www.revportcardiol.org
Revista Portuguesa de
Cardiologia
Portuguese Journal of Cardiology
ORIGINAL ARTICLE
Active acromegaly is associated with enhanced left ventricular contractility: Results from
the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study
Árpád Kormányos
a, Péter Domsik
a, Anita Kalapos
a, Nándor Gyenes
a, Zsuzsanna Valkusz
b, Csaba Lengyel
b, Tamás Forster
a,b, Attila Nemes
a,∗a2ndDepartmentofMedicineandCardiologyCentre,MedicalFaculty,AlbertSzent-GyörgyiClinicalCenter,UniversityofSzeged, Szeged,Hungary
b1stDepartmentofMedicine,MedicalFaculty,AlbertSzent-GyörgyiClinicalCenter,UniversityofSzeged,Szeged,Hungary
Received7October2018;accepted1August2019 Availableonline26May2020
KEYWORDS Acromegaly;
Echocardiography;
Function;
Leftventricle;
Three-dimensional speckletracking
Abstract
Introduction:Acromegalyisarelativelyrarechronichormonaldisease resultingindisfigure- ment.In90%ofcases,acromegalyiscausedbyabenignpituitarymonoclonalhumangrowth hormone-secretingtumor.Theaimofthepresentstudywastodeterminethepresenceofleft ventricular(LV)deformationabnormalitiesusingthree-dimensionalspeckle-trackingechocar- diographyinagroupofacromegalicpatients.
Methods:Thirty-eight acromegalic patients were involved in the study. Thirteen patients were excluded due to inadequate image quality. The mean age ofthe remaining patients was 57.2±13.6 years and seven were male. Their data were compared to an age- and gender-matched control population, which consisted of 34 healthy volunteers (mean age:
52.7±4.9years,15male).
Results:GlobalandmeansegmentalLVradialstrain(RS)(33.2±13.4%vs.25.2±10.8%,p=0.01 and 36.0±12.1% vs. 28.2±10.0%, p=0.009, respectively) proved to be significantly higher in acromegaly compared to controls. Active acromegalic patients had significantly higher global andmean segmental LV-RS (35.5±14.4% vs. 25.2±10.8%,p=0.03 and 37.9±13.3% vs.
28.2±10.0%, p=0.03, respectively) compared to controls. Between the active and inactive acromegalygroups,onlybasal LVcircumferentialstrain(-30.2±4.8%vs.-26.7±4.1%,p=0.02) wasfoundtobesignificantlydifferent.
∗Correspondingauthor.
E-mailaddress:nemes.attila@med.u-szeged.hu(A.Nemes).
https://doi.org/10.1016/j.repc.2019.08.010
0870-2551/©2020SociedadePortuguesadeCardiologia.PublishedbyElsevierEspa˜na,S.L.U.ThisisanopenaccessarticleundertheCC BY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Conclusion:Thepresentedclinical,demographic,therapeuticandechocardiographicfeatures demonstratethatactiveacromegalyisassociatedwithenhancedLVRSascomparedtohealthy controlsandthosewithinactiveacromegaly.
©2020SociedadePortuguesadeCardiologia.PublishedbyElsevierEspa˜na,S.L.U.Thisisan openaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by- nc-nd/4.0/).
PALAVRAS-CHAVE Acromegalia;
Ecocardiografia;
Func¸ão;
Ventrículoesquerdo;
Speckletracking tridimensional
Associac¸ãoentreacromegaliaativaecontractilidadeventricularaumentada:
resultadosdoEstudoMAGYAR-Pathcomecocardiografiatridimensionalporspeckle tracking
Resumo
Introduc¸ão:A acromegalia éuma doenc¸ahormonal crónicarelativamente rara,associadaa dismorfismossomáticos.Em 90%doscasos,ela écausadaporumtumorbenignomonoclonal dahipófise, secretorde hormonado crescimento. Oobjetivo dopresente estudo foidete- tarapresenc¸adealterac¸õesdadeformac¸ãomiocárdicadoventrículoesquerdo(VE)através deecocardiografia tridimensionalporspeckle trackingnumgrupodedoentesportadores de acromegalia.
Métodos: Foramincluídos38doentesportadoresdeacromegalia.Trezeforamexcluídosdevido àmáqualidadedeimagem.Aidademédiadosrestantesfoide57,2±13,6anos(7homens).Os seusdadosforamcomparadoscomumapopulac¸ãocontroloemparelhadaporidadeegénero, quecompreendeu34voluntáriossaudáveis(idademédia:52,7±4,9anos,sendo15indivíduos dosexomasculino).
Resultados: Ostrain radial(SR) global e segmentar VE (respetivamente 33,2±13,4% versus 25,2±10,8%,p=0,01e36,0±12,1%versus28,2±10,0%,p=0,009)foisignificativamentesuperior noscasosdeacromegaliaem relac¸ãoaoscasoscontrolo.Osdoentesportadoresdeacrome- galiaativativeramumSRglobalesegmentarsignificativamentesuperior(35,5±14,4%versus 25,2±10,8%,p=0,03e37,9±13,3%versus28,2±10,0%,p=0,03,respetivamente),quandocom- paradoscomosgruposcontrolo.Entreosgruposportadoresdeacromegaliaativoseinativos, sófoiconsideradosignificativamentediferenteostraincircunferencialbasalVE(-30,2±4,8%
versus-26,7±4,1%,p=0,02).
Conclusões:Combasenascaracterísticasclínicas,demográficas,terapêuticaseecocardiográ- ficasapresentadas,aacromegaliaativaapresentaSR-VEaumentadoemrelac¸ãoàpopulac¸ão saudáveleaogrupodeportadoresdeacromegaliainativa.
©2020SociedadePortuguesadeCardiologia.PublicadoporElsevierEspa˜na,S.L.U.Este ´eum artigoOpen Accesssobumalicenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by- nc-nd/4.0/).
Introduction
Acromegaly is a relatively rare chronic hormonal disease resultingin disfigurement. In 90% of cases, acromegaly is caused by a benign pituitary monoclonal human growth hormone (HGH)-secreting tumor.1 Elevation of serum HGH causes an elevation in insulin-like growth factor 1 (IGF-1),and these hormonalchanges cause a wide range ofclinicalsymptomsandcomorbiditiesincludingmetabolic, respiratory,otherendocrineandcardiovasculardiseases.2,3 Ofthesecardiovascularcomorbidities,hypertensionandleft ventricular (LV) hypertrophy are the most common, but serious valvular regurgitation and heart failure can also develop. A higherincidence of cardiac arrhythmias,most commonlyatrial fibrillation,has frequentlybeen reported inacromegaly.4,5
Three-dimensional(3D)speckle-trackingechocardiogra- phy (STE) (3D-STE) is a new, non-invasive, robust and
validated tool offeringclinicians a newimaging modality.
3D-STE is capable of measuringvarious global, segmental and regional strain values throughLV modeling.6,7 To the best of the authors’ knowledge, there have been no 3D- STEstudiesonLVdeformationabnormalitiesinacromegaly.
Therefore,theaimofthepresentstudywastodetermine thepresenceofLVdeformationalabnormalitiesusing3D-STE inagroupofacromegalicpatients.
Methods
Patientpopulation
Thirty-eight acromegalic patients were included in the present study.Thirteen wereexcluded due toinadequate image quality. The mean age of the remaining patients was 57.2±13.6 years and seven were male. Their data were compared to an age- and gender-matched control
population,whichconsistedof34healthyvolunteers(mean age52.7±4.9years,15male).Thediagnosisofacromegaly was based on current clinical standards: typical clinical features, elevated HGH levels, and elevated IGF-1 levels insuppressible with an oral glucose tolerance test.5 The acromegalicgroupwasfurtherdividedbasedontheactiv- ityofthedisease:ifserumHGHand/orIGF-1concentration wasoverthediagnosticthreshold,acromegalywasconsid- eredtobeactive.5,8Ourstudywasapprovedbythehuman researchethicscommittee ofourinstitutionandcomplied with the Declaration of Helsinki. All of the patients and healthyvolunteersgaveinformedconsent.Ouracromegalic datasetscamefromtheMAGYAR-PathStudy(MotionAnalysis oftheheartandGreatvesselsbYthree-dimensionAlspeckle- tRackingechocardiographyinPathologicalcases),whichwas developedtoassessthediagnosticandprognosticvalueof 3D-STE-derivedparameters(‘Magyar’means‘Hungarian’in theHungarianlanguage).
Two-dimensionalDopplerandtissueDoppler echocardiography
Allhealthyvolunteersandacromegalicpatientsunderwent a complete two-dimensional (2D) transthoracic echocar- diographic study using a Toshiba ArtidaTM imaging system
(ToshibaMedicalSystems,Tokyo,Japan) withaPST-30SBP phased-arraytransducer(1-5MHz).Theexaminationswere performed according to current clinical standards. In all casesLVdimensions,volumes andejectionfraction(LVEF) andleftatrial(LA)dimensionsweremeasuredandcomplete 2DDopplerandtissueDopplerstudieswereperformed.9
Three-dimensionalspeckle-tracking echocardiography
Forthe3D-STEexaminations,thesameToshibaArtidaTMsys- tem was used, with a PST-25SX matrix- array transducer (ToshibaMedical Systems,Tokyo,Japan).7 In eachcase, a pyramid-shapedfull-volume3D datasetwasobtained.The complete dataset consisted of six wedge-shaped subvol- umes, which wererecorded during six RRintervals and a singlebreath-hold.Thesubvolumesweresettobeasnar- rowaspossibletoimprovespatialresolution,thusimproving laterendocardialborderdelineation.Therecordeddatasets were analyzed offline using the supplied 3D Wall Motion Tracking software, version 2.7 (Toshiba Medical Systems, Tokyo,Japan).Fromthedatasets, thesoftwareautomati- callyreconstructedapical4-chamberand2-chamberviews, thenthe readerspecified thethreecross-sectional planes manually, using the guide planes to help standardize LV
Figure1 Three-dimensionalspeckle-trackingechocardiographicstudyofanacromegalicpatient.Apical4-chamberview(A)and apical2-chamberview(B)areautomaticallyselectedbythesoftware.Cross-sectionalplanesareattheapical(C3),midventricular (C5)andbasal(C7)leftventricular(LV)levels.Thethree-dimensionalLVmodel(D)andthecorrespondingvolumetricparameters (E)areshownalongwithsegmentalLVradialstraincurves(F).
measurements.Aftersettinguptheplanes,thereaderthen specified the septaland lateral parts of the mitralannu- lusandtheLVapex,afterwhichthesoftwareautomatically detectedtheendocardialborderandcreateda3D LVcast (Figure1).
Three-dimensionalspeckle-tracking echocardiography-derivedleftventricular volumetricmeasurements
3D-STE was used to measure LV end-diastolic volume (LVEDV),LVend-systolicvolume(LVESV), LVEFandLVmass basedontheabove-mentionedLVcast.
Three-dimensionalspeckle-tracking
echocardiography-derivedleftventricularstrain assessments
3D-STE strain measurements were performed on the LV cast using the 16-segment LV model.10 In each segment, threeunidirectionalstrainparameterswereassessed:radial (RS) (thickening and thinning of the segment), longitudi- nal (LS) (lengtheningand shortening of the segment)and
circumferential (CS) (widening and narrowing of the seg- ment). Based on these strain parameters, the software calculated thefollowing complexstrains: area(AS) (com- binationofLSandCS)and3D(3DS)(combinationofRS,LS andCS).
Statisticalanalysis
All data arereportedas mean± standard deviation. Sig- nificance wasestablished for p valueslessthan 0.05.The Shapiro-Wilks test was used to check for normal distri- bution. Levene’s test for equality of variances was used totest homogeneity of variance. Fornormallydistributed datasets,thetwo-tailedStudent’sttestwasusedforcom- parisons, however if the dataset did not follow a normal distribution,theMann-Whitney-Wilcoxontestwasused.For categorical variables, Fisher’s exact test was performed.
To establishsignificant relationshipsbetweenindependent variables, Pearson’s correlation coefficients were calcu- lated.ThestatisticalanalysiswasperformedusingRStudio (RStudio Team, RStudio: Integrated Development for R.
RStudio, Inc., Boston, MA, 2015). Foroffline data extrac- tionandanalysis,acommercialsoftwarepackagewasused (MATLAB8.6,TheMathWorksInc.,Natick,MA,2015).
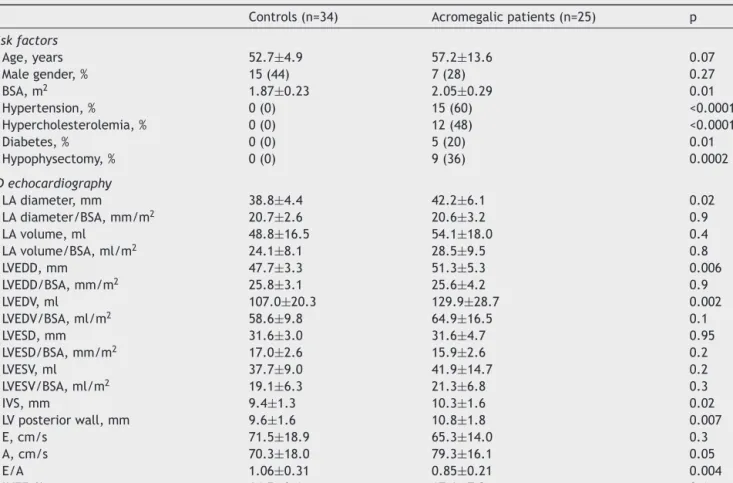
Table1 Demographic,clinical andtwo-dimensional echocardiographic characteristicsofpatients with acromegaly andof controls.
Controls(n=34) Acromegalicpatients(n=25) p
Riskfactors
Age,years 52.7±4.9 57.2±13.6 0.07
Malegender,% 15(44) 7(28) 0.27
BSA,m2 1.87±0.23 2.05±0.29 0.01
Hypertension,% 0(0) 15(60) <0.0001
Hypercholesterolemia,% 0(0) 12(48) <0.0001
Diabetes,% 0(0) 5(20) 0.01
Hypophysectomy,% 0(0) 9(36) 0.0002
2Dechocardiography
LAdiameter,mm 38.8±4.4 42.2±6.1 0.02
LAdiameter/BSA,mm/m2 20.7±2.6 20.6±3.2 0.9
LAvolume,ml 48.8±16.5 54.1±18.0 0.4
LAvolume/BSA,ml/m2 24.1±8.1 28.5±9.5 0.8
LVEDD,mm 47.7±3.3 51.3±5.3 0.006
LVEDD/BSA,mm/m2 25.8±3.1 25.6±4.2 0.9
LVEDV,ml 107.0±20.3 129.9±28.7 0.002
LVEDV/BSA,ml/m2 58.6±9.8 64.9±16.5 0.1
LVESD,mm 31.6±3.0 31.6±4.7 0.95
LVESD/BSA,mm/m2 17.0±2.6 15.9±2.6 0.2
LVESV,ml 37.7±9.0 41.9±14.7 0.2
LVESV/BSA,ml/m2 19.1±6.3 21.3±6.8 0.3
IVS,mm 9.4±1.3 10.3±1.6 0.02
LVposteriorwall,mm 9.6±1.6 10.8±1.8 0.007
E,cm/s 71.5±18.9 65.3±14.0 0.3
A,cm/s 70.3±18.0 79.3±16.1 0.05
E/A 1.06±0.31 0.85±0.21 0.004
LVEF,% 64.5±3.6 67.6±7.2 0.1
2D:two-dimensional;A:latetransmitralflowvelocity;BSA:bodysurfacearea;E:earlytransmitralflowvelocity;IVS:interventricular septalthickness;LA:leftatrial;LV:leftventricular;LVEDD:leftventricularend-diastolicdiameter;LVEDV:leftventricularend-diastolic volume;LVEF:leftventricularejectionfraction;LVESD:leftventricularend-systolicdiameter;LVESV:leftventricularend-systolicvolume.
Results
Clinicalanddemographiccharacteristicsof patientswithacromegaly
Significantdifferenceswereidentifiedbetweenacromegalic patients andhealthy controlsregarding the prevalenceof hypertension(p<0.0001),hypercholesterolemia (p<0.0001) anddiabetes(p=0.01)(Table1).
Laboratoryfindingsofpatientswithacromegaly The active acromegaly subgroup consisted of 14 patients (meanage:58.6±14.6years,fivemales).Theirmeanserum HGHlevelwas5.5±3.9ng/ml,IGF-1levelwas502.3±366.0 ng/ml and IGF-1 index (IGF-1 level/upper limit of nor- mal)was2.0±1.1.Theinactiveacromegalygroupincluded 11 patients (mean age: 54.0±12.9, 2 males). Their mean serum HGH level was 4.5±8.1 ng/ml, IGF-1 level was 216.9±129.0ng/mlandIGF-1indexwas0.9±0.6.
Two-dimensionalechocardiographicdata
Significantdifferenceswereseen inLAdiameter(p=0.02), LV end-diastolic diameter (LVEDD) (p=0.006) and vol- ume (LVEDV) (p=0.002), interventricular septal thickness (p=0.02),andLVposteriorwallthickness(p=0.007)between theacromegalic groupand healthycontrols. Body surface area(BSA)wasalsosignificantlydifferentbetweencontrols andacromegalicpatients(p=0.01).Furthermore,latetrans- mitralflowvelocityA(p=0.05)andE/Aratio(p=0.004)also differedsignificantlybetweenthetwogroups(Table1).
Three-dimensionalspeckle-tracking echocardiography-derivedleftventricular volumetricparameters
3D-STE-derived LVEDV (p=0.0003) and LVESV (p=0.005) proved to be significantly different between all the acromegalic patients and controls. LVEDV (p=0.006) and LVEF (p=0.02) differed significantly between the active acromegaly subgroup and controls, while LVEDV (p=0.01) and LVESV (p=0.01) were significantly different between the inactivedisease subgroup andcontrols. LVEF (p=0.02) wassignificantly higherin activethan in inactive disease (Tables2and3).
Three-dimensionalspeckle-tracking
echocardiography-derivedleftventricularstrain parameters
GlobalandmeansegmentalRS(p=0.01andp=0.009,respec- tively) were significantly higher in acromegalic patients compared tocontrols. Among regional strain parameters, basal and apical RS (p=0.04 and p=0.0004, respectively) were significantlydifferent between acromegalicpatients andcontrols.Patientswithactivediseasehadsignificantly higherglobalandmeansegmentalRS(p=0.03andp=0.03, respectively)comparedtocontrols;in addition,basal and
apicalRS (p=0.04and p=0.002,respectively)andbasal CS (p=0.01)weresignificantlyhighercomparedtocontrols.In patients with inactive disease, no significant differences weredetectedinLVstrainparameterscomparedtothecon- trolgroup.Betweenactiveandinactivediseasegroups,only basal CS (p=0.02) was found to be significantly different (Tables2and3).
Correlationbetweenhormonelevels andthree-dimensionalspeckle-tracking echocardiography-derivedleftventricular volumetricandstrainparameters
In the overall acromegaly group, there was a significant negative correlation between HGH levels and apical 3DS (r=-0.40,p=0.05). Nosignificant correlation wasdetected between any hormone level and LV strains in the active disease group. In the inactive disease group, IGF-1 index correlated significantly with midventricular RS (r=0.60, p=0.05), global and mean segmental RS (r=0.66, p=0.03 andr=0.66, p=0.03,respectively) and global 3DS (r=0.61, p=0.05). Furthermore, there was a significant correlation between mean segmental RS and IGF-1 levels (r=0.61, p=0.05)inthisgroup.
Discussion
Acromegalyisa severechronic hormonaldiseasethatcan affectalmostanyorgansystemthroughelevatedHGHand IGF-1 levels. These effects are especially marked in the cardiovascularsystem,resultinginnumerouscardiovascular comorbidities,themostcommonofwhichishypertension.
Although the pathophysiology is not fully understood, it is hypothesised that circulatory volume increases due to sodium retention and consequent plasma expansion.11---13 Furthermore,acromegaly is associatedwithcardiomyopa- thy, resulting in severe heart failure in terminal stages.
In the early stages of acromegaly, LV hypertrophy devel- ops due to exposure to elevated serum HGH levels. As acromegaliccardiomyopathydevelops,Ca2+hypersensitivity enhances myocardial contractility, resulting in a hyper- kinetic syndrome. As it progresses, LV hypertrophy may becomesufficientlyseveretoimpairLVdiastolicfilling.In theterminalstages,bothsystolicanddiastolicfunctionmay decrease due to extracellular collagen deposition.14---17 In addition,valvularregurgitationand arrhythmiasaremore commonamongacromegalicpatients.18,19
Inthepresentstudy,3D-STEwasusedtoassessglobaland regionalLVstrainparametersinacromegalicpatients.Strain imaging using 3D-STE has been validated in comparisons with two-dimensional speckle-tracking echocardiography (2D-STE),cardiac magnetic resonance imaging and tissue Dopplerimaging.6,10,20---22Ithasbeenreportedthat3D-STE- derivedLVstrainvaluesaresomewhatlowerthanthosefrom 2D-STE.6 Notable advantages of this methodcompared to othermodalitiesareitsnon-invasivenature,angleindepen- dence,possibilityof3Dimagingandlowercost.10
Tothebestoftheauthors’knowledge,therehavebeen no3D-STEstudiesonLV strainparametersin the acrome- galicpopulation.Therehasbeenonlyonestudywhichused 2D-STE to assess global LV longitudinal strain in patients
Table2 Comparisonofthree-dimensionalspeckle-trackingechocardiography-derivedglobalandmeansegmentalleftventric- ularpeakstrainparametersbetweenacromegalicpatientsandcontrols.
Controls(n=34) Acromegalicpatients(n=25) Activedisease(n=14) Inactivedisease(n=11) 3D-STE-derivedvolumetricparameters
LVEDV,ml 80.5±20.2 107.6±28.9a 108.5±34.6a 101.1±23.3a
LVESV,ml 34.9±10.6 45.4±14.9a 43.7±17.7 45.3±12.7a
LVEF,% 56.7±7.1 58.1±6.1 60.2±5.8a,b 55.6±5.8
Globalstrains
Radial,% 25.2±10.8 33.2±13.4a 35.5±14.4a 29.7±11.3
Circumferential,% -26.7±6.1 -28.1±4.5 -29.3±4.1 -27.0±4.9
Longitudinal,% -16.0±2.7 -15.8±3.4 -15.5±3.1 -16.1±3.9
3D,% 27.6±10.7 31.3±11.8 32.5±13.8 29.1±8.3
Area,% -39.3±5.8 -39.9±5.2 -40.8±4.7 -39.3±6.0
Meansegmentalstrains
Radial,% 28.2±10.0 36.0±12.1a 37.9±13.3a 33.1±9.9
CS,% -28.1±5.9 -29.1±4.3 -30.3±4.1 -28.2±4.4
LS,% -16.9±2.6 -17.0±3.4 -16.7±3.0 -17.5±4.0
3DS,% 30.2±9.8 33.7±10.8 34.6±12.5 31.6±7.6
AS,% -40.4±5.6 -41.2±5.3 -42.1±4.7 -40.6±6.1
ap<0.05vs.controls.
b p<0.05vs.inactivedisease.
3D:three-dimensional;3D-STE:three-dimensionalspeckle-trackingechocardiography;3DS:three-dimensionalstrain;AS:areastrain;CS:
circumferentialstrain;LS:longitudinalstrain;LVEDV:end-diastolicvolume,LVESV:end-systolicvolume,LVEF:leftventricularejection fraction;RS:radialstrain.
Table3 Comparison of three-dimensional speckle-tracking echocardiography-derived regional left ventricular peak strain parametersbetweenacromegalicpatientsandcontrols.
Controls(n=34) Acromegalicpatients(n=25) Activedisease(n=14) Inactivedisease(n=11)
RSbasal,% 34.3±14.8 44.6±19.2a 46.9±20.7a 40.2±16.8
RSmid,% 29.6±12.1 34.6±12.8 35.8±14.2 32.1±10.1
RSapex,% 16.9±8.3 25.6±10.1a 27.9±10.6a 23.9±10.4
CSbasal,% -26.1±5.8 -28.9±4.9 -30.2±4.8a,b -26.7±4.1
CSmid,% -28.4±7.4 -28.5±5.0 -29.3±5.1 -27.8±5.2
CSapex,% -30.6±12.6 -30.6±10.0 -31.7±9.2 -31.1±9.7
LSbasal,% -20.8±5.0 -19.1±5.5 -18.3±6.1 -19.3±4.4
LSmid%) -13.6±4.1 -14.7±3.6 -15.0±2.9 -15.0±4.7
LSapex,% -16.0±5.5 -17.3±7.7 -16.9±8.7 -18.4±6.4
3DSbasal,% 37.1±13.9 44.3±17.4 45.9±19.5 40.7±13.9
3DSmid,% 30.7±11.9 30.3±12.5 31.2±14.2 28.1±9.1
3DSapex,% 19.2±9.3 21.8±10.8 23.0±10.9 21.5±11.4
ASbasal,% -40.6±6.8 -41.6±7.1 -42.0±7.8 -40.0±5.5
ASmid,% -38.8±7.5 -39.0±6.2 -40.4±5.4 -38.3±7.5
ASapex,% -42.7±14.4 -43.8±12.8 -44.6±12.2 -45.0±12.5
ap<0.05vs.controls.
b p<0.05vs.inactivedisease.
3DS:three-dimensionalstrain;AS:areastrain;CS:circumferentialstrain;LS:longitudinalstrain;RS:radialstrain.
withactiveacromegaly,byVolschanetal.,whoreportedno significantdifferencesinglobalLV-LScomparedtohealthy controls.23Ourgrouphaspreviouslyreportedincreasedaor- ticstiffness in acromegaly aswell asreduced LV rotation andtwistinboth activeandinactive acromegaly.It could behypothesizedthatthesechangesaretheconsequences ofextracellularcollagendeposition.24,25
Results of the 2D echocardiography study in our pop- ulation showed that in patients with acromegaly, the LV
wasdilated, LVwalls weresignificantly thicker andthere was impaired LV diastolic function without LV systolic dysfunction.Ofthe3D-STE-derivedLVvolumetricparame- ters,LVEDVwassignificantlyincreasedregardlessofdisease activity.Inpatientswithactiveacromegaly,LVEFwashigher comparedtothecontrolgroup,andwassignificantlyhigher than in the inactive acromegaly group. Global and mean segmentalLV-RSweresignificantlyhigherincasesofactive disease compared toboth the healthy controls and those
withinactivedisease.ItshouldbenotedthatLVstrainval- ueswerenormalinpatientswithinactiveacromegaly.Based onthesefindings,itcouldbehypothesizedthat enhanced regionalstrainmaybeacompensatorymechanismoffsetting theimpairedLVrotationandtwistdemonstratedrecently.25 Our results suggest that LV deformation differences are reversiblewithpropertreatment,butnotLVdilation.Fur- therinvestigationsarewarrantedtoassesswhyLVrotation andtwist areimpaired but LV wallcontractility is not. In addition, LV-RS appears to compensate for the loss of LV twist.
Limitations
There areseveral limitations affecting the present study.
Sinceacromegalyisararedisease,thestudypopulationis relativelysmall.ThedurationofexposuretohighserumHGH andIGF-1concentrationswasnottakenintoaccountinthe analysis,andthepossibleeffectsofdrugsusedinthetreat- mentofacromegalywerenotexamined.3D-STEasanovel modalitysuffersfromseveral limitingtechnicalfactors:it haslowerspatialandtemporalresolutionthan2D-STE,and thefull-volumedatasetrequiressixidenticalRRintervals, which can resultin so-called stitchingnoise.26 It has also beenreportedthatLVstrainparametersshowsomevendor dependency.27 Finally,thegreaterproportionofwomenin thecontrolgroupcouldpartiallyexplaindifferencesinheart dimensionsbetweenthegroups.
Conclusion
The presented clinical, demographic, therapeutic and echocardiographic features demonstrate that active acromegalyisassociatedwithenhancedLV-RSascompared tohealthycontrolsandthosewithinactiveacromegaly.
Ethical standards
Theauthorsassertthatall procedurescontributingtothis work comply with the ethical standards of the relevant nationalguidelinesonhumanexperimentationandwiththe 1975Helsinki Declaration,revised in2008,and havebeen approvedbytheinstitutionalreviewboardoftheUniversity ofSzeged.
Author contributions
Árpád Kormányos --- study design, writing manuscript, patientmanagement,performing3D-STEexaminations.
PéterDomsik---patientmanagement,performing3D-STE examinations.
AnitaKalapos---patientmanagement,performing3D-STE examinations.
Nándor Gyenes--- patientmanagement,performing3D- STEexaminations.
ZsuzsannaValkusz---supervision.
CsabaLengyel---supervision.
TamásForster---supervision.
AttilaNemes---studydesign,writingmanuscript.
Conflicts of interest
Theauthorshavenoconflictsofinteresttodeclare.
Acknowledgments
Financialsupport:Thisresearchreceivednospecificgrant fromanyfundingagency,commercialornot-for-profitsec- tors.
References
1.SannoN,TeramotoA,OsamuraRY,etal.Pathologyofpituitary tumors.NeurosurgClinNAm.2003;14:25---39.
2.ColaoA, FeroneD,MarzulloP,et al.Systemiccomplications ofacromegaly:epidemiology,pathogenesis,andmanagement.
EndocrRev.2004;25:102---52.
3.ClaytonRN.Cardiovascularfunctioninacromegaly.EndocrRev.
2003;24:272---7.
4.Ramos-LeviAM,MarazuelaM.Cardiovascular comorbiditiesin acromegaly: anupdate ontheir diagnosis and management.
Endocrine.2017;55:346---59.
5.Melmed S. Medical progress: acromegaly. N Engl J Med.
2006;355:2558---73.
6.SaitoK,OkuraH,WatanabeN,etal.Comprehensiveevaluation of left ventricular strain using speckle tracking echocardio- graphy in normal adults: comparison of three-dimensional and two-dimensional approaches. J Am Soc Echocardiogr.
2009;22:1025---30.
7.Ammar KA, Paterick TE, Khandheria BK, et al. Myocar- dialmechanics:understandingandapplyingthree-dimensional speckletrackingechocardiographyinclinicalpractice.Echocar- diography.2012;29:861---72.
8.VilarL,VilarCF,LyraR,etal.Acromegaly:clinicalfeaturesat diagnosis.Pituitary.2017;20:22---32.
9.LangRM,BadanoLP,Mor-AviV,etal.Recommendationsforcar- diacchamberquantificationbyechocardiographyinadults:an updatefromtheAmericanSocietyofEchocardiographyandthe European AssociationofCardiovascular Imaging. EurHeartJ CardiovascImaging.2015;16:233---70.
10.Nemes A, Kalapos A, Domsik P, et al. Three-dimensional speckle-tracking echocardiography --- a further step in non-invasive three-dimensional cardiac imaging. Orv Hetil.
2012;153:1570---7.
11.Vitale G, Pivonello R, Auriemma RS, et al. Hypertension in acromegaly and in the normal population: prevalence and determinants.ClinEndocrinol(Oxf).2005;63:470---6.
12.Bondanelli M, Ambrosio MR, degli Uberti EC. Pathogene- sis and prevalence of hypertension in acromegaly. Pituitary.
2001;4:239---49.
13.BrevettiG,MarzulloP,SilvestroA,etal.Earlyvascularalter- ationsinacromegaly.JClinEndocrinolMetab.2002;87:3174---9.
14.ColaoA,MarzulloP,DiSommaC,etal.Growthhormoneand theheart.ClinEndocrinol(Oxf).2001;54:137---54.
15.BogazziF,LombardiM,StrataE,etal.Highprevalenceofcar- diachypertrophywithoutdetectablesignsoffibrosisinpatients withuntreatedactiveacromegaly:aninvivostudyusingmag- neticresonanceimaging.ClinEndocrinol(Oxf).2008;68:361---8.
16.SaccaL,NapoliR,CittadiniA. Growthhormone,acromegaly, and heart failure:anintricate triangulation. Clin Endocrinol (Oxf).2003;59:660---71.
17.Powlson AS, Gurnell M. Cardiovascular disease and sleep- disordered breathing in acromegaly. Neuroendocrinology.
2016;103:75---85.
18.PereiraAM,vanThielSW,LindnerJR,etal.Increasedpreva- lenceofregurgitantvalvularheartdiseaseinacromegaly.JClin EndocrinolMetab.2004;89:71---5.
19.KahalyG,OlshausenKV,Mohr-KahalyS,etal.Arrhythmiaprofile inacromegaly.EurHeartJ.1992;13:51---6.
20.Nesser HJ, Mor-Avi V, Gorissen W, et al. Quantification of left ventricularvolumesusing three-dimensional echocardio- graphic speckle tracking:comparison withMRI.Eur HeartJ.
2009;30:1565---73.
21.Kleijn SA, Brouwer WP, Aly MF, et al. Comparison between three-dimensionalspeckle-trackingechocardiographyandcar- diac magnetic resonance imaging for quantification of left ventricularvolumesandfunction.EurHeartJCardiovascImag- ing.2012;13:834---9.
22.KleijnSA,AlyMF,TerweeCB,etal.Reliabilityofleftventricular volumesand function measurementsusing three-dimensional speckle tracking echocardiography. Eur Heart J Cardiovasc Imaging.2012;13:159---68.
23.Volschan ICM, Kasuki L, Silva CMS, et al. Two-dimensional speckle tracking echocardiography demonstrates no effect of active acromegaly on left ventricular strain. Pituitary.
2017;20:349---57.
24.Nemes A, Gavaller H, Csajbok E, et al. Aortic stiffness is increasedin acromegaly--- atransthoracic echocardiographic study.IntJCardiol.2008;124:121---3.
25.KormanyosA,DomsikP,KalaposA,etal.Leftventriculartwist isimpairedinacromegaly:insightsfromthethree-dimensional speckletrackingechocardiographicMAGYAR-PathStudy.JClin Ultrasound.2018;46:122---8.
26.Seo Y, Ishizu T, Aonuma K. Current status of 3-dimensional speckletrackingechocardiography:areviewfromourexperi- ences.JCardiovascUltrasound.2014;22:49---57.
27.GayatE,AhmadH,WeinertL,etal.Reproducibilityandinter- vendorvariabilityofleftventriculardeformationmeasurements bythree-dimensionalspeckle-trackingechocardiography.JAm SocEchocardiogr.2011;24:878---85.