Chapter 2
Thermoeconomic Considerations of Sea Water Demineralization
ROBERT B. EVANS, GARY L. CRELLIN, AND MYRON TRIBUS
I. Introduction 21 II. Exergy Balances 23 III. Combining Exergy and Cost Balances 25
A . A Review of the Optimization Procedure 4 2
IV. Conclusions 4 3 A p p e n d i x A . Basic Relationships among Entropy, Exergy, Energy, and
Availability 4 4 Appendix B. On the U s e of the General Exergy Balance 6 6
Appendix C. Minimization of the Nonlinear Cost Equation 7 0
Acknowledgment 73 List of S y m b o l s 7 4 References 75
I. Introduction
T h e separation of pure liquid water from a mineral solution will not occur spontaneously. In those areas of the earth where demineralized water is needed for drinking, irrigation, or industrial purposes, it is necessary to create a controlled region of space—usually containing an interface—at which the separation may be forced to occur. T o force the separation, resources outside the controlled region must be used.
The creation and maintenance of such an interface, or zone of separa- tion, requires a capital investment. The provision for the driving agency (which is needed to force the separatory process to go against its natural tendency) requires either an additional capital investment (as in solar or wind-driven apparatus) or a continuous operating expenditure (for electric power, fuel, or the equivalent). In some cases, there must be both capital and a continuing expense, as typified by the use of transformers
21
and rectifiers required to adapt a source of motive power to the process requirements. There are often additional expenses for procurement of the raw sea water, its filtration, and chemical preparation, as well as for the disposal of the brine and the distribution of the product water.
All these matters must be given careful consideration in the design of any sea-water-demineralization scheme, regardless of the process on which the design is based. The word thermoeconomics has been coined to describe the generalized study of these factors as they appear in optimum designs. A properly conducted thermoeconomic study serves to reveal how the choices of the design variables in a given process affect the price of the water produced by that process. Thermoeconomics combines scientific disciplines (principally thermodynamics) with economic disciplines (principally cost accounting) to reveal the critical dimensionless groups which determine the cost of making fresh water from sea water.
The methods of design and analysis described in this paper are quite general and applicable to all known sea-water-conversion processes.
They are also applicable to the problem of making potable water from brackish water or waste water, but of course the resulting optimum designs will be rather different than if sea water were used.
The word "optimum" is used here in a limited technical sense to mean the most economical use of a given set of resources whose costs or values are prescribed. The question of how much value ought to be placed on water, fuel, land, labor, material, or time is too complex a question to be dealt with here. It should be noted, however, that the question is by no means trivial to the designer, particularly if he is designing a system for use in a country other than his own. The methods used to determine the cost or value of resources vary greatly over the face of the earth. In some countries the value of fuel or electric power is found by appeal to the world or the local marketplace. In other countries these values are fixed by the government as a means of influencing the use of various resources. In some places a monopoly may control prices.
Few, if any, countries use just one of these means to determine values.
Socialist and communist countries do, in part, pay heed to world markets and, of course, subsidies, price supports, and government rate setting are also well known and applied in capitalistic countries. The value of time, as reflected in interest rates, is also subject to similar controls and/or consensus.
As will be seen, the most economical design is dependent upon these costs and values, and when it is recalled that large-scale plants for sea- water demineralization are designed to operate for 2 5 years or more, it becomes evident that not only must the designer take into account his
Thermoeconomic Considerations of Sea Water Demineralization 23 uncertainty about present costs, but he should also make some provision for changing costs in the future.
In this chapter we consider a more limited approach to optimum design than ultimately required by the designer responsible for the over-all process economy. W e shall consider that all costs needed for the design calculations are known. This procedure leads to a "deterministic design." The application of modern decision theory to the rational design of plants in which these cost data are subject to uncertainty remains to be done.
II. Exergy Balances
Just as the comparison of alternative designs requires the use of a common basis for cost, so does the choice from among alternative sources of energy require a common basis for comparison. The work of Carnot and Clausius led to the understanding that the energy derived from different sources differs in its motive power. Over the intervening years, since Clausius formalized the principles of thermodynamics in the 1850s, it has been recognized that the differing forms of energy do not always possess the same ability to lift a weight or effect a change.
Various thermodynamic functions such as the Helmholtz free energy, Gibbs free energy, Keenan availability, and "lost work" have been proposed in the literature as a means for keeping the differences in view.
In the last decade the German literature has shown extensive use of such a function, called the Exergy. It has been shown that there is an important link between concepts of information theory and thermo- dynamics. It also has been shown, and the proof is reproduced in Appendix A, that our information about a departure from equilibrium may be taken as a general measure of exergy and that loss of this informa- tion is, in fact, the "lost work" (when this information is expressed in work units). This generalized exergy contains as special cases all the previously known thermodynamic functions which measure the work potential of energy, and fortunately it is a convenient function to use.
It is not necessary to understand the information-theory basis for thermodynamics to be able to use or comprehend the generalized exergy function. A n understanding of classical thermodynamics will suffice. It is necessary, however, to use information theory (or some other postulate) to demonstrate rigorously the correctness of the formulation. The use of exergy balances provides a rapid means of comparing the costs associated with various alternative sources of energy.
As shown in Appendix A, exergy is always associated with any quantity of matter, any fixed region of space (even a vacuum), or any flux across a
boundary. In each case the exergy measures the mechanical energy equivalent of the matter, space, or flux (where the mechanical energy equivalent is by definition the maximum amount of mechanical energy which can be produced upon the establishing of equilibrium with the surroundings of the system). For example, the exergy of the fresh water and brine effluent streams from a sea-water conversion plant turns out to be the Gibbs-free-energy difference between these streams and the entering sea water for the simple case where all streams are at temperature T0—this type of exergy being called chemical exergy. It should be noted that exergy is a property of the system and its surroundings. For example, an evacuated vessel, brought back to earth from outer space, may be used as a source of exergy. On the other hand, a gas-filled vessel taken to outer space becomes a source of exergy. With respect to their original environ
ments, both the evacuated vessel in outer space, or the gas-filled vessel at 1 atm at the earth's surface, have zero exergy. The importance of the exergy concept in thermoeconomics lies in the fact that as the exergy flows through a complex thermodynamic process, its dissipation and the costs associated with its dissipation may be followed and studied.
Energy and matter cannot be destroyed, hence energy and material balances are not satisfactory methods for keeping track of costs.
(The connection between exergy and the quantity known as availability is discussed in Appendix A. For the moment it may help the reader to to think of exergy as a generalization of the concept of availability, but differing from it in subtle ways discussed in Appendix A.)
From time to time entropy creation has been proposed as a quantitative measure of the dissipation of the work potential of energy. It is the measure of reversibility and therefore intimately related to the efficiency with which resources are used. Entropy, however, does not measure how much "work equivalent" is conveyed from one place to another. A n exergy balance, on the other hand, provides all the important information that is needed in the economic optimization process.
Once the equations for cost accounting and exergy accounting have been written, it is straightforward to combine these equations and find optimum combinations. Simple differentiation reveals the significant dimensionless ratios which determine the most economic designs.
These techniques have already been used to analyze several sea-water- conversion systems.
Once an accounting for exergy and capital costs has been made, one of several mathematical techniques for optimization may be employed.
The advantages of the use of exergy lie in the fact that the total exergy dissipation is directly proportional to the extra exergy required to operate the plant. Because of the additive nature of entropy creation,
Thermoeconomic Considerations of Sea Water Demineralization 25 the exergy dissipations in various parts of the plant may be added together. Therefore a certain amount of "suboptimization" on individual pieces of equipment can be accomplished without having to treat the entire plant as a whole.
Appendix Β describes a general exergy balance, including transient states. If a complete analysis is to be made, taking into account "down time" and variations in loading, or if batch processing is considered, the transient considerations must be retained. In this expository treatment of the subject we shall restrict our attention to steady-state operation, for which the exergy balance (as in Appendix B) is given by
D ^ - D W , (2.1)
b r
where Sh[ is the rate of exergy transport into a region r as measured at a station b on the boundary. T0Src is the exergy dissipation in region r.
For sea-water conversion plants, T0 represents the mean temperature of the local ocean surface waters—these waters being taken as the datum state. Src is the rate of entropy creation in region r.
In using (2.1) we recognize that, as shown in Appendix B, the symbol Sh[ represents the several ways exergy may be transported across a boundary according to the equation
= + + + + <$fC + (2.2)
where the terms on the right represent fluxes of exergy associated with heat flux, work flux, fluid mechanics, fluid thermal convection, fluid chemical convection, and diffusion.
The division of exergy flux into these various categories is dictated by principles of cost accounting. Although all forms of exergy are equivalent in a thermodynamic sense, they are not equivalent to the economic considerations of a potential user.
III. Combining Exergy and Cost Balances
Costs are introduced by use of the symbol C*; the asterisk indicates that the quantity depends on local conditions and varies from time to time and from country to country. Subscripts are used to denote particular elements of cost. A dot over a symbol (C*) means a rate of expenditure.
The use of a lower-case letter (c*) means a cost per unit of material, fuel, energy, water, or land. Final results will be presented in terms of cost ratios, so that it will not be necessary to specify whether the cost is
measured in dollars, pesos, lire, rubles, or guineas. A few examples are given in which the monetary unit is the U.S. dollar, but the equations and graphs have been prepared wherever practical for international use.
For a given zone, r, we write
Similarly, [c%fb] is a row vector of unit costs of material and (Mb) is a column vector of material fluxes. Therefore, the first term gives the values of the various forms of exergy crossing the boundaries of zone r, summed over all boundary stations b. The term Cr* represents the amortized cost of capital equipment (including interest, insurance, etc.) in zone r. The last term represents the cost of any material streams (apart from the exergy they represent) as these streams cross the bound
aries of the zone.
W e take the view that each zone must operate at such a rate as to
"break even," i.e., the sum of net income and operating expenses must cancel, which is why the terms add to zero in ( 2 . 3 ) .
Equation ( 2 . 3 ) may be written for the entire plant, or zone by zone within the plant. In each case the unit costs, i.e., (£*&), must reflect the state of affairs at that boundary. The unit costs are given the same value when viewed from either side of a boundary. This restriction bars
"profit taking" at a boundary and guarantees that when the equations for the zones are added together, all internal transactions will cancel out and the sum of the equations will represent the same equation as would be written for the plant as a whole.
The division of the plant into zones is not an entirely arbitrary operation. The various components of a sea-water-conversion plant are each included in the design for a purpose. Each zone has inputs and outputs of exergy and material. One stream, either exergy or a material, (2.3)
b b
In this equation, [cf^] is a row vector of unit costs of exergy,
lCt,b\ lCq,bcw,bcfM,bcfT,bCfc,bcd,bb
and {S’hi) is a column vector of exergy fluxes,
(2.4)
(2.5)
Thermoeconomic Considerations of Sea Water Demoralization 27 represents the principal output of that zone, i.e., the technical purpose
of the zone. The capital equipment in the zone is therefore amortized against the flux of that principal product. The cost of all other streams entering or leaving a zone is determined by the other zones and the cost of the principal product is adjusted to satisfy the zone's economic balance. In this way, all amortizations and operating costs are allocated to the functions they serve.
There will be one zone in the plant which serves as the separative zone.
This zone will have as its primary output a flux of fresh water, Sf. This
Liquid
.1 _2___ 3
Three-stream heat exchanger
3
40
4 1Sea Brine Fresh
water water
9 i J-
F I G . 2 . 1 . Vapor-compression still with zones and boundaries for purposes of exergy and economic accounting.
zone "purchases" from other zones supplies of sea water (properly conditioned) and exergy (appropriately transformed), and it also sells exergy (at cost) to whichever zones can use the exergy. It sells the water at a cost which covers the cost of the exergy dissipation plus irretrievably transformed exergy plus operating and amortization costs.
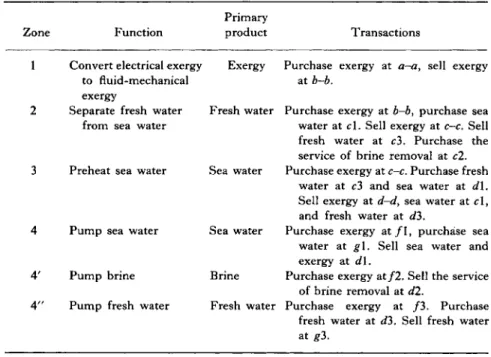
Figure 2 . 1 shows a vapor compression still divided into zones, with boundaries indicated for purposes of exergy and economic accounting.
The purposes of the zones, their principal products, and their trans
actions are given in Table 2 . 1 .
T A B L E 2 . 1
PURPOSES, PRODUCTS, AND TRANSACTIONS B Y ZONES FOR A V A P O R - C O M P R E S S I O N S T I L L
Primary
Zone Function product Transactions
1 Convert electrical exergy to fluid-mechanical exergy
Exergy Purchase exergy at a-a, sell exergy at b-b.
2 Separate fresh water from sea water
Fresh water Purchase exergy at b-b, purchase sea water at cl. Sell exergy at c-c. Sell fresh water at c3. Purchase the service of brine removal at c2.
3 Preheat sea water Sea water Purchase exergy at c-c. Purchase fresh water at c3 and sea water at d\.
Sell exergy at d-d, sea water at c l , and fresh water at d3.
4 P u m p sea water Sea water Purchase exergy a t / 1 , purchase sea water at g\. Sell sea water and exergy at dl.
4 ' P u m p brine Brine Purchase exergy at / 2 . Sell the service of brine removal at d2.
4 " P u m p fresh water Fresh water Purchase exergy at / 3 . Purchase fresh water at <f3. Sell fresh water at #3.
For each zone an exergy balance may be written, the difference between rates of entering and exiting exergies being equal to the entropy production in the zone times TQ ;
Zone Exergy balance
1 < C + < - i'n = TAC- (2-6-1)
2 <^ci — $C2 ~ ^C3 — + <^&2 = T0S2C. (2.6-2)
Thermoeconomic Considerations of Sea Water Demineralization 29
3 βί 1 βί βί ι 1 ι
0 dl 0 d2 — 0 dZ ~ 6 C l "T » C2 ~T <*cz = 7' ί^ 0 ° 3 ' c (2.6-3)
4 + ^ 1 ~~ ^dl = T0^4C (2.6-4)
4' 6 f2 \ ¨ d2 ^.92 = & (2.6-4') 4"
Note:
ι βί
6 /3 ι ^ dZ ~
gf ^ ^/M + £fT + £fC
6 93 — 0$œ. (2.6-4") (2.7) For each zone an economic balance may be written. It is helpful to study the plant as a whole and decide, by working outward from each process, whether the outputs of the zones have any economic value in other zones. Those forms of exergy which cannot be used are given zero value at the boundaries at which they first occur. For example, the chemical exergy1 in streams cl and c3 is not used anywhere else in the plant. Setting
cfc,c2 — cfc,d2 = cfc,g2 = 0 guarantees that the charge for this exergy consumption will be made in zone 2 and, therefore, will be reflected in the fresh-water cost from zone 2.
Before writing the economic balances it is useful to examine each boundary and decide upon the symbols for costs. (Lettering the bound
aries sequentially helps guarantee that none will be overlooked.) Table 2.2 gives the information used in setting the costs.
A t the boundaries there are also material costs. These are determined as follows:
c% g Unit cost of sea water at plant inlet. Includes cost of filtration, conduits, and any pretreatment.
c£>d Determined from cost of operation of zone 4.
Cy>tC From cost of operation of zone 3 . c%g Cost of brine at plant outlet.
c%c Cost of brine at boundary c, determined from the cost of operation of zone 4'.
c%c From cost of operation of zone 2.
c%t0 From cost of operation of zone 4".
1 F r o m A p p e n d i x Β it is found that the chemical exergy is merely the difference in free energy between sea water, on the one hand, and fresh water plus brine, on the other.
T A B L E 2.2
C O S T VECTORS FOR A VAPOR-COMPRESSION S T I L L
Boundary Cost vector
O-a [cZa] = [c*aCw.aC*M.aCfT.aC*C,aCd.a]
= [0
eta
0 0 0 0 ]T h e only form of exergy which m a y be used, or transmitted, at this boundary is mechanical.
b-b [c*b] = [c*bctbC*M,bCfT,bC*c.bcd.b\
= [0 0 cfMtbc*Mb0 0 ]
Only the fluid-mechanical and thermal exergy can be used by the evaporator-condenser. T h e condensation process uses the two forms of exergy indistinguishably.
C~c lct,c\ \.cq,ccw,cCfM.cCfT.ccfC,ccd.c\
= [0 0 C*M,bCfMtb0 0 ]
T h e costs in zone 2 are allocated to the fresh water. Zone 2 therefore sells exergy at the same price as it is purchased.
d-d [c*d] — [c£dct.dC*M.dC*T.dC*c.dCd.d]
= [0 0 cJM,b 0 0 0 ]
T h e costs in zone 3 are allocated to the hot sea water which leaves that zone. Therefore zone 3 sells fluid-mechanical exergy at the same price as it purchases exergy from zone 2. T h e cost of fluid-thermal exergy is zero, since there are no customers for this form beyond d-d.
-/ lct.f] = \.CQjCwjCfMjCfTjCfCjCd,f\
= [0 cl,a0 0 0 0 ]
T h e only form of exergy which m a y be utilized at this boundary is mechanical. It m u s t be bought at the same unit price as at boundary a-a.
g~g [C*a\ = [c^ctgCfM.gCfT.aC*C.gCd.g}
= [0 0 0 0 0 0 ] T h e r e are no exergy customers at g-g.
Thermoeconomic Considerations of Sea Water Demineralization 31
— C fM,b6 dl /fM + + 4 . ^ = 0 , (2.9-4)
Cw,a(^0^ ~ ^dl ^d2 + ^92 + + Cm,b^d2 + + C&.C& ~ 0>
(2.9-4')
cw,a\I0^4" 0 dZ edZ \ gZ gZ )
+ c*MJ’^ + Cp + (clc - c%,gy? = 0. (2.9-4") With these cost vectors determined, it is straightforward to write the economic balances, Eq (2.3), for the individual zones.
Zone I:
cw,aPa -TcfM,b\0b\ I © 61 — © 62 — < * 6 2 , / ~ Γυΐ ~ U- (Z.O-lJ Zone 2:
.* //fM./fT AfM jpfT ι rafM ι SfT />fM /fT /fM / cfM,b\6 &2 ~T © 62 ~~ 6 61 — © 61 "Γ © C l "Γ © C l ~"" © C2 ~ 6 C2 ~~ 6 Cz ~ ® C 3 J
+ < V + 4, c ^ - 4. c ^ - <£.c&= 0. (2.8-2) Zone 3:
. * / > / M ι ^ / Γ /fM /fT /fM />/τλ
~CfM,b\6 Cl "Γ © C l — 0 C2 — © C2 ~ " 6 C3 ~ 6 Cz)
+ cfM,b{^f<n — <* 2 — <^dz) + ^ 3 * + (^.d ~ C^,c)^ = 0· (2.8-3) Zow£ 4:
4 . 'Λ - ' / V ^ i * + < V + - 4 , , ) ^ = 0. (2.8-4) Zone 4':
cSJn + c?mJ% + C$ + clcA = 0. (2.8-4') Zone 4":
ctjfz + ^ / * M , 6 ^ 3M + Q * + ( 4. c - 4 , 9 ł = 0. (2.8-4") T h e exergy-balance equations, (2.6), are substituted into the cost- accounting equations:
* / φ N C /fM /fT ι /fM . /0/Γ\
^ w . a^ O ^ l — ^ 6 1 — © 6 ΐ Π - © 6 2 & b2)
+ cfHj&ig + < r - <r£f - ill) + £x* = o, (2.9-1)
* / rp Qf C >^/C ι *£/C ι /fC\
CfM,b\10*^2 © C l l © C2 I © C3>)
+ ^ 2 * + 4. c ^ - 4. c ^ - 4, c ^ = 0, (2.9-2) 4 o( r , Ac - iZ + *Z + + < V + ( ,Æ - 4,c)^ = 0, (2.9-3)
* (rp Λ C /fT />fM ι /fT ι /fM\
* > . C — Cy.d "I ^/M ,b — h ^/M.b
•Ν * φ ό C / δ/ Γ ι /δ/Γ / (5/Γ
U3 ι 1 0 ° 3 , 0 d2 ~ ~ 0 dZ 0 dl
>
(2.10-3)
A * J - ο c
> / Γ ι > / Μ *5/Γ 1 , * d l "Γ0 gd l ~ 0g l ~~ 0g l . * e dl
(2.10-4)
•Ν* /Τ» QfC
/ , > / Μ ^ / Γ *5/Μ /δ/Μ /.* g2 ' y ; ~ d2 ~ d2 _ /·* d2
(2.10-4')
/ΤΙ QfC /.* /·* — 4" _L r * ^ ° 4"
/of , ¸, / /
I * 0gZ Τ 0 qZ — 0 d3 0 d3 ι _ * 0 d3
Τ <V« ^ X-Cfu.b—
(2.10-4") Note that in making the substitution of the exergy-balance equation, care was taken not to eliminate the output product of each zone. That is, since zone 1 has a particular form of exergy as an output product, care was taken to see that these terms remained in the equations.
Each equation is now divided by the rate of production of the principal product, and the unit cost of that product is put on one side of the equality sign.
/*» *
c* = W
^ b2 ' ϋ 52 ω bl ω bl
+ c* a - . . Τ° ά\ r - + C*a, (2.10-1)
* ι _ * 10°2 ι „ * σ
~ ~ fM-b & ¥
, * 0 C2 Τ 0 C3 ~ ® C I ^ η ι λ - ) \
Thermoeconomic Considerations of Sea Water Demineralization 33 Each equation has now been put into the following form:
(1) The first term represents the unit cost of the principal product.
(2) The first term after the equality sign represents the amortization of the capital investment in the zone, relegated to the principal product.
(3) The second term on the right represents the cost of the irreversi- bility inside the production zone.
(4) The remaining terms represent the cost of the products "bought"
from adjacent regions and "used up" in making the principal product.
The last category of terms may require explanation. Specifically:
(1) In zone 1, the last term represents the cost of the power input.
(2) In zone 2, the third term represents the sea water "used up" and made into brine and fresh water. T h e fourth terms represents the free- energy change in making the fresh water from sea water. The last term represents the cost of removing"the brine from the plant. Note that c%c
will be negative; i.e., zone 2 must pay for this service.
(3) In zone 3, the last term represents the exergy (in thermal form) that is sent on to the pumps (and therefore cannot be used).
(4) The last two terms for zone 4 represent a "credit" for the exergy given to zone 3 by the pump (since cfMJb will be larger than c*a , as indicated in the equation for cfMh).
(5) The last term for zones 4' and 4" represents the cost of the exergy received from zone 3. The next-to-last terms represents the cost of the exergy added to the output streams by the pumps.
The equations are now ready to be optimized by identifying in each zone the entropy production rate and balancing it against capital invest- ment. For this purpose it is helpful to divide each zonal entropy produc- tion into three terms (in processes other than sea-water separation, more than three terms may be necessary). Let
Sf =S^ + S^ + S li y (2.11)
where the terms on the right represent the entropy production due to uncontrolled heat transfer, uncontrolled friction, and uncontrolled mass transfer in the ith zone. Each of these three mechanisms should be investigated separately.
The detailed optimization analysis will be applied here only for zones 2 and 3. For zones 1, 4, 4', and 4" it would require too detailed a discussion of the design of pumps and compressors to suit the purposes of this monograph. To analyze these zones carefully would require that these devices be considered to be divided into intake port, working zone,
Ob2 — ( fibl jpfM
( Gdl /
-
olt
— ( fid2
<
2
!
— < rofM
&d3
6/ 3
V*’ ^ " , " > 2 . 1 4 )
7^ g 3 , ) ( 2 1 5
For reasonably good values of the efficiencies (and there is no point in sea-water conversion in discussing inefficient devices, the energy cost being large enough as it is) the small terms representing the thermal exergy delivered by the pumps may be neglected. With these simplifica
tions the set of combined exergy-balance equations ( 2 . 6 ) and cost-accounting equations ( 2 . 1 0 ) may be written (noting that
* ' £ = 4 f = ' ί " = o)
CM . » = + < o | — · (2.16-1)
* rp AC rp AC rp AC
,C ~~ ~W ' CfM>b J T + F M'B J T
(J? ˇ ι ˇ
, * & , * &C2 ~T © C 3 ~ < C1 *
+ c<?,c^r + cfMtb — C*'c#'
/*» * rp AC rp AC
£y\c — c^,d -r cfM,b — h ^/M.b — —
(2.16-2) outlet diffuser and bearings, and the capital cost of each subunit balanced against the various fluid frictions in each. For these zones we shall rather take the position that the designer is selecting these items from a supplier and will infer the rate of entropy production by accepting the manufacturer's (or his experimentally determined) value of the adiabatic efficiency of these devices.
W e may dispense with the detailed analysis of the design of zones 1 , 4 , 4 ' , and 4", by letting
^ ø - ^ - ’ ( 2 J 2)
_ 0dl 6 ol
^ 4 = ”˘ > (2·13)
Thermoeconomic Considerations of Sea Water Demineralization 35
~ %¸ + « Æ ~ c?M,bv*) ( - ^ - ) , (2.16-4)
`Ø* dv>
4.c =-^r + (4. - Ο ^ . (2.16-4')
- c%.c = ^ " + « „ - Γ,ν»^-) • (2.16-4") For the optimization of zones 1, 4, 4', and 4", the designer selects that
combination of pump ratings, costs, and efficiencies which will minimize the unit costs. Note that in zone 4 the term ^^ ^) represents the pressure rise in the pump. T h e first term depends on the pump capacity.
In zone 2, the principal item of cost is the heat-exchanger surface.
The cost of maintaining this surface includes not only the amortization, C2*> of zone 2, but also the exergy cost cfMbT0S^2 required to circulate the water and brine over this surface. Distributing these costs over this surface is equivalent to defining a unit cost of surface c\\ by the equation
CA2 =
υ2 Τ CfM.b1 0 ° w 2 ^2 17)
The area chosen for zone 2 should be that which makes the unit cost of water a minimum, as dictated by (2.22).
If Q is the heat flux through the heat-transfer area A, and if TH
and Tc are the hot- and cold-side temperatures, the entropy generation and the unit thermal conductance are given by
^ H ? ( £ - - £ ) - 0 ( - ^ ^ ) .
(2.18)6 = UA(T„ - Tc). (2.19)
Therefore,
With these changes the unit cost of water is given by [from (2.16-2)]
In Appendix C the method of treating systems in which c%2 is not constant with respect to the area is given. If c% is constant, the value of A for which the sum of the first two terms is a minimum is given by
^
t =vSw/-
(2·
22)Substituting this value into ( 2 . 2 1 ) gives
_ 2 / cfM.b^A2 Tq Q2
" 'c V U THTC^~2
ι „ * i0 ° C 2 ι * ^ ι „ * GC2 ~T <*C3 ~ Gc i „* ^°
(2.23) where, if ˜ is the heat of vaporization,
Q = ˜˙. (2.24)
Equation ( 2 . 2 3 ) represents the cost of water from zone 2 optimized with respect to the heat-exchanger area. The designer should examine the heat exchanger for pressure drop or frictional characteristics and compute the entropy generation, S^2 > due to friction, the cost of which is included in c% . In general, attempts to reduce S^2 will tend to increase r j2 o r decrease the over-all conductance t/, the optimum brine and water velocities thereby occurring at the minimum value of c%/U. The fric
tional characteristics of heat exchangers differ so much that we shall not attempt a general treatment of the problem here. Instead we shall presume that the designer has chosen the proper tube spacing, flow- passage cross section, and turbulence promotion devices to minimize the sum of the first two terms. A n example of how to do this is given in Appendix 2 of the paper by Tribus and Evans ( 1 9 6 3 ) .
The second term in ( 2 . 2 3 ) represents the entropy creation due to mixing of the outgoing brine with incoming sea water. This can be prevented by proper baffling. The addition of baffles increases c *2; hence the proper amount of baffling will be that which minimizes the sum of the first and second terms.
If the ratio St/S^ is allowed to become indefinitely large, the fourth term of ( 2 . 2 3 ) approaches a limit which we shall denote by
cfM,hnGQ. (2.25)
Thermoeconomic Considerations of Sea Water Demineralization 37 Let us define
AG == ^ C 2 + ^ C 3 - ^ C 1 2 2) 6 (
J ( ? is the actual free-energy change and A00 would be the free-energy change if a mole of fresh water were removed from an infinite supply of sea water. Dividing all terms by c*A AG0we have (upon noting that
& = & +
c* AG V c* c* UT Τ \AG
w,a 0 0 w,a 0 ιυ,α 0
(2.27) In Fig. 2.2 we show how the second term varies with JT/Sf. Figure 2.2 was prepared using the properties of sea water given by Tribus and
0 I 1 1 1 1 L _
0.2 0.4 0.6 0.8 1.0
f/9
F I G . 2.2. M i n i m u m dimensionless exergy requirement for sea water conversion.
Evans ( 1 9 6 3 ) . T h e lower curve represents the free-energy change in producing fresh water and brine from sea water. The upper curve includes the additional exergy required to compensate for internal mixing of the vapors of the exiting brine and the fresh water. These curves are properly used for either irreversible or reversible processes, since they represent the contributions to exergy usage due to chemical effects and mixing effects, where present.
T h e minimum sum of the last three terms in ( 2 . 2 7 ) occurs when
&\S? takes on a value such that
d[(TpS£2/& AG0) + (ΔΟΙΔ00)] = cp,c - 4. c ( 2 2 8)
It is straightforward to make a graph of the ordinate of Fig. 2 . 2 and plot it against 6^/S^y and read the slope of the curve and plot that slope against A given value of ( φ € — c%c)lcfMfi AG0 through ( 2 . 2 8 ) determines
(^7^)
opt
· It is straightforward, therefore, to prepare a graph of the minimum value of the last two terms as a function of ( 4, c - c%,c)lc*M>hAO0 (Fig. 2.3). By multiplying (27) by c*A | CfMJb , we obtain the final cost equation for zone 2 :&.c = 2 / c% T0 ,AHvx2 c* AG
V
Γ/c* ^AG /L cf^AG^ &AG0 j(50JOpt c*MtbAGQm
( 2 . 2 9 )
It should be noted that the minimum dimensionless cost ratio given in the above equation has been or could be optimized with respect t o :
( 1 ) Area for heat transfer.
( 2 ) Circulation ratio, i.e.,
( 3 ) Frictional losses in the exchanger.
T w o operating parameters, however, have not been determined. These are:
( 1 ) The temperature level at which the operation occurs.
( 2 ) T h e unit cost of the sea water.
It can be seen that the lower THTc becomes, the larger will be the first term in the zone-2 cost equation, ( 2 . 2 9 ) . Referring to Fig. 2 . 1 , it
Thermoeconomic Considerations of Sea Water Demoralization 39
0 1 1 1 1 1 1 1 1 1 I I ι loo
Ο.ΟΟΙ 0.002 0.004 0.01 0.02 0.04 0.1 0.2 0.4 1.0 2.0 4.0 10.0
F I G . 2.3. O p t i m u m dimensionless exergy cost for conversion and handling of sea water.
can be seen that if Tc were sea water temperature, the heat exchanger would be unnecessary. A t low temperature in zone 2, however, it is necessary to increase c% [defined in (17)], since the walls of the zone must be increased in thickness to protect the vacuum. As Tc is increased, zone 3 must preheat the sea water. Therefore, zone 3 must be optimized for any given choice of Tc . This interzonal optimization will be taken up after zone 3 has been optimized.
Optimization of zone 3 follows the same pattern as for zone 2—subject, of course, to some appropriate suboptimization criterion such as that discussed on the last page of Appendix C. The product of 7^ and entropy generation due to heat transfer inside the exchanger is equated (with but small error) to the changes in thermal exergy of the three streams, i.e.,
TQ$%,Z ^ \&c\ ^di + ^d2~ ^cl+ $dl $1cz\ (2.30) With this approximation, the economic balance, (2.16-3), becomes
* * ^ 3 ^ 3 ι „ * ^ C 2 + <^C3 — $c\ n
CS?,C C#>,d = h CfM,b •
The term on the right is given from Appendix Β by
* s +i &; - w _ ^ _T o_ T o ln
- < V (Tci -T0-T0 ln ł-). (2.32) Treating, as a good approximation, Cp = Cp^ = and combining
terms we have
~T 0 CZ ~ 0 Cl _ I rp rp rp 1 i C2 \
— | ^ iC 2 — i C 1 — i0 in " j ^ - j
+ Cv<, ^ ( rC 3 - TC2 - T0 ln - g - ) . (2.33)
The difference, Tcs TC2, is determined by zone 2. It is small compared to TC1 TC2 and will be neglected in this simple analysis.
Since TC2 TC1 will in general be small compared to TC1 , we let ln TC2 AT
Tci TCl where ˜ == TC2 TC1. Therefore,
TC2 - TC1 - T0 ln I?L = (Ta - T0). (2.34)
1 Cl ici
In a counterflow exchanger with approximately equal thermal capacity rates in the opposing streams, ˜ remains approximately constant throughout the exchanger. Defining a conductance U through the equation,
Q = UA ΔΤ, (2.35) and the heat flux to the sea water by
Q = #CPS,(TC1 - T0), (2.36) (2.31) becomes
Thermoeconomic Considerations of Sea Water Demineralization 41 T h e optimum value of A is that which minimizes this sum, i.e.,
a I cM,b£ysr(Tcl — T0)2£f-
^ o pt = \J . * T TJ . ( 2 . 3 8)
Substituting this value of A in the cost equation gives
4’
c<rtc cse,d = z \l —jj- fr^ · V--**) As in zone 2, the choice of conductance, U3 , in zone 3, should be made in such a way as to minimize c*zj U3 . This means balancing the spacing, tube diameters, roughness, etc., to make the economic return from increased heat-transfer balance the cost of the pressure drop, each benefit weighted according to the economic value.
The analysis of each zone, as undertaken thus far, has demonstrated how to optimize the "intensity" of the irreversible processes within each zone in such a w a y as to balance the generation of entropy against the capital cost. W e summarize, in dimensionless form, the results of the zonal optimization thus far:
φ = ± [ ΐ + - ^ 1 , (2.40-1)
CF .C __ - p / έ%Ρ*ΜΛ / ΔΗν \2 T0
- IP> \J c* c* U\AG I w,a w,a *-*
ctaAOQ 2 \ c*9mc*9.U \ A0n > TC1TC2
(2.40-2)
csr,c ~ csr,d
= ζ [ ^ _ + ( l - C1MA\ 4¢º (2 4 0.4 )
* * f ^ ir + ( v*- - -^hr\ (2·40-4')
* * C4 " cfM,b * (2.40-4")
Note that in ( 2 . 4 0 - 2 ) and ( 2 . 4 0 - 3 ) we have inserted the factors F2 and F3 to account for the effects of nonlinearities. These factors are described
in Appendix C.
The final stage of the optimization process consists of balancing the operating temperatures and pressures in the various zones against one another. Starting with the most expensive zones, namely, zones 2 and 3 , the temperature level, as indicated by TC1, is optimized in such a way as to make the sum of the operating costs a minimum. It will be noted that an increase in TC1 decreases the unit costs in zone 2 and increases them in zone 3 . This optimum is easily found numerically or graphically.
Since the selection of the optimum value of J ^ / ^ is not temperature- dependent, ( 2 . 4 0 - 2 ) is multiplied by and added to ( 2 . 4 0 - 3 ) . The sum, as a function of TC1, is then plotted and the minimum noted by inspection.
A. A REVIEW OF THE OPTIMIZATION PROCEDURE
The previous example was given to illustrate the method of optimizing using exergy and economic balances. It is quite general and may be applied to any system. The steps in the analysis are straightforward and may be summarized as follows:
(1) Divide the entire plant into zones. Each zone is selected because it fulfills a function. Identify for each zone the principal product produced in that zone. Identify the boundaries between zones.
( 2 ) Identify the streams of exergy and matter crossing each boundary.
Devise a symbolic system for representing the cost vectors at each boundary, using the principle:
(a) The cost vector must be the same viewed from either side of the boundary, i.e., no profit taking in internal transactions.
(b) The cost of a unit of matter or form of exergy is constant for all streams through a zone except for the principal product of that zone. That is, the cost of capital, material, and irreversibility in a zone is allocated to the principal product of that zone, and to no other stream. Forms of matter or exergy created in a zone and having no resale value downstream are given zero value at the zone exit.
( 3 ) Write an exergy balance for each zone. The exergy balance contains terms representing exergy flows. The sign of the exergy flow
Thermoeconomic Considerations of Sea Water Demoralization 43 should be consistent with arrows drawn on the flow diagram, so that
a flux from one zone is negative and to its neighbor is positive. This guarantees that if the exergy balances are added together for all zones, the same result is obtained as if the entire plant were analyzed at once.
The general exergy-balance equation is given in (2.1).
(4) Write an economic balance for each zone.
(5) Substitute the exergy balance into each economic balance, zone by zone. Be careful not to eliminate the output exergy if the output of a zone is a form of exergy.
(6) Rearrange each equation so that the unit cost of the zonal product appears on the left of the equality sign and the terms representing capital cost, entropy generation, exergy forms used up, and material converted to useless form appear in that order.
(7) Identify the entropy generation terms in each equation, either by representing them as functions of efficiencies or conductances for rate processes.
(8) Divide the capital-investment term into terms which are allocatable to each form of entropy creation. Optimize the capital investment vs.
entropy generation, each mechanism taken one at a time.
(9) W h e n the optimization in each zone has been completed, balance the costs of the separate zones against one another, starting with the most expensive pair.
IV. Conclusions
The principal difficulty in approaching a new process is in the estima- tion of the unit costs for amortization and maintenance of equipment (the terms Or*9 r = 1, 2, 3, 4, 4', 4", in the example). The lack of such data, however, is not a barrier to the analysis, for even if the data are not precisely known, the analysis reveals how each unit cost ultimately affects the cost of the product water. The explicit elucidation of the connection between unit costs and fresh-water costs enables the designer to see which cost figures are worth knowing accurately. Furthermore, the designer may be guided by the thermoeconomic analysis in making a proper "tradeoff" of costs.
The methods described in this chapter have been applied to a number of processes and the results have been given in the paper by Tribus and Evans (1963). Additional examples will be found in the remainder of this book.
Much work remains yet to be done in simplifying further the tech
niques of analysis presented herein. Nevertheless, as of this writing, the methods shown offer a rapid and direct way to optimize the economic performance of sea-water-conversion systems.
Appendix A. Basic Relationships among Entropy. Exergy.
Energy, and Availability
In this Appendix, the basic relationships among the four fundamental quantities, entropy, exergy, energy, and availability, are developed. T h e central theme of this appendix, however, revolves around that quantity called exergy. The word exergy is a term used frequently in the current literature from Germany (Bosnjakovic, 1 9 6 1 ; Grassmann, 1 9 5 9 ; and Rant, 1 9 5 6 ) to denote the essence of the departure from equilibrium which may be said to drive all physical processes.
The notion of exergy is of course not new—this word having been used in the German literature since 1 9 5 6 (Rant, 1 9 5 6 ) . Furthermore, the free-energy functions of Gibbs ( 1 8 7 6 ) and Helmholtz (Denbigh, 1 9 5 4 ) and the availability functions of Keenan ( 1 9 4 1 , 1 9 5 1 , and Hatsopoulos and Keenan, 1 9 6 0 ) are all variations of the exergy concept.
All these functions will be shown in this paper to represent special cases of a simple exergy expression which results from considerations of entropy change. The resulting exergy function will be seen to provide a single, simple principle which automatically includes all considerations of free energy, availability, available work, and available energy.
The ideas underlying the exergy concept have been used rather extensively in the development of an engineering optimization discipline called thermoeconomics (Tribus and Evans, 1 9 6 0 , 1 9 6 2 , 1 9 6 3 ; Evans, 1 9 6 2 ; Tribus et al.y 1 9 6 0 ; Evans and Tribus, 1 9 6 5 ) . The use of exergy in the optimization of engineering systems is also being developed in Germany (Bosnjakovic, 1 9 6 1 ; Grassmann, 1 9 5 9 ; and Rant, 1 9 5 6 ) , while many workers have used the concepts of availability (Keenan, 1 9 4 1 , 1 9 5 1 , and Meyer et al., 1 9 5 9 ) , entropy increase (Fadden, 1 9 6 2 ) , and available energy (Darrieus, 1 9 3 0 , and Bruges, 1 9 5 4 ) for this purpose. It should also be mentioned that work along these lines has been developed independently by Gaggioli ( 1 9 6 1 , 1 9 6 2 ) and Obert
( 1 9 6 0 ) .
The formulation of exergy given in this paper is more general than any of the previous formulations of exergy or availability given in the literature. This formulation resulted from the information-theory approach to thermodynamics (Jaynes, 1 9 5 7 , 1 9 6 3 , 1 9 6 5 ; Brillouin,