Full Terms & Conditions of access and use can be found at
https://www.tandfonline.com/action/journalInformation?journalCode=ierp20
Expert Review of Pharmacoeconomics & Outcomes Research
ISSN: 1473-7167 (Print) 1744-8379 (Online) Journal homepage: https://www.tandfonline.com/loi/ierp20
Long-term efficacy and cost-effectiveness of infliximab as first-line treatment in rheumatoid arthritis: systematic review and meta-analysis
Zsombor Zrubka, László Gulácsi, Valentin Brodszky, Fanni Rencz, Rieke Alten, Zoltán Szekanecz & Márta Péntek
To cite this article: Zsombor Zrubka, László Gulácsi, Valentin Brodszky, Fanni Rencz, Rieke Alten, Zoltán Szekanecz & Márta Péntek (2019) Long-term efficacy and cost-effectiveness of infliximab as first-line treatment in rheumatoid arthritis: systematic review and meta- analysis, Expert Review of Pharmacoeconomics & Outcomes Research, 19:5, 537-549, DOI:
10.1080/14737167.2019.1647104
To link to this article: https://doi.org/10.1080/14737167.2019.1647104
© 2019 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
View supplementary material
Accepted author version posted online: 25 Jul 2019.
Published online: 01 Aug 2019.
Submit your article to this journal
Article views: 1226 View related articles
View Crossmark data
REVIEW
Long-term efficacy and cost-effectiveness of infliximab as first-line treatment in rheumatoid arthritis: systematic review and meta-analysis
Zsombor Zrubka a,b*, László Gulácsi a*, Valentin Brodszky a, Fanni Rencz a, Rieke Alten c, Zoltán Szekanecz dand Márta Péntek a,e
aDepartment of Health Economics, Corvinus University of Budapest, Budapest, Hungary;bDoctoral School of Business and Management, Corvinus University of Budapest, Budapest, Hungary;cRheumatology Research Center, Schlosspark-Klinik Charite, University Medicine Berlin, Berlin, Germany;dDivision of Rheumatology, Department of Medicine, University of Debrecen Faculty of Medicine, Debrecen, Hungary;eDepartment of Rheumatology, Flór Ferenc County Hospital, Kistarcsa, Hungary
ABSTRACT
Introduction: Early biological treatment of rheumatoid arthritis (RA) may reverse the autoimmune response in some patients resulting in favorable long-term outcomes. Although the cost-effectiveness of this strategy has been questioned, biosimilar entries warrant the revision of clinical and pharmaco-economic evidence.
Areas covered: We conducted a systematic review of randomized controlled trials (RCTs) published up to 24 May 2018 in Pubmed, EMBASE and Cochrane CENTRAL, comparing infliximab with non-biological therapy in patients with RA naïve to methotrexate. We performed meta-analyses for efficacy outcomes at month 6 and years 1 and 2. Six RCTs were identified, involving 1832 patients. At month 6 ACR70 response and remission, and at year 1 ACR20/ACR70 responses and remission were improved signifi- cantly with first-line infliximab versus control. The differences were not significant at year 2. We reviewed cost-utility studies, up to 31 October 2018 in PubMed, Cochrane CENTRAL and the CRD HTA databases. Four studies indicated that first-line use of originator infliximab calculated at 2005–2008 prices was not cost-effective.
Expert opinion: We demonstrated the efficacy benefits of first-line infliximab therapy up to 1 year in methotrexate-naïve RA. We highlighted the need for standardized reporting of outcomes and conduct- ing cost-effectiveness analyses of first-line biosimilar therapy in RA.
ARTICLE HISTORY Received 12 May 2019 Accepted 19 July 2019 KEYWORDS Biosimilar; cost-utility analysis; efficacy; infliximab;
meta-analysis; early rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) affects the synovial joints, causing inflammation and pain. Without treatment, progressive destruc- tion of the joints can lead to considerable disability [1–3]. Patients with early or‘recent-onset’RA can be defined as individuals with a disease duration of 2 years or less [1,2]. Of late, there has been increasing emphasis on the prompt referral of patients with suspected early RA to rheumatologists in order to prevent irre- versible joint damage [1,2]. Effective early treatment within the therapeutic ‘window of opportunity’ for RA may reverse the autoimmune response in some patients, leading to more favor- able long-term outcomes compared with patients who are trea- ted later in the disease course [4,5]. Moreover, achievement of early response may allow eventual tapering of RA treatment and potential drug-free remission in some patients [6].
Tumor necrosis factor inhibitors (TNFis) and other biological disease-modifying antirheumatic drugs (bDMARDs) have tradition- ally been used to treat patients with long-standing RA who have experienced inadequate response to previous conventional syn- thetic DMARDs (csDMARDs) such as methotrexate (MTX) or leflu- nomide. However, a number of studies have demonstrated that
TNFi treatment is also effective in patients with early RA [7–15].
Indeed, several bDMARDs, including infliximab and other TNFis, have now been approved for the first-line treatment of patients with severe, active and progressive disease that has not been treated with MTX or other csDMARDs [16]. Recent meta-analyses have demonstrated the short-term benefits of biologic agents over csDMARDs in early, MTX/csDMARD-naïve RA, suggesting that more patients may achieve remission with first-line biological therapy than with csDMARDs [3,17–19]. However, the selection of studies in these meta-analyses were diverse and sometimes incomplete [3,17,18].
At the current time, bDMARDs such as infliximab are not widely used in first-line treatment of patients with early RA [16,20]. This fact is likely to be at least partly related to the most recent (2016) guidance on this topic issued by the European League Against Rheumatism (EULAR) [21] and National Institute for Health and Care Excellence (NICE) [16], both of which do not recommend first-line treatment with bDMARDs in patients with RA. The EULAR recommendations note that the early use, and potential for subsequent with- drawal, of bDMARDs was discussed, but was not supported by the majority of members of the recommendations task force
CONTACTLászló Gulácsi laszlo.gulacsi@uni-corvinus.hu Department of Health Economics, Corvinus University of Budapest, Fővám tér 8, Budapest 1093, Hungary
*Zsombor Zrubka and László Gulácsi contributed equally to this work.
Supplemental data for this article can be accessedhere.
2019, VOL. 19, NO. 5, 537–549
https://doi.org/10.1080/14737167.2019.1647104
© 2019 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited, and is not altered, transformed, or built upon in any way.
[21]. One reason for this was the lack of evidence for super- iority of bDMARDs compared with the combination of MTX plus glucocorticoid. Furthermore, the task force commented that in the context of a treat-to-target strategy, initial use of csDMARDs has equivalent long-term efficacy. The cost- effectiveness of first-line bDMARD therapy was also ques- tioned and considered to be poor given the lack of evidence supporting its superior long-term efficacy [21]. EULAR there- fore recommend the use of MTX in combination with short- term glucocorticoid as a first-line strategy for the treatment of RA. Following treatment failure with initial csDMARD therapy, additional csDMARDs or bDMARDs/targeted synthetic DMARDs may be initiated, depending on the prognostic status of each patient. The EULAR recommendations further state that tapering of bDMARDs should be considered if the patient is in sustained remission [21]. NICE recommend the use of bDMARDs in combination with MTX for patients with severe RA (disease activity score [DAS] 28 > 5.1) following csDMARD therapy [16].
The lack of uptake of biologic agents for the first-line treatment of RA may also be due to high drug costs [20].
However, the introduction of biosimilar versions of RA- approved drugs may help to reduce treatment costs, with the availability of biosimilar infliximab since 2013 already leading to considerable price discounts in some regions [22]. Budget impact analyses have predicted large health- care savings following the uptake of biosimilar infliximab in RA across multiple markets [23–25]. There is, however, a need for more recent cost-effectiveness analyses that take into account recent reductions in the cost of some bDMARDs following the introduction of biosimilars.
Because of its comparable efficacy and lower cost versus originator infliximab, biosimilar infliximab may be an ideal candidate with which to reassess the available medical and economic evidence for first-line biological therapy in MTX- naïve, early RA. The aims of our study, therefore, were to systematically review the literature and analyze the out- comes of first-line infliximab treatment versus other
treatment strategies in patients with MTX-naïve RA, with a special focus on long-term efficacy outcomes. We also reviewed available health economic literature for infliximab cost-utility studies in this patient population.
2. Methods
2.1. Search strategy for clinical studies
We conducted a systematic literature search in PubMed, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) up to 24 May 2018. Our search included the Cochrane filter for clinical trials [26], all known development codes of infliximab [27–29], and the MeSH term for RA. Full details of the PubMed, Embase and CENTRAL search criteria are shown in Supplementary Tables S1, S2 and S3, respectively. To identify papers not yet indexed by MeSH terms, we conducted another search in PubMed without including RA as a keyword for the period of January 1 to 24 May 2018 (Supplementary Table S1).
2.2. Selection of clinical studies
Eligible studies included published randomized controlled trials (RCTs) involving patients with RA who were MTX-naïve at rando- mization. The treatment intervention of interest was infliximab.
Control treatments could include any pharmacological treatment (i.e. placebo, csDMARDs and/or steroids) with or without conco- mitant MTX that did not involve any biological therapy at study initiation, although biological treatment could be included per protocol in a later phase of the study. Multiple publications from the same RCT were retained, including RCT extension studies. We did not impose any language restrictions on our search, but we excluded case studies, pooled analyses, review articles and con- ference abstracts.
For mapping available evidence, outcomes of interest included any physician- or patient-reported clinical efficacy, economic or radiological outcome, as well as any key safety outcomes such as occurrence of adverse events (AEs), discontinuations due to AEs, deaths, infusion reactions, severe infections, tuberculosis, malig- nancy, cardiovascular events and immunogenicity (i.e. the pre- sence of anti-drug antibodies [ADAs]). For quantitative syntheses, we included studies reporting American College of Rheumatology (ACR) response criteria (ACR20/ACR70) or clinical remission (see
‘Data extraction’section below for definitions) measured at month 6, year 1 and year 2. The abstract of each potential publication was reviewed by two independent researchers to confirm relevance and, if needed, a third independent researcher resolved any differences.
2.3. Data extraction from clinical studies
All reported outcomes (detailed above) and corresponding time points for all relevant primary and secondary publications of the selected studies were recorded. For quantitative synth- esis, a Microsoft Excel spreadsheet was developed to capture the following details of relevant studies by treatment arm:
study name; reference; treatment and dosing; number of ran- domized patients, risk of bias assessment; ACR20, ACR70 and
Article highlights
● We performed a systematic search of the literature for RCTs of infliximab in early methotrexate-naïve rheumatoid arthritis, up to 24 May 2018
● We identified six RCTs with diverse designs, involving 1,832 patients.
The sample size ranged between 20-1,049, and follow-up times between 1-10 years.
● The reporting of results was heterogenous: in 26 journal articles we identified 57 endpoints for 19 time-points, allowing 244 time-point out- come combinations. 77% of outcomes were reported by a single study, 16% by at least two studies and only 7% by more than two studies. These results highlight the need for standardized reporting of outcomes.
● The meta-analysis demonstrated that first-line infliximab was more effective than delayed biological therapy at month 6 (ACR70, remis- sion) and at year 1 (ACR20, ACR70, remission). At year 2 the differ- ence was not significant.
● Economic evaluations using originator prices from years 2005–2008 did not demonstrate the cost-effectiveness of first-line infliximab therapy in early RA. The cost-effectiveness of this strategy at biosi- milar prices is yet to be determined.
remission based on any criteria (ACR, EULAR, DAS in 28 or 44 joints [DAS28/DAS44], Simple Disease Activity Index [SDAI] or Clinical Disease Activity Index [CDAI]) at month 6, year 1 and year 2. If multiple remission measures were reported, we used DAS28-erythrocyte sedimentation rate (ESR) if available, or the measure with highest number of patients in remission in the treatment arms combined.
2.4. Risk of bias assessment of clinical studies
The Cochrane Risk of Bias Tool [26] was applied to all studies. In short, based on the overall assessment of available information on randomization, treatment allocation concealment, blinding of par- ticipants, personnel and outcome assessments, the attrition of participants as well as the reporting of outcomes and other potential sources, we categorized studies as having a low, high or unknown risk of bias. Due to the transitions between double- blind and open-label observation strategies at various time points, risk of bias was assessed separately at month 6, year 1 and year 2.
2.5. Meta-analysis of clinical endpoints
All endpoints were included on an intent-to-treat basis, using the number of patients initially randomized. Calculations were per- formed using the Stata14 statistical software package (StataCorp LLC., College Station, Texas, USA). Due to the heterogenous design of studies, we chose different quantitative synthesis methods for pre-defined short- and long-term outcomes. For month 6 outcomes, a network meta-analysis using the Stata network meta package was performed comparing the infliximab plus MTX combination, multiple DMARD combinations with cor- ticosteroid with or without infliximab, steroid and MTX combina- tions, and MTX monotherapy, using MTX monotherapy as control [30]. There was no source of loop-inconsistency (i.e.
incomparable results from direct and indirect comparisons) within the network, therefore we applied the consistency net- work meta-analysis model (assuming random effects for study heterogeneity but no inconsistency between direct- and indirect comparisons). Due to the changes in applied treatments and observation strategies from month 6, treatment arms used in the network meta-analysis were not feasible for later outcomes.
Rather, treatments were dichotomized according to a first-line infliximab treatment versus other strategies (control) at rando- mization, and,–considering the heterogeneity of studies–out- comes at year 1 and 2 were assessed using a random effects meta-analysis according to the method of DerSimonian and Laird [31]. Month 18 outcomes were included in the meta- analysis at year 2, if no year 2 outcomes were available from the same study. For ACR20, ACR70 and remission, log odds ratios (ORs) with 95% confidence intervals were applied for the com- parisons. The significance threshold was set at p < 0.05.
We also investigated how the results of our analysis at year 2 would have changed if the ASPIRE trial [15], a 1-year parallel design study of first-line infliximab versus placebo in combination with MTX, had included a long-term extension study. We pro- jected hypothetical year 2 ACR70 results in the ASPIRE study using published year 1 ACR70 data and included the simulated results in our meta-analysis.
2.6. Search for cost-utility studies
We also searched for cost-utility studies of first-line infliximab in PubMed, Cochrane CENTRAL and the Centre for Reviews and Dissemination (CRD) Health Technology Assessment (HTA) data- base of the University of York up to 31 October 2018. The search- terms included keywords for economic analyses, infliximab and the MeSH terms for rheumatoid arthritis. Full details of the PubMed, CENTRAL and CRD search criteria are shown in Supplementary Tables S4, S5 and S6, respectively. Eligible studies included those that reported incremental cost-effectiveness ratio (ICER) values for infliximab in patients with MTX-naïve RA. Full text manuscripts were considered. Data extraction for main character- istics of the evaluations (publication year, country, type of model, time horizon applied, data sources used, study sample, applied treatments and comparators, year of costs, study perspective, ICER) was performed.
3. Results
3.1. Literature search of clinical studies
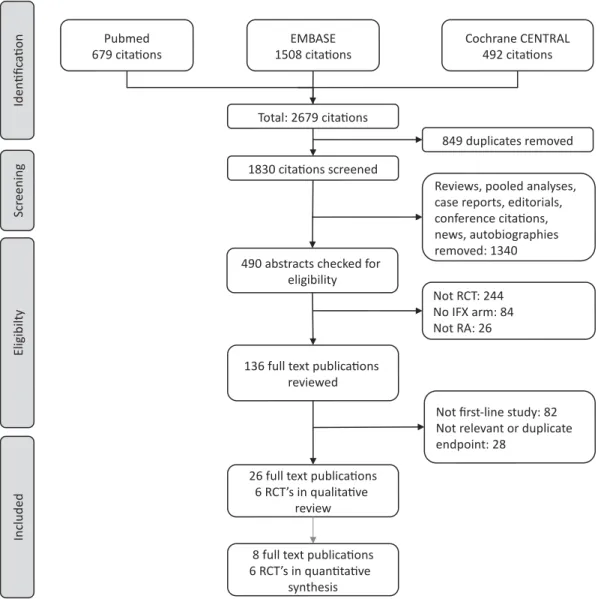
A PRISMA flow diagram for our systematic review is shown inFigure 1 and includes the number of citations found and screened. We identified six RCTs (reported in 26 full text publications) of first-line infliximab in RA: ASPIRE (3 pub- lications) [15,32,33], BeSt (15 publications) [34–48], Durez 2007 (1 publication) [49], IDEA (1 publication) [12], NEO- RACo (3 publication) [50–52] and Quinn 2005 (3 publica- tions) [53–55]. The sample size of these RCTs ranged from 20 to 1,049 patients; follow-up times were between 1 and 10 years. Key details of the studies are shown in Table 1.
Data from six studies (reported in eight publications [12,15,34,35,38,49,51,53]) were included in the quantitative synthesis. The studies included 1,832 patients at baseline, of whom 1,009 (55%) received first-line infliximab.
Inclusion criteria for RA diagnosis was based on ACR cri- teria in all six RCTs. Disease activity criteria was defined in most studies as having ≥6 tender and swollen joints.
Disease duration was limited to between 3 months and 3 years.
Results were reported for 19 different time points and for 57 relevant endpoints, allowing 244 different time point– outcome combinations (Supplementary Tables S7 and S8).
Of these, 188 (77%) were reported only by a single study, 39 (16%) by at least two studies and 17 (7%) by more than two studies. Results of ACR response and remission rates by any criteria were reported at month 6 by three [12,34,49] and four [12,34,49,51] studies, respectively, and at year 1 by five and four studies, respectively [12,15,34,49,51,53]. ACR20 and ACR70 at year 1 were reported by four studies; all other measures were reported by no more than three studies at the same time point. Apart from three studies reporting ACR70 outcomes at year 2 [35,51,53], longer-term results (>1 year) for each measure were reported by a maximum of two studies at the same time point (Supplementary Tables S7 and S8). Although the presence of ADAs was included among the pre-defined outcomes, none of the studies reported ADA results.
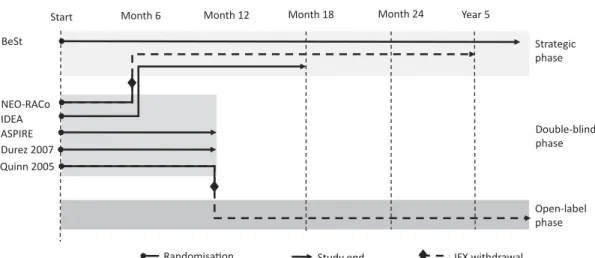
3.2. Methodological heterogeneity of clinical studies The study designs showed great heterogeneity. The metho- dological heterogeneity of studies and the treatment arms are summarized in Figure 2 and Supplementary Table S9, respectively. Patients were naïve to any DMARDs in three studies [12,51,53], and were exposed to csDMARDs other than MTX in two studies [34,49]. In ASPIRE [15], minimal exposure to MTX was allowed. All patients were naïve to bDMARDs. Two studies had a duration of 1 year [15,49] and four studies had patient follow-up beyond 1 year [12,34,51,53]. Randomized treatments changed substantially between month 6 and year 2 in all studies. The BeSt [34]
study was an open-label, strategic, treat-to-target study from the onset. In the NEO-RACo [51] and IDEA [12] studies, patients continued an open-label, treat-to-target protocol following 6 months of double-blind treatment. Patients in the Quinn 2005 et al. [53] study could continue open-label MTX treatment after cessation of infliximab at 1 year.
Infliximab was also discontinued in the NEO-RACo study after 6 months [51].
3.3. Risk of bias analysis of clinical studies
At month 6 relevant outcomes were reported from four studies.
One study was a randomized, open-label, strategic trial and was assessed as having a high risk of bias due to the lack of blinding [34]. Three double-blind, randomized controlled studies had a low risk of bias [12,49,51]. At year 1, three studies were cate- gorized as high risk of bias due to their open-label design [12,34,51], while outcomes were measured during the double- blind randomized phase of three studies categorized as having a low risk of bias [15,49,53]. At year 2, all studies were open-label and were assessed as having a high risk of bias [12,34,51,53].
3.4. Meta-analyses of clinical studies
We performed network meta-analysis for efficacy outcomes at month 6. The network map for 6-month ACR and remission outcomes are displayed in Supplementary Figure S10. There was no source of inconsistency in the network structure of the included treatments, therefore we performed the network meta-analysis using the consistency model assuming no
Figure 1.PRISMA flow diagram of the systematic review.
CENTRALCochrane Central Register of Controlled Trials;IFXinfliximab,RArheumatoid arthritis,RCTrandomized controlled trial.
Table1.RCTsoffirst-lineinfliximabtreatmentinpatientswithRA. StudyReferencePatientsRandomizedarmsN Armin network meta- analysis
Armin random effects meta- analysisDouble-blindphaseOpen-labelphase ASPIRE[15,32,33]RA(ACR1987) Age18–75years Diseaseduration≥3months,≤3years Activedisease:≥10SJC;≥12TJCandat leastoneof:RF-positiveorbone erosiononhand/feetorCRP≥20mg/L Exclusion:priorMTX(≤3doseswere allowed),anysDMARDin4weeksprior tobaseline(LEFin6months),priorTNFi treatment
3mg/kgIFX(atweek0,2,6and q8w)+MTX373IFX+ MTXFL-IFX54weeks RandomizationatBL:3groups,5:5:4 BaselinesteroidsandNSAIDswere allowed
Noopen-labelphase 6mg/kgIFX(atweek0,2,6and q8w)+MTX378 PLA+MTX298MTXControl BeSt[34–47]EarlyRA(ACR1987) Diseaseduration≤24months Age≥18years Activedisease:≥6/66SJC;≥6/68TJC;ESR ≥28mm/hour;oraglobalhealth score≥20mm(best=0, worst=100mm) Exclusion:previoustreatmentwith sDMARDsotherthananti-malarials
InitialIFXcombination:MTX+IFX (3->10mg/kg)->pre-defined sDMARDmono/combination sequence 128IFX+ MTXFL-IFXNodouble-blindphase10-yearstrategictrial RandomizationatBL:4groupsTreatmentadjustmentsevery 3months:ifDAS44≥2.4,->nextstepintheallocated treatment.IfDAS44≤2.4for6months,medicationwastapered ->1drugatmaintenancedose.PRDandIFXwerealways taperedfirst.IfDAS44≥2.4aftertapering,thelasteffectivedose wasreintroduced
Sequentialmonotherapy:MTX-> pre-definedsDMARDsequence126MTXControl Step-upcombination:MTX->MTX+ pre-definedsDMARD combinationsequence
121MTXControl InitialPRDcombination:MTX+SSZ +PRD(60->7.5mg/week)-> pre-definedsDMARDmono/ combinationsequence
133CoControl Durez2007[49]RA(ACR1987) Diseaseduration<1year Agenotspecified Activedisease:SJC≥6;TJC≥8 Exclusion:previousMTX,corticosteroids ≥3monthsor4weekspriortoBL,>2 sDMARDsorivMPD
IFX 3mg/kgiv(week0,2,6,thenq8w) +MTX
15IFX+ MTXFL-IFX52weeks RandomizationatBL:3groupsAfterweek52ivMPDandIFXwasdiscontinued.Noreportavailable aboutextensionstudy ivMPD(1g)+MTX15CSControl MTX14MTXControl IDEA[12]RA(basedonACR1987criteria) Symptomduration:3–12months Age18–80years Activedisease(DAS44>2.4) DMARD-naïve Exclusion:anypriorcsDMARD, corticosteroidusewithin1month priortobaseline
IFX(3mg/kg)+MTX MPD(250mgivatweek2,6,14,22) +MTX
55 57
IFX+ MTX CS
FL-IFX Control
26weeks RandomizationatBL:2groups IfDAS44>2.4,treat-to-target:MPD 120mgiv/scatweek6,14,22, 38,50
52weeks Pragmaticescalationprotocol:sDMARDchangeorescalation,ifDAS >5.2andtwosDMARDsfailed->otherbiologic,ifDAS44<1.6 for6months:IFXwithdrawal NEO-RACo[50,51]RA(ACR1987) Symptomduration:≤12months Age18–60years Notpermanentlyworkdisabledor retired Activedisease:≥6SJCand≥6TJCandat leastonefrom:morningstiffness ≥45min;ESR≥30mm/hr;CRP ≥20mg/L;orDMARD-naïve Exclusion:oralglucocorticoidsin previous6months;ivglucocorticoids in1month
IFX(3mg/kgatweek4,6,10,18, 16)+openintensifiedFIN-RACo (MTX+SSZ+HCQ+PRD)-> pre-definedscheduleof sDMARDs PLA(atwk4,6,10,18,16)+open intensifiedFIN-RACo(MTX+SSZ +HCQ+PRD)->pre-defined scheduleofsDMARDs
50 49
IFX+Co Co
FL-IFX Control
26weeks Double-blindinfusioninduction addedtotheopen-labelFIN- RACoprotocol.Treatment targetedtoremissionduringthe entirestudy
5years Strategictreatmenttargetedtoremission.Mandatoryswitchesif ACRremissionnotachieved,alwaysusingacombinationof threesDMARDs+PRD.If<ACR50responseafterweek26at consecutivevisits,unrestrictedtreatmentincludingswitchto otherTNFi (Continued)
treatment effect variation between designs [30]. ACR70 and remission outcomes at 6 months were improved significantly with first-line infliximab plus MTX compared with MTX control (Table 2). ACR20 at 6 months was not significantly different between the infliximab and control groups (Table 2).
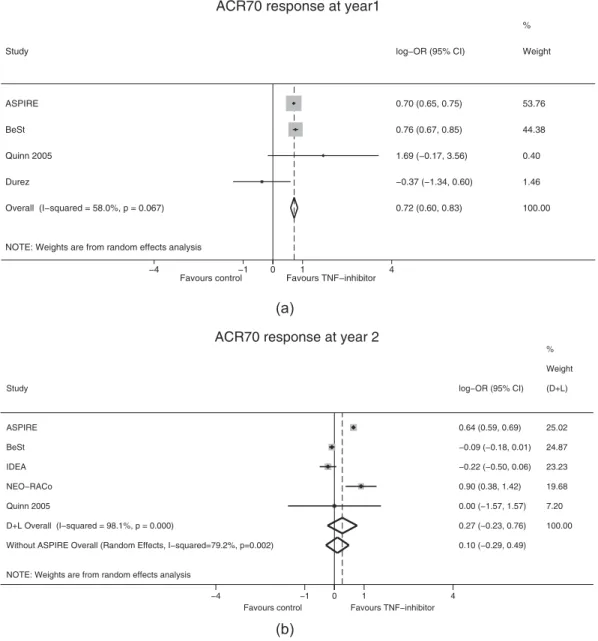
At year 1 and year 2, first-line infliximab was compared with other strategies via random effects meta-analysis. Heterogeneity between studies was low and was not significant in the year 1 models. Our analyses showed that ACR20, ACR70 and remission at year 1, significantly favored the infliximab group compared with the control group (Table 2); ACR70 response at year 1 is also shown inFigure 3(a). At year 2, there were no significant differ- ences in outcomes (ACR20, ACR70 and remission) between the infliximab and control groups (Table 2; Figure 3(b)).
Heterogeneity was significant in the year 2 ACR70 (I2= 79.2%;
p = 0.002) and remission models (I2= 75.8%; p = 0.006).
In studies with TNFis that had at least 1 year of a double- blind, parallel phase and reported year 2 results (etanercept:
COMET [9], Ebrel ERA [56]; golimumab: GO-BEFORE [57,58];
adalimumab: PREMIER [8]; infliximab: Quinn 2005 [53]), there was an additional 8.9% and 9.3% of patients with an ACR70 response at year 2 versus year 1 in the control group and TNFi group, respectively. We assumed that ACR70 response rates of the MTX alone and infliximab plus MTX combination arms in ASPIRE would change from 21% and 38% in year 1 to 21% and 35.8% by year 2, respectively. Based on these results, projected hypothetical year 2 ACR70 outcomes from the 1-year parallel design ASPIRE study were included in our meta-analysis of ACR70 response at year 2 (Figure 3(b)). Although projected year 2 ACR70 data from ASPIRE increased the between-group difference in favor of infliximab, the difference remained non-significant (Figure 3(b)).
3.5. Summary of cost-effectiveness studies
Our searches yielded 96 abstracts in PubMed, 37 abstracts in the Cochrane CENTRAL database and 123 abstracts in the CRD HTA database. We removed 27 duplicates, 171 records based on their title and two conference posters. From the remaining 56 records we identified four full text journal publications reporting cost-utility studies of infliximab as first-line treat- ment in patients with RA (Table 3). Two studies were con- ducted in the United States, and the other studies were conducted in the United Kingdom and the Netherlands.
ICERs ranged between €22,000/quality-adjusted life-year (QALY) and £650,000/QALY (Table 3). The cost effectiveness of first-line infliximab treatment was above the usually accep- table financial threshold of the respective countries in most models. All models were calculated with prices prior to the registration of biosimilar infliximab by the European Medicines Agency in 2013.
4. Conclusions
In summary, our systematic review and meta-analysis has demonstrated that 1-year efficacy outcomes (ACR20, ACR70 and remission) were improved significantly with first-line infliximab versus control strategies in patients with csDMARD- naïve RA. These efficacy differences were not significant in
Table1.(Continued). StudyReferencePatientsRandomizedarmsN Armin network meta- analysis
Armin random effects meta- analysisDouble-blindphaseOpen-labelphase Quinn2005[53]RA(ACR1987) Symptomduration:<12months PoorprognosisbyPISAscoringsystem (PISAscore≥3) NoprevioussDMARDsororal corticosteroids,MCPjoint involvement,stableonNSAID ≥2weekspriortoscreening Exclusion:inflammatoryconditionthat mightconfoundthediagnosis; previousTNFi;CPA;nitrogenmustard; chlorambucil;orotheralkylating agents
IFX(3mg/kg)+MTX PLA+MTX
10 10
IFX+ MTX MTX
FL-IFX Control
54weeks RandomizationatBL:2groups8years IFXwasdiscontinuedatweek54, non-respondersreceivedstep-upcombinationtherapy(+SSZ->+ HCQ) ACRAmericanCollegeofRheumatology,BLbaseline,ComultipleDMARDcombinationwithcorticosteroid,CPAcyclophosphamide,CRPC-reactiveprotein,CSsteroidandmethotrexatecombination,DASDiseaseActivityScore, ESRerythrocytesedimentationrate,FLfirst-line,HCQhydroxychloroquine,IFXinfliximab,ivintravenous,LEFleflunomide,MCPmetacarpophalangeal,MPDmethylprednisolone,MTXmethotrexate,NSAIDnon-steroidalanti- inflammatorydrug,PISAPersistentInflammatorySymmetricalArthritis,PLAplacebo,PRDprednisolone,q8wevery8weeks,RArheumatoidarthritis,RCTrandomizedcontrolledtrials,RFrheumatoidfactor,scsubcutaneous,(s) DMARD(synthetic)disease-modifyingantirheumaticdrug,SJCswollenjointcount,SSZsulfasalazine,TJCtenderjointcount,TNFitumornecrosisfactorinhibitor
a longer follow-up of 2 years. The diminished effect size and precision of long-term estimates may partly be explained by the highly diverse methodology of studies. The long-term effects of first-line biological therapy on disease progression should be further analyzed using all TNFi agents.
Analysis of cost-utility studies performed before the availability of lower cost infliximab biosimilars revealed that first-line use of originator infliximab was not cost effective. New cost-effectiveness analyses are needed in early RA that consider the sizable potential savings resulting from the uptake of biosimilar infliximab.
Given the potentially considerable savings due to biosimilar infliximab, the evident short-term efficacy benefits, the avail- ability of prognostic factors for progressive disease and the potential for biological-free remission if administered early, the cost-effectiveness of first-line infliximab in early, treat- ment-naïve RA should be re-assessed using biosimilar prices.
5. Expert opinion
The results of this systematic review and network meta-analysis show that ACR70 and remission outcomes at 6 months in patients with MTX-naïve RA were significantly improved with infliximab versus MTX control. At year 1, ACR20, ACR70 and remission out- comes were also significantly improved with infliximab versus control strategies. However, at year 2, no statistically significant differences in ACR20, ACR70 or remission outcomes were observed between treatment groups. Long-term (>2 years) results on clinical remission were available only from two RCTs (BeSt [34], NEO-RACo [51]) and patient-reported outcome data were presented only from the BeSt [34] and Quinn 2005 [53] studies.
We have demonstrated the need for improved standar- dized reporting of studies. Despite the 244 reported time point-outcome combinations, neither ACR response nor remis- sion rates were reported by all studies at 6 months and 1 year, and apart from ACR70, only two studies reported these effi- cacy outcomes at the same time point after 1 year. For a given time point only two outcomes were reported in four studies and 13 outcomes were reported in three studies. The diverse
reporting of results is a major barrier for effective evidence synthesis.
The included studies showed increasingly heterogenous designs over time, which partly explains the smaller effect sizes and increasing variance at year 2 compared with year 1 outcomes. However, due to the small number of studies, the decomposition of study heterogeneity via meta-regression was not feasible [26]. The randomized treatments were mod- ified substantially beyond month 6 in all studies. In NEO-RACo [51], infliximab treatment was discontinued after 6 months, and in Quinn 2005 [53], after 12 months. In the BeSt study [34,35] at year 2 nearly three quarters of initially randomized patients received infliximab plus MTX combination therapy, while in the DMARD monotherapy arm more than one quarter of patients received infliximab. In IDEA [12] two thirds of patients in the control arm received dose escalation, while DMARDs were adjusted in more than half of the patients in the infliximab arm, and infliximab was discontinued in every fourth patient due to sustained low disease activity.
While five of the six studies measured endpoints in a double- blind setting at month 6, year 2 efficacy outcomes were available only from open-label studies. We hypothesize that longer-term benefits of biologic combinations and MTX monotherapy would be demonstrated in studies that remain blinded for longer peri- ods. Therefore, using ACR70 data, we investigated how the results of our analyses at year 2 might have changed if the 1-year long ASPIRE study [15] had included a long-term extension study. The absolute risk difference in ACR70 response at year 2 between the treatment and control arms in the PREMIER [8] and COMET [9] studies were 26% and 25%, respectively. However, in our analysis of hypothetical year 2 ASPIRE results, we assumed a conservative 15% difference between infliximab plus MTX and MTX monotherapy. Although the projected year 2 ACR70 data from ASPIRE increased the between-group difference in favor of infliximab, the difference remained non-significant. Although loss of sustained clinical response due to immunogenicity [63] may be an alternative explanation for the moderate long-term effect size, we found no reports of anti-drug-antibody levels in the included studies (Supplementary Table S7).
Figure 2.Heterogeneity of studies.
IFXinfliximab.
A key strength of our study is that we provide a comprehensive evaluation of efficacy endpoints that are relevant for pharmacoeconomic analyses – ACR20, ACR70 and remission – at different time points from 6 months up to long-term outcomes at 2 years. While the benefit of immediate infliximab in preventing joint damage has been shown over other treatment strategies [15,34], radiographic progression was out of the scope of this review. Several other recent meta-analyses have compared the combination of bio- logic agents plus MTX over csDMARDs in early MTX-naïve RA [3,17–19,64]. The Cochrane report from Singh et al. [3]
included analyses of ACR50, Health Assessment Questionnaire (HAQ) scores and remission rates at <6 months, 6–12 month and >12 month time points. From the included 19 RCTs seven were infliximab studies; however, in one of them all patients received MTX at baseline [3]. Cai et al. [17]
included 20 RCTs in the analysis of ACR20, ACR50 and ACR70 endpoints. In one of the five included infliximab studies, all patients received three months of MTX induction therapy before randomization [17]. Stevenson et al. [64] in a comprehensive HTA report commissioned by NICE, synthe- sized month 6 ACR and EULAR response outcomes for seven biological drugs, including two infliximab trials. Albert et al.
[18] included one infliximab study in an indirect pairwise meta-analysis of year 1 ACR20, ACR50 and ACR70 endpoints from six RCTs of biologics + DMARD therapy. Donahue et al.
[19] reported less radiological progression and higher ACR50 response rates at year 1 with four TNFis in combination with MTX versus MTX monotherapy. Three of the 13 trials included infliximab. Taken together, data from these studies support the results of our own analysis that, in the short-term, biolo- gics combined with MTX are superior to MTX monotherapy in terms of ACR and remission endpoints in early RA. To date, no consistent evidence supports the superiority of one biologic therapy over another.
In all the six RCTs, RA diagnosis was based on the 1987 ACR diagnostic criteria for RA [65], which have been criti- cized for their lack of sensitivity in early disease [66]. The new ACR/EULAR classification system introduced in 2010, focuses on symptoms at earlier stages of the disease rather than establishing the diagnosis by its late-stage features [66]. Further studies using the new ACR/EULAR classification system may contribute to a better understanding of the effectiveness of first-line infliximab treatment in early-stage RA. Moreover, we believe that there is a need for new outcomes to detect the long-term effects of deteriorated health patients may suffer due to the lack of early elimina- tion of symptoms. These may include measures to assess the impact on internalization and legitimization of societal stereotypes of RA, subjective expectations regarding future health and treatment goals, acceptability of symptoms, uncertainty about the future, work capacity, long-term life planning and wellbeing in terms of the ability to do things that are important in patients’lives [67–71].
In our cost-utility analysis, the cost-effectiveness of origi- nator infliximab was deemed unacceptable in the published economic analyses we reviewed, with ICERs of between
€22,000/QALY and £650,000/QALY reported. Due to the broad variety of modelling assumptions of long-term
Table2.Resultsofmeta-analysisforefficacyoutcomesatmonth6,year1andyear2. Networkmeta-analysisofmonth6results First-lineIFX+MTXvsMTXmonotherapyRandomeffectsmeta-analysisofyear1results First-lineIFXvscontrolRandomeffectsmeta-analysisofyear2results First-lineIFXvscontrol StudiesRef.logOR[95%CI]pvalueStudiesRef.logOR[95%CI]pvalueStudiesRef.logOR[95%CI]pvalue ACR20BeSt Durez2007 IDEA [12,34,49]0.854[−0.337,2.045]0.160ASPIRE BeSt Durez2007 Quinn2005 [15,34,49,53]0.459[0.424,0.495]<0.0001BeSt IDEA Quinn2005
[12,35,53]−0.002[−0.108,0.104]0.602 ACR70BeSt Durez2007 IDEA
[12,34,49]1.046[0.526,1.567]<0.0001ASPIRE BeSt, Durez2007 Quinn2005 [15,34,49,53]0.716[0.598,0.835]<0.0001BeSt IDEA NEO-RACo Quinn2005
[12,35,51,53]0.103[−0.285,0.491]0.603 RemissionBeSt Durez2007 IDEA NEO-RACo
[12,34,49,51]1.058[0.556,1.561]<0.0001BeSt IDEA NEO-RACo Durez2007 [12,34,49,51]0.256[0.171,0.341]<0.0001BeSt IDEA NEO-RACo Quinn2005 [12,38,51,53]−0.070[−0.445,0.304]0.713 ACRAmericanCollegeofRheumatology,CIconfidenceinterval,IFXinfliximab,MTXmethotrexate;ORoddsratio;Ref.reference
outcomes, the results of cost-effectiveness analyses need to be interpreted with caution. Although systematic reviews did not demonstrate a substantial difference between branded TNFi drugs in terms of efficacy, ICERs between individual drugs differed considerably. In a review of six cost-utility analyses by Stevenson et al. [64], ICERs were
>US$100,000/QALY in three of four infliximab studies, two of five adalimumab studies, and five of six etanercept stu- dies. Treating biological agents as a homogenous group, Stevenson et al. estimated that the ICER for a first-line biological strategy versus MTX monotherapy in MTX-naïve, early RA was £58,300/QALY, still above the acceptable cost- effectiveness threshold.
However, the available cost-effectiveness studies with infliximab in MTX-naïve RA were performed before the mar- ket entry of biosimilar competitors. The most recent
economic evaluation of MTX-naïve, early RA reported base case results using 2013 innovator etanercept prices [72].
Reductions in the price of infliximab therapy due to the entry of biosimilar products in the RA market could improve the cost-effectiveness of first-line infliximab therapy for patients with RA. The considerable cost saving achievable with biosimilar infliximab along with the potential to improve access to RA has been demonstrated in several budget impact analyses [23–25,73]. We hypothesize that given the considerable price reductions driven by biosimilar competition, the cost-effectiveness of first-line infliximab therapy may fall under the financing threshold in many countries and allow use of this agent in the first-line setting for the treatment of patients with early RA. If proven to be cost effective, first-line therapy with biologic agents could provide a valuable opportunity to test the window of
NOTE: Weights are from random effects analysis Overall (I−squared = 58.0%, p = 0.067) Study
BeSt Quinn 2005 ASPIRE
Durez
0.72 (0.60, 0.83) log−OR (95% CI)
0.76 (0.67, 0.85) 1.69 (−0.17, 3.56) 0.70 (0.65, 0.75)
−0.37 (−1.34, 0.60)
100.00 Weight
44.38 0.40
%
53.76
1.46
Favours control Favours TNF−inhibitor
−4 −1 0 1 4
ACR70 response at year1
NOTE: Weights are from random effects analysis D+L Overall (I−squared = 98.1%, p = 0.000) ASPIRE
Quinn 2005 IDEA NEO−RACo Study
BeSt
Without ASPIRE Overall (Random Effects, I−squared=79.2%, p=0.002)
0.27 (−0.23, 0.76) 0.64 (0.59, 0.69)
0.00 (−1.57, 1.57)
−0.22 (−0.50, 0.06) 0.90 (0.38, 1.42) log−OR (95% CI)
−0.09 (−0.18, 0.01)
0.10 (−0.29, 0.49) 100.00 25.02
% Weight
7.20 23.23 19.68 (D+L)
24.87
Favours control Favours TNF−inhibitor
−4 −1 0 1 4
ACR70 response at year 2 (a)
(b)
Figure 3.Random effects meta-analysis ACR70 response (a) at year 1 and (b) at year 2 with and without simulated ASPIRE results*.
*ASPIRE 2-year ACR70 response was projected from 1 year-long double-blind, parallel-group RCTsACRAmerican College of Rheumatology,CIconfidence interval,D + LDerSimonian & Laird random effects meta-analysis,ORodds ratio,RCTrandomized controlled trial
opportunity hypothesis in a real-world setting and increase the possibility for drug-free remission in some patients with RA.
Acknowledgments
Medical writing support for this manuscript (including copyediting and fact checking) was provided to the authors by Emma Evans, PhD CMPP at Aspire Scientific Limited (Bollington, UK).
Author contribution statement
LG conceived the study, ZZ, LG, VB and MP performed the systematic literature search; ZZ, LG, VB, FR and MP contributed to data collection; ZZ performed the data analysis; ZZ, LG, VB, FR, RA, ZS and MP contributed to interpretation of data and writing the manuscript. All authors take respon- sibility for the integrity of the work as a whole, and have given final approval for the article to be published. The conception, design, analysis and interpretation of results of this study are a product of independent research performed by the authors.
Funding
Medical writing support for this manuscript (including copyediting and fact checking) was provided to the authors by Aspire Scientific Limited (Bollington, UK), and was funded by Celltrion Healthcare Co., Ltd.
(Incheon, Republic of Korea). The conception, design, analysis and inter- pretation of results of this study are a product of independent research performed by the authors.
Declaration of interest
Z Zrubka used to be a full-time employee of Egis Pharmaceuticals, Janssen Cilag, Sandoz and Pfizer. L Gulácsi has received consultancy and lecturing fee from Astellas, BMS, Celltrion, Egis Pharmaceuticals, GSK, Hikma, Hospira, Lilly Hungaria Ltd, MSD Hungary, Pfizer, Roche, Sandoz and UCB. V Brodszky has received grants and personal fees from Celltrion, Egis Pharmaceuticals, Pfizer and Sager Pharma. F Rencz has received consultancy fees from Celltrion and Hospira. R Alten has received honor- aria from Celltrion. Z Szekanecz has received consultancy and lecturing fees from Abbvie, Amgen, BMS, Lilly, MSD, Novartis, Pfizer, Roche and UCB. M Péntek has received grants and personal fees from Celltrion, Egis Pharma, Merck, Pfizer and Sager Pharma. Z Zrubka, L Gulácsi, V Brodszky, F Rencz and M Péntek received grants from Celltrion during the writing of this article.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
ORCID
Zsombor Zrubka http://orcid.org/0000-0002-1992-6087 László Gulácsi http://orcid.org/0000-0002-9285-8746 Valentin Brodszky http://orcid.org/0000-0002-6095-2295 Fanni Rencz http://orcid.org/0000-0001-9674-620X Rieke Alten http://orcid.org/0000-0002-3395-4412 Zoltán Szekanecz http://orcid.org/0000-0002-7794-6844 Márta Péntek http://orcid.org/0000-0001-9636-6012
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
Table3.Cost-utilitystudiesofinfliximabasfirst-linetreatmentinpatientswithRA. PublicationReferenceCountryModelTime horizonDatasource,sampleTreatment costComparatorPerspectiveICER Spalding& Hay2006[59]USAHAQbasedMarkovmodelLife- timeHypothetical cohortofUSfemales aged55–60yearswith RA
2005US$IFX+MTX,(ADA,ETN)vs MTXPayerUS$409,523/QALY Chenetal 2006[60]UKHAQbasedindividualsampling model(BRAM)Life- timeLiteraturereview2004£IFX+MTX(ADA,ETN)vs cDMARDPayer£650,000/QALY Daviesetal 2009[61]USAACRresponseandHAQbased individualsamplingmodelLife- timeSimulationbasedontrial data2007US$IFX+MTX(ADA,ETN)vs cDMARDSocietalUS$53,607/QALY vanden Houtetal. 2009
[62]TheNetherlandsTrialbasedanalysis2yearsTrial2008€IFX+MTXvscDMARD+ corticosteroid combination Societal€130,000/QALY(frictioncostmethod) €22,000/QALY(humancapitalapproach) ACRAmericanCollegeofRheumatology,ADAadalimumab,BRAMBirminghamRheumatoidArthritisModel,csDMARDconventionalsyntheticdisease-modifyingantirheumaticdrug,ETNetanercept,HAQhealthassessment questionnaire,IFXinfliximab,MTXmethotrexate,QALYquality-adjustedlifeyear,RArheumatoidarthritis,UKUnitedKingdom,USUnitedStates,USAUnitedStatesofAmerica