Investigating thermal stability based on the structural changes of lactase enzyme by several orthogonal methods
Márton Király
a, Borbála Dalmadi Kiss
a, Péter Horváth
b, László Drahos
c, Arash Mirzahosseini
b, Gyula Pálfy
d,e, István Antal
a, Krisztina Ludányi
a,*
aDepartmentofPharmaceutics,FacultyofPharmacy,SemmelweisUniversity,HÅgyesEndreu.7.,1092,Budapest,Hungary
bDepartmentofPharmaceuticalChemistry,FacultyofPharmacy,SemmelweisUniversity,HÅgyesEndreu.7.,1092,Budapest,Hungary
cMSProteomicsResearchGroup,ResearchCentreforNaturalSciences,MagyarTudósokkörútja2.,H-1117,Budapest,Hungary
dLaboratoryofStructuralChemistryandBiology,InstituteofChemistry,EötvösLorándUniversity,PázmányP.sétány1/A,1117,Budapest,Hungary
eProteinModelingGroupHAS-ELTE,InstituteofChemistry,EötvösLorándUniversity,1538,Budapest,P.O.B.32,Hungary
ARTICLE INFO
Articlehistory:
Received20April2021 Accepted24May2021
Keywords:
NMR Proteomics β-Galactosidase Aggregation Massspectrometry
ABSTRACT
Thermalstabilityoflactase(β-galactosidase)enzymehasbeenstudiedbyavarietyofphysico-chemical methods.β-galactosidaseis themain active ingredientof medicationsforlactoseintolerance.Itis typicallyproducedindustriallybytheAspergillusoryzaefilamentousfungus.Lactasewasusedasa model to help understand thermal stability of enzyme-type biopharmaceuticals. Enzyme activity (hydrolyzationoflactose)ofβ-galactosidasewasdeterminedafterstoringthesolidenzymesubstanceat varioustemperatures.Forabetterunderstandingoftherelationshipbetweenstructureandactivity changeswedeterminedthemassandsizeofthemoleculeswithgelelectrophoresisanddynamiclight scatteringanddetectedaggregationprocesses.Abottom-upproteomicprocedurewasusedtodetermine theprimaryaminoacidsequenceandtoinvestigatechangesintheN-glycosylationpatternoftheprotein.
NMRandCDspectroscopicmethodswereusedtoobservechangesinhigherorderstructuresandto revealrelationshipsbetweenstructuralandfunctionalchanges.
©2021TheAuthor(s).PublishedbyElsevierB.V.ThisisanopenaccessarticleundertheCCBYlicense (http://creativecommons.org/licenses/by/4.0/).
1.Introduction
Biologicals produced bybiotechnology are becoming widely used. Qualityassurance and stability testing of these complex protein type molecules demand extensive knowledge of their structure in order to provide effective and safe medications.
Stabilitytestingofimmunoglobulin-basedbiologicalsisrelatively wellestablished,butforotherbiologicals,likelarge,enzymetype drugsithasnotyetbeendefined.Stabilityandqualityassessments areusuallybasedonsimpleactivitytests,withoutanyinformation onpossiblechanges inthestructure orin theposttranslational modificationsoftheenzyme.Stressmeasurementsoftheenzyme are usually performed in the form of a solution, although the formulationsandtechnologicalprocessesaremainlypresentinthe solidform.
β-galactosidase,alsocalledlactaseisahydrolyticenzymethat decomposeslactose,adisaccharidefoundindairyproducts.Italso catalysesthereverseofthereaction,calledtransglycosylation[1].
β-Galactosidasesofvariousorigins belongtofamiliesofglycosyl hydrolasesproducedbyalmosteverylivingorganism.Thelackor decreasedproductionofthisdigestiveenzymeinthehumansmall intestine results in the inability to properly break down and assimilatemilkcontainingproducts.Thesedairyproductsplayan important role in gastronomy in many parts of the world, irregularityofitsmetabolism(insufficienthydrolysis oflactose) mayresultinaseriousdeteriorationofthequalityoflife[2,3].The prevalence ofthe lactoseintoleranceis over 60% amongst the humanpopulation,causing health problemsof varyingseverity [4].Thetreatmentofthisdiseaseinvolvestheadministrationof various medications containing lactase. These pharmaceutical products:tablets,chewing-tablets,capsules,solutionsetc.usually containone specific streamof lactase derivedfrom Aspergillus oryzae (Yellow koji mold), also called Tilactase [5]. The beta- galactosidaseproducedbythismedicallyandindustriallyimpor- tantfilamentousfungusisarelativelybigandcomplexglycopro- tein, which contains 1009 amino acids, and has an estimated molecular mass of 109kDa without the post translational modifications[6].
Enzymemoleculeslikelactasehavemanydifferentstructural variantswithdifferentpropertiesandresistancetoexternaleffects
*Correspondingauthor.
E-mailaddress:ludanyi.krisztina@pharma.semmelweis-univ.hu(K.Ludányi).
https://doi.org/10.1016/j.btre.2021.e00637
2215-017X/©2021TheAuthor(s).PublishedbyElsevierB.V.ThisisanopenaccessarticleundertheCCBYlicense(http://creativecommons.org/licenses/by/4.0/).
ContentslistsavailableatScienceDirect
Biotechnology Reports
j o u r n al h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / b t r e
(pH, temperatureetc.), dependingon its source.[7]. Structural differencesbehindthisphenomenonhavenotbeenexploredyet, butitseemslikelythattheglycanstructuresplayanimportantrole inthestabilityandefficiencyoftheprotein[8–11].Enzymetype activeingredientsareusuallysensitivetopH,moisture,pressure, temperature, applied excipients and reactants (metal ions) occurring during manufacture. There is a possibility that they maylosetheireffectivenessbeforeorduringoraladministration, although various technologiestryto overcomethese disadvan- tages[12–15].Forceddegradationstudiesareoftenperformedon active pharmaceutical ingredients to determine and predict possible degradationpathways under variousstress conditions.
Alargevarietyofdegradationprocessesmayresultinthelossof functionorlossofefficiency,andtheseareverycomplextoanalyse [16].Currently,therearenoexact[17]guidelinesfortheindustry defininghowtoperformstabilitytestingincase ofproteintype activeingredients.[18].
Despitethepharmaceuticalandcommercialrelevanceofthe lactasecontainingproducts[19],ourknowledgeislimitedabout the relationship between their structure and activity. In the presentstudywewilluseacombinationofanalyticaltoolsinorder todiscoverstructuralchangescausedbystressconditions,which influence enzyme activity and function. The lactase enzyme in pulverizedformwillbestudied(i.e.,inthesolidform),whichis typicalinandproductionstorage.Bottom-upproteomicprocedure utilizingnanoUHPLC-MSmethodwasusedtoobservethepossible alterations intheprimary aminoacidsequence andchanges in PTMs, such as N-glycan structures. Beside mass spectrometric measurementsSDS-PAGE,DLS,NMR,CDmethodswereappliedto observe thepossiblechanges in theprimary and in thehigher orderedstructuresandtolookforpossibleaggregation.NMRand CDtechniqueswereusedtodeterminealterationsinthesecondary and tertiary structures. Parallel ortho-nitrophenyl-β-galactoside (ONPG)basedactivity studieshavebeenperformedtoestablish structure-activityrelationship.
The main objective of the research is to reveal structural changesofthelactaseenzymeunderheat-stressconditions.Thisis importantbothforunderstandingfundamentalaspectsofenzyme activitychanges under storage,and forpractical considerations suchasimprovingdosing,manufacturing,shippingandstorageof biopharmaceuticals,likelactasecontainingproducts[20].
2.Materialsandmethods 2.1.Materials
The powder of β-galactosidase enzyme (Opti-lactase A-50 powder) derived from Aspergillus oryzae was purchased from Optiferm Gmbh(Oy-Mittleberg,Germany). O-nitrophenyl-D-gal- actopyranoside (ONPG), MU-Gal (4-Methylumbelliferyl β-D-gal- actopyranoside)andthebroadrangeSDS–PAGEmolecularweight standardswereobtainedfromVWRInternational(Radnor,PA,US).
ForthetrypticdigestionTrypsinGold,MassSpectrometryGrade (Promega Corporation,Madison, WI, US)and Rapigest(Waters, Milford,MA,US)wereused.Ultrapurewaterobtainedfromthe MilliRO-hMilliQ-system(MerckMillipore,Burlington,MA,US)was usedthroughoutallmeasurements.Allotheringredientslikethe reagents, solvents,eluentswere obtainedfrom SigmaChemical Company(St.Louis,MO,US).
2.2.Stressstabilitytests
Theessenceofthestressstabilitytestistomodelthebehaviour of the sample underextreme conditions, suchas hightemper- aturestodetermineandmodelextremestorageandproduction parameters[21],andpredictprocessesoccurringamongnormal
conditions.Duringthetemperature-inducedstresstestthenative proteinpowdersamples(1000mgeach)wereincubatedatseveral temperaturesfordifferenttimeperiodsandwerekeptinlocked glassvials.Theheatloadtookplaceinastabilitytestchamberkept and controlled at a constant temperature and humidity. Two controlsampleswerestoredatroomtemperatureandrefrigerated.
TheexactexperimentalconditionsaresummarisedinTable1.
2.3.Activitymeasurement
The hydrolysing activity of theexamined enzyme stored at varioustemperatures forvarioustime periodslistedabovewas estimatedusing o-nitrophenyl-β-D-galactopyranoside (ONPG) as substrate[22].ONPGisacolourlesssubstance,whichiscleavedby β-galactosidasetogalactoseando-nitrophenol(ONP),thereaction isindicatedbyayellowcolourchange.Thereagentwassolvedin pH=4.5phosphate-citrate(McIlvaine)buffersolution[23].5ng/
mLconcentrationsamplesweremadewithMilliQwaterandthe twosolutionsweremixedinaratioof1:3[24,25].Thesamples wereincubatedinwaterbathat37Cfor30min.Aliquotswere withdrawnfromthereactionmixtureandpipetteddirectlyinto 1mLNa2CO3 solution tostop the enzymatic hydrolysisand to producethemaximumpossibleabsorptionduetotheliberatedo- nitrophenolate ion. The solution was left to cool to room temperatureandtheabsorbanceofo-nitrophenolwasdetermined by UV–vis spectroscopy using an ATi Unicam UV2 UV/VIS Spectrometer (UNICAM,Budapest,Hungary)at420nm inthree parallelmeasurementswithfivedifferentstressedsamples.
2.4.DLS
Theaveragediameteroftheproteinsinthedifferentsamples wasdeterminedbydynamiclightscattering(DLS)usingZetasizer NanoZSTM(MalvernPanalyticalLtd.,Malvern,UK).Absorbancesof a sample havebeen investigatedbeforeeach particlesizing by single beam UV–vis spectrophotometer, (8453 type; Agilent Technologies,USA)at
l
max=633nm,todetermine thecorrectamount of sample scatterings vs. absorbances. Samples were illuminatedbyaHeNelaser(wavelengthof632.8nm,4.0mW),an avalanchephotodiode(APD)detectionanglewas173(backscatter mode). Protein samples were dissolved in MilliQ water in the concentration of 1mg/mL to reduce nonlinearity effects on measurements by increased viscosity of solvent with higher concentrations.Measurementsweremadeperforming15runsof 10seach,anequilibrationtimeofatleast5minwassetbeforethe measurementstarted.Sizemeasurementsweremadeintriplicate and wereusedimmediately afterpreparation.The experiments were carried out at a constant room temperatureof 25C. All solutions were analysed without prior filtration to assess if aggregateswerepresent upto10,000nm (detectionlimit).The diameterofthesolvedmoleculesisrelatedtothepropertiesofthe solvent, so refractive index and viscosity were tested. The refractive index was measuredby a clinical refractometer, the viscositybyaBrookfiledAmetekDVEviscometer(Middleboro,MA, US)anditprovedtobe1.031and0.8991cP.
Table1
Experimentlayoutofthermalstabilitystresstests.
Conditions StorageTime
40C 1day 3days 5days 1week 2weeks
60C 1day 3days 5days 1week 2weeks
80C 1day 3days 5days 1week
Control1(25Croomtemp.) 1day 2weeks
Control2(5 8Crefrigerated) 1day 2weeks
20Cdeepfrozen 1day 2weeks
2.5.Electrophoreticanalysis
SDS-PAGEwascarriedoutusinga10%Tris-Glycingelanda verticalNovexMinigelelectrophoreticsystem.100
m
Lof1mg/mLlactasesolutionwasmixedto100
m
LSDSsamplebuffer(62.5mMTris–HCl,2%SDS,25%glycerol,0.01%bromophenolblue,5% mercapto-ethanol or100mM DTT,pH=6.8)denaturedat80C for5min.15
m
Lofeachsamplewasusedandtheelectrophoresis was carried out at 35mA and 150V for 90min until the bromophenol blue has reached the lower edges of the gel.Protein bands were visualised by staining with Coomassie Brilliant Blue R-250 in 50 % methanol, and 10 % acetic acid stainingsolution,distainedbyasolutionof5%methanoland10% acetic acid for overnight. Broad-range SDS–PAGE molecular weightstandardspurchasedfromVWRwereusedasmolecular mass standards (Myosin 200kDa, β-Galactosidase 120kDa, Bovine Serum Albumine 91kDa, Glutamic dehydrogenase 62kDa,Ovalbumin47kDa,CarbonicAnhydrase37kDa,Myoglo- bin28kDa,Lysozyme19kDa,Aprotinin9kDa).
FortheZymography(correlationbetweenactivityandprotein position)thegelissubjectedtofluorometricanalysisusingMU-Gal (4-Methylumbelliferylβ-D-galactopyranoside)totestforβ-galac- tosidase activityof the separated bands. Thisgel is soaked for 15min in 100mM sodium acetate buffer, pH=5.0 with lateral shaking,100rpm.Then,itisincubatedwiththeMU-Galsolutionat 37Cfor 15min.Thezymogram gelis analyzedunderUVlight,
l
=365nm to detect enzyme activity due tothe release of 4-methylumbelliferone.Thisstepshouldbeperformedquickly,as the fluorescent product diffuses within the gel, obscuring the bands.
NotethatwhileSDSandreducingagentareusedintheSDS- PAGEgelandrunningbuffer,non-denaturingelectrophoresiswas carried outwiththeomissionofSDSfromthegelrunningand loading buffers, and the sample was pre-treated only under moderatetemperature.
2.6.NMR
DatawereacquiredfromtwosampleswithtwodifferentNMR methods:1D1Hand2Ddiffusion-orderedspectroscopy(DOSY) NMR.Thesampleswere:lactasestandard100mgsolvedinMilliQ water, 10 % D2O, 1% DSS (standard) added, pH=7.45. Heated lactaseat80Cfor14days100mgsolvedinMilliQwater,10% D2O, 1% DSS (standard) added, pH=6.96. Before the NMR measurements the samples were filtered five times with Eppendorf centrifuge tube equipped with a membrane of 10kDa cut. After the filtration, the samples were diluted to 500
m
L with MilliQ Water then measured immediately. NMR measurementswereperformedonaBrukerAvanceIII700MHz spectrometerequippedwitha5mmProdigyTCIH&F-C/N-D,z- gradientprobeheadoperatingat700.05MHzfor1H.Allspectra wereprocessedwithBrukerTOPSPINsoftware.1HNMRmeasure- mentsDOSYexperimentswereperformedat14Cinaccordance withapreviousNMRstudywhichcomparesdiffusionconstants with the size of proteins [26], using the stebpgp1s19 pulse sequence with water suppressionfor diffusionmeasurements.The lengthsofdiffusion delaysand pulseswereoptimizedto
d
=5ms,
D
=300ms. The strength of the diffusion gradient waslinearlyincrementedin32equalsteps,varyingbetween5%and95
%ofitsmaximumvalue;theappliedmaximumgradientstrength was45.4G/cm.Thenumberofscanswasadjustedforeachsample toobtainreliableS/Nratios(16for 1Hand 40 or160forDOSY experiments). Each measurement was repeated at least three times.Toinvestigatethediffusionconstantssignalswerechosen fromseveralregionsinthealiphaticprotonrange,andthedecay wasprocessedwith2DFouriertransform.
2.7.CD
Circulardichroism(CD)measurementswereperformedona JascoJ-815CD spectrometerequippedwitha thermostable cell holder.TemperaturewascontrolledandmonitoredwithaPeltier typeJascoCDF-426Lthermostatunit.Thesubstrateanddifferent stressed enzyme mixtures were measured together for kinetic studies,todeterminethereductionofactivityintime.Thepossible manifestationinsecondarystructurechangesduetothesupposed temperatureinduceddegradationwasalsointendedforanalyza- tion.Conformationalchangesinthesecondarystructureofprotein were monitored in the region of 190–250nm with a protein concentration of 50
m
g/mL (0.60m
mol) in a quartz cuvette(Hellma) with a path length of 1mm. The scanning speed, bandwidth and data pitch were set to 50nm/min, 1nm and 0.5nm,respectively,whilethenumberofaccumulationsforeach recordedspectrumwassetto3(within600HTvoltagerange)and averagedtogetthecompletespectrum.
TheactivitymeasurementswerefollowedbyCDspectroscopy alternatively, where the ligand ONPG hydrolysis could be measured selectively online, and the kinetic curve could be acquired directly. Kinetic measurements were performed as follows: ONPG (0.050g / 10mL) +1
m
L enzyme stock solution(10mg/mL)dissolvedin3mLofMcIlvainecitrate-Na2HPO4buffer, pH=4.6,temperature37C0.05.Detectionwavelengthwasset at356nm(ONPGCDmaximum).
2.8.PeptideandN-glycosylationanalysis
Forthesequenceandglycanidentificationbottom-upanalysis wasused.Fivedifferentparallelstressedsampleswereidentified toinspectthepermanencelevelofthechanges.Allreagentswere used in relevant volume, comparable to theprotein level. The samplescorrespondingto100
m
gofproteinweresolvedinMilliQwater.FortheS-Sbridgereductionammoniumbicarbonatebuffer atpH8.0containing200mMofDTTandRapigestwasadded.The samplesweresuccessivelyincubatedat60Cfor30min,andthen cooledat roomtemperature. Alkylationof cysteineresidues by adding 200mM IAA in 100mM ammonium bicarbonate was carried out in the dark at room temperature for 30min. The sampleswereleft tobedigested for90minat 37C byadding trypsin1:50w/wratioconsideringthetotalproteincontent.Lastly, sampleswereacidifiedwithcc.formicacidtostopthedigestion.In theendthesampleswerecentrifugedat13447G-forcefor10min andthephaseswerecarefullyseparated.Thetrypsindigestionwas carriedoutinsolutionwithoutTFAinordertoavoidtheMSsource contamination. The mass spectrometric measurements started rightafterthepreparationasfollows:6
m
LsamplewassubjectedtonanoLC-MS/MS analysisusing a Dionex Ultimate3000 RSLC nanoLC(Dionex,Sunnyvale,CA,USA)coupledtoaBrukerMaxisII Q-TOF (Bruker Daltonics, Bremen, Germany) via CaptiveSpray nanoBoosterionsource.PeptideswereseparatedonanAcquityM- ClassBEH130C18 analyticalcolumn(1.7
m
m,75m
m250mm Waters,Milford,MA) usinggradientelution(4–50%eluentBin 120min)followingtrappingonanAcclaimPepMap100C18,5m
m,100
m
m20mm(ThermoFisherScientific,Waltham,MA)trap column.SolventAconsistedofwatercontaining0.1%formicacid, while SolventB was acetonitrile containing 0.1 % formic acid.Spectra were collectedusing a fix cycle time of 2.5s and the following scan speeds: MS spectra at 3Hz, while CID was performedonmultiplychargedprecursorsat16Hzforabundant ones and at 4Hz for the ones with low abundance. Internal calibrationwasperformedbyCompassDataAnalysissoftware4.3 (BrukerDaltonics,Bremen,Germany)basedoninfusingsodium- formate. Data were processed by the Byonic software (Protein Metrics,Cupertino,CA,USA).Peptideswereidentifiedbysearching
againsttheAspergillusOryzae(W5ZSH9_ASPOZ)in theUNIprot database.GlycanswerequantifiedwiththeGlycoPatternsoftware [27].
3.Resultsanddiscussion
The enzyme activity and various structural aspects of the lactasesamplestoredundervariousconditions(Table1)hasbeen studied in detail. No observable change was found among the control and the frozen samples, so these will not be further discussed.Asexpected,thelargestandmostsignificantchangesin enzyme activity and property was observed at the highest temperaturesandlongestperiods.Structuralchangescorrespond- ingtotheobservedchangeinenzymeactivityhavebeenstudiedby a number of analytical methods. In the following discussion structureoflactase,andinparticularchangesinitsstructurehave beenevaluatedusinganumberofanalyticalmethods.Changesin the molecularmass has been studied by SDS-PAGE and NMR.
Changes in themolecularsize and polydispersity werestudied usingDLS.Informationaboutthestructurechangeswereobtained byMSandCDspectrometry.
Theseresultswillbepresentedbelowindetail.
3.1.Activity
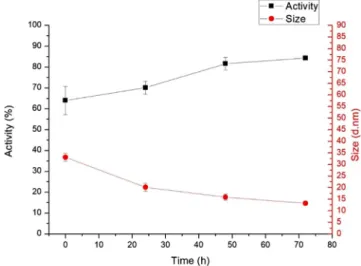
Thechangesinlactaseactivityduetothermalstressareshown inFig.1.Thisclearlyshowsthatenzymeactivityisconsiderably reduced underheat stress.Degradationof thestressedenzyme sampleswascalculatedcomparedtotheactivityofthestandard sampletakenas100%.Enzymeactivityofthesamplestoredat 80Cforoneweekdecreasedto64%thatofthecontrolsample.
This is,however, a smallerdecreasethan occursis solution(in whichcaseactivitydecreasedtoca.40%thatofthecontrolsample) [7,28]. It is important, that after dissolving the heat-stressed sample, lactase activityslowly regeneratesin time (inhoursor days,from64to80%thatoftheoriginalactivity).Thisisshownin Fig.2.Thissuggestschangesinthesampleduetoheat-stressare partiallyreversible.
3.2.DLS
Havingobservedchangesinlactaseactivityduetoheat-stress, wehavetriedtounderstandthecorrespondingchangesinprotein structure.First,wehaveperformeddynamiclightscattering(DLS) measurements to characterize molecular size and possible
aggregation. We have determined the average molecular size (hydrolytic diameter using a hard sphere model) and the polydispersityindex.Note,polydispersityindicatesthevariability ofthemolecularsizebasedontheDLSdistribution[29,30].The DLSdistributionisshowninFig.3,boththatofthestandardand theheat-stressedsample.Thehydrolyticdiameteroftheparticles inthestandardenzymesolutionwasbetween9and10nmineach measurement, and polydispersity index was less than 0.2, suggesting a narrow distribution. These indicate, that under normal conditions the enzyme in solution is in the form of monomers.Fig.1showsthatheatstress(whichdecreasesenzyme activity)increasestheaverageparticlesize,indicatingaggregation andproteinunfolding.Dissolvingtheheat-stressedsample,itwas shownthatenzyme activityslowlyrecovers (Fig.2).In parallel, particle size decreases, indicating that aggregation (and/or unfolding)ispartiallyreversible.
Increaseofparticlesize issmall atlow temperaturesand is likely due to proteinunfolding. At higher temperatures (80C) large,2 3-foldincreaseinaverageparticlesizecanbeobserved.
Fig.3showsthatinthiscaseaslargeasmicrometersizedparticles alsoappear,clearlyindicatingproteinaggregation.Thisfigurealso showsthatthefirstpeakintheparticledistributionoftheheat- stressedsample iswiderthanthatofthestandardsample.This smallerchangeinparticlesizesuggestsproteinunfolding.
Fig.1.Theeffectoftemperatureofheatstressontheβ-galactosidaseactivity(n=3) andtheaveragehydrolyticdiameter(n=4)oftheparticlesinthestandardand differenttemperaturestressedenzymesolutions,immediatelyaftersolvation.
Fig.2.Changesinactivity(n=3)andaveragemolecularsize(n=4)overtimeinthe 80Cstressedlactasesampleinsolution.
Fig.3.Sizedistributionofthestandardandheatstressedproteindeterminedby DLSmeasurement.
3.3.SDS-PAGE
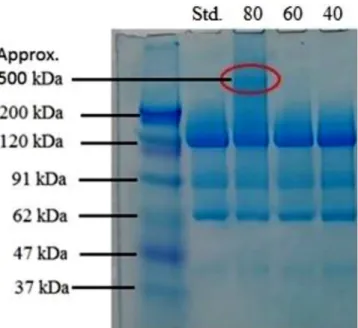
AccordingtotheinformationintheUNIprotdatabase[6],the weightofβ-galactosidasefromAspergillusoryzaeis109kDa.This informationisbasedontheaminoacidsequenceanddoesnottake into account possible structural modifications. Our preliminary MALDI-TOFmeasurementindicatesamolecularmassof123kDa, whichsuggestsextensivepost-translationalmodifications(PTMs).
We havestudiedchanges in themolecularmassof thecontrol sampleandheatstressedsamplesstoredat40,60and80Cforone weekusingSDS-PAGE.TheresultisshowninFig.4.
In the first column a standard protein ladder was used to determinewherethedifferentmassesofmaterialsarelocatedon thegel.Thereisnovisiblechangeinmolecularweightinthecaseof 40C,60Cheatstressedsamples,butat80Cabandappearedat higher(approximately500kDa)molecularweight,fromwhichit canbeconcludedthattheproteinstartstoaggregateand likely formstri-ortetramericstructures,assuggested[31].Itcanalsobe seenonthegelthattheloadedsampledrewacontinuousstrip, presumably due tothepresence oflarger butnot well-defined particlesincomparisontothemaincomponent.Boththestandard andstressedsamplecontainmoleculeswithlowermolecularmass at90,60and40kDa(Fig.4).Thepresenceofthesebandssuggests contaminantsordegradationproducts.Asthepresenceofthese bandsdidnotchangeduetoheatstress,thesewerenotfurther studied.
3.4.NMR
TheNMRmeasurementsprovidedinformationonthestructure andsizeoftheprotein.Thelargemolecularsizeandheterogeneous composition of the lactase samples caused some practical difficulties regarding NMR measurements. For this reason, the classicalStejksal-Tannerequationfittingdidnotgiveusefulresults, as regression always resulted in a multicomponent fitting.
Informationonvarioussizemolecularcomponentsinthelactase sample(i.e.smallmolecules,intactlactaseandlactaseaggregates wereobtainedusing2DDOSYspectroscopy).Thisrevealedthat multiplediffusionconstantscanbedetectedinthesampleswith
differencesbothinsizeanddistribution.Thespecifieddiffusion constantsandthecalculatedmassesareextrapolated(basedonthe workofDudásandBodor[26])aresummarizedinTable2.
The27and 58kDasizerangesinthemeasurementsarenot causedbythethermal stress;theyare presentinboth samples fromthebeginninglikepresentedearlierinFig.4.Thesearelikely tobecontaminantsor degradationproducts in thecommercial lactasesample. Theparticlesobservedfollowingheat stressare likelydi-andtetrameraggregatesofthelactasemolecule(257and 550kDa).
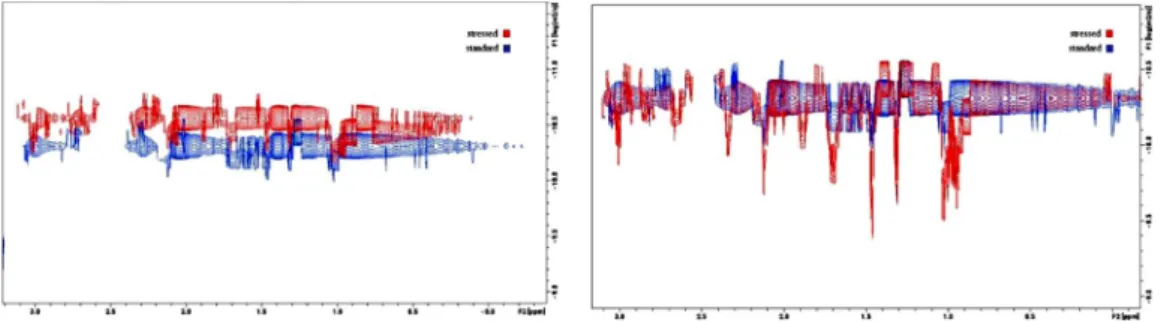
BoththeDOSYand1H-NMRpatternschangeovertimeinthe samples.BasedontheDOSYspectra,thesampleswerefoundtobe mixtures characterized by multiple diffusion constants. The moleculesizesdeterminedbasedonDOSYaresignificantlylarger inthestressedsamples(Table2).Thedissolvedproteinaggregates areunstableamongthesecircumstancesinaccordancewiththe formerobservations.AdefinitealterationinDOSYpatterncouldbe observedovertimeincaseoftheheatstressedsamplesdissolved forNMRmeasurement.Interestingly,thefractionofDOSYsignals correspondingtotheproteinaggregatesdecreasesovertime,and after72htheDOSYpatternoftheheatedsamplesfinallyreaches that of the untreated sample. This indicates that aggregation occurs in the solid state during heat stress, but it is mostly reversibleinsolution(Fig.5).
Theintensityofsharpsignalsofsmallmoleculesissignificantly growing in the1H spectra of the heated samples (Fig.6).The increaseintheintensityofthesmallmoleculesignalsinthe1H- NMRspectraispresumablyassociatedwiththesmallcarbohydrate moleculesarisingduetothedegradationoftheglycanchainsfrom theprotein.Glycandegradation hasbeen confirmedbytheMS study,andwillbediscussedbelowindetail.
3.5.Circulardichroism
Lactase activity changes were observed/confirmed by CD kineticstudies,whereenzymekineticswasstudiedbyobserving bothlactaseand itssubstrate.Directmonitoringofthereaction wasaccomplishedbydualCD/UVdetection.Itwaspossible,asthe amountofsubstrates, which formedasa resultofthecatalytic reaction of ONPG can be selectively measured in the solution, becausegalactoseischiraland theortho-nitrophenolisagood chromophore. During the hydrolysis, the disruption of the glycosidic bond causes thedisappearanceof CD signal and the strengtheningofUVsignal.TheCDcurveselectivelymeasuresthe hydrolysingsubstrate,whiletheincreaseintheUVcurveindicates the formation of dinitrophenol (Fig. 7). Results obtained from enzymekineticCDstudiescorrelatewellwithactivitymeasure- ments(Fig.1).
Thepossibleconformationalchangesinthesecondarystructure werealsoanalysed.Thedifferencesbetweenthespectramaybe causedbythedifferentinitialconcentrations,butifwecalculate theDissymmetryFactor(g),whichistheratiooftheCDandtheUV signals and a dimensionless parameter,then the concentration differences disappear. Furthermore, if there is a slight but significant difference in either the CD or the UV spectrum, it
Fig.4. SDS-PAGEelectropherogramoftheStandardandheatstressedenzymes.The contentsofthelanesfromlefttorightarethefollowing:Lane1:molecularmass markers,massindicatedalongside.Lane2:Standardβ-gal.enzyme.Lane3-5:Heat stress.
Table2
Thediffusionconstantvaluesofthedifferentparticlesinthestandardand80C stressedsamplesandtheequivalentmasses.
Lactasestandard: Stressedsample(80C):
6.471011m2s 1(27kDa) N/A
4.8610 11m2s 1(58kDa) 4.881011m2s 1(57kDa) 3.6310 11m2s 1(124kDa) 3.661011m2s 1(121kDa)
N/A 2,751011m2s 1(257kDa)
N/A 2.7510 11m2s 1(550kDa)
Fig.5.The2DDOSY1HNMRspectraofthesamesampledetected2h(left)and72h(right)afterdissolvingthesolidsamplesinwater.TheDOSYprofilebelongingtothe stressedsample(red)returntothestandard’sprofile(blue)after72h.
Fig.6. The1Hspectrumofthestandardandstressedsamples.
Fig.7.LactoseandONPGCD(Upper)andUV(Bottom)kineticcurvesofsameconcentrationat37C.
appearsontheg-spectrum[32].ThefollowingFig.8showstheCD andUVspectraofsamplesontheleftandtheg-spectraofstandard andtreatedlactaseat80Contherightside.Theintensitiesdiffer duetothedifferentconcentrations.However,thevalue'g'(CD/UV ratiospectrum),whichisindependentofconcentration,doesnot differ significantlyin thecase of thestandardand thesamples storedat40and60C,whichmeanstheresidualproteinstilldoes not loseitsstructure. Achangein theintensityof g-spectrum indicatessomestructuraldifferencescomparingthestandardand thetreatedsampleat80C.Realevidenceforthestructuralchange istheratioofthepeaksat241/222.4nm,wherethevalueis1.086 forthestandardand1.307forthetreatedsample.
3.6.PeptideandN-glycosylationanalysis
Thelactaseenzymes(thecontrolandheat-stressedsamples) were studied,after tryptic digestion,using LC–MS/MS analysis.
Peptides were identified using the Byonicsoftware. Very high, 95.02%sequencecoveragewasobservedforthecontrolsample, while91.4%forthe80Cstressedsample.Recentstudiesonother proteins [9,33] suggest glycans hasa largeinflenceonenzyme activity.Forthisreason,wehavestudiedN-glycosylationoflactase
indetail,for whichverylittleinformationisavailable. Wehave found8differentglycosylationsites; each wassubstitutedbya large number of glycans (Table 3). The glycans contained N- acetylhexosamine, hexose, sialic acid and fucose residues, and mosthadahigh-mannosestructure(Figs.9and10).Note,ourLC– MSanalysisallowedsite-specificglycosylationanalysis(i.e.tohave informationontheglycansobservedatthevariousglycosylation sites).Mostglycans wereso-called complexand highmannose glycans.
Heat-stress caused a large (and irreversible) change in glycosylation.Thiswasrevealedbyquantitativemassspectromet- ricanalysis.Forquantitativecomparison5replicatesofthecontrol sample,and5replicatesofthe80C heatstressedsamplewere analysed.Theglycopeptidesfoundinbothsampleswereselected for further investigation. A t-test was used to identify those glycans,thatshowedsignificant(p=0.05)differencebetweenthe controlandtheheat-stressedsample(Table3).Theglycosylation pattern(relativeglycoformabundances)fortwoglycosylationsites are shown in Figs. 9 and 10. The glycopeptide in Fig. 9 (NLTTGVYTDTSDLAVTPLMGDSPGSFFVVR) shows a very large changeduetoheat-stress:Thelargestchangeoccurredinglycan size,inparticularthenumberofhexoseunitspresent.Duetoheat- Fig.8.CDandUVspectraoffreshlypreparedlactasesamples(left)andg-spectraoflactasestandardandlactasestoredat80C(right).
Table3
Thetableshowstheexaminedpeptides,thenumberoffoundglycans,thenumberofstatisticallysignificantdifferencesbetweenthesamples.
Peptide Numberoffoundglycans SignificantDifferences
VNGTLR 25 21
SNVTIIEGSDSGIVSTR 26 20
GWDVPLYFNFGNNTQAAR 13 5
LPTSAGNLTIPQLEGTLSLNGR 24 17
LKLPTSAGNLTIPQLEGTLSLNGR 20 12
IHVVDYNVSGTNIIYSTAEVFTWK 19 19
NLTTGVYTDTSDLAVTPLMGDSPGSFFVVR 16 16
GAHLDGADLHLTADFNATTPIEVIAPTGAK 5 5
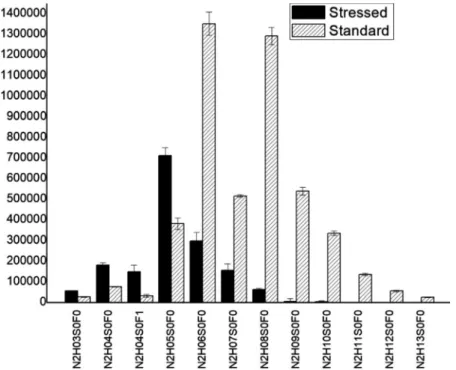
stresstheaveragenumberofhexoseunitsdecreasedformca.8to 5.Intheotherexample(VNGLTR)thechangeinglycancomposi- tion was not so large, but still observable, and statistically significant(Fig.10andTable3).Notethatthebuildupofcomplex glycansoccursinlivingcells,sotheirdegradationisirreversible.
4.Conclusion
Our investigations indicate various changes occur when submittingthesolid enzymetoheat stress.Heat stress causes significant decrease in enzyme activity. However, after Fig.9. SectionoftheglycosylationpatternofNLTTGVYTDTSDLAVTPLMGDSPGSFFVVR,comparingtheaverageabundanceoftheglycansofthestressedandstandardsamples ontheNLTTGVYTDTSDLAVTPLMGDSPGSFFVVRglycopeptide.(N:N-acetylhexosamine,H:hexose,S:sialicacid,F:fucose).
Fig.10.SectionoftheglycosylationpatternofVNGLTR,comparingtheaverageabundanceoftheglycansofthestressedandstandardsamplesontheVNGLTRglycopeptide.
(N:N-acetylhexosamine,H:hexose,S:sialicacid,F:fucose).
dissolution,inseveralhours,enzymeactivitysomewhatrecovers, suggestingtheactivitydecreaseispartlyreversible.Thiscanbe explained by reversible aggregation of lactase molecules, suggested by the results of DLS and NMR measurements. In thecaseoforalmedication,partialrecoveryofenzymeactivityis notrelevant,aslactasemoleculesdonotspendsufficienttimein dissolved form in the body. The aminoacidsequence andthe secondarystructureoftheenzymedidnotshowanychangedue toheatstress.Lactaseisheavilyglycosylated,andheatstressdid changeglycosylation significantly.LC–MSexperimentsrevealed thatvariouspeptide-boundglycanchainswereshorteneddueto heat stress. These are irreversible changes, changeing the physico-chemical properties of the enzyme and may increase tendencyofaggregation.
Thesestudiessuggestthatbothstructuralchangesinthehigher order structure of the protein and changes due to altered glycosylation are responsible for the decrease of activity.
Understanding behaviourunderheat stressmayhelp todesign better manufacturing, shipping, and storage conditions and improve our understanding of different types of lactases, like Kluyveromyceslactisusedindairyindustryorevenhumanlactase.
The methodology developed here may serve as a model for investigatingotherenzymesofpharmaceuticalinterest.
CRediTauthorshipcontributionstatement
MártonKirály:Writing-originaldraft,Visualization,Formal analysis, Investigation, Methodology. Borbála Dalmadi Kiss:
Writing-review&editing,Methodology,Conceptualization.Péter Horváth: Investigation, Writing - original draft, Visualization.
László Drahos: Software, Formal analysis, Writing - review &
editing. Arash Mirzahosseini: Funding acquisition, Writing - originaldraft.GyulaPálfy:Investigation,Visualization,Writing- review & editing. István Antal: Resources. Krisztina Ludányi:
Conceptualization, Supervision, Funding acquisition, Writing - review&editing,Methodology.
DeclarationofCompetingInterest
The authors declare that they have no known competing financial interests or personal relationships that could have appearedtoinfluencetheworkreportedinthispaper.
Acknowledgements
TheresearchwasfinancedbytheHigherEducationInstitutional Excellence Programmeof the Ministryof Human Capacities in Hungary,withintheframeworkofthemolecularbiologythematic programmeoftheSemmelweisUniversity.
AppendixA.Supplementarydata
Supplementarymaterialrelatedtothisarticlecanbefound,in the online version, at doi:https://doi.org/10.1016/j.btre.2021.
e00637.
References
[1]S.Zárate, M.H. López-Leiva, Oligosaccharideformation duringenzymatic lactosehydrolysis:aliteraturereview,J.FoodProt.53(1990)262–268,doi:
http://dx.doi.org/10.4315/0362-028X-53.3.262.
[2]T.M. Bayless, E. Brown, D.M. Paige, Lactase non-persistence and lactose intolerance,Curr.Gastroenterol.Rep.19(2017)23,doi:http://dx.doi.org/
10.1007/s11894-017-0558-9.
[3]B.Misselwitz,D. Pohl,H.Frühauf,M.Fried,S.R.Vavricka,M.Fox,Lactose malabsorptionandintolerance:pathogenesis,diagnosisandtreatment, UnitedEur.Gastroenterol.J.1(2013)151–159,doi:http://dx.doi.org/10.1177/
2050640613484463.
[4]C.L.Storhaug,S.K.Fosse,L.T.Fadnes,Country,regional,andglobalestimatesfor lactosemalabsorptioninadults:asystematicreviewandmeta-analysis, LancetGastroenterol.Hepatol.2(2017)738–746,doi:http://dx.doi.org/
10.1016/S2468-1253(17)30154-1.
[5]S.Saqib,A.Akram,S.A.Halim,R.Tassaduq,Sourcesofβ-galactosidaseandits applicationsinfoodindustry,3Biotech7(2017)79,doi:http://dx.doi.org/
10.1007/s13205-017-0645-5.
[6]Y.Ito,T.Sasaki,K.Kitamoto,C.Kumagai,K.Takahashi,K.Gomi,G.Tamura, Cloning,nucleotidesequencing,andexpressionofthebeta-galactosidase- encodinggene(lacA)fromAspergillusoryzae,J.Gen.Appl.Microbiol.48 (2002)135–142,doi:http://dx.doi.org/10.2323/jgam.48.135.
[7]L.F.Atyaksheva,O.S.Pilipenko,E.S.Chukhrai,O.M.Poltorak,Similarityofand differencesbetweenthemechanismsofthermalinactivationofβ- galactosidasesofdifferentorigins,Russ.J.Phys.Chem.A,FocusChem.82 (2008)864–869,doi:http://dx.doi.org/10.1134/S0036024408050300.
[8]N.G.Jayaprakash,A.Surolia,Roleofglycosylationinnucleatingproteinfolding andstability,Biochem.J.474(2017)2333–2347,doi:http://dx.doi.org/10.1042/
BCJ20170111.
[9]P.Goettig,Effectsofglycosylationontheenzymaticactivityandmechanisms ofproteases,Int.J.Mol.Sci.17(2016)1969,doi:http://dx.doi.org/10.3390/
ijms17121969.
[10]D.Shental-Bechor,Y.Levy,Effectofglycosylationonproteinfolding:aclose lookatthermodynamicstabilization,Proc.Natl.Acad.Sci.U.S.A.105(2008) 8256–8261,doi:http://dx.doi.org/10.1073/pnas.0801340105.
[11]R.J.Solá,K.Griebenow,Effectsofglycosylationonthestabilityofprotein pharmaceuticals,J.Pharm.Sci.98(2009)1223–1245,doi:http://dx.doi.org/
10.1002/jps.21504.
[12]I.Wagner,Z.K.Nagy,P.Vass,C.Fehér,Z.Barta,T.Vigh,P.L.Sóti,A.H.Harasztos, H.Pataki,A.Balogh,G.Verreck,I.VanAssche,G.Marosi,Stableformulationof protein-typedruginelectrospunpolymericfiberfollowedbytabletingand scaling-upexperiments,Polym.Adv.Technol.26(2015)1461–1467,doi:http://
dx.doi.org/10.1002/pat.3569.
[13]P.Vass,E.Hirsch,R.Kóczián,B.Démuth,A.Farkas,C.Fehér,E.Szabó,Á.
Németh,S.K.Andersen,T.Vigh,G.Verreck,I.Csontos,G.Marosi,Z.K.Nagy, Scaled-upproductionandtabletingofGrindableelectrospunfiberscontaining aprotein-typedrug,Pharmaceutics.11(2019)329,doi:http://dx.doi.org/
10.3390/pharmaceutics11070329.
[14]B.S.Dionizio,D.V.deBabos,D.H.Souza,E.R.Pereira-Filho,Evaluationofthe QualityofFormulationsContainingLactaseβ-Galactosidase)EmployingGel ElectrophoresisandCellPhone,(2019).
[15]M.J.Hernaiz,D.H.G.Crout,Immobilization/stabilizationonEupergitCoftheβ- galactosidasefromB.circulansandanα-galactosidasefromAspergillus oryzae,EnzymeMicrob.Technol.27(2000)26–32,doi:http://dx.doi.org/
10.1016/S0141-0229(00)00150-2.
[16]D.Kishore, S.Kundu,A.M.Kayastha,Thermal,chemical andpH induced denaturationofamultimericβ-galactosidaserevealsmultipleunfolding pathways,PLoSOne7(2012)e50380,doi:http://dx.doi.org/10.1371/journal.
pone.0050380.
[17]H.Khan,M.Ali,A.Ahuja,J.Ali,Stabilitytestingofpharmaceuticalproducts- comparisonofstabilitytestingguidelines,Curr.Pharm.Anal.6(2021)142–
150.n.d.https://www.ingentaconnect.com/content/ben/cpa/2010/00000006/
00000002/art00006.
[18]A.Hawe,M.Wiggenhorn,M.vandeWeert,J.H.O.Garbe,H.-C.Mahler,W.
Jiskoot,Forceddegradationoftherapeuticproteins,J.Pharm.Sci.101(2012) 895–913,doi:http://dx.doi.org/10.1002/jps.22812.
[19]N.Stourman,J.Moore,Analysisoflactaseinlactoseintolerancesupplements, Biochem.Mol.Biol.Educ.46(2018)652–662,doi:http://dx.doi.org/10.1002/
bmb.21185.
[20]L.P.L. Lim, E. Garnsey, M. Gregory, Product and process innovation in biopharmaceuticals:anewperspectiveondevelopment,R&DManag.36 (2006)27–36,doi:http://dx.doi.org/10.1111/j.1467-9310.2006.00413.x.
[21]C.P.Chan,Forceddegradationstudies:currenttrendsandfutureperspectives forprotein-basedtherapeutics,ExpertRev.Proteomics13(2016)651–658,doi:
http://dx.doi.org/10.1080/14789450.2016.1200469.
[22]R.Kwapiszewski,J.Szczudlowska,K.Kwapiszewska,M.Chudy,Z.Brzozka, Determinationofacidβ-galactosidaseactivity:methodologyandperspectives, IndianJ.Clin.Biochem.29(2014)57–62,doi:http://dx.doi.org/10.1007/
s12291-013-0318-z.
[23]J. Lederberg, The beta-d-galactosidaseof Escherichia coli, strain K-12, J.
Bacteriol.60(1950)381–392.https://www.ncbi.nlm.nih.gov/pubmed/
14784466.
[24]B. Rotman, Measurement of activity of single molecules of beta-D- galactosidase,Proc.Natl.Acad.Sci.U.S.A.47(1961)1981–1991,doi:http://
dx.doi.org/10.1073/pnas.47.12.1981.
[25]W.Li, X.Zhao,S.Zou,Y.Ma,K.Zhang,M.Zhang,Scanningassayofβ- galactosidaseactivity,Appl.Biochem.Microbiol.48(2012)603–607,doi:
http://dx.doi.org/10.1134/S0003683812060075.
[26]E.F.Dudás,A.Bodor,Quantitative,diffusionNMRbasedanalytical toolto distinguishfolded,disordered,anddenaturedbiomolecules,Anal.Chem.91 (2019)4929–4933,doi:http://dx.doi.org/10.1021/acs.analchem.8b05617.
[27] O.Ozohanics,L.Turiák,A.Puerta,K.Vékey,L.Drahos,High-performanceliquid chromatographycoupledtomassspectrometrymethodologyforanalyzing site-specificN-glycosylationpatterns,J.Chromatogr.A1259(2012)200–212, doi:http://dx.doi.org/10.1016/j.chroma.2012.05.031.
[28]A.Bosso,L.R.I.Morioka,L.F.dosSantos,H.H.Suguimoto,Lactosehydrolysis potentialandthermalstabilityofcommercialβ-galactosidaseinUHTand
skimmedmilk,FoodSci.Technol.36(2016)159–165,doi:http://dx.doi.org/
10.1590/1678-457X.0085.
[29]Z.Yu,J.C.Reid,Y.-P.Yang,Utilizingdynamiclightscatteringasaprocess analyticaltechnologyforproteinfoldingandaggregationmonitoringin vaccinemanufacturing,J.Pharm.Sci.102(2013)4284–4290,doi:http://dx.doi.
org/10.1002/jps.23746.
[30]K. Shiba, T. Niidome,E. Katoh, H. Xiang,L. Han, T. Mori, Y. Katayama, Polydispersityasaparameterforindicatingthethermalstabilityofproteinsby dynamiclightscattering,Anal.Sci.Int.J.Jpn.Soc.Anal.Chem.26(2010)659–
663,doi:http://dx.doi.org/10.2116/analsci.26.659.
[31]C.Sagné,M.F.Isambert,J.P.Henry,B.Gasnier,SDS-resistantaggregationof membraneproteins:applicationtothepurificationofthevesicular
monoaminetransporter,Biochem.J.316(Pt3)(1996)825–831,doi:http://dx.
doi.org/10.1042/bj3160825.
[32]B.R.Baker,R.L.Garrell,g-Factoranalysisofproteinsecondarystructurein solutionsandthinfilms,FaradayDiscuss.126(2004)209–222,doi:http://dx.
doi.org/10.1039/B305291E.
[33]G.T.Beckham,Z.Dai,J.F.Matthews,M.Momany,C.M.Payne,W.S.Adney,S.E.
Baker,M.E.Himmel,Harnessingglycosylationtoimprovecellulaseactivity, Curr.Opin.Biotechnol.23(2012)338–345,doi:http://dx.doi.org/10.1016/j.
copbio.2011.11.030.