Polypeptides and Proteins as Inhibitors*
Edward J. Modest, George E. Foley, and Sidney Farber
I . Introduction 76 I I . Polypeptide Antibiotics 76
A . Actinomycins 77 B . Antibiotics of Bacterial Origin 86
C. D i - and Tripeptide Antibiotics 91 I I I . Natural Protein and Polypeptide Inhibitors 95
A . Viruses as Inhibitors 95 B. Antigen-Antibody Phenomena 96
C. Enzymes as Inhibitors 96 D . Natural Inhibitors of Proteolytic Enzymes 97
E. Antimicrobial Tissue Polypeptides and Proteins 100
F. Inhibitory Properties of Protamines 103 I V . Synthetic Poly-a-Amino Acids 104
A . Enzyme Inhibition 104 B. Inhibition of Blood Coagulation 105
C. Antiviral Properties 105 D . Antibacterial Properties 105 V . Analogues of the Polypeptide and Protein Hormones 107
A . Inhibitors of Hormone Activity 108 B. Inhibitors of Hormone-Inactivating Systems I l l
V I . Modified Enzymes as Inhibitors I l l V I I . Miscellaneous D i - a n d Tripeptide Inhibitors 113
A . Folic Acid Analogues 113 B. Peptides of Aromatic Nitrogen Mustards 114
C. Pantothenic Acid Analogues 115 D . Glutathione Analogues 116 E. Synthetic Antibacterial Tripeptides 116
V I I I . Concluding Remarks 116
References 118
* The preparation of this chapter was supported in part by grants CY-3335 and C-6516 from the National Cancer Institute, National Institutes of Health, United States Public Health Service.
75
I. INTRODUCTION
Inhibitors which contain protein or the structural units of protein have been categorized by Chain (1) as follows: (a) amino acids, (6) peptides, and (c) polypeptides. The arbitrary distinction usually made between polypeptides and proteins is based upon size; proteins are broadly classi
fied as nondialyzable polypeptides with molecular weights in excess of 10,000. The present review is confined primarily to inhibitors of polypep
tide and protein nature, and only brief mention will be made of certain di- and tripeptides.
The polypeptide inhibitors may be divided into two broad categories, those of natural and those of chemically modified or synthetic origin, and will be discussed in this order. Inhibitory activity concerned with poly
peptides embraces areas of biological antagonism as divergent as the role of virus as a metabolic inhibitor, antigen-antibody phenomena, and the activity of polypeptide antibiotics. Among the natural polypeptide in
hibitors major emphasis will be placed on the antibiotics and certain microbial and enzyme inhibitors. The principal synthetic or chemically modified polypeptides include certain hormone inhibitors and poly-a- amino acids. The inhibitory influences of one naturally occurring hormone upon the biological activity of another are not considered to be within the scope of this review.
The polypeptide antibiotics are of particular interest not only because they represent unique, complex structures, but also because many of them contain amino acid residues in the unnatural D-configuration, or amino acid derivatives which are not ordinarily encountered in nature.
These unnatural configurations may contribute to the untoward toxicity, which in certain instances approaches that characteristic of bacterial toxins, exhibited by many of these products.
The necessity for structural considerations in the interpretation of metabolite-antimetabolite relationships need not be emphasized here. It is evident, however, from review of the available data, that with few ex
ceptions knowledge of polypeptide inhibitors does not permit extensive consideration of molecular antagonism, even if configurational specificity is viewed in the broadest physicochemical sense, as suggested by Roblin (β) and Schueler (8).
Except for notations to the contrary, all amino acids capable of optical isomerism are of the L-configuration. Polypeptide structures are written in abbreviated form after the Brand and Edsall system (4).
II. POLYPEPTIDE ANTIBIOTICS
There are well over one hundred products known to be polypeptides in the ever-expanding catalogue of antibiotics. These structures, and dériva-
tives thereof, exhibit biological activity against a vast array of aerobic and anaerobic bacteria, viruses, bacteriophages, Fungi Imperfecti, fungi, certain protozoa, helminths, rickettsia, phytopathogens, plants (5, 6), mammalian cells in culture (7-13), and neoplasia (6). However, even in those instances where chemical structure has been delineated, remarkably little is known concerning the precise mechanism of action in vitro, and there is even less information concerning mechanism of action in vivo.
Since the available information on the chemistry of these antibiotics has been reviewed recently (6,14-19a), only the salient points concerning those for which mechanism of action studies have been reported will be con
sidered here. These polypeptide antibiotics, to be discussed in Sections I I , A and I I , B, are tabulated in Table I .
T A B L E I
POLYPEPTIDE ANTIBIOTICS WITH REPORTED MECHANISM OP ACTION
Antibiotic Source Reference
Actinomycin A Streptomyces antibioticus
m
Actinomycin C Streptomyces chrysomallus
m)
Actinomycin D Streptomyces parvullus (22)
Bacitracins Bacillus subtilis, Bacillus licheniformis (23)
Circulin Bacillus circulans (U)
Gramicidins Bacillus brevis (25)
Polymyxins Bacillus poly my xa, Bacillus aerosporus Greer (26, 27)
Subtilin Bacillus subtilis
m
(29)Tyrocidines Bacillus brevis (26, SO)
Among the other polypeptide antibiotics worthy of mention but not considered individually here are such agents as the nisins (6, 15), etamycin (6, 15, 17, 80a), telomycin (80a), and echinomycin (15).
A. Actinomycins
1. S T R U C T U R E
The actinomycins are elaborated by various species of the family Strep- tomycetaceae. The first descriptions of the actinomycins were reported by Waksman and Woodruff (20, 81, 82), and since then a great deal of re
search has been done with these polypeptides, particularly in the labora
tories of Waksman, Brockmann, and Johnson (88), on the isolation and characterization of several members of the actinomycin family. The
actinomycins are quinonoid chromopeptides possessing a phenoxazone chromophore in common but differing in the amino acid composition of the two cyclic pentapeptide lactone groupings attached to the chromo
phore (84-86). Structure ( I ) has been proposed for actinomycin D. Actino-
0 = C -
C H ( C H3)2 C H ( C H3)2
- C H HC C = 0
C H C H3
(I)
mycin C has been shown to contain three components, Ci, C2, and C3 (87-40), one of which (Ci) is identical with actinomycin D (87-89, 41).
The amino acid sequence of each peptide chain of actinomycin D and C3 is shown in the accompanying tabulation (89, J$, 4$)·
Actinomycin D ( C i ) Actinomycin C3
iV-Methylvaline 1
Sarcosine 1 L-Proline
1 D-Valine
1 L-Threonine
JV-Methylvaline 1
Sarcosine 1 L-Proline
1
D-Alloisoleucine 1
L-Threonine
Actinomycin C2 is a "hybrid" of actinomycin D and C3, possessing, in place of two molecules of D-valine or D-alloisoleucine, one molecule each of D-valine and D-alloisoleucine (39, 44)·
The amino acid content of actinomycin A is the same as that of actino
mycin D. A structure has not been proposed, since actinomycin A is not a pure compound.
The literature is complicated by the facts that several of the actino
mycins proved to be mixtures on closer examination and that nomen
clature is even now inconsistent. The reader is referred to the symposium previously cited (33) for further details.
2. M E C H A N I S M O F A C T I O N
The inhibition of Ceratostomella ulmi by actinomycin A may be reversed by the addition of excess pyridoxine or peptone to a synthetic substrate
(45). It was not determined whether reversal by peptone was due to its pyridoxine content (45). It is of interest to note that the strain of C. ulmi used in these studies was unable to synthesize its pyridoxine re
quirement (46). Waksman and Bugie, in this same report (45), cite un
published observations (by H. B. Woodruff) to the effect that ascorbic acid partially reversed the inhibitory activity of actinomycin A in unspeci
fied bacterial systems. Inhibition by actinomycin C has been reported to be reversed by p-aminobenzoic acid, tyrosine, and phenylalanine in other bacterial systems (47), and is reported to interfere with the conversion of pyridoxine to pyridoxal phosphate in the liver of rats (48). The effects of actinomycin C on mammalian cells in vivo have been described as resem
bling those of X-irradiation, i.e., the induction of chromosomal abnor
malities in preprophase cells (49).
Actinomycin D was found to be a competitive inhibitor of pantothenate in several bacterial assay systems utilizing microorganisms with a require
ment for exogenous pantothenic acid (50). The noncompetitive reversal by excess concentrations of certain amino acids, dicarboxylic acids, orotic acid, or adenine (50) suggested the hypothesis that in such dependent bacterial systems actinomycin D interferes with pantothenate-dependent reactions concerned with the biosynthesis and/or utilization of amino acids. Unpublished observations (by G. E. Foley) in these laboratories indicate that certain synthetic polypeptides* (poly-DL-alanine and a co
polymer of DL-alanine and ^-alanine) also are effective in the noncom
petitive reversal of actinomycin D inhibition in pantothenate-dependent bacterial systems. Other unpublished observations (by G. E. Foley) in
* Synthesized by E. R . Blout, The Children's Cancer Research Foundation, Boston, Mass.
these laboratories [cited by Farber (51) and Maddock et al. (52)], as well as the observations of others (53), record the failure of excess pantothenate to alter either the toxicity or antitumor activity of actinomycin D in mice.
On the other hand, actinomycin D effects what appears thus far to be an irreversible inhibition in nonpantothenate-dependent Bacillus subtilis systems (54). In other studies in B. subtilis systems, actinomycin D was reported to interfere with the assimilation of ammonia and the formation of certain inducible enzymes (55, 56). The addition of inhibitory concen
trations of actinomycin D to cultures of B. subtilis in the log phase of growth has been observed to inhibit protein and ribonucleic acid ( R N A ) synthesis, with a disproportionate production of deoxyribonucleic acid (DNA) (56, 57) similar to that reported in Escherichia coli when inhibited by 0-2-thienylalanine (58). Wheeler and Bennett (59) found that actino
mycin D interferes with the synthesis of R N A and D N A by bacterial and Ehrlich ascites cells.
Actinomycin D has been reported to suppress the assimilation of gluta
mate by Streptococcus faecalis, B. subtilis, and Saccharomyces cerevisiae (56), and to inhibit the response of β-alanine-dependent S. cerevisiae to sub- optimal concentrations of β-alanine (56). It is of interest to note that when jfl-alanine was replaced by pantothenate as the growth-limiting metabolite in this latter system, there also was evidence of inhibition by actinomycin D (56). In other bacterial systems, inhibition by actinomycin D (and C) has been reported to be reversed by p-aminobenzoic acid
(PABA), tyrosine, and phenylalanine (47). Actinomycin D has been re
ported to inhibit R N A synthesis in Micrococcus (59a), Neurospora (59b), B. subtilis (59c, 59d), mammalian cell assay systems (59, 59e, 59f, 60), and cell-free systems (59a, 59c, 59g). The addition of actinomycin D to log- phase cultures of Micrococcus inhibits R N A synthesis immediately, re
sulting in the prompt inhibition of protein synthesis, and, later, the partial inhibition of D N A synthesis (59a). Similar conclusions were reached in other studies in which mammalian cell cultures exposed to actinomycin D were studied in situ by cytochemical methods which allowed the meas
urement per cell of RNA, DNA, and protein content (59h). Inhibition of Lactobacillus arabinosus by actinomycin D is reversed by 2'-deoxy- guanosine or DNA, but not by RNA, purines, pyrimidines, amino acids, etc., whereas the inhibition of Lactobacillus leichmannii and HEP-2 cells in vitro seems to result from interference with the de novo synthesis of purine ribonucleotides (59f). Wheeler and Bennett (59f) suggested that since inhibition of the utilization of guanine nucleotides for the synthesis of R N A could explain their observations in a number of bioassay systems, this may be the locus of inhibition of actinomycin D. Hackmann (49), on the other hand, found that the most marked effect of actinomycin D (as well as C, Fi, and F3) on mammalian cells in culture was depletion of DNA,
with little or no effect on the mitochondria of treated cells. Kirk (59a) reported that the combination of D N A and actinomycin D results in a spectral change in the inhibitor. These observations suggest the formation of a complex between these two compounds, which may be relatively specific for D N A , since Kawamata and Imanishi (60a) found no evidence of interaction with R N A . Rauen et al. (60b) have reported complex forma
tion between actinomycin D and R N A only with concentrations of R N A far in excess of the concentration of D N A required for the reaction.
The difficulties and complexities of such mechanism of action studies are evidenced by the variability among observations made with the same antibiotic in different bioassay systems. Such apparent multiplicities of mechanism of action may well be the result of differences in biochemical detail which are inherent in different bioassay systems. The failure of microorganisms lacking a clear-cut requirement for a specific metabolite to respond to inhibitors which are highly effective as growth inhibitors, even of other strains of the same genus which exhibit a specific require
ment for the metabolite in question, is well known (61, 62). Therefore, it is not surprising, for example, that pantothenate antagonism by actino
mycin D (50) could not be demonstrated with nonpantothenate-dependent strains of B. subtilis (54, 56).
The logical consequence of the introduction of a metabolic inhibitor into a living system is the disruption of the over-all biosynthetic economy or
"harmony" of the cell in such manner that, more often than not, forma
tion of the end products of all major biosynthetic pathways will be af
fected, irrespective of the precise locus of inhibition characteristic of a given inhibitor. It is evident that (1) the sensitivity of a bioassay system is dependent upon the factor (or factors) which limit the degree of meta
bolic activity or growth obtainable in a given substrate; (2) these limiting factors vary in different substrates, even though the same (to say nothing of different) microorganisms or mammalian cell lines are used for bio
assay; (8) the reactions which are growth-limiting in a particular bio
synthetic sequence in a particular substrate will be the most susceptible to inhibition; (4) the efficiency of a metabolic inhibitor will vary with the accessibility of a particular locus to effective concentrations of inhibitor, the relative concentration of the product of a particular biosynthetic reaction required for the survival of the cell in that particular substrate, and, of course, the available effective concentration of the inhibitor [the difference in the concentration of methotrexate required for the effective inhibition of pteroylglutamic acid (PGA) and N
5
-formyltetrahydropteroyl- glutamic acid (N
5
-formyltetrahydro-PGA) respectively, even in the same bioassay system, is a well-known illustration] ; and (5) at best, inhibitory activity in a bioassay system can be defined precisely only in terms of specific reversibility in a specific system.
In the case of actinomycin D, many of the reported studies on mech
anism of action have not included sufficiently detailed reversal data to permit precise definition of the locus of inhibition. On the other hand, if this deficiency, together with the inherent shortcomings and difficulties of the experimental method, are kept in mind, these studies are not incom
patible with the general hypothesis that actinomycin D interferes with the synthesis of nucleoprotein and protein. More recent studies on the mecha
nism of action of actinomycin D have been concerned primarily with the question as to whether or not this compound specifically inhibits the messenger R N A which is involved in the transfer of information from the nucleus to the loci of protein synthesis in bacteria (62a). Information per
tinent to this question has been derived from a variety of biological sys
tems, including mammalian cell systems, although a fraction analogous to messenger R N A has not been demonstrated unequivocally in mammalian cells. There are, however, numerous reports suggesting that a variety of mammalian cells may contain such a fraction (62a-62k). There is evidence, too, that mammalian cell lines maintained in vitro also may contain an analogous R N A fraction (621, 62m).
Reich et al. (59e) reported that actinomycin D inhibits the synthesis of R N A by mammalian cells in vitro and decreases the yield of the D N A - containing vaccinia virus but does not inhibit the synthesis of cellular D N A or the replication of the RNA-containing Mengo virus. These ex
periments were interpreted (59e) to mean that the replication of virus R N A and the synthesis of cellular RNA, controlled by virus and cellular D N A respectively, can be distinguished by inhibition of those portions of R N A synthesis which are dependent upon cellular or viral DNA. Levinthal et al. (59d) have interpreted the inhibition of R N A synthesis in B. subtilis by actinomycin D to be due to interference with a DNA-dependent reac
tion, since R N A virus can multiply in the presence of the inhibitor, and since DNA-dependent R N A synthesis is suppressed completely by actino
mycin D in cell-free systems. The fact that total R N A synthesis is in
hibited completely was interpreted as implying that ribosomal as well as messenger R N A synthesis is DNA-dependent, rather than the result of synthesis by a system wherein R N A is replicated from an R N A template.
On the other hand, Nakata et al. (62n) have reported that the replication of T2 phage and the synthesis of phage protein are inhibited by actino
mycin D, whereas the synthesis of phage D N A is unaffected.
Kirk (59a) has reported the inhibition of D N A polymerase by actino
mycin D. Hurwitz et al. (59c) described the competitive inhibition of the synthesis of R N A by a DNA-dependent R N A polymerase with actino
mycin D in cell-free systems. Inhibition of R N A polymerase appeared to be dependent upon the type of D N A used to prime the reaction, and
T A B L E I I
SUMMARY OF REPORTED BIOCHEMICAL INHIBITIONS B Y ACTINOMYCINS I N V A R I O U S BIOLOGICAL S Y S T E M S
0
Coenzyme reactions Incorporation of :
Precursor ! •pantothenate j •products (56) (50)
Amino acids- -•proteins P A B A - ->iV
10
-formyltetrahydrofolic acid Purines-
(47, 50, 55, 56)
— ! >RNA (47) (60, 59, 59b, 59f, 60) Pyridoxine-
M, 48)
->pyridoxal phosphate Pyrimidines- + D N A
(50, 59, 69b, 59f)
D N A
(49, 60, 59, 59a, 59c, 59f, 59g, 60b) R N A
(59d, 59e)
DNA-dependent R N A synthesis (59c, 69d, 59g, 59h)
RNA-mediated protein synthesis (56, 57, 69, 69a-59h, 60, 60b, 62n)
° Vertical broken lines represent points of inhibition deduced from reported inhibitions and reversals of inhibition in various systems.
Numbers in parentheses refer to references.
S AND PROTEINS AS INHIBITORS 83
inhibition could be reversed by the addition of D N A . D N A synthesis catalyzed by D N A polymerase was inhibited to a lesser extent by actino
mycin D, and this inhibition also could be reversed by the addition of DNA. Goldberg and Rabinowitz (59g) observed that actinomycin D inhibits the synthesis of R N A by nuclear extracts of HeLa cells in a RNA- synthesizing system which is dependent upon D N A and requires the presence of all four ribonucleoside triphosphates. Inhibition in this system also could be reversed by the addition of D N A .
These studies in cell-free systems, in which inhibition is reversed by the addition of DNA, support the mechanism of action proposed for actino
mycin D in the intact cell by Reich et al. (59e). These observations, as well as this proposed-mechanism of action, may prove to be related to the formation of a complex between actinomycin D and D N A (59a, 60a, 60b).
Thus it appears that inhibition by actinomycin D may be specific for DNA-linked R N A synthesis. However, the studies referred to earlier (59h), in which mammalian cell cultures exposed to actinomycin D were studied in situ by cytochemical methods which allowed the measurement of RNA, D N A , and protein content, per cell, clearly indicate the total inhibition of R N A and protein synthesis at concentrations of actinomycin D which exhibit little or no effect upon the continued synthesis of D N A in individual cells. Thus, there is the possibility that actinomycin D spe
cifically inhibits messenger RNA, but such specificity has not yet been established unequivocally.
The areas of metabolic activity concerned with the various inhibitory activities attributed to the actinomycins are summarized in Table I I , wherein each reported inhibition, with reference to the appropriate pub
lished report, is indicated. These proposed loci cannot be located more precisely with respect to the intermediates in the various biochemical pathways because of lack of detailed reversal data. It is evident that, e.g., the reported inhibition of the synthesis and/or biological function of RNA, protein, or D N A is not necessarily incompatible with reversal of inhibition by amino acids, dicarboxylic acids, pantothenate, purines, pyrimidines, or even PABA; the apparent differences in all probability are the result of inherent differences in the bioassay systems employed and the lack of sufficiently detailed information.
3. A N T I T U M O R P R O P E R T I E S
Perhaps the most exciting aspect of antibiotics research today has to do with the isolation and characterization of those antibiotics which exhibit antitumor activity. Research in this area has expanded rapidly in recent
years both in this country and abroad, as attested by the increasing num
ber of reports describing new antibiotics exhibiting such activity. There are now some two dozen antibiotics which exhibit antitumor activity in experimental tumor systems (89), and àt least six of these agents exhibit interesting activity in human neoplasia (83, 68). In addition, there are now in various stages of development more than twenty new products endowed with antitumor activity, which have resulted from the antibiotics programs supported by the Cancer Chemotherapy National Service Center of the National Cancer Institute during the past several years (64)·
The structures of many of these agents, like their mechanisms of action, are unknown. Although antitumor activity is not peculiar to the polypeptide antibiotics (88, 89, 64, 65), those of most current interest in the chemo
therapy of human neoplasia are the actinomycins. The available informa
tion concerning the mechanism of action of these polypeptide inhibitors already has been discussed. The chronological development and differen
tiation of the actinomycins has been reviewed elsewhere (38, 63).
a. Actinomyin A. Actinomycin A was isolated by Waksman and Wood
ruff (20, 31, 32), but was considered to be too toxic to be useful as a chemo
therapeutic agent. Stock (66) and Reilly et al. (67) reported slight in
hibition of Sarcoma 180 in vivo, but only at toxic doses.
b. Actinomycin C. Actinomycin C was isolated by Brockmann and Grubhofer (21) and has been studied extensively by Brockmann and his as
sociates (89). Hackmann (68) described the carcinolytic effects of this agent in man in 1952, and it has since been studied extensively in a variety of experimental tumors (49, 69, 70).
The most extensive experience in the chemotherapy of human neoplasia has been reported from European clinics. Actinomycin C is of most interest in the chemotherapy of Hodgkin's disease and other lymphomas (51, 63), although it is occasionally effective in other forms of neoplasia (71-78).
Actinomycin C is a potent agent, an average, daily adult dose being only 50-100 Mg. Its use in combination with X-irradiation bas been reported to be more effective than either agent alone in the therapy of Hodgkin's disease (78-75).
c. Actinomycin D. Actinomycin D (I) was isolated by Waksman and his colleagues (22), and is one component (actinomycin Ci) of actinomycin C (87-89, 41)- Farber et al. first described its antitumor activity in experi
mental tumor systems (52, 76, 77) and in man (51, 68, 78, 79).
Actinomycin D also is a potent agent, the usual daily dose in man being 60-75 Mg/kg. Preliminary studies indicated that there was sufficient evidence of clinical improvement in a variety of human neoplasia to
warrant extensive clinical trial against a spectrum of human tumors (63).
Although marked clinical effects have been observed occasionally in a variety of human tumors, actinomycin D is most effective in the chemo
therapy of the lymphomas and Wilms' tumors (51, 63, 71, 78-84). The effectiveness of actinomycin D also is potentiated by X-irradiation (51, 73, 78-80).
d. Actinomycin F\. Actinomycin F i , a product of "directed" biosyn
thesis, was reported by Schmidt-Kastner (85), and its inhibitory activity in experimental tumor systems was described by Sugiura and Schmid (86), and Burchenal et al. (70).
The activity of actinomycin Fi in a variety of human neoplasia has been reported by Tan et al. (71). Its clinical usefulness and limitations in general appear to be similar to those of actinomycin C.
B. Antibiotics of Bacterial Origin
The antibiotics considered in this section are elaborated by various species of the family Bacillaceae.
1. B A C I T R A C I N S
The most probable configuration for bacitracin A, one member of this family of polypeptides, is ( I I ) . It is likely that the terminal L-isoleucine and L-cysteine residues are joined in a thiazoline ring (16, 87-90).
j — S — j ^ ^ D - O r n — L - I l e u ^
L - I l e u - L - C y - L - L e u - D - G I U -L-Ileu - L - L y s ^ D - P h e L -A s p — L - H i s
D- A s p ( I I )
The bacitracins are surface active, disorganizing the bacterial cell wall following even brief exposure. At suboptimal concentrations, morphological aberrations and protoplast formation are induced (91). Uridine nucleotides accumulate in damaged cells (92). The inhibition of glutamic acid uptake by Micrococcus pyogenes, var. aureus (M. aureus or Staphylococcus aureus) also has been reported (92). In some respects, the antibacterial activity of the bacitracins resembles that of penicillin (16).
2. ClRCULI N
The molecula r formul a o f th e basi c polypeptid e circuli n i s
(CœH^OsN^)* !
(93, 94). Th e structur e i s unknown . Th e molecul e contain s D-leucine , L-threonine, L - α , 7-diaminobutyric acid, and an optically active isomer of pelargonic acid (24, 93, 94). Circulin exhibits properties similar to those of the polymyxins (14, 15, 18).
Circulin attacks the bacterial cell wall, causing the leakage of cellular constituents into the surrounding medium (95).
3. G R A M I C I D I N S
The gramicidins, A , B, and C, which have been crystallized, occur in the tyrothrycin complex (14) (cf. Section I I , B, 6). These neutral poly
peptides are characterized by the presence of 2-aminoethanol (96, 97) and the absence of free amino and carboxyl groups (98), and they contain the constituents (98-101) given in the accompanying tabulation.
GRAMICIDINS A , B , AND C
Constituent A B C
Ethanolamine
+ + +
D-Leucine
+ + +
L-Tryptophan
+ + +
DL-Valine
+ + +
L-Alanine
+ + +
Glycine
+ + +
Phenylalanine
- + -
Tyrosine
— — +
Gramicidin S is also produced by a strain of B. brevis (102, 103). Grami
cidin S ( I I I ) is a basic cyclic decapeptide containing D-phenylalanine, two free amino groups, and no free carboxyl groups (IO4-IO6), and has been
POLYMYXINS A ( E ) , B , C , AND D
Constituent A B C D D-Leucine + + — + L-Threonine + + + + D-Serine — — — - { -
D-Phenylalanine — + + —
Circulin (14, 15, 18) and polypeptin (18) are considered to belong to the polymyxin family. Polymyxin Β has been separated by countercurrent distribution into two fractions, Bi and B2, which differ only with respect to the fatty acid component (121). The B2 component contains an iso- octanoic acid of undetermined structure instead of Ipel (21). Polymyxin
Bi
contains 6 molecules of L - D A B , 2 of ^threonine, 1 of L-leucine, 1 of D-phenylalanine, and 1 of Ipel (121, 122). One of the structures proposed for polymyxin B: is (IV) (14, 15, 122).synthesized (107, 108). Gramicidin J has been reported to be a basic cyclic heptapeptide of undetermined structure (15).
L -Leu— D -Phe L- O r n
L- V a l
L
~ Y
AL
L- P r o L-OTÏÏ
D - Ph e — L - L e u '
(m)
These polypeptide s als o ar e surfac e active . Inhibitor y activit y i s re - duced b y cephalin-typ e phospholipid s (109) an d cationi c detergent s (110).
Inhibition o f th e synthesi s o f capsula r polysaccharid e ha s bee n reporte d i n Klebsiella pneumoniae (111), an d interferenc e wit h phosphat e uptak e ha s been observe d wit h M. aureus (112).
4. P O L Y M Y X I N S
The polymyxin s ar e a closel y relate d grou p o f strongl y basi c cycli c polypeptides (113), al l o f whic h contai n (+)-6-methyloctanoic(isopelar - gonic, Ipel ) (114, H6) an d L - α , 7-diaminobutyric ( L - D A B ) acids (116, 117). The amino acid composition of individual polymyxins varies, as shown in the accompanying tabulation (27, 118-120).
D - P h e — L - L e u L - D A B L - D A B L - D A B
( + ) - I p e l - L - D A B L - T h r - L - D A B
L - D A B — L - T h r
( I V )
The antibacterial spectrum of all polymyxins is essentially similar.
These polypeptides have been reported to inhibit a variety of enzyme systems, e.g., esterase, the oxidation of acetate, pyruvate, oxalacetate, 2-ketogluconate, etc., but only at concentrations beyond those required for bacterial effects (128,124), which probably result from the surface activity of these structures. Few (125) presented evidence that the polymyxins interact with the protoplasmic membrane, probably by complexing with lipids and/or cephalin. There is some evidence (123, 124) that sensitivity to the polymyxins can be correlated with phospholipid content, but there is no evidence that the loci of interaction and activity are necessarily identical.
Other studies indicate that polymyxins form water-insoluble complexes with R N A and mononucleotides, as well as phospholipids (126). The re
lease of soluble constituents from sensitive cells has led to the concept that the polymyxins disorient those structures in or on the cell wall which maintain osmotic equilibrium (127, 128). The inhibition of gram-positive microorganisms may be antagonized by the addition of magnesium ribo- nucleate (129), while with certain gram-negative microorganisms (e.g., Pseudomonas aeruginosus), inhibition may be as effectively reversed by the addition of Mg+ +, Mn+ +, Ca+ +, or Fe+ to the medium (ISO).
5. S U B T I L I N
The empirical formula of this basic polypeptide is still uncertain, and its amino acid composition is not yet completely known (181, 132).
The mode of action of subtilin is similar to that of tyrocidine (cf. Sec
tion I I , B, 6) and other surface active agents, resulting in the lytic de
struction of bacteria (188,184).
6. T Y R O C I D I N E S
The tyrocidines, occurring in the tyrothrycin complex, are cyclic basic decapeptides with no free α-amino or carboxyl groups (185). The sequence of amino acids in tyrocidine A is indicated in (V) (186, 187). The amino acid sequence in tyrocidine Β is similar to that of tyrocidine A, except that L-phenylalanine is replaced by L-tryptophan (187, 188). The optical
configurations of tyrocidines A and Β appear to be identical (137). The structure of tyrocidine C has not been determined.
L - L e u — D - P h e
L - O r n L - P r o / \ L - V a l L - P h e
ι I
ι
LL - T y r D - P n e L - G I U — L - A s p
( V )
The tyrocidines are primarily surface active agents and, like other agents of this class, their activity is decreased in the presence of an ionic detergent (129) or magnesium (130). The decrease of activity in the presence of serum has been attributed to binding by serum lipids (112). Inhibition of glucose phosphorylation has been observed with certain anaerobic bac
teria (139). Inhibition in such systems, however, appears to be secondary to the rapid lysis of cells by these polypeptides, with the consequent liberation of adenosine triphosphate ( A T P ) and phosphoric acid (139).
7. M E C H A N I S M S O F A C T I O N
The properties, with respect to structure, of these and other naturally occurring polypeptides have been reviewed by Bricas and Fromageot
(140); and these, as well as other surface active bactericides, have been reviewed by Newton (141)» The relationship between structure and activity in the case of gramicidin S, for example, is not yet apparent, although this compound is perhaps the most extensively studied of the cyclic polypeptide antibiotics ( 1 4 1 ) · Harris and Work (142) concluded, from the study of synthetic pentapeptides containing the same amino acid sequence, that the biological activity of gramicidin S was not di
rectly dependent upon the presence of D-phenylalanine or L-ornithine, but rather appeared to be related to cyclic structure. On the other hand, Erlanger, Sachs, and Brand (148) concluded from their studies with synthetic linear decapeptides that the activity of gramicidin S may not be dependent upon cyclic structure unless a different mechanism of action is presumed for the acyclic and cyclic forms of this decapeptide (cf. Sec
tion IV, D ) . Greater resistance to enzymic degradation (144) and greater molecular rigidity (resulting in better absorption) (143) have been postu
lated in explanation of the greater biological activity of the cyclic as compared to the acyclic forms of these polypeptides. Hotchkiss (112) and Erlanger et al. (14$) present evidence that free amino groups are involved in the attachment of polypeptide antibiotics to cells, while Newton (146)
presented evidence that at least one of the four available free amino groups in polymyxin can be blocked without loss of biological activity.
The question posed by Newton (141) as to whether the disruption of cell membranes by the surface active polypeptides is a primary or sec
ondary effect remains to be answered. There is evidence that surface active agents dissolve, denature, or disrupt macromolecules and that such agents dissociate the prosthetic groups from enzyme proteins (141)*
There is some evidence (147) that denaturation of a specific enzyme protein might be the primary mechanism of action, with changes in cell permeability being secondary phenomena. Chapman (147a, 147b) reported electron microscopic evidence that exposure to the cyclic polypeptide antibiotic colistin (19a, 147c) results in the loss of nuclear material as well as cytoplastic changes in gram-positive and gram-negative bacteria, sug
gesting that this antibiotic may penetrate the cell. On the other hand, Hotchkiss (148) calls attention to the greater concentration required for protein denaturation than is necessary for cell death and expresses doubt that the usual surface active agent penetrates the living cell.
Failure to penetrate the living cell, however, does not preclude the possibility that enzyme (or substrate) inhibition is the primary mechanism of action of these surface active agents, since it is known that enzymes may be associated with the surface structures of the bacterial cell. Certain phosphatases and an invertase, for example, are known to be associated with the surface of yeast cells (149, 150); various dehydrogenases, acid phosphatase, and the cytochrome complex are in or on the protoplast membrane of M. aureus (151); and an adaptive enzyme concerned with lactose transport appears to be associated with the cell membrane of E. coli (152). Salton (158), in studies concerned with other surface active agents, suggested that a primary metabolic inhibition might result in a sequence of reactions resulting in secondary effects such as membrane or cell wall damage and the consequent loss of cellular constituents to the substrate. Newton (141) suggests that the bacterial protoplast membrane is a dynamic system, and proposed that surface active agents may inhibit enzymes concerned with the maintenance of its dynamic equilibrium. The morphology, structure, and function of the surface layers of the bacterial cell, the synthesis of enzymes by protoplasts, and the localization of enzymes in bacterial cells have been the subjects of other reviews (154)- C Di- and Tripeptide Antibiotics
The metabolic inhibitors considered in this section are not polypeptides in the strict sense of the definition (1) used here. However, these selected agents are of sufficient interest to the general problems of metabolic
inhibition by peptidic inhibitors to warrant brief mention here. There are several other antibiotics of known structure which contain peptide bonds, such as amicetin and cycloserine. Detailed information concerning such agents has been summarized elsewhere (6, 19a).
1. P E N I C I L L I N S
The chemistry of the natural and synthetic penicillins (VI) has been reviewed by Regna (14) and Rebstock (16). There have been numerous
R C O N H C H — Ç H C(CH3)2
C O - N C H C O O H
( V I )
reports suggesting that penicillin interferes with the formation of peptide bonds. Simmonds and Fruton (166) observed reversal of the bacterio
static effect of penicillin by leucylglycine, whereas mixtures of the com
ponent amino acids were ineffective. Interference with transpeptida- tion also has been reported (166), and Binkley and Olson (167) observed inhibition of the hydrolysis of glutathione. On the other hand, reports (168) describing the profound effects of penicillin on nucleic acid metab
olism and the cell wall are even more numerous. Such differing reports are not necessarily contradictory in view of the interrelationships of nucleic acid metabolism and protein synthesis, but it is evident that statements as to the precise mechanism of action of penicillin cannot be made at the present time.
a. Newer Synthetic Penicillins. The isolation of 6-aminopenicillanic acid (169), the fundamental ring system of the penicillins, provides the basis for the synthesis of new, modified penicillins by variation of the side chains which may be introduced into the molecule. Among the first of these new synthetic penicillins is sodium 6-(2
/ ,6
,
-dimethoxybenzamido)-penicillanate monohydrate (VI, R = 2
/ ,6
/
-dimethoxyphenyl) (169).
The interesting antimetabolic activity of this analogue of penicillin in microbiological assay systems has been described by Stewart (160), and in man by Douthwaite and Trafford (161), and Stewart, Nixon, and Coles (162). A unique biological property of this compound is its apparent effectiveness in inhibiting all strains of M. aureus, irrespective of their re
sponse to the other penicillins (168), including those strains which pro
duce penicillinase (164-166). Despite its resistance to penicillinase, this synthetic penicillin, like cephalosporins Ν and C (cf. Section I I , C, 2) is a potent inducer of this enzyme (166). Other details concerning mechanism of action have not yet been reported.
Almost simultaneously with the announcement of studies on the struc
ture of (VI), a conference under the auspices of the Bristol Laboratories was held September 7, 1960, to announce similar studies with the identical structure (sodium dimethoxyphenylpenicillin, "Staphcillin"*) (167).
Chemical attachment of a variety of side chains to the fundamental ring system of penicillin has resulted in a series of structures which ex
hibit varying degrees of resistance to penicillinase (167a, 167b). The desirability of a penicillin which is resistant to the destructive activity of penicillinase, particularly staphylococcal penicillinase, requires no elabora
tion. That penicillinase itself is amenable to inhibition is indicated by the studies of Saz et al (167c), who reported that the penicillinase produced by M. pyogenes, var. aureus is inhibited by various dipeptides, particularly D-valyl-D-valine. This and other peptides containing D-cysteinyl or D-valyl residues are of particular interest in view of the presence of D-cysteine and D-valine in the nucleus of (natural) penicillin, hydrolysis of the β-lactam ring of penicillin (by penicillinase) representing essentially the hydrolysis of a cyclic dipeptide. Certain dipeptides formed by the condensation of amino acids and metal-binding compounds appear to be more active inhibitors of penicillinase than dipeptides containing only amino acids (167 c).
2. C E P H A L O S P O R I N S
The cephalosporins comprise two families of antibiotics elaborated by Cephalosporium: (1) the cephalosporin Ρ group, which are not peptides, and (2) cephalosporins Ν and C. The chemistry of the cephalosporins has been reviewed recently (6, 14-16). Cephalosporin Ν and C are both peni
cillin analogues containing D-a-aminoadipic acid in the side chain. Cephalo
sporin Ν (synnematin B) is (D-4-amino-4-carboxyl-n-butyl)penicillin (VI, R = D-a-aminoadipic acid). The structure of cephalosporin C has not been announced.
Although the spectrum of activity differs from that of the other penicil
lins in that cephalosporin Ν is reported to be effective in Salmonella infec
tions (168,169), the mechanism of action is as yet unknown. Cephalosporin C is of particular interest in that it is more resistant to penicillinase than either the common penicillins or cephalosporin N , and has been reported to be a competitive inhibitor of certain penicillinases (170) and to exhibit synergism with benzylpenicillin versus penicillin-resistant M. aureus (171). Cephalosporin C and Ν are potent inducers of penicillinase in B. cereus (172).
* Trade-mark, Bristol Laboratories, Syracuse, N e w York.
3. A L A Z O P E P T I N
This antibiotic, produced by Streptomyces griseoplanus, has been re
ported to be a peptide containing one mole of α-alanine and two moles of 6-diazo-5-oxo-L-norleucine (DON), or isomers of the latter structure (178).
Like DON, which is a glutamine antagonist (174, 175), alazopeptin ex
hibits an interesting spectrum of experimental antitumor activity (178).
4. A C T I T H I A Z I C A C I D
This antibiotic, (—)-4-oxo-2-thiazolidinehexanoic acid ( V I I ) , resembles desthiobiotin, and is antagonized competitively by biotin in vitro (176) ; this
CHa CH(CHa)eCOOH
CO N H
( V I I )
suggests a metabolite-antimetabolite relationship. Mycobacterium tuber
culosis is antagonized in vitro, but actithiazic acid appears to be inactive in vivo (177, 178).
Actithizaic acid has been synthesized (177, 179, 180). The methyl and ethyl esters are more active than actithiazic acid in vitro, but, like the parent compound, also are inactive in vivo (180, 181).
5. L Y C O M A R A S M I N
The fungus associated with tomato wilt, Fusarium oxysporum f. lyco- persici (Snyder and Hansen), produces an antibiotic, lycomarasmin, a peptide derivative of asparagine, glycine, and α-hydroxyalanine with the probable structure ( V I I I ) (182, 188), which is antagonized by the poly
peptide growth factor strepogenin (184).
OH H2N C O C H2C H N H C O C H2N H C C H 3 I
I I
COOH COOH ( V I I I )
Strepogenin has been isolated from liver extract (185, 186) and other protein sources (187). Strepogenin activity also has been found in insulin hydrolyzates, synthetic oxytocin (188), and arginine-vasopressin (189).
The peptides derived from insulin and oxytocin are not identical despite
their strepogenin activity, and several of the smaller synthetic polypep
tides representing the sequences in the parent compounds are devoid of such activity. This evidence suggests that strepogenin activity probably does not reside in a specific amino acid configuration, but may be due to the polypeptide structure itself.
A synthetic tripeptide, serylglycylglutamic acid, exhibits strepogenin- like activity as a growth factor and also antagonizes lycomarasmin (190, 191). The growth-promoting activity of serylglycylglutamic acid in turn is antagonized by another tripeptide, serylglycylaspartic acid, in micro
biological assay systems (192). The mechanisms involved in these an
tagonisms are poorly understood.
III. NATURAL PROTEIN AND POLYPEPTIDE INHIBITORS The protein and polypeptide inhibitors discussed in this section either exist as such in nature or are derived from natural sources. They are treated here as a class distinct from the naturally occurring polypeptide antibiotics and hormones. In general, these inhibitors have not yet been adequately characterized. Except for the basic polypeptide and protein inhibitors, which are thought to act via electrostatic interaction with anionic polymeric sites, little is known of the mechanism of inhibition of this class of substances. The coverage of the voluminous literature pertinent to these inhibitors is representative rather than exhaustive.
A. Viruses as Inhibitors
Virus particles may be considered to be polypeptide inhibitors in the broad sense by virtue of their protein content. The biochemistry, biology, and consequences of viral infection to the cell have been the subjects of several excellent reviews (193-197). Polypeptide structures also play a role in the blocking of hemagglutination (198) and virus adsorption (194, 199, 200), as well as in interference phenomena between viruses (196, 201-203).
Isaacs and Lindenmann (203a) described a soluble factor called inter
feron, which exhibits the properties of a protein and is produced by cells consequent to exposure to infective virus or viral components. These substances appear to be viral inhibitors which enhance cell resistance to virus. The production, biological behavior, and potential significance of the interferons has been reviewed recently (203b).
Β. Antigen-Antibody Phenomena
That many forms of biological antagonism are intimately associated with the periodicity of amino acids and the surface characteristics of poly
peptide compounds is evidenced by the vast range of antigen-antibody phenomena. Sevag (204) considers antigenic proteins to be enzymic, their specific biological activity being the production of specific antibody.
The biological aspects of antibody formation have been the subject of a recent review (205).
The substrate for these reactions is γ-globulin, which, in the presence of antigen, is synthesized in stable configurations determined by the periodicity and surface characteristics of the antigen. Pauling (206) pro
posed that the synthesis of antibody results from a folding of the poly
peptide precursors into a configuration complementary to that of the antigen. The studies of Landsteiner and van der Scheer (207-210) more than thirty years ago with hapten peptides demonstrated that the in
hibition of antigen-antibody reactions represents a direct displacement phenomenon.
More detailed reviews of these topics may be found in Sevag (204),
Martin (211), Landsteiner (212), Burnet (213), and current textbooks on classical immunology and immunochemistry.
C. Enzymes as Inhibitors
1. E N Z Y M E S A S T O X I N S
The classic toxins and endotoxins of many species of bacteria are pro
teins, to which enzyme activity has not yet been attributed (214, 215).
However, bacteria secrete a variety of enzymes, such as hyaluronidases, collagenases, proteases, peptidases, phospholipases, and phosphatases (216), which contribute to the physiological effects of toxins and endotoxins by means of their adverse effects upon the host and thus, in a sense, may be considered to be metabolic inhibitors. The venoms secreted by bees, scorpions, poisonous spiders, snakes, toads, and fish also contain a variety of enzymes which contribute to physiological shock, interfere with blood coagulation, lyse erythrocytes, etc. (216).
There are examples in which enzyme protein appears to function as a toxin. The parallel relationship between the proteolytic and anticoagulant activity of snake venoms is well known (217). Eagle (218) observed a similar relationship between the anticoagulant activity and protease activity of a variety of poisons. Since hematological effects are prominent in snake bite, such proteases must be considered to be toxins. Similarly, those phospholipases which lyse erythrocytes are considered to function as toxins (216).
Among bacterial products, the α-toxin secreted by various species of Clostridium welchii is a lecithinase (phospholipase) {216, 219), the activity of which can be inhibited or blocked by displacement with cerebrosides (220) or antibody (219). The enzymes secreted by other species of the
"gas gangrene" group are antigenically distinct (221), and can be associ
ated only in varying degrees with toxicity, although antibody or substrate appears to be bound at the same locus on the enzyme protein (222).
The role of enzymes as toxins or as components of toxins has been re
viewed more extensively by Zeller (216).
2 . O T H E R I N H I B I T O R Y E N Z Y M E S
The inhibition of enzymes by other enzymes has been reported. Sang -(228) found the inhibitor isolated from the body wall of the round worm, Ascaris lumbricoides, to be a protease. Urease blocks the action of trypsin without losing its own enzymic activity (224).
Lysozyme, a basic mucopolysaccharidase isolated from a wide variety of animal, plant, and bacterial sources, exhibits bacteriolytic activity against both gram-positive and gram-negative bacteria. It is particularly effective against Micrococcus lysodeikticus, B. megaterium, and Sarcina flava. The properties of lysozyme have been reviewed (225). Other basic enzymes reported to possess antibacterial activity are ribonuclease, de- oxyribonuclease, and hyaluronidase. It is possible that the antibacterial properties of these enzymes, active only in high concentrations, were due to the presence of impurities (226). Other inhibitory properties of ribo
nuclease have been summarized by Bergel (226a). Trypsin and chymo- trypsin have been reported to inhibit penicillinase (226b).
Bergel (226a, 227) has reviewed preliminary work on the inhibition of experimental tumors in vivo with milk xanthine oxidase (228), ribonuclease (229), and deoxyribonuclease (229a).
D. Natural Inhibitors of Proteolytic Enzymes
1. N A T U R A L T R Y P S I N I N H I B I T O R S
Trypsin, a proteolytic pancreatic enzyme that hydrolyzes peptide bonds of basic character, is specifically inhibited by several natural proteins which may be involved in the stabilization of various biological fluids
(280-282). These trypsin inhibitors possess molecular weights in the range of 6000-34,000, and have been isolated from pancreas, soybean, lima bean, egg white (ovomucoid), and colostrum. Table I I I , abstracted from Des- nuelle (280), gives some of the properties of the natural trypsin inhibitors.
The inhibition of trypsin seems in most instances to be stoichiometric and
T A B L E I I I
NATURALLY OCCURRING TRYPSIN INHIBITORS
0
Origin
Approximate molecular
weight Isoelectric point
Pancreas I 6500-16,000 8.7-10.0
Pancreas I I 9600 4.5-5.9
Soybean 17,000-24,000 4.5
Lima bean 8000-18,700 < 3 . 6
Egg white (ovomucoid) 9100-34,000 3.8-4.4
Colostrum 10,500 4.2
° Abstracted from P. Desnuelle ref. 280.
reversible. The pancreatic, ovomucoid, and soybean inhibitors are com
petitive. Since the natural inhibitor-trypsin complex is unstable in acid or base and can be destroyed by dilution, the binding evidently is not covalent. By analogy with the inhibition of trypsin by acidic poly-a-amino acids (cf. Section I V , A ) , this binding may be electrostatic in nature.
Trypsin is also inhibited by serum protein (282) and by protein ex
tracts of the body wall of the round worm, A. lumbricoides (223, 233) (cf. Section I I I , C, 2 ) .
2. T H E N A T U R A L P E P S I N I N H I B I T O R
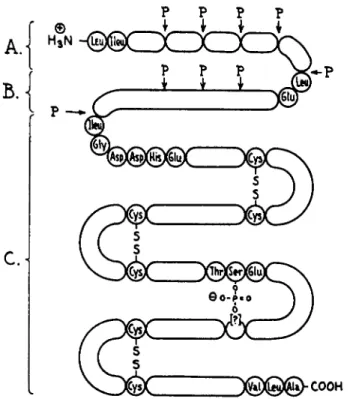
Pepsinogen is the zymogen of pepsin, and must be cleaved by acid or pepsin itself before the proteolytic action of pepsin can be demonstrated (234). The activation of pepsinogen (mol. wt. ca. 43,000) involves the generation of pepsin (mol. wt. ca. 36,000), several neutral peptides (total mol. wt. ca. 4000), and the so-called "pepsin inhibitor" (mol. wt. ca. 3200)
(235). The pepsin inhibitor, a polypeptide with 29 amino acids, appears to be located in the middle of the pepsinogen molecule. Before pepsinogen can be activated, the pepsin-pepsin inhibitor amide bond must be broken.
Figure 1, by Bovey and Yanari (234), is a schematic representation of the location of the pepsin inhibitor in the pepsinogen molecule. Further de
tails are given by Bovey and Yanari (234). This polypeptide inhibits pepsin between pH 5 and 6.
The natural pepsin inhibitor and the synthetic basic poly-a-amino acids, such as polylysine (236) (cf. Section I V , A ) , are effective com
petitive inhibitors of pepsin. Extracts of Ascaris (223, 233) also show antipeptic activity.
FIG. 1. The structure of pepsinogen. The principal attack by pepsin is at points marked P, releasing miscellaneous peptides (A), pepsin inhibitor ( £ ) , and pepsin (C). The undetermined sequences are actually much larger in relation to the known sequences than is indicated. The location of the phosphoserine residue with respect to the di
sulfides is also uncertain. [Reproduced from F . A . Bovey and S. S. Yanari, ref. (234).]
3. N A T U R A L I N H I B I T O R S O F B A C T E R I A L A N D M O L D P R O T E A S E S
Several proteolytic enzymes elaborated by microorganisms are inhibited by naturally occurring protein inhibitors (237). Proteinaceous inhibitors active against B. subtilis alkaline protease, Aspergillus alkaline protease, and trypsin are widely distributed in cereals, beans, and potatoes, but not in fruits or vegetables. Barley, rye, kidney beans, broad beans, and po
tatoes are especially rich in these proteins, which inhibit B. subtilis alka
line protease, Aspergillus alkaline protease, and trypsin, but not Asper
gillus acid protease, pepsin, or papain (238). Soybean trypsin inhibitor and ovomucoid from egg white, which inhibit trypsin (cf. Section I I I , D , 1) do not inhibit B. subtilis or Aspergillus alkaline proteases. The ovoinhibitor component of egg white (289) inhibits all three of these proteolytic en
zymes. Serum contains an inhibitor of Aspergillus protease, which is probably different from serum trypsin inhibitor.
Ε. Antimicrobial Tissue Polypeptides and Proteins
It has long been known that antimicrobial substances can be obtained from various animal sources, notably blood, leucocytes, and lymphatic tissues. Because of incomplete chemical characterization and periodic i
'rediscovery/ ' there is considerable confusion as to the identification of such substances and their biological significance as metabolic inhibitors of infectious processes. Extensive consideration of these substances is beyond the scope of this chapter. The reader is referred to the admirable review of these inhibitors by Skarnes and Watson (226) for further details and references.
1. N A T U R A L A N T I B O D I E S
The substances discussed in this section are complex proteins derived from serum, and are inhibitory to bacteria and to viruses.
a. Complement ("Alexin" "Opsonin"). Complement is a euglobulin- carbohydrate-albumin complex which, in conjunction with normal (non
immune) antibody, accounts for the bactericidal, viricidal, lytic, and phagocytosis-enhancing effects attributable to normal (nonimmune) serum. Although early differences of opinion appeared to have been re
solved and the current consensus is that complement, "alexin," and
"opsonin" represent a single substance (226), Tullis and Surgenor (240) have described two serum proteins which enhance phagocytosis and may be neither complement nor properdin.
b. Normal Antibody. It is generally accepted that, in addition to comple
ment, the antimicrobial effects characteristic of normal (nonimmune) serum require the participation of a second component, the nonspecific normal antibody, as opposed to antibodies elicited following exposure to a specific antigen. The origin and specificity of natural antibody has been the subject of extensive debate (226). Mackie and Finkelstein (241, 242) suggested that normal serum contains specific antibodies which result from natural immunization, whereas Landsteiner (243) suggested the natural existence of a variety of globulins which, by accidental affinity to different antigens, appear to be specific. As pointed out by Skarnes and Watson (226), the problem of the nature of natural antibody un
doubtedly has been confused in many instances by the presence of usually undemonstrable levels of specific antibody in normal (nonimmune) serum.
c. Properdin. Properdin (244) is a euglobulin contained in normal (non
immune) serum, which is concerned with nonspecific resistance to in
fectious agents. Properdin requires the presence of complement and
M g +
+ ions for activity, which is a nonspecific antibacterial and antiviral effect. The biological nature and activity of properdin have been reviewed
(245) >
a n
d Skarnes and Watson (226) have presented the not incon
siderable evidence for the identity of natural antibody and properdin, together with the interrelationships of the various components par
ticipating in the antimicrobial and lytic activities of normal serum. The relationship of properdin and noncellular resistance in general to the problems of neoplastic disease has been reviewed by Southam (246).
2. B A S I C P O L Y P E P T I D E S A N D P R O T E I N S
Most of the polypeptide and protein inhibitors of tissue origin are basic in nature, and have been derived principally from cellular elements.
These substances generally are most active against gram-positive bacteria.
Their inhibitory effects are probably the result of electrostatic interaction with negatively charged bacteria or viruses, and the mechanism of action is presumably similar to that of the synthetic basic polypeptides discussed in Section IV.
a. Nucleins, Historiés, and Protamines. The nucleins, complexes of nucleic acids with simple basic proteins such as histones or protamines, are active against gram-positive bacteria (226).
The histones contain large amounts of the two basic amino acids, lysine and arginine, whereas the protamines are rich in arginine and usually low in lysine content. The antibacterial properties of the histones and the protamines have been reported (226, 247-249). The antibacterial activity of a calf thymus histone containing a large amount of lysine has been described (250). Other antibacterial histones have been derived from the basic tissue polypeptides discussed below.
Protamines from various sources have been shown to inhibit bacteria, viruses, a trypanosome, and a yeast. Clupeine, a protamine, has been reported to inactivate bacteriophage (251). Clupeine sulfate suppresses the activity of vaccinia virus (252). Both vaccinia virus and bacteriophage have negatively charged surfaces, allowing combination with basic sub
stances (253).
Skarnes and Watson (254) isolated leukin, an arginine-rich protamine with activity against gram-positive bacteria, from rabbit polymorpho
nuclear leucocytes. Leukin or leukin-like substances have been obtained from the leucocytes of several species, and are presumably protamine or histone fractions of nucleoprotein origin (226).
b. Basic Tissue Polypeptides. Bloom et al. (255) reported the inhibition of B. anthracis by a basic polypeptide, containing a large proportion of
lysine, which was isolated from several animal tissues. Similar basic tissue polypeptides are active against a number of other bacteria (266, 257) and against a bacteriophage (258). Watson and Bloom (258) have correlated antibacterial activity with the high lysine content of the molecule. By fractionation of histone preparations, Crampton, Moore, and Stein (259, 260) obtained a lysine-rich fraction (histone A ) and an arginine-rich frac
tion (histone B). Skarnes and Watson (261) showed the tissue peptide of Bloom et al. (255) to be identical with histone A (259), which is most active at alkaline pH.
A basic polypeptide from calf thymus containing about 40% of lysine and arginine by weight was found to exhibit activity against M. tuber
culosis (262, 268). Hirsch (264) reported that under certain in vitro condi
tions, the arginine-rich histone Β (259, 260) exerted bactericidal activity against various coliform bacilli and micrococci, but that the lysine-rich histone A (259, 260) manifested no significant antibacterial action.
3. M I S C E L L A N E O U S P R O T E I N S W I T H A N T I B A C T E R I A L A C T I V I T Y
The proteinaceous inhibitors discussed in this section are difficult to classify because of even less certain structure, purity, and mechanism of action. Although these substances are discussed in the singular form, each one is probably a mixture and undoubtedly varies in kind according to the source and method of isolation. Except for phagocytin, these sub
stances are all effective against gram-positive bacteria. With the excep
tion of substance M and the antistaphylococcal serum factor, this group of inhibitors has been adequately reviewed by Skarnes and Watson (226).
Phagocytin, a bactericidal substance obtained from rabbits, and limited in distribution mainly to the polymorphonuclear leucocyte, appears to be a protein with general properties characteristic of a globulin. It is different from properdin and lysozyme (265, 266).
β-Lysin is a poorly characterized bactericidal substance isolated from human, horse, and dog serum. It is presumably a protein.
Plakin, an antibacterial material from the blood platelets of the horse, is thought to be a protein and may be related to leukin.
Lactenin is a bactericidal protein found in the whey of human, cow, and goat milk.
Mascherpa (267) has described the isolation of substance M from the organs of tuberculosis-free mammals. This product, a mixture containing a polypeptide component, is reported to exhibit activity against M. tuber
culosis in vitro and in vivo.
Yotis and Ekstedt (268, 269) have described a partially purified, heat- stable, antistaphylococcal serum factor, which occurs primarily in a water-
soluble globulin fraction of normal human, rabbit, and horse serum. It has not yet been demonstrated in bovine serum. This antibacterial factor, which appears to be distinct from lysozyme, exerts a nonspecific (partially lytic), lethal effect upon several species of gram-positive bacteria. Anti
bacterial activity, except that exhibited against M. lysodeikticus, can be blocked by exposure of the cells to staphylococcus coagulase (268, 269).
This factor has been shown to interfere with the oxidation of glucose (269), but otherwise its mechanism of action is unknown.
There are a number of protein-like compounds known as bacteriocins and bacteriocin-like substances elaborated by several bacterial species which are inhibitory for the cells which produced them, i.e., their bio
synthesis by the cell is self-lethal. Some of these substances exhibit in
hibitory activity against a limited spectrum of related bacterial species.
The reader is referred to the recent review by Ivancvics (269a) for further details.
As pointed out recently by Nigrelli (269b), it is now well established that many marine organisms produce growth-promoting and growth- inhibiting substances, including antibiotics in the classic sense. Although information concerning the biochemical nature and mechanism of action of such substances in well-defined biological systems is incomplete, certain of these substances will undoubtedly prove to be peptides or proteins. Li et al. (269c), for example, have described a class of compounds designated as paolins which have been isolated from abalone and oysters. These substances are nondialyzable, appear to be proteins (probably muco- proteins), and exhibit antiviral as well as antibacterial activity.
F. Inhibitory Properties of Protamines
The cationic nature, basic amino acid content, and antimicrobial proper
ties of the protamines have been discussed (cf. Section I I I , E, 2) and have been reviewed elsewhere (226, 247, 248). In this section, other inhibitory properties of the protamines are described.
1. H E P A R I N A N T A G O N I S M
Protamine, a basic protein, and heparin, an acidic polysaccharide, are both anticoagulants. However, the clotting time of heparinized blood can be restored to normal by the inhibition of heparin with protamine (270).
This stoichiometric reaction of protamine with heparin is the basis for the so-called heparin-protamine titration. The inhibition is presumably through electrostatic forces. Similar antiheparin effects have been observed with synthetic basic polypeptides (cf. Section IV, B ) .