ContentslistsavailableatSciVerseScienceDirect
Journal of Pharmaceutical and Biomedical Analysis
j o ur na l ho me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / j p b a
Analysis of submicron-sized niflumic acid crystals prepared by electrospray crystallization
Rita Ambrus
a, Norbert Radacsi
b, Tímea Szunyogh
a, Antoine E.D.M. van der Heijden
b,c, Joop H. ter Horst
b, Piroska Szabó-Révész
a,∗aDepartmentofPharmaceuticalTechnology,UniversityofSzeged,Eötvös6,H-6720Szeged,Hungary
bProcess&EnergyLaboratory,DelftUniversityofTechnology,Leeghwaterstraat44,2628CADelft,Netherlands
cTechnicalSciences,TNO,2280AARijswijk,Netherlands
a r t i c l e i n f o
Articlehistory:
Received26June2012
Receivedinrevisedform31October2012 Accepted2December2012
Available online xxx
Keywords:
Nanoparticles
Electrospraycrystallization Anti-solventcrystallization Solventevaporation Niflumicacid
Physico-chemicalanalysis
a b s t r a c t
Interestinsubmicron-sizeddrugparticleshasemergedfrombothlaboratoryandindustrialperspectives inthelastdecade.Productionofcrystalsinthenanosizescaleoffersanovelwaytoparticlesfordrug formulationsolvingformulationproblemsofdrugswithlowsolubilityinclassIIoftheBiopharmaceutical ClassificationSystem.Inthisworkniflumicacidnanoparticleswithasizerangeof200–800nmwerepro- ducedbythenovelcrystallizationmethod,electrospraycrystallization.Theirpropertieswerecompared tothosefromevaporativeandanti-solventcrystallizations,usingthesameorganicsolvent,acetone.
Thereisaremarkabledifferenceintheproductcrystalsizedependingontheappliedmethods.Thesize andmorphologywereanalyzedbyscanningelectronmicroscopyandlaserdiffraction.Thestructureof thesampleswasinvestigatedusingdifferentialscanningcalorimetry,Fourier-transformedinfraredspec- troscopyandX-raypowderdiffraction.Theparticlesproducedusingelectrospraycrystallizationprocess wereprobablychangingfromamorphoustocrystallinestateaftertheprocedure.
© 2012 Elsevier B.V. All rights reserved.
1. Introduction
Niflumicacid(NIF)isanimportantanti-inflammatorydrugand hasaweakanalgesiceffect.Itisprimarilyusedtotreatdifferent formsofrheumatism,likerheumatoid arthritisorarthrosis,and tocureotherinflammatorydiseases[1].However,itspooraque- oussolubilityanddissolutionratearedisadvantages[2].Toachieve optimalpharmacodynamicpropertiessuchasarapidonsetofthe drugeffect,fastdissolutionisimportantforthistypeofdrug.Inour earlierstudies,theaimwastoimprovethesolubilityanddissolu- tionrate,viathepreparationofternarysystemsofNIF,cyclodextrin (CD)andpolyvinylpirrolidone(PVPK-25)indifferentNIFtoCDto PVPratiosbyphysicalmixing,kneading,microwaveirradiationand micronization[3–6].
The interest for nano/micron particles in pharmaceutical research hasemerged recently,since studies have proven that particlesizereductioncaninfluencethemechanismsofdrugdeliv- eryinmanyways[7–14].Thedifferentmethodsusedforparticle sizereductioncanbedividedintotwomaincategories:top–down method,where theraw material is subsequently broken down byusingmillingmethodsuntilmicro-ornanosizedparticlesare
∗Correspondingauthor.Tel.:+3662545575;fax:+3662545571.
E-mailaddress:revesz@pharm.u-szeged.hu(P.Szabó-Révész).
producedandthebottom–upapproach(crystallizationprocedure), thebasicprincipleofwhichinvolvingdissolutionofthedrugina solvent,followedbyadditionofthesolutiontoanon-solvent,asa consequenceofwhichthedrugprecipitates.Thenanosuspension engineeringprocessescurrentlyusedincludeprecipitationusing high-pressurehomogenizationinwater,inmixturesofwaterand water-miscibleliquids,orinnon-aqueousmedia[15–19].Electro- spraycrystallizationasanovelpossibilityinbottom–upprocedure, hasthepotentialtobecomeanefficient,cost-effectivemethodfor theproductionofsubmicron-sizedorganiccrystals[20],offeringa simpleformulationwayofpharmaceuticalingredientswithbene- ficialproperties[21–23].
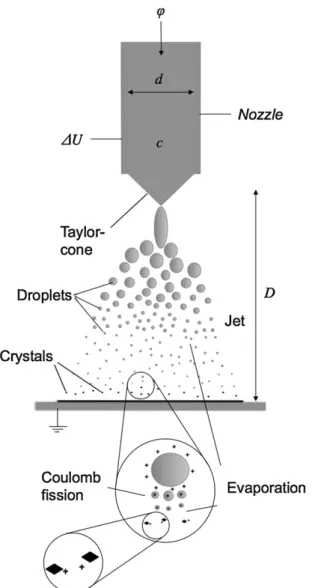
Inelectrospraycrystallizationahigh,constantpotentialdiffer- enceisappliedtoanozzle,throughwhichaconductivesolution ispumped(Fig.1).Ifthepotentialdifferenceissufficientlyhigh, electrostaticforcesovercomethesurfacetensionandajetofliq- uidisemittedfromtheso-calledTaylor-coneformedatthenozzle.
Atsomedistancefromthenozzle,thejetbecomesunstableand breaksintodroplets,whichareacceleratedtowardthegrounded platebytheelectricfield.Coalescenceofthedropletsisprevented becauseoftheunipolarcharge[21](whichenablesproductionof nano-sizedcrystals).Uponusingasufficientlyvolatilesolventsuch asacetone,solventevaporationoccurs,whichincreasesthespecific surfacechargedensitybecauseofthedecreaseindropletvolume andsurfacearea.Asthesurfacechargedensityreachesacritical 0731-7085/$–seefrontmatter© 2012 Elsevier B.V. All rights reserved.
http://dx.doi.org/10.1016/j.jpba.2012.12.001
Fig.1.Schematicsoftheelectrospraycrystallizationprocess.Usingasmallnozzle diameter(d),alowconstantflowrate(ϕ),relativelylowsoluteconcentration(c) ajetofhighlychargedsmalldropletswillbeemittedfromtheconeappearingat thenozzletipwhenapplyingahighenoughpotentialdifference(U)atacertain workingdistance(D).
value(Rayleigh-limit)[24]electrostaticforcesovercomethesur- facetensionandthedropletdisruptsintosmallerdropletstoreduce thesurfacechargedensitybycreatingmoresurfacearea.Thisdis- ruptionprocessiscalledCoulomb-fission.Atsomepointduringthis processofdropletevaporationanddisruption,thedrivingforcefor crystallizationbecomessufficientlylargeforcrystalnucleationand growthtooccur.Itisassumedthatcrystallizationisconfinedtothe volumeofthedroplet[25].Therefore,ifthedropletsaresufficiently small,typicallyonecrystalperdropletisformed.Thesecharged submicroncrystalsaccumulateatthegroundedsurfacewherethey losetheirsurfacecharge.
ItisknownthattheelectrospraycrystallizationresultsinNIF particleswithsignificantlyimproveddissolutionrate[26],andthis paperismeanttocharacterizetheproducedsubmicroncrystals.
Thegoalofthisresearchwastoprepareandanalyzesubmicron- sizedNIFcrystals.Herebyelectrospraycrystallizationisintroduced asapotentialinexpensiveandsimplemethodfortheproduction of such submicron-sized drug crystals and compared to con- ventionalevaporative anti-solvent crystallizations.The effectof three differentcrystallizationmethods (electrospray crystalliza- tion,anti-solventcrystallizationandevaporativecrystallization)on
thecrystalstructure,productandsizemicrometricpropertiesis investigatedandcomparedwiththeconventionalNIF.
2. Materialsandmethods
2.1. Materials
Conventional niflumic acid (NIF) (2-[[3-(trifluoromethyl) phenyl]amino]-3-pyridinecarboxylicacid)withbroadcrystalsize distributionandameansizeofaround80mwaspurchasedfrom G.RichterPharmaceuticalFactory,Budapest,Hungary.Forallthe crystallization processes thesolution was prepared with99.8%
acetone,purchasedfromMerck.
2.2. Methods
2.2.1. Preparationprocedure
2.2.1.1. Electrospray crystallization. An eight-nozzle electrospray crystallizationsetupwithdifferentNIFconcentrations(10,20and 30mgml−1)wasusedtoproducethesubmicron-sizedNIF.The setupconsistedofeightnozzles,anozzleholder,agroundedstain- lesssteelcollectorplate,aMeredosTL-EADperistalticpump,which providedanequaldistributionofthesolutionflowoverthenoz- zles,andaWallis±10kVDCpowersupplythatwasusedinpositive DCmodetoprovidethepotentialdifferencebetweenthetipofthe nozzlesandthegroundedplate.Theusednozzleshadalengthof 25.4mmandtheinnerdiameterof0.33mm.Theywerepurchased fromEFD,USA.TheproductdesignatedinthetextasNIF-NANO referstothesampleafter2weeksstorage.
2.2.1.2. Anti-solventcrystallization(AC). Inthismethod,asolution of500mgofNIFin5mlofacetonewasaddedto25mlofanaqueous mediaat roomtemperature (25◦C)that wasacting asan anti- solvent(Fig.2).Duetotheanti-solventeffect(acetoneismiscible withwater,and wateractsasananti-solventforNIF),thedriv- ingforceforcrystallizationwasreachedrapidly,andasuspension ofprecipitatedcrystalswasproducedinwater.Thisprocesswas carriedoutbyusingaT-25typeofUltra-Turraxdispersersimul- taneously(IKA, Staufen,Germany) at 24,000rpm for 10min,as energyinputforsizedecreasing(thegrowthoftheprecipitated crystalcouldbecontrolled).Thisveryhighstirringrateresultsina slighttemperatureincrease(upto33◦C)andenhancestheevapora- tionrateoftheacetonefromthemixture.Theproductwasfiltered anddriedatroomtemperature,theninvestigatedimmediately.The productislabeledasNIF-ACinthepaper.
2.2.1.3. Preparation ofthe sampleby solventevaporation(SE). An amountof250mgNIFwasdissolvedin5mlacetone,andthenthe solventwasevaporatedwiththehelpofacolddryer(HM3Dryer,
Fig.2.Schematicdrawingoftheanti-solventcrystallizationmethod.
MH9150201,Siemens,Germany)at20◦Cuntilalltheacetoneevap- orated. Thenthedry powderwaspulverized and homogenized manuallyinamortartoensureanarrowcrystalsizedistribution.
Thentheproductwasinvestigatedimmediately.TheNIFproduced byevaporativecrystallizationislabeledinthetextasNIF-SEinthe paper.
2.3. Analysis
2.3.1. Scanningelectronmicroscopy(SEM)
Thecrystalsizeand shapewereexaminedbyscanningelec- tronmicroscopy(HitachiS4700,HitachiScientific Ltd.,Japan).A sputtercoatingapparatus(Bio-RadSC502,VGMicrotech,England) wasappliedtoinduceelectricconductivityonthesurfaceofthe samples.Theairpressurewas1.3–13.0mPa.Sampleswerefixed ontoametallicstubwithdouble-sidedconductivetape(diame- ter12mm,Oxon,OxfordInstruments,UK).Imagesweretakenin secondaryelectronimagemodeat10kVaccelerationvoltage.The particlediameterdistributionswereobtainedbyanalyzingseveral SEMimageswiththeImageJsoftwareenvironment[27].Over150 individualparticlemeasurementsweremadeusingatleastfive differentimagesinordertodeterminetheparticlesizedistribution.
2.3.2. Particlesizeanalysis
TheparticlesizedistributionoftheconventionalNIFwasmea- suredbyalaserdiffractometer(MastersizerS,MalvernInstruments Ltd.,Worcestershire,UK)withthefollowingparameters:300RF lens, small volume dispersion unit rotated at 2000rpm, 1.510 refractiveindexfordispersedparticles,and1.330refractiveindex forthedispersionmedium.
2.3.3. Differentialscanningcalorimetry(DSC)
DSCwasemployedtoinvestigatethecrystallizationbehavior andthemeltingbehavioroftheconventionalandsubmicron-sized NIF. The DSCmeasurements weremade witha Mettler Toledo DSC821ethermalanalysissystemwiththeSTARethermalanalysis programV9.1(MettlerInc.,Schwerzenbach,Switzerland).Approx- imately2–5mgofproductwasexaminedinthetemperaturerange between25◦Cand300◦C.Theheatingratewas5◦Cmin−1.Argon wasusedasacarriergas,ataflowrateof10lh−1duringtheDSC investigation.
ThecrystallinityindexofthedifferentNIFsampleswascalcu- latedfromtheheatsoffusion:theratiobetweenthenormalized enthalpyoftheNIFsamplesandthenormalizedenthalpyofthe conventionalNIFindicatestheproductcrystallinity[28].
2.3.4. X-raypowderdiffraction(XRPD)
XRPDwascarriedoutinordertodeterminethecrystallineform and crystallinityoftheproducedmaterials.Samplesweremea- suredwithaBrukerD8Advancediffractometer(BrukerAXSGmbH, Karlsruhe,Germany).Datacollectionwascarriedoutatroomtem- peratureusingmonochromaticCuK˛1radiation(˛=0.154060nm) inthe2regionbetween3◦ and50◦.Formeasurementsdirectly afterelectrospraycrystallization,about10 milligramsofsample wasdirectlydepositedonazerobackgroundholder(Sisinglecrys- tal<510>wafer)andplacedintotheXRDdirectlyafterproduction.
Therecording of thepatternwas relativelyfast,it lasted180s.
Diffractionpatternswererecordedfrom15mintill20hafterpro- ductionatdifferenttimeintervals.
2.3.5. Fouriertransforminfraredspectroscopy(FT-IR)
FT-IRspectroscopywasusedtoinvestigatethechemical sta- bilityof thedrug.FT-IR spectraweremeasuredonan AVATAR 330 FT-IR apparatus (Thermo Nicolet, USA), in the interval 400–4000cm−1,at4cm−1opticalresolution.StandardKBrpellets
Fig.3.Therelationbetweenworkingdistance(D)andpotentialdifference(U).
werepreparedfrom150mgofKBrpressedwith10tonandsamples containing0.5mgofNIFwereused.
3. Resultsanddiscussion
3.1. Electrospraycrystallizationprocessparameters
Theelectrospraycrystallizationprocessparametersweredeter- minedfortheproductionofthedesiredsubmicronproduct.Three processparameterswereidentifiedtohaveamajoreffectonthe product:initialsoluteconcentration(c),potentialdifference(U) and nozzlediameter(d).Theflowrate(ϕ)couldnot beinvesti- gated,sincetheoperationwindowoftheflowratewastoolow,and changesinflowrateresultedininstablejet.Theworkingdistance (D)stronglydepends ontheappliedpotentialdifference:larger distancesdemandhigherpotentialdifference.Fig.3showstherela- tionshipbetweenworkingdistanceDandpotentialdifferenceU.
Whenhigherinitialconcentrationswereused,theaveragecrys- talsizeincreased:thesamedropletwithahigherconcentration containsmoresolutethatcancrystallize,resultinginlargercrystals (Fig.4).Thecrystalshapealsodependsontheinitialconcentra- tion.Athigher concentrations,theobtainedcrystalsweremore needle-like,whileatlowerconcentrationsthecrystalshadsome- whatsphericalshape(Fig.4).
Alowerpotentialdifferenceresultedinagglomerationofcrys- tals.Theincreaseofthenozzlediameteralsoresultsinagglomerate
Fig.4.Therelationshipbetweenthecrystalsizeandshapeandusedsolutioncon- centrationofNIFcrystals,producedbyelectrospraycrystallization.
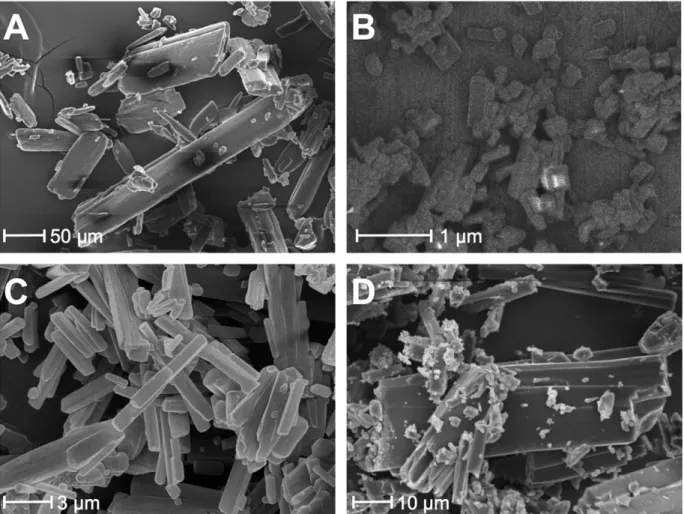
Fig.5.SEMimagesof(a)conventionalNIF,(b)NIFproducedbyelectrospraycrystallization(NIF-NANO),(c)NIFproducedbyanti-solventcrystallization(NIF-AC)and(d)NIF producedbysolventevaporation(NIF-SE).
formation[26].Alowerpotentialdifferenceorlargernozzlediam- eter results in a lower charge density at the Taylor-cone and thereforeatthedropletsurface.Atalowersurfacechargedensity Coulomb-fissionisoccurringlaterorisabsentandcrystallization mightoccurinlargerdroplets,resultinginagglomeratedcrystals.
Theoptimalprocessparametersforproducingsubmicron-sized NIFcrystals(NIF-NANO)withacompactshape withoutagglom- erationwerefoundtobe:solutionconcentrationof20mgml−1, apotentialdifferenceof+4.7kV,anozzlediameterof0.33mm,a workingdistanceof17mmandaflowrateof1.8mlh−1.Withthese parameterssomewhatprismaticshapeNIFcrystalswithamean sizeofaround500nmwereproduced.Theproductionrateunder theseconditionswas150mgh−1.
3.2. Productsizeandshape
TheconventionalNIFcrystalshadasmoothsurfacewithpris- maticshapewitharound80mmeansizeandabroadcrystalsize distribution(Fig.5A).ThisshapewasalsoseenfortheNIF-SEcrys- tals(Fig.5D).Thecrystalshape ofNIF-SEwasprismaticaswell, anditssizedistributionwasverybroadwithanestimatemean sizeofaround46m,probablyduetotheappliedhomogenization bymanualpulverization.Byusingtheanti-solventcrystallization method(NIF-AC), needle-likeNIF crystalsin thesize ofaround 10mwereproduced(Fig.5C).Thiscrystallizationmethodcom- bined withhighshear mixing isan effectiveprocedure forthe particlesizedecreasingtowardthemicronrange.Theelectrospray crystallizationprocedurecausedthemostremarkablealterations
from allthe used crystallization methods. Afterthe procedure, somewhatsphericalshapedNIFparticleswerefoundwithamean sizeof500nm(Fig.5B).Thesesubmicronparticlesareclustered intoaggregatesupto20minsize,possiblyduetothestrongcohe- siveinteractionsbetweenthehydrophobiccrystalsurfaceswiththe increasedspecificsurfacearea.
Table1presentsthemeanparticlesizeofNIFdeterminedusing aSEMimagesanalysis.Itcanbeseenthatthesolventevaporation withpulverizationofthecrystals(NIF-SE)decreased thecrystal sizetonearlyhalfthatoftheconventionalNIFsize(80m)deter- mined.Theproductproduced bytheanti-solventcrystallization process(AC)resultedincrystalsaround7m.Electrospraycrystal- lizationresultedinnano-sizedparticles,around500nm.Itseems thattheincreaseinsupersaturationintheanti-solventcrystalliza- tionprocessisnotenoughtoobtainsubmicroncrystals.Alsothe crystallizationvolumeshouldbedecreased,asinelectrospraycrys- tallizationthemainreasonofsizedecreasingisthatmicron-sized dropletsarecreatedintheprocess,andcrystallizationcommences inthesmall,confinedvolumeofferedbythedroplets.
Table1
ThecalculatedmeancrystalsizeoftheconventionalNIFandtheproducedcrystals.
Sample Meansize(m)±SD
NIF 80±22.6
NIF-NANO 0.5±0.2
NIF-AC 7.4±3.9
NIF-SE 46.2±26.0
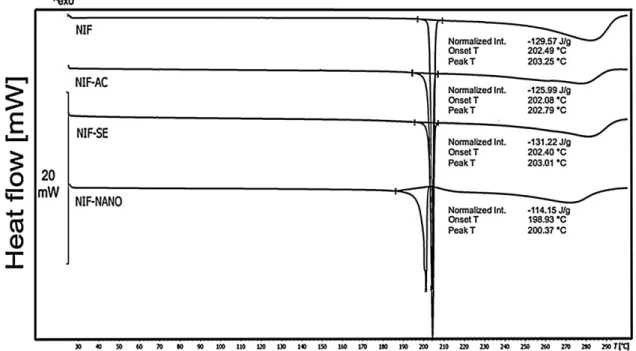
Fig.6. DSCcurveoftheconventionalNIFcomparedwiththerecrystallizedNIFobtainedfromthesolventevaporation(NIF-SE),therecrystallizedNIFobtainedfromthe antisolventcrystallization(NIF-AC)andthesubmicron-sizedNIFfromelectrospraycrystallization(NIF-NANO).
3.3. Structuralanalysis(DSC,XRPDandFT-IR)
The presence of amorphous fraction could be expected by spraying crystallization techniques,since the driving forces for crystallizationarehigh[29,30].Thepresenceofamorphousfrac- tionhasaneffectonthestabilityofthesamplesaswell.Dueto therapidevaporationofthesolventinelectrospraycrystallization, thecrystalstructure might notbuildupcompletely(kinetically formedmoreslowly)duringthecrystallizationprocess[31].Thus structural characterizations wereperformed to checkthe crys- tallinestateoftheproducedNIFcrystals.
3.3.1. Differentialscanningcalorimetry
DSCwasusedtomeasurethemeltingpoint(T)andtheheatof melting.TheonsetToftheconventionalNIFat202.49◦Creflected itsmeltingpoint(Fig.6).TheNIF-ACandNIF-SEsamplespresented similar DSCcurves with a melting point at around 202◦C. The submicron-sizedNIF (NIF-NANO)had awelldetectablemelting pointatalowertemperature(198.93◦C).Thelowermeltingpoint andbroaderpeakofthesubmicron-sizeddrugcanbeexplainedby therelativelylargeproportionofsurfacemoleculescomparedto thebulk.Thevibrationalandpositionalenthalpyandentropyof surfacemoleculesofsubmicron-sizedmaterialsaredifferentfrom thatofmoleculesinsidethebulkofthecrystals,whichcanalter physicalproperties,includingthermalproperties[32].Onewayto testthecrystallinityofthesamplesistodeterminethenormalized heatofmelting,sincethereisarelationbetweentheheatofmelting andthecrystallinefractionofthesample[28].
ThecrystallinityindexofNIFwascalculatedfromthenormal- izedheatofmelting ofthesamples(Table2).Theconventional NIF sample wasassumed to have 100% crystallinity. The sam- pleproducedbysolventevaporation(NIF-SE)showedhigherheat of meltingthan theconventional NIF,probably due tothepul- verizationimpactduringthepreparationprocedure.Thesample fromtheanti-solventcrystallizationmethod(NIF-AC)showed97%
crystallinity.Theelectrospraycrystallizationproduct(NIF-NANO) showedthelowestcrystallinityfromalltheproducedsamples,88%
ofthematerialwasmeasuredtobecrystalline,whichimpliesthat 12%ofthesampleisamorphous.Thecrystallinityindexwasalso
Table2
Thecrystallinityindex(CI)ofthesamplesaccordingtotheDSCmeasurements.
Sample Normalizedintegral(Jg−1) CI(%)
NIF 129.57±0.23 100
NIF-SE 131.22±0.45 100
NIF-AC 125.99±0.36 97
NIF-NANO(2weeksafter preparation)
114.15±0.82 88
ElectrosprayedNIF(15min aftertheprocedure)
105.32±0.39 81
calculatedforasamplethatwasmeasured15minafterthepro- ductionbytheelectrospraycrystallizationprocess.Itscrystallinity indexwas81%,7%lessthantheelectrosprayedsampleafter2weeks (NIF-NANO).ThisshowsthatapartofNIFchangedfromamorphous phasetocrystallinephaseduringthestorage.
TosummarizetheDSCresults,thereasonofmeltingdepression isapartofamorphousstateandparticlessizedecreaseofNIF.
3.3.2. X-raypowderdiffraction
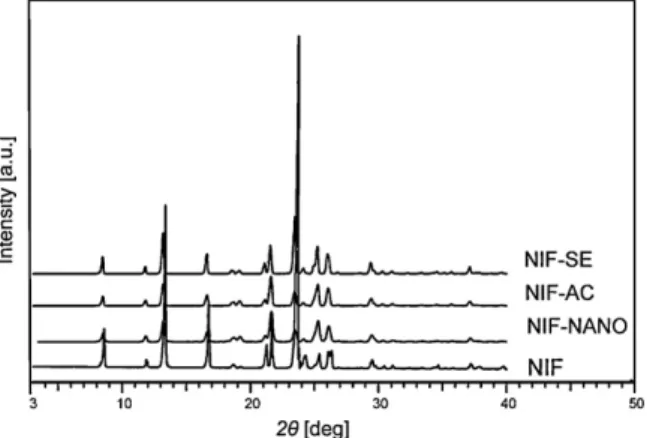
XRPDanalysiswasusedtoexaminethecrystalstructureandit wasalsousedtoassessthecrystallinityoftheproducts.Thechar- acteristicintensityvaluesoftheNIFcanbefoundat8.2,12.9,16.2, 23.2, 25.72 values.Alltheproducedsamplesshowedpeaksat thesamepositionsastheconventionalNIF,buttheirintensitywas lower(Fig.7).Whenthethreeproducedsamplesarecomparedwith eachother,itcanbeseenthattheNIF-SEsamplehasmoreintense peaks,thantheNIF-ACorNIF-NANOsamples,indicatingthatthe NIF-SEsamplehashighercrystallinitythantheothertwoproducts.
ThepeakintensityoftheNIF-ACsampleisinbetweentheNIF-SE andtheNIF-NANOsamples.Fromthethreeproducedmaterials,the NIF-NANOsamplepresentsthelowestpeakintensities,indicating thelowestcrystallinefractionfromthemeasuredproducts.Sincein generaltheevaporativecrystallizationresultsinhighlycrystalline product,itwasassumedthattheNIF-ACandNIF-NANOsamples arepartiallyamorphous.TheparticlesizedecreaseoftheNIF-AC andNIF-NANOsamplesalsocanbethereasonforthemeasured lowerintensitiesintheXRPDpatterns.
Fig.7.XRPDpatternsoftheNIFsamplesproducedbysolventevaporation(NIF-SE), anti-solventcrystallization(NIF-AC)andelectrospraycrystallization(NIF-NANO), comparedwiththeconventionalNIF(NIF).
Fig.8.XRPDpatternoftheproducedNIF-NANOsampletill20haftertheprocedure.
Toinvestigatea possibletransitionbetweenamorphous and crystallinestateoftheNIF-NANOsample,diffractionpatternsof thesamplewasrecordeddirectlyaftersprayingfor20h.Thecrys- tallinitydidnotchangeinthefirst20h(Fig.8).SincetheXRPD patternoftheNIF-NANOsample(whichmeansthenanoparticles 2weeksafterelectrospraycrystallization)showedhigherintensi- tiesthancanbeseenonthe20hdiffractionpattern,probablythe crystallinityincreasedfurtherinthefollowingtwoweeks.
3.3.3. FT-IRanalysis
IR spectroscopy was used to study the chemical stability of the NIF samples. The IR spectrum of NIF displays strong
Fig.9.FT-IRspectraofconventionalNIF,theelectroprayedNIF-NANO,NIF-ACby anti-solventcrystallizationandNIF-SEbysolventevaporationmethods.
absorptionat1607cm−1thatisduetothecharacteristicas(COO) ands(COO)stretchingmodesofcarboxylgroups.Amiddle-strong peakat3163cm–1canbeascribedtotheN–Hvibrationofimine [33,34],andthestrongabsorptionpeakat1523cm–1isowingto theframeworkvibrationofthephenylandpyridinerings.TheFT-IR spectra(Fig.9)of NIF-SEand NIF-NANOdidnot presentdiffer- encescomparedwithNIF.Newbondswerenotdevelopedandold bondsdidnotdisappear.AccordingtotheFT-IRanalysisnochem- icalchangeinthestructurewasdetected,whichmeansthatthe producedsamplesdidnotshowpolymorphictransitionorchemical decomposition.ThisimpliesthattheproducedNIF-NANOcrystals arechemicallystablebeforeandaftertheprocedure.
4. Conclusions
Thisstudyhasshownthatelectrospraycrystallizationasanon- conventionalmethodcanbeusedtoformulatesubmicron-sized NIFcrystalswithameansizeofaround500nm.Carefulselection ofpreparationparametersiscriticaltoachievethesuitablesizeand shape.Lowerinitialsolutionconcentrationsleadtocompact,some- whatsphericalcrystalshape,whilehigherconcentrationsresult in needle-like crystals.The crystallization procedurewas com- paredtotwoconventionalcrystallizationmethods,evaporativeand anti-solventcrystallization,wheremacro-andmicro-sizedcrystals wereprepared.XRPDmeasurementsoftheimmediatelyproduced NIFcrystalsshowedthatbyevaporativeandanti-solventcrystal- lizationtheproductswerehighlycrystallinerightafterproduction andalsoafter2weeks.Howevertheelectrosprayedproductwas partlyamorphousrightaftertheproduction,andonly81%ofthe samplewascrystalline.Thecrystallinityoftheelectrosprayedsam- plehasincreasedduringstorage,accordingtotheDSCresultsthe two-week-old electrosprayedNIFsampleswere88% crystalline.
AccordingtotheFT-IRmeasurementstheNIFcrystalswerechem- icallystablebeforeandaftertheprocedure.
Acknowledgement
The publication/presentation is supported by the European UnionandcofundedbytheEuropeanSocialFund.Projectnumber:
TÁMOP-4.2.2/B-10/1-2010-2012.
Projecttitle:“Broadeningtheknowledgebaseandsupporting thelongtermprofessionalsustainabilityoftheResearchUniversity CenterofExcellenceattheUniversityofSzegedbyensuringthe risinggenerationofexcellentscientists.”
References
[1]The Merck Index, 14th ed., Merck & Co. Inc., Rahway, NJ, 2012 (http://themerckindex.chemfinder.com).
[2]Martindale,TheExtraPharmacopoeia,37thed.,TheRoyalPharm.Society,Lon- don,2011,pp234-282.
[3]R.Ambrus,Z.Aigner,C.Dehelean,C.Soica,P.Szabó-Révész,Physico-chemical studiesonsoliddispersionsofniflumicacidpreparedwithPVP,Rev.Chim.58 (2007)60–64.
[4]R.Ambrus,Z.Aigner,C.Soica,C.Peev,P.Szabó-Révész,Amorphisationofnif- lumicacidwithpolyvinylpyrrolidonepreparedsoliddispersiontoreachrapid drugrelease,Rev.Chim.58(2007)206–209.
[5]M.Kata,R.Ambrus,Z.Aigner,Preparationandinvestigationofinclusioncom- plexescontainingniflumicacidandcyclodextrins,J.Inc.Phenom.44(2002) 123–126.
[6]T.Szunyogh,R.Ambrus,P.Szabó-Révész,Importanceofparticlesizedecrease inthepreformulation,ActaPharm.Hung.81(2011)29–36.
[7]J.Kanzer,S.Hupfeld,T.Vasskog,I.Tho,P.Hölig,M.Mägerlein,G.Fricker,M.
Brandl,Insituformationofnanoparticlesupondispersionofmeltextrudate formulationsinaqueousmediumassessedbyasymmetricalflowfield-flow fractionation,J.Pharm.Biomed.Anal.53(2010)359–365.
[8]J.Zhang,H.Lv,K.Jiang,Y.Gao,Enhancedbioavailabilityafteroralandpul- monaryadministrationofbaicaleinnanocrystal,Int.J.Pharm. 420(2011) 180–188.
[9] T.W. Prow, J.E. Grice, L.L. Lin, R. Faye, M. Butler, W. Becker, E.M.T.
Wurm,C. Yoong,T.A. Robertson, H.P.Soyer, M.S. Roberts, Nanoparticles
andmicroparticlesforskindrugdelivery,Adv.DrugDeliv.Rev.63(2011) 470–491.
[10]H. Chen, X. Kou, Z. Yang, W. Ni, J. Wang, Shape- and size-dependent refractive index sensitivity of gold nanoparticles, Langmuir 24 (2008) 5233–5237.
[11]D.Missirlis,N.Tirelli,J.A.Hubbel,Amphiphilichydrogelnanoparticlesprepa- rationcharacterizationandpreliminaryassessment asnewcolloidaldrug carriers,Langmuir21(2005)2605–2613.
[12]J.-W.Kim,M.-S.Shin,J.-K.Kim,H.-S.Kim,K.-K.Koo,Evaporationcrystallization ofRDXbyultrasonicspray,Ind.Eng.Chem.Res.50(2011)12186–12193.
[13]R.Ambrus,P.Kocbek,J.Kristl,R.Sibanc,R.Rajkó,P.Szabó-Révész,Investigation ofpreparationparameterstoimprovethedissolutionrateofpoorlysoluble meloxicam,Int.J.Pharm.318(2)(2009)153–159.
[14] V.A.Saharan,V.Kukkar,M.Kataria,M.Gera,P.M.Choudhury,Dissolution enhancementofdrugs.PartI:technologiesandeffectofcarriers,Int.J.Health Res.2(2009)107–124.
[15]G.G.Liversidge,P.Conzentino,Drugparticlesizereductionfordecreasinggas- tricirritancyandenhancingabsorptionofnaproxeninrats,Int.J.Pharm.125 (1995)309–313.
[16] P.Liu,X.Rong,J.Laru,B.vonVeen,J.Kiesvaara,J.Hiruonen,T.Laaksonen,L.Pel- tonen,Nanosuspensionsofpoorlysolubledrugs:preparationanddevelopment bywetmilling,Int.J.Pharm.411(2011)215–222.
[17]R.H.Müller,A.Akkar,Drugnanocrystalsofpoorlysolubledrugs,Encyclop.
Nanosci.Nanotechnol.2(2004)627–638.
[18]P.Kocbek,S.Baumgartner,J.Kristl,Preparationandevaluationofnanosuspen- sionforenhancingthedissolutionofpoorlysolubledrugs,Int.J.Pharm.312 (2006)179–186.
[19]T.Yasuji,H.Takeuchi,Y.Kawashima,Particledesignofpoorlywater-soluble substancesusingsupercriticalfluidtechnologies,Adv.Drug.Dev.Rev.60(2008) 388–398.
[20]N.Radacsi,A.I.Stankiewicz,Y.L.M.Creyghton,A.E.D.M.vanderHeijden,J.H.ter Horst,Electrospraycrystallizationforhigh-qualitysubmicron-sizedcrystals, Chem.Eng.Technol.34(2011)624–630.
[21]C.U.Yurteri,R.P.A.Hartman,J.C.M. Marijnissen,Producingpharmaceutical particlesviaelectrosprayingwithanemphasisonnanoandnanostructure particles– areview,KONAPowderParticleJ.28(2010)91–115.
[22]A.Jaworek,Micro-andnanoparticleproductionbyelectrospraying,Powder Technol.176(2007)18–35.
[23]B.Almería,W.Deng,T.M.Fahmy,A.Gomez,Controllingthemorphologyof electrospray-generatedPLGAmicroparticlesfordrugdelivery,J.ColloidInterf.
Sci.343(2010)125–133.
[24]L.Rayleigh,Comparisonofmethodsforthedeterminationofresistancesin absolutemeasure,Phil.Mag.14(1882)184–186.
[25] E.Revalor,Z.Hammadi,J.P.Astier,R.Grossier,E.Garcia,C.Hoff,K.Furuta,T.
Okutsu,R.Morin,S.Veesler,Usualandunusualcrystallizationfromsolution,J.
Cryst.Growth312(2010)939–946.
[26]N.Radacsi,R.Ambrus,T.Szunyogh,P.Szabó-Révész,A.I.Stankiewicz,A.E.D.M.
vanderHeijden,J.H.terHorst,Electrospraycrystallizationfornano-sized pharmaceuticalswith improved properties,Cryst. GrowthDes.12(2012) 3514–3520.
[27]M.D.Abramoff,P.J.Magelhaes,S.J.Ram,Imageprocessingwithimage,J.Bio- photon.Int.11(2004)36–42.
[28]M.Wagner,Thermalanalysisinpractice,in:DSCevaluations,MettlerToledo, Schwerzenbach,2009,pp.90–141.
[29]M.J.Arias-Blanco,J.R.Moyano,J.I.Perez-Martinez,J.M.Gines,Studyoftheinclu- sionofgliclazidein␣-cyclodextrin,J.Pharm.Biomed.Anal.18(1998)275–279.
[30] H.A.Crisp,J.C.Clayton,L.G.Elliott,E.M.Wilson,Preparationofahighlypure substantiallyamorphousformofcefuroximeaxetil,U.S.Patent4,820,1833, (1989).
[31]S.Nakayamaa,T.Watanabec,M.Sennaa,Rapidamorphizationofmolecular crystalsbyabsorptionofsolventmoleculesinthepresenceofhydrophilic matrices,J.Alloy.Compd.483(2009)217–221.
[32]G.C.Yang,F.D.Nie,J.S.Li,Preparationandcharacterizationofnano-NTOexplo- sive,J.Energ.Mater.25(2007)35–47.
[33]K.Takács-Novák,A.Avdeef,K.J.Box,B.Podányi,GySzász,Determinationof protonationmacro-andmicroconstantsandoctanol/waterpartitioncoeffi- cientofantiinflammatorydrugniflumicacid,J.Pharm.Biomed.Anal.12(1994) 1369–1377.
[34]H.M.K. Murthy, M. Vijayan, 2[[3-(Trifluoromethyl)phenyl]amino]-3- pyridinecarboxylic acid (niflumic acid), Acta Crystallogr. 35 (1979) 262–263.