PROCEEDINGS OF THE
25 th International Symposium
on Analytical and Environmental Problems
Szeged, Hungary October 7-8, 2019
University of Szeged
25th International Symposium on Analytical and Environmental Problems
Edited by:
Tünde Alapi István Ilisz
Publisher:
University of Szeged, H-6720 Szeged, Dugonics tér 13, Hungary
ISBN 978-963-306-702-4
2019.
Szeged, Hungary
25th International Symposium on Analytical and Environmental Problems
The 25
thInternational Symposium on Analytical and Environmental Problems
Organized by:
SZAB Kémiai Szakbizottság Analitikai és Környezetvédelmi Munkabizottsága
Supporting Organizations
Institute of Pharmaceutical Analysis, University of Szeged
Department of Inorganic and Analytical Chemistry, University of Szeged Institute of Environmental Science and Technology, University of Szeged
Hungarian Academy of Sciences
Symposium Chairman:
István Ilisz, DSc
Honorary Chairman:
Zoltán Galbács, PhD
Organizing Committee:
István Ilisz, DSc associate professor
University of Szeged, Institute of Pharmaceutical Analysis ilisz@pharm.u-szeged.hu
Tünde Alapi, PhD assistant professor
University of Szeged, Department of Inorganic and Analytical Chemistry
alapi@chem.u-szeged.hu
25th International Symposium on Analytical and Environmental Problems
Lecture Proceedings
25th International Symposium on Analytical and Environmental Problems
THE STUDY OF PHOTOCATALYTIC DEGRADATION OF ANIONIC DYES BY 1D COORDINATION POLYMERS BASED ON CADMIUM(II)
Ildiko Mariana Bută, Maria Andreea Nıstor, Simona Gabriela Muntean, Otilia Costişor
„Coriolan Drăgulescu” Institute of Chemistry Romania, 24 Mihai Viteazu, Timisoara e-mail: ildiko_buta@acad-icht.tm.edu.ro
Abstract
Organic dyes resulted from industries such as textile, food, printing, and pharmaceuticals are the main contamination in wastewater. [1] In the last years, several physical, biological and chemical methods have been investigated for the treatment of industrial colored wastewaters. Among them, photocatalysis proved to be a useful method due to the degradation of colored pollutants into smaller, non-toxic compounds. [2] Coordination polymers may exhibit photocatalytic properties under UV or visible light irradiation due to their nature and structure properties [3].
Three new cadmium(II) coordination polymers [Cd3L(CH3COO)4]·H2O (CP-1), [Cd5L2(CH3COO)6] (CP-2) and [Cd2L(NO3)2]·CHCl3 (CP-3), where H2L stands for N,N’- bis[(2-hydroxybenzilideneamino)-propyl]-piperazine were synthetized by direct metal-ligand reaction of the respective ligand with the corresponding cadmium(II) salts. The nature of the compounds was established based on elemental analysis, and structural information were obtained by IR and UV–Vis spectroscopy and single-crystal X-ray diffraction.
The photocatalytic activity of the CPs 1 - 3 was investigated on the degradation of two anionic: Congo Red (CR), and Methyl Orange (MO). Photocatalytic studies were carried out, at room temperature, in a visible chamber with 500 W Hg lamp providing 546 nm irradiation, and the dye concentrations were determined by a UV/VIS spectrophotometry.
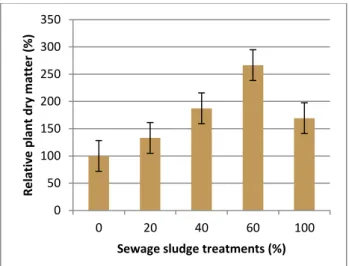
Figure 1. Photodegradation efficiency of CPs for colored pollutants, under visible irradiation (50 mg/L dye, 1 g/L CP, 25oC, solution pH)
For all investigated dyes the photocatalytic degradation efficiency increased in order: CP-1<
CP-2< CP-3. The CPs were capable of photodegradation of anionic dyes, as evidenced by their decomposition efficiency up to: 88% for CR and 83% for MO.
Acknowledgements
This work was supported by Project 2.4 and Project 4.1 of the “Coriolan Drăgulescu” Institute of Chemistry.
References
[1] M. Li, X. Wang, C.J. Porter, W. Cheng, X. Zhang, L. Wang, M. Elimelech, Environ. Sci.
Technol. 53 (2019) 3078.
[2] A. Kumar, G. Pandey, Mater. Sci. Eng. C 1 (2017) 18.
[3] Gaur, R. Inorg. Chem. Front. 6 (2019) 278.
CR MO
0 20 40 60 80 100
Photocatalytic degradation efficiency (%)
Pollutant
CP-1 CP-2 CP-3
25th International Symposium on Analytical and Environmental Problems
CARBONATION OF SOME CONCRETE MIXTURES USING RECYCLED CONCRETE AGGREGATES
Remus Chendes 1, Corneliu Bob 1, Liana Iures1, Sorin Dan1, Catalin Badea1 , Dan-Cristian Tănasie*2
1 Politehnica University Timisoara, Faculty of Construction, 2nd Traian Lalescu, 300223, Timisoara, Romania
2 National Institute of Research & Development for Electrochemistry and Condensed Matter, 144th Dr. Aurel Paunescu Podeanu, 300569, Timisoara, Romania
e-mail: remus.chendes@upt.com , *tase@incemc.ro
Abstract
Typical C16/20 concrete class has been studied in an accelerated carbonation experiments carried out under high CO2 concentration. The 100x100x100 mm cube specimens, prepared with natural aggregates and recycled aggregates, were stored for 28 days in water and have been tested (physical-mechanical characteristics). After 60 days of accelerated carbonation conditions test, in a protected environment - 50% carbon dioxide concentration, temperature 25 ° C and humidity 75-80%, the specimens were cleaved to determine the carbonation depth by phenolphthalein test on the faces in the splitting zone, measuring the minimum and maximum carbon dioxide penetration values. Correlation was made between the compressive strengths obtained for the studied specimens and the carbonation depth after the accelerated carbonation experiments in the protected environment.
Introduction
The construction industry plays an important role in the social and economic development. According to Eurostat, in July 2019, the construction sector in Romania registered an increase of 39.5%, being the first in the European Union. Only Hungary is approaching, with an increase of almost 33%, while in Bulgaria or Poland the increases are only a few percent and the average increase of the European Union is only 1.7%. [1]
But, the construction sector has a negative impact on the environment, by large CO2 emissions (contributing to global warming), by large amount of construction and demolition processes wastes generated (uncontrolled disposal), also leading to the depletion of natural resources by over-exploiting them. The only ways to reduce this negative impact of this sector is reuse, recycling and waste reduction [2].
An option, for the concrete resulted from the demolition process is to be used as aggregates into a new concrete, instead of using as coarse aggregate and filler in road construction industry. [3].
By recycling the materials are changed into new products, preventing this way, the waste of potentially useful materials, reducing the consumption of fresh raw materials, the energy usage and the air and water pollution. [4].
Results and discussions
The study on the durability of recycled concrete was carried out in accelerated carbonation experiments, continuing the previous work on topic of RCA influence on carbonation depth, due to higher porosity (20-30%) of the concretes than those cast with natural aggregates in the mix. ([7],[8]).
25th International Symposium on Analytical and Environmental Problems
The usual concrete class C16 / 20 cast with natural aggregate in the mixture, replaced by the recycled aggregate concrete (different granulometric fractions), obtained after an industrial building demolition has been studied into the experimental program (Table 1.) [8].
Table 1. Experimental mixture
Concrete Class
Mixture
Water/cement ratio CEM I 42,5R
[kg/m3]
Mixture water [kg/m3]
Admixture [kg/m3]
Aggregate [kg/m3]
C16/20 292 205 1,46 1694 0,7
The concrete mixtures belong to the class of consistency S3, the maximum size of the aggregate of 16 mm and a super-plasticizing additive was used.
It was proposed to replace 100% of the natural aggregate (NAT) with RCA (R1). The other mixtures R2, R3, R4 and R5 replace only certain granulometric fractions in the natural aggregate with the RCA, keeping the others granulometric fractions unchanged (Table 2).
Table 2. Granulometric fractions
(100% NAT) (100% RCA) (NAT + RCA)
M1 R1 R2 R3 R4 R5
NAT: 0,0-16,0 mm - 0,0-0,5 mm 1,0-16,0 mm
0,0-1,0 mm 2,0-16,0 mm
0,0-4,0 mm 8,0-16,0 mm
0,0- 8,0 mm
RCA: - 0,0-16,0 mm 0,5-1,0 mm 1,0-2,0 mm 4,0-8,0 mm 8,0-16,0 mm
As the previous experimental work, the 100x100x100mm cube specimens were stored for 28 days in water, tested (physical-mechanical characteristics) and subjected to accelerated carbonation experiments (fig.1) in a protected environment - carbon dioxide concentration (50%), temperature (25 °C) and relative humidity (75-80%) (figure 3).
The three parameters - carbon dioxide concentration, relative humidity and temperature - were measured with the Testo 350XL analyzer (Fig. 2). [8]
Figure 1.Experimental setup Figure 2. Testo 350XL analyzer
25th International Symposium on Analytical and Environmental Problems
Figure 3.Temperature and relative humidity evolution
After being stored for 60 days under accelerated carbonation conditions, the specimens were cleaved to determine the carbonation depth after phenolphthalein test of the faces in the splitting zone, drawing the carbonated surface and measuring the minimum and maximum carbon dioxide penetration values (figure 4 and table 3).
Figure 4. Phenolphthalein test - Carbonated surface [8]
In the calculation of the average depth of theoretical carbonation, the developed C.Bob formula presented below [6] was used:
- average carbonation depth [mm]
fc - concrete compression strength [MPa]
t - time [years]
c,k,d - coeficients
0 10 20 30 40 50 60 70 80 90 100
0 17,5 83 89,5 107 114 131 138 186 251 258 275 282 299 306 323 330 347 354 419 426 443 450 467 474 498 515 522 594 611 618 635 642 659 666 683 690 755 762 779 786 803 810 851 858 923 930 947 954 971 978 995 1002 1019 1026 1091 1098 1115 1122 1139 1163 1169 1187 1194 1259 1283 1289 1427
Timp [ ore ]
Temperatura [ grd ]
0 10 20 30 40 50 60 70 80 90 100
Umiditatea [ % ]
Temperatura Umiditatea
25th International Symposium on Analytical and Environmental Problems
Table 3. Experimental results
Samples
Compression strength fc [Mpa]
c k d
Time [years]
x theoretic [mm]
x-min exp.
[mm]
x-max exp.
[mm]
M1 (100% natural) 29 1 0,7 2,7 0,164 3,96 4,0 11,0
R1 (100% RCA) 32,0 1 0,7 2,7 0,164 3,59 4,0 13,0
R2 (natural+RCA) 23,0 1 0,7 2,7 0,164 4,60 8,0 13,0 R3 (natural+RCA) 25,0 1 0,7 2,7 0,164 4,26 12,5 16,0 R4 (natural+RCA) 26,7 1 0,7 2,7 0,164 4,15 11,0 16,0 R5 (natural+RCA) 27,7 1 0,7 2,7 0,164 4,12 9,3 17,2
Figure 5. Carbonation depth vs. compression strength evolution Conclusions
The additive added additionally in the case of studied mixtures, positively influences the carbonation depth.
As the grain size fraction replaced by RCA is larger, the compression strength is higher, but also the carbonation depth is greater.
Due to a quality control, difficult to manage, the results obtained for compression strength correlated with the carbonation depth, exhibit a different behavior from the results obtained in other studied cases.
References
[1] https://stirileprotv.ro/stiri/actualitate/constructiile-din-romania-cea-mai-mare-crestere-din- ue-ce-spun-economistii.html
[2] Evaluations of existing waste recycling methods: A Hong Kong study. Vivian W.Y. Tam, C.M. Tam. s.l. : Building and Environment, 2006, Vol. 41.
[3] C.Badea, The Time Behaviour of Self Compacting Concrete With Fly Ash, 17th International Multidisciplinary Scientific GeoConference SGEM 2017, Energy and Clean
25th International Symposium on Analytical and Environmental Problems
Technologies, Issue 43, 27-29 November, pp. 259-265, Vienna, Austria, 2017
[4] M. F. Prada, S. Brata, D. F. Tudor, D. E. Popescu, Energy Saving in Europe and in the World – a Desideratum at the Beginning of the Millenium Case Study for Existing Buildings in Romania, Proceedings of the 11th WSEAS International Conference on Sustainability in Science Engineering, p.246 – 251, ISBN: 978-960-474-080-2, Timisoara, Romania, 2009.
[5] F.T. Olorusongo, Early Age Properties of Recycled Aggregate Concrete., Proceeding of the International Seminar on Exploiting Wastes in Concrete, University of Dundee, 1999.
[6] Bob, C., Verificarea calității siguranței și durabilității construcțiilor, s.l.Facla, 1989.
[7] Chendes R., Iures L., Popa R., Bob C., Dan S., Tănasie C. – Comparison between recycled concrete aggregates and natural aggregates density and water absorption, The national technical-scientific conference (international participation) „Modern technologies for the 3rd millennium” - the 17th edition, Oradea, Romania, 2018
[8] Chendes R., Iures L., Bob C., Dan S., Badea C., Tănasie C. - Experimental determination of recycled aggregates concrete carbonation, 24th International Symposium on Analytical and Environmental Problems, Szeged, Hungary, 2018
25th International Symposium on Analytical and Environmental Problems
SELF-ASSEMBLY IN WATER OF BULKY OCTAHEDRAL COORDINATION COMPLEXES BASED ON RH(III) METAL CENTER
Carmen Cretu1, Maria A. Sprirache1, Adél Len2,3, Zoltán Dudás4, Alessandra Crispini 5, Elisabeta I. Szerb1
1“Coriolan Dragulescu” Institute of Chemistry, Romanian Academy, 24 Mihai Viteazu Bvd.
300223 – Timisoara, Romania, e-mail: cretucami@yahoo.com
2 Nuclear Analysis and Radiography Department/Centre for Energy Research, Konkoly-Thege 29-33, 1121 Budapest, Hungary
3Faculty of Engineering and Information Technology/University of Pécs, Boszorkány út 2, 7624 Pécs, Hungary
4Neutron Spectroscopy Department/Wigner Research Centre for Physics, Konkoly-Thege 29- 33, 1121 Budapest, Hungary
5Università della Calabria, Dipartimento di Chimica e Tecnologie Chimiche, 87030 Arcavacata di Rende, Italy
Abstract
Metal complexes able to self-assembly in water into chromonic-type liquid-crystalline phases are progressively attracting attention because of their unique features consisting in the combination of properties deriving from liquid crystals (self-ordering, ease of alignment, sensitivity to changing conditions and additives) coupled with the optical and electro-optical properties brought by the metal centre. [1,2]
In the last decade, rhodium (III) cyclometallated complexes were researched for their electrocatalytic activity, fluorescent properties and especially for medicinal applications as drug delivery and therapeutic agents. [3(a-d)] Therefore, supramolecular organized aqueous systems built up with these compounds should bring important developments in bio-related fields. Herein we report new Rh(III) cyclometallated complexes that show the ability to self- assemble into chromonic-like phases at relatively low concentration in water. Their supramolecular arrangements in mesophase will be proposed based on optical polarization microscopy (POM), X-ray diffraction studies on single crystals and Small Angle Neutron Scattering (SANS) measurements in the mesophase. The role of different molecular moieties will be discussed in the self-assembling with respect to previous reported structurally analogues Ir(III) complexes [1,2].
Acknowledgements: The Romanian authors acknowledge the support of the “Coriolan Dragulescu” Institute of Chemistry, Project 4.1.
References
[1] Y.J. Yadav, B. Heinrich, G. De Luca, A.M. Talarico, T.F. Mastropietro, M. Ghedini, B.
Donnio, E.I. Szerb, Adv. Optical Mater. 1 (2013) 844.
[2] L. Ricciardi, T.F. Mastropietro, M. Ghedini, M. La Deda, E.I. Szerb, J. Organomet. Chem.
772-773 (2014) 307.
[3] a) J. Kima, E. Rajkumara, S. Kima, Y.M. Parka, Y. Kima, S.-J. Kima, H.J. Leeb, Catal.
Today 295 (2017) 75; b) A. Nano, A.N. Boynton, J.K. Barton, J. Am. Chem. Soc. 139(48) (2017) 17301; c) J. Markham, J. Liang, A. Levina, R. Mak, B. Johannessen, P. Kappen, C.J.
Glover, B. Lai, S. Vogt, P.A. Lay, Eur. J. Inorg. Chem. 12 (2017) 1812; d) C.-H. Leung, H.-J.
Zhong, D.S.-H. Chan, M. Dik-Lung, Coord. Chem. Rev. 257 (2013) 1764.
25th International Symposium on Analytical and Environmental Problems
LIQUID CRYSTAL PHASES BASED ON FLUORENONE CORE
Daniela Haidua, Ya-Xin Lib, Xiang-Bing Zengb, Goran Ungara,c and Liliana Cseha
a”Coriolan Dragulescu” Institute of Chemistry, 300223-Timisoara, Romania lilianacseh@gmail.com
b Department of Materials Science and Engineering, University of Sheffield, Sheffield S1 3JD, U.K.
c School of Materials, Xi’an Jiaotong University, Xi’an 710049, P.R. China
Dynamic chirality synchronization of supramolecular aggregates in liquids and liquid crystals is a new mode of mirror symmetry breaking, providing chiral fluids of non-chiral compounds with long-term stability even at high temperatures [1]. Formation of networks and, particularly, network junctions seems to be the key to the long-range propagation of homochirality [2, 3]. Depending on the lattice symmetry the cubic phases of rod-like and polycatenar molecules are achiral (double gyroid phase - Ia3d) or chiral triple network - Im3m); therefore, the molecular design play an important role for the achievements of new supramolecular structures with specific required properties. Here, we present the design, synthesis and characterization of first examples of fluorenone derivatives displaying bicontinuous cubic phases. The synthetized compounds show columnar, double gyroid and the triple network phase. The Ia3d seems to be preferred at higher, and Im3m at lower temperatures.
These fluorescent compounds also have the potential to be used as electro- or photoluminescent materials in devices that may emit circularly polarized light.
Acknowledgements: This work was supported by a grant of Ministry of Research and Innovation, CNCS-UEFISCDI, project number PN-III-P4-ID-PCE-20160720, within PNCDI III.
[1] Tschierske C., Ungar G., Chem. Phys. Chem., 17 (2016), 9-26.
[2] Dressel C., Liu F., Prehm M., Zeng X.B., Ungar G., Tschierske C., Angew. Chem. Int.
Ed., 53 (2014), 13115-20.
[3] Lu H., Zeng X.B., Ungar G., Dressel C., Tschierske C., Angew. Chem. Int. Ed., 57 (2018), 2835-2840.
25th International Symposium on Analytical and Environmental Problems
DEVELOPMENT OF BAKERY PRODUCT WITH SEA BUCKTHORN POMACE (HIPPOPHAE RHAMNOIDES L.)
Zsuzsanna Kiss1, Beatrix Szabó-Nótin1, Rentsendavaa Chaagnadorj1, Mónika Stéger- Máté1, Éva Stefanovits-Bányai2, Diána Furulyás1
1Department of Food Preservation, Szent István University, H-1118 Budapest, Villányi Street 29-43, Hungary
2Department of Applied Chemistry, Szent István University, H-1118 Budapest, Villányi Street 29-43, Hungary
e-mail: furulyas.diana@etk.szie.hu
Abstract
In our modern world, avoid wasting or keep it at low level is important for the food industry too. After the fruits pressing stay a lot of pomace, which is rich in vitamins, antioxidant, and polyphenols. It has a few further usages, like pectin commodity or soil conditioners. After drying and grinding, it becomes seed meal.
In our experiment we try out the buckthorn (Hippophae rhamnoides L.) pomace further usage, in our case, we mixed with flour and baked cookie. We tested the level of polyphenol and antioxidants in the cookies. Furthermore, the whole polyphenol capacity (TPC), ferric reducing antioxidant power assay (FRAP) and the texture of biscuits have been tested.
The results are favorable in case of TPC and FRAP. The buckthorn pomace has a positive impact regard to substance.
Introduction
There more and more proof confirm the theory of the oxidative progress by radicals are contribute to arteriosclerosis, additionally, it was reported that antioxidant nutrients influence cell response and gene expression, which gives a new perspective to the mechanism of biological antioxidant activity [1-3]. Due to the modern way of life the consumption of the right quantity and quality antioxidant are essential to mitigate the harmful impact of radicals.
In our busy world, there is increasing demand to fast food. As a result, the food industry tends to develop and produce such foods, which are good choice for health-conscious customer [4- 6].
The sea buckthorn (Hippophae rhamnoides L.) is a rich source of antioxidant, polyunsaturated fatty acids, vitamins and minerals [7-10]. It is consumed in many forms in the food industry such as raw, syrup, canned, soda, jam and vitamin-rich processed concentrate.
After squeezing the crop of sea buckthorn, the 75-85% of the weight of the berry is juice, other remaining after the press, like seed and peel in the most cases are used for forage or soil conditioner. However only a little percentage of total pomace mass is used in the food industry [11-14]. The sea buckthorn pomace in form of grist can be suitable for the mix with flour. While harvest the favorable physiological effect, it can be made a pleasant taste product. In today’s world, the usage of the higher and higher rate of material became very important, while mitigating the weight of waste.
For this reason, the aim of our research is to successfully use the byproduct of buckthorn in the biscuits in order to make antioxidant-rich product.
Experimental
The „Ascola” sea buckthorn was collected from agricultural plots of Hungary in 2018.
Chemicals were purchased from Sigma-Aldrich Chemie Ltd. All reagents used were of analytical grade.
25th International Symposium on Analytical and Environmental Problems
Sea buckthorn was destemmed and then heated to 80°C, to inactivate enzymes. The material was squeezed, resulting in juice and pomace. Drying the pomace was the next step by an atmospheric dryer (LMIM, Hungary) at 80°C [15] until moisture content became lesser than 10%. After this step, the pomace was grinded.
In this experiment, three recipes were made with increasing sea buckthorn content (Fig. 1.)
sugar (g) coconut oil (g) flour BL 55 (g) sea buckthorn (g) baking powder (g) water (ml)
control 100 100 250 0 12 100
2.5% pomace 100 100 243.75 6,25 12 100
5% pomace 100 100 237.5 12.5 12 100
The biscuits were baked for the same time (10 min, 190℃).
Samples were stored at room temperature for 2 weeks in a sealed package, during which the following were examined at 3 sampling times:
Water content was determined by drying until constant weight at 121 °C using a MAC-50 moisture analyzer (Radwag Waagen GMBH, Hilden, Germany).
Various spectrophotometric measurements were carried out. All spectrophotometry measurements were performed in triplicate:
o TPC: Total Polyphenol Content was evaluated using a method by Singleton and Rossi [16]. The absorbance was measured at 765 nm. Results were specified in mg Gallic acid equivalent/ 100 g biscuit (mg GAE/100g).
o FRAP: The ferric reducing antioxidant power method of the samples was determined by Benzie and Strain [17]. The reduction is followed by the measurement of absorption change at 593 nm. FRAP value was defined in ascorbic acid equivalent (mg Ascorbic acid equivalent/ 100g biscuit; mg AA/100g).
Texture was investigated by Brookfield, LFRA 4500 Texture Analyser. In the course of texture examination, hardness, adhesion, and elasticity were measured, because in the evaluation of the quality of biscuits these parameters are important aspects.
Results and discussion
Water content results during the two weeks storage period show on the Figure 1.
Figure 1.Water content of the biscuits during the storage period
At the beginning the control sample showed the highest level of water content, the 2.5 % pomace had lower and the 5% pomace had the lowest level of water content. After the one-
0 2 4 6 8
1 2 3
Water content, m/m %
Storage peroid, week at 25°C
control 2.5 % pomace 5 % pomace
25th International Symposium on Analytical and Environmental Problems
water content of the 5% pomace showed a decrease and water content of the other two samples grew a little bit.
Figure 2. Results of total polyphenol content from biscuits during the storage period
The figure 2 shows the changes of total polyphenol content of biscuits during the storage period. At the first measurement, the 2.5% pomace sample had the lowest level of polyphenol out of the three sample (21.90 mg GAE/100g). In the case of control sample had a little higher value (23.70 mg GAE/100g), the 5% pomace sample had even higher value (26.75 mg GAE /100g). After one week the control sample had the lowest polyphenol content, the 5% pomace sample had a little higher value, while the 5% pomace sample had the highest measured value.
At the next week, the measurement the ratio of the samples remained the same as before, but the values were raised a little bit.
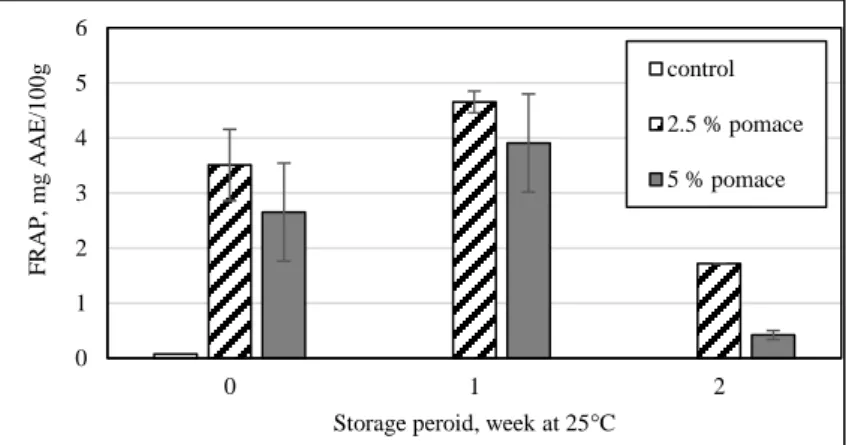
Figure 3. Results of antioxidant capacity (FRAP) from biscuits during the storage period
On the figure 3. the results of antioxidant capacity can see. The control sample barely contained antioxidants and those were completely decomposed during the weeks of storage.
The initial sample, which contained 2.5% pomace, had the highest measured value (3,512mg AAE/100g), the 5% sample had less FRAP value (2,654mg AAE/100g). After one week storage, the antioxidant level was raised in both of the samples meanwhile there ratio stayed the same.
At the second week measurement, we experienced decreasing level in the 2.5% sample as well as in the 5% sample. Measured value of the 2.5% pomace sample was (1.720mg AAE/100g), while the 5% pomace had very low level FRAP (0.423mg AAE/100g).
0 5 10 15 20 25 30
0 1 2
TPC, mg GAE/100g
Storage peroid, week at 25°C
control 2.5 % pomace 5 % pomace
0 1 2 3 4 5 6
0 1 2
FRAP, mg AAE/100g
Storage peroid, week at 25°C
control 2.5 % pomace 5 % pomace
25th International Symposium on Analytical and Environmental Problems
Figure 4. Hardness values of samples during the storage period
The results of texture examination are on the Figure 4.The hardness of the control sample had grown more during the storage, than the other two samples hardness. After one-week the value of hardness had double and after that, it continued to rise. In the beginning, we measured higher value in the case of 2.5% sample, unlike the control sample. A little growing could be observed after the first and second week. We measured double value in the 5%
sample than in the beginning status of the control, the 2.5% sample had a little higher value as well. As the time moved forward, the hardness had grown a little bit of this sample.
Figure 5. Adhesiveness values of sample during the storage period
The adhesion had decreased as the time has passed in the control sample. In the 2.5% pomace sample this value doubled after the first week and after the second week, it has started to decrease.
In the 2.5% pomace sample first got higher the adhesion level and after the second week, it has started to decrease. The adhesion level of 5% sample had moved just a little and mostly decreased a little.
Conclusion
One possible usage of the buckthorn pomace is to mix in products of the bakery.
Whit that the antioxidant content is enriching and this antioxidant level can be preserved at a high level during one-week store in 25℃ as we observed in the research.
The polyphenol concentration of the cookies was almost the same after two weeks at 25℃.
25th International Symposium on Analytical and Environmental Problems
For those who follow health-conscious nutrition, the favorable physiological effect from the buckthorn is available by the cookies. It easily could become an enjoyable but healthy everyday snack for families with children.
Acknowledgements
The authors thank Gábor Juhász for help and support. This work was supported by the Hungarian Government through project No. EFOP-3.6.3-VEKOP-16-2017-00005.
References
[1] C. Eccleston, Y. Baoru, R. Tahvonen, H. Kallio, G.H. Rimbach, A.M. Minihane, The Journal of nutritional biochemistry, 13(6) (2002) 346-354.
[2] M.G. Hertog, D. Kromhout, C. Aravanis, H. Blackburn, R. Buzina, F. Fidanza, M.
Pekkarinen, Archives of internal medicine, 155(4) (1995) 381-386.
[3] R. Puupponen‐Pimiä, L. Nohynek, C. Meier, M. Kähkönen, M. Heinonen, A. Hopia, K.M.
Oksman‐Caldentey, Journal of applied microbiology, 90(4) (2001) 494-507.
[4] M. Sayegh, C. Miglio, S. Ray, Intl. J. Food Sci. Nutr. 65 (2014) 521–528.
[5] A.A.M. Botterweck, H. Verhagen, R.A. Goldbohm, J. Kleinjans, P.A. Van den Brandt Food and Chemical Toxicology, 38(7) (2000) 599-605
[6] Haminiuk, C.W.I., Maciel, G.M., Plata-Oviedo, M.S.V., Peralta, R.M. 2012. Phenolic compounds in fruits –an overview. Int. J. Food Science and Technology, 47:2023–2044.
[7] J. Bernáth (Eds.) Gyógy és aromanövények. Budapestp, Mezőgazda Kiadó, 2000, pp. 350- 354.
[8] X.Y. Xu, B.J. Xie, S.Y. Pan, L. Liu, Y.D. Wang, C.D. Chen, Asia Pac. J. Clin. 16, (2007) 234–238.
[9] N.K. Upadhyay, R. Kumar, S.K. Mandotra, R.N. Meena, M.S. Siddiqui, R.C. Sawhney, A.
Gupta, Food Chem. Toxicol. 47 (2009) 1146–1153.
[10] G. Ficzek, G. Matravolgyi, D. Furulyas, C. Rentsendavaa, I. Jocsak, D. Papp, M. Steger- Mate, European Journal of Horticultural Science, 84(1) (2019) 31-38.
[11] T. Beveridge, J.E. Harrison, J. Drover, J. Agric. Food Chem, 50 (2002) 113-116
[12] T. Beveridge, T.S.C. Li, B.D. Oomah, A. Smith, J. Agric. Food Chem., 47 (1999) 3480- 3488
[13] J. Krisch, L. Galgóczy, T. Papp, Cs. Vágvölgyi, Journal of engineering. (2009) 1584 – 2665
[14] A. Mirzaei-Aghsaghali, N. Maheri-Sis, World J. Zool, 3(2) (2008) 40-46.
[15] D. Furulyás, R. Chagnaadorj, F. Kis, K. Bíró, M. Stéger-Máté, É. Stefanovits-Bányai, Proceedings of the International Symposium on Analytical and Environmental Problems, 23 (2017) 392-396.
[16] V.L. Singleton, J.A. Rossi, American journal of Enology and Viticulture, 16(3) (1965) 144-158.
[17] I.I.F. Benzie, J.J. Strain, Annalitical Biochemistry, 239. (1966) 70-76
25th International Symposium on Analytical and Environmental Problems
RECOVERY OF PLATINUM FROM LEACHING SOLUTIONS BY INTERACTION WITH PORPHYRINS
Anca Lascu1*
1Institute of Chemistry “Coriolan Dragulescu”, M. Viteazul Ave. 24, 300223- Timisoara, Romania, Tel: +40256/491818; Fax: +40256/491824
*email: ancalascu@yahoo.com
Abstract
Two differently substituted base porphyrins, one containing aliphatic unsaturated groups and one functionalized with basic effect at the periphery: 5,10,15,20-tetrakis-(4-allyloxyphenyl)- porphyrin and 5,10,15,20-tetrakis-(4-aminophenyl)-porphyrin, were investigated for their capacity to complex with hexachloroplatinic acid from leaching solutions. Their different nature makes them interact differently with the hexachloroplatinic acid in solution, as aminophenylporphyrin is more capable to form more stable complexes, therefore it is suitable for the recovery of platinum from diluted solutions (removal capacity is large 86.6 %) whereas the amount of platinum that can be recovered by allyloxyphenylporphyrin is lower, only 74.07 %.
Introduction
The automotive industry uses large amounts of platinum salts as catalysts. As platinum is a rare metal and the natural resources are scarce, the recovery of platinum from leaching solutions has to be taken into serious consideration. This can be achieved based on the capacity of some materials to generate complexes with hexachloroplatinic acid. Some of the compounds capable to perform this task are base porphyrins, properly substituted at the periphery with functional basic groups, or metalloporphyrins, due to their capacity to coordinate ligands at the metal centers [1]. The following step is to obtain platinum colloid, by reducing the already coordinated systems.
A couple of aliphatic- and amino-substituted porphyrin bases were investigated for their capacity of platinum absorption.
Figure 1. Structure of the investigated compounds: 5,10,15,20-tetrakis-(4-allyloxyphenyl)- porphyrin (1); 5,10,15,20-tetrakis-(4-aminophenyl)-porphyrin (2)
Experimental
Materials and methods. N,N-Dimethylformamide was purchased from Merck (Darmstadt, Germany), chloroplatinic acid hexahydrate was acquired from Sigma-Aldrich (St. Louis,
25th International Symposium on Analytical and Environmental Problems
tetrakis-(4-aminophenyl)-porphyrin (2) were synthetized and characterized as published in previous papers [2].
The experiments were performed in 5 mL porphyrin solutions in DMF, to which increasing amounts of hexachloroplatinic acid solution in water (c = 1.03 x 10-3 M) were added. The mixtures were stirred for 30 seconds and then the UV-vis spectrum was recorded for each step.
Apparatus. For recording UV-visible spectra, standard 1 cm pass quartz cells were used on a JASCO UV- V-650 spectrometer (Japan).
Results and Discussions
In order to have a precise measurement, without the effect of volatile solvents, the porphyrins were solved in DMF, a polar, nonvolatile solvent compatible with water. The platinum- containing agent of use in the experiment was hexachloroplatinic acid solution in water (c=1.03 x 10-3 M).
The absorption domain of the chloroplatinic acid solution does not interfere with the absorption wavelengths of the investigated porphyrins, as can be observed in Figure 2.
Figure 2. Comparative UV-vis spectra of the investigated compounds
The overlapped spectra for the successive adding of chloroplatinic acid to the solutions of the two porphyrin-base compounds of interest are presented in Figure 3.
Figure 3. Comparison of the UV-vis spectra after adding chloroplatinic acid to 5,10,15,20- tetrakis-(4-allyloxyphenyl)-porphyrin (1) and 5,10,15,20-tetrakis-(4-aminophenyl)-porphyrin (2). Details present magnification of Q bands and of isosbestic points.
It can be noticed that the allyloxyphenyl porphyrin can only interact with small amounts of platinum from the solution, the intensity of the Soret band decreases drastically after adding 2.2 mL chloroplatinic solution (Figure 3(1)). Nevertheless, some interaction between the
25th International Symposium on Analytical and Environmental Problems
porphyrin molecule and the platinum ions takes place, as isosbestic points show: one on the descending branch of the Soret band, at 435 nm and one at 564 nm, on the Q3 band.
The aminophenylporphyrin is able to better interact with platinum ions in solution, many intermediate species are present, as proven by the presence of numerous isosbestic points: one at 458 nm, on the Soret band, then on the Q bands, in increasing wavelength: 528 nm, 570 nm and 665 nm respectively (Figure 3(2)). The isosbestic point at 570 nm corresponds to the H2PtCl6 concentration interval of 2.023 x 10-5 M to 7.644 x 10-5 M. Also, a new peak appears at 784 nm, probably due to self- aggregation phenomena [3]. Besides, these isosbestic points, together with the appearance of the new peak, indicate a possible acid-base complexation between the weak hexachloroplatinic acid and the four amino groups at the periphery of the porphyrin molecule, that can be protonated. The presumed final complex can have the structure presented in Figure 4.
Figure 4. The presumed structure for the complex formed between the tetra- aminophenylporphyrin and the hexachloroplatinic acid
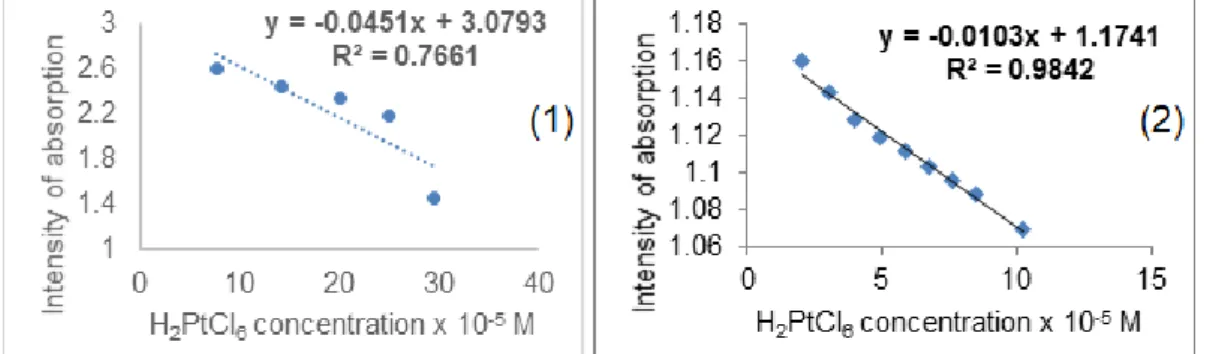
The linear dependence between the intensity of absorption read at the Soret wavelength maxima and the H2PtCl6 concentration for the two porphyrins under investigation are presented in Figure 5.
Figure 5. Linear dependence between the intensity of absorption read at Soret maximum wavelength and the H2PtCl6 concentration for allyloxyphenylporpyrin (1) and for aminophenylporphyrin (2)
It can be observed that the capacity to recognize platinum ions is very poor in the case of allyloxyphenylporphyrin, as the intensity of absorption read at 422 nm is linear with the concentration of chloroplatinic acid only in the concentration domain of 7.6 x 10-5M to 29.5 x 10-5 M, with a poor correlation coefficient of 76.61% and with low sensitivity. In comparison, the chloroplatinic acid concentration interval (2.023 x 10-5 M to 10.227 x 10-5 M) for which
25th International Symposium on Analytical and Environmental Problems
the dependence between the intensity of absorption of aminophenylporphyrin and platinic acid concentration is linear, is represented by a high correlation coefficient of 98.42%.
The capacity to remove platinum ions from a solution, with the aid of porphyrin molecules, was the purpose of this study. Therefore, according to [1] the quantity of platinum that can be retrieved by the porphyrins can be evaluated according to the equation:
𝑄𝑒 = (𝐶0− 𝐶𝑒) 𝑉
𝑚 (𝑚𝑔/𝑔)
Where, adapting the formula for our specific case and taking into consideration the variation of platinic acid:
C0 is the concentration of Pt in the porphyrin solution at the end of the experiment expressed in ppm
Ce is the concentration of the Pt in the porphyrin solution at the beginning of the experiment expressed in ppm
V is the volume of the solution in L
m is the quantity of porphyrin in the solution expressed in g
The distribution coefficient (KD) shows the affinity of the porphyrin solution toward Pt colloidal particles and can be calculated according to the equation:
𝐾𝐷 =𝑄𝑒
𝐶𝑒 (𝐿/𝑔)
The removal capacity of Pt colloidal particles from solution can be calculated according to the formula:
𝑅𝑒𝑚𝑜𝑣𝑎𝑙 𝑐𝑎𝑝𝑎𝑐𝑖𝑡𝑦 (%) =(𝐶0− 𝐶𝑒)
𝐶0 𝑥 100 Table 1 presents the results obtained for the two porphyrins investigated.
Porphyrin Qe [mg Pt/g porphyrin] KD [L/g] Rc [%]
5,10,15,20-tetrakis-(4-allyloxyphenyl)-porphyrin 1175.208 78.809 74.07 5,10,15,20-tetrakis-(4-aminophenyl)-porphyrin 581.75 293.8 86.6
If the capacity to remove platinum from dilute solutions is the desired target, then the 5,10,15,20-tetrakis-(4-aminophenyl)-porphyrin is the better material as given in Table 1, each NH2 base unit being capable to interact with the hexachloroplatinic acid.
The platinum can be recovered as colloid or as platinum particles by reducing the novel generated complexes either with sodium citrate or with excess NaBH4, a more potent reducing agent and subsequent centrifugation of the solution.
Conclusion
Two differently substituted porphyrin-base compounds, one containing aliphatic unsaturated groups and one containing basic groups at the periphery: 5,10,15,20-tetrakis-(4- allyloxyphenyl)-porphyrin and 5,10,15,20-tetrakis-(4-aminophenyl)-porphyrin, were investigated for their capacity to complex hexachloroplatinic acid from leaching solutions.
Their different nature makes them interact differently with the platinum ions in solution. So, aminophenylporphyrin is more capable to bind hexachloroplatinic acid, therefore it is suitable for the recovery of platinum from diluted solutions.
Acknowledgements
The authors are acknowledging UEFISCDI PN-III-P1-1.2-PCCDI-2017-1-Project ECOTECH-GMP 76PCCDI/2018 and the Romanian Academy for financial support in the frame of Programme 3/2019 from ICT.
25th International Symposium on Analytical and Environmental Problems
References
[1] D. Vlascici, I. Popa, V.A. Chiriac, G. Fagadar-Cosma, H. Popovici, E. Fagadar-Cosma, Chem. Cent. J.7 (2013) 111-118.
[2] B.O. Taranu, I. Popa Sebarchievici, I. Taranu, M.I. Birdeanu, E. Fagadar-Cosma, Rev.
Chim. -Bucharest 67(5) (2016) 892-896.
[3] E. Tarabukina, E. Fagadar-Cosma, C. Enache, N. Zakharova, M. Birdeanu, J. Macromol.
Sci. B 52(8) (2013) 1092–1106.
25th International Symposium on Analytical and Environmental Problems
DETERMINATION OF HYDROXYL RADICALS USING COUMARIN AND COUMARIN-3-CARBOXYLIC ACID DURING GAMMA RADIOLYSIS AND
HETEROGENEOUS PHOTOCATALYSIS
Máté Náfrádi1, László Wojnárovits2, Erzsébet Takács2, Tünde Alapi1
1Department of Inorganic and Analytical Chemistry, University of Szeged, H-6720 Szeged, Dóm tér 7, Hungary
2Institute for Energy Security and Environmental Safety, Centre for Energy Research, Hungarian Academy of Sciences, Budapest, Hungary
e-mail: nafradim@chem.u-szeged.hu
Abstract
Coumarin and 3-carboxycoumarinic acid, two fluorescent probes commonly used for HO•
detection has been used during gamma radiolysis and heterogeneous photocatalysis. The O2 dependency and the radiation yield of their hydroxylated flurescent products (7-hydroxy- coumarin and 7-hydroxy-3-carboxycoumarinic acid) has been investigated during gamma radiolysis. The radiation yields were found to be 1.2(±0.2) % in O2-free solutions, while it was 2.9 (±0.06) % in the presence of O2, proving the importance of peroxyl radicals in the formation of these products. The results obtained from radiolysis experiments, were employed during heterogeneous photocatalysis performed with commercial TiO2 catalyst. The effect of dissolved O2 was also investigated, as its electron scavanging role during photocatalyis is also important. The formation rate of HO• during photocatalysis was calculated from the formation rate of the fluorescent products, and were found to be 1.8×10-7 mol dm-3 s-1, while the quantum efficiency for its formation is 0.0038.
Introduction
Advanced oxidation processes (AOPs) have been investigated in the last few decades for their possible use as an additional wastewater purification method. During AOPs different reactive species form, the most important one is the hydroxyl radical (HO•), due to its high reaction rate with most organic pollutants. However several methods can be employed, like time- resolved spectroscopy, ESR, the determination of HO• formation rates is a complicated task.
Fluorescent probes, like terephtalic acid or coumarines, have also been applied during heterogeneous photocatalyis to evaluate the formation rate of HO•. [1-5]
In this study coumarin (COU) and coumarin-3-carboxylic acid (3-CCA) have been used as a fluroescent probe for determination of the HO• formation rate. They are reported to form highly fluorescent hydroxylated prodcts in their reaction with HO•, 7-hydroxy-coumarin (7- HO-COU) and 7-hydroxy-3-carboxycoumarinic acid (7-HO-3-CCA), respectively. The formation rate of both hydroxylated products are reported to be dependant on dissolved O2. In the presence of O2 they form via peroxyl type radicals, while in the abscence of O2 they form via dismutation, significantly reducing their formation rate. [1-3]
The application of COU and 3-CCA for HO• detection was investigated during two, different AOPs. In the case of gamma radiolysis the formation rate of all reactive species (HO•, eaq-
, H•) is well determined, since the values of their radiation yields (G value) are well known.
Heterogeneous photocatalysis is also a highly researched field of AOPs, but the reacton mechanisms are often not clear. The transformation of organic compounds is mostly related to the reactions with HO•. In addition, the reactions initiated directly by the photogenerated charges (hvb+ and ecb-) has to be taken into consideration too. The reactions take place on the surface or close to the surface of photocatalyst, and consequently the interactions between the photocatalyst and substrate may have an important role.
25th International Symposium on Analytical and Environmental Problems
The aim of this study is to determine the radiation yields of 7-HO-COU and 7HO-3-CCA, and investigate the effect of dissolved O2 during gamma radiolysis. Based on these result, the formation rate of HO• and the quantum yield of the HO• formation (ΦHO•) may be determined during the heterogeneous photocatalysis. By comparing the transformation of the non- adsorbed COU, and the well adsorbed 3-CCA, we may investigate the importance of adsorption on the reactions of these substrates with HO•.
Experimental
In the gamma-radiolysis experiments a 60Co gamma source was used in a panoramic type irradiator, the dose rate was 1.48 Gy min-1. The solutions of COU and 3-CCA were irradiated in sealed ampulles, which were saturated with either O2, N2O or N2. All experiments were performed in 10-4 mol dm-3 solutions of COU and 3-CCA in pH = 7.0 (in 0.01 mol dm3 phosphate buffer).
Photocatalysis experiments were performed in a glass reactor. 1.0 g dm-3 TiO2 Aeroxide P25 (Acros Organics) was added to the 250 cm3 solutions, and irradiated using a fluorescent UV light source (GCL303T5/UVA, Lighttech) emitting in the 300-400 nm range. The photon flux of the lamp was 1.20×10-5 molphoton min-1, determied by ferrioxalate actinometry. Since 3- CCA adsorbed on the photocatalyst surface (≈30 % adsorbed on TiO2), NaF was added to the samples, for the desorption of the analytes. All samples were centrifuged at 15000 RPM, and filtered using 0.22 µm syringe filters (FilterBio PVDF-L).
The transformation of COU and 3-CCA (λCOUmax = 277 nm, λ3-CCAmax = 291 nm, εCOU=10300 dm3 mol-1 cm-1, ε3-CCA=12170 dm3 mol-1 cm-1) has been followed using UV-Vis spectrophotometry (Agilent 8453). The formation of 7-HO-COU and 7-HO-3-CCA were followed using fluorescence spectroscopy (Hitachi F4500) at 455 and 447 nm, respectively.
The initial transformation rates of COU and 3-CCA were determined from linear regression fits to the actual concentration versus the duration of irradiation, up to 15 % conversion. The initial formation rates of 7-HO-COU and 7-HO-3-CCA were obtained from the linear regression fits to the actual concentration versus the duration of irradiation.
Results and discussion
First the effect of O2 on the formation of 7-HO-COU and 7-HO-3-CCA was investigated. In the case of radiolysis mainly HO• and eaq-
forms from water. In the presence of O2 eaq-
transforms into O2•-
. Since both O2•-
and its protonated form, HO2•
have a low reactivity towards organic compounds, mainly HO• is responsible for the transformation of COU in this case. Moreover, from carbon centered radicals peroxyl radicals form immediately. The formation of peroxly radicals opens a new pathway for the formation of hydroxilated products via unimolecular HO2• elimination. In O2-free solutions both the HO• and the eaq- are able to initiate the transformation of COU. Without O2, the formation of 7-HO-COU happens via bimolecilar dismutation. The formation rate of HO• in the presence of O2, air or N2 can be calculated (r0HO• = 6.91×10-9 mol dm-3 s-1). In N2O saturated solutions eaq-
transforms into HO•, and doubling the HO• yield (r0HO• = 1.33×10-8 mol dm-3 s-1).
25th International Symposium on Analytical and Environmental Problems
Figure 1. Concentration of COU (A) and 3-CCA (B) as a function of dose in O2 (■), N2O (■)) and N2 (■) saturated solutions, and the formation of 7-HO-COU during gamma radiolysis in O2 (◊), N2O (◊) and N2 (◊) saturated solutions.
The effect of the different gases on the transformation rate of COU and 3-CCA were negligible. Despite the similar transformation rates, the formation rate of the hydroxylated products show significant differences. The lowest formation rate can be observed in O2-free solution, due to the lack of the possibility of peroxyl radical formation, and probably because of the significant contribution of eaq-
to the transformation of COU/3-CCA, which do not result in hydroxilated products. The formation rates in N2O saturated solutions are greatly increased, due to the increased HO• formation rate. The formation rates are even higher in the presence of O2, despite the lower HO• formation, proving the importance of peroxyl radicals in the formation of the hydroxylated products. (Figure 1. and Table 1)
From the formation rates of the hydroxylated products and the formation rate of HO•, the radiation yield for both fluorescent product can be calculated. In N2 saturated solutions 0.94 and 1.35 %, in N2O saturated solutions 1.16 and 1.29 %, while in O2 saturated solutions 2.99 and 2.86% of HO• produces 7-HO-COU from COU and 7-HO-3-CCA from 3-CCA, respectively.
Table 1. Initial transformation rates of COU and 3-CCA and initial formation rates of 7-HO- COU and 7-HO-3-CCA during gamma radiolysis
r0 COU
(× 10-9mol dm-3 s-1)
r0
7-HO-COU
(× 10-10mol dm-3 s-1)
r0 3-CCA
(× 10-9mol dm-3 s-1)
r0
7-HO-3-CCA
(× 10-10mol dm-3 s-1)
O2 3.35 2.06 3.23 1.98
Air 4.33 1.55 2.40 1.71
N2 3.38 0.64 2.73 0.93
During heterogeneous photocatalysis the main reactive species is the HO•. The photogenerated hvb+
and ecb-
pair may also react with organic compounds, especially when there is special interaction between the substrate and the catalyst surface. COU and 3-CCA have different adsorption properties, as COU do not adsorb on the catalyst surface, as opposed to 3-CCA, due to the strong interaction between its carboxyl groups and TiOH surface groups of phtocatalyst.
A B
25th International Symposium on Analytical and Environmental Problems
Figure 2. Transformation of COU (A) and 3-CCA (B) as a function of time, in O2 (■), air (■) and N2 (■) saturated suspensions, and the formation of 7-HO-COU during heterogeneus photocatalysis in O2 (◊), air (◊) and N2 (◊) saturated suspensions.
O2 plays a crucial role as an electron scavenger, hindering charge recombination, and helping HO• formation via O2•-. In the case of COU there was no difference in the transformation rates determined in O2 saturated and airated suspensions. At the same time, the formation rate of 7-HO-COU is nearly 1.5 times greater in the case of higher dissolved O2 concentration. In the case of 3-CCA the transformation rate is also higher with 25% in the case of O2 saturated suspension, while the formation rate of 7-HO-3-CCA increased with 33 %. In N2 saturated suspensions the transformation of COU and the formation of 7-HO-COU is negligible. This suggests, that direct charge transfer reactions have a low probability, and HO• formation is negligible. The transformation rate of the well adsorbed 3-CCA is 47 % of the value measured in O2 saturated suspension, and there is no 7-HO-3-CCA formation. This suggest that, 3-CCA transformation can happen via direct charge transfer, but this way does not results in hydroxilated products.
Table 2. Initial transformation rates of COU and 3-CCA and initial formation rates of 7-HO- COU and 7-HO-3-CCA during heterogeneous photocatalysis
r0COU
× 10-8 (mol dm-3 s-1)
r07-HO COU× 10-9 (mol dm-3 s-1)
r03-CCA× 10-8 (mol dm-3 s-1)
r07-HO-3-CCA× 10-9 (mol dm-3 s-1)
O2 6.15 2.55 8.98 2.77
Air 6.08 1.73 7.10 2.08
N2 0.29 - 4.23 -
Using the results obtained from gamma radiolysis, the formation rate of HO• in the case of O2 saturated TiO2 suspension was found to be 1.78×10-7 and 1.86×10-7 mol dm-3 in the case of COU and 3-CCA respectively. Assuming that, TiO2 absorbs completly the emitted photons from the light source, the quantum yield for the formation of HO• is 0.0037 and 0.0039, respectively.
A B
25th International Symposium on Analytical and Environmental Problems
Conclusion
The formation of 7-HO-COU from COU and 7-HO-3-CCA from 3-CCA requires HO•, while dissolved O2 highly enhances their formation rate.
The radiation yield of 7-HO-COU from COU and 7-HO-3-CCA from 3-CCA were determined in the case of gamma-radiolysis
Based on radiation yields of the hydroxylated products, and the photon flux of the light source, the formation rate of HO• and its quantum efficiency has been determined during heterogeneous photocatalysis, using TiO2 as photocatalyst
Acknowledgements
This work was financially supported by Industrial Research and development Projects of Hungarian-Indian cooperation (TÉT_15_IN-1-2016-0013). This publication was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences, ÚNKP-19-3- SZTE-207andUNKP-19-4-SZTE-115,new national excellence programs of the Ministry for Innovation and Technology.
References
[1] Louit, G., Foley, S., Cabillic, J., Coffigny, H., Taran, F., Valleix, A., Renault, J.P., Pina, S., Radiation Physics and Chemistry 72 (2005) 119–124.
[2] Manevich, Y., Held, K., Biaglow, J.E., Radiation Research 148 (1997) 580–591.
[3] Yamashita, S., Baldacchino, G., Maeyama, T., Taguchi, M., Muroya, Y., Lin, M., Kimura, A., Murakami, T., Katsumura, Y., Free Radical Research 46 (2011) 861–871.
[4] Zhang, J., Nosaka, J., Applied Catalysis B: Environmental 166–167 (2015) 32–36.
[5] Nosaka Y., Nishikawa, M., Nosaka, A.Y., Molecules 19 (2014) 18248–18267.