RegulatoRy pRocesses
of hungeR motivated behavioR*
L. Lénárd* * and Z. Karádi
neurophysiology Research group of the hungarian academy of sciences and institute of physiology, pécs university, medical school, h-7624 pécs, hungary
(Received: november 15, 2011; accepted: december 12, 2011)
While food intake and body weight are under homeostatic regulation, eating is a highly motivated and reinforced behavior that induces feelings of gratification and pleasure. The chemical senses (taste and odor) and their evaluation are essential to these functions. Brainstem and limbic glucose-monitoring (gm) neurons receiving neurochemical information from the periphery and from the local brain milieu are important controlling hunger motivation, and brain gut peptides have a modulatory role on this func- tion. The hypothalamic and limbic forebrain areas are responsible for evaluation of reward quality and related emotions. They are innervated by the mesolimbic dopaminergic system (MLDS) and majority of GM neurons are also influenced by dopamine. Via dopamine release, the MLDS plays an essential role in rewarding-reinforcing processes of feeding and addiction. The GM network and the MLDS in the limbic system represent essential elements in the neural substrate of motivation.
Keywords: hunger motivation – glucose-monitoring network – taste information processing – dopamine – reward
intRoduction
the most important argument of the drive-reduction hypothesis of learning and rein- forcement is the feeding per se [14]. The hunger drive is the consequence of a “need- state”: the lack of nutrients and/or excess of certain metabolites generate an excita- tory stage. Driven by hunger the organism exploring the environment finally discov- ers the food (reward) and during eating (consumption) the hunger-drive is reduced.
Because of its plausibility and attractiveness the drive-reduction theory has influ- enced neuroscience for decades even if it could explain only the positive reinforce- ment. The discovery of dual “hunger” and “satiety centers” in the hypothalamus (the lateral hypothalamic area and the ventromedial hypothalamic nucleus, respective- ly) [2] than in the amygdaloid body (the centromedial and basolateral parts of the amygdala, respectively) [7] also supported the drive-reduction hypothesis. Namely, it
* Dedicated to Professor József Hámori on the occasion of his 80th birthday.
** Corresponding author; e-mail: Laszlo.Lenard@aok.pte.hu
has been suggested that feeding is induced by the activation of the lateral hypotha- lamic “hunger center”. During feeding, due to stomach distension and elevation of blood glucose level, the ventromedial hypothalamic “satiety center” increases its activity which in turn inhibits the lateral hypothalamus.
the glucostatic [26], aminostatic [27] and lipostatic [19] theories of feeding are based on the concept of the hypothalamic dual centers and their regulatory processes correspond to a simple negative feed-back. These theories did not concern soma- tosensory and other perceptual events, only a viscerosensory – humoral feed-back regulation was taken into account. It should be emphasized, however, that eating is a highly motivated and reinforced behavior that not only provides nutrients needed for survival, but also induces feelings of gratification and pleasure. The sight, smell and taste of foods, the presence of secondary (learned) cues can evoke feeding, or – under certain circumstances – can stop feeding even in hungry animals. Therefore, it is obvious that feeding behavior is under multifactorial control of different cns mech- anisms.
peRipheRal feeding Related mechanisms
the cns, however, should continuously monitor the peripheral mechanisms, the events of the internal environment, especially within the gastrointestinal system. It has been shown that glucose infusion into the portal vein inhibits feeding even in hungry animal and it modifies the firing activity of the afferent vagal fibers and hypothalamic neurons [30]. Glucose-sensitive unit activity can be recorded from the portal and pancreatic vagal afferents [30] and vagal fibers originating from the small intestine carry glucose related information. The glucose and free fatty acid sensing system of the liver, pancreas and small intestine and mechanoreceptors of the stom- ach send their information up to the nucleus of the solitary tract and the dorsal vagal complex (NTS). It is remarkable that taste related information from the tongue and mouth also arrives to the NTS. Stomach distension stimulating stretch receptors leads to the release of gastrin releasing peptide (gRp) which triggers the peripheral release of other brain gut peptides such as cholecystokinin and glucagon involved in satie- ty [20]. Via the blood stream GRP acts on the CNS at the chemosensitive zone of the 4th ventricle (the area postrema) and via the afferent vagal nerve fibers it influences the activity of the NTS. It is important to emphasize that GRP containing neurons are located in the nts per se, and they send their axons to the hypothalamus, amygdaloid body and other limbic structures involved in the regulation of feeding. It has been shown that GRP microinjections into the 4th ventricle or into the hypothalamus inhibit feeding [21]. Microstructural analysis of feeding showed that GRP and its fragment neuromedin C injected directly into the amygdaloid body evoke character- istic behavioral satiety sequence of progression and this effect can be prevented by prior application of GRP receptor antagonists [5]. On the basis of these data it is clear that the cns receives humoral input via the area postrema and neural input via the vagal nerve and NTS in order to monitor the actual status of the internal environment.
it is also remarkable that brain gut peptides represent peripheral feeding related sig- nals and that the same neuropeptides within the cns – especially within the limbic system – form distinct neuronal circuits regulating hunger and satiety.
the glucose-monitoRing netWoRk
based on our previous results and related data of literature we propose a new model explaining hunger motivated behavior and food rewarded learning. The central core of this model is the glucose-monitoring (GM) network. In 1969 Oomura and his co workers [31] discovered glucose-sensitive neurons in the lateral hypothalamic
“feeding center” and glucose-receptor neurons in the ventromedial hypothalamic
“satiety center” of anaesthetized rats. Activity of glucose-sensitive neurons is sup- pressed by local electrophoretic application of d-glucose while glucose receptor neurons are facilitated by d-glucose. These two types of the so-called GM neurons were described later in the monkey hypothalamus and it has been shown that they exhibit characteristic activity changes during alimentary conditioning and are influ- enced by peripheral manipulation of blood glucose concentration [3].
gm neurons have been discovered in the area postrema [8], the nts, the amygdaloid body, ventral globus pallidus, nucleus accumbens (nac), mediodorsal prefrontal cortex (mdpfc) and the orbitofrontal cortex (obf) [16–18, 25, 28], the brain areas involved in the regulation of feeding and feeding related learning. The GM neurons are specific chemosensory cells monitoring neurochemicals of the local brain milieu and they receive humoral and viscerosensory information from the periphery via the area postrema, the vagal nerve and NTS, respectively. Activities of GM neurons are influenced by electrophoretically applied insulin, glucagon, free fatty acids, opioids, various other hormones, orexigenic and anorexigenic peptides. On the basis of these results one can suppose that peripheral mechanisms and different areas of the CNS form a “glucose-monitoring”, chemosensitive neuronal network and that the most important part of this network is located within the limbic system.
elements of this hierarchically organized network (area postrema, nts, hypo- thalamus, amygdaloid body, ventral globus pallidus, nac, mdpfc and obf) inte- grate neurochemical signals from the periphery, the local brain milieu and from other brain loci. Via feed-back and feed-forward regulation (anticipatory responses) they have efferent influences to the metabolism and endocrine events at the periphery. The first two key elements of the GM system are the chemosensitive zone of the area postrema and the NTS. Receiving humoral and neural inputs these brain sites direct- ly control the metabolic constituents and neurochemicals of the blood and cerebrospi- nal fluid. At the same time the NTS is the first relay station of the central taste system.
it is remarkable that the gm network and a central representation of the taste system (i.e. the NTS, hypothalamus, amygdala and OBF) highly overlap and it is obvious that they share common functions controlling metabolic processes, hunger motivation and food rewarded learning. The GM network includes those hypothalamic and lim-
bic-forebrain areas which are well known structures involved in learning and rein- forcement and which are innervated by the mesolimbic dopaminergic (da) system (MLDS).
taste and odoR Related infoRmation pRocessing
the hypothalamic and limbic forebrain areas are responsible for evaluation of reward quality and related emotions. The amygdaloid body has for long been associated with the control of emotions, motivation and hedonic tone [15]. It has been characterized as a region where representations of the internal and external world overlap, permit- ting the organism to assess and fulfill its physiological needs in relation to the external resources available [36]. The amygdaloid body plays a critical role in the initiation and guidance of feeding, which relies on an integration of autonomic and exterocep- tive information. Amygdaloid lesions interrupt both taste aversion and food rewarded learning. Different kinds of neurochemical lesions of the amygdaloid body, the lat- eral hypothalamus, nucleus accumbens and ventral globus pallidus lead to feeding disturbances and to serious learning deficits [22, 23]. In the hypothalamus and amyg- daloid body taste and odor sensitive neurons have been described in the rhesus mon- key [16, 18]. Similar neurons have been found in the ventromedial globus pallidus and OBF [17, 18, 25]. These taste and odor sensitive neurons exhibited characteristic activity patterns to changes of quality of food reward, during alimentary conditioning, during extinction of alimentary conditioned responses and during satiation. There is no doubt that these neurons are involved in the neural mechanisms of emotional and hedonic evaluation of food reward.
It has also been shown that majority of GM neurons localized in the above brain regions exhibit remarkable activity changes to taste and odor stimulations and are involved in the detection of familiar foods and in the evaluation of reward quality [16–18, 25]. The OBF has been identified as the “secondary taste cortex” and it is interconnected with the insular cortex (the primary taste cortex), the amygdaloid body, the globus pallidus and the hypothalamus. In the OBF the primary reinforcing value of taste and odor is represented, the texture of food is detected and bimodal olfactory and taste quality is evaluated creating the perception of flavor of foods [34].
these evaluations converge with visual information and earlier experiences related to food and are associated with the endogenous chemosensory information represented by the GM neuronal network.
dopamine, feeding and addiction
the mlds originating from the ventral tegmental area (the dopaminergic cell groups a10 and a8) innervates the hypothalamus, ventral pallidum, other limbic system structures and different cortical areas. Specific neurochemical (DA) lesions at differ-
ent levels of the mlds result in serious disturbances in hunger motivated behavior, food rewarded learning and conditioned taste aversion [23]. The nigrostriatal dopaminergic and the ascending noradrenergic (na) pathways were also supposed to play an important role in the regulation of feeding [1, 22, 37]. It has been shown that in different parts of the limbic system the specific catecholaminergic microlesions reducing the na content produce hyperphagia and body weight increase, while da depletions cause hypophagia and weight decrease [23]. The direction of body weight changes well correlated with the actual da/na ratio measured in the lesioned limbic areas. It has been hypothesized, therefore, that the MLDS and the NA systems repre- sent a balance mechanism influencing feeding behavior and maintaining physiologic body weight [23].
numerous experimental data suggest that the activation of the mlds is associated with arousal, stress, reflex facilitation, self-stimulation, reward, positive reinforce- ment and drug abuse [39]. It has been shown that electrical self-stimulation directly increases the activity of MLDS neurons [6]. Inhibition of DA synthesis or application of da receptor antagonists decreases the reinforcing value of electrical self-stimula- tion [38]. In the nucleus accumbens chemical self-addiction can be elaborated by amphetamine or by DA [13]. In place preference test and in other behavioral para- digms psychostimulants (such as cocaine or phenylcyclidine), morphine or nicotine significantly increase DA level [11]. Specific DA lesion in the nucleus accumbens inhibits cocaine self-addiction and in morphine dependent animals the morphine antagonist naloxon decreases the extracellular DA level [33]. At the onset of normal feeding or after neurochemically induced feeding by NA microinjections into the paraventricular nucleus of the hypothalamus, da level increases in the nucleus accumbens [10]. During food deprivation DA level dramatically drops while during eating the extracellular concentration of da increases in the amygdaloid body and the prefrontal cortex, too [9, 12]. Glucose infusion elevated and insulin injection decreased DA level in the amygdaloid body [9]. Rewarding effects of sweet solutions are related to da because during their consumption the extracellular level of da increases [12].
in our single unit recording experiments in rhesus monkeys it was shown that dur- ing elaboration of bar press feeding responses lateral hypothalamic da and morphine sensitive neurons were activated at the conditioned light stimulus and during the reward phase [24, 32]. These activity changes disappeared during satiation or after application of DA antagonists. During electrical stimulation of the ventral tegmental area monosynaptic responses (with 1–2 ms latency) were recorded from these cells showing that there is a direct influence of the MLDS on hypothalamic DA sensitive, reward related neurons [24]. Similar conclusion has been made by Schultz and coworkers [35] showing that due to the effect of the reinforcement predicting stimu- lus in the mlds neurons a characteristic bursting activity was recorded for several milliseconds. Based on these results it is obvious that the activity of the MLDS and that the da per se play essential roles in rewarding – reinforcing processes including feeding behavior and drug addiction [11–13, 24, 29]. It can be supposed that under
certain circumstances – via da release – eating acts as a psychostimulant and that release of rewarding DA during eating can explain bulimic symptoms. It is also sug- gested that binge-eating is a kind of addiction where food represents a natural reward- ing “drug” [11]. One may suppose, therefore, that in bulimic patients and in binge eaters eating is driven not for calories but for the rewarding DA.
It is remarkable that majority of GM neurons in the hypothalamus, ventral globus pallidus, amygdaloid body, NAC, mdPFC and OBF are influenced by local electro- phoretic application of DA [16, 17, 25]. It has been shown that hypothalamic and amygdaloid gm neurons also exhibit characteristic activity changes to morphine and these responses are attenuated by naloxone [28, 32]. These data clearly indicate that activity of the GM network is influenced by the MLDS and opiates suggesting the integrative role of GM neurons in feeding and reward related processes.
geneRation of adaptive behavioRal Responses
the emotional-motivational behavior depends on the mutual relationship of the actual physiological state of the organism and the environmental incentive stimuli [4].
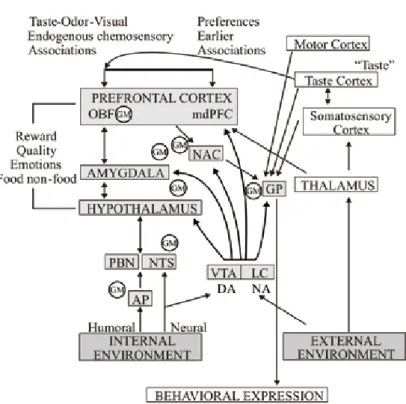
the animal will eat if it is hungry but food deprivation per se can lead to feeding only if the food or the appropriate incentives signaling the presence and availability of food are present. The actual physiological state of the organism is controlled by a peripheral viscerosensory mechanism which sends updated information to the central GM network. The GM network consists of chemosensitive neurons which highly integrate information of the peripheral metabolism with local brain neurochemical signals and taste and odor related information. Somatosensory (oropharyngeal), taste- and odor-related signals, as well as visual and acoustic cues are detected in the pri- mary cortices (localization and intensity discrimination) however, via serial and par- allel information processing they are sent to the limbic system and OBF, too. The hypothalamic and limbic forebrain structures are interconnected and tuned up by the rewarding DA which is released from the MLDS. These areas are responsible for evaluation of reward quality and related emotions and they form a special network which is the substrate of motivation, learning and reinforcement (Fig. 1). The output of the system generating the adaptive behavioral responses is activated by the nucle- us accumbens, amygdaloid body and hypothalamus, thus, via the globus pallidus, brainstem extrapyramidal motor regions, the endocrine system and vegetative/vis- ceral efferents behavioral responses are executed. Detailed cellular mechanisms of the network and identification of local interconnections of GM neurons remain to be elucidated, however.
acknoWledgements
the authors would like to express their thanks to andrás belvárácz and anikó lengyel for their technical contribution to this work. This study was supported by NKTH-OTKA K 68431, SROP-4.2.2/B-10/1-2010-0029, SROP-4.2.1/B-10/2/KONV-2010-0002, the Ajinomoto Co. Inc.
(51064/2009) and by the Hungarian Academy of Sciences.
RefeRences
1. Ahlskog, J. E. (1974) Food intake and amphetamine anorexia after selective forebrain norepinephrine loss. Brain Res. 82, 211–240.
2. Anand, B. K., Brobeck, J. R. (1951) Localization of a “feeding center” in the hypothalamus of the rat.
Proc. Soc. Exp. Biol. Med. 77, 323–324.
3. Aou, S., Oomura, Y., Lénárd, L., Nishino, H., Inokuchi, A., Minami, T., Misaki, H. (1984) Behavioral significance of monkey hypothalamic glucose-sensitive neurons. Brain Res. 302, 69–74.
Fig. 1. Schematic illustration of central regulatory processes of motivation. For more explanation see the text. Abbreviations: gm: population of glucose-monitoring neurons; ap: area postrema; da: dopamine;
na: noradrenaline; vta: ventral tegmental area; lc: locus coeruleus; pbn: parabrachial nucleus; nts:
nucleus of the solitary tract; nac: nucleus accumbens
4. Bindra, D. (1968) A unified interpretation of emotion and motivation. Ann. N. Y. Acad. Sci. 159, 1071–1083.
5. Fekete, É., Bagi, É. E., Tóth, K., Lénárd, L. (2007) Neuromedin C microinjected into the amygdala inhibits feeding. Brain Res. Bull. 71, 386–392 .
6. Fibiger, H. C., Phillips, A. G. (1987) Role of catecholamine transmitters in brain reward systems:
Implication for neurobiology of affect. In: Engel, J., Oreland, L. (eds) Brain Reward Systems and Abuse. Raven Press, New York, pp. 61–74.
7. Fonberg, E. (1974) Amygdala functions within the alimentary system. Acta Neurobiol. Exp. (Wars) 34, 435–466.
8. Funashi, M., Adachi, A. (1993) Glucose-responsive neurons exist within the area postrema of the rat:
In vitro study on the isolated slice preparation. Brain Res. Bull. 32, 531–535.
9. Hajnal, A., Lénárd, L. (1997) Feeding-related changes in extracellular dopamine in the amygdala of freely moving rats. Neurorep. 8, 2817–2820.
10. Hajnal, A., Mark, G., Rada, P., Lénárd, L., Hoebel, B. G. (1997) Norepinephrine microinjections in the hypothalamic paraventricular nucleus increase extracellular dopamine and decrease acetylcholine in the nucleus accumbens: Relevance to feeding behavior. J. Neurochem. 68, 667–674.
11. Hernandez, L., Hoebel, B. G. (1988) Food reward and cocaine increase EC dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 42, 1705–1712.
12. Hernandez, L., Hoebel, B. G. (1990) Feeding can enhance dopamine turnover in the prefrontal cortex.
Brain Res. Bull. 25, 978–979.
13. Hoebel, B. G., Monaco, A. P., Hernandez, L., Stanley, B. G., Aulisi, E. F., Lénárd, L. (1983) Self- injection of amphetamine directly into the brain. Psychopharmacol. 81, 158–164.
14. Hull, C. L. (1943) Principles of Behaviour. Appleton-Century-Crofts, New York.
15. Jones, B., Mishkin, M. (1972) Limbic lesions and the problem of stimulus-reinforcement associa- tions. Exp. Neurol. 36, 362–377.
16. Karádi, Z., Oomura, Y., Nishino, H., Scott, T. R., Lénárd, L., Aou, S. (1992) Responses of lateral hyptohalamic glucose-sensitive and glucose-insensitive neurons to chemical stimuli in behaving rhe- sus monkeys. J. Neurophysiol. 67, 389–400.
17. Karádi, Z., Faludi, B., Vida, I., Czurkó, A., Niedetzky, Cs., Sándor, P., Lénárd, L., Nishino, H. (1995) Glucose-sensitive neurons of the globus pallidus: II. Complex functional attributes. Brain Res. Bull.
37, 157–162.
18. Karádi, Z., Scott, T. R., Oomura, Y., Nishino, H., Aou, S., Lénárd, L. (1998) Complex functional attributes of amygdaloid gustatory neurons in the rhesus monkey. Ann. N. Y. Acad. Sci. 855, 488–
493.
19. Kennedy, G. C. (1953) The role of depot fat in the hypothalamic control of food intake in the rat. Proc.
Roy. Soc. 140, 578–592.
20. King, B. F. (1991) Bombesin and satiety. NIPS 6, 177–180.
21. Ladenheim, E. E., Ritter, E. C. (1988) Low dose fourth ventricular bombesin selectively suppresses food intake. Am. J. Physiol. 255, R988–R992.
22. Lénárd, L. (1977) Sex-dependent body weight loss after bilateral 6-hydroxydopamine injection into the globus pallidus. Brain Res. 128, 559–568.
23. Lénárd, L., Hahn, Z. (1982) Amygdalar noradrenergic and dopaminergic mechanisms in the regula- tion of hunger and thirst-motivated behavior. Brain Res. 233, 115–132.
24. Lénárd, L., Oomura, Y., Nishino, H., Aou, S., Nakano, Y., Yamamoto, T. (1986) Activity in monkey lateral hypothalamus during operant feeding. Modulation by catcholamines and opiate. In: Oomura, Y. (ed.) Emotions: Neuronal and Chemical Control, Japan Sci. Soc. Press, Karger S, AG, Tokyo/
Basel, pp. 45–53.
25. Lénárd, L., Karádi, Z., Faludi, B., Czurkó, A., Niedetzky, Cs., Vida, I., Nishinoo, H. (1995) Glucose- sensitive neurons of the globus pallidus: I. Neurochemical characteristics. Brain Res. Bull. 37, 149–
26. Mayer, J. (1955) Regulation of energy intake and the body weight. The glucostatic theory and the 155.
lipostatic hypothesis. Ann. N. Y. Acad. Sci. 63, 15–43.
27. Mellinkoff, S. M., Frankland, M., Boyle, D., Greipel, M. (1956) Relationship between serum amino acid concentration and fluctuation in appetite. J. Appl. Physiol. 8, 535–538.
28. Nakano, Y., Oomura, Y., Lénárd, L., Nishino, H., Aou, S., Yamamoto, T., Aoyagi, K. (1986) Feeding- related activity of glucose- and mophine-sensitive neurons in the monkey amygdala. Brain Res. 399, 167–172.
29. Nakano, Y., Lénárd, L., Oomura,Y., Nishino, H., Aou, S., Yamamoto, T. (1987) Functional involve- ment of catecholamines in reward-related neuronal activity of monkey amygdala. J. Neurophysiol. 57, 72–91.
30. Niijima, A. (1969) Afferent impulse discharges from glucoreceptors in the liver of the guinea pig.
Ann. N. Y. Acad. Sci. 157, 690–700.
31. Oomura, Y., Ono, T., Ooyama, H., Wayner, W. J. (1969) Glucose and osmosensitive neurons of the rat hypothalamus. Nature 222, 282–284.
32. Oomura, Y., Nishino, H., Aou, S., Lénárd, L. (1986) Opiate mechanism in reward related neuronal responses during operant feeding behavior of the monkey. Brain Res. 365, 335–339.
33. Pothos, E., Rada, P., Mark, G. P., Hoebel, B. G. (1991) Dopamine microdialysis in the nucleus accum- bens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment.
Brain Res. 566, 348–350.
34. Rolls, E. T., Critchley, H. D., Browning, A. S., Hernádi, I., Lénárd, L. (1999) Responses to the sen- sory properties of fat of neurons in the primate orbitofrontal cortex. J. Neurosci. 19, 1532–1540.
35. Schultz, W., Apicella, P., Ljundberg, T. (1993) Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning and delayed response task. J. Neurosci. 13, 900–913.
36. Turner, B. H., Mishkin, M., Knapp, M. (1980) Organization of the amygdalopetal projections from modality-specific cortical association areas in the monkey. J. Comp. Neurol. 191, 515–543.
37. Ungerstedt, U. (1971) Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigrostriatal dopamine system. Acta Physiol. Scand. Suppl. 367, 95–122.
38. Wauquier, A., Niemegeers, C. J. (1981) Effects of clopheramine, pyrilamine and astemizole on intrac- ranial self-stimulation. Eur. J. Pharmacol. 72, 245–248.
39. Willner, P., Scheel-Krüger, J. (1991) The Mesolimbic Dopamine System: From Motivation to Action.
Wiely Publishing Co., Chichester.