Morphological Differences among Pseudopodia of Various Small Amebae and Their
Functional Significance
1EUGENE C. BOVEE
Department of Zoology, University of California, Los Angeles, California
The many kinds of smaller amebae have pseudopodial morphologies and ameboid movements which are as varied as the amebae themselves.
I am much intrigued by these variations and also by the probable fact that these are facets of a basic plan of protoplasmic movement.
Most theories of ameboid movement are based on the very large amebae—Chaos Carolin en sis, Pelomyxa palustris, and Amoeba proteus and its relatives Amoeba discoïdes and Amoeba dubia (Allen, 1961a, b;
Bingley and Thompson, 1962; Buchsbaum et al., 1944; Dellinger, 1906;
Goldacre, 1961a; Hyman, 1917; Kavanau, 1963; Mast, 1926; Rhumbler, 1898; Rinaldi and Jahn, 1962, 1963; Schaeffer, 1920; Schulze, 1875; Wal- lich, 1863). Their movements are somewhat alike and readily observ- able, and these amebae have long been cultivated in the laboratory.
Other studies have been made of movement in smaller amebae (Abé, 1961, 1962; Bovee, 1956, 1960a, b; Pantin, 1923; Penard, 1905; Ray and Hayes, 1954; Schaeffer, 1920, 1926a, 1931), but these are scarcely noted in the battles about the movements of the giant species.
Among smaller amebae, however, great variety of speed, degree, and finesse of protoplasmic flow and of functional form may be seen. Com- pared to them the movements of the giant species seem stodgy.
I shall discuss some smaller amebae, particularly from Schaeffer's (1926a) family Mayorellidae. There are some interesting locomotive and pseudopodial formations shown by his genera Mayorella, Vexillifera, and Flabellula, and by the genera I have added to the family: Subula- moeba (Bovee, 1953a), Oscillosignum (Bovee, 1953b), Flagellip odium (Bovee, 1951, 1953c), and Vannella (Bovee, 1963b).
Subulamoeba saphirina (Penard, 1902) forms the clear conical pseu- dopods of the Mayorellidae (Fig. 1C and E). These serve as piers over and between which the body advances at moderate speed. In rapid
ι These studies have been supported by Research Grants USPHS # 6 4 6 2 and NIH-AI-01158-06.
189
advance, however, it throws forward a tubular extension which has a clear conical tip (Fig. IF and G). This tube becomes the main channel of flow and drives the conical tip forward.
Another ameba, Oscillosignum proboscidium, also uses such a pseu- dopod (Fig. 2), but it bends it and anchors the tip to the substrate (Fig.
2A and C). It then expands that pseudopod and flows into it, riding
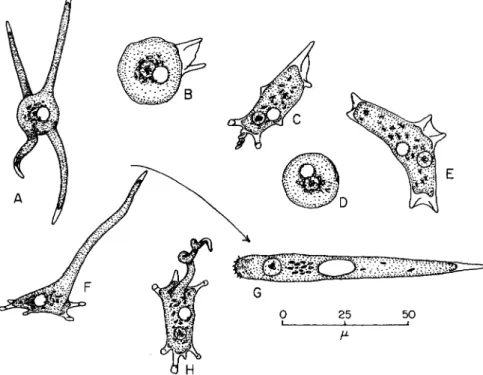
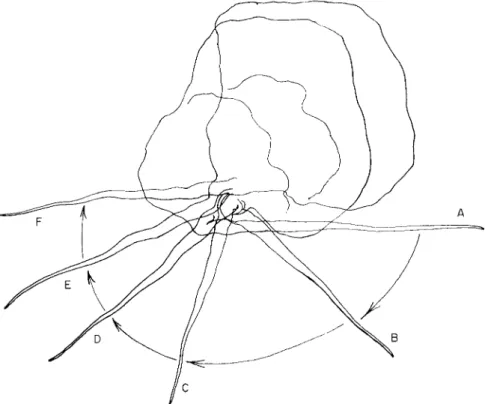
FIG. 1. Subulamoeba saphirina. (A) Radiate and afloat; (B) beginning locomotion;
(C) in moderate progress, showing conical, mayorellid, determinate pseudopods; (D) at rest, spherical; (E) changing direction, with a new pair of pseudopods formed near the nucleus indicative of the new route; ( F ) with a long tubular pseudopod thrown forward, to become (G) the rapidly locomotive organism; (H) dorsal view of the organism, with the elongate pseudopod. (After Bovee, 1953a.)
forward over the anchored tip while extending a new pseudopod (Fig.
2B). It also forms shorter pseudopods rapidly, appearing to walk on them (Fig. 2B). T h e tubular pseudopod is of larger diameter at the rear than at the front end in both Subulamoeba and Oscillosignum.
Sometimes a mayorellid ameba extends one or more pseudopods ahead of the body and into the water above it (Fig. 3A-E). If the nu- cleus moves up into the pseudopod, the ameba erects itself, supported on
Morphological Differences among Pseudopodia 191 its rear (Fig. 3C-E). It stays erect for some time, and an Oscillosignum may extend and wave pseudopods (Fig. 3D). The erect ameba does not resume locomotion until the nucleus descends to the base of the body, from where a new pseudopod then extends along a new route of loco- motion, followed by the nucleus and the body (Fig. 3B). T h e site of the nucleus thus seems related to the site of a new pseudopod. (See p. 194.) If a mayorellid ameba stops, a new pair of pseudopods often forms on one side or the other of the body near the nucleus (Fig. IE). New advance is then in the direction of the new pseudopods.
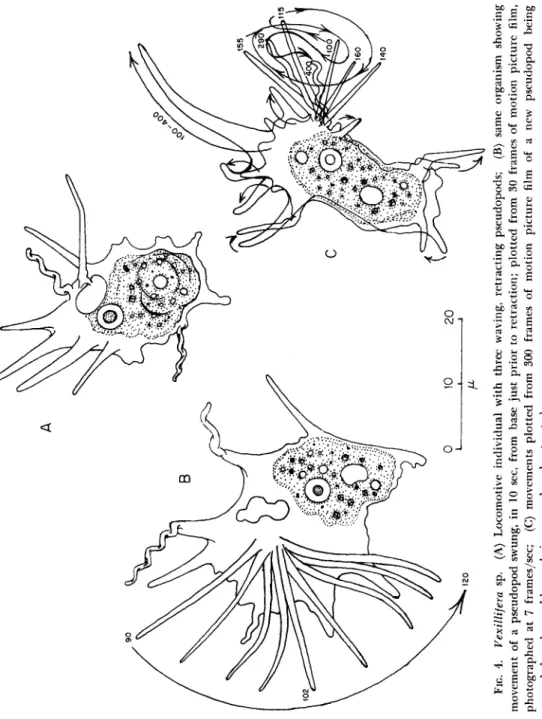
The conical waving pseudopods of Vexillifera sp. (Bovee, 1951, 1956) are of clear protoplasm (Fig. 4). These wave slowly just prior to re- traction (Fig. 4B and C). When speeded up by cinematographic pro- jection, these appear impressively active. (See p. 195.)
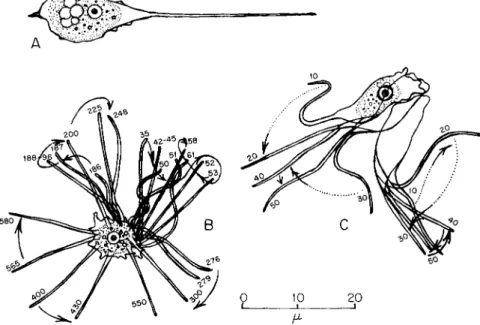
Amebae of the genus Flagellipodium flip out, in 0.5 sec or less, a flagellum-like pseudopod which is vibratile, but does not beat in sine wave patterns seen in typical flagella (Jahn and Harmon, 1963; Jahn et ai, 1962). The base of this "flagellipodium" may move along the sur- face of the organism more or less continuously while it is motile (Fig. 5B).
It may be yanked in as fast as it is cast out; it, or one like it, may be flipped out again from any point of the body surface (Fig. 5B and C).
The flagellipod may shoot out from the ventral surface of the organism as well as from the dorsal surface, or from the margin. In rapid advance the flagellipod is held rigidly ahead from a clear conical base, like a bowsprit (Fig. 5A). When the ameba halts, the pseudopod may again become motile; may be jerked in again; and may be flipped out again promptly or later. (See p. 196.)
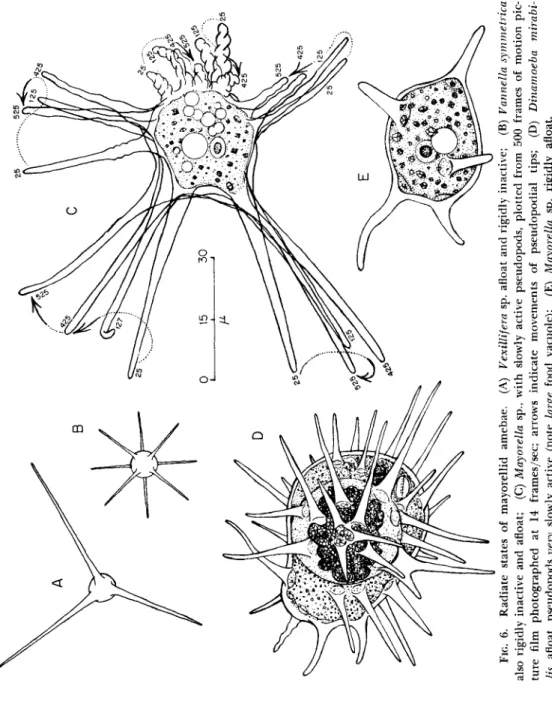
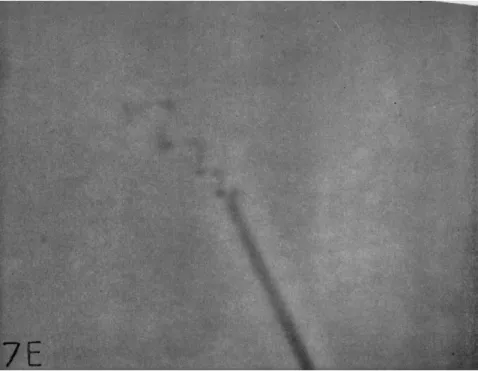
Mayorellid amebae also form radiate floating stages with more or less, long, clear, conical pseudopods (Figs. 6 and 7). These are rigid in some species (Fig. 6A, B, D, and E) but may bend and oscillate in others (Figs. 6C and 7). Sometimes the tips of such pseudopods may be coiled;
and this coil moves, seemingly independently of the rest of the pseu- dopod (Fig. 7A-D). These pelagic stages are the "radiosa" amebae of the literature. (See pp. 197-99.)
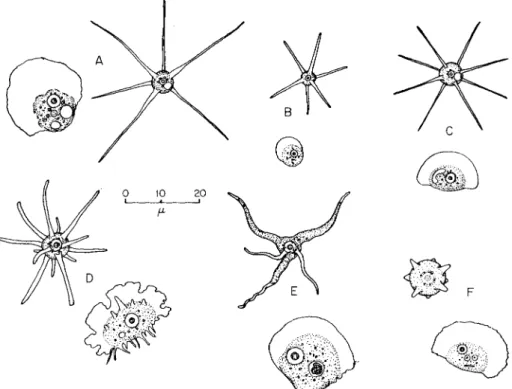
Amebae of the genera Flabellula and Vannella form clear conical pseudopods in the radiate state; and Schaeffer (1926a) placed them in the family Mayorellidae. They are fan-shaped in locomotion with a clear anterior border (Fig. 8). The pattern of protoplasmic flow is active, but difficult to follow in that clear wave. (See p. 200.)
The flabellulid ameba, Vannella miroides (Bovee, 1963b) has proto- plasmic currents which force their way, seemingly under pressure, through the semisolid parts of the margin, adding to and advancing the
Morphological Differences among Pseudopodia 193 marginal wave (Fig. 9E). The lateral margins of the clear wave appear
to contract and retract into the sides of the main body (Fig. 9E). T h e body trails as a more or less ovate mass (Fig. 9D). (See pp. 201-204.)
On descending from afloat, Vannella miroides trails and waves some of the old radiate pseudopods for a time (Fig. 10); and it may form new ones from the upper surface of the body (Fig. 9A-C). Each new pseu-
FIG. 2. Oscillosignum sp. showing movements plotted from 4 0 0 frames of motion picture film (see p. 192). (A) Pseudopod denoting new locomotive path at left, a waving pseudopod at lower right, a supporting pseudopod at upper right, and body movement above; speed of photography, 5 frames/sec. (B) "Walking" movements of pseudopods and forward movements; (C) with formation of a directional pseudopod, lower right;
waving pseudopods shown extending above the body. Dotted lines and arrows indi- cate direction of movement of pseudopodial tips. (D) Photograph of the organism showing waving and directional pseudopods.
dopod starts as a clear half-round bulge, which then extends, fingerlike, 4 to 5 times longer than its diameter, with a clear tip (Fig. 9A). It lengthens spirally until 4 to 6 times that first length, becoming tapering (Fig. 9B). Its distal two-thirds is clear and conical; and the proximal one-third also tapers, but may be granular (Fig. 9C). When extended it may be rigid; or may oscillate by basal movements; or may undulate slowly from base to tip. (See p. 202.)
Other small amebae have other types of locomotion. T h e Hartman- nellidae resemble the flabellulids in gross appearance. They also have a clear advancing margin and a trailing body; but the anterior wave is not so flat as in the flabellulids, nor so fan-shaped (Fig. 11). As a hart-
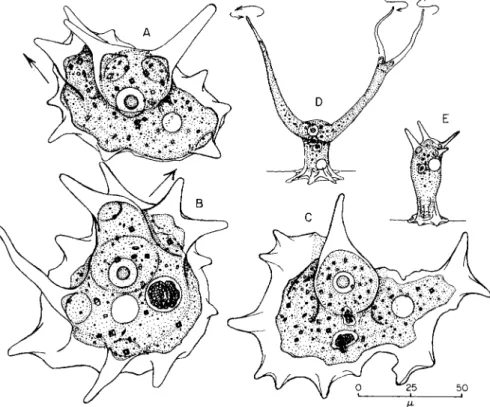
FIG. 3. Erect states of mayorellids—(A)-(C) Mayorella bigemma. (A) Nucleus has descended from upright pseudopods; new pseudopods at upper left in direction of arrow; (B) similar, with new pseudopods at upper right; (C) with nucleus in the upright pseudopod; no new pseudopods being formed for locomotion. (D) Oscillosig- num proboscidium in standing form, with extended pseudopods waving as indicated by the arrows; nucleus in the upright portion; no locomotion. (E) A static, polyplike, erect state of Mayorella clavabellans; nucleus in upright body; no locomotion. [(D) After Bovee, 1953a.]
mannellid ameba advances eruptive waves of clear protoplasm burst for- ward from the granular body mass into the clear semirigid anterior border, spreading it and adding their substance to the anterior margin of it as they, in turn, appear to gelate (Fig. 11C, D, G, and H).
Hartmannellid amebae may form filose pseudopods in groups of 2 to 4 from the upper surface of the body (Ray and Hayes, 1954; Bovee,
FIG. 4. Vexillifera sp. (A) Locomotive individual with three waving, retracting pseudopods; (B) same organism showing · movement of a pseudopod swung, in 10 sec, from base just prior to retraction; plotted from 30 frames of motion picture film, οχ photographed at 7 frames/sec; (C) movements plotted from 300 frames of motion picture film of a new pseudopod being extended and an old one being waved and retracted.
1963a). These aid in feeding. Bacteria are dragged down and engulfed (Fig. I I B ) . (See p. 205.)
In soft agar, hartmannellid amebae burrow (Bovee, 1963a). Acantha- moeba castellani then becomes elongated. It pushes new eruptive waves ahead into the agar, adding to the anterior length of the body and to the length of the tunnel (Fig. 12C-F). T h e body is pressed against and adheres to the wall of the tunnel (Fig. 12C), except along the terminal
FIG. 5. Flagellipodium sp. ( A ) Locomotive stage with the "flagellipod" rigidly an- terior in the direction of locomotion; (B) nonlocomotive organism, showing various positions and movements of the active flagellipod, plotted from 600 frames of motion picture film photographed at 17 frames/sec; ( C ) "casting" movements of the flagel- lipod plotted from two separate sequences of 50 frames of motion picture film.
one-third of the body, which is wrinkled and seems to be forcibly con- tractile (Fig. 12C). Presumably they burrow this way in moist soil where they live. This burrowing form of A. castellani resembles the form taken by Amoeba proteus in a capillary tube (Goldacre, 1961a) (Fig. 12G).
(See p. 206.)
Ameboid stages of some mastigamebae are like the hartmannellid amebae, but they also form pear-shaped biflagellate to tetraflagellate stages (Bovee, 1959; Willmer, 1961) (Fig. 13A-D). Two examples are Naegleria gruben and Trimastigamoeba philippinensis. (See p. 207.)
Thick-pellicled thecamebae have a dense, clear ectoplasm. Semipar- allel ridges appear on the dorsal surface of the moving ameba (Fig. 14B
FIG. 6. Radiate states of mayorellid amebae. (A) Vexillifera sp. afloat and rigidly inactive; (B) Vannella symmetrica also rigidly inactive and afloat; (C) Mayorella sp., with slowly active pseudopods, plotted from 500 frames of motion pic- ture film photographed at 14 frames/sec; arrows indicate movements of pseudopodial tips; (D) Dinamoeba mirabi- lis, afloat, pseudopods very slowly active (note large food vacuole); (E) Mayorella sp. rigidly afloat.
and C). Abé (1961, 1962) reports that the ridges are piers of semisolid protoplasm. T h e moving protoplasm between these carries along inclu- sions. T h e contractile vacuole is pressed against these ridges and de- formed during locomotion as is also the nucleus (Abé, 1961, 1962; Leidy, 1879; Penard, 1902). Similar ridges are formed by the larger species Thecamoeba papyracea and Thecamoeba sphaeronucleolus, as well as by Thecamoeba striata (Fig. 14A-C). T h e rolling movement described by
Fig. 7 (A)-(D).
Jennings (1904) does not seem to occur (Penard, 1905; Schaeffer, 1920;
Goldacre, 1961a). (See p. 208.)
The ameboid stages of some mastigamebae resemble those of mayorel- lid amebae (Fig. 15B); that of Mastigamoeba aspera is somewhat like Dinamoeba mirabilis (Fig. 15C); but they are clearly distinct species.
The likeness, however, led the Swiss protozoologist, Penard (1936), to believe that they were the same organism. As an ameboflagellate, M.
aspera crawls with the flagellum ahead of it, snapping the tip in a hook- like stroke similar to that of the euglenid flagellate Peranema tricho- phorum (Fig. 15B), meanwhile forming and retracting pseudopods. (See p. 209.)
Morphological Differences among Pseudopodia 199 Mastigamoeba setosa is enclosed in a thick plasmalemma studded with chitinoid spines (Fig. 16B). Within this jacket the ameboid move- ment is like that of pelomyxid amebae. Often, at the same time, its long anterior flagellum is extended and beats in a pattern similar to that of Mastigamoeba aspera (Fig. 16A-C). (See p. 210.)
Some of the mastigamebae also beat their flagella in sine waves (Bovee et al., 1963) like those of true flagellates (Jahn et al., 1963; Jahn et al., 1962); and the sine waves may run from base to tip during the swimming
FIG. 7. A radiate, floating mayorellid with six pseudopods (see p. 1 9 8 ) . ( A ) - ( D ) Oscillating pseudopods with varying degrees of terminal coiling; each pseudopod de- scribed a circle at the tip, as indicated, counterclockwise, in 2 5 frames of motion picture film photographed at 1 2 frames/sec. ( E ) Photograph of coiled pseudopodial tip (above).
beat (Bovee et al., 1963) (Fig. 16D-K). Mastigamoeba steinii also uses its flagellum in this way (Fig. 17). (See pp. 210-212.)
The mastigamebae may produce a flagellum in 0.5 sec or less—and retract it as swiftly; and by their movements these appear to be true flagella. I do not know of any studies which have been made by electron microscopy of their fine structure.
T o assume a different basic mechanism for each bizarre protoplasmic movement by these amebae is unnecessary. There is much more reason to consider the similarities, and then try to account for the differences.
It was popular a century ago to consider protoplasmic flow to be basically similar in character wherever found—in plants, ameba, or muscle. Dujardin (1835, 1838), Ecker (1849), Engelmann (1879), and others held such views. Later, as chemistry and physics developed, this postulation became naive. Surface-tension theories bloomed late in the nineteenth century (Berthold, 1886; Bütschli, 1882, 1892; Rhumbler, 1898), waxed, and waned in the first quarter of the twentieth (Furth,
FIG. 8. Radiate and locomotive stages of flabellulid amebae. (A) Vannella miroides;
(B) Rugipes vivax; (C) Vannella symmetrica', (D) Flabellula citata; (E) Vannella mira;
( F ) Vannella sensilis. [(B) and (D) After Schaeffer, 1926a; (C) after Bovee, 1953c]
1922; Schäfer, 1910; Tiegs, 1928a, b). An early denial of the surface- tension concepts was Jennings' (1904) "rolling sac" theory, itself promptly denied (Penard, 1905) and countered with a "walking" concept (Dellinger, 1906).
Studies of colloids and their properties led to syneretic gel-sol con- cepts of ameboid movement before 1925 (Hyman, 1917; Pantin, 1923).
These were promptly developed into a contractile-hydraulic gel-sol ex- planation of the events of ameboid movement by Mast (1926), one which is still accepted, with modification, by many. Meanwhile, and since,
Morphological Differences among Pseudopodia 201 many physical and chemical factors have been employed experimentally
to test and postulate bases for the visible events of ameboid locomotion (reviews De Bruyn, 1947; Heilbrunn, 1958; Allen, 1961b).
Szent-Györgyi (1949, 1953), and others especially studying muscle (review, Weber, 1958), and many others studying contractility of cells in
the past 25 years (Child, 1961; Hoffmann-Berling, 1954, 1960; Kriszat, 1949, 1954; Loewy, 1952; Nakajima, 1956; Ts'o et al, 1956, 1957) have shown the presence of and importance of actomyosin-like proteins and phosphate energy reservoirs in protoplasmic movements. Still, as late as 1952, it may have been premature to explain ameboid movement, as I then did (Bovee, 1952) via an actomyosin-adenosine triphosphate (ATP) system. At least my paper was generally ignored, but it was cited by Noland (1957) as an attractive hypothesis, still then only an assump- tion.
More recent information shows plainly that it is no longer premature to assume the generality of such a system; others have already done so (Kamiya, 1959, 1960; Landau, 1959; Oosawa, 1963).
Landau (1959), dealing with ameboid movement, thoroughly reviewed and discussed the literature to that date, and Kamiya has done likewise for protoplasmic movements of mycetozoans and plant cells. Landau gives a sound assumption for an actomyosin-like complex energized by A T P in amebae, having a spatial gradient, and responsible for ameboid movement, and assuming a dynamic equilibrium within the ameba.
Kamiya (1959) says this in his review:
"It seems after all that the predominant feature of protoplasmic streaming is based upon the mechanochemical system represented by myosin-like proteins which have by themselves an ATP-ase activity. It looks as if nature had a predilection for using actomyosin-like proteins and A T P as a mechanochemical system for carrying out mechanical work in many cells beside muscle . . . it may be tentatively assumed without denying other possibilities in special cases, that this is the basis of the mechanism of movement widely used in protoplasm in general. It is through it that there are formed different dynamic structures which deter- mine the various types of protoplasmic movement and streaming/'
Abé (1961, 1962) also notes the dynamic interaction of morphology and physiology of amebae. Others have stressed the dynamic morphology of amebae in locomotion in relation to their taxonomy and the identity of their species (Bovee, 1953c, 1960a, b; Bovee and Jahn, 1960; Jepps, 1956;
Leidy, 1879; Penard, 1902; Schaeffer, 1920, 1926a). Many who have studied ameboid movement also remark on the dynamic interdependence of ameboid form and function (Bovee, 1960b; De Bruyn, 1947; Goldacre,
Morphological Differences among Pseudopodia 203 1961a; Griffin, 1961, 1962; Jahn et al, 1960; Jahn and Rinaldi, 1959;
Rinaldi and Jahn, 1963; Kamiya, 1959; Noland, 1957).
This dynamicity is a challenge for study. T h e basic actomyosin-ATP system seems simple enough, but as Kamiya (1959) indicates, there is likelihood that it is not a single stereotyped machine. It seems, instead,
FIG. 9. Vannella miroides (see p. 202). (A)-(C) T h e development of a radiate, waving pseudopod by an actively locomotive individual, plotted from 3000 frames of motion picture film, photographed at 14 frames/sec. (D) T h e fully locomotive fan-shaped indi- vidual. (E) T h e movements of protoplasmic currents in the anterior border of the locomotive individual; tonguelike flow directly to the anterior border indicates the path of flow of sol from the body to the anterior border; other arcs in sequence as indicated by arrows mark successive positions (each 35 frames) of gel islands formed pre- viously, showing their migrations laterally and back to the body mass; plotted from a sequence of 400 frames of motion picture film photographed at 8 frames/sec. ( F ) A photograph showing the central sol flow and laterally moving gel islands in the fan-shaped border.
to have many variations. Szent-Györgyi (1960) contends that the machinery of life is based on varied and statistically improbable reactions, because they are the more easily controlled. Self-regulation (i.e., the adaptation of naturalistic terms) is of course the cornerstone of survival.
One deficiency of the theories concerning ameboid movement is that they do not agree on the site for contraction (Weiss, 1961; Rinaldi and
FIG. 10. Vannella miroides. Swinging movement of a radiating pseudopod just before its retraction. Movement from positions (A) through (F) occurred in 20 sec.
Plotted from 175 frames of motion picture film photographed at 8 frames/sec.
Jahn, 1963). Another difficulty is that they do not take into consideration, and therefore question, whether polymers of actomyosin simply contract, fold, or coil (Goldacre, 1952; Goldacre and Lorch, 1950) or whether as they do that, they shear and slide past one another as well (Allen 1961a;
Huxley and Hanson, 1955; Jahn and Rinaldi, 1959), perhaps with a ratchet action (Hayashi, 1961; Noland, 1957). According to one current theory muscle now slides and shears instead of contracting (Huxley and Hanson, 1955, 1960; Weber, 1958); and another recent theory asserts that foraminiferans cast forth self-sliding filaments that glide out from and back to the body along themselves (Jahn and Rinaldi, 1959).
Morphological Differences among Pseudopodia 205
FIG. 11. Various hartmannellid amebae. ( A ) Acanthamoeba castellani (after Vol- konsky, 1931); (B) the same, with filose pseudopods; (C) Acanthamoeba sp., from orig- inal motion picture film; (D) Hartmannella sp. (after Chatton and Brodsky, 1909);
(E) Vahlkampfia sp. (after Wherry, 1913); ( F ) Hartmannella sp., from an original mo- tion picture film; (G) and (H) vahlkampfian hartmannellids from an original motion picture film.
1939, 1942); and the ways in which an ameba uses its machinery, in Jepps' words (1956) "have to fit the circumstances."
However, any ameba adapts and controls its mechanisms only within the limits of its capabilities. Even if its physical mechanisms are highly improbable according to hydrodynamics and thermodynamics, they still must conform to the rules thereof. T h e difficult, but more durable, types of machinery are perhaps the more often statistically and evolu-
I do not disparage these ideas. I cite them to note some of the intri- cacy of the problems the organisms have solved. T h e biomechanochemical systems involved are considerably mediated by the amebae, however, a concept that I and others have stressed elsewhere (Bovee, 1959, 1960a;
Kepner and Taliaferro, 1913; Schaeffer, 1917, 1920, 1926a,b; Seifriz,
tionarily available. The spontaneously functional, physically probable types are fewer and less controllable.
T o the point of triteness, textbooks of general biology state that the nucleus of an ameba or any other cell is vital, and directs its growth, form, and function. Yet only rarely is the nucleus mentioned in theories of ameboid movement, or in discussions of pseudopodial form and func- tion. It appears that the machine is assumed to operate efficiently with-
\ΟΌμ G
FIG. 12. Acanthamoeba castellani. (A) Active trophozoite in fluid overlay on agar;
(B) active trophozoite, flattened, in motion between agar and the glass surface of petri dish; (C) active trophozoite burrowing in agar, body pressed against the tunnel wall except for contracting terminal "tail," eruptive waves pushed forward into agar to enlarge the tunnel. ( D ) - ( F ) Various tunnels constructed by acanthamebae in agar.
T h e incomplete tunnels were formed by amebae which encysted at the enlarged terminus of the tunnel within the agar. T h e tunnels running from top to bottom of the agar were made by amebae which moved off between the agar and the glass of the petri dish after completing the tunnel. (After Bovee, 1963a.)
out guidance. I believe that this is a sin of omission. Even though ame- boid cytoplasm, as has been shown (Allen, 1959, 1961c; Allen et al., 1960), will continue to run like an "engine" when disengaged and dissociated from the ameba, I am inclined to regard such a circumstance as analo- gous to the idling motor of the automobile, which provides energy and force to drive the machine only when it is engaged with the other functional parts of the mechanism, at the direction of the operator.
There is, of course, abundant evidence of nuclear involvement in
Morphological Differences among Pseudopodia 207 ameboid cytoplasmic form and function (review, Brächet, 1959, 1961;
Brächet, 1955; Hirschfield, 1959). All genetics is based on the idea of nuclear control of cytoplasmic function, and there is no need to belabor the genetic point of view here.
Grafting experiments on irradiated and nonirradiated amebae (Dan- iels, 1952, 1955, 1959) and nuclear transplantations are especially en-
FIG. 1 3 . Polyflagellate amebae. (A) Ameboid state of Naegleria gruben; (B) bi- flagellate stage of N. gruben; ( C ) ameboid stage of Trimastigamoeba philippinensis;
( D ) tetraflagellate stage of T. philippinensis. [(A) and (B) After Singh, 1 9 5 2 ; ( C ) and ( D ) after Bovee, 1 9 5 9 . ]
lightening as to the closeness of relationship between cytoplasmic and nuclear forms and functions (reviews, Danielli, 1959; Brächet, 1959,
1961).
Daniels shows (1952, 1955, 1959; Daniels and Vogel, 1959) that the organization of ameboid cytoplasm, once severely damaged by radiation, cannot be repaired by a normal nucleus, unless undamaged cytoplasm is also present to provide, presumably, some undamaged cytoplasmic tem- plates to be copied. He has also shown that interspecific infusion of cytoplasm is quickly lethal to the recipient, because of probable specific differences in protein polymers (Daniels, 1962).
Danielli and his co-workers show that the cytoplasmic morphology
of Amoeba proteus may be affected by the nucleus of Amoeba discoïdes.
The hybrid is morphologically and functionally intermediate. However, the cytoplasm of the recipient A. proteus never becomes entirely like that of the A. discoïdes from which the donor nucleus came, and the A. discoïdes nucleus, in size at least, becomes more nearly that which it should be were it normally an A. proteus nucleus (Danielli, 1955;
Lorch and Danielli, 1950, 1953). Also it has been observed that there
FIG. 14. Thecamebid amebae. (A) Thecamoeba papyracea—note distortion of the nucleus under pressure against gel islands; (B) Thecamoeba sphaeronucleolus—
note parallel gel ridges in anterior portion of the body and the distortion of both nucleus and contractile vacuole by pressure against the gel and the packed inclusions;
(C) Thecamoeba striata, nucleus and contractile vacuole distorted, and parallel gel ridges prominent in the anterior portion.
is a critical-phase relationship between growth of the nucleus and cyto- plasm as evidenced in cross-nuclear transplants from old and young amebae (Comandon and deFonbrun, 1939, 1942), and that certain bio- chemical capabilities are lost by starved cytoplasm and nucleus (Mug- gleton and Danielli, 1958). Cross-nuclear transplants have failed com- pletely in A. proteus and A. dubia, which are presumably close taxonomic relatives (Comandon and deFonbrun, 1939; Rudzinska and Chambers,
1951; Lorch and Danielli, 1953). There must be in ameboid cytoplasm
Morphological Differences among Pseudopodia 209 some durable molecules which only a compatible nucleus can modify,
and which no nucleus alone can replace.
A long-ignored paper by Heathman (1932) shows that amebae which develop morphologically distinct pseudopods and patterns of ameboid movement, are also antigenically distinct. In an order of arrangement showing both similarities and differences, her studies show that, anti-
FIG. 1 5 . (A) Ameboid stages of Mastigamoeba aspera; (B) ameboflagellate stage of the same organism; (C) Dinamoeba mirabilis, showing resemblance to, but also difference from M. aspera.
genically: (1) Amoeba proteus and Amoeba dubia are distinct species, but closely related; (2) Mayorella conipes and Mayorella bigemma are distinct amebae in a second group intermediate between the Amoeba spp. and a third group (3) composed of two more separate species, Fla- bellula citata and Flabellula mira. T h e third group is clearly distinct from, but closer than groups (1) and (2) are, to group (4) composed of varieties of the parasitic Entamoeba histolytica (Table I). These relations exactly par- allel the morphological and taxonomic distinctions proposed for those ame- bae by Schaeffer (1926a). Heathman (1932) suggested that there are in ame-
FIG. 1 6 . (A)-(C) Mastigamoeba setosa. (A) Shows the hooking power stroke and anterior-posterior wave of the flagellum, in 6 successive frames of motion picture film;
(B) the whole organism, showing pelomyxid body form and the pellicular spicules, with part of the recovery stroke of the flagellum plotted from the next 6 frames of motion picture film; (C) the remainder of the recovery stroke in the succeeding 8 frames of film; photographed at 8 frames/sec. (D)-(K) Flagellar movements of Mas- tigamoeba sp.; (D)-(F) three successive sine waves which progressed from tip to base
Morphological Differences among Pseudopodia 211
T A B L E I
ANTIGENTIC SIMILARITIES AND DIFFERENCES BETWEEN S P E C I E S ^ M
Immune serum A. A. M. M. F. F. E.
(0.05 ml) proteus dubia b ige mm a conipes mira citata histolytica
A. proteus 4 3 2 2 2 0 0
F. mira 2 0 1 1 4 2 Trace
F. citata 1 0 3 3 3 4 CM
Ε. histolytica 0 X Χ X Trace 2 4
α After Heathman, 1932.
ö Amount of material used: 0.1 ml antigen of ameba plus 0.5 ml guinea pig complement.
c Key: 4, very strong reaction; 3, strong reaction; 2, moderate reaction; 1, weak reaction; trace, very slight reaction; X , not tested.
ment and locomotion are not only valid, but are the most valuable means by which amebae may be quickly and accurately identified. As Schaeffer (1926a) succinctly puts it: "In amebas the shape is dynamic;
that is, movement is a function of shape." T h e converse is also true;
and both are ultimately the products of inherent cytoplasmic-nuclear organizations and interactions.
Landau (1959) states that in the presumed actomyosin-ATP system for ameboid motion "the role of the nucleus is multifold." Goldacre (1961a) postulates a self-regulatory feedback system in the cytoplasm of the locomoting Amoeba proteus. Thus, he says, "The tail has a means of 'knowing' whether there are other contracting regions in the cell or not." He states elsewhere (Goldacre, 1958a) that the nucleus is intri- cately involved in the locomotive feedback system, and in other systems which function in the cell, and that the surface membranes assist the feedback operation (Goldacre, 1958b, 1961b).
The feedback systems not only exist from front to rear of the ameba
of the flagellum, as flagellar movement began. Plotted from 75 frames (each second frame) of motion picture film photographed at 300 frames/sec with a missile-tracking camera; (H)-(K) sine-wave paths in progress along the flagellum (each fourth frame) from a succeeding sequence of 150 frames of the same film.
bae one or more common antigenic properties, perhaps lipoid in nature;
and she further suggested that the specific differences are due to specific differences in the protein-carbohydrate complexes which vary among the amebae.
Apparently, then, even visibly slight differences between amebae may reflect significant physiological differences in organization which estab- lish the differing organisms as distinct species. Therefore, even slight, but constant visible differences in the morphological patterns of move-
Fie. 17. Mastigamoeba steinii. (A) Sine waves in progress from tip to base of the flagellum from 10 successive frames of motion picture film photographed at 17 frames/sec; (B) and (C) paths of granules which moved along the beating flagellum from tip to base, 80 frames, positions of granules shown (each second frame). Arrows indicate sine-wave paths of granules. (D) and (E) Casting and recovery strokes of the flagellum employed in orienting the anterior end of the body in a new direction;
eighteen successive frames.
Morphological Differences among Pseudopodia 213 but oscillate from side-to-side and probably up and down as well. The
cross-feed oscillation is evident in the formation of pseudopods during continuous locomotion. These arise in many amebae in an alternating pattern at left and then right anterolateral positions (Fig. 18), resulting in a sinusoidal pathway on a flat surface, as repeatedly mentioned by Schaeffer (1916, 1917, 1926a, b, 1931). T h e dorsoventral feedback rela- tionship is apparent from the fact that new pseudopods form dorsally and old ones are diverted to ventral positions or posterior ones.
These feedbacks may involve even the characteristic structure of the specific carbohydrate-protein complexes of the internal machinery. Ac- cording to Katchalsky (1963), it may be demonstrated both mathemati- cally and experimentally that certain irreversible, but cyclic, routes for energy exist via these large bio-organic polymers, amounting to rudi- mentary "memory" patterns within the cell. Also, Chance (1963) sug- gests some such differences in the structures concerned with energy trans- fers of the cell, particularly the mitochondrial surfaces and the aggre- gates attached to them; he indicates that they, too, participate in the regulatory feedback mechanisms.
We might, then, presume that the large molecules, probably proteins which are responsible for the specific antigenic differences among amebae, are also among the molecules that operate differentially and specifically in the feedback and information systems of the various amebae. They and others specific to the locomotor mechanism interact and develop the locomotor morphology of the ameba. Therefore, we should expect each kind of ameba to have some proteins common to all cells, with perhaps minor variation in them, and others more specifically its own.
These should interact to produce certain visible features of locomotion and morphology common to all amebae, and still others characteristic only of a certain limited group of amebae, or of even a particular species.
These visible features do exist, and they are of both taxonomic and evo- lutionary import.
An ameba is plainly in control of its activities, and is a self-competent organism. It exhibits an awareness of and responds to its environment with normality and regularity. This has led some writers to use anthro- pomorphic terms in describing ameboid activities such that they suggest an incipient wisdom on the part of the ameba (Jepps, 1956; Kepner and Taliaferro, 1913; Leidy, 1879; Schaeffer, 1917).
An ameba's responses are not "frozen" into invariable and irrevoca- ble sequences of events. It is keenly attuned, through its informational feedback mechanisms, to the nuances of its environment, and it responds in patterns which are normally and distinctly its own (Bovee, 1960a;
Schaeffer, 1926a). These responses not only identify the ameba; but they
Tic. 18.
Morphological Differences among Pseudopodia 215 are exactly those criteria by which the experimenter determines whether or not, and how, the ameba responds to and is affected by experiment.
Abnormality rarely appears in sublethal situations (Bovee, 1960a). T o those the self-regulatory mechanisms of the ameba are able to adapt (Bovee, 1960b; Seifriz, 1939, 1942; Schaeffer, 1926a).
The formation of a single pseudopod is often adequate to demon- strate the individuality and normality of an ameba. Although a new pseudopod forms initially in all amebae, so far as I can tell, as a hemi- spherical bulge at some point on the surface, it always assumes the mor- phology characteristic of a pseudopod of its species, as it elongates. There is plainly an orderly flow of and incorporation of materials into it which are specifically and sequentially organized into the pseudopodial form characteristic of the species. Otherwise no two pseudopods formed by an ameba should resemble one another; but they always do.
Somehow the notion still generally persists that amebae are shape- less, ever and erratically altering their contours, and move randomly.
Such a concept is obviously false. Goldacre (1961a) has called this "the sickly child of literary inbreeding" through a succession of textbooks.
I believe it is a useful business to debate the mechanisms of ameboid movement. However, it often seems to me that some theorists try assid- uously to force amebae to conform to their preconceptions of mathemat- ical and physical principles and to formulas (Buchsbaum et ah, 1944).
Perhaps they are still bemused by this embalmed error of presumed ameboid randomness. I find it singular, if provocative, for example, to contemplate the role of surface films in ameboid movement by consid- ering the random water currents in a flushing toilet, as one theorist suggests (Kavanau, 1962). This neither substantiates nor denies the pos- sible role of surface films in ameboid movement. They may be, and probably are involved. I do not belittle such exercises. Good ideas often come from observations of common events. Theorizing and debate attract attention to the problem, whether or not the debated theories offer a solution. I find both the problem and the debate exciting, and I find the amebae most fascinatingly complex.
We should not, however, lose sight of the organism in the excitement of the debate. As Mazia (1956) suggests, if we are to find out what the cell does and how it does it, we must devise answerable questions, and
FIG. 1 8 . Paths of locomotion of a vahlkampfian ameba. (A) Positions of new pseudopods, showing alternating and oscillating sequence resulting in a sinuous path- way of progress. Plotted from a sequence of 1 2 0 0 frames of motion picture film (each twentieth frame), photographed at 8 frames/sec; (B) the same (each hundredth frame) from a sequence of 3 5 0 0 frames, showing the tighter sinuous pathway superimposed on the greater sinuous track.
pose them most politely to the cell. Perhaps it will then answer. I be- lieve we will find thereby that we are still describing protoplasmic movement as the blind men described the elephant—in portions with partial truth from each, depending on how and where we have touched the problem. We must grope further, by stages, as Noland (1957) says.
What, then, is the significance of the ameboid movements in smaller amebae? I believe it is that of theme and variation, demonstrating the great variability of a fundamental mechanism of protoplasmic move- ment, and the specific evolutionary segregation and survival of some of the variants. It is clear that the various amebae are distinct animals.
It is evident that they may use their locomotive mechanism in a variety of ways. T h e elegant and seminal simplicity of the basic parts of the machine and the various bizarre applications of it by amebae are mar- velous. They far exceed in inventiveness the theories purporting to ex- plain them.
Material pertinent to this paper will be discussed in the Free Discussions of Parts III and IV.
REFERENCES Abé, T. H. (1961). Cytologia 2 6 , 378.
Abé, T . H. (1962). Cytologia 2 7 , 111.
Allen, R. D. (1959). Biol. Bull 1 0 9 , 339.
Allen, R. D. (1961a). Exptl Cell Res. Suppl. 8 , 17.
Allen, R. D. (1961b). In "The Cell" (J. Brächet and A. E. Mirsky, eds.), Vol. II, pp. 135-216. Academic Press, New York.
Allen, R. D. (1961c). In "Biological Structure and Function" (T. W. Goodwin and O. Lindberg, eds.), Vol. II, pp. 549-556. Academic Press, New York.
Allen, R. D., Cooledge, J . W., and Hall, P. J . (1960). Nature 1 8 7 , 896.
Berthold, G. (1866). "Studien über Protoplasmamechanik." A. Felix, Leipzig, Germany.
Bingley, M. S., and Thompson, C. M. (1962). / . Theoret. Biol. 2 , 16.
Bovee, E. C. (1951). Proc. Am. Soc. Protozool. 2 , 4.
Bovee, E. C. (1952). Proc. Iowa Acad. Sei. 5 9 , 428.
Bovee, E. C. (1953a). Trans. Am. Microscop. Soc. 7 2 , 17.
Bovee, E. C. (1953b). Trans. Am. Microscop. Soc. 7 3 , 328.
Bovee, E. C. (1953c). Proc. Iowa Acad. Sei. 6 0 , 599.
Bovee, E . C. (1956). / . Protozool. 3 , 155.
Bovee, E. C. (1959). / . Protozool. 6 , 69.
Bovee, E. C. (1960a). Am. Midland Naturalist 6 3 , 257.
Bovee, E. C. (1960b). / . Protozool. 7, 55.
Bovee, E. C. (1963a). Am. Midland Naturalist 6 6 , 173.
Bovee, E. C. (1963b). J. Protozool. Suppl. 1 0 , 10.
Bovee, E. C , and Jahn, T . L. (1960). / . Protozool. Suppl. 7, 8.
Bovee, E. C , Jahn, T . L., Fonseca, J . , and Landman, M. (1963). Biophys. Soc. Abstr.}
p. MD2.
Brächet, J . (1955). Biochem. Biophys. Acta. 1 8 , 247.
Brächet, J . (1959). Ann. N. Y. Acad. Sei. 7 8 , 688.
Morphological Differences among Pseudopodia 217
Brächet, J . (1961). In "The Cell" (J. Brächet and A. E. Mirsky, eds.), Vol. II, pp. 771- 841. Academic Press, New York.
Buchsbaum, R., Rashevsky, N., and Stanton, H. E. (1944). Bull. Math. Biophys. 6 , 61.
Bütschli, O. (1882). In "Klassung und Ordnung des Thier-Reichs" (H. G. Bronn, ed.), Vol. 1(1), pp. 1-1097. Winter, Leipzig, Germany.
Bütschli, Ο. (1892). "Untersuchungen über mikroskopische Schaume und das Proto- plasma." Leipzig, Germany.
Chance, B. (1963). In "Control Mechanisms in Biological Systems," Symp. Biophys. Soc.
In preparation.
Chatton, E., and Brodsky, A. (1909). Arch. Protistenk. 1 7 , 1.
Child, F. M. (1961). Exptl. Cell Res. Suppl. 8 , 47.
Comandon, J . , and deFonbrun, P. (1939). Compt. Rend. Soc. Biol. 1 3 0 , 740.
Comandon, J . , and deFonbrun, P. (1942). Compt. Rend. Soc. Biol. 1 3 6 , 763.
Danielli, J . F. (1955). Exptl. Cell Res. Suppl. 3 , 98.
Danielli, J . F. (1959). Ann. N. Y. Acad. Sei. 7 8 , 675.
Daniels, E. W. (1952). / . Exptl. Zool. 1 2 0 , 525.
Daniels, E. W. (1955). / . Exptl. Zool. 1 3 0 , 183.
Daniels, E. W. (1959). Ann. N. Y. Acad. Sei. 7 8 , 662.
Daniels, E. W. (1962). / . Protozool. 9 , 183.
Daniels, E. W., and Vogel, H. H., Ir. (1959). Radiation Res. 1 0 , 584.
De Bruyn, P. P. H. (1947). Quart. Rev. Biol. 2 2 , 1.
Dellinger, Ο. P. (1906). / . Exptl. Zool. 3 , 337.
Dujardin, F. (1835). Ann. Sei. Nat. Zool. 4 , 343.
Dujardin, F. (1838). Ann. Sei. Nat. Zool. 1 0 , 230.
Ecker, A. (1849). Z. Wiss. Zool. 1, 218.
Engelmann, T . W. (1879). In "Hermann's Handbuch der Physiologie," Vol. I, p. 343.
Vogel, Leipzig.
Furth, O. (1922). Arch. Néerl. Physiol. 7, 39.
Goldacre, R. J . (1952). Intern. Rev. Cytol. 1, 135.
Goldacre, R. J . (1958a). Proc. Intern. Congr. Cybernetics, 1st, Namur, 1956, 1, 715.
Goldacre, R. J . (1958b). In "Surface Films in Chemistry and Biology" (J. F. Danielli, K. G. A. Pankhurst, and A. C. Riddiford, eds.), pp. 278. Pergamon Press, London.
Goldacre, R. J . (1961a). Exptl. Cell Res. Suppl. 8 , 1.
Goldacre, R. J . (1961b). In "Biological Structure and Function" (T. W. Goodwin and O. Lindberg, eds.), Vol. II, pp. 633-643. Academic Press, New York.
Goldacre, R. J . , and Lorch, I. J . (1950). Nature 1 6 6 , 497.
Griffin, J . L. (1961). Proc. Am. Soc. Cell. Biol. 1, 77.
Griffin, J . L. (1962). Proc. Am. Soc. Cell. Biol. 2, 61.
Hayashi, T . (1961). Set. Am. 2 0 5 , 184.
Heathman, L, (1932). Am. J. Hyg. 1 6 , 97.
Heilbrunn, L. V. (1958). Protoplasmatologia 2 , 1.
Hirschfield, H. I. (1959). Ann. N. Y. Acad. Set. 7 8 , 64.
Hoffmann-Berling, H. (1954). Biochim. Biophys. Acta 1 4 , 182.
Hoffmann-Berling, H. (1960). Comp. Biochem. 2 , 341.
Huxley, H. E., and Hanson, J . (1955). Symp. Soc. Exptl. Biol. 9 , 228.
Huxley, H. E., and Hanson, J . (1960). In "Structure and Function of Muscle" (G. H.
Bourne, ed.), Vol. I, pp. 183-225. Academic Press, New York.
Hyman, L. H. (1917). / . Exptl. Zool. 2 4 , 55.
Jahn, T . L., and Rinaldi, R. A. (1959). Biol. Bull. 1 1 7 , 100.
Jahn, T . L., Bovee, Ε. C , and Small, Ε. Β. (1960). / . Protozool. Suppl. 7, 8.
Jahn, T. L., Fonseca, J . , and Landman, M. (1962). Proc. Am. Soc. Cell. Biol. 2 , 79.
Jahn, T. L., Harmon, W., and Landman, M. (1963). / . Protozool. 1 0 , 358.
Jennings, H. S. (1904). Carnegie Inst. Wash. Puhl. 1 6 , 129.
Jepps, M. (1956). "The Protozoa. Sarcodina." Oliver and Boyd, Edinburgh.
Kamiya, N. (1959). Protoplasmatologia 8 , 1.
Kamiya, N. (1960). Ann. Rept. Sei. Works, Fac. Sei. Osaka Univ. 8 , 13.
Katchalsky, A. (1963). In "Non-muscular Contractions in Biological Systems" Symp.
Biophys. Soc. (in press).
Kavanau, J . L. (1962). Science 136, 652.
Kavanau, J . L. (1963). / . Theoret. Biol. 4 , 124.
Kepner, W. Α., and Taliaferro, W. (1913). Biol. Bull. 2 4 , 411.
Kriszat, G. (1949). Arkiv. Zool. 1, 81.
Kriszat, G. (1954). Arkiv. Zool. 6 , 195.
Landau, J . V. (1959). Ann. Ν. Y. Acad. Sei. 7 8 , 487.
Leidy, J . (1879). U. S. Geol. Sun;. Territories 1 2 , 1.
Loewy, A. (1952). / . Cellular Comp. Physiol. 4 0 , 127.
Lorch, I. J . , and Danielli, J . F. (1950). Nature 1 6 6 , 329.
Lorch, I. J . , and Danielli, J . F. (1953). Quart. J. Microscop. Sei. 94, 445; ibid. 9 4 , 461.
Mast, S. O. (1926). / . Morphol. and Physiol. 41, 347.
Mazia, D. (1956). Am. Scientist 44, 1.
Muggleton, Α., and Danielli, J . F. (1958). Science 181, 1738.
Nakajima, H. (1956). Seitai No Kagaku 7, 256.
Noland, L. E. (1957). / . Protozool. 4, 1.
Oosawa, F. (1963). In "Non-muscular Contractions in Biological Systems." Symp. Bio- phys. Soc. (in press).
Pantin, C. F. A. (1923). / . Marine Biol. Assoc. (U. K.) 13, 24.
Penard, E. (1902). "Faune rhizopodique du Bassin du Leman." Kundig, Geneva.
Penard, Ε. (1905). Arch. Protistenk. 6 , 175.
Penard, Ε. (1936). Bull. Soc. Franc. Microscop. 5 , 136.
Ray, D. L., and Hayes, R. E. (1954). J. Morphol. 9 5 , 159.
Rhumbler, L. (1898). Arch. Entwicklungsmech. Organ. 7, 103.
Rinaldi, R. Α., and Jahn, T . L. (1962). / . Protozool. Suppl. 9 , 16.
Rinaldi, R. Α., and Jahn, T. L. (1963). / . Protozool. 10, 344.
Rudzinska, M., and Chambers, R. (1951). Proc. Am. Soc. Protozool. 1, 13.
Schaeffer, A. A. (1916). Arch. Protistenk. 37, 204.
Schaeffer, A. A. (1917). / . Animal Behavior 7, 220.
Schaeffer, A. A. (1920). "Amoeboid Movement." Princeton Univ. Press, Princeton, New Jersey.
Schaeffer, A. A. (1926a). Carnegie Inst. Wash. Publ. 345, 1.
Schaeffer, A. A. (1926b). Quart. Rev. Biol. 1, 95.
Schaeffer, A. A. (1931). Science 74, 47.
Schafer, E. A. (1910). Quart. J. Exptl. Physiol. 3, 285.
Schulze, F. Ε. (1875). Arch. Mikroskop. Anat. Entwicklungsmech. 1 1 , 329.
Seifriz, W. (1939). Protoplasma 32, 538.
Seifriz, W. (1942). "The Structure of Protoplasm." Iowa State College Press, Arnes, Iowa.
Singh, Β. Ν. (1952). Phil. Trans. Roy. Soc. London B236, 405.
Szent-Györgyi, A. (1949). Science 110, 411.
Szent-Györgyi, A. (1953). "Chemical Physiology of Body and Heart Muscle." Academic Press, New York.
Morphological Differences among Pseudopodia 219
Szent-Györgyi, A. (1960). "Introduction to Submolecular Biology." Academic Press, New York.
Tiegs, O. W. (1928a). Australian J. Exptl. Biol. Med. Sei. 3, 1.
Tiegs, O. W . (1928b). Protoplasma 4, 88.
Ts'o, P. O. P., Eggman, L., and Vinograd, J . (1956). / . Gen. Physiol. 39, 801.
Ts'o, P. O. P., Eggman, L., and Vinograd, J . (1957). Biochim. Biophys. Acta 25, 532.
Volkonsky, M. (1931). Arch. Zool. Exptl. Gen. 72, 317.
Wallich, G. C. (1863). Ann. Mag. Nat. Hist. 11, 365.
Weber, H. H. (1958). "The Motility of Muscle and Cells." Harvard Univ. Press, Cambridge, Massachusetts.
Weiss, P. (1961). Exptl. Cell Res. Suppl. 8, 260.
Wherry, W. B. (1913). Arch. Protistenk. 31, 77.
Willmer, E. W. (1961). Exptl. Cell Res. Suppl. 8, 32.