Mineral Nutrition of Plants in Soils and in Culture Media
C . B O U L D A N D E . J. H E W I T T
Part 1. Mineral Nutrition of Plants in Soils by C. Bould 16
I. Historical: Soils and Plant Nutrition 16
A. Early Developments 16 B. Period of Rapid Scientific Development 18
II. Factors in the Occurrence and Distribution of Mineral Nutrients in the
Soil 22 A. Geochemistry 22
B. Pedology 25 III. Nature and Content of Soil Nutrients 27
A. Macronutrient Elements 27 B. Micronutrient Elements 32 IV. Factors in the Retention and Availability of Nutrients 38
A. Nature of the Soil Colloids 38 B. Cation and Anion Exchange 44 C. Fixation of Plant Nutrients 47 D. Soil pH and Acidity Complex 51 V. Nutrient Uptake from Soil 52
A. Soil Solution 53 B. Exchangeable Cations 56
C. Cation Exchange Properties of Roots 61
D. Bonding Energy of Clays 63 E. Phosphorus Nutrition 63
F. Ion Uptake 65 VI. Methods for Deterniining the Nutrient Requirement of Crops in the Field 65
A. Manurial Experiments 65
B. Soil Analysis 72 C. Plant Analysis 79 VII. Foliar Nutrition 91
A. Factors that Affect the Absorption of Foliar-Applied Nutrients. . 92 Part 2. Mineral Nutrition of Plants in Culture Media by E. J . H e w i t t . . . . 97 VIII. Early Experiments and the Development of Nutrient Culture Methods. 97
IX. The Essential Plant Micronutrients 99
A. Terminology 99 B. Discoveries of Essential Micronutrients 99
C. Criteria for the Determination of Essential Nutrient Requirements. 106 X. Experimental Methods for the Study of Micronutrient Requirements. . 109
A. Culture Containers 109 B. Rooting Media 110
15
C. Water 110 D. Nutrient Reagents 111
E. Cell Nutrient Reserves 113 F. Atmospheric Sources of Nutrients 113
G. Problems in the Exclusion of Specific Elements 114 H. Tests for Contamination and for Evaluation of Results 115 XL The Application of Culture Methods in the Study of Edaphic Factors. . 116
A. Soil Acidity and Crop Production 117 B. Adaptation to Edaphic Factors 117 References for Parts 1 and 2 120
P A R T 1. M I N E R A L N U T R I T I O N O F P L A N T S I N S O I L S by C. B O U L D
1. Historical: Soils and Plant Nutrition
A. E A R L Y D E V E L O P M E N T S
Speculation on t h e factors t h a t control p l a n t growth can be traced to R o m a n times a n d earlier. Cato ( 2 3 4 - 1 4 9 B.C.) was one of the earliest R o m a n agricultural writers. H e considered t h a t good ploughing was m o r e i m p o r t a n t t h a n m a n u r i n g b u t urged careful conservation of m a n u r e . A m o n g t h e practices advocated b y t h e Romans m a y be listed t h e following: (1) thorough tillage, (2) green m a n u r i n g , (3) crop rotation, (4) u s e of lime, (5) application of m a n u r e , a n d (6) growth of legumes for soil improvement. W i t h the fall of the R o m a n E m p i r e , agriculture, as well as other forms of civilization, lost ground for m a n y years. T h e historical account b y Russell (205) traces the gradual emergence of rational ideas of p l a n t n u t r i t i o n until, in t h e n i n e t e e n t h century, t h e y could be based on a n adequate knowledge of chemistry.
T h e period between 1630 a n d 1750 was taken u p w i t h a search for
" t h e principle of vegetation" w h e n , from t i m e to time, a n y one of the five " e l e m e n t s , " fire, water, air, earth, a n d niter, w a s considered to be t h e active ingredient of vegetable m a t t e r . It was d u r i n g this period t h a t V a n H e l m o n t (1577-1644) conducted his classic experiment w i t h a willow {Salix sp.) tree, although it is t h o u g h t t h a t h e w a s preceded in this t y p e of experiment b y Nicholas of Cusa ( 1 4 0 1 - 1 4 4 6 ) (see H a r v a r d Case Histories N o . 5, 1952). V a n H e l m o n t placed 200 pounds of oven- dried soil i n a pot, moistened it w i t h r a i n water, a n d planted i n it a willow shoot weighing 5 p o u n d s ; this h e allowed to grow for a period of five years. A t t h e end of this period h e weighed the tree a n d found it to weigh 169 pounds a n d about 3 ounces. T h e tree h a d received n o t h i n g b u t r a i n water, a n d the soil was covered w i t h a sheet to exclude dust.
A t t h e e n d of the experiment the soil weighed 200 pounds less about 2 ounces. V a n H e l m o n t concluded, therefore, t h a t t h e additional 164
pounds of wood, bark, a n d root arose from t h e w a t e r alone. T h e ex- p e r i m e n t w a s simple a n d convincing a n d satisfied a n o t h e r e m i n e n t chemist, Robert Boyle, w h o repeated t h e experiment w i t h " s q u a s h "
{Cucurbita sp.) a n d obtained similar results. Both experimenters con- cluded t h a t t h e substance of t h e p l a n t was, in each instance, produced from water. Nevertheless, the conclusion w h i c h t h e n appeared sound is entirely w r o n g because t h e y both overlooked t h e p a r t p l a y e d b y air a n d t h e missing 2 ounces of soil ( 2 0 5 ) .
Glauber, in 1656, obtained saltpeter from cattle m a n u r e a n d found t h a t it h a d great stimulating effect on p l a n t growth. H e concluded t h a t fertility of t h e soil a n d t h e value of m a n u r e s are entirely d u e to saltpeter. T h i s view was supported b y J o h n M a y o w , w h o estimated the a m o u n t of niter in the soil at different times of the year. H e showed t h a t it occurs i n greatest q u a n t i t y in t h e spring w h e n plants a r e just beginning to grow, b u t t h a t it is not to be found " i n soil on w h i c h plants grow a b u n d a n t l y , the reason being t h a t all t h e n i t e r of t h e soil is sucked out b y the p l a n t s . "
J o h n W o o d w a r d (283) published the results of a n interesting experi- m e n t in 1699. H e g r e w s p e a r m i n t {Mentha sp.) in (a) r a i n water, (b) w a t e r from t h e river T h a m e s , (c) effluent from H y d e P a r k conduit, a n d (d) effluent plus g a r d e n mold. A t t h e end of 77 days the plants w e r e weighed; t h e weights w e r e found to be in ascending order from (a) to ( d ) . Since all plants h a d a n a b u n d a n c e of water, the growth m u s t h a v e been related to t h e a m o u n t of sediment. W o o d w a r d concluded, there- fore, t h a t w a t e r could not be the principle of growth. H e ascribed growth to absorption of a "certain peculiar terrestial m a t t e r . " For m a n y years no outstanding advance was m a d e , except t h a t b y Stephen Hales (ca. 1727), who showed t h a t air is " w r o u g h t into the composition" of plants ( 2 0 5 ) .
Advances, however, w e r e being m a d e in agricultural practice, owing largely to t h e writings from 1731 o n w a r d of J e t h r o T u l l , a n English- m a n . H e held that: (a) all plants live on t h e same food, n a m e l y , fine soil particles; (b) pressure caused b y t h e swelling of t h e roots forced m i n u t e soil particles t h r o u g h t h e "lacteal m o u t h s of t h e roots" a n d hence to enter the circulatory system; (c) cultivation of t h e soil in- creased its fineness a n d t h u s t h e feeding o p p o r t u n i t y of p l a n t s ; (d) a rotation of crops is a convenience b u t not a necessity; (e) a n y soil will nourish a n y p l a n t if t e m p e r a t u r e a n d moisture supply a r e in proper adjustment; (f) applications of m a n u r e w e r e effective because t h e y brought about a fine, c r u m b l y soil condition. T h e position about this time can be s u m m e d u p i n Tull's o w n words: " I t is agreed t h a t the following materials contribute in some m a n n e r to the increase of plants,
but it is disputed w h i c h of t h e m is t h a t v e r y increase or food: (1) niter, (2) water, (3) air, (4) fire, (5) e a r t h " ( 2 0 5 ) .
D u r i n g the latter half of the eighteenth c e n t u r y great interest was taken i n agriculture. Beginning i n 1755 H o m e , a n English chemist, m a d e pot experiments to determine t h e effect of various substances on p l a n t growth. H e found t h a t saltpeter, Epsom salts, a n d potassium sul- fate all lead to increased growth, yet t h e y are t h r e e distinct salts. This w a s a big a d v a n c e because it showed t h a t p l a n t n u t r i t i o n depends on several factors. H o m e also established two methods for studying plant nutrition, n a m e l y , pot culture a n d p l a n t analyses.
Between 1770 a n d 1800 w o r k was done on the effects of vegetation on air. Joseph Priestley, i n 1775, investigated t h e effects of sprigs of m i n t {Mentha sp.) on vitiated air. H e found t h a t t h e m i n t m a d e the air purer, a n d concluded " t h a t plants, instead of affecting t h e air in t h e same m a n n e r as a n i m a l respiration, reverse t h e effects of breathing, a n d tend to keep t h e atmosphere p u r e a n d wholesome." But h e h a d not yet discovered oxygen a n d so could n o t i n t e r p r e t his discovery. I t w a s left to Ingen-Housz, i n 1779, to show t h a t purification goes on in light only, whereas vitiation takes place in darkness a n d plants h a d t h e n the same effect on air as did animals. J e a n Senebier, of Geneva, w e n t further a n d showed t h a t plants extract something from the air w h i c h h e des- ignated as "fixed air." F u r t h e r m o r e h e explained the growth of V a n H e l m o n t ' s willow tree on t h e basis of the absorption of this fixed air
( 2 0 5 ) .
B. P E R I O D O F R A P I D S C I E N T I F I C D E V E L O P M E N T
D u r i n g this period (1800-1880) m u c h progress was m a d e in t h e knowledge of chemistry, a n d t h e information obtained was applied to the study of soils a n d p l a n t growth, i n particular b y de Saussure a n d Liebig. T h e initiation of m o d e r n experimental methods i n p l a n t n u -
trition is largely d u e to de Saussure. H e showed: (a) t h a t t h e elements of w a t e r a r e fixed i n the p l a n t at the same time as the carbon; (b) t h a t t h e r e is n o n o r m a l n u t r i t i o n of t h e p l a n t without t h e u p t a k e of nitrates a n d m i n e r a l m a t t e r ; (c) t h a t the nitrogen in the p l a n t comes, not from t h e air as advocated b y Liebig, b u t from t h e soil. F u r t h e r , h e showed t h a t t h e root is n o t a m e r e filter; it takes in w a t e r m o r e readily t h a n dissolved matter, a n d it absorbs n o n n u t r i e n t elements. H e showed t h a t the composition of plant ash is not constant, b u t varies w i t h t h e n a t u r e of the soil a n d w i t h the age of the plant. U n f o r t u n a t e l y de Saussure's discoveries w e r e not accepted b y his contemporaries, w h o adopted the prevailing view t h a t plants d r a w their carbon a n d other n u t r i e n t s from soil h u m u s ( 1 9 5 ) .
T h i s latter view was ridiculed b y Liebig ( 1 4 6 ) in a report w h i c h h e prepared for t h e British Association for t h e A d v a n c e m e n t of Science in 1 8 4 0 , i n w h i c h h e s u m m a r i z e d t h e state of knowledge at t h a t time.
C o n t r a r y to general opinion at t h e time, h e stated quite categorically
" t h a t h u m u s i n t h e form i n w h i c h it exists in the soil does not yield the smallest n o u r i s h m e n t to p l a n t s . " T h i s was a reference to h u m u s as a direct source of p l a n t carbon, a view c u r r e n t l y held b y p l a n t physi- ologists. H e w e n t on to a r g u e " t h a t carbon m u s t be derived from other sources; a n d as soil does not yield it, it can only be extracted from the atmosphere." H e argued t h a t because t h e quantities of carbonic acid a n d oxygen i n t h e atmosphere r e m a i n u n c h a n g e d " a cause m u s t exist w h i c h prevents t h e increase of carbonic acid b y removing t h a t w h i c h is constantly forming, a n d there m u s t be some m e a n s of replacing the oxygen. Both these causes a r e u n i t e d i n the processes of vegetable life."
H e held, incorrectly, t h a t h u m u s nourishes plants " b y presenting a slow a n d lasting source of carbonic acid w h i c h is absorbed b y the roots, a n d is the principal n u t r i m e n t of y o u n g plants at a time w h e n , being destitute of leaves, t h e y a r e u n a b l e to extract food from the atmosphere."
H e m a i n t a i n e d t h a t all the h y d r o g e n necessary for t h e formation of organic compounds is derived from w a t e r a n d t h a t nitrogen is taken from the air, or soil, as a m m o n i a . W i t h r e g a r d to m i n e r a l n u t r i t i o n h e thought t h a t "all substances in solution in a soil a r e absorbed b y t h e roots of plants, exactly as a sponge inbibes a liquid, a n d all t h a t it con- tains, without selection." M a g n e s i u m a n d phosphate w e r e necessary for seed formation; alkalis w e r e needed to neutralize organic acids, a n d common salt, sulfate of potash, chloride of potassium, a n d other m a t t e r s w e r e necessary constituents of m a n y plants. I n order to m a i n t a i n soil fertility h e m a i n t a i n e d t h a t all those substances removed b y crops m u s t be r e t u r n e d to the soil. H e predicted t h a t " a t i m e will come w h e n fields will be m a n u r e d w i t h a solution of glass (silicate of p o t a s h ) , w i t h t h e ashes of b u r n t s t r a w a n d w i t h salts of phosphoric acid." Acting on this last conclusion Liebig m a d e u p a m i n e r a l fertilizer a n d placed it on t h e market. However, it failed because t h e m i n e r a l s w e r e first rendered insoluble b y fusion.
Liebig's report attracted a great deal of attention a n d finally killed t h e h u m u s theory, i.e., as a direct source of p l a n t carbon. L a t e r h e developed his m i n e r a l t h e o r y of n u t r i t i o n a n d gave it a quantitative form: " T h e crops on a field diminish or increase in exact proportion to the diminution or increase of t h e m i n e r a l substances conveyed to it in m a n u r e , " a n d still later h e added w h a t became k n o w n as t h e l a w of the m i n i m u m : " B y the deficiency or absence of one necessary con- stituent, all the others being present, the soil is r e n d e r e d b a r r e n for all
those crops to t h e life of which t h a t one constituent is indispensable."
Liebig's insistence that plants derive their nitrogen from a m m o n i a in the atmosphere a n d that the low content of phosphorus in the ash of t u r n i p proved t h a t the t u r n i p h a d a low phosphorus r e q u i r e m e n t , w e r e shown to be erroneous b y Lawes a n d Gilbert, w h o started their famous field experiments at Rothamsted, England, in 1843. These experiments w e r e conducted on the same general lines as those of Boussingault in Alsace. By 1855, t h e following points h a d been definitely settled: (a) crops r e q u i r e phosphates a n d salts of t h e alkalis, b u t t h e composition of p l a n t ash does not afford reliable information as to t h e a m o u n t s of each needed; (b) nonleguminous crops r e q u i r e a supply of some nitrogenous compound, nitrates a n d a m m o n i u m salts being equally good; (c) soil fertility m a y be m a i n t a i n e d for some years at least b y m e a n s of artificial m a n u r e s ; a n d (d) t h e beneficial effect of fallowing lies in the increase brought about in the available nitrogen compounds in t h e soil ( 2 0 5 ) .
A t about this t i m e W a y (273) carried out his i m p o r t a n t investiga- tion on t h e base-exchange properties of soil, the results of w h i c h will be described in Section IV, B. W a y noted t h a t t h e active ingredient in soil was clay a n d t h a t the exchangeable bases w e r e associated with the clay fraction.
T h e nitrogen n u t r i t i o n of plants r e m a i n e d a controversial problem for m a n y years. Liebig held t h a t a m m o n i a , b u t not gaseous nitrogen, was taken u p b y plants, a view confirmed b y Lawes, Gilbert, a n d P u g h
(142) i n 1861. Leguminous plants, however, still puzzled the in- vestigators. L a c h m a n n , 1858, first noted the presence of organisms in the nodules of leguminous plants, b u t his p a p e r attracted little attention.
I n 1885, Berthelot showed t h a t certain soil microorganisms could fix atmospheric nitrogen, a n d in t h e following y e a r Hellriegel a n d W i l - farth established the relationship between t h e root nodule organisms a n d t h e power of leguminous plants to fix nitrogen. Beijerinck, 1888, isolated t h e organism; t h u s another l a n d m a r k in the nutrition of plants in soils h a d been established.
By 1880 the following facts concerning the nutrition of plants had been established: (a) soils h a v e the ability to support vegetation for a n u n d e t e r m i n e d t i m e ; (b) soils a r e derived from rocks, yet pulverized rock is not soil; (c) w e a t h e r i n g has produced i m p o r t a n t changes in t h e chemistry of t h e derived soils; (d) m i n e r a l constituents are necessary for plants; (e) m i n e r a l constituents a r e obtained from the soil; (f) t h e m i n e r a l constituents absorbed b y plants come chiefly from a fraction of t h e soil; (g) t h e osmotic properties of t h e contents of root cells are related to their ability to absorb w a t e r from the soil; ( h ) the ratio of t h e m i n e r a l constituents absorbed b y t h e p l a n t differs from t h e ratio
existing i n the soil; (i) p l a n t growth is related i n t i m a t e l y to rainfall a n d t e m p e r a t u r e ; a n d (j) soil a m e n d m e n t s sometimes increase p l a n t growth ( 1 9 5 ) .
I n t h e early p a r t of t h e twentieth c e n t u r y considerable attention w a s paid to the soil solution, in particular b y K i n g a n d his co-workers at Wisconsin, a n d b y W h i t n e y a n d Cameron, of t h e U n i t e d States Depart- m e n t of Agriculture. C a m e r o n developed (54) the f u n d a m e n t a l ideas of t h e soil solution considered as a liquid phase, w h e r e i n t h e roots find ions which t h e y m a y absorb. H e showed t h a t the c o m m o n l y occurring m i n - erals in the soil are far m o r e soluble t h a n h a d been assumed a n d t h a t the dissolved substances reach a m o r e or less constant concentration. H e held t h a t no soil is ever in a state of final equilibrium, because of t h e effects of manifold physical a n d chemical factors. L a t e r Burd intro- duced (49) the concept of the " s u p p l y i n g p o w e r " of the soil. A v e r y dilute solution, accordingly, could afford enough n u t r i e n t s , if t h e sup- plying power w e r e adequate. For example, the absolute a m o u n t s of potassium or phosphate m i g h t not be adequate at a n y one m o m e n t , yet t h e y could suffice if the rate of release to the soil solution was sufficient to m a i n t a i n a fairly constant concentration. T h e formulation of the concept of the soil as a d y n a m i c system took place early in the twentieth c e n t u r y a n d liberated soil science from t h e domination of Liebig.
T h e work of W a y on base exchange in the previous century, al- though creating a m a r k e d impression at the time, was not followed u p u n t i l Gedroiz, in 1918, discovered t h a t t h e r e are differences in the readiness w i t h w h i c h soils absorb different ions ( 1 3 0 ) . For example, t h e potassium ion is a somewhat m o r e powerful replacer t h a n the sodium ion a n d is m o r e readily adsorbed b y the clay. T h e state of knowledge in 1924 concerning base exchange w a s s u m m a r i z e d b y Hissink (107) as follows: " T h e exchangeable bases are located on the surface of t h e soil particles; in other words t h e y occur in the adsorbed condition. T h e cause of this adsorption is to be sought in the chemical attraction be- t w e e n t h e bases a n d t h e soil acids (clay a n d h u m u s acids). W h e n the soil is treated with water, a soil suspension is formed. A p a r t of the surface molecules t h e n become ionized, forming a r o u n d the surface of the absorbing clay a n d h u m u s particles a n electrical double layer.
I n t h e i n n e r p a r t of this double l a y e r a r e found t h e anions of t h e soil acids, in the outer p a r t the cations; H+, M g+ +, C a+ +, K+, N a+. " Hissink also postulated t h e presence of free ions in true solution. Shortly after- w a r d it was shown b y Ross a n d S h a n n o n , b y Hendricks a n d F r y , a n d b y others, t h a t clays are composed of crystalline minerals h a v i n g a lattice structure (see Section IV, A, 1 ) . This enabled Kelley et al. (131) in 1931 to advance t h e idea t h a t easily replaceable ions, like calcium, are held on the outside of the crystal lattice of t h e clay particles and
hence a r e readily exchangeable. Others, like m a g n e s i u m a n d potassium, a r e held to some extent on t h e inside of the lattice a n d h e n c e are not so readily exchangeable unless t h e lattice is broken b y g r i n d i n g ; some m a g n e s i u m a n d potassium is, however, easily exchangeable a n d readily available for plant nutrition.
T h e discovery of t h e crystalline lattice structure of clay m i n e r a l s w a s a n i m p o r t a n t l a n d m a r k i n soil science a n d has helped in solving m a n y problems concerned w i t h t h e fixation of p l a n t n u t r i e n t s in soil.
T h e s e problems will be dealt w i t h in later sections.
II. Factors in the Occurrence and Distribution of Mineral Nutrients in the Soil
A. G E O C H E M I S T R Y
E a r l y i n t h e evolution of t h e earth, a condensation of m a t t e r from a hot gaseous stage to liquid a n d solid phases m u s t h a v e taken place.
Goldschmidt (83) advances t h e hypothesis t h a t equilibria in t h e partition of elements a m o n g metallic, semimetallic, a n d silicate phases h a v e been responsible for t h e distribution a n d m o r e or less effective elimination of certain elements from the m a t e r i a l of t h e earth's crust.
T h e distribution of t h e elements w a s essentially controlled b y their chemical affinities for oxygen a n d sulfur. As a suitable m e a s u r e of these affinities one can take t h e free energy of oxidation per g r a m atom of oxygen. E l e m e n t s t h a t h a v e a h i g h e r free e n e r g y of oxidation t h a n iron, e.g., silicon, a l u m i n u m , alkali, a n d alkaline earth metals, m a y be expected to concentrate i n the silicate crust d u r i n g primordial dif- ferentiation. Elements w i t h a lower free e n e r g y of oxidation, e.g., nickel a n d cobalt, are associated w i t h metallic iron.
T h e second step involves redistribution d u r i n g crystallization from liquid m a g m a s a n d t h e building u p of space lattices of atoms, or ions, depending on their atomic or ionic radii. Into such a lattice only those particles can enter w h i c h a r e of a size appropriate to t h e lattice spacings. Therefore the crystals act as a kind of sorting or sieving m e c h a n i s m allowing certain particles to enter a n d excluding others of unsuitable size. Radii a n d v a l e n c y t h u s regulate t h e distribution of elements i n t h e p r i m a r y m a g m a t i c rocks a n d in m i n e r a l s derived from t h e m . G e n e r a l l y the possibility of large-scale isomorphous substitution in minerals (see Section IV, A, 1) will be limited to such pairs of ions, t h e radii of w h i c h agree w i t h i n a tolerance of 1 0 - 1 5 % of t h e larger radius of the pair. F o r instance, m a g n e s i u m (0.78 A ) a n d ferrous iron (0.83 A ) freely replace each other in ionic crystals, but not m a g n e s i u m and calcium (1.06 A ) .
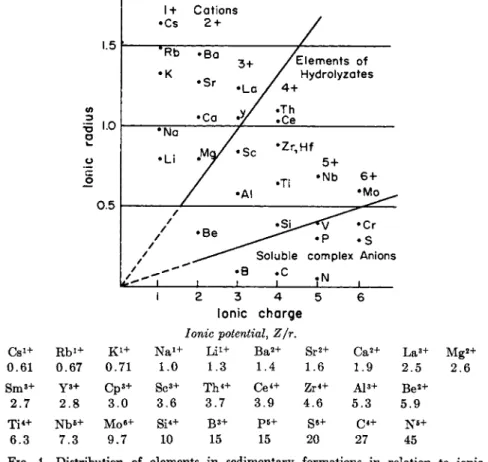
T h e t h i r d stage, i.e., t h e distribution of elements i n s e d i m e n t a r y formations is regulated b y t h e ionic potential ( t h e quotient between ionic charge a n d ionic r a d i u s ) . Substances w i t h low ionic potential (large ions w i t h small c h a r g e ) , such as sodium, potassium, calcium, a n d m a g n e s i u m , r e m a i n i n solution or m a y be adsorbed d u r i n g t h e process
1.5
0.5
•Cs 1 + Cations 2 + 'Rb
•K
•Bo
•Sr •La .
/Elements of / Hydrolyzates / 4+
•Ca V •Th
• Ce
•Να
•Li . M a / •Sc
•AI
•Zr,Hf 5+
•Ti -N b 6 +
• M o ^ .
/ /
/ / •Be
1
• S i ^ - ^ / ^Cr
^ ^ ^ ^ ·Ρ ^ S Soluble complex Anions
;B ;C I N ,
Ionic charge Ionic potential, Ζ jr.
C s1 + R b1 + K1 + N a1 + L i1 + B a2 + S r2 + C a2 + L a3 + Mg«-
0.61 0.67 0.71 1.0 1.3 1.4 1.6 1.9 2.5 2.6
S m3 + Y 3 + C p3 + S c3 + T h4 + C e4 + A l3 + B e2 +
2.7 2.8 3.0 3.6 3.7 3.9 4.6 5.3 5.9
T i4+ Nb*+ M oe + S i4 + B3 + p 5 + S«+ C4 + N5 +
6.3 7.3 9.7 10 15 15 20 27 45
FIG. 1. Distribution of elements in sedimentary formations in relation to ionic potential. From Goldschmidt (83).
of w e a t h e r i n g a n d transportation, those w i t h i n t e r m e d i a t e ionic po- tentials (between 2 a n d 12) a r e precipitated b y hydrolysis, a n d those w i t h still h i g h e r ionic potentials (above 12) form anions w i t h oxygen (Fig. 1 ) . Goldschmidt (84) likens t h e process of w e a t h e r i n g a n d t h e cycle of formation of s e d i m e n t a r y rocks to t h a t of a gigantic semi- quantitative chemical analysis i n w h i c h t h e following products a r e separated:
1. Insoluble residues such as sand or sandstone, w i t h such resistant minerals as q u a r t z a n d zircon
2. Hydrolyzates, such as bauxite, clay, a n d shales, w i t h h y d r a t e d oxides a n d hydrosilicates of a l u m i n u m
3. Oxidates such as m a n y sedimentary iron a n d m a n g a n e s e ores
4. Reducents such as coal, bituminous sediments, s e d i m e n t a r y sulfides a n d sulfur
5. Carbonates, such as limestone a n d dolomite
6. Evaporates containing such salts as chlorides, sulfates, a n d borates of alkali a n d alkaline earth metals.
T h e effect of this separation on t h e distribution of major a n d trace elements is shown in Table I.
T A B L E I
DEPOSITION OF SEDIMENTARY ROCKS WITH THEIR ASSOCIATED MAJOR AND TRACE ELEMENTS"
Process of Major Type of Main rock Associated trace
sedimentation constituents product types constituents
Si Al Si Κ
Fe Μη
Cu Mg Fe
Κ Na Ca Mg
Résistâtes Hydrolyzates
Oxidates
Carbonates
Evaporates
Sandstones Shales and bitumi- nous shales Bauxites Iron ores Manganese
ores Limestones,
dolomites Salt
deposits
Zr, Ti, Sn, rare earths Th, Au, Pt, etc.
V, U, As, Sb, Mo, Cu, Ni, Co, Cd, Ag, Au, Pt, B, Se
Be, Ga, Nb, Ti V, P, As, Sb, Se Li, K, Ba, B, Ti, W,
Co, Ni, Cu, Zn, Pb Ba, Sr, Pb, Mn
B , I
eF r o m Mitchell (168).
T h e distribution of m a n y m i n o r elements depends v e r y m u c h on their stage of oxidation. Iron, m a n g a n e s e , a n d cobalt are often im- mobilized as a result of processes of oxidation; sulfur, selenium, arsenic, v a n a d i u m , c h r o m i u m a n d m o l y b d e n u m m a y be mobilized b y oxidation to readily soluble complex anions, in accordance w i t h the rules of ionic potential. I n the processes of oxidation a n d reduction i m p o r t a n t bio-
chemical reactions participate a n d often predominate.
T h e fourth stage i n distribution of elements is represented b y bio- logical accumulation, as illustrated b y t h e accumulation of elements u n d e r forest covers. T h e elements a r e t h e n dissolved in t h e soil solution, t a k e n u p b y t h e roots, translocated to t h e leaves w h i c h in t u r n fall on to t h e soil surface. H e r e , d u r i n g t h e process of decomposition, t h e soluble n u t r i e n t s are leached out a n d the insoluble elements r e m a i n in t h e surface layer. Elements accumulated in this m a n n e r include boron, m a n g a n e s e , nickel, a n d cobalt.
B . P E D O L O G Y
Pedology originated in Russia w i t h t h e classic researches of Doku- chaev (1877 a n d thereafter) a n d his pupils ( 1 2 4 ) . Like other n a t u r a l sciences pedology started out w i t h the descriptive phase. T h e soil body was cut open vertically a n d t h e exposed surfaces w e r e described. I n a m a t u r e state, the soil body revealed a definite construction consisting of distinct layers, k n o w n as horizons, w h i c h a r e specific in their morphological characters irrespective of t h e geographic position of the soil a n d of t h e u n d e r l y i n g geological formation, provided it is located in identical climatic zones. T h e genetically related exposed horizons of a vertical cut in t h e soil body, taken as a unit, comprise w h a t is k n o w n as t h e soil profile. Pedology begins, therefore, w i t h a profile study of t h e soil body a n d aims to u n r a v e l t h e f u n d a m e n t a l laws w h i c h govern the processes of soil formation in relation to weathering, one of t h e p r i m a r y physicochemical forces of n a t u r e responsible for t h e genesis of the soil. A knowledge of these laws of soil formation enables one to predict t h e geographical distribution of soils. T h u s t h e zone of the podsol soils is typical of the temperate h u m i d regions; the chernozem is found in less h u m i d regions, such as the steppes of Russia;
t h e lateritic soils are typical of the tropics. One of the f u n d a m e n t a l laws of pedology, formulated b y Dokuchaev is 4 4 t h e l a w of t h e adaptability of soil types of t h e globe to n a t u r a l ( p r i m a r i l y climatic) conditions"
( 1 2 4 ) .
T h e factors concerned in soil formation are p a r e n t material, topog- r a p h y (the configuration of the soil surface), climate, a n d organisms.
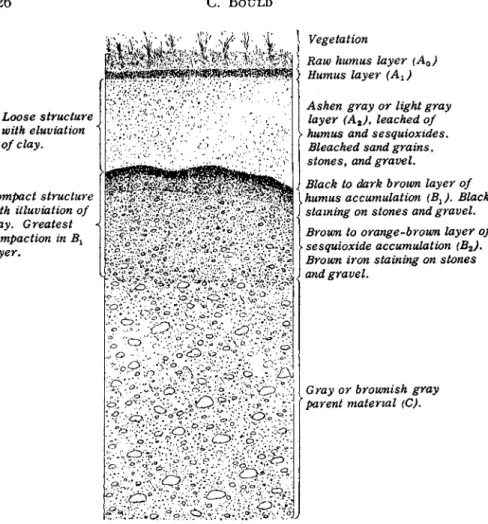
Soil is a n independent, d y n a m i c body of n a t u r e t h a t acquires properties in accordance w i t h the forces w h i c h act u p o n it. It m a y be defined as t h a t m a t e r i a l w h i c h occupies the outermost p a r t of the earth's crust a n d the character of w h i c h varies from t h e surface to the p a r e n t m a - terial (see Fig. 2 ) .
One of the most i m p o r t a n t changes in the minerals of the p a r e n t material d u r i n g soil development is their diminution in particle size.
T h i s in t u r n leads to greater chemical activity, since most of the chemi- cal reactions involve p r i m a r i l y t h e u n i t cells exposed at t h e surface of
Loose structure with eluviation ^ of clay.
Compact structure with illuviation of clay. Greatest compaction in Bl
layer.
Vegetation
|$| } Raw humus layer (A0) Humus layer (Ax) Ashen gray or light gray
layer (A2), leached of
\ humus and sesquioxides.
Bleached sand grains, stones, and gravel.
\ Black to dark brown layer of
\ humus accumulation (B1 ) . Black staining on stones and gravel.
Brown to orange-brown layer of sesquioxide accumulation (B2).
Brown iron staining on stones and gravel.
ο -
.ill . . · < * · · » . ·' ο
Gray or brownish gray parent material (C).
FIG. 2. Diagrammatic representation of a podsol profile, showing soil horizons.
From Robinson (204).
a crystal particle. T h e reactions t h a t lead to changes i n composition a n d availability of n u t r i e n t s include: ion exchange, hydrolysis, solution, dif- fusion, oxidation-reduction, a n d adsorption a n d swelling d u e to h y d r a - tion. W a t e r is essential i n all these reactions. O n e of t h e most i m p o r t a n t soil reactions is illustrated b y t h e loss of K+ from biotite, w h i c h m a y be considered as a n e x c h a n g e reaction, t h u s :
Κ biotite + H+—• Η biotite + K+
or as t h e result of a hydrolysis reaction, t h u s :
Κ biotite + H20 -> Η biotite + K+ + OH~
but since p a r t of t h e biotite goes into solution, t h e reaction m a y also be viewed as solution.
T h e s e t h r e e reactions, exchange, hydrolysis, a n d solution, constitute t h e p r i m a r y loss of bases from minerals.
Diffusion of cations into a n d out of t h e interior of crystal particles is responsible for converting one t y p e of m i n e r a l to a n o t h e r type.
Oxidation reactions are of p r i m a r y i m p o r t a n c e i n m i n e r a l s t h a t con- tain ferrous iron. Because t h e electrostatic n e u t r a l i t y of a crystal struc- t u r e m u s t be m a i n t a i n e d , oxidation of ferrous to ferric iron brings about t h e expulsion of some other cation. Such departures leave e m p t y posi- tions, w h i c h cause t h e structure to become unstable, t h e r e b y accelerat- ing w e a t h e r i n g .
Adsorption of water, H+ or OH", is t e r m e d h y d r a t i o n , a n d is t h e first step i n t h e release of oxides from crystal particles ( 1 8 ) .
T h e adsorption of organic substances, such as a m i n o acids a n d pro- teins, w i t h i n t h e spacings between successive layers of clay causes large particles to disintegrate, besides giving a degree of protection to the organic substance from microbiological decomposition.
III. Nature and Content of Soil Nutrients
A. M A C R O N U T R I E N T E L E M E N T S
Ï. Nitrogen
N i t r o g e n occurs p r i m a r i l y i n organic combination. I t is present p a r t l y i n t h e form of original nitrogenous p l a n t constituents, somewhat modified, a n d p a r t l y as microbial cells. F r e e a m i n o acids h a v e also been isolated from certain soils. M u c h of t h e so-called " h u m u s " consists of a lignin-protein complex w h i c h on acid hydrolysis gives approximately one-third of t h e total nitrogen as α-amino acids. F r o m this it is deduced t h a t at least one-third, or m o r e , of soil organic nitrogen is in protein form ( 4 4 ) . I n addition to a m i n o acids, soils contain a m i n o sugars. T h e r e is also some evidence for t h e occurrence of nucleic acids ( 2 7 ) .
Complex organic nitrogen compounds m u s t first be mineralized be- fore t h e nitrogen can be utilized b y plants. T h e biological transforma- tion occurs thus: organic nitrogen -> a m m o n i u m - » nitrite -> nitrate, t h e a m o u n t of n i t r a t e finally produced depending on the relative a m o u n t s of easily decomposable carbonaceous materials present ( 2 2 4 ) . If t h e carbon : nitrogen ratio is high little, if a n y , nitrogen will appear as nitrate, t h e intermediate compounds being utilized b y microorgan- isms for further decomposition of organic substances. T h e mineraliza- tion a n d immobilization of nitrogen i n soil has been reviewed recently
b y W i n s o r ( 2 7 9 ) . Inorganic nitrogen occurs in soils as nitrous and nitric oxides, a m m o n i u m , nitrite, a n d nitrate. T h e first two are gases a n d are present only in trace amounts. A m m o n i u m ion is usually adsorbed on the soil colloids, b u t it is n o w k n o w n that, like potassium ions, it m a y be fixed in the expanding crystal lattices of clay minerals such as illite, vermiculite, a n d montmorillonite. Recent studies h a v e shown t h a t 3 - 8 % of the total nitrogen in surface soils, a n d 9 - 4 4 % of the nitrogen in subsoils, was i n the form of fixed a m m o n i u m ( 4 5 ) . Nitrite is present only in trace a m o u n t s as a transition compound, a n d practically all the nitrate is present in the soil solution.
T h e surface layer of cultivated soils contains between 0.02 a n d 0 . 4 % nitrogen, the a m o u n t depending largely on soil type, t e m p e r a t u r e , a n d rainfall. Climate plays a d o m i n a n t p a r t in d e t e r m i n i n g t h e nitrogen status of soils. W i t h i n belts of u n i f o r m moisture conditions a n d com- parable vegetation, t h e average nitrogen a n d organic m a t t e r contents of the soil decrease exponentially as the a n n u a l t e m p e r a t u r e rises ( 1 2 0 ) .
Some of t h e earliest work on t h e absorption of nitrogen b y plants was carried out b y Boussingault, a n d b y Ville from 1837 o n w a r d ( 1 9 5 ) , but the source of p l a n t nitrogen was probably first demonstrated b y J o h n Lawes of Rothamsted about 1840. H e collected r a i n a n d determined its nitrogen content. Since this a m o u n t e d only to 3-5 pounds per acre a n n u a l l y h e assumed t h a t plants obtained the r e m a i n d e r from the soil.
H e t h e n tried applying m i n e r a l forms of nitrogen to his w h e a t (Trit- icum) crops. T h e enhanced growth a n d increased yield resulting from the minerals demonstrated t h a t crops derive most of their nitrogen from the soil ( 1 4 2 ) .
2. Phosphorus
Soil phosphorus m a y be divided into two p r i m a r y classes, organic a n d inorganic. Organic phosphorus occurs in t h e form of phospholipids, nucleic acids a n d inositol phosphates. Phospholipid phosphorus seldom exceeds 3 parts per million ( p p m ) , b u t values as high as 34 p p m h a v e been reported ( 2 7 ) . Values t h a t r a n g e from 17 to 5 8 % have been found for nucleic acid phosphorus, a n d in certain Iowa soils from 41 to 4 9 % of the organic phosphorus was present in the form of p h y t i n a n d lower phosphoric acid esters of inositol. Organic phosphorus, expressed as a percentage of total phosphorus, m a y r a n g e from 2.6 to 7 5 % . It would appear t h a t the phosphorus in these organic compounds m u s t first be mineralized before it can be absorbed b y plants.
Inorganic phosphorus occurs in m a n y forms, t h e n a t u r e a n d extent depending largely on soil p H . A small fraction, n o r m a l l y less t h a n 1 p p m , is present in the soil solution a n d is in equilibrium w i t h adsorbed
phosphorus. I n acid soils, most of t h e inorganic phosphorus is associated with iron a n d a l u m i n u m compounds a n d to a lesser degree w i t h clay minerals, such as dufrenite, vivianite, a n d wavelite. Recently the presence of certain dihydroxy-dihydrogen phosphates of iron a n d alumi- n u m , k n o w n as strengite [ F e ( O H )2H2P 04] a n d variscite [ A l ( O H )2- H2P 04] , h a v e been identified (138, 143). I n alkaline a n d calcareous soils phosphorus is present largely as apatite, hydroxyapatite, and carbonatoapatite.
T h e total phosphorus content of the lithosphère (outer crust of the earth) averages 0 . 2 8 % P205, b u t most surface soils contain from 0.022 to 0 . 0 8 3 % of phosphorus. Total phosphorus values are of little use in predicting t h e response to phosphatic fertilizer. For t h e purpose of estimating " a v a i l a b l e " phosphorus, a n u m b e r of chemical (278) and biological tests (257) h a v e been devised. Chemical methods involve the use of dilute organic a n d m i n e r a l acids, a n d biological methods include t h e N e u b a u e r , Mitscherlich, a n d Aspergillus niger tests (see Section V I , B ) .
Liebig, in 1840, a n d W a y , in 1850, w e r e a m o n g the first investigators to observe t h a t phosphate was retained b y soils. Lawes a n d Gilbert (1843-1855) demonstrated t h e response of field crops to soluble phos- phates a n d thus laid t h e foundation of the phosphatic fertilizer industry.
Ville, 1861, appears to h a v e been one of t h e first investigators to show t h a t phosphorus is necessary for all p l a n t growth. H e also showed that, i n order to serve as a p l a n t n u t r i e n t , t h e phosphorus m u s t b e in the form of phosphate ( 1 9 5 ) .
3. Potassium
Potassium occurs as p r i m a r y a n d w e a t h e r e d minerals, a n d in non- exchangeable, exchangeable, a n d water-soluble forms. T h e most impor- t a n t potassium-containing minerals a r e orthoclase a n d microcline feldspar ( K A l S i308) , muscovite [ K ( A l S i3O1 0) A l2( O H , F )2] , biotite [ K ( A l S i3O1 0) M g 3( O H , F )2] , a n d t h e clay m i n e r a l , illite. T h e n a t u r e of these soil minerals has been changed somewhat from the original; t h e y h a v e lost some interlayer potassium a n d gained some w a t e r of h y d r a - tion.
F o r p l a n t n u t r i t i o n t h e exchangeable a n d water-soluble forms a r e the most readily available, t h e nonexchangeable potassium acting as a reserve. T h e u p p e r lithosphère contains 2 . 5 9 % potassium ( 8 5 ) . Bear et al. (19) found t h e average distribution of potassium in 20 N e w Jersey soils as follows: exchangeable, 0.2 m e q ; nonexchangeable 46.3 m e q ; total, 46.5 m e q / 1 0 0 g m soil.
It has been shown b y a n u m b e r of studies t h a t exchangeable and
"available" potassium are b y no m e a n s identical. As m u c h as 5 0 % of t h e total potassium absorbed b y crops m a y come from t h e nonexchange- able fraction. Exchangeable potassium m a y be determined b y leaching the soil w i t h n e u t r a l salts. " A v a i l a b l e " potassium m a y be determined b y extraction w i t h 1 % citric acid, n e u t r a l n o r m a l a m m o n i u m acetate, sodium acetate-acetic acid buffer solution ( M o r g a n ' s r e a g e n t ) , or b y biological tests ( N e u b a u e r ) .
Although Birner a n d L u c a n u s , 1866, experimenting w i t h oats in w a t e r culture, gave t h e first proof t h a t potassium is essential for flower- ing plants, potassium deficiency in field crops w a s first described b y W i l f a r t h a n d W i m m e r in 1902 ( 1 5 4 ) , a n d for fruit crops b y W a l l a c e
(265) in 1921.
4. Calcium
T h e most i m p o r t a n t p r i m a r y calcium minerals in t h e soil are anorthite ( C a A l2S i208) a n d pyroxenes of t h e t y p e C a M g ( S i 03)2. I n addition, small a m o u n t s of calcium m a y be present as borosilicates.
Calcite ( C a C 03) m a y be t h e d o m i n a n t source in some soils, a n d dolo- m i t e [ C a M g ( C 03)2] in others. A variety of calcium phosphates gen- erally occur in soil. T h e most i m p o r t a n t are apatites, C a5( P 04)3F and C a5( P 04)3C l , a n d hydroxyapatite, C a5( O H ) ( P 04)3. Calcium sulfate, or g y p s u m , m a y be present in surface soils or subsoils to some extent.
T h e black e a r t h soils, including chernozems (black e a r t h soils of Russia) a n d rendzinas ( h u m u s soils arising from limestone or g y p s u m ) , are v e r y high in calcium carbonate, reaching values of 4 0 - 5 0 % .
T h e calcium most readily available for p l a n t n u t r i t i o n includes the water-soluble a n d exchangeable fractions. I n fertile soils the exchange- able calcium should constitute from 70 to 8 0 % of the total exchange- able bases. It is usually determined b y leaching t h e soil w i t h n e u t r a l salts.
Calcium deficiency is usually associated w i t h acidity effects, and it is often difficult to differentiate one from t h e other ( 1 0 0 ) . T h e effects of calcium deficiency on tobacco u n d e r field conditions a n d solution culture are identical ( 1 5 4 ) . Stohman, 1862, concluded t h a t calcium w a s necessary for green plants, a n d Wolf, 1864, appears to h a v e dis-
covered t h a t calcium h a d a stimulating effect on root growth ( 1 9 5 ) . Excess calcium, associated with alkaline p H , m a y lead to deficiencies of iron, m a n g a n e s e , copper, boron, a n d zinc.
5. Magnesium
M a g n e s i u m is present in soil as water-soluble, exchangeable, fixed a n d p r i m a r y m i n e r a l forms. It is found chiefly i n t h e c o m m o n l y occurring aluminosilicate minerals, such as biotite, augite, hornblende,
a n d montmorillonite. I n regions of limited rainfall, dolomite [ M g C a ( C 03)2] , magnesite ( M g C 03) , a n d epsomite ( M g S 04- 7 H20 ) m a y con- stitute appreciable sources of this element.
T h e lithosphère contains a n average of 2 . 6 8 % of m a g n e s i u m . Soils v a r y considerably i n their m a g n e s i u m content according to their geo- logical origin. Jacob (116) gives t h e r a n g e of m a g n e s i u m in soils from E u r o p e a n countries as 0.006 to 1.0% of M g O . T h e total m a g - n e s i u m content of a soil is not necessarily a reliable guide to its availability. T h i s m a y be determined b y biological or chemical methods.
T h e availability of m a g n e s i u m depends not only on t h e total a m o u n t present, b u t on the a m o u n t in relation to the exchange capacity of t h e soil colloids, a n d on t h e n a t u r e of t h e c o m p l e m e n t a r y ions. According to P r i n c e et ah ( 1 9 1 ) , if m a g n e s i u m constitutes less t h a n 6 % of the exchange cations of the soil, crops growing on t h a t soil a r e likely to respond to applications of m a g n e s i u m . T h e ideal a m o u n t of m a g n e s i u m is believed to be about 1 0 % of the total exchange capacity of the soil.
F u r t h e r m o r e t h e ratio K : M g should not greatly exceed 2 : 1 .
Although t h e researches of Willstätter, 1906, h a d shown t h e presence of m a g n e s i u m i n chlorophyll, m a g n e s i u m deficiency in plants in the field w a s not diagnosed u n t i l G a r n e r et al. (81) showed it to be re- sponsible for t h e condition k n o w n as " s a n d d r o w n " in tobacco {Nico- tiana tabacum). T h e symptoms of m a g n e s i u m deficiency in a wide r a n g e of crops a r e described b y Jacob (116) a n d W a l l a c e ( 2 6 6 ) . Deficiency is clearly linked w i t h soil type: it occurs m a i n l y on podsolic soils b u t is not to be expected on black earths.
6. Sulfur
Sulfur is present in soils i n both inorganic a n d organic forms. I n h u m i d soils the bulk is present as p y r i t e ( F e S2) , sphalerite ( Z n S ) , chalcopyrite ( C u F e S2) , cobaltite (CoAsS), a n d v a r y i n g a m o u n t s of g y p s u m a n d epsomite. Field soils of h u m i d t e m p e r a t u r e regions h a v e 5 0 - 5 0 0 p p m of sulfate soluble in w a t e r or w e a k acids. T h e total sulfur in these soils ranges from 0.01 to 0 . 1 5 % . I n arid a n d semiarid soils m u c h of t h e total sulfur is present as soluble sulfates of calcium, m a g n e s i u m , potassium, a n d sodium. I n glasshouse soils accumulation of soluble sulfates m a y cause root i n j u r y a n d depression of p l a n t growth.
F r o m t h e time of Liebig it has been k n o w n t h a t sulfates are necessary for p l a n t growth.
Sulfur deficiency is v e r y r a r e in industrial countries. T h e so-called
"yellows" disease of tea {Camellia sinensis [Thea sinensis'}) has been reported b y Storey a n d Leach (238) to be d u e to sulfur deficiency.
U n d e r field conditions it has also been reported in tobacco {Nicotiana tabacum), sugar cane {Saccharum officinarum), soybean {Glycine
max), citrus {Citrus spp.) a n d s u b t e r r a n e a n clover {Trifolium sub- terraneum).
B. M I C R O N U T R I E N T E L E M E N T S
1. Iron
Iron is present in appreciable a m o u n t s in minerals, h y d r a t e d oxides similar to goethite and limonite, a n d as the sulfide. It is also present in organic complexes. T h e total F e203 content of soils varies from about 2 - 6 % in n o r m a l t e m p e r a t e soils to as m u c h as 6 0 % in t h e ferruginous latosols (tropical soils).
It would appear t h a t the ferrous form is most available for p l a n t nutrition. As t h e soil becomes m o r e alkaline t h e iron becomes oxidized to the ferric form, w h i c h is relatively unavailable to plants. U n d e r alkaline soil conditions plants m a y suffer from iron deficiency often referred to as lime-induced iron deficiency. H e a v y metals w h e n present in excess a m o u n t s m a y also induce iron deficiency. U n t i l recently h e a v y metal-induced iron deficiency w a s difficult to control u n d e r field con- ditions, b u t w i t h the introduction of synthetic iron chelates, such as iron ethylenediaminetetraacetic acid ( F e - E D T A ) , t h e control of m e t a l toxicity u n d e r acid soil conditions is n o w possible. T h i s was first demonstrated b y Stewart a n d L e o n a r d ( 2 3 4 ) .
T h e first n u t r i e n t deficiency ever to be reported, b y Gris in 1844, appears to be t h a t of iron ( 9 0 ) . Excess m a n g a n e s e is reported to in- duce iron deficiency in pineapple {Ananas comosus), a n d excess copper causes chlorosis of citrus in Florida. Bennett (20) was the first to treat lime-induced chlorosis in fruit trees b y t r u n k injection of iron salts.
This m e t h o d has been superseded b y the use of soil dressings a n d foliar sprays of iron chelates, t h e most successful being iron-ethylenediamine bis (o-hydroxyphenylacetic acid).
2. Copper
Chalcopyrite ( C u F e S2) is the most i m p o r t a n t copper compound in p r i m a r y rocks, a n d n a t u r a l deposits of copper sulfide h a v e probably originated from it. Field experiments h a v e shown t h a t copper sulfide
acts as a source of copper for p l a n t growth.
T h e average copper content of the lithosphère is 70 p p m ( 2 4 1 ) . T h e total copper content of soils ranges from 2 to 100 p p m of w h i c h about 1 p p m m a y be extracted b y dilute hydrochloric acid. Availability depends on t h e relative a m o u n t s of copper in t h e exchangeable, m i n e r a l , a n d organically complexed form. It is assessed b y extraction in dilute m i n e r a l acids, b y buffered salt solutions, or biologically using the
fungus Aspergillus niger (see Section V I , B ) . Extraction of D a n i s h soils w i t h H C l at p H 2 gave values v a r y i n g from < 0 . 0 5 to > 1 p p m , organic soils generally giving l o w values. T h e earliest instances of copper deficiency u n d e r field conditions occurred i n citrus i n Florida i n 1 8 7 5 ( 2 3 5 ) . T h e deficiency gave rise to symptoms k n o w n as
" e x a n t h e m a " or die-back. Copper deficiency i n other fruit trees w a s recorded i n t h e U n i t e d States i n 1 9 2 8 , a n d Sjollema in 1 9 3 3 attributed the " r e c l a m a t i o n " disease of cereals, a n d other crops, to a deficiency of copper. Excess copper induces iron deficiency, as noted b y Reuther a n d Smith ( 1 9 7 ) i n citrus.
3 . Zinc
Z i n c occurs i n ferromagnesian minerals, magnetite, biotite, a n d hornblende. Most zinc-bearing minerals a r e readily weathered, t h e zinc so released is probably adsorbed onto colloids as a divalent cation or is complexed b y organic m a t t e r . Nelson a n d Melsted ( 1 7 2 ) investigated t h e fate of zinc added to n e u t r a l a n d acid soils. W i t h a n acid soil, practically all t h e zinc could be replaced b y a m m o n i u m acetate, whereas w i t h a calcium soil p a r t of t h e zinc could be recovered only b y re- peated leachings w i t h dilute hydrochloric acid. This acid-soluble zinc did n o t occupy exchange sites because t h e r e was no reduction in t h e exchange capacity of t h e soil.
S w a i n e ( 2 4 1 ) gives t h e a b u n d a n c e i n t h e lithosphère as 8 0 p p m . N o r m a l soils contain 1 0 - 3 0 0 p p m of total zinc. Total zinc content of soils is n o t necessarily related to availability. T u c k e r a n d K u r t z ( 2 5 4 ) compared several methods of extracting available zinc a n d found that t h e bioassay, dithizone, a n d 0 . 1 TV H C l procedures w e r e t h e most satisfactory. Dilute acids, such as 0 . 5 Ν acetic acid, m a y remove u p to 3 0 p p m , a n d n e u t r a l n o r m a l a m m o n i u m acetate < 1 0 p p m . By t h e Aspergillus niger method it w a s found t h a t n o r m a l soils contained
> 1 0 p p m a n d deficient soils < 2 p p m Z n ( 3 4 ) .
Little leaf, a n d rosetting of fruit trees, w e r e t h e first field symptoms to be related to zinc deficiency. P e c a n (Carya illinoinensis) rosette w a s recognized b y growers as early as 1 9 0 0 , although it w a s n o t diagnosed as zinc deficiency till 1 9 3 2 . W h i t e b u d of m a i z e (Zea mays), a n d mottle leaf, or frenching, of citrus a r e other field disorders k n o w n to be due to zinc deficiency ( 2 3 5 ) .
4. Manganese
Soil m a n g a n e s e c a n b e divided into t h e bivalent ion—existing i n t h e soil solution, or as a n exchangeable ion, or i n a nonexchangeable f o r m — a n d t h e insoluble higher oxides, minerals, a n d organically combined
forms, all of w h i c h are in d y n a m i c equilibrium w i t h one another ( 1 4 4 ) . T h e average content i n the lithosphère is 1000 p p m , a n d the total a m o u n t in soils varies from 200 to 3000 p p m ( 2 4 1 ) . Exchangeable m a n g a n e s e is usually determined b y extraction w i t h n e u t r a l salt solutions, values < 2 p p m being regarded as indicative of deficiency.
A value w h i c h is m a r g i n a l or low for a soil w i t h a p H of 7 becomes satisfactory if t h e p H is lowered to 6. T h e m a n g a n i c forms act as a reserve; the ease w i t h w h i c h t h e y can be reduced to the m a n g a n o u s state varies considerably. T h e quinol-soluble m a n g a n e s e is taken as a n index of potential availability: in n o r m a l soils it should exceed 100 p p m . T h e m a i n soil factors t h a t d e t e r m i n e availability are p H a n d t h e oxi- dation-reduction conditions. p H values a r o u n d 6-6.5 a p p e a r to be critical, lower values favoring reduction a n d higher values, oxidation.
Quastel (192) believes t h a t organisms a r e m a i n l y responsible for oxidation from p H 6 to 7.9 p H a n d t h a t nonbiological oxidation is m a r k e d only above p H 8. H e suggests t h a t w h e n biological oxidation takes place i n n e u t r a l or slightly acid soils, tervalent m a n g a n e s e is formed. This in t u r n dismutes forming m a n g a n e s e dioxide a n d bivalent manganese, w h i c h undergoes biological oxidation once m o r e (cf.
Chapter 6 ) .
" G r a y speck" disease of oats (Avena sativa) has been k n o w n for m a n y years a n d could be controlled b y t r e a t m e n t w i t h m a n g a n e s e salts, but t h e proof t h a t " g r a y speck" w a s actually related to m a n g a n e s e deficiency was provided b y Samuel a n d P i p e r in 1928 ( 2 0 9 ) . M a r s h spot of peas (Pisum sativum), p a h a l a blight of sugar cane (Saccharum officinarum), speckled yellows of sugar beet {Beta vulgaris), a n d frenching of t u n g (Aleurites fordii) trees a r e other c o m m o n field dis- orders caused b y m a n g a n e s e deficiency.
5. Boron
Boron occurs as tourmaline, a v e r y insoluble fluorine-containing borosilicate, as calcium a n d m a g n e s i u m borates, a n d as iron a n d a l u m i n u m complexes ( 1 6 8 ) . Total boron ranges from 2 to 100 p p m . T h e boron status of plants is related to t h e a m o u n t of boron removed from soil b y extraction w i t h boiling water. T h e a m o u n t s r a n g e from
< 0 . 0 5 to > 5 0 p p m , t h e majority of soils h a v i n g values > 3 p p m . T h e deficiency level depends on conditions of extraction, p H , a n d organic m a t t e r status. A limiting value would be in t h e region of 0.5 p p m air-dry soil.
T h e sunflower {Helianthus annuus) is v e r y sensitive to boron deficiency a n d has been used b y Stephenson a n d Schuster a n d b y Col well for determining plant-available boron ( 2 5 7 ) .
Light, acid soils in h u m i d regions are likely to be deficient in avail- able boron because of t h e ease w i t h w h i c h boron is leached. H i g h e r a m o u n t s of boron a r e found in organic soils. P l a n t u p t a k e of boron is reduced b y increasing t h e soil p H b y liming. Colwell a n d C u m m i n g s (59) h a v e d r a w n attention to the possible significance, in this respect, of the differences in molecular structure between calcium metaborate a n d the corresponding sodium a n d potassium salts.
Boron toxicity can arise in arid areas in w h i c h sodium a n d calcium borates accumulate in t h e surface soils. Irrigation waters containing
> 2 p p m of boron a r e reported to be undesirable ( 1 6 8 ) .
According to Miller ( 1 6 6 ) , A g u l h o n ( 1 ) , in 1910, w a s the first to recognize t h e essential n a t u r e of boron in p l a n t life, b u t his work was not generally accepted u n t i l W a r i n g t o n ' s (272) w o r k on the bean
{Phaseolus vulgaris) was published. Since t h e n a voluminous literature on boron in relation to p l a n t growth has developed. H e a r t rot of sugar beet, mangolds {Beta vulgaris) a n d other root crops, b r o w n i n g of cauliflower {Brassica oleracea var. botrytis), cracked stem of celery {Apium graveolens var. dulce), l u c e r n e {Medicago sativa), yellows, top sickness of tobacco {Nicotiana tabacum), a n d i n t e r n a l cork of apples
{Malus sylvestris) a r e b u t a few of the economic diseases associated w i t h boron deficiency.
6. Molybdenum
M o l y b d e n u m occurs in igneous rocks as molybdenite, M o S2, a n d as t h e p r i m a r y molybdates powellite, C a M o 04, a n d wulfenite, P b M o 04. T h e average content of m o l y b d e n u m i n t h e lithosphère is 2.3 p p m
( 8 5 ) . Total m o l y b d e n u m i n soils varies from 0.2 to 5 p p m t h e average value is about 2 p p m . Dilute acids or n e u t r a l n o r m a l a m m o n i u m ace- tate usually extract < 0 . 2 p p m .
D a vies (63) classifies soil m o l y b d e n u m as follows: unavailable, held w i t h i n t h e crystal lattice of p r i m a r y a n d secondary m i n e r a l s ; condi- tionally available, retained as t h e M o 04 anion b y clay minerals a n d available to a greater or lesser degree depending on p H a n d phosphate status; i n organic form; a n d w a t e r soluble. U p to 9 0 % of the total m o l y b d e n u m m a y occur in the unavailable category. Available molyb- d e n u m m a y be assessed b y analysis of indicator plants such as sweet vernal grass {Anthoxanthum odoratum), b y bioassay using Aspergillus niger, a n d b y chemical extractants such as n e u t r a l n o r m a l a m m o n i u m acetate a n d T a m m ' s acid oxalate, p H 3.3. U s i n g acid oxalate, Davies (63) found t h a t a fair prediction of response could be m a d e if inter- pretation was modified according to soil p H . A t p H 5 t h e response level was 0.2 p p m a n d at p H 6.5, 0.05 p p m m o l y b d e n u m .
T w o field disorders, w h i p tail of cauliflower a n d broccoli (two forms of Brassica oleracea var. botrytis) a n d yellow spot of citrus, w e r e described about 30 to 45 years before the responsibility for the dis- orders w e r e attributed to a m o l y b d e n u m deficiency b y Mitchell and b y Davies in 1945 for brassicae, and b y Stewart a n d Leonard in 1952 for citrus ( 1 0 1 ) . T h e first instance of m o l y b d e n u m deficiency in higher plants growing in t h e field in the United States was recorded b y W a l k e r in 1948 ( 1 0 1 ) .
Excess m o l y b d e n u m in soils was first investigated b y Ferguson, Lewis, a n d W a t s o n (72) in relation to a cattle disease k n o w n locally in southwest E n g l a n d as " t e a r t " a n d characterized b y e x t r e m e diarrhea.
This condition is favored b y alkaline soils a n d high phosphate status.
Deficiency is usually associated w i t h acid soils. H e a v y liming a n d molybdate application frequently bring about equal i m p r o v e m e n t in yield. P l a n t (188) showed that 5 tons of crushed limestone per acre a n d 4 pounds of a m m o n i u m molybdate w e r e equally effective in con- trolling 4' w h i p t a i l " in cauliflower in English soils, but in N e w Zealand absolute deficiency m a y respond only to dressings of molybdate.
7. Other Elements of Biological Interest
Although not essential for t h e growth of higher plants, cobalt is essential for animals a n d for some symbiotic nitrogen-fixing micro- organisms a n d v a n a d i u m is essential for certain microorganisms. In addition, iodine a n d fluorine are concerned w i t h a n i m a l h e a l t h ; arsenic a n d selenium m a y be toxic to animals, a n d nickel a n d c h r o m i u m to plants. Sodium is beneficial to some plants but not to others, a n d chlorine deficiency has not been observed u n d e r field conditions. All these elements occur in soils to v a r y i n g extents.
I n r u m i n a n t animals, t h e production of vitamin B1 2 necessitates adequate supplies of cobalt for r u m e n bacteria. M a n y instances of cobalt deficiency h a v e been reported on soils of diverse character in Australia, N e w Zealand, Great Britain, N o r w a y , the U n i t e d States, Canada, a n d other countries. T h e largest areas probably occur on sand- stones, a n d symptoms are particularly pronounced if the sandstones are calcareous. I n deficient soils there is generally a n i n h e r e n t l y low content of cobalt, total contents of < 0 . 5 - 3 p p m being c o m m o n l y reported w h e r e - as contents of u p to 30 p p m a r e usual in soils which support h e a l t h y stock ( 1 6 8 ) . Owing to differences in availability from soil to soil, no di- rect correlation is noted between total cobalt content of t h e soils a n d of t h e herbage. I n soils, cobalt m a y occur in a n available or extractable form as adsorbed Co in the exchange complex, as phosphate, or as sulfate.
T h e a m o u n t of cobalt extracted from Scottish arable soils b y 2 . 5 % acetic acid varies from < 0 . 0 5 p p m in v e r y deficient sands to > 1 p p m
in m a n y soils derived from basic igneous rocks. I n cultivated m i n e r a l soils w i t h m o d e r a t e acidity ( p H 5.6), t h e deficiency level is in t h e region of 0.25 p p m of acetic-soluble cobalt ( 1 6 8 ) , or 5 p p m of total cobalt ( 2 8 5 ) .
V a n a d i u m occurs in greatest a m o u n t in shales a n d the less basic igneous rocks. T h e total contents of eight Scottish soils varied from 20 to 250 p p m ( 1 6 8 ) . I m p e d e d drainage results i n increased a m o u n t s of v a n a d i u m w h i c h is soluble in acetic acid. B e r t r a n d (21) determined the v a n a d i u m content of sixty-two plant materials a n d found a r a n g e of 0.27 to 4.2 p p m i n d r y matter. Seeds of legumes w e r e particularly poor in v a n a d i u m . T h e gallium content of t w e n t y soils ranged from 0.4 to 6 p p m ( 2 2 ) .
Fluorine is m u c h m o r e a b u n d a n t t h a n iodine in soils. T h e bulk of the fluorine occurs in the crystal lattices of silicates a n d phosphate minerals. T h e total content of fluorine in soils ranges from about 10 to 1000 p p m , whereas t h e r a n g e for iodine is about 0.6 to 8 p p m with exceptional values u p to 70 p p m , most of w h i c h is w a t e r soluble ( 1 6 8 ) . Fluorine toxicity sometimes occurs in t h e neighborhood of smelting works.
Arsenic occurs n a t u r a l l y in most soils i n a m o u n t s between 1 a n d 70 p p m ( 2 4 1 ) . I n fruit orchards it m a y a c c u m u l a t e in the surface soil;
Greaves (88) found values r a n g i n g from 7.2 to 367 pounds per acre- foot of soil. Water-soluble arsenic is not related to total content. For- t u n a t e l y p l a n t growth appears to be restricted before a m o u n t s dangerous to animals are absorbed.
M a n y soils are seleniferous a n d thus bear crops injurious to animals.
T h e first account of a disease of horses, n o w k n o w n to be due to selenium poisoning, w a s given in 1857. Crops w h i c h accumulate selenium include species of Astragalus, Machaeranthera (Xylorrhiza), Haplopappus (Oonopsis), a n d Stanleya. I n seleniferous areas, Creta- ceous shales a n d other s e d i m e n t a r y rocks contain a b n o r m a l l y high a m o u n t s of selenium v a r y i n g from 1 to 10 p p m . A n area of seleniferous soils in I r e l a n d contains 30—300 p p m selenium, a n d herbage contents u p to 500 p p m h a v e been reported ( 2 7 1 ) . Soils from h e a l t h y areas con- tained < 2 p p m selenium. T h e average selenium content of accumula- tor plants was found b y Trelease (249) to be 500 p p m , and the highest recorded is 15,000 p p m .
Of the r e m a i n i n g elements t h a t occur in toxic amounts, nickel is the most important. Contents of u p to 8000 p p m total nickel a n d 100 p p m nickel soluble in acetic acid have been recorded in Scotland in poorly drained soils derived from ultrabasic rocks ( 1 6 8 ) .
T h e r e are m a n y well-authenticated cases i n w h i c h additions of sodium salts h a v e increased the yield of, or otherwise improved, various
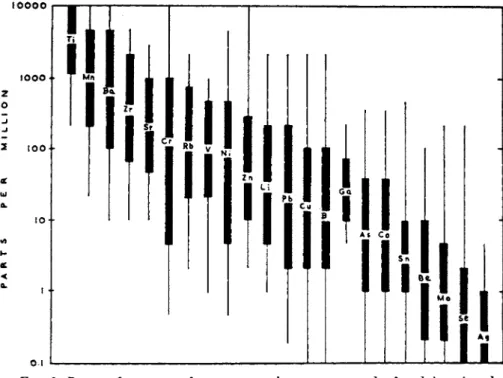
FIG. 3. Range of contents of some trace elements commonly found in mineral soils. Thin lines indicate more unusual values; certain extremely high contents re- ported from abnormal localities influenced, for instance, by ore deposits have been ignored. From Mitchell (168).
N o r m a l soils contain m a n y other elements w h i c h m a y h a v e a bene- ficial effect on p l a n t growth, but their essentiality has not y e t been established (see Fig. 3 ) .
IV. Factors in the Retention and Availability of Nutrients
A . N A T U R E O F T H E S O I L C O L L O I D S
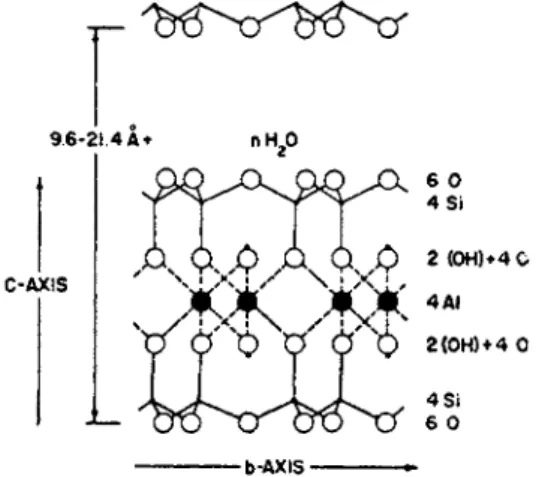
T h e colloidal fraction of soils is p a r t l y inorganic (clay) a n d p a r t l y organic ( h u m u s ) , t h e two forming in most soils a c l a y - h u m u s complex.
crops. Nevertheless t h e evidence is not sufficient to prove t h a t t h e element is essential for n o r m a l growth of plants. Crops w h i c h respond well to applications of sodium salts include celery, mangold, sugar beet, table beet, a n d t u r n i p . Sodium, like potassium, is present as a n exchangeable cation in n o r m a l soils a n d ranges in a m o u n t from 100 to 200 pounds per acre (6-inch surface l a y e r ) . It is present as sodium
carbonate in alkali soils.