PANNON EGYETEM
GEORGIKON MEZŐGAZDASÁGTUDOMÁNYI KAR Állat- és Agrárkörnyezet-Tudományi Doktori Iskola
az Interdiszciplináris Doktori Iskola jogutódja
DOKTORI (PhD) ÉRTEKEZÉS
Sebestyén István
Investigation of Incidences and Developement of Spontaneous Tumours Typical of Crl: CD BR Rat Strain in Two Carcinogenecity Studies
Performed Under Similar Circumstances
Témavezető: Dr. habil.Várnagy László DSc,
Keszthely 2008
Investigation of Incidences and Developement of Spontaneous Tumours Typical of Crl: CD BR Rat Strain in Two Carcinogenecity Studies
Performed Under Similar Circumstances
A Crl: CD BR patkánytörzsre jellemző spontán tumorok kifejlődésének és incidenciájának vizsgálata két hasonló körülmények között elvégzett kétéves
karcinogenitási kísérlet alapján
Írta:
Sebestyén István
Készült a Pannon Egyetem Állat- és Agrárkörnyezet-Tudományi Doktori Iskola, mint az Interdiszciplináris Doktori Iskola jogutódja keretében
Témavezető: Dr. habil. Várnagy László D.Sc.
Elfogadásra javaslom (igen / nem)
(aláírás)
A jelölt a doktori szigorlaton …... % -ot ért el,
Az értekezést bírálóként elfogadásra javaslom:
Bíráló neve: …... igen /nem
……….
(aláírás) Bíráló neve: …... igen /nem
……….
(aláírás)
A jelölt az értekezés nyilvános vitáján …...% - ot ért el
Veszprém/Keszthely, ……….
a Bíráló Bizottság elnöke A doktori (PhD) oklevél minősítése…...
………
Az EDT elnöke
CONTENTS
1. Abstracts...4
2. Review of literature ...7
3. Materials and Methods ...32
3.1. Introduction...32
3.2. Experimental Animals...33
3.2.1. Hygiene and Health Conditions ... 34
3.2.2. Husbandry ... 34
3.2.3. Food and Feeding... 35
3.2.4. Water Supply... 37
3.2.5. Identification of Animals ... 37
3.2.6. Randomization ... 38
3.4. Observations...38
3.4.1. Clinical Observaions and Mortality ... 38
3.4.2. Body Weight ... 41
3.4.3. Food Consumption... 41
3.5. Laboratory Examinations...41
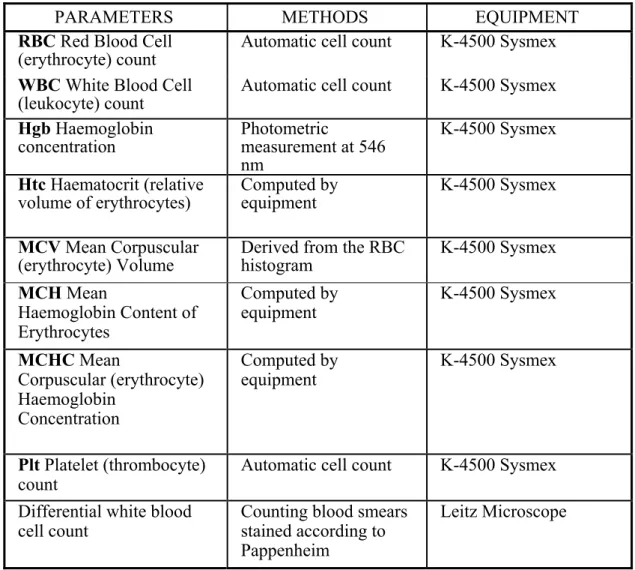
3.5.1 Haematology ... 41
3.6. Pathology and Histopathology ...42
3.6.1. Necropsy ... 42
3.6.2. Histopathology ... 43
3.7. Statistical Evaluation...45
3.8. Good Laboratory Practice (GLP) and Quality Assurance ...46
4. Results ...47
4.1. Clinical Observations and Mortality ...47
4.2. Body Weight ...50
4.3. Food Consumption...52
4.4. Laboratory Examinations...54
4.4.1 Haematology ... 54
4.5. Pathology ...54
4.6. Organ Weight...56
4.7. Histopathology ...57
5. Summary ...64
6. References ...71
7. Theses...79
Appendix 1 Individual mortality data 82
Appendix 2 Mean Body Weight 86
Appendix 3 Food Consumption 92
Appendix 4 Results of Haematological Investigation 98 Appendix 5 Summary of Organ Weight Investigation 100 Appendix 6 Summary Incidence of Histological Findings 102
Appendix 7 Breeder’s Historical Control Data 106
1. Abstracts
Uncontrolled body weight of experimental animals due to ad libitum overfeeding is the most significant variable affecting the long-term or carcinogenicity studies performed for regulatory purposes. The author in this assay summarize the observations, development and incidence of spontaneous tumours of the control groups of two carcinogenicity studies in Crl:CD Br rats performed under similar conditions, in the same laboratory and by the same staff. The most important difference between the two studies was the composition of the diet. The control group in the first study received
„normal” diet (ME: 12.3 MJ/kg), the control group of the second study was fed with
„low energy” diet (ME: 9.5 MJ/kg).
At starting 50 rats of both genders were used in the control groups of the studies.
Animals were observed for 104 weeks, particular attention was paid to observe the tumour development during the palpation examinations. The body weight and food consumption of animals were regularly measured. Haematological investigations were performed at 12th, 18th and 24th months. At termination detailed necropsy was performed and the weight of selected organs was measured. All organs and tissues fixed were subjected to histopathological examinations. At histopathological evaluations the observed neoplastic and non-neoplastic alterations were identified and their frequency was evaluated.
Summarising the results of our evaluations, the advantage of the feeding the experimental animals in the long term and carcinogenicity studies with Low Energy Diet occurred in the better survivals, mainly in male animals, in the better general health state of animals. The body weight gain of animals fed with Low energy diet was balanced in spite of the higher food consumptions. The higher food consumption can help to reach higher dose levels in the feeding studies. The feeding with the Low Energy Diet did not influence the measured haematological parameters, but the liver weights were higher than in the Normal Diet groups.
The total number of tumours was slightly higher in male and female animals fed with Low Energy Diet, than in the male and female groups fed with Normal Diet.
This operationally simple procedure significantly improves survival, exposure time, and statistical sensitivity for these bioassays to detect a true treatment effect in a 2-yr period.
A Crl: CD BR patkánytörzsre jellemző spontán tumorok kifejlődésének és incidenciájának vizsgálata két hasonló körülmények között elvégzett kétéves karcinogenitási kísérlet
alapján
KIVONAT
A dolgozat szerzője két hasonló körülmények között egymás után elvégzett kétéves karcinogenitási kísérlet kontroll csoportjaiban vizsgálta a normál (ME: 12,3 MJ/kg) és a csökkentett energiatartalmú (ME: 9,5 MJ/kg) táp etetése során a klinikai megfigyelésekben és a spontán daganatok kifejlődésében észlelhető különbségeket. Az eredmények összefoglalásaként megállapította, hogy kedvező hatás jelentkezett különösen a hím állatok túlélésében, a patkányok egészségi állapotában az alacsony energiatartalmú táp etetésekor, de a hematológiai paramétereket nem befolyásolta az eltérő összetételű táp fogyasztása. A megfigyelt tumorok összes száma azonban magasabb volt a csökkentett energiatartalmú tápot fogyasztó csoportokban.
Eine Untersuchung für Entstehung und Inzidenz der für die Crl:CD Br Ratten charakteristischen Spontantumoren aufgrund zwei, unter ähnlichen Bedingungen durchgeführten
zweijährigen Karzinogenität-Experimenten.
Auszug
Der Autor der Dissertation untersuchte in den Kontrollgruppen von zwei, unter ähnlichen Umständen durchgeführten Karzinogenität-Experimenten die bei der klinischen Beobachtung sowie bei den Spontantumoren entstandenen Unterschiede, die während Fütterung mit Futter von normalem (ME: 12,3 MJ/kg) Energiegehat und Fütterung mit Futter von niedrigem (ME: 9,5 MJ/kg) Energiegehalt entstanden sind.
Als Zusammenfassung der Ergebnisse stellte der Autor fest, daß sich eine günstige Wirkung zeigte, besonders beim Überleben der männlichen Tiere, bzw. beim Gesundheitsstatus der Ratten im Falle der Fütterung mit Futter von niedrigem Brennwert. Die hematologischen Parameter wurden aber infolge Einnahme von Füttern mit verschiedenen Zutaten nicht beeinflußt. Die Gesamtzahl der Tumoren war aber höher bei den Gruppen, die das Futter mit niedrigem Brennwert genommen haben.
2. Review of literature
Human cancer is probably as old as the human race. It is obvious that cancer did not suddenly start appearing after modernization or industrial revolution. The oldest known description of human cancer is found in an Egyptian seven papyri or writing written between 3000-1500 BC.
Hippocrates, the great Greek physician (460-370 B.C), who is considered the father of medicine is though to be the first person to clearly recognize difference between benign and malignant tumours.
Hippocrates noticed that blood vessels around a malignant tumour looked like the claws of crab. He named the disease karkinos (the Greek name for crab) to describe tumours that may or may not progress to ulceration. In English this term translates to carcinos or carcinoma.
During the Renaissance, beginning in the 15th century, physicians acquired greater knowledge of the anatomy and physiology of the human body. This started an era, which has seen the advancement of surgery and development of rational therapies based on clinical observations.
Jean Astruc and chemist Bernard, two 18th century physicians conducted research to confirm or disprove then current theories related to the origin of cancer. These efforts were the very first steps of experimental oncology. The art and science of seeking better diagnosis, treatments and understanding of the causes of cancer evolved from many who followed their path.
The early 20th century saw great progress in our understanding of microscopic structure and functioning of the living cells. Researchers pursued different theories to the origin of cancer, subjecting their hypotheses to systematic research and experimentation. A virus causing cancer in chickens was identified in 1911.
Existence of many chemical and physical carcinogens was conclusively identified during later part of the 20th century. Later part of the 20th century showed tremendous improvement in our understanding of the cellular mechanisms related to cell growth and division. Many factors that suppress and activate the cell growth and division were identified. [1]
Cancer progresses from the uncontrolled growth of cells to the formation of a primary tumour mass, vascularisation and subsequent spread (metastasis) of cancer cells to other parts of the body where secondary tumours may form.
Cells on their way to becoming cancerous accumulate a number of genetic and chromosomal abnormalities, which push the cell towards unrestricted growth. At first, clusters of genetically identical cells are formed, each cell dividing more rapidly than its normal neighbours.
This dysplasia, or dysplastic changes include atypical changes in the nuclei of cells, the cytoplasm, or in the growth pattern of cells. They are considered to be pre-cancerous and divide into two categories, depending on how far the cells have progressed: low-grade dysplasia and high-grade dysplasia. This abnormal cell growth in humans is almost always monoclonal [2] [3]
In the context of cancer, monoclonal refers to the fact that tumours arise from a single damaged cell
Low-Grade Dysplasia – Atypical changes in less than 50% of nuclei. Growth patterns in tissue appear to be normal. Also called ‘Hyperplasia’.
High-Grade Dysplasia – Atypical changes (enlarged nuclei and frequent divisions) in more than 50% of cells, together with distorted/irregular growth patterns.
A benign tumour is a slow-growing, non-life threatening tumour that is often surrounded by a fibrous capsule, either formed from healthy cells or the tumour itself. Although usually easy to remove surgically, these tumours can put pressure on neighbouring tissues and organs, resulting in compression and atrophy, occasionally becoming lethal through impairment of critical function.
Tumours are usually named after their tissue of origin, e.g. Adenomas occur in connective tissues, myomas in muscles, lipomas in adipose tissue.
The most frequent types of Benign tumours:
Cysts: lumps filled with fluid. Common types include sebaceous cysts on the skin, filled with greasy sebum, and ovarian cysts.
Nodules: formed in inflammatory conditions such as arthritis, sarcoid and polyarteritis.
Fibromas and fibroademonas: lumps of fibrous or fibrous and glandular tissue.
Haematoma: lump formed by blood escaping into the tissues - simply a large bruise.
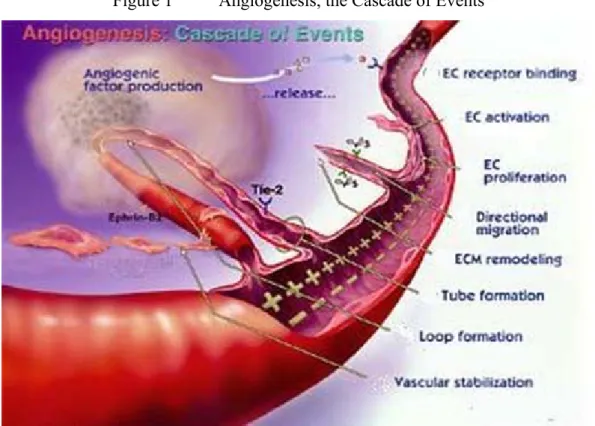
When the cell mass reaches a sufficient size, the cells release chemicals to recruit surrounding connective tissue and vascular cells to the tumour and induce them to grow into blood vessels, effectively growing their own blood supply from existing blood vessels, a process called angiogenesis (See Figure 1).
Figure 1 Angiogenesis, the Cascade of Events
The Angiogenesis Process:
• Tumour cells release angionec growth factors (e.g. Angiogenin, Angiopoietin-1, Del-1, Fibroblast growth factors: acidic (aFGF) and basic (bFGF) , Follistatin, Leptin, Placental growth factor, Tumour necrosis factor-alpha (TNF-alpha)) that diffuse into the nearby tissues.
• The angiogenic growth factors bind to specific receptors located on the endothelial cells (EC) of nearby pre-existing blood vessels.
• Once growth factors bind to their receptors, the endothelial cells are activated and signals are sent to the nucleus. Enzymes are released which create small pores in the basement membrane surrounding the existing blood vessels.
• The endothelial cells begin to proliferate, and migrate out through the pores of
the existing vessel towards the tumour tissue, whilst specialized adhesion molecules, or ‘integrins’ help to pull the new blood vessel sprout forward.
• Metalloproteinases (protein-degrading enzyme with a metal ion (Ca2+ or Zn2+) bound within its active site) are produced to disperse the tissue in front of the growing vessel, and as it extends, to remould it around the vessel.
• Endothelial cells proliferate to form the blood vessel tube, whilst individual vessels connect to form loops that can circulate blood.
• Finally, the newly formed blood vessels are provided structural support by specialized muscle cells. Blood flow then begins.
There is evidence that angiogenesis is initiated because cells in the interior of the tumour become starved of oxygen and the nutrients they need to continue growing. [4] [5]
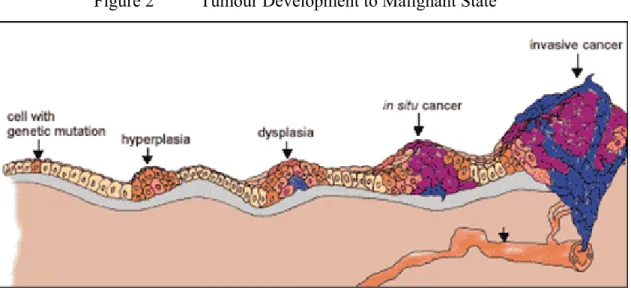
Whilst most benign tumours will remain contained indefinitely, some undergo a series of changes to develop from a pre-malignant to a malignant state (See Figure 2). This process is known as tumour progression, and is characterised by a new, irreversible and inheritable change in the cells that leads to tumour heterogeneity (a tumour with two or more differing populations of cells).
Figure 2 Tumour Development to Malignant State
If some cells experience additional mutations that allow them to escape from the surrounding capsule and migrate, as only white blood cells are designed to do, to invade neighbouring tissues and release cells into the blood or lymph, the tumour is said to have become malignant.
The escaped cells may establish new tumours (metastases) at other locations in the body.[6] [7] [8]
Since the vast majority of cells are designed to remain fixed within their tissue of origin, metastasis requires the cancer cells to escape the tumour mass and migrate through the connective tissue capsule to reach a blood vessel large enough to carry them to a new organ. Once there, they must pass through the blood-vessel wall into the new organ and there form new connections. Here angiogenesis has an additional consequence. Connective tissue and vascular cells release factors that stimulate the growth and motility of cancer cells, meaning that the new tumour can only grow effectively if it’s supported by the surrounding healthy tissues.
This new, metastatic tumour is often more damaging than the original primary tumour. The metastatic cancer cells act to displace functional cells and deprive them of nutrients, resulting in lethality if the affected organ(s) can no longer perform vital functions. Secondary tumours can also occur in large numbers, and spread out into numerous organs and body systems. One of the major sites for these metastatic tumours is in the lung, due to cancerous cells travelling through the pulmonary circulation and lodging in alveolar capillaries.
Malignant tumours are graded in four degrees of severity – 1 to 4 – in terms of the differentiation of the tumour cells. If the cells appear well differentiated (i.e. more normal), then the less aggressive the tumour, and the lower the grade. Grade 3 and 4 tumours are (nearly) undifferentiated and consequently, more aggressive/invasive. [9] [10]
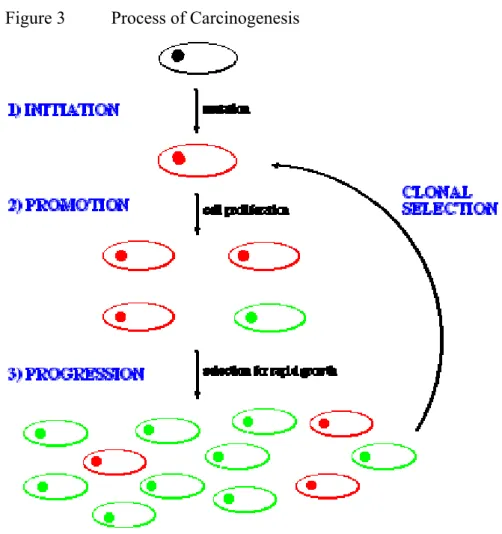
The initial neoplasm that forms is not malignant. Carcinogenesis occurs in a three- stage process (See Figure 3).
Figure 3 Process of Carcinogenesis
Initiation involves the formation of a mutant cell line through a change in the genetic information of a cell line. Promotion involves the division of the parent cell to produce many copies (NOTE: these are identical to the parent, and therefore termed clones). During this process, some of the clones pick up additional mutations and grow more rapidly than the others, a process termed progression. These cells therefore begin to outnumber the others. This process is repeated, the tumour cells becoming more aggressive after each cycle, in a process termed clonal selection. After 4-6 cycles the neoplasm becomes aggressive enough to become malignant. The loss of the usual characteristics of these cancerous cells is termed anaplasia.
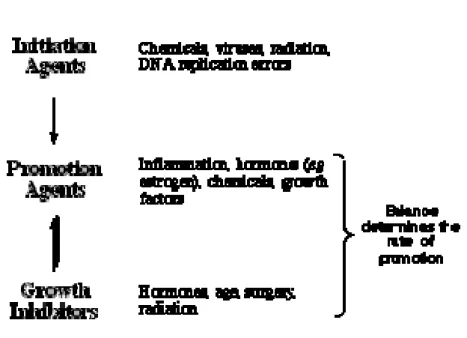
Factors influencing cancer development
Agents which promote tumour initiation are known as mutagens
Agents which promote malignancy (promotion and progression) are known as carcinogens (See Figure 4).
Figure 4 Factors Influencing Cancer Development
The chemical agents can be Alkylating agents, polycyclic aromatic hydrocarbons (PAHs), or naturally some of the pesticides. [11]
In recent years, chemical pesticides have become the most important consciously- applied form of pest management. This is a generalization of course; for some crops in some areas, alternative forms of pest control are still used heavily.
In case of pesticides, the other largest field of dangerous substances beside the pharmaceuticals The Plant Protection Products Directive (91/414/EEC), 'The Authorisations Directive', was adopted by the Council of Ministers on 15 July 1991 and published on 19 August 1991 (OJ L230, ISSN 0378 6978). It came into force on 26 July 1993 and is implemented in Hungary by the Plant Protection Law, „2000. évi XXXV. Törvény”, and the regulation „89/2004. (V. 15.) FVM rendelet, a növényvédő szerek forgalomba hozatalának és felhasználásának engedélyezéséről, valamint a növényvédő szerek csomagolásáról, jelöléséről, tárolásáról és szállításáról”. [12] [13] [14]
The main elements of the Directive are as follows:
To harmonise the overall arrangements for authorisation of plant protection products within the European Union. This is achieved by harmonising the process for considering the safety of active substances at a European Community level by establishing agreed criteria for considering the safety of those products. Product authorisation remains the responsibility of individual Member States
The Directive provides for the establishment of a positive list of active substances (Annex I), that have been shown to be without unacceptable risk to people or the environment
Active substances are added to Annex I of the Directive as existing active substances are reviewed (under the European Commission (EC) Review Programme) and new ones authorised.
Member States can only authorise the marketing and use of plant protection products after an active substance is listed in Annex I, except where transitional arrangements apply.
Before an active substance can be considered for inclusion in Annex I of Directive 91/414/EEC, companies must submit a complete data package (dossier) on both the active substance and at least one plant protection product containing that active substance. The data required:
• identify an active substance and plant protection product;
• describe their physical and chemical properties;
• their effects on target pests, and;
• allow for a risk assessment to be made of any possible effects on workers, consumers, the environment and non-target plants and animals.
Comprehensive lists of the data required to be evaluated to satisfy inclusion in Annex I of the Directive, or the authorisation of a plant protection product are set out in the Directive, (Annexes II and III). Annex II data relate to the active substance and Annex III to the plant protection product. These data are submitted to one or more Member States for evaluation. A report of the evaluation is submitted to the European Food Safety Authority (EFSA). Following peer review of the report EFSA makes a recommendation to the European Commission on whether Annex I inclusion is acceptable. This recommendation is then discussed by all Member States in the framework of the Standing Committee on the Food Chain and Animal Health (SCFA) previously the Standing Committee on Plant Health (SCPH). Where necessary, the Scientific Panel is consulted before the SCFA can deliver an opinion on whether an active substance should be included in Annex I of 91/414/EEC.
The "Uniform Principles" (Annex VI of Directive 91/414/EEC) establishing common criteria for evaluating products at a national level were published on 27 September 1997 (OJ L265, p.87).
Application of the Uniform Principles ensures that authorisations issued in all Member States are assessed to the same standards. [15]
The objectives of carcinogenicity studies are to identify a tumourigenic potential in animals and to assess the relevant risk in humans. Any cause for concern derived from laboratory investigations, animal toxicology studies, and data in humans may lead to a need for carcinogenicity studies. The practice of requiring carcinogenicity studies in rodents was instituted for pharmaceuticals that were expected to be administered regularly over a substantial part of a patient's lifetime.
The design and interpretation of the results from these studies preceded much of the available current technology to test for genotoxic potential and the more recent advances in technologies to assess systemic exposure. These studies also preceded our current understanding of tumorigenesis with nongenotoxic agents. Results from genotoxicity studies, toxicokinetics, and mechanistic studies can now be routinely applied in preclinical safety assessment. These additional data are important not only in considering whether to perform carcinogenicity studies but for interpreting study outcomes with respect to relevance for human safety. Since carcinogenicity studies are time consuming and resource intensive they should only be performed when human exposure warrants the need for information from life-time studies in animals in order to assess carcinogenic potential. In Japan, according to the 1990 “Guidelines for Toxicity Studies of Drugs Manual”, carcinogenicity studies were needed if the clinical use was expected to be continuously for 6 months or longer. If there was cause for concern, pharmaceuticals generally used continuously for less than 6 months may have needed carcinogenicity studies. In the United States, most pharmaceuticals were tested in animals for their carcinogenic potential before widespread use in humans. According to the US Food and Drug Administration, pharmaceuticals generally used for 3 months or more required carcinogenicity studies. In Europe, the Rules Governing Medicinal Products in the European Community defined the circumstances when carcinogenicity studies were required. These circumstances included administration over a substantial period of life, i.e., continuously during a minimum period of 6 months or frequently in an intermittent manner so that the total exposure was similar. (ICH Harmonised Tripartite Guideline S1A, Guideline on the Need for Carcinogenicity Studies of Pharmaceuticals) [16] [17] [18]
In Europe according to the 91/414/EEC Directive the long-term studies should be conducted and reported, taken together with other relevant data and information on the active substance, must be sufficient to permit the identification of effects, following repeated exposure to the active substance, and in particular must be sufficient to:
— identify adverse effects resulting from exposure to the active substance,
— identify target organs, where relevant,
— establish the dose-response relationship,
— identify changes in toxic signs and manifestations observed, and
— establish the No Adverse Effect Level (NOAEL).
Similarly, the carcinogenicity studies taken together with other relevant data and information on the active substance, must be sufficient to permit the hazards for humans, following repeated exposure to the active substance, to be assessed, and in particular must be sufficient:
— to identify carcinogenic effects resulting from exposure to the active substance,
— to establish the species and organ specificity of tumours induced,
— to establish the dose-response relationship, and
— for non-genotoxic carcinogens, to identify the maximum dose eliciting no adverse effect (threshold dose).
The long-term toxicity and carcinogenicity of all active substances must be determined. If in exceptional circumstances, it is claimed that such testing is unnecessary, that claim must be fully justified, i.e. toxicokinetic data demonstrates that absorption of the active substance does not occur from the gut, through the skin or via the pulmonary system.
A long-term oral toxicity and carcinogenicity study (two years) of the active substance must be conducted using the rat as test species; these studies can be combined. [19] [20]
A carcinogenicity study of the active substance must be conducted using the mouse as test species.
Where a non-genotoxic mechanism for carcinogenicity is suggested, a well argued case, supported with relevant experimental data, including that necessary to elucidate the possible mechanism involved, must be provided.
While the standard reference points for treatment responses are concurrent control data, historical control data, may be helpful in the interpretation of particular carcinogenicity studies. Where submitted, historical control data should be from
the same species and strain, maintained under similar conditions and should be from contemporaneous studies. The information on historical control data provided must include:
— identification of species and strain, name of the supplier, and specific colony identification, if the supplier has more than one geographical location,
— name of the laboratory and the dates when the study was performed,
— description of the general conditions under which animals were maintained, including the type or brand of diet and, where possible, the amount consumed,
— approximate age, in days, of the control animals at the beginning of the study and at the time of killing or death,
— description of the control group mortality pattern observed during rat the end of the study, and other pertinent observations (e.g. diseases, infections),
— name of the laboratory and the examining scientists responsible for gathering and interpreting the pathological data from the study, and
— a statement of the nature of the tumours that may have been combined to produce any of the incidence data.
In the collection of data and compilation of reports, incidence of benign and malignant tumours must not be combined, unless there is clear evidence of benign tumours becoming malignant with time.
Similarly, dissimilar, un-associated tumours, whether benign or malignant, occurring in the same organ, must not be combined, for reporting purposes. In the interests of avoiding confusion, terminology such as that developed by American Society of Toxicologic Pathologists, or the Hannover Tumour Registry (RENI) should be used in the nomenclature and reporting of tumours. The system used must be identified.
As mentioned in the EEC Directive, the role of historical control data is very important during the evaluation of the results of the carcinogenicity studies.
Caloric intakes are now recognized to be important uncontrolled variables in bioassays because rodents chronically fed ad libitum become obese, reproductively senile and have increased incidences of age-related diseases, higher tumour burdens and decreased survival. The available literature suggests that ad libitum feeding neither optimizes the health and well-being of rodents nor
provides the best model for use in evaluation of pharmacological and toxicological profiles. Use of an optimized diet, restricted in terms of caloric intakes, has been proposed for chronic toxicity and carcinogenicity studies in rodents. [80, 81]
Caloric restriction and experimental tumorigenesis are evaluated in the article of Nutrition Today by David Kritchevsky as cited below:
Although dietary guidance directed to the reduction of cancer risk emphasizes reduction in fat intake, this article documents evidence from animal studies that caloric balance either through reduced intake or increased expenditure of energy without a reduction in fat is even more effective in controlling tumour growth.
Dietary fat has been correlated positively with incidence of human tumours, especially those of the colon and breast. Diets high in fat have also been shown to accelerate the growth of chemically induced tumours in mice and rats. Diets high in fat are generally diets high in calories. In 1975 Berg[21] and Howell[22]
suggested that it might be pertinent to correlate tumour risk with overall dietary life-style. Indeed, in the 1920s Frederick L. Hoffman, a consulting statistician with the Prudential Insurance Company, suggested that cancer was associated with over-nutrition.[23]
In 1909 Moreschi reported that underfeeding significantly retarded the growth of transplanted tumours in mice.[24] His finding stimulated interest in this phenomenon and for the next 15 years there was considerable activity in this area of research. The research showed that underfeeding inhibited the growth of spontaneous and transplanted tumours in rodents. This area of research was then quiescent until 1940 when Tannenbaum began further work on the effects of underfeeding.
In underfeeding studies the test animals are fed a fraction of the same diet that is being fed to the controls. This feeding mode can result in deficiencies of micronutrients if the intake of the control just satisfies the control's needs. This was recognized by Tannenbaum, who began to feed a synthetic diet after demonstrating that underfeeding reduced significantly the appearance of spontaneous mammary tumours in mice. Tannenbaum's diet consisted of commercial dog or fox chow, skim milk powder and starch. Calories were reduced by deleting various amounts of starch. Using this diet he showed that
caloric restriction reduced the incidence of induced or spontaneous tumours in several strains of mice. Tannenbaum published extensively in the 1940s and summarized his findings in a review.[25]
In 1943 Lavik and Baumann[26] studied the influences of fat and calories on the incidence of chemically induced skin tumours in mice. They found (Table 1) that rats fed a high-fat, low-calorie diet exhibited 48% fewer tumours than those fed a low-fat, high-calorie diet. Recently Albanes [27] summarized the results of 82 studies in mice and also found that diets high in calories but low in fat were distinctly more carcinogenic than those low in calories but high in fat (Table 1).
Table 1 Relative Effects of Calories and Fat on Methylcholanthrene- Induced Skin Tumours in Mice(*) Regimen
Fat Calories Tumour Incidence (%)
Low Low 0
Low High 54
High Low 28
High High 66
(*) After Lavik and Baumann.[26]
The authors [28,29] have shown that the incidence of both colon and mammary tumours is significantly lower in rats whose caloric intake is 40% lower than that of ad libitum fed controls. The fat content of the calorically restricted diet was more than double that of the ad libitum diet. Boissonneault et al.[30] confirmed earlier findings that a low-fat (5%) diet was 41% less carcinogenic than a high-fat (30%) diet in rats treated with a mammary carcinogen. However, when a high-fat (30%) diet was fed on a restricted basis, so that the calorie-restricted rats ingested 10.5% less fat a day (2.2 g versus 2.7 g), tumour incidence was reduced by 90%.
Most of the foregoing studies were carried out using diets that contained a low level of fat. What would happen in rats fed large amounts of fat? To answer this, three groups of rats were given a mammary carcinogen and fed ad libitum diets containing either 5, 15 or 20% corn oil. Two other carcinogen-treated groups of rats were fed diets in which calories were restricted by 25% and which contained either 20% or 26.7% fat. Thus, each rat fed the calorie-restricted 20% fat diet ingested exactly the same amount of fat daily as did the ad libitum-fed group given 15% fat. The same is true for the groups fed 20% fat (ad libitum) or 26.7%
fat (25% restricted). [32] It is clear that in the ad libitum-fed rats increasing fat from 5 to 15% increased tumour incidence, tumour multiplicity and tumour burden. At 20% fat intake there was no further increase in tumour incidence, but tumour multiplicity rose by 37% and tumour burden by 79% when compared with rats fed 15% fat. In rats fed the same level of fat but 25% fewer calories, tumour incidence and other tumour characteristics were reduced significantly.
How early must caloric restriction be instituted to be effective? Tannenbaum studied inhibition of spontaneous mammary tumorigenesis as a function of time at which caloric restriction was begun. Restriction started at 2, 5 or 9 months resulted in inhibition of 100, 95 and 80%, respectively. Weindruch and Walford [33] placed tumour-prone mice on a restricted regimen when they were 1 year old.
This action increased their life span by 12% and decreased incidence of hepatomas, lymphomas, lung or other tumours by 7, 34, 50 and 63%, respectively.
Ross and Bras [34] found that drastic caloric restriction (60+%) for only 7 weeks after weaning did not influence the life span of rats but reduced the incidence of spontaneous tumours by 38%. A study of caloric restriction instituted at various times after carcinogen administration showed that tumour incidence was correlated with feed efficiency.[35]
Another way of influencing caloric flux is exercise. Data now available show that exercise reduced the incidence of chemically induced tumours in rats. In man it has been shown that men who work at other than sedentary jobs exhibit a decreased risk of colon cancer. [36, 37]
The foregoing suggests that insofar as risk is concerned total caloric intake may be more culpable than fat intake per se. Overweight has been shown to present a risk for any number of tumours in both women and men.[38] Dietary guidelines exhort us to eat a variety of foods and to maintain desirable weight. Variety and moderation may be the most prudent and most workable suggestions. In a study of the relationship of energy intake to colon cancer risk Lyon et al. [39] stated,
"Total energy intake must be evaluated before attempting to assign a causal role to any food or nutrient that may be postulated to play a role in colon cancer." In 1945 Potter [40] recommended that we eat less and exercise more. Nothing has happened since then to contradict this advice
It is well accepted that hormonal, dietary and genetic factors each influence breast
cancer risk. However, the underlying mechanisms and the extent to which these factors interact are largely unknown. The authors on AICR's 11th Annual Research Conference on Diet, Nutrition and Cancer [41] have demonstrated that the female ACI rat exhibits a unique genetically conferred propensity to develop mammary cancers when treated with physiological levels of 17ß-estradiol (E2).
More recently, they mapped to rat chromosome 5 a strong genetic modifier of susceptibility to E2-induced mammary cancers, termed estrogen-induced mammary cancer 1 (Emca1), and have identified potential Emca1 candidate genes. Because estrogens have been inextricably linked to the genesis of breast cancer in humans, the ACI rat model has the potential to reveal novel physiologically relevant insights into how the contributory actions of E2 are modified by specific dietary factors. In the present study, the authors examined the ability of a 40% restriction of dietary energy consumption to inhibit E2- induced mammary carcinogenesis. The hypothesis tested was that energy restriction will inhibit mammary carcinogenesis even when circulating E2 remains elevated through administration of exogenous hormone. The data presented strongly suggest that energy restriction inhibits E2-induced mammary carcinogenesis in the ACI rat at least partly by retarding progression of atypical hyperplastic foci to carcinoma.
In overwiev of Roger Segelken [42] laboratory rats, fed diets composed of substantially reduced intakes of protein, consume more energy but gain slightly less weight and exhibit increased thermogenesis due both to enhanced metabolic body heat and to diet-driven physical activity," said Campbell at the recent Conference on the Role of Diet and Caloric Intake in Aging, Obesity and Cancer, in Reston, Va. Also, he reported, these same rats show sharply reduced blood cholesterol concentrations and tumour development. Details of the theory will be published in the American Journal of Clinical Nutrition by Campbell, the Jacob Gould Schurman Professor of Nutritional Science at Cornell, and by Dr. Junshi Chen of the Chinese Academy of Preventive Medicine's Institute of Nutrition and Food Hygiene.
The Cornell rat studies, in which lab animals ate diets ranging from 5 percent to 20 percent protein in the form of casein from cow milk, found another remarkable result: Rats on low-protein diets voluntarily exercised more. Given a choice of
lounging around their cages or climbing on exercise wheels, the low-protein-fed rats spent more time burning calories compared with rats on moderate- and high- protein diets.
In 1980 Silvermann [43] studied the effect of dietary fat levels on the induction of mammary cancer by 350 rads total-body x-radiation given to noninbred albino Sprague-Dawley rats at 50 days of age. Compared to rats on a low-fat (LF) diet (5% lard), rats on a high-fat (HF) diet (20% lard) from 30 days of age had more tumours, with a higher multiplicity of carcinomas per rat. LF-fed groups exhibited a longer median tumour latency period than did HF-fed groups. A similar trend toward more tumours with an earlier time of death was seen in rats given single iv doses of 50 mg 1-methyl-1-nitrosourea/kg and fed an HF diet as compared to an LF diet.
French scientists [44] investigated whether the oxidative status of an 18:3(n-3) polyunsaturated fatty acid (PUFA)-enriched diet could modulate the growth of chemically induced rat mammary tumours, three independent experiments were performed. Experiments I and II examined the variation of tumour growth by addition of antioxidant (vitamin E) or a prooxidant system (sodium ascorbate/2- methyl-1,4-naphthoquinone) to a 15% linseed oil diet rich in 18:3(n-3).
Experiment III addressed the role of PUFA in the tumour growth modulation by vitamin E. For this purpose, we compared the effect of vitamin E in 15% fat diets containing a high level of 18:3(n-3) (linseed oil, high-PUFA diet) or devoid of 18:3(n-3) (hydrogenated palm/sunflower oil, low-PUFA diet). In Experiments I- III, tumour growth increased in the presence of vitamin E compared with control (without vitamin E). Furthermore, it decreased when prooxidant was added. In contrast, no difference was observed when the diet was low in PUFA, suggesting that sensitivity of PUFA to peroxidation may interfere with tumour growth. This observation was supported by growth kinetic parameter analysis, which indicated that tumour growth resulted from variations in cell loss but not from changes in cell proliferation. These data show that, in vivo, PUFA effects on tumour growth are highly dependent on diet oxidative status.
A low-calorie diet slows the progress of prostate cancer in animals, new research showed in Ohio State University Comprehensive Cancer Center—Arthur G.
James Cancer Hospital and Richard J. Solove Research Institute [45]. The slowing of tumour progression occurred whether the calories were reduced by cutting fat, carbohydrates, or the overall diet. The results further suggested the way that the lower-calorie diet slowed tumour growth in rats and mice -- it retarded the development of new blood vessels in the tumour.
In addition, these results can help clinical investigators design studies of the relationship between diet and prostate cancer in humans.
The paper by Clinton and a team of researchers [47] appeared in the March 17 issue of the Journal of the National Cancer Institute. It involved three sets of experiments, two using rats and one using mice.
In the first set of experiments, malignant cells from a form of rat prostate cancer were transplanted into four groups of cancer-free rats. One group of rats was allowed to eat as much as desired. Castrated rats made up a second group that was also allowed to eat freely. The third and fourth groups were fed diets containing 20 percent and 40 percent fewer calories, respectively, than the groups with restricted diets.
The researchers used castrated rats because depriving prostate tumours of male hormones, androgens, is one of the most effective ways of slowing prostate tumour growth. Clinton wanted to know how diet would affect tumour growth compared to the gold standard of androgen deprivation.
The second experiment also involved groups of castrated and non-castrated rats that were allowed to eat at will, plus three other groups fed 30 percent fewer calories. The groups differed in the source of caloric restriction. One group had fewer calories from fat, another had fewer calories from carbohydrates, and a third had fewer calories in the overall diet.
After 16 weeks, the researchers removed, weighed, and measured the tumours.
They also examined tumour structure, rates of cell proliferation and cell death, and numbers of blood vessels within each tumour.
Last, they measured the activity of the gene for vascular endothelial growth factor (VEGF), a substance produced by tumour cells that stimulates blood vessel growth.
In the third set of experiments, the researchers transplanted human prostate- tumour cells into mice lacking an immune system (i.e., severe combined immunodeficient [SCID] mice). This approach allowed the investigators to
determine if human prostate cancer cells might also be sensitive to the dietary intervention.
Four groups of mice were used. One group was allowed to eat at will, while the other three were fed diets containing 30 percent fewer calories. As in the preceding rat experiment, one group received total dietary restriction, while the second and third groups were restricted by fat and carbohydrates.
All experiments ran for 16 weeks. Non-castrated rats from the first experiment had tumour diameters averaging 2.2 cm. Castrated rats, on the other hand, had tumour diameters only about one-fourth that size on average.
Animals fed 40 percent fewer calories had tumours averaging about 60 percent the size of castrated rats, while those fed 20 percent fewer had tumours that were three-quarters the size of those in castrated animals.
The second rat experiment showed that the type of caloric restriction -- whether overall, or from fat or carbohydrate -- had no significant influence on tumour size.
All slowed tumour growth to a similar degree.
The mouse experiment using transplanted human prostate-tumour cells also showed that a diet lower in calories slowed prostate tumour growth, regardless of where the calories were cut.
The study also suggested that both castration and a low- calorie diet reduced angiogenesis -- the growth of new blood vessels -- in prostate tumours, and the production of growth factors by tumour cells that promote angiogenesis.
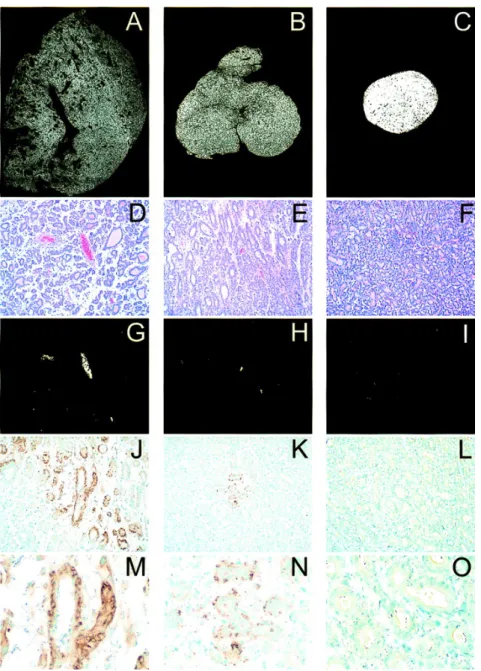
Mukherjee et al., presented the representative histology and immuno- histochemistry pictures from the Dunning R3327-H prostate adenocarcinoma in rats in US Journal of the National Cancer Institute [46] (See Figure 5).
Figure 5 Representative histology and immunohistochemistry from the Dunning R3327-H prostate adenocarcinoma in rats
In Figure 5, Panels A, B, and C: tissue density images of prostate tumours from a rat fed ad libitum, a rat fed at 30% total diet restriction, and a castrated rat, respectively. Tumour sections from ad libitum fed rats show heterogeneously sized glands and ducts with extensive intraluminal secretions. Numerous large and small vascular channels are readily observed. The space between glands and ducts is composed of a loose connective tissue matrix. Tumours from energy-restricted rats show smaller and more homogeneous glands with less conspicuous luminal secretions and with fewer identifiable vascular structures. The matrix is more cellular and dense. Tumours from castrated rats show numerous, small homogeneous glands separated by a thin rim of dense matrix. Panels G, H, and I:
computerized images generated to show the areas composed of red blood cells within vascular channels of sections shown in D, E, and F, respectively.
Examination of high-power fields of tissue obtained from ad libitum fed rats revealed several large vessels and a large number of microvessels. In contrast, only rare sections of tumours from rats fed energy-restricted diets contained large vessels, and the number of microvessels was reduced. Tumours from castrated animals showed few vascular structures containing red blood cells. Calculations from multiple sections show a mean ± standard deviation (SD) for vascular area of 2.6% ± 0.2% for ad libitum fed rats, 0.3% ± 0.2% for energy-restricted rats, and
<0.1% (below resolution) for castrated rats. Panels J, K, and L:
immunohistochemical staining for vascular endothelial growth factor (VEGF) at low magnification, revealing the extensive staining of the glandular elements of the tumours from ad libitum fed rats in J, while K shows the only focus of staining observed in any tumour section from rats fed energy-restricted diets. Tumours from castrated rats showed no evidence of VEGF staining. Image analysis indicates VEGF staining in 24.5% ± 7.5% (mean ± SD) of the tumour area in ad libitum fed rats compared with 0.4% ± 0.8% for energy-restricted rats and no staining in tumours from castrated rats. Panels M, N, and O: a high-power image illustrating the intense immunohistochemical localization of VEGF to the intracellular compartment of the glandular cells from an ad libitum fed rat (M) and less intense staining in the small focus in a tumour from a rat fed an energy- restricted diet (N). They did not observe VEGF staining in any tumour section from castrated rats.
Cutting Calories Reduces Prostate Cancer Growth in Laboratory Animals
The growth of prostate cancers in rats and mice can be substantially reduced by cutting calories in the animals' diets. These results, which the authors believe to be the first to show the effects of energy intake on prostate tumour growth, are presented in the March 17 issue of the Journal of the National Cancer Institute by Purna Mukherjee, Ph.D., of the Dana Farber Cancer Institute, Steven Clinton, M.D., Ph.D., of the Ohio State University, and colleagues.[47]
In a series of experiments with rats, slices of prostate tumour tissue (Dunning R3327-H prostate adenocarcinoma derived from a spontaneous rat tumour) were transplanted into male rats fed a variety of calorie-restricted diets. The tumours were allowed to grow for 16 weeks, then were removed, weighed, and examined for changes in tumour structure and gene expression. A similar set of experiments was completed with a human prostate carcinoma grown in immunodeficient mice.
In one set of experiments, the control rats were allowed to eat at will while others, consuming the same diet, had energy consumption cut by 40% or 20%. Compared with controls, tumour weight was reduced 76% in rats with a 40% energy restriction and 62% in rats with a 20% energy restriction. In another experiment, total energy was cut by 30% by specifically removing calories from dietary fat or carbohydrate. Interestingly, each type of energy restriction (total diet, fat calories, or carbohydrate calories) produced a similar inhibition of prostate tumour growth.
There was no evidence that high-fat diets promote prostate tumour growth under energy-restricted conditions.
The authors also examined tumour tissues and blood to determine mechanisms whereby diet restriction may influence tumour growth. Energy restriction reduced the concentration of circulating insulin-like growth factor-I, a hormone that has been associated with increased prostate cancer risk in human epidemiologic studies. An examination of tumours showed that energy restriction produced a pattern of changes in tumour cell proliferation, tumour apoptosis (programmed cell death), and tumour blood vessel density. These latter changes suggest that the dietary effects are mediated by inhibiting the growth of tumour blood vessels (angiogenesis), a process required for tumour growth. Thus, the authors conclude that biomarkers of tumour angiogenesis may be useful tools in identifying effective dietary strategies for reducing prostate cancer etiology or progression.
In an accompanying editorial, Bosland, of New York University School of Medicine, and Oakley-Girvan and Whittemore, of Stanford University School of Medicine, say that Mukherjee et al. describe the first carefully designed experiments addressing the relationship between fat intake and energy intake and prostate cancer. Because of uncertainties in extrapolating results from rodents to humans and because of a lack of understanding of the mechanisms that underlie the observed relationships between diet and prostate cancer, the editorial writers question the direct application of these findings to people.
Cutting Fat Calories May Reduce Breast Cancer Risk in Humans
A meta-analysis of 13 studies indicates that reducing dietary fat lowers blood levels of an estrogen believed to influence breast cancer risk. This result is presented in the March 17 issue of the Journal of the National Cancer Institute by Anna Wu, Ph.D., and associates at the University of Southern California Medical School, Los Angeles.[48]
The analysis raises the possibility that substantial reductions in fat consumption could offer an approach to breast cancer prevention, according to the authors.
The authors searched the English-language literature for studies that related estradiol (the most active type of estrogen) levels in blood to dietary fat interventions. Estrogen levels are of interest because there is substantial evidence that they are a critical determinant of breast cancer risk. For example, in a recent pooled analysis of six prospective studies, postmenopausal women who developed breast cancer had a 15% higher serum concentration of estradiol than women who did not.
Wu and her colleagues found 13 relevant studies published in English from January 1966 to June 1998. In each study, hormonal levels were determined before and after dietary intervention. Studies of premenopausal women involved cutting dietary fat to 21%-25% of total calories in five studies, 18%-20% in four, and 12% in one. Typical study periods were 2-3 months. In postmenopausal women, studies lasted from 3 weeks to 5 months, with fat calories being 24% of total in one study, 18%-20% in two, and 10% in one.
The authors derived pooled estimates from the 13 reported changes in estradiol levels associated with fat reduction. They calculated statistically significant reductions in estradiol level of -7.4% for premenopausal women and -23% for postmenopausal women, with an overall reduction of -13.4% for all women. The two studies that cut the percentage of calories from fat to the lowest levels (12%
for premenopausal women and 10% for postmenopausal women) produced the largest decreases in estradiol levels.
The authors state that there are no epidemiologic studies of breast cancer and fat consumption that effectively address the question of whether reducing fat consumption below 20% of calories will reduce breast cancer risk. Since very few human intervention trials involve fat levels much below 20%, the authors
conclude that reducing fat consumption below 20% of total calories may still have a role in reducing the risk of breast cancer.
In an editorial, Rachel Ballard-Barbash, M.D., M.P.H., and colleagues at the National Cancer Institute, note that many factors must be considered in interpreting the results of dietary intervention studies. These factors include the changes that occur in other dietary components in association with dietary fat reduction, the effect of other dietary components on serum estrogens, and measurement errors for both the diet and serum estrogens. The authors propose that future meta-analyses consider a statistical model that includes the effects of such covariates as intakes of fat, fiber, and total energy and perhaps changes in subject weight. They also recommend that, in future meta-analyses, the results of studies in which food intake is strictly supervised might be considered separately from those in which food records, at times less reliable, are submitted by the participants.
In Biomembrane Research Laboratory, Oklahoma, [49] the levels of γ- glutamyltranspeptidase (GGTP) (EC 2.3.2.2) were measured in 7,12- dimethylbenz(a)anthracene (DMBA)-induced mammary adenocarcinomas, in control mammary gland tissue, and in sera from tumour-bearing and control rats.
The carcinogenic process was modulated by diets differing in the content and degree of unsaturation of their lipid component. GGTP activity levels were elevated up to 18-fold in mammary tumours as compared to corresponding control mammary gland tissue. Also, GGTP activity in sera from tumour-bearing rats was up to 4-fold higher than in the sera of the corresponding controls. GGTP activity levels in the mammary gland and in the sera of control rats from low-fat dietary group were up to 6-fold higher than the control values in either of two high-fat dietary groups.
Researchers examined the effects of feeding a high-sucrose diet on body weight gain, plasma triglycerides, and stress tolerance in rats [50]. Feeding a high-sucrose (60%) diet for 2 weeks did not induce a greater body weight gain compared with that of standard diet when caloric intake was similar in ventromedial hypothalamic-lesioned obese and sham-operated lean animals. The high-sucrose diet elevated plasma triglycerides by increasing the triglycerides secret/on rate and decreasing the fractional catabolic rate in both groups. In response to stress, feeding a high-sucrose diet for one week induced enhanced gene expressions of heat shock proteins (HSP 70 and 27) and suppressed NOx production in the brain,
whereas the standard diet did not. Results suggest that feeding a high-sucrose diet does not induce obesity in lean rats or enhance weight gain in obese rats, if caloric intake is appropriate. The diet does elevate plasma triglycerides in lean and obese rats, but it may have the potential to improve stress tolerance.
The lifetime (2 year) carcinogenic bioassays are generally conducted in both sexes of rats and/or mice. In most studies, inbred rodent strains are used in order to reduce experimental variables and to enhance the interpretability of the results.
The control of experimental variables and the large experience with common laboratory strains of rats and mice represent great advantages for risk assessment.
[51, 52, 53]
On the basis of the availability of historical control database on tumours, the following strains are commonly used. Rats: Fischer 344, Sprague-Dawley, Wistar.
Mouse: B6C3F1, ICR Swiss (CD-1), BALB/c. In a recent study, Britton et al (2004) reports that of the three rat strains studied (Harlan Hsd:Sprague-Dawley SD, Harlan Wistar Hsd:BrlHan:WIST, Charles River Crl:CD), Harlan Wistar strain survived in much greater numbers in 104-week carcinogenicity study. The improved survival rate, according to the authors, appeared to be independent of body weight and food consumption and is reflected in the spontaneous pathology profile.[54, 55]
In the course of data analysis from a carcinogenicity study, statistical tests will occasionally indicate that the incidence of a particular neoplasm is significantly greater in a treated group than in the concurrent control. Since statistical differences can occur as a matter of chance alone, using a positive statistical difference as the sole or definitive evaluation tool could produce a false positive result. [56]
Alternatively, a slight increase in the incidence of a rare neoplasm would be unlikely to achieve statistical significance by the tests typically employed in toxicology studies. In this type of situation, the use of historical control data could justify the biological significance of even a slight increase in the incidence of an uncommon neoplasm. [57]
In the lifetime carcinogenic bioassay, most of the carcinogenic agents that induce tumours through genotoxic mechanisms can be detected. Agents, which produce metabolites and thereby react with the DNA forming mutagenic DNA adducts and trans-species carcinogens, can be detected in long-term rodent bioassays. Within the current guidelines and testing practices, some degree of flexibility exists in designing a study that is best for a given compound. Selection is possible for rodent strains, housing conditions, group numbers above a minimum, dose levels, route of administration, diagnostic criteria, investigative techniques, types of tissues preserved for microscopic examination, data analysis, and presentation within a particular study. [58]
The Sprague Dawley CDR IGS rat strain of Charles River laboratories is an outbred strain, which has a significant incidence of hypophysis adenomas and mammary carcinomas. Data on their website (www.criver.com) compiled from 31 studies, reports that in two year old females, there is a 71% incidence of hypophysis adenomas, 6% incidence of hypophysis carcinomas, and a 22%
incidence of spontaneous mammary carcinomas. If the hypophysis pathology in this strain occurs early and the adenomas are prolactin producing, it would explain the lactational morphology and the early incidence of mammary tumours can be observed in control rats. At some time in the past in the Charles River breeding stock, an increased susceptibility to hypophysis hyperplasia, adenoma, and carcinoma developed. [59, 60]
3. Materials and Methods
3.1. Introduction
The environment of the living organisms has changed with the spreading of the chemicalisation of agriculture. The pesticides and other chemicals used during the agricultural production mean hazard either for the plants or animals, and through the food-chain, or during the work for the peoples. So the stipulation of use and circulation of these chemicals is authority registration.
The determination of the risk or hazard represented by the chemical product is performed by the toxicology, as interdisciplinar science. The one of the main fields of the usage of toxicological data obtained during the in vivo or in vitro toxicological studies is the authority registration for using and circulation of the pesticides and other agricultural chemicals. The organic part of the registration documentation is the data of animal experiments including the 2-year carcinogenicity study on rats or mice, which is necessary for the final permission.
On the basis of this study the potential carcinogenic effect of the chemical product during a long-term, even life-span repeated exposition can be estimated.
The most important symptom in the carcinogenicity studies is the tumour (benign or malign) and very important the decision if the tumour is spontaneously developed, which is age-dependent process in the rodents, or induced by the exposition to the test item.
The presence and evaluation of the historical control data are also very important during the evaluation of the carcinogenicity study in dividing the spontaneous and the treatment-related tumours and expressing an opinion about the test item, which is the basis of the risk assessment during the registration procedure and releasing for trading.
The objective of the carcinogenicity studies were to observe test animals for a major portion of their life span for the development of neoplastic lesions, as part of a program of animal toxicity experiments designed to provide information which will allow the test material to be safe for a long-term use. The
carcinogenicity studies taken together with other relevant data and information on the active substance, must be sufficient to permit the hazards for humans, following repeated exposure to the active substance, to be assessed, and in particular must be sufficient:
— to identify carcinogenic effects resulting from exposure to the active substance,
— to establish the species and organ specificity of tumours induced,
— to establish the dose-response relationship, and
— for non-genotoxic carcinogens, to identify the maximum dose eliciting no adverse effect (threshold dose).
The long-term toxicity and carcinogenicity of all active substances must be determined. If in exceptional circumstances, it is claimed that such testing is unnecessary, that claim must be fully justified, i.e. toxicokinetic data demonstrates that absorption of the active substance does not occur from the gut, through the skin or via the pulmonary system.
While the standard reference points for treatment responses are concurrent control data, historical control data, may be helpful in the interpretation of particular carcinogenicity studies. Where submitted, historical control data should be from the same species and strain, maintained under similar conditions and should be from contemporaneous studies.
3.2. Experimental Animals
Species and strain: Crl:CD BR (Sprague-Dawley) rats
Source: CHARLES RIVER WIGA Gmbh
Sandhofer Weg 7
D-97633 Sulzfeld
Justification of strain: Rat is an internationally accepted species for carcinogenecity studies. The Crl:CD BR rat is widely used for this purpose , therefore historical data are available.
Number of animals: 50 animals/sex/group Age of animals to be received: 4 - 6 weeks
Quarantine and
Acclimatization period: 14 days
Gender: male and female
Study 1 Study 2
Body weight range Males Females Males Females at randomisation (g): 120-148 112-131 88-130 87-124
3.2.1. Hygiene and Health Conditions
The studies were started with animals of SPF state (certified by supplier).
The animals were maintained behind a specified barrier system, in separated room equipped with sterile air- conditioning system, air filtered by bacterial filter EU9.
The animal unit had a clean corridor separated from a dirty corridor by a barrier system with personnel and material lock.
Cages and bedding were changed as required. Water bottles were cleaned on a rota basis as required during the course of the study.
Room sanitation: at the end of each working day the floor was swept and then mopped with an acceptable disinfectant.
Only animals in acceptable health condition were used in the study. The health condition was certified by the responsible veterinarian on the basis of external clinical examination and the results of base level examinations. Animals that were evidently diseased were culled.
3.2.2. Husbandry
Room No.: TC/B2 SPF room in both studies
Housing: In first study group caging (5 animals/cage) at start of examination, 3 animals/cage from the 6th month and individual caging from the end of the first year.
In the second study animals were housed in plastic cages Techniplast T III., with 2 animals/cage
Cage type: Animals were housed in plastic cages Techniplast T III. In both studies, (18 cm high, floor dimensions 18cm x 36cm).
Bedding: laboratory bedding Light: 12 hours daily from 6 am to 6 pm Temperature: 22 ± 3 °C
Relative humidity: 50 ± 20 %
Air changes: 10-15 changes per hour
The cages were replaced to the place of the next cage as a circulation in every two weeks during the study.
There was regular monitoring of temperature and humidity.
3.2.3. Food and Feeding
In the first study the animals received SSNIFF R/M-Z+H 15 MM AUTOKLAVABLE COMPLET DIET FOR RATS AND MICE - BREEDING + MAINTENANCE. In the assay this diet was called as Normal Diet.
In the second study the animals were fed with SSNIFF R/M-H, ERED LOW ENERGY COMPLETE DIET FOR RATS AND MICE. In the assay this diet was called as Low Energy Diet.
Both diets were produced by Ssniff Spezialdiäten GmbH, D-59494 Soest Germany. The food was offered ad libitum. Before the terminal necropsy animals were without food for a night.
The check of ingredients and analytical examinations of diet were done simultaneously. Besides, the diet was analyzed for possible contamination (aflatoxin, pesticide residue, heavy metals) and microbiological sampling was also made from every batch of mixing. Quality control of food is performed by National Institute for Agricultural Quality Control (Budapest Remény u. 42.
Hungary).
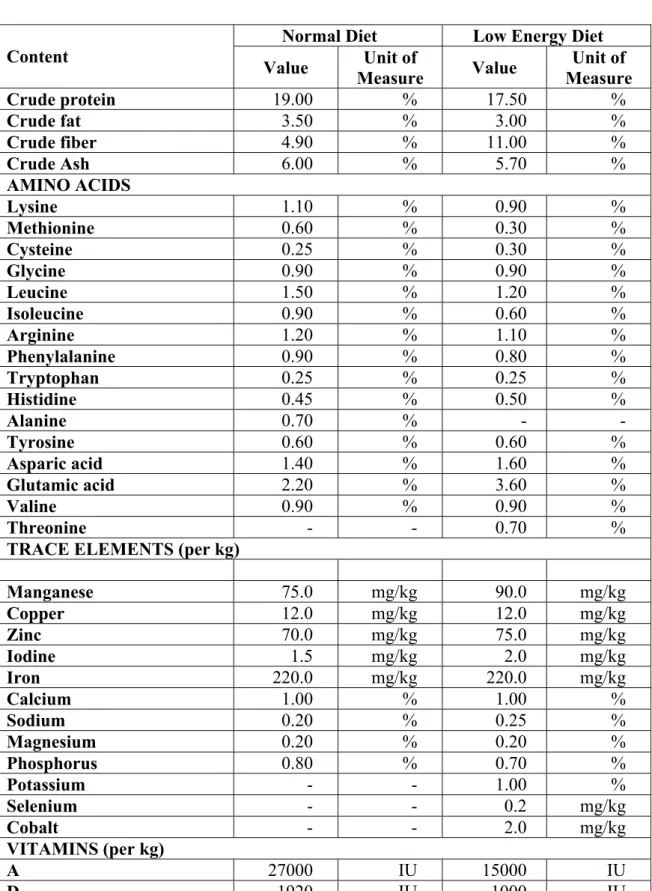
The composition of the diet was the most important difference between the two evaluated studies. The nominal contents of Normal Diet and Low Energy Diet see in Table 2.
Table 2 Nominal Contents of the Diets
Normal Diet Low Energy Diet Content
Value Unit of
Measure Value Unit of Measure
Crude protein 19.00 % 17.50 %
Crude fat 3.50 % 3.00 %
Crude fiber 4.90 % 11.00 %
Crude Ash 6.00 % 5.70 %
AMINO ACIDS
Lysine 1.10 % 0.90 %
Methionine 0.60 % 0.30 %
Cysteine 0.25 % 0.30 %
Glycine 0.90 % 0.90 %
Leucine 1.50 % 1.20 %
Isoleucine 0.90 % 0.60 %
Arginine 1.20 % 1.10 %
Phenylalanine 0.90 % 0.80 %
Tryptophan 0.25 % 0.25 %
Histidine 0.45 % 0.50 %
Alanine 0.70 % - -
Tyrosine 0.60 % 0.60 %
Asparic acid 1.40 % 1.60 %
Glutamic acid 2.20 % 3.60 %
Valine 0.90 % 0.90 %
Threonine - - 0.70 %
TRACE ELEMENTS (per kg)
Manganese 75.0 mg/kg 90.0 mg/kg
Copper 12.0 mg/kg 12.0 mg/kg
Zinc 70.0 mg/kg 75.0 mg/kg
Iodine 1.5 mg/kg 2.0 mg/kg
Iron 220.0 mg/kg 220.0 mg/kg
Calcium 1.00 % 1.00 %
Sodium 0.20 % 0.25 %
Magnesium 0.20 % 0.20 %
Phosphorus 0.80 % 0.70 %
Potassium - - 1.00 %
Selenium - - 0.2 mg/kg
Cobalt - - 2.0 mg/kg
VITAMINS (per kg)
A 27000 IU 15000 IU
D3 1920 IU 1000 IU
E 180 mg/kg 100 mg/kg
C 500 mg/kg - -
B1 24 mg/kg 10 mg/kg
B2 45 mg/kg 20 mg/kg
B6 25 mg/kg 12 mg/kg
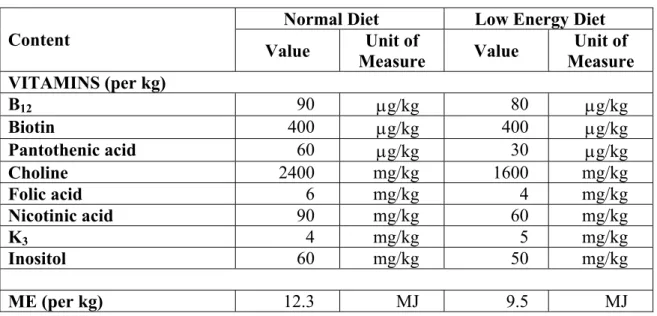
Table 2. Nominal Contents of the Diets (cont.)
Normal Diet Low Energy Diet Content
Value Unit of
Measure Value Unit of Measure VITAMINS (per kg)
B12 90 μg/kg 80 μg/kg
Biotin 400 μg/kg 400 μg/kg
Pantothenic acid 60 μg/kg 30 μg/kg
Choline 2400 mg/kg 1600 mg/kg
Folic acid 6 mg/kg 4 mg/kg
Nicotinic acid 90 mg/kg 60 mg/kg
K3 4 mg/kg 5 mg/kg
Inositol 60 mg/kg 50 mg/kg
ME (per kg) 12.3 MJ 9.5 MJ
3.2.4. Water Supply
The animals received filtered tap water, as for human consumption ad libitum from 500 ml bottles (water was filtered by bacterial filter).
Quality control of water was performed in every three months by Central Laboratory of Water and Canalization Company of the Capital (H-1134 Budapest, Váci út 23-27, Hungary.).
The food and water used was not considered to contain any additional item in sufficient concentrations to have a deleterious influence on the outcome of the study.
3.2.5. Identification of Animals
The individual identification was performed by ear tags or tattooing the tail of the rats. The numbers were given as following:
MALES FEMALES
1. Study 1 X001-X050 X051-X100
2. Study 2 001-050 301-350
The cages were identified by identity cards, with information about study code, sex, dose group, cage number and individual animal numbers.
3.2.6. Randomization
After individual identification all animals were sorted according to body weight by computer. There was an equal number of animals from each weight group in each of the experimental groups during the randomization. The grouping was controlled by SPSS/PC Plus computer program according to the actual body weight verifying the homogeneity and deviations among the groups and cages.
3.4. Observations
3.4.1. Clinical Observaions and Mortality
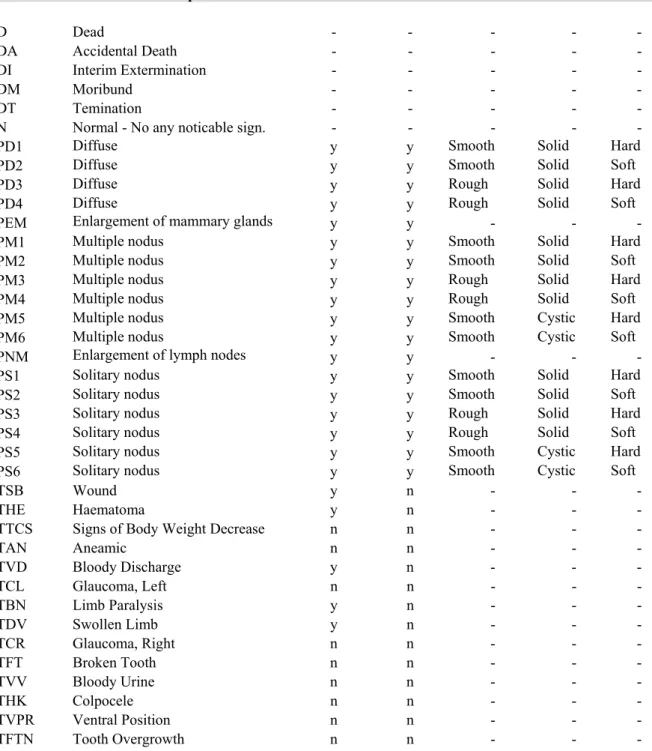
A careful clinical examination was made and recorded once prior to the initiation of treatment and once weekly during treatment in all animals. Individual observations included the check of the appearance, condition, behaviour, activity, excretory functions, respiration, orifices and eyes.
All animals were observed daily to detect onset and progression of all toxic effects as well as to minimise loss due to diseases, autolysis, or cannibalism.
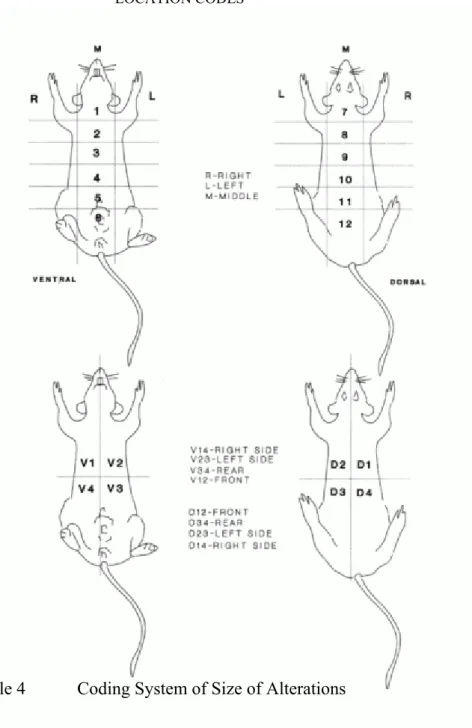
Special attention was paid to tumour development: the time of onset, location, dimension, appearance and progression of each grossly visible or palpable tumour were recorded monthly from the start to 6 month, in every fortnight between 7 and 12 months and weekly thereafter.
Animals were examined twice each day for being moribund state and mortality.
The date of death was recorded. Animals found moribund were isolated and/or sacrificed. They were processed in the same way as the animals of the terminal necropsy.
The clinical signs were collected using the coding system showed in Tables 3 and 4, Figure 6.