Ph.D. THESIS
Maha Mohamed Soliman Mohamed Refaey
VESZPRÉM
2013
b
c
THESIS OF THE DOCTORAL (Ph.D.) DISSERTATION
ASSESSMENT OF WASTEWATER TOXICITY CHANGES DUE TO BIODEGRADATION PROCESSES
by:
Maha Mohamed Soliman Mohamed Refaey MSc. in Environmental Engineering
Doctoral School of Chemical Engineering and Material Sciences University of Pannonia
Supervisor: Dr.
Nora Kováts
Institute of Environmental Engineering and Radiochemistry Faculty of Engineering
University of Pannonia
Veszprém
2013
d ASSESSMENT OF WASTEWATER TOXICITY CHANGES DUE TO
BIODEGRADATION PROCESSES Thesis for obtaining a PhD degree in the Doctoral School in Chemical Engineering
of the University of Pannonia.
Written by:
Maha Mohamed Soliman Mohamed Refaey
Written in the Chemical Engineering and Material Sciences doctoral school of the University of Pannonia
Supervisor: Dr. Nora Kováts
I propose for acceptance (yes / no)
(signature) The candidate has achieved …... % at the comprehensive exam,
I propose the thesis for acceptance as the reviewer:
Name of reviewer: …... …... yes / no
……….
(signature) Name of reviewer: …... …... yes / no
……….
(signature) *** Name of reviewer: …... …... yes / no
……….
(signature) The candidate has achieved …... % at the public discussion.
Veszprém, ……….
Chairman of the Committee Labelling of the PhD diploma …...
………
President of the UCDH
e
Acknowledgment
First and foremost, I must thank Allah the most Gracious and the most Merciful to whom my loyalty will remain forever for his bless and granting me this humble study.
I would like to express my sincere appreciation to Dr. Nora Kováts my supervisor for her kindness and support during the work and for guiding me into the exciting world of ecotoxicology.
Words stand short when coming to express my deepest thanks and gratitude to Prof. Dr.
Rédey Ákos, Head of Env. Eng. Department, University of Pannonia, for his kind encouragement, support and guidance.
My deepest thanks and appreciation to Prof. Dr. Hesham Shafik for his help, guidance and advice through writing my thesis.
I would like to express my thanks and gratitude to all my professors, colleagues and friends for their cooperation and encouragement.
Finally, all my gratitude, appreciation, and my sincere love for my family especially my parents, for their generous unlimited love, support, encouragement, guidance and pushing me toward success.
i
Contents
ةصلاخلا ةماعلا
...
...
...
1
General Abstract………2
Kivonat ………...…………..3
General Introduction ………..…..………4
1. Objectives ……….………...…...….……4
2. Introduction ………..………...…4
2.1. Biodegradability testing ..………...….7
2.1.1. Tests on ready biodegradability………8
i) DOC Die-Away Test (OECD 301A, EC C.4.A, ISO 7827)……….8
ii) CO2 Evolution Test (OECD 301B, EC C.4.C, ISO 9439)……….….9
iii) Modified MITI Test (I) (OECD 301C, EC C.4.F)………10
iv) Closed Bottle Test (OECD 301D, EC C.4.E, ISO 10707)………..…..11
v) Modified OECD Screening Test (OECD 301E, EC C.4.B)…………...……12
vi) Manometric Respirometry (OECD 301F, EC C.4.D ISO 9408)……...……13
vii) Biochemical Oxygen Demand (EC C.5)………...……14
2.1.2. Tests on Inherent Biodegradability:………...……..14
i) Modified Semi-Continuous Activated Sludge (SCAS) Test (OECD 302A, ISO 9887)………...……….14
ii) Modified Zahn-Wellens/EMPA Test (OECD 302B, ISO 9888) …...………15
2.1.3. Simulations Tests: Activated Sludge Simulation Tests: Coupled Units Test (OECD 303A, EC Activated Sludge Simulation Tests, ISO 11733)……….17
2.2. Ecotoxicity testing………...18
2.2.1. Algal inhibition test………18
2.2.2. Daphnia-based toxicity testing………19
2.2.3. Fish-based toxicity tests……….…20
a) Tests based on mortality as an endpoint………20
b) Tests based on sub-lethal endpoints……….20
2.2.4. Vibrio fischeri bioluminescence inhibition assay………..21
2.3. Wastewaters of high organic (biodegradable) matter content………..28
ii
Materials and Methods………..………...31
1. Sample selection and sampling………..…..31
2. Sample preparation, adding inoculum and Experimental conditions:…………....33
3. Ecotoxicity testing………...36
3.1 ToxAlert test………...36
3.2. Flash assay (kinetic determination)………..….37
4. Chemical analysis………....38
4.1. Chemical Oxygen Demand (COD) measurement……….38
4.2. Total Organic Carbon (TOC) measurement………..39
Results and Discussion………40
1. Assessment of toxic effects of municipal wastewater for recipient freshwater systems using different inocula………..40
2. Assessment of toxic effects of municipal wastewater for recipient freshwater systems under different temperature regimes……….42
3. Biodegradation assessment of liquid manure disposal………..49
4. Biodegradation studies on paper mill effluent………..54
Bahr El-Baqar Drain: An Egyptian case study………..…….61
Abstract………...……....61
Introduction ……….…...61
Bahr El-Baqar ……….…...62
Materials and Methods ……….……..65
1. pH measurement ……….…...…65
2. Total Soluble Salts……….………65
3. Biological Oxygen Demands (BOD5)……….……...65
4. Chemical Oxygen Demand (COD) measurement………..………65
5. Total Organic Carbon (TOC) measurement ………..………65
6. Total nitrogen (TN) ……….……..65
7. Total soluble phosphate ……….…...…65
8. Ecotoxicological test ……….…65
Results and Discussion ………..66
References ……….…….…………71
1
يويحلا ريسكتلا ةيلمع للاخ فرصلا هايم ةيمس يف ريغتلا مييقت
ةصلاخلا ةماعلا
نإ تارابتخا ةيمسلا
يه ةادأ ةديفم مييقتل رطاخملا يتلا
طيحت ةئيبلاب نم ءارج فيرصت هايم
فرصلا لكشب
ماع ,
يهو بعلت ارود اماه يف معد ةيلمع عنص رارقلا . دقل مت مادختسا رابتخا طيبثت ةءاضلإا ةيئايحلاا ايريتكبلل
ليلحت عم ةينوزولحلا جايتحلاا
يئايميكلا نيجيسكولأل
و نوبركلا يوضعلا لكلا
مييقتل ي تارييغت ةيمسلا
هايمل فرصلا
,)حاقل نودب وأ عم( ةفلتخم فورظ يف )ةيعانصلا و ةيعارزلا و ةيرشبلا(
اهتيمهأو ةبسنلاب
تايلمعل ةجلاعم هايم
فرصلا
، فرعتلاو ؤبنتلاو
راثلآاب ةيئيبلا ةبترتملا اهيلع . ترهظأو جئاتنلا
نأ تارابتخا
،ةيمسلا ىلع سكع ليلاحتلا
ةيئايميكلا ,
يطعت ارشؤم ىلع ةيفيك ريغت ةيمس فرصلا هايم ةجيتن
للحتلا
،
،اذكهو نكمي ؤبنتلا كولسب هايم فرصلا
يف مظنلا ةيجولوكيلاا
،ةيئاملا يلاتلابو نكمي ؤبنتلا اهتاريثأتب ىلع
ةئيبلا
،اضيأ اهتيمهلأ يف ةيامح تاطحم ةجلاعملا
ةيجولويبلا نم
دفاورلا اسلا .ةم
:ةيسيئرلا تاملكلا تارابتخا
ةيمسلا ,ةينوزلحلا ايريتكبلا ,فرصلا هايم , جايتحلاا
يئايميكلا نيجيسكولأل
, نوبركلا
يوضعلا يلكلا
2
Assessment of wastewater toxicity changes due to biodegradation processes
General Abstract
The ecotoxicological tests identifying the ecological hazard of wastewater discharges are useful tools for the identification of environmental impacts. Direct toxicity assessment plays an important role in supporting decision-making. Vibrio fischeri bioluminescence inhibition test was used combined with COD and TOC analysis to evaluate the whole effluent (municipal, agricultural and industrial) toxicity changes at different conditions (with/without inocula and/or different temperature regimes), its relevance to the wastewater treatment operations, and to identify and predict its environmental impacts.
The results showed that toxicity tests, unlike the chemical end-points analysis, give an indication on how effluent toxicity will change due to degradation, therefore, predict the behavior of wastewater in the aquatic ecosystem and in turn its environmental impacts.
Also, their importance in protecting the biological treatment plants from toxic influents.
Key words: Ecotoxicological tests, Vibrio fischeri, whole effluent, wastewater treatment, COD, TOC
3
Szennyvízek ökotoxikológiai változása a biológiai tisztítási folyamat során
Kivonat
Az ökotoxikológiai vizsgálatok, melyek meghatározzák a szennyvíz kibocsátások ökológiai veszélyeit, hasznos eszközök a környezeti hatások azonosítására. A közvetlen toxicitás értékelés fontos szerepet játszik a döntéshozatal támogatásában. Vibrio fischeri biolumineszcencia gátlás tesztet alkalmaztunk KOI és TOC analízissel kombinálva a szennyvizek (kommunális, mezőgazdasági és ipari) toxicitásának vizsgálatára különböző körülmények között (oltóanyaggal vagy a nélkül, ill. különböző hőmérsékletű rendszerekben), a szennyvíztisztítók működésére vonatkozóan, hogy meghatározzuk, és előre jelezzük a környezeti hatásokat. Az eredmények azt mutatták, hogy a kémiai végpont elemzésekkel ellentétben a toxikológiai vizsgálatok azt jelzik, hogy a szennyvíz toxicitása hogyan változik a lebomlás következtében, ezentúl előre jelzi a szennyvíz viselkedését a vízi ökoszisztémákban, valamint a környezeti hatásait. Továbbá jelentős szerepe van a biológiai szennyvíztisztító telepek mérgező befolyóktól való védelmében.
Kulcsszavak: ökotoxikológiai vizsgálatok, Vibrio fischeri, szennyvízkezelés, KOI, TOC, teljes kifolyó.
4
General Introduction
1. Objectives
The objectives of this thesis are to study how the whole effluent toxicity changes during degradation of discharged wastewater (treated and untreated), test how the degradation processes, especially biodegradation, affect the toxicity of different types of wastewater (municipal, agricultural and industrial) and to predict its behavior, verify the importance of toxicity test in evaluating the wastewater treatment operation, evaluate the wastewater treatment plant efficiency and its capability in decreasing the hazard of wastewater before discharging it into the environment, emphasize the importance of the acute toxicity parameters incorporation into the environmental regulations for wastewater discharge to protect the receiving ecosystems, study the differences between capacities of different microbial communities to degrade wastewater samples, with special respect to microbial community of the recipient water, test toxic effects of municipal wastewater on recipient freshwater systems under different temperature regimes, and developing an extensive database that may help in create an innovative treatment technology.
2. Introduction
Although water is essential for all life forms, water pollution and the destruction of ecosystems continue to increase. Due to the rapid increase in population growth, urbanization, industrialization, globalization and warfare combined with increased wealth and more luxurious lifestyles, huge amounts of pollutants and contaminants are produced and discarded into the water system (UN-Water, 2006). Water pollution is considered one of the main reasons for our modern fresh water crisis. Billions of gallons of wastewater and sewage are produced every day. Wastewater constitution varies widely from one site to another depending on the surrounding activities, but most wastewaters contain organic, inorganic, biological pollutants, solid wastes and various toxins. With the limited and diminishing sources of fresh water and the negative effect of all these wastes and pollutants on the surrounding environment, wastewater treatment became a must to decrease the discharged load of such organic, inorganic and biological wastes to the
5 environment. Wastewater treatment plants use chemical, physical and biological processes in removing nutrients, solids, and harmful compounds from the wastewater before discharging to the recipient water. However, the discharged treated wastewaters still contain a wide variety of potentially harmful substances despite the extensive treatment processes.
In many countries, both chemical and biological analysis/testing of effluents is required by regulations either before discharging to sewage treatment plants or before discharging to the recipient. However, most legislation is directed towards regulation of discharging to the receiving environment. The most comprehensive is the EU Water Framework Directive (2000), which aims at maintaining and improving the aquatic environment in the community, and is concerned primarily with protecting receiving waters from pollution. National (Hungarian) legislation (27/2005 Decree of the Minister for Environmental Affairs and Water Management on the control of discharging used and wastewaters) (XII. 6.) KvVM rendelete a használt- és szennyvizek kibocsátásának ellenőrzésére vonatkozó részletes szabályokról) definitely states, that effluents should be checked for chemical parameters such as COD, BOD, defined toxic compounds. Also, the same regulation states that toxicity of the effluent should be estimated, using standard bioassays such as the alga growth inhibition test (ISO 14442:2006), the Daphnia immobility test (ISO 6341:1996) or the Vibrio fischeri bioluminescence test (ISO 11348-1-2-3:2007). It is important to note, however, that these tests or analyses give only a static overview on the composition and chemical quality of the effluent and are not able to predict its environmental behaviour.
The hazard which toxic industrial effluents may pose to sewage treatment works has also been widely recognised, as several incidents have occurred. Toxicity can have an inhibitory effect on biodegradation, which in turn may result in longer treatment time or in reduced efficiency of treatment (e.g. Rebhun and Galil, 1988). Extremely high toxic shock can kill off the secondary tanks.
In order to avoid such incidents, Annex 1 of the EU Urban Waste Water Treatment Directive (1991) states ‘Industrial wastewater entering collecting systems and urban waste water treatment plants shall be subject to such pre-treatment as is required in order
6 to: … ensure that the operation of the waste water treatment plant and the treatment of sludge are not impeded’.
Numerous authors have used ecotoxicological tests to assess the effectiveness of wastewater purification/treatment processes (e.g. Cēbere et al., 2009; Daniel et al., 2004;
Emmanuel et al., 2005; Libralato et al., 2006; Metcalf & Eddy, 2003, Blinova, 2001, Kennedy et al., 2000). Toxicity, however, is not a static parameter: toxic potential of an effluent will change due to degradation processes such as photolysis, hydrolysis, oxidization and biodegradation. The risk of toxic effects in the recipient depends primarily on the time-related variation of toxicity of the effluent.
Ready biodegradability of an effluent is a key parameter to assess hazard an effluent poses to its environment either it is treated in a municipal treatment plant or discharged to a recipient surface water (e.g. Tisler et al., 1999).
The latest version of OECD tests for ready biodegradability (OECD, 1995) aims at predicting whether a chemical has the potential to be easily biodegraded in the environment. In these protocols usually oxygen uptake is measured, involving long-term (14 to 28+ day) respirometer testing. However, these tests which use chemical end-points such as COD do not give an indication on how toxicity of the chemical will change due to the formation of intermediate products. These tests were not designed to predict the behaviour of waste water in the aquatic ecosystem. Therefore although some methods have been described to evaluate the biodegradability of chemicals in environmental water (e.g. Means et al., 1981), no relevant OECD Test Guidelines have been proposed so far.
Instead, simulation tests exist such as the stream model of Shimp et al. (1989) or the die- away test of Anderson et al. (1990).
Biodegradation in the environment is affected by many factors such as concentration of the effluent (e.g. references), exposure and the composition of microbial communities.
In recipient water presence of competent bacteria can be expected (e.g. Ward and Brock, 1976). For testing biodegradability, it has been recognised that a mixed community of natural origin is more capable of degrading a wide range of compounds than pure cultures of selected strains (OECD, 1995).
In order to represent real-world conditions, incubation time should be long enough.
The minimum incubation time is considered 8 weeks (e.g. Shelton and Tiedje, 1984,
7 Strevett et al., 2002.). However, Strevett et al. (2002) report that for some chemicals this time period may be insufficient, requiring an incubation time period of 100 days.
During degradation processes not only concentration of the chemicals (and therefore exposure) will change but also, photo-degradable, hydrolytically unstable, oxidizable and biodegradable substances in addition may form such breakdown products which can be even more toxic than the parent substance was.
Evaluation of the biodegradability is a key element of hazard identification of whole effluents, and it should comprise toxicity testing as well (e.g. Zgajnar Gotvajn and Zagorc-Koncan, 1998, 2003). The PEEP (Potential Ecotoxic Effects Probe) index developed by the Fraser Pollution Abatement Office (Environmental Management Associates, 1993) aggregates the toxicity measured using different tests before and after biodegradation of the sample.
However, during degradation such intermediate products might appear which are more toxic: this is not only a hypothetical suggestion (Cairns, 1983) but has been demonstrated experimentally (Kováts et al., 2002). Illés et al. (2013) and Szabó et al.
(2011) studies biodegradation of non-steroid anti-inflammatory drugs in clinical wastewater. In the frame of this work the toxicity of a clinical wastewater sample was followed during three months, using Vibrio fischeri as an indicator organism. An analytical method (SPE, HPLC-MS) was developed for the determination of two non- steroidal anti-inflammatory drugs, Ibuprofen and Ketoprofen, in wastewaters. The results showed that during three months, at the beginning of the degradation process toxicity increased, due to analytically demonstrated presence of intermediate products.
In the following parts first biodegradability testing, ecotoxicity testing then the used wastewater types in this thesis are discussed.
2.1 Biodegradability testing
For determining the biodegradation, a tiered approach is recognized. In the first level, ready biodegradability tests (stringent screening tests), performed under aerobic conditions, in which a high concentration of test substance (in the range of 2 to 100 mg/l) is used and biodegradation is determined by non-specific parameters like Biochemical Oxygen Demand (BOD), Dissolved Organic Carbon (DOC) and CO2 production. A
8 positive result in this test can give indication of rapid and ultimate degradation in most environments including biological STPs. Therefore the substance is classified as “readily biodegradable”. In the second level, Inherent biodegradability tests with optimized conditions, e.g. a low ratio of test substance to biomass and long exposure time of the test substance to microorganisms, are performed to the test substances which couldn’t pass the ready biodegradability tests. Therefore gives a better chance to obtain positive results.
These chemicals are classified as “inherently biodegradable”. In the third level, simulation tests are conducted to assess the rate and extent of biodegradation in a laboratory system. They allow setting environmentally relevant conditions that are also optimum for biodegradability. Chemicals that do not pass the ready or inherent biodegradability level can reach appropriate biodegradation rates in this realistic test.
Various guidelines are available for these tests such as OECD and EC guidelines and ISO standard methods:
2.1.1. Tests on ready biodegradability:
Table 1: Applicability of test methods
Test Analytical method Suitability for compounds which are:
Poorly soluble volatile adsorbing
DOC Die-Away Dissolved organic carbon - - +/-
CO2 Evolution Respirometry: CO2
evolution + - +
MITI (I) Respirometry: oxygen
consumption + +/- +
Closed Bottle Respirometry: dissolved
oxygen +/- + +
Modified OECD
Screening Dissolved organic carbon - - +/-
Manometric
Respirometry Oxygen consumption + +/- +
i) DOC Die-Away Test (OECD 301A, EC C.4.A, ISO 7827)
This test is used for non-volatile substance which has solubility in water of at least 100 mg/l. The carbon content and the major components purity or relative proportions are preferred to be known. It’s similar to the Modified OECD Screening test (301 E) but allows using much higher microbial cell densities.
9 A measured volume of inoculated mineral medium with a known concentration of the test substance (10-40 mg DOC/l), is aerated in the dark or diffuse light at 22 ± 2oC. DOC analysis at frequent intervals over a 28-day period is used to follow the degradation. The biodegradation degree is calculated by expressing the concentration of removed DOC as a percentage of the concentration initially present.
The percentage degradation (Dt): [
] where:
Dt = % degradation at time t,
Co = the initial concentration of DOC in the inoculated culture medium containing the test substance (mg DOC/l),
Ct = the concentration of DOC in the inoculated culture medium containing test substance at time t (mg DOC/l),
Cbl(o) = the initial concentration of DOC in blank inoculated mineral medium (mg DOC/l), Cbl(t) = concentration of DOC blank inoculated mineral medium at time t (mg DOC/l).
When abiotic sterile control is used, the percentage abiotic degradation is calculated using:
% abiotic degradation =
where:
Cs(o) = DOC concentration in sterile control at day 0, Cs(t) = DOC concentration in sterile control at day t.
ii) CO2 Evolution (Modified Sturm Test) (OECD 301B, EC C.4.C, ISO 9439)
This test is also used for non-volatile substance. The carbon content and the major components purity or relative proportions are preferred to be known.
A known concentration of the test substance (10-20 mg DOC or TOC/l) in a measured volume of inoculated mineral medium is aerated by controlled rate-passage of carbon dioxide-free air in dark or in diffuse light. Degradation is followed over 28 days by determining the produced CO2 which is expressed as a percentage of ThCO2. The
10 absorbed CO2 in barium or sodium hydroxide, in the absorption bottle, is measured by titration of the remaining hydroxide or as inorganic carbon.
When 0.0125 M Ba(OH)2 is used as the absorbent, the amount remaining is assessed by titrating with 0.05 M HCl. (Thus 50 ml HCl would be needed to titrate 100 ml Ba(OH)2).
Since 1 mmol of CO2 is produced for every mmol of Ba(OH)2 reacted to BaCl2 and 2 mmol of HCl are needed for the titration of the remaining Ba(OH)2, and given that the molecular weight of CO2 is 44 g, the weight of CO2 produced (mg) is calculated by:
The weight of CO2 produced from the test substance is the difference between the weights of CO2 produced from the inoculum plus test substance and from the inoculum alone.
The biodegradation percentage is calculated from:
% degradation = CO CO2
2
% degradation = CO2
Where, 3.67 is the conversion factor (44/12) for carbon to CO2. The percentage of ThCO2
values calculated for each of the days, up to that time, on which it was measured.
When NaOH is used as the absorbent, calculate the amount of CO2 produced after any time interval from the concentration of inorganic carbon and the volume of absorbent used. Calculate the percentage degradation from:
% of ThCO2 =
Display the course of degradation graphically and calculate the percentage removal achieved at the plateau at the end of the test.
When an abiotic control is used, the percentage abiotic degradation is calculated by:
% abiotic degradation = CO2
CO2
iii) Modified MITI Test (I) (OECD 301C, EC C.4.F)
In this test, insoluble and volatile substances can be measured with certain precautions.
The insoluble substances should be well dispersed physically not with chemical means.
11 While for the volatile substances, the volume of "dead" gas space in the automatic respirometer should be kept to a minimum. The ThOD is calculated.
The oxygen uptake by an inoculated solution of the test substance in a mineral medium is measured automatically in a darkened, enclosed respirometer at 25 + 1°C over a period of 28 days. Evolved CO2 is absorbed by soda lime. Biodegradation is expressed as the percentage oxygen uptake of the theoretical uptake (ThOD). By using additional specific chemical analysis made at the beginning and end of incubation, the primary biodegradation percentage can be calculated, and the ultimate biodegradation can be calculated by DOC analysis.
BOD is expressed as mg oxygen/mg test substance can be calculated by dividing the oxygen uptake (mg) by the test substance (mg) after a given time, corrected for that taken up by the blank inoculum control after the same time, by the weight of the test substance used.
BOD = mg O2
= mg O2 / mg test substance The percentage biodegradation is then obtained from:
% degradation = % ThOD = O2. /
The primary biodegradation percentage can be calculated from the loss of specific (parent) chemical:
Dt = where:
Dt = % primary degradation at time t, normally 28 days;
Sa = residual amount of test chemical in inoculated medium at end of the test (mg);
Sb = residual amount of test chemical in the abiotic control at the end of the test (mg).
iv) Closed Bottle test (OECD 301D, EC C.4.E, ISO 10707)
In this test, volatile and insoluble substances can be measured with taking proper precautions such as shaking the bottles periodically during the incubation in the insoluble substances cases.
12 The test substance solution, usually at 2-5 mg/l, is inoculated with a relatively small amount of mixed population micro-organisms and kept in completely closed bottles at constant temperature in the dark. By analyzing the dissolved oxygen over a 28-d period, the degradation can be followed. The percentage of ThOD is the amount of biological oxygen uptake during the test substance biodegradation, corrected by the blank inoculum uptake run in parallel, or, less satisfactorily COD.
To calculate the ThOD, the substance formula and its purity, or major components relative proportions, should be known. The COD should be measured if the ThOD can’t be calculated, but sometimes, false high values of biodegradation percentage may be obtained if the test substance is not oxidized completely in the COD test.
BOD = mg O2/l uptake by test substance - mg O2/l uptake by blank
mg test substance/l in vessel = mg O2/mg test substance The percentage biodegradation is then obtained from:
% degradation = O2. /
% degradation = O2. /
v) Modified OECD Screening test (OECD 301E, EC C.4.B)
This test is used for non-volatile test substances with solubility in water of at least 100 mg/l. The carbon content and the purity of major components are preferred to be known.
A relatively low concentration of micro-organisms is used.
A measured volume of the medium containing a known test substance concentration of (10-40 mg DOC/l) is inoculated with 0.5 ml effluent per liter of medium. The mixture is aerated at 22 + 2°C in dark or diffused light. DOC analysis at frequent intervals over a 28 d period is used to follow the degradation. The biodegradation degree is calculated by expressing the concentration of removed DOC as a percentage of the concentration initially present. Primary biodegradation can be calculated from additional chemical analysis for a specific compound at the beginning and end of the test.
[
] where:
13 Dt = % degradation at time t,
Co = the initial concentration of DOC in the inoculated culture medium containing the test substance (mg DOC/l),
Ct = the concentration of DOC in the inoculated culture medium containing test substance at time t (mg DOC/l),
Cbl(o) = the initial concentration of DOC in blank inoculated mineral medium (mg DOC/l), Cbl(t) = concentration of DOC blank inoculated mineral medium at time t (mg DOC/l).
When abiotic sterile control is used calculate the percentage abiotic degradation using:
% abiotic degradation =
where,
Cs(o) = DOC concentration in sterile control at day 0, Cs(t) = DOC concentration in sterile control at day t.
vi) Manometric Respirometry (OECD 301F, EC C.4.D ISO 9408)
In this test, volatile and insoluble substances can be measured with taking proper precautions.
To calculate the ThOD, the substance formula and its purity, or major components relative proportions, should be known. The COD should be measured if the ThOD can’t be calculated, but sometimes, false high values of biodegradation percentage may be obtained if the test substance is not oxidized completely in the COD test.
A known test substance concentration (100 mg test substance/l giving at least 50-100 mg ThOD/l) in a measured volume of inoculated mineral medium is stirred in a closed flask for up to 28 days at a constant temperature (+1°C or closer). The oxygen consumption is evaluated either by assessing the quantity of oxygen (produced electrolytically) necessary needed to keep constant gas volume in the respirometer flask, or from volume or pressure change (or a combination of the two) in the apparatus. KOH solution or another suitable absorbent is used to absorb the evolved CO2. The amount of microbial oxygen uptake during the test substance biodegradation (corrected for uptake by blank inoculum, run in parallel) is expressed as a percentage of ThOD or, less acceptably, COD.
14 Primary biodegradation can be calculated from additional chemical analysis for a specific compound at the beginning and end of the test, and ultimate biodegradation by DOC analysis.
BOD = mg O2/l uptake by test substance - mg O2/l uptake by blank
mg test substance/l in vessel = mg O2/mg test substance The percentage biodegradation is then obtained from:
% degradation = O2. /
% degradation = O2. /
vii) Biochemical Oxygen Demand (EC C.5)
This test is applied to water-soluble organic compounds; however, it may be applied for volatile and low water soluble compounds by taking special measures and precautions.
A measured amount of the substance is dissolved or dispersed in a suitable medium, inoculated with micro-organisms, well-aerated, and incubated in the dark at a constant defined temperature.
The BOD is measured by determining the difference in dissolved oxygen content at the beginning and at the end of the test. The duration of the test must be at least five days and not more than 28 days.
A blank must be measured in parallel with the test sample.
2.1.2. Tests on Inherent Biodegradability:
i) Modified Semi-Continuous Activated Sludge (SCAS) Test (OECD 302A, ISO 9887)
This method was adapted from the Soap and Detergent Association semi-continuous activated sludge (SCAS) procedure for assessing the primary biodegradation of alkyl benzene sulphonate. It is used to evaluate the potential ultimate biodegradability of non- volatile, water soluble (at least 20 mg dissolved organic carbon/liter) organic substances under exposure to relatively high concentrations of microorganisms over a long time
15 period (at least 12 weeks). The microorganisms viability is maintained over this time period by daily addition of a settled sewage feed.
The test is applicable to water soluble and non-volatile organic chemicals that do not significantly adsorb within the test system, have negligible vapour pressure, are not lost by foaming from the test solution and are not inhibitory to bacteria at the test concentration.
In an aeration (SCAS) unit, activated sludge from a sewage treatment plant is placed, the test compound and settled domestic sewage are added, and the mixture is aerated for 23 hours. Then the aeration is stopped, the sludge is allowed to settle and the supernatant liquor is removed. The sludge remaining in the aeration chamber is then mixed with more aliquot of test compound and sewage and the cycle is repeated. Biodegradation is evaluated by the determination of the dissolved organic carbon content of the supernatant liquor. This value is compared with the one of the liquor obtained from a control tube dosed with settled sewage only.
The dissolved organic carbon results in the supernatant liquors of the test units and the control units are plotted against time.
% Degradation = Where,
OT = concentration of test compound as organic carbon added to the settled sewage at the start of the aeration period.
Ot = concentration of dissolved organic carbon found in the supernatant liquor of the test at the end of the aeration period.
Oc = concentration of dissolved organic carbon found in the supernatant liquor of the control.
The level of biodegradation is therefore the percentage elimination of organic carbon.
ii) Modified Zahn-Wellens/EMPA Test (OECD 302B, ISO 9888)
In this method, the measurement of DOC or COD is used to assess the ultimate biodegradability of the test substance.
16 The test substance, mineral nutrients and a relatively large amount of activated sludge in aqueous medium are mixed. The mixture is agitated and aerated in the dark or in diffuse light at 20-25°C for up to 28 days. Blank controls (without test substance) are run in parallel. Monitoring the biodegradation process is done by evaluating the DOC (or COD) in the filtered samples taken at daily or other time intervals. The biodegradation percentage at the sampling time is the ratio of eliminated DOC (or COD), corrected for the blank, after each time interval, to the initial DOC (or COD) value. The biodegradation percentage is plotted against time to give the biodegradation curve.
The chemical structure, water solubility and vapour pressure of the test substance should be known and its foaming properties also. The toxicity of the test substance to bacteria is advantageous for selecting the proper test concentrations and in understanding the results that show poor biodegradability. This test is usually executed only after the test substance fail to pass ready biodegradability test.
Chemicals which are soluble in water to at least 50 mg DOC/l, non-volatile, do not significantly adsorb within the test system, are not lost by foaming from the test solution and are not inhibitory to bacteria at the test concentration may be determined by this test.
[
] where:
Dt = percentage degradation at time t;
CA = concentration (mg/l) of DOC or COD in the test suspension measured after 3h ± 30 min of incubation;
Ct = mean concentration (mg/l) of DOC or COD in the test suspension at time t;
CBA = mean concentration (mg/l) of DOC or COD in the blanks measured after 3h ± 30 min of incubation;
CB = mean concentration (mg/l) of DOC or COD in the blanks at time t.
17 2.1.3. Simulations Tests:
Activated Sludge Simulation Tests: Coupled Units Test (OECD 303A, EC Activated Sludge Simulation Tests, ISO 11733)
This test is applicable only to the organic substances which are soluble in water, have negligible vapour pressure, and are not inhibitory to bacteria, under the test conditions.
Determination of the substance toxicity to the micro-organisms contributes in the interpretation of the results with low values and in the selection of appropriate test concentrations.
The simulation tests aim at evaluating the extent and rate of biodegradation and the fate of chemicals in a designed laboratory system to represent either the aerobic treatment stage of STP or certain environmental compartments, such as fresh or marine surface water.
Two continuously operated test units are used. Each unit consists of an aeration vessel, a separator and a sludge recirculation. The system is designed to determine the removal and the primary and/or ultimate biodegradation of water-soluble organics by monitoring the changes in DOC and/or COD. One unit is used as control and the other as test item treatment.
The test units are run in parallel under the same conditions. Generally, the mean hydraulic retention time is 6 h, the mean sludge age is 6 to 10 days and the test duration should not exceed twelve weeks after adding the test item. The addition of the test substance is at a concentration of between 10 mg/l DOC and 20 mg/l DOC to only one of the units.
Running and sampling are classified into three main periods:
Stabilization Period: to allow the system to reach a steady state of efficient DOC removal. At least one sample is taken per week.
Running-In Period: starts from the addition time of test substance till its removal reaches a plateau. Three samplings per week are taken.
Evaluation Period: A three weeks period starts when the plateau phase is reached. If the substance shows little or no degradation in the first six weeks, the evaluation period is performed in the following three weeks. Around 15 valid values in the plateau phase are necessary for the test results evaluation.
18 The difference between the concentrations of DOC/COD in the effluent from the test and control units is compared with the added test substance concentration to calculate the elimination of the test substance.
The percentage of removal is plotted versus time. From the elimination curve, the conclusions about the test substance removal process can be drawn.
2.2 Ecotoxicity testing
It is a well-known paradigm in ecotoxicology that bioassays (and test organisms) show different sensitivity to different contaminants. A bioassay will not be equally sensitive to all toxic compounds, its applicability should always be determined for a given purpose. Toxicity assessments are generally based on a battery of tests, where the bioassays represent (1) different trophic levels; (2) exposure routes and (3) might have different sensitivity. Such a minimum battery should include a primary producer (generally an alga), a zooplankton organism (primary consumer) and a fish (secondary consumer). It is also recommended to include a bacterial bioassay.
2.2.1. Algal inhibition test
This test determines the effects of a substance on the unicellular green algal species growth and it takes 72 hours, so the test can measure the effects over several generations.
In this test, the fast-growing green alga species are preferred to be used such as Selenastrum capricornutum or Scenedesmus subspicatus, which are put into the test vessels in a known density. Every 24h the density is measured, and after 72h the test is terminated. Growth is expressed as specific growth rate (r), and the effect as inhibition relative to control growth. The algae density is evaluated by using fluorescence measurements and is confirmed by cell counts.
A cellular fluorescence capacity (CFC) index can be used as another endpoint (Thompson, 1997). The algal fluorescence before (F) and after (Fd) the addition of the toxicant is used to calculate it. The index is expressed as a fraction [(Fd-f)/Fd].
The ISO 8692:2004 standard identifies a growth inhibition of unicellular green algae method applicable for the water soluble substances. This method was modified as
19 described in ISO 14442 and ISO 5667-16, so, the poorly soluble organic and inorganic materials, heavy metals, volatile compounds and waste water can be tested. ISO 10253:2006 describes a method for evaluating the growth inhibition of the unicellular marine algae Skeletonema costatum and Phaeodactylum tricornutum by substances and mixtures in the sea water.
Algae can be employed in on-line water quality testing. The Algae Toximeter is based on the measurements of chlorophyll fluorescence (i.e. toxicants react with the chloroplasts of green algae causing photosynthetic inhibition).
2.2.2. Daphnia-based toxicity testing
Daphnids are the most widely used zooplankton as test organisms. They represent the primary consumers in the aquatic ecosystem. Daphnia-based toxicity test is an acute toxicity test, where the exposing period is 24-48 hours. ISO 6341:1996 standard employs Daphnia magna and the test end-point is the motility inhibition (practically mortality).
Daphnids are specific in the way that they can show other symptoms which are relatively easy to evaluate and give an earlier warning of the toxic effect. Many Sub-lethal Daphnia tests were developed. Some were based on measuring the changes in the heart beat rates (Baylor, 1942), another on grazing intensity (Lotocka et al., 2001), moulting disruption (Rohrlack et al., 2004), inhibition of feeding activity (Ács et al., 2009), and life-history traits such as somatic (individual) growth (Burks et al., 2000), time to first reproduction, number of newborns (Lürling and van der Grinten, 2003). Lürling (2003) used population growth as the measure of toxic effect.
De Mott and Dhawale (1995) studied and used in vitro protein phosphatase activity inhibition as a biochemical end-point.
Daphnia can be used in on-line water quality testing. The Daphnia Toximeter is based on the movement pattern of daphnids. These movements are recorded and the live images are analyzed online. Any behavioral changes are immediately estimated.
20 2.2.3. Fish-based toxicity tests
a) Tests based on mortality as an endpoint
These tests are often used to determine the substance acute lethal toxicity to fish. Zebra fish and rainbow trout are the most preferred test species. The fishes are exposed to different concentrations of the toxic sample for a period of 96 hours. Mortalities are recorded at 24-hour intervals. There are three types of mortality tests can be used:
1. Static test: test solution remains unchanged during the test period.
2. Semi-static test: in which a regular batch of test solution is renewed after long periods (e.g. 24 hours).
3. Flow-through test: in which the test solution is renewed regularly in the test chambers.
ISO 7346/1, /2 and /3 -Water Quality -Determination of the acute lethal toxicity of substances to a fresh water fish (Brachydanio rerio Hamilton-Buchanan -Teleostei, Cyprinidae) test protocols are used in the EU.
b) Tests based on sub-lethal endpoints
Behavioural endpoints in fish are easily noted. The earliest automated systems were based on rheotaxis (Besch et al., 1976). When a toxicant damages the nerve system of the fish, they will sweep away by the current. Following the fish movement can be recorded and converted into x,y coordinate data (Vogl et al., 1999; Beauvais et al., 2000), and transformed into relevant endpoints which include velocity, swimming activity, water column position, angular change, or total distance travelled (Little and Finger, 1990;
Steinberg et al., 1995). van der Schalie et al., (1988) and Diamond et al., (1990) studied the changes in fish ventilator response pattern. U.S. Army Center for Environmental Health Research (USACEHR has developed a system to detect and record the ventilatory movements for continuous automatic evaluation (Sarabun et al., 1999). various test designs have been developed based on detecting during embryonic/larval development abnormalities as well (e.g. Oberemm et al., 1997; Palíková et al., 2007).
Fish can be used in on-line water quality testing. The Fish Toximeter is based on the changes in the fish behaviour. The measurements of speed, swimming activity such as turns or circular motions and number of active fish are used in the analysis.
21 2.2.4. Vibrio fischeri bioluminescence inhibition assay
Vibrio fischeri is a marine, bioluminescent bacterium. Bioluminescence is a natural phenomenon in which visible light is generated by an organism as a result of a chemical reaction. These reactions can be reconstructed outside the organisms from which they originate, thereby enabling exploitation of this natural process. There are diverse types of organisms that display bioluminescence: bacteria, protozoa, fungi, sponges, crustaceans, insects, fish, squid, jellyfish, and lower plants. Bioluminescent organisms occur in a variety of habitats, particularly the deep sea, where light is employed for functions including defence, reproduction and feeding. The enzymes involved in the luminescent (lux) system, including luciferase, as well as the corresponding lux genes, have been most extensively studied from the marine bacteria in the Vibrio and Photobacterium genera and from terrestrial bacteria in the Xenorhabdus genus. It has been found that the light-emitting reactions are quite distinct for different organisms with the only common component being molecular oxygen.
V. fischeri can be found in small amounts in the ocean and in large amount in isolated areas such as the light organs of a squid, Euprymna scolopes with which it has a symbiotic relationship (Brovko, 2010). When the squid is young, it draws in free-living bacteria from the ocean into its light organ. Here they are provided with all of the nutrients that they need to survive. Light emittance is activated only within the squid, as in the ocean cell density is app. 102 cells/ml, and this low concentration of cells is not enough to cause the luminescence genes to be activated. Cell density-dependent control of gene expression of lux genes is activated by autoinduction that involves the coupling of a transcriptional activator protein with a signal molecule (autoinducer) that is released by the bacteria into its surrounding environment (this „communication” is called quorum- sensing1). When in the light organ of a squid, the cell concentration is about 1010 cells/ml, and the autoinducer causes the bacteria to emit light. The squid is even capable of controlling light emittance: during the day, it keeps the bacteria at lower concentrations
1 The regulation of density-dependent behaviour by means of quorum sensing is widespread in bacteria. It can be used for example by opportunistic/infectous bacteria such as Pseudomonas aeruginosa to overcome the host’s immune system: bacteria grow to a certain concentration without any warning sign and they become aggressive only when reaching a critical mass.
22 by expelling some of them into the ocean during regular intervals. At night, as the squid is night-feeder, the bacteria are „allowed in”.
The light output of luminescent microorganisms which emit light as a normal consequence of respiration is read by a luminometer. Chemicals or chemical mixtures, which are toxic to the bacteria, cause changes in some cellular structures or functions such as the electron transport system, cytoplasmic constituents or the cell membrane (Fig.
1), resulting in a reduction in light output proportional to the strength of the toxin (Fig.
2). As bioluminescence of V. fischeri is directly linked to respiratory activity, it provides a good indicator of the metabolic status and has been found to be well correlated with in vivo toxicity tests using higher organisms (e.g. Kaiser et al., 1994).
Fig. 1: The biochemical mechanism of bioluminescence.
Bioluminescence
100
50
15 min 30 min
0
a b c d e
Control
The most toxic sample/
dilution
Samples/dilutions 50 % light reduction
Time (min)
Fig.2: Luminescence inhibition is roughly proportional to the concentration of the toxic compound
23 This principle is used by several commercially available systems as the Microtox, LumisTox, BioTox or ToxAlert. Most experiments were conducted using the Microtox version. It is the most referred in the literature. Numerous authors studied and compared the toxicity values obtained using the Microtox ® system (for a wide range of organic and inorganic chemicals, … etc) with values obtained using other live organisms such as fish, crustaceans, and algae. These studies proved an excellent correlation (Farré and Baceló, 2003). Microtox® offers great sensitivity, cost effective, accuracy and is time saving (Curtis et al., 1982; Gutiérrez, 2002; Dalzell, 2002; Wang, 2002).
One main benefit of the test is that it requires very short exposure: maximum exposure is 30 minutes but an indicative value is given even after 5 minutes. This fact and the easy-to-perform nature of the test make it suitable to use when a very high number of samples are to be processed.
Many field portable devices are based on the measurement of biolumescence inhibition either using Vibrio fischeri or another test organism2. Commercially available for example the ToxScreen system developed by CheckLight Co. On-line versions of the bioluminescent bactera test are also available such as the TOXcontrol On-line Biomonitor developed by microLAN On-line Biomonitoring Systems.
Despite its advantages, the Vibrio fischeri bioluminescence inhibition assay is still rather controversial in ecotoxicology. Some authors question its ecological relevance, emphasizing that Vibrio fischeri, being a marine bacterium, cannot be used to reflect the behaviour of terrestrial or freshwater organisms. New trends in ecotoxicology might help to resolve this problem. The mechanism of bioluminescence is already well-known (e.g.
Nunes-Halldorson and Duran, 2003), purified luciferase enzyme was made available already in the 1950’s (Mc Elroy and Green, 1955). Bioluminescent reporter bacteria can be genetically engineered by placing a lux gene under the control of an inducible promoter, making originally non-luminescent organisms capable to show bioluminescence. The lux system consists of five genes: luxA, luxB, luxC, luxD and luxE.
LuxAB bioreporters contain only the luxA and luxB genes and due to methodological
2 Another system uses the natural bioluminescence of the microscopic marine dinoflagellate algal Pyrocystis lunula. The principle, trade-named Lumitox, was developed into a portable, hand-held instrument (TOX-BOX), in response to a solicitation by the United States Army for a rapid field toxicity test for water supplies. As dinoflagellates are eukaryotic, they might be better models for human risk assessments. Also, it can detect the presence of toxins in the ppb range.
24 constraints not discussed here are not as widely used as luxCDABE reporters. Ben-Israel et al. (1998) used the luxCDABE bioluminescence genes of the Vibrio fischeri lux system as a reporter system for different stress promoters of Escherichia coli, making qualitative and quantitative analysis possible. Riether et al. (2001) constructed two plasmids in which the metal-inducible zntA and copA promoters from Escherichia coli were fused to a promoterless Vibrio fischeri luxCDABE operon and studied the specific response given by heavy metals induction. They found that in optimized assay conditions, metals could be detected at threshold concentrations ranging from nanomolar to micromolar. These tests are referred to as “bioreporters” or “biosensors”: living microbial cells have been genetically engineered to produce a measurable signal. By „transplanting luminescence” to such organisms which are native and common in our environment ecological relevance of the test is ensured. In addition to E. coli, Ralstonia eutropha and Pseudomonas sp. are used as recipient organisms3. A Ralstonia eutropha AE2515 strain was produced for detecting Ni2+ and Co2+ (Tibazarwa, 2001), and a Pseudomonas fluorescens DF57 strain for bioavailable copper indication (Tom-Petersen et al., 2001).
Another novelty is the increased specificity of the bacterial test. Specificity is achieved by the controlled expression of the lux operon genes. In this controlled expression a transcriptional regulatory protein takes part which specifically recognizes the target analyte. The target analyte triggers an increased bioluminescence even at very low concentration. (Please note that this mechanism is just the opposite of the standard Vibrio fischeri test where toxicity induces a decrease in bioluminescence!)
Up to now, a wide range of bacterial biosensors are available, developed and tested for a given heavy metal, such as cadmium and lead (e.g. Tauirainen et al., 1998), mercury (e.g. Ivask et al., 2001, 2002, Ivask and Bernaus, 2004), zinc and chromium (Ivask et al., 2002), as well as nickel (e.g. Tibazarwa et al., 2001). Many of them have
3 Genetically modified luminescent bacteria are not only produced for ecotoxicity testing but also for detecting a targeted microbe marked with bacterial lux genes in a mixed culture for example in bioremediation systems. For example, when special degraders are introduced into the system, their interaction with the indigenous microorganisms can be followed (Xing et al., 2000).
25 proven applicable in field conditions (e.g. Kahru et al., 2005). Specific biosensors are already commercially available in a kit form such as the BIOMET®.
The Vibrio fischeri bioluminescence inhibition test was originally developed for wastewater toxicity testing but has gained ground in water and sediment toxicity testing as well. For water analysis, the following standards apply:
ISO/CD 11348-2: Water quality -- Determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (Luminescent bacteria test) -- Part 2: Method using liquid-dried bacteria
ISO/CD 11348-3: Water quality -- Determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (Luminescent bacteria test) -- Part 3: Method using freeze-dried bacteria
As V. fischeri is a marine bacterium, the conventional protocol uses aqueous samples (in compliance with ISO 11348-3). The test has been widely used for assessing wastewater and activated sludge toxicity (Bailey and Beney, 2000; Gutiérrez et al., 2002;
Manusadzianas et al., 2003; Ren, S., 2004; Barbusiński, 2005), monitoring pesticides ecotoxicity in water (Köck et al., 2010), assessing insecticides degradation and degradation intermediates toxicity (Šojić et al., 2012), and is often incorporated in national regulations.
However, turbidity and colour of the sample might reduce the light output recorded by the luminometer due to physical effects, creating the potential for false- positives (Campisi et al., 2005). Wastewater samples are very often turbid and/or coloured, which might result in overestimating their toxicity if they are tested unfiltered.
Filtering can be an option, in fact, ISO 11348-3 prescribes that samples should be filtered if turbid. However, toxicity can be underestimated if the toxic effect depends mostly on particle-bound compounds (Harkey and Young, 2000).
Lappalainen et al. (1999, 2001) presented a novel protocol for testing the toxicity of solid and/or coloured samples. Here luminescence intensity is evaluated in a kinetic mode. As the bacterial suspension is injected to the sample, the luminous intensity
26 increases, to a peak (maximum) within 30 s (that is why the system is called Flash). The results are expressed as the ratio of luminescence at 30s normalized to the peak value.
The method has several advantages. First, the light output in the sample is assessed independently from the control, taking peak luminescence measured during the first 30s interval and comparing it to the value recorded after the present exposure time.
The protocol has been standardised, the ISO standard (ISO 21338:2010: Water quality - Kinetic determination of the inhibitory effects of sediment, other solids and coloured samples on the light emission of Vibrio fischeri /kinetic luminescent bacteria test/) was issued recently, in 2010. The test has been successfully used for testing contaminated soils or sediments and compost toxicity (Pollumaa et al., 2000, 2004; Degli-Innocenti et al., 2001; Heinlaan et al., 2007; Karlsson et al., 2010), nanoparticle toxicity (Mortimer et al., 2008; Farré et al., 2009) and for assessing the potential ecological hazard of the red mud accident of Devecser, Hungary (Gelencsér et al., 2011). However, no data are available yet on its efficacy in wastewater/whole effluent toxicity testing.
Expression of ecotoxicity:
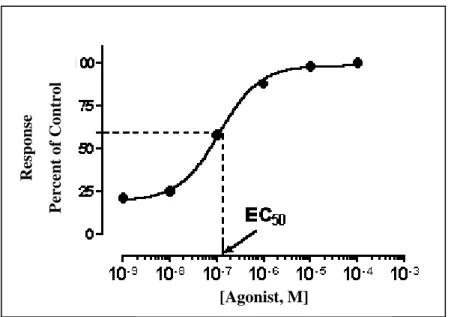
The test end-point (which was defined as a quantifiable biological response such as mortality, growth inhibition, enzymatic inhibition, etc.) is measured at a range of effluent concentrations to develop a concentration-response curve. This curve is typically sigmoidal (Fig. 3), but is linearized by data transformation methods, e.g. probit transform.
From the resulting linearized concentration-response curve, a point estimate effect concentration can be calculated (Fig. 4), most often expressed as EC504. The EC50 of a graded dose response curve represents the concentration of a compound where 50% of its maximal effect is observed or represents the concentration of a compound where 50% of the population exhibits a response. The EC50 generally substitutes LC where the half lethal concentration is estimated, supposing that the effect observed is mortality. In addition, the term IC50 can be used which is a measure of a compound's inhibition (50%
inhibition).
4 A free software developed for probit calculation can be downloaded from: András, EPA
27 Fig.3 Sigmoidal concentration-response curve
Concentration measures typically follow an S-shaped curve, increasing rapidly over a relatively small change in concentration. The point at which the effectiveness slows with increasing concentration is the IC50. This can be determined mathematically by derivation of the best-fit line. However, it is more easily observed from a graph and estimated rather than through complex calculus equations.
Fig. 4 Determination of point estimates from a linearized concentration-response curve.
[Agonist, M]
Response Percent of Control
Concentration Response (percent mortality)
28 The toxic compound or sample can also be characterised by NOEC and LOEC values (NOEC is the highest concentration of toxicant in which the values for the observed responses are not statistically significantly different and LOEC is the lowest concentration of toxicant in which the values for the observed responses are statistically significantly different from the controls).
2.3. Wastewaters of high organic (biodegradable) matter content
In this thesis, the ecotoxicity of different types of wastewater and their degradation under different conditions were assessed, but, they were of high organic (biodegradable) matter content in common.
Municipal wastewater used came from municipal wastewater treatment plant.Municipal wastewater is consisting mainly of water (99.9%) and relatively small concentrations of inorganic and suspended and dissolved organic solids. Organic matters are carbohydrates, lignin, proteins and their decomposition products, fats, soaps and synthetic detergents, several natural and synthetic organics from the process industries, anddisease-causing pathogens. The inorganic substances may include a number of potentially toxic elements such as arsenic, cadmium, chromium, copper, lead, mercury, zinc, etc. Even if they are not at toxic-level concentrations to humans, they might be at phytotoxic levels, which would limit their agricultural use or at toxic levels to animals or to any other ecosystem compartment.
Apart from municipal wastewater, an industrial and an agricultural wastewater were tested. Agricultural wastewater used came from liquid manure. Ammonia concentration is posing the highest threat to water quality. The running Hungarian National Environmental Program (2003-2008, number 132/2003; X.11. OGy) is emphasizing that the liquid manure produced by agriculture in huge quantities can cause serious environmental risks. Reduction of the environmental impacts of liquid manure can be done by building up disposal ponds with up-to-date technological protection.
Natural formations (compact sand layers or rock) can be considered as technological protection, as well.