release.
This is the most investigated biodegradable polyester for biomedical application. Its preparation is easy, and it is used in sutures (since 1974) [60], applicable for tissue engineering scaffolds and for drug delivery (e.g. in chemotherapy) [61, 62], for the delivery of antibiotics [63-65], anti-inflammatory drugs [66, 67], and analgetics [68, 69].
The most common representative of the poly(hydroxyalkanoate) group is the poly(3- hydroxybutyrate), which is a good candidate for a long-term tissue engineering, but not really suitable for controlled delivery. The undesired character could be improved by copolymerization with PHB. This results in polyesters applicable in tissue engineering of bones [70-72], tendon [73], cartilage [74], and skin [75, 76]. For tissue engineering, the
collagen based poly(2-hydroxybutyrate-co-4-hydroxybutyrate) could be used where the collagen enhances the hydrophilicity.
Poly(caprolactone): Due to the very low in vivo degradation of this semi-crystalline polyester and the high drug permeability, it is often used as long-term implant delivery device [77]. The PCL has low tensile strength and very high elongation at break, this characteristic makes it a good elastic biomaterial [78]. It can be used as it is or in composites as tissue engineering scaffolds for bone regeneration [79-81], cartilage [82, 83], and skin [84, 85]. Surface plasma treatment of the polycaprolactone membrane can improve the adhesion and proliferation of human cells [86].
Poly(propylene fumarate): As this polyester has cross-linking ability, the polymer degradation depends on the cross-linking density and the molecular weight. This favorable property makes it suitable for biomedical application (bone filling, andlong- term delivery of ocular drugs [87-89].
The poly(anhydrides), which are surface eroding polymers, were originally developed as textile fibers in 1930s, but the hydrolytic instability did not allow its wide-spread usage.
Extensive study on the degradation (which depends on the backbone chemistry) led to polymers, where the deterioration rate could vary by orders of magnitude, as a result of co-monomers used. It helped in the drug delivery application such as chemotherapeutics [90, 91], antibiotics [92, 93], and vaccines [94-96].
The poly(sebacic anhydride) has limited application because of the rapid degradation.
Polyester blended by biomacromolecules: Other possibility to enhance the polyester’s biodegradability is the blending by biomacromolecules, like in the case of hyperbranched poly(acrylic acid-co-3-hydroxypropionate),, which matrix is good for drug delivery or hydrogel scaffold for tissue engineering.
It is essential to mention that not only chemical, physical and mechanical properties should be taken into account in the healthcare and medical textiles but other
considerations, as their engineering and application is also very important [97]. Also major factors are the biocompatibility, biodegradability in the healthcare application as well as the hygiene, wound care, grafts, and implantable. The characteristics of the biopolyesters are also affected by the structure of textiles (woven, knots, and nonwoven), which is related to specific medical uses.
8. Summary
This chapter summarizes the naturally occurring and synthetic polyesters. These synthetic biodegradable polyesters that could be used in the medical field are discussed in detail.
The synthetic routes via these biodegradable polyesters could be prepared are described and on the basis of the advantages and disadvantages, one can decide which is favorable for the production of the desired product. For example, by ring opening synthesis materials with high purity can be synthesized, but the yield is very low because of the many synthetic steps. The direct poly-condensation is a simple, one-pot technique with a disadvantage that the product is not homogeneous and needs further purification. Detailed information about the most important representatives of the synthetic polyesters summarizes not only the chemical, physical, and mechanical characteristics but the application possibilities together with the field, where they are used. In that case when the basic polyester does not have the proper attribute (mechanical, physical etc.) it could be further modified and improved by co-polymerization, irradiated) as long as the material with the proper characteristic is produced. There are important representatives for suture, drug delivery, prosthetic devices, and implants just to mention some of the application possibilities of these very important biodegradable polyesters.
References
[1] Scheirs, J., Long, T. E., (2003). Modern Polyesters: Chemistry and technology of polyesters and copolyesters. Wiley Series in Polymer Science. Series editor:
John Scheirs. John Wiley & Sons Ltd, Chichester, England. ISBN 0471498564.
[2] Zhang, C., (2015). Biodegradable polyesters: synthesis, properties, applications.
In: Biodegradable Polyesters. Editor: Stoyko Fakirov. Wiley-VCH Verlag Weinheim, Germany. ISBN 9783527330867.
[3] Albertsson, A.-C., Varma, I. K., (2002). Aliphatic Polyesters: Synthesis, Properties and Applications. In: Advances in Polymer Science. Volume 157.
Degradable Aliphatic polyesters. Volume editor: Ann-Christine Albertsson.
Springer-Verlag Berlin Heidelberg. ISBN 3540422498.
[4] Albertsson, A.-C., Karlsson, S., (1996). Macromolecular Architecture-Nature as a Model for Degradable Polymers. J. Macromol. Sci. Pure. Appl. Chem. A.
33(10), 1565-1570.
[5] Vert, M., Li, S. M., Spenlehauer, G., Guerin, P., (1992). Bioresorbability and biocompatibility of aliphatic polyesters. J. Mater. Sci. Mater. Med. 3(6), 432- 446.
[6] Rydz J., Zawidlak-Węgrzyńska B., Christova, D., (2015). Degradable polymers.
In: Mishra M.K., editor. Encyclopedia of Biomedical Polymers and Polymeric Biomaterials. CRC Press; Boca Ratón, FL, USA, ISBN 9781439898796.
[7] Lenz, R. W., (1993). Biodegradable polymers. In: Biopolymers I. Advances in Polymer Science. Volume 107. Editors: Peppas, N. A., Langer, R. S. Springer- Verlag Berlin, Heidelberg. ISBN 354056148X.
[8] Avérous, L., (2008). Polylactic acid: Synthesis, properties and applications. In:
Monomers, Polymers and Composites from Renewable Resources. Editors:
Belgacem M.N., Gandini A. Elsevier, Oxford, UK, 433–450, ISBN 9780080453163.
[9] Tian, H., Tang, Z., Zhuang, X., Chen, X., Jing, X., (2012). Biodegradable synthetic polymers: Preparation, functionalization and biomedical application.
Prog. Polym. Sci. 37, 237-280.
[10] Nair, L. S., Laurencin, C. T., (2007). Biodegradable polymers as biomaterials.
Prog. Polym. Sci. 32, 762-798.
[11] Khemani K., Scholz C., (2012). Introduction and overview of degradable and renewable polymers and materials. In: Degradable Polymers and Materials:
Principles and Practice. Second edition. Editors: Khemani K., Scholz C.
American Chemical Society, Washington, DC, USA, ISBN 9780841228221.
[12] Gomes, M., Azevedo, H., Malafaya, P., Silva, S., Oliveira, J., Silva, G., Sousa, R., Mano, J., Reis, R., (2008). Natural polymers in tissue engineering applications. Chapter 6 in: Tissue Engineering. Academic Press Series in Biomedical Engineering. Academic Press, Burlington, USA. ISBN 9780123708694.
[13] Brandl, H., Gross, R. A., Lenz, R. W., Fuller, R. C., (1990). Plastics from bacteria and for bacteria: Poly(β-hydroxyalkanoates) as natural, biocompatible, and biodegradable polyesters. In: Microbial Bioproducts, Advances in Biochemical Engineering/Biotechnology, Volume 41. Springer, Berlin, Heidelberg, 77-93.
[14] Ueda, H., Tabata, Y., (2003). Polyhydroxyalkanonate Derivatives in Current Clinical Applications and Trials. Adv. Drug. Deliv. Rev., 55, 501–18.
[15] Borden, M., Attawia, M., Khan, Y., Laurencin, C. T., (2002). Tissue Engineered Microsphere-based Matrices for Bone Repair: Design and Evaluation.
Biomaterials, 23, 551–559.
[16] Middleton, J. C., Tipton, A. J., (2000). Synthetic Biodegradable Polymers as Orthopedic Devices. Biomaterials, 21, 2335–2346.
[17] Goepferich, A., (1995). Polymer Bulk Erosion. Macromolecules, 30, 2598–
2604.
[18] Piskin, E., (1995). Biodegradable Polymers as Biomaterials. J. Biomat. Sci.
Polym. 6, 775–795.
[19] Tormala, P., (1992). Biodegradable Self-reinforced Composite Materials:
Manufacturing Structure and Mechanical Properties. Clin. Mater., 10, 29–34.
[20] Huang, Z. W., Laurent, V., Chetouani, G., Ljubimova, J. Y. Holler, E., Benvegnu, T., Loyer, P., Cammas-Marion, S., (2012). New functional degradable and bio-compatible nanoparticles based on poly(malic acid) derivatives for site-specific anti-cancer drug delivery. Int. J. Pharm., 423(1), 84–92.
[21] Ljubimova, J. Y., Fujita, M., Khazenzon, N.M., Lee, B.-S., Wachsmann-Hogiu, S., Farkas, D. L., Black, K. L., Holler, E., (2008). Nanoconjugate based on polymalic acid for tumor targeting. Chem. Biol. Interact., 171(2), 195–203.
[22] Holler, E., (1997). Poly (malic acid) from natural sources. In: Handbook of Engineering Polymeric Materials. Editor: Cheremisinoff, N.P., New York (NY), Marcel Dekker, 93–103, ISBN 9780824797997.
[23] Telegdi, J., Trif, L., Mihály, J., Nagy, E., Nyikos, L., (2015). Controlled synthesis and characterization of biodegradable, stereomer copolycondensates of L-malic acid. J. Therm. Anal. Calorim., 121, 663-674.
[24] Telegdi, J., Trif, L., Nagy, E., Molnár, N., Mihály, J., (2017). New comonomers in malic acid polyesters. J. Therm. Anal. Calorim., 129, 991-1000.
[25] Ferruti, P., Pence, M., D’Addato, P., Ranucci, E., Deghenghi, R., (1995).
Synthesis and properties of novel block copolymers containing poly(lactic- glycolic acid) and poly(ethyleneglyco1) segments. Biomaterials, 16, 1423-1428.
[26] Williams, C. K., (2007). Synthesis of functionalized biodegradable polyesters.
Chem. Soc. Rev., 36, 1573-1580.
[27] Cameron, D. J., Shaver, M. P., (2011). Aliphatic polyester polymer stars:
synthesis, properties and applications in biomedicine and nanotechnology.
Chem. Soc. Rev., 40, 1761-1776.
[28] Guérin, P., Francillette, J., Braud, C., Vert, M., (1986). Benzyl esters of optically active malic acid stereocopolymers as obtained by ring-opening polymerization of (R)-(+)- and (S)-(-)-benzyl malolactonates. Makromolekulare Chemie, Macromolecular Symposia, 6, 305-314.
[29] Cammas, S., Renard, I., Langlois, V., Guerin, P., (1996). Poly(β-malic acid):
obtaining high molecular weights by improvement of the synthesis route.
Polymer, 37, 4215-4220.
[30] Lenz, R. W., Vert, M., (1981). Malic acid polymers. US 4265247.
[31] Vert, M., (1998). Chemical routes to poly(β-malic acid) and potential applications of this water-soluble bioresorbable poly(β-hydroxy alkanoate).
Polym. Degrad. Stab., 59, 169-175.
[32] Dong, J., Bian, Z., (2011). Method for preparing biodegradable poly(malic acid) material. CN102002148.
[33] Kobayashi, N., Kajiyama, A., Taguchi, S., Tanaka, J., (2004). Manufacture of high-molecular weight poly(malic acid) with high yield. JP2004175999.
[34] Coulembier, O., Degee, P., Cammas-Marion, S., Guerin, P., Dubois, P., (2002).
New Amphiphilic Poly[(R,S)-β-malic acid-b-ε-caprolactone] Diblock Copolymers by Combining Anionic and Coordination-Insertion Ring-Opening Polymerization. Macromolecules, 35, 9896-9903.
[35] Kajiyama, T., Taguchi, T., Kobayashi, H., Kataoka, K., Tanaka, J., (2003).
Synthesis of high molecular weight poly(α,β-malic acid) for biomedical use by direct polycondensation. Polym. Degrad. Stab., 81, 525-530.
[36] Kajiyama, T., Kobayashi, H., Taguchi, T., Kataoka, K., Tanaka, J., (2004).
Improved synthesis with high yield and increased molecular weight of poly(α,β- malic acid) by direct polycondensation. Biomacromolecules, 5, 169-174.
[37] Wang, Z.-Y., Luo, Y.-F., Ye, R.-R., Song, X.-M., (2011). Synthesis of novel biodegradable material poly(lactic acid-trimesic acid) via direct melt copolycondensation and its characterization. J. Polym. Res. 18(4), 499-508.
[38] Vert, M., (2005). Aliphatic polyesters: Great degradable polymers that cannot do everything. Biomacromol., 6, 538–546.
[39] Iván, B., Telegdi, J., Bóta, A., (2009). Yearly report on ALMAACID. Project Number: OM-00161-164/2007; Hungary.
[40] Kobayashi, N., Kajiyama, A., Taguchi, S., Tanaka, J., (2005). Manufacture of malic acid copolymers. JP2005320426.
[41] Holler, E., (2010). Production of long chain unbranched β-poly(L-malic acid) by large scale Physarum cultivation and high-grade purification of the same.
US20100216199.
[42] Chi, Z., Liu, G. L., Liu, C. G., Chi, Z. M., (2016). Poly (β-L-malic acid) (PMLA) from Aureobasidium spp. and its current proceedings. Appl. Microbiol.
Biotechnol., 100, 3841–3851.
[43] Wan, Y., Cao, W., Su, Y., Shen, F., Chen, X., Yi, S., Zhao, F., (2010).
Fermentation process for manufacturing poly(β-malic acid) using Aureobasidium pullulans. CN101696434.
[44] Zhang, H., Cai, J., Dong, J., Zhang, D., Huang, L., Xu, Z., Cen, P., (2011).
High-level production of poly (β-L-malic acid) with a new isolated Aureobasidium pullulans strain. Appl. Microbiol. Biotechnol., 92, 295-303.
[45] Commandeur, S., van Beusekom, H. M. M., van der Giessen, W. J., (2006).
Polymers, Drug Release, and Drug-Eluting Stents. J. Interv. Cardiol. 19(6), 500-506.
[46] Jain, J. P., Ayen, W. Y., Domb, A. J., Kumar, N., (2011). Biodegradable polymers in drug delivery. Chapter 1 in: Biodegradable Polymers in Clinical Use and Clinical Development. Editors: Domb, A. J., Kumar, N., Ezra, A. John Wiley & Sons, Inc., Hoboken, New Jersey. ISBN 9780470424759.
[47] Shalaby, S. W., Burg, K. J. L., Absorbable and Biodegradable Polymers. In:
Advances in polymeric biomaterials series. CRC Press LLC. Boca Raton, Florida. ISBN 0849314844.
[48] Lenz, R. W., (1993). Biodegradable Polymers. In: Biopolymers I. Advances in Polymer Science, Volume 107. Editors: Peppas, N. A., Langer, R. S. Springer- Verlag Berlin, Heidelberg. ISBN 354056148X.
[49] Karlsson, S., Albertsson, A.-C., (1995). Degradation Products in Degradable Polymers. J. Macromol. Sci. Pure. Appl. Chem. A., 32(4), 599-605.
[50] Sisson, A. L., Schroeter, M., Lendlein, A., (2011). Polyesters. Chapter 1 in:
Handbook of Biodegradable Polymers, Synthesis, Characterization and Applications. Editors: Lendlein, A., Sisson, A. Wiley-VCH Weinheim, Germany. ISBN 9783527324415.
[51] Ikada Y., Tsuji, H., (2000). Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun., 21, 117–132.
[52] Azevedo, H. S., Reis, R. L., (2005). Understanding the enzymatic degradation of biodegradable polymers and strategies to control their degradation rate. In:
Biodegradable Systems in Tissue Engineering and Regenerative Medicine.
Editors: Reis R.L., Roman J.S. CRC Press, Boca Ratón, FL, USA, 177–201, ISBN 0849319366.
[53] Murthy, N., Wilson, S., Sy, J. C., (2012). Biodegradation of polymers. In:
Polymer Science: A Comprehensive Reference. Editors: Matyjaszewski K., Möller, M. Elsevier, Amsterdam, The Netherlands. 547–560, ISBN 9780080878621.
[54] McIntire, J. E., (1998). Polyester fibers. In: Handbook of Fiber Chemistry.
Second Edition. Editors: Lewin, M., Pearce, E. M. CRC Press, New York, NY, USA, 1–69, ISBN 0824794710.
[55] Swift, G., (1998). Requirements for biodegradable water-soluble polymers.
Polym. Degrad. Stab., 59, 19–24.
[56] Mueller, R.-J., (2006). Biological degradation of synthetic polyesters—
Enzymes as potential catalysts for polyester recycling. Process. Biochem., 41, 2124–2128.
[57] Walter, T., Augusta, J., Muller, R.-J., Widdecke, H., Klein, J., (1995).
Enzymatic degradation of a model polyester by lipase from Rhizopus. delemar.
Enzym. Microb. Technol., 17, 218–224.
[58] Ulery, B. D., Nair, L. S., Laurencin, C. T., (2011). Biomedical application of biodegradable polymers. J. Polym. Sci. B., Polym. Phys., 49(17), 832-864.
[59] Leung, L., Chan, C., Baek, S., Naguib, H., (2008). Comparison of morphology and mechanical properties of PLGA bioscaffolds. Biomedical Materials. 3, 025006.
[60] Conn, J., Oyasu, R., Welsh, M., Beal, J. M., (1974). Vicryl (polyglactin 910) synthetic absorbable sutures. Am. J. Surg., 128, 19–23.
[61] Betancourt, T., Brown, B., Brannon-Peppas, L., (2007). Nanomedicine. 2, 219–
232.
[62] Liu, J., Qiu, Z., Wang, S., Zhou, L., Zhang, S., (2010). A modified double- emulsion method for the preparation of daunorubicin-loaded polymeric nanoparticle with enhanced in vitro anti-tumor activity. Biomed. Mater., 5, 065002.
[63] Jeong, Y.-I., Na, H.-S., Seo, D.-H., Kim, D.-G., Lee, H.-C., Jang, M.-K., Na, S.- K., Roh, S.-H., Kim, S.-I., Nah, J.-W., (2008). Ciprofloxacin-encapsulated poly(dl-lactide-co-glycolide) nanoparticles and its antibacterial activity. Int. J.
Pharm., 352, 317–323.
[64] Jhunjhunwala, S., Raimondi, G., Thomson, A. W., Little, S. R., (2009).
Delivery of rapamycin to dendritic cells using degradable microparticles. J.
Contr. Rel., 133, 191–197.
[65] Farazuddin, M., Alam, M., Khan, A. A., Khan, N., Parvez, S., Dutt, G. U., Mohammad, O., (2010). Efficacy of amoxicillin bearing microsphere formulation in treatment of Listeria monocytogenes infection in Swiss albino mice. J. Drug Targeting., 18, 45–52.
[66] Zolnik, B. S., Burgess, D. J., (2008). Evaluation of in vivo–in vitro release of dexamethasone from PLGA microspheres. J. Contr. Rel., 127, 137–145.
[67] Eperon, S., Bossy-Nobs, L., Petropoulos, I. K., Gurny, R., Guex-Crosier, Y., (2008). A biodegradable drug delivery system for the treatment of postoperative inflammation. Int. J. Pharm., 352, 240–247.
[68] Tang, Y., Singh, J., (2008). Controlled delivery of aspirin: Effect of aspirin on polymer degradation and in vitro release from PLGA based phase sensitive systems. Int. J. Pharm., 357, 119–125.
[69] Vega, E., Gamisans, F., Garcia, M. L., Chauvet, A., Lacoulonche, F., Egea, M.
A., (2008). Journal of Pharmaceutical Biomedical Applications of Biodegradable Polymers
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3136871/35 of 68 5/3/2018, 5:06 PM Sciences. 2008;97:5306–5317.
[70] Ahmed, T., Marcal, H., Lawless, M., Wanandy, N. S., Chiu, A., Foster, L. J. R., (2010). Polyhydroxybutyrate and its Copolymer with Polyhydroxyvalerate as Biomaterials: Influence on Progression of Stem Cell Cycle. Biomacromol., 11, 2707–2715.
[71] Cool, S. M., Kenny, B., Wu, A., Nurcombe, V., Trau, M., Cassady, A. I., Grondahl, L., (2007). Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) composite biomaterials for bone tissue regeneration: In vitro performance assessed by
osteoblast proliferation, osteoclast adhesion and resorption, and macrophage proinflammatory response. J. Biomed. Mater. Res. A., 82A, 599–610.
[72] Ke, Y., Wang, Y. J., Ren, L., Zhao, Q. C., Huang, W., (2010). Modified PHBV scaffolds by in situ UV polymerization: Structural characteristic, mechanical properties and bone mesenchymal stem cell compatibility. Acta Biomaterialia., 6, 1329–1336.
[73] Rathbone, S., Furrer, P., Lubben, J., Zinn, M., Cartmell, S., (2010).
Biocompatibility of polyhydroxyalkanoate as a potential material for ligament and tendon scaffold material. J. Biomed. Mater. Res. A., 93A, 1391–1403.
[74] Liu, J., Zhao, B., Zhang, Y., Lin, Y., Hu, P., Ye, C., (2010). PHBV and predifferentiated human adipose-derived stem cells for cartilage tissue engineering. J. Biomed. Mater. Res. A., 94A, 603–610.
[75] Suwantong, O., Waleetorncheepsawat, S., Sanchavanakit, N., Pavasant, P., Cheepsunthorn, P., Bunaprasert, T., Supaphol, P., (2007). In vitro biocompatibility of electrospun poly(3-hydroxybutyrate) and poly(3- hydroxybutyrate-co-3-hydroxyvalerate) fiber mats. Int. J. Biol. Macromol., 40, 217–223.
[76] Ji, Y., Li, X.-T., Chen, G.-Q., (2008). Interactions between a poly(3- hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) terpolyester and human keratinocytes. Biomater., 29, 3807–3814.
[77] Darney, P. D., Monroe, S. E., Klaisle, C. M., Alvarado, A., (1989). Clinical evaluation of the Capronor contraceptive implant: Preliminary report. Am. J.
Obstet. Gynecol., 160, 1292–1295.
[78] Gunatillake, P., Mayadunne, R., Adhikari, R., (2006). Recent developments in biodegradable synthetic polymers. Biotechnol. Annual Rev., 12, 301–347.
[79] Pankajakshan, D., Philipose, L. P., Palakkal, M., Krishnan, K., Krishnan, L.K., (2008). Development of a fibrin composite-coated poly(ε-caprolactone) scaffold for potential vascular tissue engineering applications. J. Biomed. Mater. Res. B., 87B, 570–579.
[80] Plikk, P., Malberg, S., Albertsson, A.-C., (2009). Design of Resorbable Porous Tubular Copolyester Scaffolds for Use in Nerve Regeneration. Biomacromol., 10, 1259–1264.
[81] Zuo, Y., Yang, F., Wolke, J. G. C., Li, Y., Jansen, J. A., (2010). Incorporation of biodegradable electrospun fibers into calcium phosphate cement for bone regeneration. Acta Biomater., 6, 1238–1247.
[82] Vaquette, C., Kahn, C., Frochot, C., Nouvel, C., Six, J.-L., Isla, N. D., Luo, L.- H., Cooper-White, J., Rahouadj, R., Wang, X., (2010). Aligned poly(L-lactic- co-e-caprolactone) electrospun microfibers and knitted structure: A novel composite scaffold for ligament tissue engineering. J. Biomed. Mater. Res. A., 94A, 1270–1282.
[83] Li, J., Li, L., Yu, H., Cao, H., Gao, C., Gong, Y., (2006). Growth and Metabolism of Human Hepatocytes on Biomodified Collagen Poly(lactic-co- glycolic acid) Three-Dimensional Scaffold. ASAIO Journal., 52, 321–327.
[84] Garkhal, K., Verma, S., Tikoo, K., Kumar, N., (2007). Surface modified poly(L- lactide-co-ε-caprolactone) microspheres as scaffold for tissue engineering. J.
Biomed. Mater. Res. A., 82A, 747–756.
[85] Li, W.-J., Chiang, H., Kuo, T.-F., Lee, H.-S., Jiang, C.-C., Tuan, R. S., (2009).
Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: a pilot study. J. Tissue Eng. Regener. Med., 3, 1–10.
[86] Lengalova, A., Vesel, A., Feng, Y., Sencadas, V., (2016). Biodegradable Polymers for Medical Applications. Int. J. Polym. Sci., Volume 2016, Article ID 6047284, http://dx.doi.org/10.1155/2016/6047284
[87] Haesslein, A., Ueda, H., Hacker, M. C., Jo, S., Ammon, D. M., Borazjani, R. N., Kunzler, J. F., Salamone, J. C., Mikos, A. G., (2006). Long-term release of fluocinolone acetonide using biodegradable fumarate-based polymers. J. Contr.
Rel., 114, 251–260.
[88] Ueda, H., Hacker, M. C., Haesslein, A., Jo, S., Ammon, D. M., Borazjani, R. N., Kunzler, J. F., Salamone, J. C., Mikos, A. G., (2007). Injectable, in situ forming poly(propylene fumarate)-based ocular drug delivery systems. J. Biomed.
Mater. Res. A., 83A, 656–666.
[89] Hacker, M. C., Haesslein, A., Ueda, H., Foster, W. J., Garcia, C. A., Ammon, D. M., Borazjani, R. N., Kunzler, J. F., Salamone, J. C., Mikos, A.G., (2009).
Biodegradable fumarate-based drug-delivery systems for ophthalmic applications. J. Biomed. Mater. Res. A., 88A, 976–989.
[90] Agueros, M., Ruiz-Gaton, L., Vauthier, C., Bouchemal, K., Espuelas, S., Ponchel, G., Irache, J. M., (2009). Combined hydroxypropyl-β-cyclodextrin and poly(anhydride) nanoparticles improve the oral permeability of paclitaxel. Eur.
J. Pharm. Sci., 38, 405–413.
[91] Agueros, M., Zabaleta, V., Espuelas, S., Campanero, M. A., Irache, J. M., (2010). Increased oral bioavailability of paclitaxel by its encapsulation through complex formation with cyclodextrins in poly(anhydride) nanoparticles. J.
Contr. Rel., 145, 2–8.
[92] Krasko, M. Y., Golenser, J., Nyska, A., Nyska, M., Brin, Y. S., Domb, A. J., (2007). Gentamicin extended release from an injectable polymeric implant. J.
Contr. Rel., 117, 90–96.
[93] Brin, Y. S., Golenser, J., Mizrahi, B., Maoz, G., Domb, A. J., Peddada, S., Tuvia, S., Nyska, A., Nyska, M., (2008). Treatment of osteomyelitis in rats by injection of degradable polymer releasing gentamicin. J. Contr. Rel., 131, 121–
127.
[94] Salman, H. H., Irache, J. M., Gamazo, C., (2009). Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination. Vaccine., 27, 4784–4790.
[95] Carrillo-Conde, B., Schiltz, E., Yu, J., Minion, F. C., Phillips, G. J., Wannemuehler, M. J., Narasimhan, B., (2010). Encapsulation into amphiphilic polyanhydride microparticles stabilizes Yersinia pestis antigens. Acta Biomater., 6, 3110–3119.
[96] Tamayo, I., Irache, J. M., Mansilla, C., Ochoa-Reparaz, J., Lasarte, J. J., Gamazo, C., (2010). Poly(Anhydride) Nanoparticles Act as Active Th1 Adjuvants through Toll-Like Receptor Exploitation. Clinical and Vaccine Immunology., 17, 1356–1362.
[97] Zhong, W., (2013). An Introduction to Healthcare and Medical Textiles, DEStech Publications, Inc. Lancaster, Pennsylvania, USA, ISBN 9781605950204.
[98] Malafaya, P. B., Silva, G. A., Reis, R. L., (2007). Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 59(4-5) 207-233.
[99] Lee, S.-H., Shin, H., (2007). Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 59(4- 5) 339-359.
Table of content
page Biodegradable polyesters for medical applications
Abstract 1
1.Polyesters; a short historical overview 1
2.Biodegradable polymers 3
3.Classification of biodegradable polyesters: natural; synthetic 4
4.The most common synthetic polyesters 6
4.1Poly(α-hydroxy esters) 7
4.1.1 Poly(Glycolic acid) (PGA) 7
4.1.2 Poly(Lactic Acid) (PLA) 8
4.1.3 Poly(Lactic-co-Glycolic Acid) (PLGA) 8
4.1.4 Poly(Malic acid) (PMA) 9
4.1.5 Polydioxanone 10
4.1.6 Polycaprolactone (PCL) 10
4.1.7 Poly (trimethylene carbonate) (PTMC) 10 4.1.8 Poly(butylene succinate) (PBS) and its copolymers 11
5. Synthetic routes to polyesters 11
5.1 Ring-opening polymerization 11
5.2 Direct poly-condensation 12
5.3 Biotechnological synthesis 13
6. Degradation of natural and synthetic polyesters 13
6.1 Routes of polymer degradation 15
6.1.1 Alkali-catalyzed polyester hydrolysis 16 6.1.2 Acid-catalyzed polyester hydrolysis 17 6.1.3 Enzymatic degradation of polyesters 17 7. Medical application of biodegradable polyesters 17
8. Summary 21
References 21
![Figure 6.2. Alkaline-catalyzed hydrolysis of polyesters [53]](https://thumb-eu.123doks.com/thumbv2/9dokorg/1429229.121448/16.918.132.777.827.924/figure-alkaline-catalyzed-hydrolysis-of-polyesters.webp)
![Figure 6.3. Acid-catalyzed hydrolysis of polyesters [53]](https://thumb-eu.123doks.com/thumbv2/9dokorg/1429229.121448/17.918.145.779.341.533/figure-acid-catalyzed-hydrolysis-of-polyesters.webp)