PROCEEDINGS OF THE
26 th International Symposium
on Analytical and Environmental Problems
Szeged, Hungary November 23-24, 2020
University of Szeged
2
Edited by:
Tünde Alapi Róbert Berkecz
István Ilisz
Publisher:
University of Szeged, H-6720 Szeged, Dugonics tér 13, Hungary
ISBN 978-963-306-771-0
2020.
Szeged, Hungary
3
The 26
thInternational Symposium on Analytical and Environmental Problems
Organized by:
SZAB Kémiai Szakbizottság Analitikai és Környezetvédelmi Munkabizottsága
Supporting Organizations
Institute of Pharmaceutical Analysis, University of Szeged
Department of Inorganic and Analytical Chemistry, University of Szeged
Symposium Chairman:
István Ilisz, DSc
Honorary Chairman:
Zoltán Galbács, PhD
Organizing Committee:
István Ilisz, DSc professor of chemistry
University of Szeged, Institute of Pharmaceutical Analysis Tünde Alapi, PhD
assistant professor
University of Szeged, Department of Inorganic and Analytical Chemistry Róbert Berkecz, PhD
assistant professor
University of Szeged, Institute of Pharmaceutical Analysis
Scientific Committee:
István Ilisz, DSc Tünde Alapi, PhD Róbert Berkecz, PhD Daniela Sojic Merkulov, PhD
associate professor
University of Novi Sad, Faculty of Sciences, Department of Chemistry, Biochemistry and Environmental Protection
4
Lecture Proceedings
5
EMISSIVE Zn(II) METALLOMESOGEN BASED ON TRIDENTATE TERPYRIDINE LIGAND
Adelina A. Andelescu1, Benoît Heinrich2, Emilie Voirin2, Evelyn Popa1, Massimo La Deda3, Giuseppe Di Maio3, Otilia Costişor1, Bertrand Donnio2,Elisabeta I. Szerb1,*
1Institutul de Chimie “Coriolan Drăgulescu”, B-dul. Mihai Viteazu nr. 24, 300223- Timișoara, România.
szella73@gmail.com
2Institut de Physique et Chimie des Matériaux de Strasbourg, 23 Rue du Loess, Strasbourg, 67034, France
3Dipartimento di Chimica e Tecnologie Chimiche, Universita della Calabria, via P. Bucci, Cubo 14/C, 87036 Arcavacata di Rende, Italy
Abstract
A low temperature liquid crystal based on luminescent terpyridine Zn(II) complex is presented.
The induction of the mesomorphic properties was achieved using a lipophilic gallate unit as ancillary ligands. The mesomorphic properties were investigated by polarised optical microscopy (POM), differential scanning calorimetry (DSC), thermogravimetric analysis (TA) and X-ray scattering (SWAXS) of bulk materials, while the optical properties of the complex were investigated in solution and in condensed liquid crystalline states.
Introduction
Metallomesogens (metal-containing liquid crystals) combine the supramolecular ordering of liquid crystals with the properties imparted by the metal centre (magnetic, electrical, optical and electro-optical),[1] thus leading to advanced materials with potential applications in optoelectronics.[2,3] Due to their excellent light emitting efficiencies and high thermal stabilities, Zn(II) complexes are of particularly high interest.[4] On this background, we report the synthesis and characterization of a terpyridine (tpy) Zn(II) liquid crystal.
Experimental
The structural characterization of the compounds was realized by employing a variety of analytical and spectroscopic methods: elemental analysis, Nuclear Magnetic Resonance - NMR, Fourier-Transform Infrared - FT-IR, and Ultraviolet–visible - UV-Vis spectroscopies.
The thermal behaviour and liquid-crystalline properties were investigated by polarised optical microscopy (POM), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and small- and wide-angle X-ray scattering (SWAXS). Photophysical investigations were carried out in different solvents and condensed states.
Results and discussion
The ligand L_tpy [5] and precursor AgGal [6] were obtained following reported procedures.
The reaction of L_tpy with ZnCl2 afforded the neutral complex Zn_1, whereas the final complex Zn_2 was obtained by the displacement of the chlorine ligands with AgGal lipophilic unit,[7] as described in Scheme 1.
6
Scheme 1. Synthesis of Zn(II) complexes: (i) ZnCl2, MeOH/CHCl3, r. t., 1.5 hours; (ii) CHCl3, r. t., 2 hours.
The neutral pentacoordinated complex Zn_2 self-assembles in a columnar hexagonal phase already at room temperature, as determined by POM, DSC and SWAXS analysis.
The absorption and emission properties were studied in dichloromethane solutions. Both complexes resulted to be fluorescent, with an emission quantum yield of 6.4 % for Zn_1 and 24.5 % for Zn_2. Also, both complexes presented emission in condensed state at room temperature.
The luminescent properties of the liquid crystalline Zn_2 were also measured in condensed states at various temperatures and found to be kept in the liquid crystalline states, but lost in the isotropic states due to the increase of the vibrational modes by increasing the temperature.
Conclusion
A luminescent liquid crystal based on Zn(II) metal centre was obtained by a straightforward synthetic approach, using a lipophilic gallate derivative as ancillary ligand. The complex was found to be luminescent in solution and in condensed states. In the mesophase, Zn_2 emits showing a broad band whose maximum depends on the temperature.
Acknowledgements
M. LD and G. DM acknowledge the help granted by Regione Calabria (POR Calabria FESR 2014/2020-Azione 1.2.2) through the MERAVIGLIE project. B.D. and B.H. thank the CNRS and University of Strasbourg for support. A.A.A. P.E, E.I.S. and O.C. acknowledge the Romanian Academy, Program 4. A.A.A. is grateful for an “Ion Heliade Radulescu” mobility scholarship.
References
[1] B. Donnio, D.W. Bruce, Struct. Bonding 95 (1999) 193.
[2] X. Wu, G. Xie, C.P. Cabry, X. Xu, S.J. Cowling, D.W. Bruce, W. Zhu, E. Baranoff, Y.
Wang, J. Mater. Chem. C 6 (2018) 3298.
[3] X. Wu, M. Zhu, D.W. Bruce, W. Zhu, Y. Wang, J. Mater. Chem. C 6 (2018) 9848.
[4] A. Crispini, M. Ghedini, D. Pucci, Beilstein J. Org. Chem. 5 (2009) 54.
[5] U.S. Schubert, S. Schmatloch, A. Precup, Des. Monomers Polym. 5 (2002) 211.
[6] E. I. Szerb, D. Pucci, A. Crispini, M. La Deda, Mol. Cryst. Liq. Cryst. 573 (2013) 34.
[7] A.A. Andelescu, B. Heinrich, M.A. Spirache, E. Voirin, M. La Deda, G. Di Maio, E.I. Szerb, B. Donnio, O. Costisor, Chem. Eur. J. 26 (2020) 4850.
7
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHIC ENANTIOSEPARATION OF SOME AMINO CMOPOUNDS WITH PHARMACEUTICAL RELEVANCE ON
ION-EXCHANGER-BASED CHIRAL STATIONARY PHASES
Attila Bajtai1, Dániel Tanács1, Enikő Forró2, Ferenc Fülöp2, Wolfgang Lindner3, Antal Péter1, István Ilisz1
1Institute of Pharmaceutical Analysis, Interdisciplinary Excellence Centre, University of Szeged, H-6720 Szeged, Somogyi utca 4, Hungary
2Institute of Pharmaceutical Chemistry, Interdisciplinary Excellence Centre, University of Szeged, H-6720 Szeged, Eötvös u. 6, Hungary
3Department of Analytical Chemistry, University of Vienna, Währingerstrasse 38, 1090 Vienna, Austria
e-mail: bajtai@pharm.u-szeged.hu
New, pharmacologically interesting chiral amino compounds, namely, tetrahydroisoquinoline (THIQ)- and tetrahydro-β-carboline (THβC)-core containing alkaloids ([1, 2], Figure 1) have been separated by high-performance liquid chromatography on novel strong cation exchangers and Cinchona alkaloid-based zwitterionic (ZWIX(+)™ and ZWIX(−)™) ion-exchanger stationary phases. Separation of the stereoisomers was optimized by investigating the effects of the composition of the bulk solvent, the impact of the counter- and co-ion concentration and the influence of the temperature on the chromatographic behaviour. In addition, the relationship between the compound’s structure and the chromatographic parameters were also investigated.
Experiments were performed in the temperature range 10–50 °C. Thermodynamic parameters were calculated from plots of lnα versus 1/T. The separations were generally enthalpy- controlled, but entropy-controlled separation was also observed. The enantiomer elution order was determined in all cases and most of the time was observed to be opposite on the ZWIX(+)™
and ZWIX(−)™ columns. Our results contribute to a better understanding of the enantiorecognition mechanism of chiral bases with chiral zwitterionic and cation-exchanger selectors.
Figure 1. Structure of analytes Acknowledgements
SUPPORTED BY THE ÚNKP-20-3-NEW NATIONAL EXCELLENCE PROGRAM OF THE MINISTRY FOR INNOVATION AND TECHNOLOGY FROM THE SOURCE OF THE NATIONAL RESEARCH,DEVELOPMENT AND INNOVATION FUND.
References
[1] B. Kovács; R. Megyesi; E. Forró; F. Fülöp Tetrahedron: Asymmetry 2017, 28, 1829-1833.
[2] B. Kovács; E. Forró; F. Fülöp Tetrahedron, 2018, 74, 6873-6877.
8
RECOGNITION OF RENEWABLE ENERGY AMONG BUSINESS STUDENTS László Berényi1, Nikolett Deutsch2
1Institute of Management Science, University of Miskolc, H-3515 Miskolc-Egyetemváros, Hungary
2Department of Strategy and Project Management, Corvinus Business School, Corvinus University of Budapest, No. 8. Fővám Square, H-1093 Budapest, Hungary
e-mail: szvblaci@uni-miskolc.hu Abstract
Using renewable energy sources is in the mainstream of environmental protection, including climate change. Non-professional opinions in the field are essential to explore for enhancing the acceptance and utilization level. This paper shows the evaluation of Hungarian business students (n=632) about renewable energy sources and their utilization. The results show that the students feel that the use of renewable energy lags behind the EU level, but there is a trust in convergence in the medium term.
Introduction
Energy dependence is a complex social and technical challenge of the present age [1]. Forcing the use of renewable energy sources is beneficial to climate, but technical problems and availability must be managed [2], including the local access to them [3]. Moreover, there are human aspects to consider. The lack of knowledge and social acceptance [4] may deflect the use of technically right solutions.
EU has embraced the topic that gives legal justification for development and research efforts.
The renewable energy directives [5-6] require the EU to fulfill at least 20% of its total energy needs with renewables by 2020 (at least 10% for each member country) and 32% for 2030. The statistics [7] show a remarkable increase in renewable energy use, but the target vales seem to be ambitious. In the case of Hungary, a decline can be detected (Figure 1). Among others, this result draws attention to comprehensive research on renewable energy use. The problem goes far beyond one study; our paper aims to contribute to a better understanding of the possible development directions.
Figure 1. Share of renewable energy in gross final energy consumption in some countries Source: based on [7]
9 Experimental
Data collection is performed by a voluntary online survey among higher education students between 2018 and 2019. The research sample includes 563 respondents who are studying business. 61.6% of them are females, and 38.4% are males. 54.2% of the respondents are studying bachelor or higher vocational students, 45.8% of them are at the master level. There are students without any work experience (38.4%), others with internship experience (20.4%) and employees (41.2%). It is to note that the responses are collected from business students of various Hungarian universities, but the representativeness of the sample structure is not ensured.
Despite a large number of responses, the generalization of the results and conclusions are limited.
The presented results are based on two items of the survey. It includes a list of some energy sources (Figure 2) and asks to mark whether the respondents consider it as renewable or not.
This part of the survey allows us to check the knowledge level and non-professional opinions about the perception of renewable characteristics. Besides, there are four questions formulated with a 5-point scale evaluation about the present and future position of renewable energies:
- Q1: How do you think we currently use renewable energy sources compared to other European countries?
(1: much less, 5: in a much greater extent);
- Q2: Do you think people would pay more for energy if it were definitely from a ‘green’ source? (1: surely not, 5: surely yes);
- Q3: How much do you agree with the statement that people are increasingly striving to save energy in their everyday lives? (1: not at all, 5: fully agree);
- Q4: Do you think that in 10-15 years, we will use renewable energy sources compared to other European countries? (1: much less, 5: in a much greater extent).
The mean values of the responses represent the results of the evaluation.
Results and discussion
Figure 2: Evaluation of renewable characteristic of energy sources (marked by % of the sample) The items of energy sources include both evident and questionable items. Compared to the professional opinion about the renewable characteristic, the results show some differences (Figure 2). Nuclear energy (6.4%) and natural gas (5.4%) are considered renewable by a small but remarkable minority. The results of solar, wind, and hydropower energy are in line with professional opinions. Biomass (66.3%) and geothermal (65.5%) energy sources are less
10
considered renewable. Energy plantations (38.6%) represent a surprisingly low share, especially that about half of the respondents marked it who marked biomass.
According to the evaluation of the present and future of renewable energy, ANOVA tests were conducted in order to check the grouping effect of gender, level of studies, and work experience.
The analysis found significant effects in 2 of 12 cases (Table 1) by the non-parametric Kruskal- Wallis test. Various statistical tests were run for finding clustering the results to draw up characteristic patterns of opinions and to develop targeted strategies, but the outcomes were not significant or interpretable.
Table 1: Significant differences by grouping factors
Item Factor Mean values Kruskal- Wallis H
df sig.
How much do you agree with the statement that people are increasingly striving to save energy in their everyday lives?
Level of Studies
xbachelor =3.10 xmaster=3.34
8.274 1 .004
How do you think we currently use renewable energy sources compared to other European countries?
Work experience
xnone=2.03 xemployee=1.84 xinternship=2.02
6.709 2 .035
Other results are presented for the entire sample (Figure 3). The mean values of the responses suggest that the students are critical to present, but hey have trust in the future. They think that the use of renewable energy sources will reach the EU level that in 10-15 years. Paying for greener energy does not seem to be an acceptable way; saving energy received a higher mean value.
Figure 3: Mean values of evaluation (5-point scale)
Consequently, it is also worth examining the distribution of the evaluations due to the medium level of mean values (Table 2 and Figure 4). The present use of renewables compared to the EU is evaluated rather worse by 78.7% of the respondents, while 2.7% think that Hungary has a better performance. According to the future, the ratio of skeptics (evaluated 1 or 2) is 26.8%, while there are 28.9% optimists in the sample.
11
Table 2: Distribution of evaluations on the 5-point scale (% of the sample)
1 2 3 4 5
How do you think we currently use renewable energy sources compared to other European countries?
30.17 48.55 18.07 2.60 0.61 Do you think people would pay more for energy if
it were definitely from a ‘green’ source?
11.79 34.76 29.71 21.29 2.45 How much do you agree with the statement that
people are increasingly striving to save energy in their everyday lives?
5.36 17.76 34.61 35.07 7.20 Do you think that in 10-15 years, we will use
renewable energy sources compared to other European countries?
5.51 21.29 44.26 23.74 5.21
Figure 4: Distribution of evaluations on the 5-point scale (% of the sample)
Those who think that people would pay more for green energy represent 23.7% of the sample, while 46.6% consider still the opposite. At the same time, 42.3% rather trust that people will strive to save energy. This suggests that ‘saving’ covers both energy and cost-saving by the students.
The correlation between the responses (Table 3) shows weak and medium level but significant results. The highest value (0.372) is found between the present and the future use of renewable energy sources. Future use significantly correlates with strive to save energy (0.206) and willingness to pay more for green energy with strive to save it (0.217).
12 Table 3: Spearman’s correlation between the questions
Q1 Q2 Q3 Q4
Q1 (present use) Corr. Coef. .113** .121** .372**
Sig. (2-tailed) 0.004 0.002 0.000 Q2 (pay more for green
energy)
Corr. Coef. .113** .217** .141**
Sig. (2-tailed) 0.004 0.000 0.000 Q3 (strive to save energy) Corr. Coef. .121** .217** .206**
Sig. (2-tailed) 0.002 0.000 0.000 Q4 (future use) Corr. Coef. .372** .141** .206**
Sig. (2-tailed) 0.000 0.000 0.000
**: correlation is significant at the 0.01 level (2-tailed) Conclusion
Professional and non-professional (student) opinions are not entirely overlapped. While solar, wind, and hydropower energy are considered renewable by most students, geothermal energy, biomass, and especially energy plantation are already in the background. A conclusion for teaching and education is given by the results that a higher emphasis should be given to the technical issues of renewable energy.
The positive approach to the future improvement in using renewable energies and convergence to the EU level is encouraging, but an essential experience of the survey is that progress is rather supported by energy savings than paying more for greening. Since business students are expected to become company decision-makers in the near future, consequently, their opinions and attitudes are prognostic.
References
[1] P. Högselius, A. Kaijser, Energy dependence in historical perspective: The geopolitics of smaller nations. Energy Policy 127 (2019) 438-444.
[2] V.V. Quaschning, Renewable Energy and Climate Change, 2nd edition, Wiley, Hoboken, 2019, pp. 351.
[3] L. Berényi, Z. Birkner, N. Deutsch, A Multidimensional Evaluation of Renewable and Nuclear Energy among Higher Education Students, Sustainability, 12, 2020, 1449.
[4] M. Wolsink, Contested environmental policy infrastructure: Socio-political acceptance of renewable energy, water, and waste facilities. Environmental Impact Assessment Review 30 (2010) 302-311.
[5] Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC
[6] Directive (EU) 2018/2001 of the European Parliament and of The Council of 11 December 2018 on the promotion of the use of energy from renewable sources
[7] Share of renewable energy in gross final energy consumption (T2020_31). Available online:
https://ec.europa.eu/eurostat/databrowser/view/t2020_31/default/table?lang=en (accessed on 10 07 2020).
13
AMMONIA TRANSPORT ACCIDENT EXPOSURE ANALYSIS Jovana Bondžić1, Maja Petrović1
1Department of Environmental Engineering and Occupational Safety and Health, Faculty of Technical Sciences, University of Novi Sad, Trg Dositeja Obradovića 6, Novi Sad, Serbia
e-mail: jovanasimic@uns.ac.rs Abstract
Anhydrous ammonia proved its efficiency and has been used extensively in the food and processing industry as a refrigerant. Between production and utilization locations it is necessary to organize transport of this substance. Due to its physicochemical properties, there is a possibility for the endangering population and environment in urban areas if the tank traffic accident occurs during the transport. Therefore, this research analyses exposure to ammonia transport accidents through the use of two software applications.
Introduction
Ammonia is, under normal conditions, a colorless gas with a pungent odor [1]. It is lighter than air and soluble in water. In industry, ammonia is used in pure form, without water and is called anhydrous ammonia. It can be liquefied under pressure, or at a temperature below its boiling point. Its boiling point, under atmospheric pressure, is -33,3℃. The low boiling point and the ability to absorb a large amount of heat enable the use of ammonia as a refrigerant. As industrial refrigerant ammonia has been used extensively since chlorofluorocarbons evolved as less toxic refrigerants. Still, it is widely used in industrial refrigeration, because of its low cost and high efficiency. Ammonia is toxic with an 8-hour exposure limit of 25 ml/m3 and a 15-minute exposure limit of 35 ml/m3 [2]. The flammable limit of ammonia is between 16-27% by volume [3], while the auto-ignition temperature is 651℃.
In the Republic of Serbia, anhydrous ammonia is produced in HIP Azotara Pančevo, and it is transported to the locations of use by road in tanks. Considering the physicochemical characteristics of ammonia, in the case of an accidental release during transport in populated areas, the human population and environment could be exposed to the toxic and flammable impact of ammonia. In this research, for the exposure analysis of ammonia transport accident, two software ALOHA and Quantum GIS (QGIS) were used. A case study was developed for ammonia release from a tanker truck in the urban area of Novi Sad for three possible hazard scenarios.
Experimental
The quantity of ammonia transported criss-cross the Republic of Serbia differs according to demands, therefore, there is an expectancy of a small tank with ammonia to be driven through the urban area of Novi Sad. Considering the probability of traffic accidents during the transport of ammonia, a location with a high traffic frequency was chosen for the experimental analysis.
Based on the ammonia properties, three possible scenarios were identified: dispersion of toxic vapor cloud, ignition of vapor cloud and explosion of the vapor cloud. For the determination of dispersion for each scenario, ALOHA (Areal Locations of Hazardous Atmospheres) software, as a hazard modeling program, was used.
ALOHA is a computer program designed to model chemical releases for emergency responders and planners [4]. It allows user to enter details about a real or potential chemical release, and then it will generate threat zone estimates for various types of hazards such as toxic release
14
dispersion, flammable area of the gas cloud, vapor cloud explosion, BLEVE (Boiling Liquid Expanding Vapor Explosion), jet fire, etc. For the purpose of this research, hazard scenarios were modeled in ALOHA based on input parameters’ values listed in Table 1.
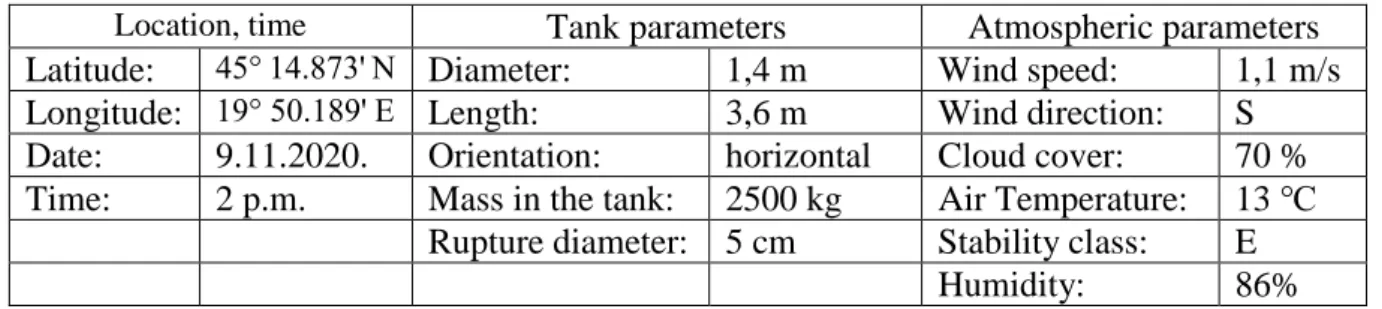
Table 1. Input parameters’ values for hazard model in ALOHA software
Location, time Tank parameters Atmospheric parameters
Latitude: 45° 14.873' N Diameter: 1,4 m Wind speed: 1,1 m/s Longitude: 19° 50.189' E Length: 3,6 m Wind direction: S Date: 9.11.2020. Orientation: horizontal Cloud cover: 70 % Time: 2 p.m. Mass in the tank: 2500 kg Air Temperature: 13 ℃
Rupture diameter: 5 cm Stability class: E
Humidity: 86%
The results of the simulation experiments were obtained in the form of graphs showing the threat zones. Threat zones represent the area within which the ground-level exposure exceeds the user-specified level of concern at some time after the beginning of a release [5]. ALOHA will display up to three threat zones, yellow, orange and red, overlaid on a single picture. Using ALOHA’s KML export feature, outputs were imported into the Quantum GIS project in order to analyze the overall spatial context of the accident.
Quantum GIS is a user-friendly and open-source Geographic Information System that enables viewing, editing, and analysis of geospatial data. It supports numerous vector, raster, and database formats and functionalities [6]. It is suitable for representing objects from the real system on the spatial model.
Results and discussion
Two of three possible hazard scenarios simulated within the experiment gave results that exceed user-specified levels of concern (LOC). Level of Concern is a threshold value of a hazardous impact (toxicity, flammability, or overpressure) above which a threat to people or property may exist.
For the Toxic Area of Vapor Cloud scenario, Acute Exposure guideline Level (AEGL) was used as a LOC. In Figure 1a, it can be seen that all three LOCs were exceeded in this scenario.
In the red zone, it is predicted that the population exposed to more than 1 000 ppm concentration of ammonia could experience life-threatening health effects or death. In the orange zone, people could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape. In the yellow zone, the effects are not disabling and are temporary.
Flammable Area of Vapor Cloud scenario uses 60% of LEL (Lower Explosive Limit) as a LOC for a red zone and 10% of LEL as a LOC for a yellow zone. Both concentrations were exceeded as it is shown in Figure 1b. If there is an ignition source, fire hazard could occur between 90 000 and 15 000 ppm ammonia concentration in the air.
Comparing two graphs it can be noticed that far more area will be endangered in the case of toxic than flammable exposure. In order to analyze the exposed population and property both graphs were imported into the QGIS project (Figure 2). In addition, OpeenStreet Map was used as a base map layer in the project. By visual examination of both scenarios represented within the spatial model, it can be concluded that significant urban areas could be exposed to the hazard of this type. The most vulnerable objects detected in both exposed zones are primary, secondary
15
and higher schools because of high frequency of children presented during the whole day. Also, it should be emphasized that a primary school for children with disabilities will be exposed within the red zone of both scenarios.
a b
Figure 1. Results obtained by ALOHA software: a – Toxic area; b – Flammable area
a b
Figure 2. Results imported into QGIS project: a – Toxic area; b – Flammable area Conclusion
The described method for the analysis of potential accidental situations during the transport of toxic substances enables responders to generate necessary information almost immediately and to conduct appropriate reactions promptly. Therefore, it is extremely important to analyze hazards that could occur in the populated areas in advance, to be as prepared as possible for the unwanted consequences to people and the environment.
References
[1] Janković Z., 2016. Razvoj modela za proračun rizika u logističkim sistemima opasnih materija. PhD thesis. Faculty of Technical Sciences, University of Novi Sad
[2] Eckhoff R., 2016. Explosion Hazards in the Process Industries, Chapter Three - Boiling Liquid Expanding Vapor Explosions (BLEVEs), University of Bergen, Norway
16
[3] HIP Azotara, 2010. MSDS, Available at: http://hip-azotara.rs/wp-content/uploads /2016/10/amonijak-sr.pdf. (Accessed 10th November 2020)
[4] NOAA, 2020. Available at: https://response.restoration.noaa.gov/sites/default/files/
aloha.pdf (Accessed 11th November 2020)
[5] QGIS, 2020. Available at: https://qgis.org/en/site/about/index.html (Accessed 12th November 2020)
[6] Jones, R., W. Lehr, D. Simecek-Beatty, R. Michael Reynolds. 2013. ALOHA® (Areal Locations of Hazardous Atmospheres) 5.4.4: Technical Documentation. U. S. Dept. of Commerce, NOAA Technical Memorandum NOS OR&R 43. Seattle, WA: Emergency Response Division, NOAA. 96 pp.
17
STRUCTURAL AND MAGNETIC PROPERTIES OF THREE 1D COPPER(II) COORDINATION POLYMERS
Ildiko Buta1*, Peter Lönnecke2, Evamarie Hey-Hawkins2, Marius Andruh3, Otilia Costisor1
1Romanian Academy ‘‘Coriolan Dragulescu” Institute of Chemistry, 24 Mihai Viteazu Bvd., 300223-Timisoara, Romania
2Leipzig University, Faculty of Chemistry and Mineralogy, Institute of Inorganic Chemistry, Johannisallee 29, 04103 Leipzig, Germany
3University of Bucharest, Faculty of Chemistry, Inorganic Chemistry Laboratory, 23 Dumbrava Rosie Str, 020464-Bucharest, Romania
e-mail: ildiko_buta@acad-icht.tm.edu.ro Abstract
The design of coordination polymers is controlled by the nature of the ligands and metal ions involved [1]. The electronic structure, size and stereochemical preference of the metal ion, along with the number and the relative position of the coordinating groups of the ligand, determine the dimensionality and topology of the resulting compounds [2].
Here, we report three coordination polymers, 1∞[Cu3L2(NO3)]NO3·2MeOH·2H2O (1),
1∞[Cu3L2(N3)]CH3COO (2) and 1∞[Cu3L2(H2O)](ClO4)2 (3), based on the Schiff base H2L (H2L
= N,N’-bis[(2-hydroxybenzilideneamino)propyl]piperazine). X-ray single-crystal analysis shows that compounds 1 and 2 are isostructural and crystallize in orthorombic system, space group, P212121, while complex 3 crystallizes in a monoclinic system, space group P21/c.
Compounds 1-3 consist of trinuclear complex entities, [Cu3L2]2+, connected via different bridges, nitrato (1), azido (2) and phenoxido (3), depending on the nature of the counterion. The cryomagnetic measurements showed weak ferro- (1) and antiferromagnetic (2 and 3) interactions between the copper(II) ions (Figure 1).
Figure 1. MT vs T curves for compounds 1-3 Acknowledgements
We thank the Romanian Academy, Institute of Chemistry “Coriolan Dragulescu” (Project 4.1.3) for financial support.
References
[1] F.A.A. Paz, J. Klinowski, S.M.F. Vilela, J.P.C. Tomé, J.A.S. Cavaleiro, J. Rocha, Chem.
Soc. Rev. 41 (2012) 1088.
[2] W.L. Leong, J.J. Vittal, Chem. Rev. 111 (2011) 688.
18
COULD HUMIC SUBSTANCES BE GOOD ANTIOXIDANTS?
A. Csicsora, E. Tombáczb
aHymato Products Ltd., H-8225 Szentkirályszabadja, Kossuth u 33., Hungary
bUniversity of Pannonia, Soós Ernő Water Technology Research and Development Center, H- 8800 Zrínyi M. str. 18. Nagykanizsa, Hungary
email: csicsor.attila@gmail.com Abstract
Humic substances (HS) are natural macromolecules that can be found everywhere (e.g., soils, waters, air, peat, coal deposits) in the environment. Humic substances (HS) are natural organic colloids built up randomly from the decay products of plant originated biomass in humification process. [1]
In today’s modern world, processed foods, medicines, cosmetics, and electro smog produce so much free radical in living organisms that antioxidants taken from the outside will become increasingly important to neutralize this increased load.
Antioxidants are substances that inhibit the oxidation in a broader sense oxidation retardants. The most important physiological role of antioxidant substances is to deactivate the free radicals continuously formed in the Szent-Györgyi-Krebs cycle, and to counteract the radicals with different oxidizing properties that enter the body from the outside.
There are well known antioxidants like vitamin-C; vitamin-A; flavonoids; resveratrol, ect. These antioxidants like flavonoid and the resveratrol are formed in plants. The reason plants produce these materials is to protect themselves for the effect of the UV radiation. When the UV light beam hits the plant it produces free radicals. By producing antioxidant molecules the plants can protect themself from this effect [2].The reason we think that the humic substances could be great antioxidant is this. Because they are formed from plant residues. So these materials are like the essence of the decomposed plant. [3]. Humic acid also has antioxidant properties via phenolic and polyphenolic hydroxyl groups, and behaves as free radical scavengers. Secondary substituents on the polyphenolic structure that support the electron- donating ability of phenolic OH groups by inductive and mesomeric effects also enhance the antioxidant property. [4]
First of all we extracted different fractions (humic acid, fulvic acid and hymatomelic acid) from a raw material the Leonardite. Then we measured the antioxidant capacity of these fractions. Although it is not an easy task. We measured the total phenol content (TPC) of the samples. We used gallic acid as a reference molecule. The values are as follows: while gallic acid (GS) gave 982 mg/g TPC, the values of fulvic acid 9 mg/g TPC and hymatomelic acid 52 mg/g TPC are much lower. We also measured the antioxidant capacity by the DPPH and CUPRAC methods, and the values were as follows. For the DPPH radical scavenging activity for the fulvic acid was 20,4 % for the hymatomelic 31,4 % in an interval concertation from 0-1 mg/ml. From the results we can conclude that humic substances has antioxidant, free radical inhibitory effects.
References
[1] Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, Wiley, New York, 1994, pp. 188-210.
[2] Gould, K. S., and Lister, C. (2006). “Flavonoid functions in plants,” in Flavonoids:
Chemistry, Biochemistry and Applications, eds Ø. M. Anderson and K. R. Markham (Boca Raton: CRC Press), 397–442.
19
[3] Şeyda Karadirek at al. (2016): Determination of total antioxidant capacity of humic acids using CUPRAC, Folin-Ciocalteu, noble metal nanoparticle- and solid-liquid extraction- based methods, DOI: 10.1016/j.talanta.2016.03.006
[4] O.V. Smirnova, at al. (2012): Antioxidant and pro-oxidant activity of ascorbic and humic acids in radical-chain oxidation processes, Russ. J. Appl. Chem. 85 (2012) 252-255.
DOI: https://doi.org/10.1134/S1070427212020164
20
POULTRY WASTEWATER TREATMENT USING PORPHYRIDIUM SPP.
Zamfira Dincă1, Anamaria Iulia Török1, Ana Moldovan1,2, Emilia Neag1, Cecilia Roman1
1INCDO-INOE 2000, Research Institute for Analytical Instrumentation, 67 Donath Street, 400293, Cluj-Napoca, Romania
2Technical University, Faculty of Materials and Environmental Engineering, 103-105 Muncii Boulevard, 400641 Cluj-Napoca, Romania
e-mail: emilia.neag@icia.ro Abstract
Wastewater contains various nutrients that can be used by microalgae for their growth.
Microalgae are capable of removing nitrogen, phosphorus, heavy metals, as well as some toxic compounds from wastewater [1]. Microalgae species must present the ability to adapt in wastewater and the capability of growing to high cell density [2]. Several species, such as Chlorella vulgaris, Nannochloropsis spp., Rhizoclonium spp., Scenedesmus intermedius were used for wastewater treatment, while few studies reported the potential of Porphyridium spp., a red microalgae, to grow in wastewater [3].
The main objective of the present study was to investigate the capability of the marine microalgae, Porphyridium spp. to grow in poultry wastewater containing heavy metals and other contaminants in various concentrations. The composition of wastewater was determined before and after the microalgae cultivation, in order to monitor the ability of Porphyridium spp.
to reduce the metals concentration. The initial pH of the wastewater as a growth medium was 6.6. After ten days of growth period, the pH value increased up to 8.6 suggesting that the Porphyridium spp. adapted to the new conditions. Also, the conductivity of the medium increased to 8.2 mS/cm after treatment compared with the initial value (before treatment) of 0.73 mS/cm. The highest removal efficiency exceeded 98 % in case of Al, followed by 95 % in case of Zn, 92 % for Fe and 90 % for B, while the lowest removal efficiency of 7 % was obtained for Mg. The results revealed a removal percentage of 41 % for Cd, 67 % for Cr, 54 % for Co, 10 % for Cu, 44 % for Mn, 30 % for Ni, 7 % for Mg, 79 % for Si, 8 % for K and 67 % for Ca.
However, Porphyridium spp. exhibited low biomass productivity after wastewater treatment, compared with the control biomass grown in modified F/2 (Guillard's) medium. Even if the growth rate was low compared to the control, poultry wastewater has the potential to be used as an alternative growth medium for microalgae with simultaneous uptake of the metals and organic contaminants.
Acknowledgements
This work was funded by the Core Program, under the support of ANCS, project no. PN 19- 18.01.01 (contract no. 18N/08.02.2019).
References
[1] N. Abdel-Raouf, A.A. Al-Homaidan, I.B.M. Ibraheem, Saudi J. Biol. Sci. 19 (2012) 257–275.
[2] W. Zhou, B. Hu, Y. Li, M. Min, M. Mohr, Z. Du, P. Chen, R. Ruan, Appl. Biochem. Biotechnol. 168 (2012) 348–363.
[3] H.B. Ulusoy Erol, M. L. Menegazzo, H. Sandefur, E. Gottberg, J.Vaden, M. Asgharpour, C.N.
Hestekin, J.A. Hestekin, Energies 13, 2020, 3194.
21
IMPROVING THE PERFORMANCE OF THE POLYSULFONE MEMBRANES INDUCED BY THE PRESENCE OF IONIC LIQUIDS: RHEOLOGICAL
INVESTIGATIONS
Adina Maria Dobos1, Mihaela Dorina Onofrei1, Lavinia Lupa2, Anca Filimon1
1”Petru Poni” Institute of Macromolecular Chemistry, Aleea Grigore Ghica Voda 41 A, 700487 Iasi, Romania
2University Politehnica Timisoara, Faculty of Industrial Chemistry and Environmental Engineering, 6 Vasile Parvan Blv, 300223, Timisoara, Romania
e-mail: necula_adina@yahoo.com Abstract
The various environmental problems of air or water pollution recently are trying to be solved by membrane technology. For this reason, finding the right compounds to obtain reverse osmosis, nanofiltration, and ultrafiltration membrane with improved properties is always a challenge for the researchers. In this context, the polysulfones containing quaternary ammonium side groups (PSFQ) are considered to be suitable for a wide range of applications from environmental field as result of their specific properties, such as hydrophylicity, flexibility and film forming capability. However, experience in the operation of polysulfonic membrane shows that there are some problems caused by membrane fouling. Thus, the aim is to improve their performance by mixing them with ionic liquids. In the obtaining process of ionic liquids- based membranes these could act as carrier of the final product which leads to the increasing of the membrane functionality and selectivity. For the present study two types of ionic liquids were chosen to be mixed in various rations (0.03 – 0.25wt.) with PSFQ:
trihexyltetradecylphosphonium chloride (Cyphos) (ILp) and methyltrialkylammonium chloride, Aliquat 336 (ILq). The flow behavior of these mixtures was analyzed by rheology, in order to obtain information concerning the flexibility, conformation and also, to identify the specific interactions (Figure 1). Based on these parameters were established the compatibility of compounds and implicitly, the optimal composition of the used ionic liquids, aspects which will allow the obtaining of membranes suitable for treatment or water purification.
Figure 1. Log–log plots of dynamic viscosity vs. shear rate for PSFQ/ILp and PSFQ/ILq at different mixing ratio
As is observed from Figure 1, the two ionic liquids act like as plasticizers, manifesting a thinning behavior, visualized by a decrease of the dynamic viscosity of blends compared with pure solution of PSFQ. The obtained results are useful in identifying of the most suitable mixing ratio of PSFQ/ILp and PSFQ/ILq blends that will have an impact on membranes obtaining with the performance required by water purification technology.
Acknowledgements
This work was supported by a grant of the Romanian Ministry of Education and Research, CCCDI - UEFISCDI, project number PN-III-P2-2.1-PED-2019-3013, within PNCDI III.
22
MODELING THE FUNCTIONALIZED POLYSULFONE FIBERS BY THE
ELECTROSPINNING PROCESS AND CONTROL OF SOLUTIONS PARAMETERS Anca Filimon1, Niculae Olaru1, Florica Doroftei1
1”Petru Poni” Institute of Macromolecular Chemistry, Aleea Grigore Ghica Voda 41 A, 700487 Iasi, Romania
e-mail: capataanca@yahoo.com Abstract
Industrial activities and increase of population worldwide have led to severe water and air contamination that result in major environmental concerns and cause adverse health effects.
The development of nanostructured materials by electrospinning technique destine for use in environmental applications is considered to be of importance in the effective removal of water and air contaminants. Among those, long and continuous polymer fibers with tunable properties (e.g., high surface-to-volume ratio, high porosity and permeability), and tailored functionalities are highly promising in environmental applications (e.g., in water remediation and in air filtration processes). In this context, one of the most versatile polymeric materials is functionalized polysulfones (e.g., quaternized polysulfones, PSFQ) that have found industrial and medical applications as advanced membranes due to many useful characteristics, such as hydrophilicity, antimicrobial properties, higher permeability, and better separation. Therefore, the solutions of the functionalized polysulfone, with a tunable density of quaternary ammonium functional groups, were processed by electrospinning to create new fibrous materials that can modulate membrane properties. In the present study the relationship between processable solution properties and morphological aspects was assessed by scanning electron microscopy (SEM) technique (Figure 1). Images were conducted to visualize and compare the differences in morphology and characteristics of nanofibers attribute to the effects of the structural features of PSFQ and concentrations of the solution used in electrospinning process.
Figure 1. SEM images of PSFQ fibers obtained in N,N- dimethylformamide (DMF) at the polymer concentration of 40% (A) and 45 % (B), respectively.
The results have shown that the morphology of fibers formed with different forms and dimensions can be attributed to the combined effects of the solution parameters associated with polymer and solvent properties (concentrations, viscosity, boiling point of the solvents, and the surface tension) and also, processing parameters related with the operation of electrospinning apparatus and environmental parameters (temperature, humidity, and local atmospheric conditions). Therefore, the combining these factors assure the PSFQ fibers performance in terms of morphological and surface characteristics and implicitly, the possibility of fibrous material applicability in environmental field.
Acknowledgements
This work was supported by a grant of the Romanian Ministry of Education and Research, CCCDI - UEFISCDI, project number PN-III-P2-2.1-PED-2019-3013, within PNCDI III.
23
CHARACTERIZATION OF NATURAL ZEOLITE (CLINOPTILOLITE) AS ONE OF THE HIGH CATION EXCHANGE CAPACITY GEOPOLYMER MATERIAL Nenad Grba1, Marina Šćiban2, Dejan Krčmar1, Sanja Panić2, Mirjana Petronijević2,
Slaven Tenodi1, Đurđa Kerkez1, Kristiana Zrnić Tenodi1, Dragan Radulović3 and Božo Dalmacija1
1University of Novi Sad, Faculty of Sciences, Department of Chemistry, Biochemistry and Environmental Protection, Trg Dositeja Obradovica 3, Novi Sad, Serbia,
2Faculty of Technology, University of Novi Sad, Novi Sad, Bulevar cara Lazara 1, Serbia
3Institute for Technology of Nuclear and other Mineral Raw Materials, Franse d’ Esperea 86, Belgrade, Serbia
e-mail: nenad.grba@dh.uns.ac.rs Abstract
The aim of this research is to subject one of the specific and locally used natural zeolite (clinoptilolite) from Vranjska Banja, Serbia with high cation exchange capacity. Mineralogical - X-Ray Diffraction Analysis (XRD), Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) and determination of cation exchange capacity (CEC) were investigated. Results showed homogenous structure with dominant clinoptilolite - heulandite type zeolites as most abundant minerals. The important aspect of this research is possibility of wider usage of natural zeolite-clinoptilolite due to cost-efficiency aspects of this natural material that can be exploited in large amount from several Serbian deposits e.g.
“Zlatokop” (Vranjska Banja) and “Igroš Vidojević” (Brus), Serbia. The present of higher, but also extremely concentration of heavy metals in Pannonian, Internal Dinarides and wider European region lead us to boost novel high performance but economically viable techniques.
The starting points are geochemical characterization of novel geo- materials before further water-treatment implementation.
Introduction
Natural zeolites are hydrated aluminosilicate minerals with valuable physicochemical properties, such as cation exchange, molecular sieving, catalysis and sorption. In the past decades, natural zeolites have found a variety of applications in adsorption, catalysis, building industry, agriculture, soil remediation, and energy. The use of natural zeolites for environmental applications is gaining new research interests mainly due to their properties and significant worldwide occurrence. Natural zeolite have also been reported for removal of anions and organics from water systems [1, 2, 3]. These materials are abundant and low-cost resources and have been found in many areas of the world. In this paper we will examine the characterization of zeolite (clinoptilolite) from the territory of the Republic Serbia.
The aim of this work is to determine the consistent, homogenity and high CEC capacity for local and economicly most affordable materilas in order to be use on fileds investigations on groundwater and industrial wastewater most suitable purification/remediation material in order to recommend it for best avaible water treatment technology as main adsorbent.
The need for new geomaterial with high to superhigh cation exchange capacity (CEC) have been stated in many scientific papers and fields investigations [1]. The aim of this study is to characterizes zeolites from the investigated area of Serbia, in this case specific geochemical composition from natural zeolite (clinoptiolite) from Vranjska Banja, Serbia.
24 Experimental
The primary sample of zeolite, natural zeolite (clinoptiolite) from Vranjska Banja, Serbia ca. 1 kg, was prepared and dried by Standard methods for sample preparation (SRPS B.B8.080.) in Dryer - "Binder" (sample distributor Jones). The sample was analyzed for mineralogical - XRD analysis, SEM/EDS and determination of CEC. XRD and SEM analysis of the matrices were done in order to elucidate the microscopic structures and morphology of surfaces and CEC analysis will show specific content of exchangeable cations in clinoptilolite sample.
Examination of the mineral composition of the sample was investigated by X-ray automatic powder diffractometer PHILIPS, model PW-1710. X-ray diffraction analysis was used to determine and monitor the phase composition of the sample. The intensities of diffracted CuK
X-rays radiation (=1.54178Å) were measured at room temperature at intervals of 0,02 2
and a time of 1 s in the range from 4 do 65 2. The X-ray tube was loaded with a voltage of 40 kV and a current of 30 mA, while the slots for directing the primary and diffracted beam were 1 and 0.1 mm. Method for determination of exchangeable cations Ca2+, Mg2+, Na+ and K+ and cation exchange capacity was DM 10-0/40.
Results and discussion
The sample was examined by X - ray diffraction on a polycrystalline sample (powder). The diffractogram of the tested sample is presenting the clinoptilolite - heulandite type zeolites as most aboundant minerals.
Figure 1. The diffractogram of the clinoptilolite (natural zeolites) from Vranjska Banja The presence of the following minerals was determined in the analyzed sample: clinoptilolite- heulandite type zeolites, feldspar, quartz, interstarted clays, carbonates and mica. The most common minerals are zeolite and then feldspar, while quartz is significantly less common. Of the feldspar, plagioclase is predominantly represented, relative to K-feldspar. Semiquantitative share crystalline phases (minerals) is as follows: zeolites ≈ 85%, feldspars ≈ 10%, clays ≤ 5%, quartz 2-3%. Carbonates, respectively calcite and mica are present in the trace.
25
The next important targeted analysis of sample surfaces is Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) method and results can be seen from Figure 2. The SEM-micrograph shoves from several to tents micrometers semi-homogenius structure and macro/meso-porosity. The brighter areas in the crystallites represent feldspar and darker areas clinoptilolite as in study from [4].
a) b)
Figure 2. SEM-micrograph (a) and (b) EDS spectrum of the natural zeolite (clinoptilolite) sample
Generally, the important ratio for clasification of zeolite from clinoptilolite type is Si/Al and according to the EDS analysis it was round 5.3 and accordingly higher then prescribed value of 4.5 [2] for this type. Additinaly, previous studies support this examination with also similar Ca, Fe, K, Mg and Na content (Table 1) and similar chemical composition on globescale1. Table 1. Results of EDS analysis from natural zeolite (clinoptilolite) from Vranjska Banja, Serbia compared with other related zeolites (clinoptilolite) samples
The results of determining the content of exchangeable cations are shown in Table 2:
Element [norm. wt.%] [norm. wt.%]4 [norm. wt.%]3
Silicon 69.98 65.63 70.90
Aluminium 13.19 12.97 12.40
Calcium 7.49 3.08 2.54
Iron 4.19 1.48 1.21
Potassium 3.57 1.33 4.46
Magnesium 1.12 1.41 0.83
Sodium 0.44 0.95 0.28
CEC (meq/g) 1.2 / 1.6–1.8
26
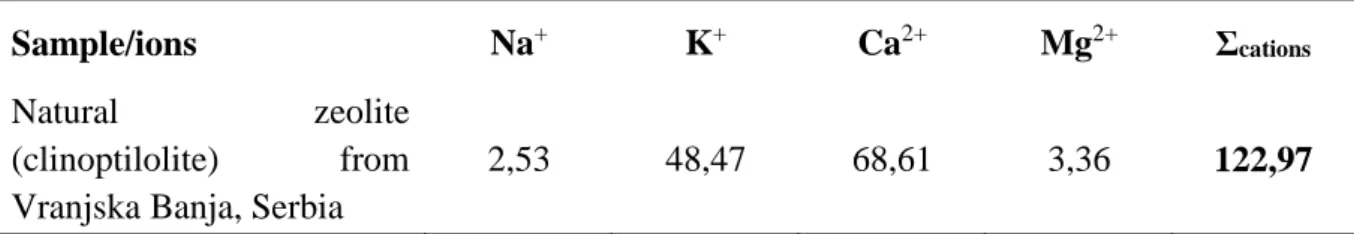
Table 2. Results of determining the content of variable cations of zeolite samples (meq/100g)
Sample/ions Na+ K+ Ca2+ Mg2+ Σcations
Natural zeolite
(clinoptilolite) from Vranjska Banja, Serbia
2,53 48,47 68,61 3,36 122,97
Due to many study observed in paper from Wang and Peng, 2008 the CEC value could clasify this zeolite as higher in the class with high potential for local but also comercial near region used as adsorbent with superhigh cation exchange capacity regarding previous [4,5] but also ongoing and future studies.
Conclusion
This research shows good potential and high CEC capacity of zeolite (clinoptilolite) from Vranjska Banja, Serbia. Future application will be based on this geochemical scaning of clinoptilolite as potentialy well structure and geo-chemicaly powerful purification material.
Acknowledgements
The authors acknowledge financial support of the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451-03-68/2020-14/200125 and No. 451-03-68/2020-14/200134) and the Innovation Fund of the Republic of Serbia (Grant No. 5717). The authors also acknowledge PhD Goran Kitić and MSc Jovana Stanojev from BioSense Institute (Novi Sad, Serbia), for the technical support from SEM/EDS analysis.
References
[1] S. Wang, Y. Peng: Natural zeolites as effective adsorbents in water and wastewater treatment, Chemical Engineering Journal 156 1 (2010) 11-24.
[2] C. Orha, F. Manea, A. Popi, G. Burtica, I. Fazakas Todea: Materials with Antibacterial Properties, Rev. Chim. (Bucuresti) 59 (2008) 173–177.
[3] S.K. Alpat, O. Ozbayrak, S. Alpat, H. Akcay: Journal of Hazardous Materials 151 (2008) 213–220.
[4] Š. C. Stefanović, N. Z. Logar, K. Margeta, N. No. Tušar, I. Arčon, K. Maver, J. Kovač, V.
Kaučič: Microporous and Mesoporous Materials, 105 3 (2007) 251-259
[5] Z. T. Sekulić, A.S. Daković, M. Kragović, M.A. Marković, B. Ivosević and B.M. Kolonja:
Hemijska Industrija, 67 (2013) 4.
27
PREPARATIVE PURIFICATION OF OCHRATOXIN A BY LIQUID-LIQUID CHROMATOGRAPHY
Zsófia Hegedüs1,2, Dominik Szabó1, Csaba Vágvölgyi1, András Szekeres1
1University of Szeged, Faculty of Science and Informatics, Departement of Microbiology, Közép fasor 52. Szeged H-6726
2Doctoral School in Biology, Faculty of Science and Informatics, University of Szeged, Szeged, Hungary
e-mail: hegedus.zsofia95@gmail.com Abstract
Ochratoxin A is an important mycotoxin, produced by different Aspergillus and Penicillium species hence there are strict regulations on its level in foods and feeds. Qualitative and quantitative measurements of this compound require relatively high amounts of pure ochratoxin A as a standard. This large amount of pure compound can be obtained by purifying the fermentation medium of the producing microorganisms. Liquid-liquid chromatography (LLC) seems to be a suitable method, which is becoming more common in the purification of natural compounds.
Introduction
Mycotoxins are toxic secondary metabolites produced by certain filamentous fungi. Among them ochratoxins have outstanding importance due to their high-level toxicity, which could cause remarkable problems in food and feed industry [1,2]. One of the most important member of this metabolite group is ochratoxin A, which was classified as possible human carcinogen (group 2B) by IARC [3]. In food safety laboratories, numerous methods are available for measuring these compounds from various matrices [4-6] requiring relatively high amounts of pure ochratoxins as standard compounds for both qualification and quantification. Generally, the chemical synthesis of ochratoxins can be accomplished with low yield [7], but higher amount of pure compound can be obtained by the purification of the fermentation environment of the producer microorganisms. Liquid-liquid chromatography may be a suitable method, whose application is becoming frequently used in the purification of natural compounds [8,9]. One of the technical implementations of this technique is the Centrifugal Partiton Chromatography (CPC), which was applied in our work for the separation of ochratoxins from the fermentation product and from each other.
Experimental
Aspergillus albertensis (SZMC 2107) was cultivated on yeast extract, sucrose media in dark at 28 °C. The incubation time and the sucrose content of the culture media was optimized for maximum ochratoxin production. For sample preparation a three-step acid-base extraction was used with ethyl acetate and sodium-bicarbonate as solvents. The crude extract was dispensed into 1,5 ml vials and were evaporated to dryness. For solvent system testing numerous three- and four-component biphasic systems were examined with the “Shake-flask” method. The selected systems were assembled and mixed in a test tubes, thereafter equal volumes of the phases were added to the vials containing the extract. The concentrations in the upper- and lower phase were measured by HPLC-UV technique. Partition coefficients (P) and separation factors (α) were calculated based on the concentrations, and the best system was selected for the purification procedure. During instrumental optimization suitable flow direction, flow rate and rotational speed was chosen. The purity of ochratoxin A and B in the collected fractions was calculated based on the areas of HPLC-UV chromatogram on 333 nm.
28 Results and discussion
At the beginning of our work the cultivation parameters of Aspergillus albertensis (SZMC 2107) were optimized. The maximum amount of ochratoxin A was measured after an incubation period of 8 days on liquid media containing 2 % yeast extract and 15 % sucrose. The crude extract obtained after liquid-liquid extraction contained ochratoxin A (OTA), ochratoxin B (OTB) and 9 major impurities. The extract was used for solvent system testing to find an appropriate biphase for the purification. Several compositions of one quaterner (hexane-ethyl- acetate-methanol-water) and 19 different type ternary systems were studied. The distribution coefficients and the separation factors of both OTA and OTB were in the proper range in a hexane-isopropanol-water system. After instrumental optimization the separation was carried out in ascendent mode at 10 ml/min flow rate and 2000 rpm rotational speed. The purities of OTA and OTB were more than 99% and 55 %, respectively.
Conclusion
The separation of ochratoxin A and B was accomplished using Centrifugal Partition Chromatography with the purities of more than 99 % and 55 %, respectively. Based on the results the developed method may be suitable for large scale purification of ochratoxin A in high purity, which is required for quantitative and qualitative measurements. Further investigation is necessary in order to increase the purity of OTB, and to confirm the purities by HR-MS and NMR techniques.
Acknowledgements
This work was supported by the Hungarian Scientific Research Fund by grants NKFI K-115690 and this work was connected to the project GINOP-2.3.2-15-2016-00012. The infrastructural background was established with the support of GINOP-2.3.3-15-2016-00006.
References
[1] Miraglia, M., De Dominicis, A., Brera, C., Corneli, S., Cava, E., Menghetti, E., and Miraslia, E. (1995). Ochratoxin a levels in human milk and related food samples: An exposure assessment. Nat. Toxins 3, 436–444.
[2] Duarte, S.C., Pena, A., and Lino, C.M. (2010). A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiology 27, 187–
198.
[3] IARC. Ochratoxin A. In IARC Monographs on the Evaluation of Carcinogenic Risk to Humans: Some Naturally Occurring Substances; Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; Volume 56, 489–521.
[4] Valenta, H. (1998). Chromatographic methods for the determination of ochratoxin A in animal and human tissues and fluids. Journal of Chromatography A 815, 75–92.
[5] Aboul-Enein, H.Y., Kutluk, Ö.B., Altiokka, G., and Tunçel, M. (2002). A modified HPLC method for the determination of ochratoxin A by fluorescence detection: Determination of ochratoxin A. Biomed. Chromatogr. 16, 470–474.
[6] Li, J., Liu, X., Han, S., Li, J., Xu, Q., Xu, H., Wang, Y., Liu, F., and Zhang, Z. (2012).
Analysis of Ochratoxin A in Wine by High-Resolution UHPLC-MS. Food Anal. Methods 5, 1506–1513.
[7] Kraus, G.A. (1981). A facile synthesis of ochratoxin A. J. Org. Chem. 46, 201–202.
[8] Endre, G., Hegedüs, Z., Turbat, A., Škrbić, B., Vágvölgyi, C., and Szekeres, A. (2019).
Separation and Purification of Aflatoxins by Centrifugal Partition Chromatography. Toxins 11, 309.
29
[9] Szekeres, A., Lorántfy, L., Bencsik, O., Kecskeméti, A., Szécsi, Á., Mesterházy, Á., and Vágvölgyi, Cs. (2013). Rapid purification method for fumonisin B1 using centrifugal partition chromatography. Food Additives & Contaminants: Part A 30, 147–155.
30
THE MECHANOCHEMICAL IMPLEMENTATION OF THE ENVIRONMENTALLY FRIENDLY ASYMMETRIC TRANSFER
HYDROGENATION OF KETONES Vanessza Judit Kolcsár,1* György Szőllősi2
1Department of Organic Chemistry, University of Szeged, Dóm tér 8, Szeged, 6720, Hungary,
2MTA-SZTE Stereochemistry Research Group, Dóm tér 8, Szeged, 6720, Hungary
*Corresponding author: kolcsar.vanessza@chem.u-szeged.hu Abstract
Optically pure compounds are essential in the synthesis of pharmaceuticals, fragrances and pesticides. Asymmetric catalytic reactions are the most favorable methods to achieve high conversions and enantiomeric excesses in fast reactions, using only catalytic amount of the chirality sources. Hydrogenations and transfer hydrogenations are well-studied procedures to obtain optically pure chiral alcohols. The transfer hydrogenation is convenient, as it ensures the possibility to use a hydrogen donor compound instead of hydrogen gas to provide the necessary H atom. In the past few decades several synthetic chiral compounds were used as the ligand of the catalyst complex, however these days more environmentally friendly implementations are favored. The use of natural chiral compounds would provide great opportunities to carry out asymmetric catalytic reactions using less organic solvents and producing less hazardous waste.
In our previous studies, we have developed an asymmetric catalytic system for the transfer hydrogenation of prochiral ketones using an in situ prepared Ru-chitosan complex in aqueous media. The catalyst prepared using the readily and easily available, biodegradable and inexpensive biopolymer provided good result in the transfer hydrogenation of various prochiral ketones in aqueous-phase reactions. In order to increase the preparative value of this method for preparing optically pure alcohols, we decided to use mechanical energy transmission instead of conventional thermally activated reactions carried out in magnetically stirred batch reactors.
With the former method the reaction time can be reduced, as well as the volume of the used solvent and the produced waste. After the optimization of the reaction conditions in the transfer hydrogenation of 4-chromanone, we examined the reaction of various ketones and the obtained results were compared with those reached in reactions carried out conventionally. All the examined ketones were transformed in similar degrees and the chiral alcohols were obtained in high enantiomeric excesses (ee). The reactions were scaled up as well, to prepare the optically enriched compounds in mmol quantities.
Introduction and aims
During the last few decades the protection of the environment, the application of sustainable and economical solutions play major role in science. Although the synthesis of optically pure compounds is well studied and successful as well, researchers have to develop greener and more sustainable methods to meet the standards of the environmental regulations. Optically pure compounds are widely used in pharmaceuticals, agrochemicals, flavors and fragrances.
Asymmetric catalytic transfer hydrogenations are convenient methods to transform prochiral unsaturated compounds into essential optically pure chemicals. Large variety of chiral complexes have been developed to catalyze these reactions [1]. Although excellent results were achieved, synthetic ligands do not meet the requirements of the recent economic and ecologic trends.
The use of chiral ligands from natural sources became essential, to develop new, economically and environmentally advantageous methods. Shell food industry produces great volume of chitin as waste, which could be further used in catalysis. In alkali solution chitin can