Morphogenetic Phases in Development
EDGAR ZWILLING
Department of Biology, Brandeis University, Waltham, Massachusetts INTRODUCTION
It is most appropriate that the material of my talk be presented at a symposium dedicated to Professor Viktor Hamburger. Almost all the ideas which I shall discuss are derived from experiments with chick embryo limbs. A good deal of the early experimental knowledge of the development of this structure is due to the pioneering researches of Professor Hamburger on the development and innervation of limbs in foreign sites (1938, 1939). I should like to acknowledge now my grat
itude to Professor Hamburger for providing the basic information on which my experiments have been built and for his continued interest in my work. We also owe him a debt of gratitude for compiling, along with Dr. Howard Hamilton ( 1951 ), the series of normal stages which are invaluable for people who work with chick embryos. Acknowledg
ment is due also to John Saunders, organizer of this symposium, whose insight into the importance of the apical ridge of the limb bud's ecto
derm and other aspects of limb development was largely responsible for many of the approaches which we have taken.
Questions about the activation and regulation of genomic materials which control particular syntheses of cytodifiFerentiation have acquired a new importance in recent years. Several studies have revealed that many of the synthetic events involved in differentiation of a structure occur some time prior to the typical cytodifiFerentiation of its cells. One of the most striking sets of observations has been made on the develop
ing pancreas of mouse, rat, and chicken. It is now clear that pancreatic amylase, lipase, chymotrypsin, insulin and carboxypeptidase B are present in the earliest gland rudiment of mouse and rat at levels which are low by comparison with a fully elaborated gland, but significantly higher than those found in nonpancreatic tissues (Rutter et ah, 1967, 1968). Further, high resolution studies have revealed the presence of typical secretory granules in a and ß cells of the pancreas of chick em-
184
MORPHOGENETIC PHASES 185 bryos as early as the fourth day of development ( Dieterlen-Lièvre, 1965). Most of the cells of the early pancreas of the mouse at stages when Rutter and his group have detected the several enzymes appear to have no particular indication of precocious cy to differentiation. How
ever, electron dense ß cells may be recognized as early as the 23 somite stage (9 days of development), and typical ß cell secretory granules may be seen in them at this time (Wessells and Evans, 1968). Recent studies of the relation between the appearance of a number of enzymes and the morphogenetic events with which they are associated in a cellular slime mold reveal that low-level activities of some enzymes are detected prior to the visible morphogenetic event to which they are related. The specific activities of some enzymes increase markedly prior to or at the time of the associated morphogenesis (Sussman, 1966;
Roth et al., 1968). Other examples may be cited. It seems that in many (not necessarily all) developmental situations which have been exam
ined with sufficiently sensitive methods, synthesis of small quantities of substances recognized as products of differentiation appear prior to active cytodifferentiation. The active phase of the cy to differentiation is marked by an increased synthetic activity related to the particular product being monitored. Whether the initial synthesis involves few cells which are fully active or many cells which are only partially active remains to be determined in each case.
In what follows I should like to bring together data which show that a similar situation exists with respect to synthesis of chondroitin sulfate in limb mesoderm and that all mesodermal tissues of early limb buds, whether destined to form muscle or cartilage, engage in this synthesis. In addition the data indicate that the direction of differ
entiation of both types of tissue is labile until a fairly advanced stage of development. Finally I shall attempt to relate these findings to the morphogenetic events involved in the elaboration of a limb.
EARLY CHONDROITIN SULFATE SYNTHESIS
Two of my associates, Searls and Medoff, have studied chondro- genesis in the chick limb with the objective of learning when the products of typical cartilage matrix can be detected. Cartilage matrix consists largely of a chondromucoprotein, a mucopolysaccharide about whose protein moiety relatively little is known. The sulfated poly- saccharide chondroitin sulfate has been characterized and can be as
sayed quite readily (Dorfman, 1962, 1963). Searls started this work
186 EDGAR ZWILLING
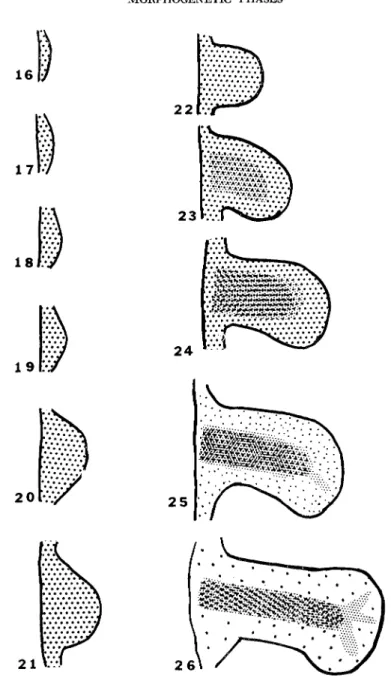
with an autoradiographic study of the binding of isotopic sulfate by limb bud mesoderm (1965a). In brief, his work revealed that sulfate was incorporated uniformly by all the limb mesoderm until stage 22.
After that the rate of incorporation of 35S increased in the central chondrogenic areas of the limb until active histogenesis of cartilage was evident ( Fig. 1 ). However, the nonchondrogenic areas of the limb mesoderm fixed sulfate until a relatively late stage, albeit at a slower rate than the chondrogenic areas. Searls' analyses of the sulfated ma
terial in limb-buds revealed that more than 90% of it at all stages was chondroitin sulfates A and C, with possibly some chondroitin sulfate B (1965b). Independent assays, kindly performed by Dr. Karl Meyer on large numbers of whole embryos of the earliest stages used by Searls, confirmed that chondroitin sulfates A and C were present as well as small quantities of other sulfated mucopolysaccharides ( keratosulfate, chondroitin sulfate B), and we presume that the latter represent the remaining 10% or so of the sulfated materials in Searls' assays.
The analysis was extended by Medoff (1967), who looked for chondroitin sulfate and some of the enzymes associated with its syn
thesis at earlier stages than the earliest studied by Searls. Medoff found low levels of chondroitin sulfate in the earliest limb buds (stages 16-18) and in the presumptive limb tissue of stage 15 embryos. Limb mesoderm from stage 19 was subjected to extensive analysis which re
vealed that UDPG dehydrogenase, UDPGNAc-epimerase, the sul- fating enzyme PAPS, as well as chondroitin sulfate were present at this stage. In addition she demonstrated the presence of one of the more specific enzymes related to chondrogenesis, a polymerase respon
sible for the formation of polysaccharide from uridine nucleotide sugars, in stage 20 limb buds. To extend her studies, she took advantage of Moscona's 1961 observation that virtually all aggregates of dissoci
ated limb mesoderm from early stages become cartilage. Stage 19 limb mesoblasts were dissociated and allowed to reaggregate, and the activities of the above enzymes were assayed over a period of 7 days while the aggregates were grown in shaking flask cultures. Since prac
tically all the tissue chondrified, the events during chondrogenesis could, in effect, be amplified. The dissociation procedure lowered the levels of all the enzymes but did not eradicate them. During the course of the culture period the activity of all the enzymes rose gradually until about 24 hours before cartilage was detected histologically. An
ticipation of the appearance of histogically detectable cartilage was
MORPHOGENETIC PHASES 187
1 6 i
1 7 l
1 81>.
191
2oi·.·;
2 1 Vv
FIG. 1. Diagram of limb stages with shading to represent relative rates of isotopic sulfate incorporation in the various regions of the limb mesoderm. Limb outlines are those for leg buds based on the Hamburger and Hamilton (1951) stages. Incorporation data based on Searls (1965a).
188
marked by an increase in specific activity of all the enzymes. The curve for presence of chondroitin sulfate over this period roughly par
alleled those for the enzymes.
These data tell us at least two important things. The presence of enzymes which participate in chondroitin sulfate synthesis from very early stages means that the genomic activation required for cartilage differentiation (at least for the sugar moiety) has occurred a long time before active cytodifferentiation (i.e., production of evident matrix) has begun. Furthermore the data indicate that a key event which precedes cytodifferentiation of cartilage is a marked increase in the specific activities of the enzymes involved in chondroitin sul
fate synthesis. The immediately critical regulatory mechanisms which fix the fate of cells which become cartilage are those involved in the dramatic augmentation of activity of the chondrogenic enzymes, not in their initiation. This statement receives added emphasis from evidence that all the mesodermal tissue synthesizes chondroitin sulfate in the early limbs even though a considerable proportion of it (i.e., the myogenic tissue) does not form cartilage. That is, genomic activation for cartilage synthesis does not mean that cartilage will differentiate from the tissue in which it occurs; only those tissues which undergo the augmentation of these synthetic activities chondrify.
Support for the contention that the chondroitin sulfate of early tissue is related to, if not identical with, that of cartilage comes from experiments which reveal that all the early limb mesoderm is equiv
alent with respect to ability to differentiate into cartilage. The obser
vations of Moscona (1961) and Medoff (1967), already cited, reveal that virtually all aggregates of dissociated limb mesoderm cells form cartilage. Experiments (Fig. 2) in which blocks of both presumptive myogenic and chondrogenic tissue were isolated and grown in culture revealed that even as late as stage 24, when there is differential uptake of 35S in the two areas, pieces of equivalent size from the two regions form cartilage in like amounts and at equal rates (Zwilling, 1966).
These data lend strong support to the likelihood that the chondroitin sulfate identified in both myogenic and chondrogenic tissues is the same and is, in turn, the same as the chondroitin sulfate of cartilage.
One consequence of the above observations should be that the stabilization of muscle and cartilage formation from limb mesoderm occurs relatively late. That is, if all tissues synthesize low levels of chondroitin sulfate and have a strong tendency to differentiate into
MORPHOGENETIC PHASES 189
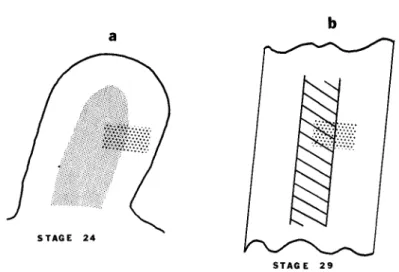
C a r t i l a g e 46/68(67%) 10/14(71%) 36/56(64%) FIG. 2. Diagram that shows the equivalence with respect to cartilage forma
tion of the limb mesoderm. Cut surface represents base of a stage 24 limb bud.
Stippled area = chondrogenic tissue. Clear peripheral tissue = myogenic tissue.
After Zwilling (1966).
cartilage under various culture conditions, then the synthesis of mus
cle proteins either has not begun or may be overshadowed by syn
thesis of cartilage products until at least stage 24. Late stabilization of cytodifferentiation is demonstrated by evidence that even the con
tinuation of cartilage differentiation from advanced chondrogenic tis
sue of this stage is still subject to local conditions. This was demon
strated when Searls (1967) grafted a block of either thymidine-3H or
35S-labeled chondrogenic mesoderm so that part of it was in chondro
genic and part in myogenic tissue of an unlabeled host limb of the same stage (Fig. 3). Only that part of the graft which was in register with the host's chondrifying tissue actually formed matrix. The rest of it did not chondrify and could still be identified by its label. The differentiation of muscle from this tissue was difficult to demonstrate with certainty since the label became greatly diluted over the time in
terval required. However, there were some instances in which striated muscle could be demonstrated in soft tissue which still retained sulfate
label
STAGE 29
FIG. 3. Diagram representing lability of chondrogenic tissue of stage 24 limb mesoderm. Light stippled area = chondrogenic tissue. Heavy stippled rectangle = isotope ( either thymidine-3H or sulfate-35S ) labeled chondrogenic tissue from stage 24 donor limb. Hatched area = area in which cartilage matrix has formed. Only that part of the labeled graft tissue in register with the host cartilage formed matrix. Based on Searls ( 1967 ).
MORPHOGENETIC ACTIVITIES
Despite their low level synthesis of substances associated with the differentiation of cartilage the cells of limb mesoderm do not have a distinctive morphology during the early limb stages. The cells are small, have relatively large nuclei, are tightly packed, and have the round, or more probably duodecahedral, shape common to tightly packed cells (Saunders, 1948; Milaire, 1962a, 1966; Jurand, 1965). Cer
tainly they cannot be recognized as cartilage or procartilage cells.
Distinctive properties of this tissue can best be described in terms of developmental or behavioral capabilities. A considerable body of in
formation reveals that the major properties of limb mesoderm tissue, evinced prior to active cytodifferentiation, are concerned with the elaboration of the form of the limb and with the general disposition of its skeletal elements, i.e., with its morphogenesis. Since the experi
mental evidence for these properties has been reviewed in several places in recent years (Zwilling, 1961; Milaire, 1962b; Amprino, 1965;
Goetinck, 1966), I shall present only a summary based on what has been called the Saunders-Zwilling interpretation of limb development.
MORPHOGENETIG PHASES 191 This interpretation is not subscribed to by all who do research on limb development (Amprino, 1965), but I shall not present a defense of these views at the present.
According to our interpretation the initial growth of the limb as an elongated structure with a particular morphology is basically the con
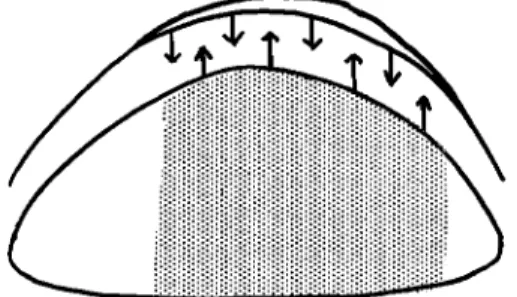
sequence of interaction between limb mesoderm and ectoderm (Fig.
4). The apical ectodermal ridge, initially induced by mesoderm of the early limb field (Kieny, 1960), evokes the outgrowth of the under
lying mesoderm. That tissue in turn provides some as yet unidentified factor (maintenance factor) which is required for the persistence of the ridge as a thickened morphological entity with capacity to induce
FIG. 4. Diagram to represent reciprocal interaction between limb bud meso
derm and the apical ectodermal ridge.
mesodermal outgrowth. There is evidence which indicates that the distinctive asymmetries of limbs result from asymmetric distribution of the maintenance factor and possible involvement of a "polarizing"
factor recently discovered by Saunders and Gasseling (1968). The sequence of events is: initial asymmetry of maintenance factor deter
mines asymmetry in thickness of ridge, which in turn induces asym
metrical outgrowth of the mesoderm. This oversimplification of the events in limb development shall suffice for present purposes. Proper
ties of the limb mesoderm may be summarized:
1. It responds to the ectodermal ridge by active proliferation and outgrowth (Saunders, 1948; Saunders et al, 1957; Zwilling, 1956a;
Hampe, 1957, Goetinck, 1964).
2. It maintains or supports the re-formation of the ridge (Saunders et al, 1957, 1958; Zwilling, 1956a; Searls and Zwilling, 1964).
3. The mesoderm may transmit maintenance factor, largely in a
m
EDGAR ZWILLINGproximodistal direction (Amprino and Camosso, 1958; Saunders et al, 1958; Saunders and Gasseling, 1963; Zwilling, unpublished).
4. Under appropriate conditions it may begin to generate factor after its normal production has ceased (Amprino and Camosso, 1965;
Zwilling, unpublished).
5. It forms jointed skeletal elements which have a particular relation to each other—i.e., long bones form proximally and are followed by digits.
6. Limb type (i.e., leg or wing) characteristics are stable in the mesoderm from early stages (Cairns and Saunders, 1954; Zwilling, 1955; Saunders et al, 1957; Crosby, 1967).
AU these characteristics are evinced as a consequence of the inter
action between the mesoderm and the limb ectoderm. Intact limb mesoblasts which are grown, either as organ cultures or grafts, without ectoderm, without an ectodermal ridge or covered with non-limb ecto
derm, may form more or less of typical proximal structures depending on the stage which is deprived of the ridge (Fell and Canti, 1934;
Saunders, 1948; Zwilling, 1955). However, when equivalent tissue is fragmented or formed into pellets following dissociation of its con
stituent cells, only nodules, rods or sheets of cartilage develop during subsequent growth of the mesoderm either alone, or in association with non-limb ectoderm. There is no trace of patterned limb structure unless such fragments or pellets are reassociated with intact limb bud ectoderms (Zwilling, 1964).
The bones of the vertebral column and chondrocranium are readily distinguished by shape from each other and from those of the ap
pendages. Their associated muscles have unique shapes and character
istics which make their gross identification relatively easy. On the other hand, the tissues from which the bones (or precursor cartilages) and muscles are constructed are quite similar. One would be hard pressed to distinguish vertebral cartilage from femoral cartilage or trunk from thigh muscle by their histological appearance or by chemical analyses.
These structures, so different in form yet composed of similar tissues, are derived from different embryonic sources. Vertebral cartilage and trunk musculature are derived from somites. Limb skeleton and mus
culature come from limb buds. Ribs and costal musculature are de
rived from the flank mesoderm (Fell, 1939). As a consequence of the similarity of cell types, molded into different forms, which are derived from different sources, the skeletomuscular system is very favorable
MORPHOGENETIC PHASES 193 for investigations into possible relations between morphogenetic and histogenetic capabilities. Techniques which provide the means for separation of layers or dissociation of tissues enable the investigator to substitute various types of tissues for the original at will. It is pos
sible to place mesoderm from somites, from the flank or from other regions into isolated limb bud ectoderms, complete with ridges, and to challenge these his to genetically equivalent tissues to see whether they have limb properties. If such composite assemblies are grafted to a nonlimb site, they must, if they have independent limb properties, be able to respond to a ridge by formation of an outgrowth and to main
tain a ridge. Failing this, if they are placed on the dorsal surface of a host limb, their mesoderm can be assayed for ability to transmit the maintenance factor from host tissue to the graft's ectodermal ridge.
When such experiments were done with either fragmented or dissoci
ated somite and flank mesoderms the experiments revealed that these tissues have no limb properties (Zwilling, 1964). Despite their histo
genetic equivalence, the other mesodermal tissues are completely de
void of limb properties. Any cartilage which develops under these cir
cumstances is atypical in form (vertebral, rib?) but definitely not characteristic of any of the limb cartilages. [An exception was found when presumptive flank mesoderm from stage 14 embryos was placed in stage 19 limb ectoderm. There is no limb bud at stage 14. Limb structures formed from mesoderm of both presumptive limb area and its adjacent flank region. This has been interpreted (Crosby, 1967) to indicate that the limb field at stage 14 extends beyond the presumptive limb area.]
CELL SORTING BASED ON MORPHOGENETIC PROPERTIES Additional experiments, performed to learn whether tissues with histogenetic equivalence may participate with limb mesoderm in the formation of chimeric structures, have been most revealing. Somites and limb mesoderm have been dissociated into cell suspensions and the cells from both rudiments have been thoroughly randomized. One or the other of the two mesoderms was labeled with isotopic thymidine so that the origin of individual cells could be recognized in auto- radiographs. Somites were taken from stage 13-15 embryos and limb mesoderm (in all cases leg buds) from stages 18-19. Following ran
domization the cells were centrifuged until they formed a pellet and were then placed into intact unlabeled ectodermal pouches from limb
194
MORPHOGENETIC PHASES 195 buds. The "assemblies" were grafted to unlabeled host embryos, either on the dorsal surface of a wing bud or over some of the somites. Rep
resentative grafts were sacrificed during the first, second, and subse
quent days and subjected to autoradiography for analysis (Zwilling, unpublished).
The results clearly indicated that, despite their histogenetic equiv
alence, the somite and limb cells did not cooperate in the formation of chimeric structures. Instead the cells sorted so that somite cells came together and limb cells associated with other limb cells. Such sorting was quite clear within 18-20 hours after the cells had been randomized.
Somite cells usually moved (or were forced) to a central position while limb cells were arranged peripherally (Figs. 5-9). Limb bud ectoderm (with a ridge) was not required for the sorting, since the same kind of nonrandom association of cells of like origin occurred when pellets were wrapped in flank ectoderm.
When grafts of mixed pellet in limb ectoderm were allowed to grow for a sufficient time, outgrowths from the limb tissue in association with the ectodermal ridge eventually formed digits. The somite tissue remained at the base of the grafts, and in some cases formed small distorted (atypical vertebral?) cartilages which did not articulate with the long bones of the limb outgrowth.
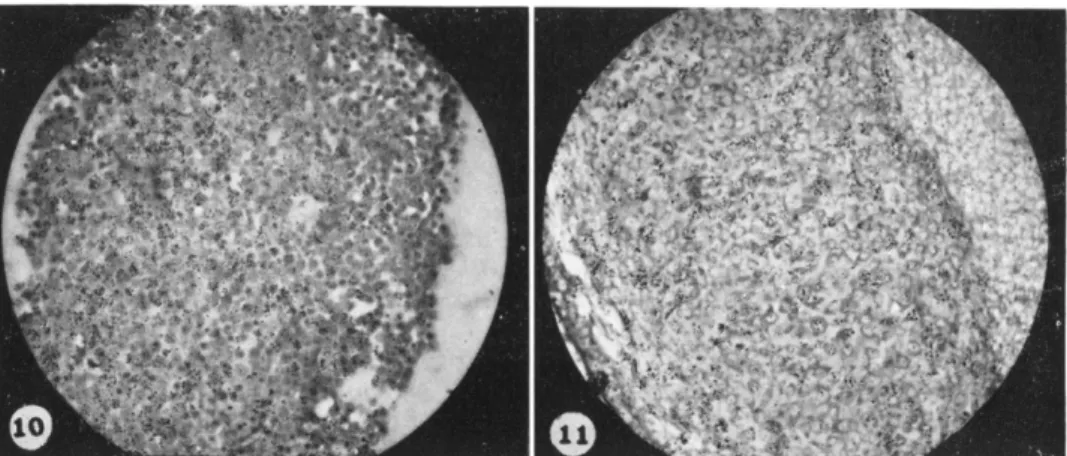
Sclerotome (presumptive cartilage) was separated from somites of FIG. 5. Photograph of autoradiograph of mixture of thymidine-3H labeled limb cells which have been randomized among unlabeled somite cells. Limb cells from stage 19, somite cells from stage 13-15. Pellet was fixed 3.5 hours after centrifugation.
FIG. 6. Photograph of graft of sample of pellet shown in Fig. 5. Graft was grown for 18 hours on the dorsal surface of an unlabeled host limb bud. Labeled limb cells have sorted to the periphery. Many are in contact with the graft ectodermal ridge. Central unlabeled cells are the somite cells.
FIG. 7. Photograph of labeled limb cells randomized with unlabeled somite cells. Limb cells are from stage 19, somite cells from anterior somites of stage 14-15 embryos. Pellet was fixed 4 hours after centrifugation.
FIG. 8. Photograph of graft of sample of pellet shown in Fig. 7. Again all the labeled limb cells have sorted after 20 hours on an unlabeled host wing bud:
labeled cells to left bottom; unlabeled somite cells to right.
FIG. 9. Illustrates sorting of segmental plate cells from limb mesoderm. In this case the presomite cells of the segmental plate of stage 13-14 embryos were labeled and randomized with stage 19 limb mesoderm. After 22 hours as a graft on an unlabeled host wing bud, it can be seen that the somite cells have sorted to a central position and are surrounded by unlabeled limb cells of the graft.
196
the mid-trunk region of thymidine-3H labeled stage 18-19 embryos.
This and the combined presumptive muscle-connective tissue part of the somites were separately randomized with leg bud mesoderm from unlabeled stage 18-19 embryos, pelleted, placed in ectodermal jackets and grafted in the manner described. Sorting occurred in both cases;
that is the labeled chondrogenic cells from the sclerotome of the somites came together as did the myotome-dermatome cells.
Evidence for sorting of limb mesoderm cells from flank mesoderm cells, also histogenetically equivalent, has been described by Crosby (1967). Sorting between flank and limb cells from stages 14, 15, and 18 in various combinations was not as dramatic as in the case of somite and limb mixtures, but was definite. The labeled component tended to form islands of cells which were surrounded by the unlabeled com
ponent. This difference is not surprising since the cells of flank and limb are more similar than those of somite and limb (see below).
Crosby also has evidence that leg and wing mesoderm cells tend to sort when randomized in a common mixture. In such mixtures the direction of sorting depended on the relative proportion of the two components. The numerically minor component, regardless of limb type, moved to the basal position in the graft. This observation in the autoradiographs was confirmed by the finding that digits which de
veloped were always of the type expected of the majority component.
That is, in a mixture of 25% labeled wing mesoderm cells with 75% un
labeled leg cells the labeled cells were largely in the basal portion of early grafts and the digits which developed from older grafts were typical foot digits. Distal wing parts formed when the ratio in the mix
ture was reversed.
Cell sorting is a phenomenon which has been encountered, in the past, when histogenetically dissimilar cell types have been mixed in random association (Moscona, 1957, 1960; Steinberg, 1963, 1964).
Histogenetically similar cells from different species did not segregate on the basis of species of origin; randomized chondrogenic cells from chick and mouse limb buds remained randomized to form a chimeric cartilage mass (Moscona, 1957). Yet here we have a situation in which histogenetically equivalent cells segregate on the basis of their rudi
ment of origin despite their ability to differentiate into the same cell types. The surface distinctions responsible for the segregation of these cells, be they in the plasma membranes or surface-associated materials, are related to the morphogenetic properties which prevail at the time
MORPHOGENETIC PHASES 197 that the mixtures were made, rather than to the eventual cytodifferen- tiation of the cells. Thus chondrogenic cells from one origin segregate from chondrogenic cells of another origin. The same is true for the myogenic cells. Aside from the segregation of cells from different regions of early embryos described by Holtfreter and his associates
(Holtfreter, 1944; Townes and Holtfreter, 1955), the only other re
corded example of sorting based on morphogenetic properties of tis
sues from relatively advanced stages is that described by Nöthiger ( 1964 ). In randomized mixtures of cells from two kinds of Drosophila imaginai discs the cells which originated from each of the discs sep
arated into discrete masses which then developed in the manner ex
pected of the original disc. Genetic markers were used to identify the individual cells.
After it became clear that the segregation of his to genetically equiv
alent cells from different embryonic sources was a consistent phenom
enon we undertook to assess whether fully differentiated cells retained any of the earlier distinctive properties. Chondrocytes were digested from the matrices of limb cartilages and vertebral cartilages of 8-day embryos and combined in random mixtures. One or another of the constituent cells was labeled with thymidine-3H. The mixed cells were treated as before; that is they were pelleted by centrifugation and the pellets were stuffed in limb bud ectoderm and grafted to unlabeled host embryos. Under these circumstances there was no indication of segregation of chondrocytes from the two sources. Vertebral and limb chondrocytes remained randomized and differentiated into a chimeric mass of cartilage (Figs. 10 and 11). It is possible that rapid synthesis of matrix may have interfered with sorting. However, the results as they now stand indicate that the early surface distinctions which are properties of the morphogenetic phase of the limb and vertebral cells are no longer present once active cytodifferentiation has been under way. Precisely when the surface distinctions become altered still re
mains to be established.
COMPARISON OF LIMB AND SOMITE MESODERMS
Segregation of limb from somite cells has a number of interesting aspects aside from those already discussed. There is evidence that cells in young somites of Ambystoma may all be capable of forming car
tilage (Holtzer and Detwiler, 1953). The same may be true for chick embryo somites, but there is no evidence to this effect. However, even
198 EDGAR ZWILLING
FIG. 10. Autoradiograph of pellet in which unlabeled chondrocytes from limb cartilages were mixed with labeled chondrocytes from vertebral cartilages. Em
bryos were 8 days old when cells were removed from cartilage matrices. Pellet was fixed 4.5 hours after centrifugation.
FIG. 11. Sample of pellet in Fig. 10 after it had grown for 48 hours as a graft to the dorsal surface of an unlabeled host wing bud. Note that the labeled cells are still randomly distributed among the unlabeled ones. Metachromatic matrix has been secreted between the cells by this time. The cartilage is a chimera of limb and vertebral chondrocytes.
somites of stage 11 chick embryos have a sulfated mucopolysaccharide which has the same electrophoretic mobility as chondroitin sulfate A ( Franco-Browder et al., 1963). Lash (1968) has confirmed this obser
vation and has reported that somites at early stages stain meta- chromatically with stains which give this reaction with matrix and have enzymes associated with chondroitin sulfate synthesis. However, it is the early morphology and sequence of events of differentiation of the two structures that bear comparison. Limb mesoderm, as indicated above, consists of rounded cells, neither mesenchymal nor epithelial.
Somites are epithelial during their early stages, and then the scleroto- mal region becomes mesenchymal (Trelstad et al., 1967). Thus the distinct segregation of limb and somite is not very surprising since they represent different morphological cell types at the time that they were intermixed despite the eventual similarities of the differentiated cells. For this reason, sorting between limb and flank or leg and wing cells, in both cases morphologically quite similar, represents the more striking evidence for surface distinctions even though the sorting was not as complete as that between somite and limb cells.
MORPHOGENETIC PHASES 199 Another point worthy of mention is that the relative rates and se
quence of differentiation of different cell types is different in somite and limb. Cartilage condensation and active matrix production appear before muscle differentiation is evident in the limb. In somites muscle begins to acquire striated myofilaments very early, before chondro- genesis is under way. Myotomes of anterior somites of stage 13 em
bryos show definite indications of myogenesis, and by stage 18 more than 20 of the 30 pairs of somites contain cells with distinct myo
filaments ( Holtzer, 1961 ). Histologically detectable vertebral cartilage is not seen until one or two days later. Somites, with their distinctive early morphology, the particular relation which exists between them and nerve cord with regard to induction and spacing of somite-derived cartilage (Grobstein and Holtzer, 1955), and their particular sequence of cytodifferentiation, obviously have distinct morphogenetic proper
ties of their own. The similarity to limb development resides only in two features; there are indications of precocious syntheses of substances which appear as major products of the fully differentiated cells, and the tissue types which form from somites are the same as those which develop from limb mesoderm.
TEMPORAL RELATIONS
An important aspect of attempts to relate morphogenetic events to those of cytodifferentiation concerns the time of termination of one set of properties and that of initiation of another. We have already seen that low-level synthesis of a typical product associated with the differentiation of cartilage is present in the earliest limb tissue. When can the morphogenetic properties be detected, and how long do they persist?
Rudnick ( 1945 ) has shown that distinctive limb structures may de
velop when presumptive limb regions (in association with adjacent tissues) of very young embryos (stage 6 for wings, stage 8-9 for legs) are isolated as expiants to the body cavity of host embryos. Chaube ( 1959 ) has mapped the position of the presumptive limb areas in early embryos and has confirmed the observation that such areas can differ
entiate into limb structures in foreign sites and has demonstrated that axial relations are established by stage 11. Crosby's experiments (1967) have revealed that limb properties are sufficiently stable in the meso
derm so that well developed and recognizable limb structures appear after completely dissociated and randomized cells of that tissue are placed in stage 19 ectoderm and grown as grafts. Leg properties are
200 EDGAR ZWILLING
stable enough to withstand this treatment by stage 14 and wing pro
perties by stage 16. It is thus evident that at least the major morpho- genetic properties of the limb mesoderm are established prior to the appearance of definite limb buds.
Although the precise stage when general limb properties are no longer present has not been rigorously clarified, there is evidence that they persist until relatively late stages. Presumptive thigh tissue from limbs in stage 24 (Saunders et al, 1957) and possibly from limbs in stage 26 (Amprino and Bonetti, 1964) is still capable of responding to an ectodermal ridge from a younger limb to form digits. Such tissue from embryos older than stage 26 will not respond to or maintain a ridge. Since the limb forms in a proximodistal sequence (Saunders, 1948) the presumptive thigh tissue is morphologically the oldest in the limb. Differential sulfate fixation ( Fig. 1 ) by the central chondro- genic tissue has already occurred in these regions of the limb by stage 24 ( Searls, 1965a ). Additional data are required to clarify when central as well as peripheral tissues, and distal as well as proximal tissues lose morphogenetic capabilities. Nevertheless, available information sup
ports the general statement that the properties of limb mesoderm that are related to its morphogenetic activities decline at about stage 24-26.
Since metachromatic staining of matrix in the proximal chondrogenic tissues of limbs can be detected by stage 25 (Searls, 1965a), it appears that the decline in morphogenetic properties may coincide with the onset of active cytodifferentiation.
RELATION BETWEEN MORPHOGENESIS AND HISTOGENESIS Higher organisms are not haphazard collections of cells and tissues.
The organism has a definite shape, and for it to function normally the constituent organs and structures must have definite form and relation to each other. A major problem for the developmental biologist is to decipher the manner in which form is established. It is evident to stu
dents of development that study of the elaboration of form has been a major preoccupation of people in the field from its earliest days. It is equally evident that current activities have emphasized events which result in the synthesis of characteristic products of adult cell types.
Many people have sought the molecular order which must be at the base of the organization visible in the fully formed structure. It may be well to recall R. G. Harrison's attempts to assess whether a par
ticular molecular orientation could be involved in the development of
MORPHOGENETIC PHASES 201 typical polarity properties in the ear and other embryonic structures by means of X-ray diffraction techniques (Harrison et al., 1940). Such an excursion was premature, but most of us share the conviction that a complete understanding of the ontogeny of functional form will eventually relate the molecular, cellular, or supracellular phenomena responsible for elaboration of form to the synthetic activities of the fully differentiated cells. This is a large problem, and possibly it is again premature to pose it at this time.
Any discussion - of biological form must recognize that there are many levels of involvement. Macromolecular constituents of cells have particular form, cells may have distinctive shapes and structure, and organs and structures have distinct morphologies. To what extent is the elaboration of form at these various levels an obligate reflection of the molecular structure of the typical products of cy to differentiation?
To what extent may the form at one or another of the levels be inde
pendent of eventual cellular differentiation? There is probably a com
plete spectrum of interrelations of form and typical cell product. There are cases in which the shape of a cell may be radically altered as a consequence of a single amino acid substitution in its major protein.
The typical plump oval shape of the erythrocyte becomes converted to the crescent-shaped sickle cell when such a substitution occurs in the primary sequence of the hemoglobin molecule (Ingram, 1957).
There is beautiful evidence that the shape of some algal cells depends on whether the cellulose macromolecules of the wall are highly ordered or exist in randon array ( Green, 1963 ). When the cellulose is ordered turgor pressure forces the cell to the form of an elongated cylinder;
when orientation of the molecules is random the cells are spherical.
The material presented in this paper deals with a structure whose basic form at the supracellular level appears to be established rela
tively independently of the differentiation of its constituent cells. In fact the differentiation of the cells appears to be related to the pre
ceding morphogenesis somewhat like the development of an exposed photographic plate. The cytodifferentiation brings out the latent re
lations established by the earlier events.
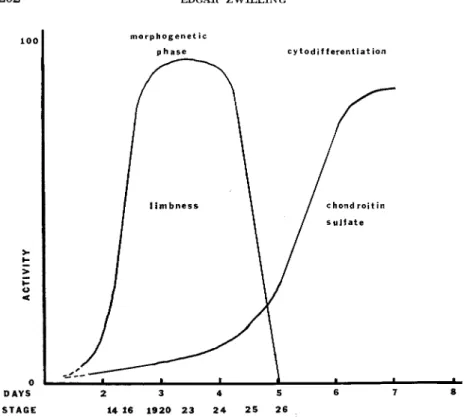
A summary of the events of limb development may be represented by Fig. 12, a diagram based on a number of facts and approximations, in which early events are related to the eventual differentiation of cartilage cells. At first there is a morphogenetic phase during which the limb tissues have a definite set of properties responsible for the
202 EDGAR ZWILLING
<
1 ---■- -- J L -
morphogenet ic p hase
f l i m b n e s s
1 ^ ^ _
cytodif ferentiation
/ chondroitin / s u l f a t e
* ■ ' '
DAYS 2 3 4 5 6 7 8 STAGE 14 16 1920 23 2 4 25 26
FIG. 12. Diagram which represents relative activities of both morphogenetic phase and synthesis of chondroitin sulfate against a time axis. Based on data presented and discussed in the text.
interaction between limb components and elaboration of major limb form. These properties are distinctive and are not shared by other tis
sues, even though these tissues are capable of elaborating the same cell types as those found in a limb. This morphogenetic phase is not only distinct, but easily identified and must involve its own set of genetic factors. There are mutants which affect the morphogenetic properties without apparent effect on the capabilities of the tissue to form muscle and cartilage. The "wingless" mutations, in which the ability of the mesoderm to support a ridge is deficient, but in which cartilage and muscle may differentiate, are the most striking of these (Waters and Bywaters, 1948; Zwilling, 1949, 1956b). The morphoge
netic phase is reflected in a set of cell surface properties that bring cells of like morphogenetic type together after they are intermixed with cells
MORPHOGENETIC PHASES 203 of equivalent histogenetic capability, but from another embryonic source. Furthermore the morphogenetic phase is transitory. It termi
nates at about the time that cells enter into active cytodifferentiation.
During this phase limb cells cannot be characterized as chondroblasts or myoblasts. They are limb cells and have" a set of properties which may be summarized as limbness. The particular pathway of cyto
differentiation of the constituent cells is probably imposed on the tis
sues as a consequence of the position they come to occupy because of the prior morphogenetic events despite the low level synthesis of chondroitin sulfate by all the limb mesoderm throughout the mor
phogenetic phase. Cytodifferentiation of cartilage is not set off by the initial activation of the genome related to chondromucoprotein syn
thesis. That event has occurred early in all the cells. The regulatory mechanism which is responsible for the marked augmentation of the synthesis of the cartilage substances should be sought as the key to active differentiation of cartilage cells.
I have cited two extremes of the spectrum of relations between form and molecular structure: one in which the primary amino acid se
quence of a protein affects the shape of the cell which produces the protein; the other in which supracellular form and histogenetic events in a structure are quite independent of each other. It is possible that within a structure in which form elaboration and histogenetic events are independent at the supracellular level the morphology of in
dividual cells depends on the configuration of their characteristic proteins. There is reason to believe that important aspects of the form of muscle cells reflect the structure and arrangement of myosin and actin. Thus there are many levels of involvement and many questions to be answered. Do the precocious products of pancreas cell differ
entiation influence the development of the form of the pancreas? Does an early morphogenetic phase, in which branching of the early rudi
ment is the chief activity, have a similar relation to these syntheses as the early limb phase to chondroitin sulfate synthesis? Many structures which differ in form but have the same characteristic protein are de
rived from skin. Claws, hair, feathers, scales, for example, all have ker
atin as a major constituent. Are unknown minor components responsible for the different forms of the structures, or are the differences due to subtle variations in the structure of the keratin molecules? Or is the form independent of the keratin and the consequence of a set of early
204
morphogenetic properties? Do the migrating neural crest cells rep
resent a morphogenetic phase characterized by precocious low level syntheses of some or all of the many substances which they may elab
orate? The task of answering such questions is formidable—but this may be an appropriate time to reformulate them.
ACKNOWLEDGMENTS
The original research reported in this paper was supported by grant HD-03465 from the National Institute for Child Health and Human Development of the National Institutes of Health. Former associates whose research is mentioned were supported by training grant Tl-HD-0022 from the same Institute.
REFERENCES
AMPRINO, R. (1965). Aspects of limb morphogenesis in the chicken. In
"Organogenesis" (R. L. DeHaan and H. Ursprung, eds.), p p . 255-281. Holt, New York.
AMPRINO, R., and BONETTI, D . A. (1964). Effect of the implantation site on the development of grafted limb bud mesoderm in chick embryos. Nature 204, 298.
AMPRINO, R., and CAMOSSO, M. (1958). Analisi sperimentale dello sviluppo dell'ala nell'embrione di polio. Arch. Entwicklungmech. Organ. 150, 509-541.
AMPRINO, R., and CAMOSSO, M. E. (1965). Developmental fate of heterotopically grafted proximal pre-axial material of the chick embryo limb bud. Ada. Anat.
61, 259-288.
CAIRNS, J. M., and SAUNDERS, J. W., JR. (1954). The influence of embryonic mesoderm on the regional specification of epidermal derivatives in the chick.
/. Exptl Zool. 127, 221-248.
CHAUBE, S. (1959). On axiation and symmetry in transplanted wing of the chick.
/. Exptl. Zool. 140, 29-78.
CROSBY, G. M. ( 1967). Developmental capabilities of the lateral somatic mesoderm of early chick embryos. Ph.D Thesis, Brandeis Univ., Waltham, Massachusetts.
DIETERLEN-LIEVRE, F . (1965). Étude morphologique et expérimentale de la dif
férenciation du pancréas chez l'embryon de poulet. Bull. Biol. France Belg. 99, 3-116.
DORFMAN, A. (1962). Biosynthesis and metabolism of acid mucopolysaccharides of connective tissue. Federation Proc. 2 1 , 1070-1074.
DORFMAN, A. (1963). Polysaccharides of connective tissue. J. Histochem. Cyto- chem. 11, 2 - 1 3 .
FELL, H. B. (1939). T h e origin and developmental mechanics of the avian ster
num. Phil. Trans. Roy. Soc. London B229, 407-463.
FELL, H. B., and CANTI, R. G. (1934). Experiments on the development of the avian knee-joint. Proc. Roy. Soc. B116, 316-351.
FRANCO-BROWDER, S., D E R Y D T , J., and D O R F M A N , A. ( 1 9 6 3 ) . T h e identification
of a sulfated mucopolysaccharide in chick embryos, stages 11-13. Proc. Natl.
Acad. Sei. U. S. 49, 643-647.
MORPHOGENETIC PHASES 2 0 5 GoETiNCK, P. F. (1964). Studies on limb morphogenesis. II. Experiments with
the polydactylous mutant Eudiplopodia. Develop. Biol. 10, 71-91.
GOETINCK, P. F . (1966). Genetic aspects of skin and limb development. Current Topics Develop. Biol 1, 253-283.
GREEN, P. B. (1963). On mechanisms of elongation. In "CytodiiFerentiation and Macromolecular Synthesis/' Troc. Symp. Soc. Develop. Biol. 2 1 , pp. 203-234.
Academic Press, New York.
GROBSTEIN, C., and HOLTZER, H. (1955). In vitro studies of cartilage induction in mouse somite mesoderm. /. Exptl. Zool. 128, 333-358.
HAMBURGER, V. (1938). Morphogenetic and axial self-differentiation of trans
planted limb primoïdia of 2-day chick embryos. /. Exptl. Zool. 77, 379-399.
HAMBURGER, V. (1939). The development and innervation of transplanted limb primordia of chick embryos. /. Exptl. Zool. 80, 347-389.
HAMBURGER, V., and HAMILTON, H. L. ( 1951 ). A series of normal stages in the development of chick embryo. / . Morphol. 88, 49-92.
H A M P E , A. (1957). Recherches sur la régulation des déficiences et des excédents du bourgeon de la patte du Poulet. Arch. Anat. Microscop. Morphol. Exptl. 46, 268-282.
HARRISON, R. G., ASTBURY, W. T., and RUDALL, K. M. ( 1940 ). An attempt at an
X-ray analysis of embryonic processes. /. Exptl. Zool. 85, 339-363.
HOLTFRETER, J. ( 1944 ). Experimental studies on the development of the proneph- ros. Rev. Can. Biol. 3, 220-249.
HOLTZER, H. (1961). Aspects of chondrogenesis and myogenesis. In "Synthesis of Molecular and Cellular Structure," Proc. Symp. Soc. Develop. Biol. 19, pp. 3 5 - 87. Ronald Press, New York.
HOLTZER, H., and DETWILER, S. R. (1953). An experimental analysis of the de
velopment of the spinal column. III. Induction of skeletogenous cells. /. Exptl.
Zool. 123, 335-370.
INGRAM, V. M. (1957). Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature 180, 326-328.
JURAND, A. (1965). Ultrastructural aspects of early development of the fore-limb buds in the chick and mouse. Proc. Roy. Soc. B162, 387-405.
KIENY, M. (1960). Rôle inducteur du mésoderme dans la différenciation précoce du burgeon de membre chez l'embryon de Poulet. J. Embryol. Exptl. Morphol.
8, 457-467.
LASH, J. W. (1968). Somitic mesenchyme and its response to cartilage induction.
In "Epithelial-Mesenchymal Interaction" (R. Fleischmajer and R. Billingham, eds.), pp. 165-172. William & Wilkins, Baltimore, Maryland.
MEDOFF, J. (1967). Enzymatic events during cartilage differentiation in the chick embryonic limb bud. Develop. Biol. 16, 118-143.
MILAIRE, J. ( 1962a ). Détection histochimique de modifications des ébauches dans les membres en formation chez la souris oligosyndactyle. Acad. Roy. Belg., Bull.
Classe Sei. [5] 48, 505-528.
MILAIRE, J. (1962b). Histochemical aspects of limb morphogenesis in vertebrates.
Advan. Morphogenesis 2, 183-209.
MILAIRE, J. (1966). Étude histochimique des premiers stades du développement des membres chez le Poulet, Çompt. Rend. Assoc. Anat 51, 688-698.
206
MOSCONA, A. (1957). T h e development in vitro of chimeric aggregates of dis
sociated embryonic chick and mouse cells. Proc. Natl. Acad. Sei. U. S. 43, 184- 194.
MOSCONA, A. A. (1960). Patterns and mechanisms of tissue reconstruction from dissociated cells. In "Developing cell systems and their control," Proc. Soc.
Develop. Biol. 18, pp. 45-70. Ronald Press, New York.
MOSCONA, A. (1961). Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interaction in vitro. Exptl. Cell Res. 22, 455-475.
NÖTHIGER, R. ( 1964 ) Differenzierungsleistungen in Kombinaten, hergestellt aus Imaginalscheiben verschiedener Arten, Geschlechter und Körpersegmente von Drosophila. Arch. Entwicklungsmech. Organ. 155, 269-301.
ROTH, R., ASHWORTH, J. M., and SUSSMAN, M. (1968). Periods of genetic tran
scription required for the synthesis of three enzymes during cellular slime mold development. Proc. Natl. Acad. Sei. U. S. 59, 1235-1242.
RUDNICK, D . (1945). Limb-forming potencies of the chick blastoderm: Including notes on associated trunk structures. Trans. Conn. Acad. Arts Sei. 36, 353-377.
RUTTER, W . J., BALL, W . D., BRADSHAW, W . S., CLARK, W . R., and SAUNDERS,
T. G. (1967). Levels of regulation in cytodifferentiation. Exptl. Biol. Med. 1, 110-124.
RUTTER, W . J., CLARK, W . R., K E M P , J. D., BRADSHAW, W . S., SAUNDERS, T. G.,
and BALL, W . D . (1968). Multiphasic regulation in cytodifferentiation. In
"Epithelial-Mesenchymal Interaction" (R. Fleischmajer and R. Billingham, eds. ), p p . 114-131. Williams & Wilkins, Baltimore, Maryland.
SAUNDERS, J. W., JR. (1948). T h e proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J. Exptl. Zool. 108, 363-404.
SAUNDERS, J. W., JR., and GASSELING, M. T. ( 1 9 6 8 ) . Ectodermal-mesenchymal interactions in the origin of limb symmetry. In "Epithelial-Mesenchymal Inter
action" (R. Fleischmajer and R. Billingham, eds.), p p . 78-97. Williams &
Wilkins, Baltimore, Maryland.
SAUNDERS, J. W., JR., and GASSELING, M. T. (1963). Trans-filter propagation of apical ectoderm maintenance factor in the chick embryo wing bud. Develop.
Biol. 7, 64-78.
SAUNDERS, J. W., J R . , CAIRNS, J. M., and GASSELING, M. T. ( 1 9 5 7 ) . T h e role of
the apical ridge of ectoderm in the differentiation of the morphological structure and inductive specificity of limb parts in the chick. / . Morphol. 101, 57-87.
SAUNDERS, J. W., J R . , GASSELING, M. T., and GFELLER, M. D . ( 1 9 5 8 ) . Interactions
of ectoderm and mesoderm in the origin of axial relationships in the wing of the fowl. J. Exptl. Zool. 137, 39-74.
SEARLS, R. L. (1965a). An autoradiographic study of the uptake of S35-sulfate during the differentiation of limb b u d cartilage. Develop. Biol. 11, 155-168.
SEARLS, R. L., (1965b). Isolation of mucopolysaccharide from the precartilaginous embryonic chick limb bud. Proc. Soc. Exptl. Biol. Med. 118, 1172-1176.
SEARLS, R. L. (1967). T h e role of cell migration in the development of the embryonic chick limb bud. J. Exptl. Zool. 166, 39-50.
SEARLS, R. L., and ZWILLING, E. (1964). Regeneration of the apical ectodermal ridge of the chick limb bud. Develop. Biol. 9? 38-55..
MORPHOGENETIC PHASES 207 STEINBERG, M. S. (1963). Reconstruction of tissues by dissociated cells. Science
141, 401-408.
STEINBERG, M. S. (1964). The problem of adhesive selectivity in cellular inter
action. In "Cellular Membranes in Development," Troc. Symp. Soc. Develop.
Biol. 22, pp. 321-366. Academic Press, New York.
SUSSMAN, M. (1966). Some genetic and biochemical aspects of the regulatory program for slime mold development. Current Topics Develop. Biol. 1, 61-83.
TOWNES, P. L., and HOLTFRETER, J. (1955). Directed movements and selective adhesion of embryonic amphibian cells. /. Exptl. Zool. 128, 53-120.
TRELSTAD, R. L., HAY, E. D., and REVEL, J. P. (1967). Cell contacts during early morphogenesis in the chick embryo. Develop. Biol. 16, 78-106.
WATERS, N. F., and BYWATERS, J. H. (1948). A lethal embryonic wing mutation in the domestic fowl. /. Hered. 34, 213-217.
WESSELLS, N. K., and EVANS, J. (1968). Ultrastructural studies of early mor
phogenesis and cytodifferentiation in the embryonic mammalian pancreas.
Develop. Biol. 17, 413-446.
ZWILLING, E. (1949). The role of epithelial components in the developmental origin of the "wingless" syndrome of chick embryos. /. Exptl. Zool. I l l , 1 7 5 - 187.
ZWILLING, E. (1955). Ectoderm-mesoderm relationship in the development of the chick embryo limb bud. /. Expl. Zool. 128, 423-441.
ZWILLING, E. (1956a). Interaction between limb bud ectoderm and mesoderm in the chick embryo. II. Experimental limb duplication. /. Exptl. Zool. 132, 173-187.
ZWILLING, E. (1956b). Interaction between limb bud ectoderm and mesoderm in the chick embryo. IV. Experiments with a wingless mutant. /. Exptl. Zool.
132, 241-253.
ZWILLING, E. (1961). Limb morphogenesis. Advan. Morphogenesis 1, 301-330.
ZWILLING, E. (1964). Development of fragmented and of dissociated limb bud mesoderm. Develop. Biol. 9, 20-37.
ZWILLING, E. (1966). Cartilage formation from so-called myogenic tissues of chick enbryo limb buds. Ann. Med. Exptl. Biol. Fenniae (Helsinki) 44, 134-139.