. . . .
. . . .
. . . .
Rationale and design of the BUDAPEST-CRT Upgrade Study: a prospective, randomized, multicentre clinical trial
Bela Merkely
1* , Annamaria Kosztin
1, Attila Roka
1, Laszlo Geller
1, Endre Zima
1, Attila Kovacs
1, Andras Mihaly Boros
1, Helmut Klein
2, Jerzy K. Wranicz
3,
Gerhard Hindricks
4, Marcell Clemens
5, Gabor Z. Duray
6, Arthur J. Moss
2, Ilan Goldenberg
2,7†, and Valentina Kutyifa
1,2†1Heart and Vascular Center, Semmelweis University, 68 Varosmajor St, H-1122 Budapest, Hungary;2University of Rochester Medical Center, Heart Research Program, Rochester, NY, USA;3Department of Electrocardiology, Medical University of Lodz, Lodz, Poland;4Leipzig-Heart Center, University of Leipzig, Leipzig, Germany;5Department of Cardiology, University of Debrecen, Debrecen, Hungary;6Department of Cardiology, Medical Centre, Hungarian Defence Forces, Budapest, Hungary; and7Sheba Medical Center, Tel Hashomer, Israel
Received 29 February 2016; accepted after revision 22 May 2016;
Aims There is lack of conclusive evidence from randomized clinical trials on the efficacy and safety of upgrade to cardiac re- synchronization therapy (CRT) in patients with implanted pacemakers (PM) or defibrillators (ICD) with reduced left ventricular ejection fraction (LVEF) and chronic heart failure (HF). The BUDAPEST-CRT Upgrade Study was designed to compare the efficacy and safety of CRT upgrade from conventional PM or ICD therapy in patients with intermittent or permanent right ventricular (RV) septal/apical pacing, reduced LVEF, and symptomatic HF.
Methods and results
The BUDAPEST-CRT study is a prospective, randomized, multicentre, investigator-sponsored clinical trial. A total of 360 subjects will be enrolled with LVEF≤35%, NYHA functional classes II – IVa, paced QRS≥150 ms, and a RV pacing≥20%. Patients will be followed for 12 months. Randomization is performed in a 3:2 ratio (CRT-D vs. ICD).
The primary composite endpoint is all-cause mortality, a first HF event, or less than 15% reduction in left ventricular (LV) end-systolic volume at 12 months. Secondary endpoints are all-cause mortality, all-cause mortality or HF event, and LV volume reduction at 12 months. Tertiary endpoints include changes in quality of life, NYHA functional class, 6 min walk test, natriuretic peptides, and safety outcomes.
Conclusion The results of our prospective, randomized, multicentre clinical trial will provide important information on the role of cardiac resynchronization therapy with defibrillator (CRT-D) upgrade in patients with symptomatic HF, reduced LVEF, and wide-paced QRS with intermittent or permanent RV pacing.
Clinical trials.gov identifier
NCT02270840.
- - - -
Keywords Cardiac resynchronization therapy upgrade † Right ventricular pacing † Dyssynchrony † Randomized controlled trial † Study design † Pacing-induced heart failure
*Corresponding author. Tel:+361 458 68 10; fax:+361 458 68 17.E-mail address:merkely.bela@kardio.sote.hu
†These authors contributed equally to this article.
&The Author 2016. Published by Oxford University Press on behalf of the European Society of Cardiology.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact
journals.permissions@oup.com
online publish-ahead-of-print 6 October 2016
Introduction
Cardiac resynchronization therapy (CRT) reduces morbidity and mortality in selected patients with heart failure (HF) and broad QRS complex (≥130 ms). Large randomized clinical trials have shown improvement in cardiac function and decreased HF events or all-cause mortality, mainly in patients withde novoCRT device im- plantations.1–4However, 28% of CRT implantations in Europe are upgrade procedures after previously implanted cardiac devices.5To date, there are no conclusive data on the superiority of cardiac re- synchronization therapy with defibrillator (CRT-D) over implanta- ble cardioverter defibrillator (ICD) in patients with previously implanted pacemaker (PM) or ICD devices with reduced left ven- tricular (LV) function and symptomatic HF who require intermittent or permanent ventricular pacing. Furthermore, recent data indi- cated that upgrade procedures to biventricular pacing are asso- ciated with a relatively high complication rate,6suggesting that more conclusive data are required before a general recommenda- tion for an upgrade to a CRT system can be made.
Study design
Study objectives
The aim of the BUDAPEST-CRT Upgrade Study is to investigate the safety and efficacy of upgrading to CRT from existing previously im- planted PM or defibrillators, during a 12-month follow-up time in patients with reduced ejection fraction (LVEF≤35%), symptomatic heart failure (NYHA II – IVa), and intermittent or permanent right ventricular (RV) pacing (≥20%, with paced QRS complex≥150 ms), compared with therapy with ICD only.
The primary endpoint of the study is the composite of clinical and echocardiographic parameters including the first occurrence of
a non-fatal HF event, or all-cause mortality, or less than 15% re- duction in LVESV from baseline to 12 months, determined by echocardiography.
Secondary endpoints are all-cause mortality, the composite end- point of death or HF events, and changes in left ventricular ejection fraction (LVEF) and left ventricular end-diastolic volume from base- line to 12 months.
Tertiary endpoints are safety endpoints related to procedure suc- cess as well as changes in functional (NYHA) class, biomarker para- meters (NT-pro-BNP) after 1 year, quality of life, and 6 min walk test.
Study design
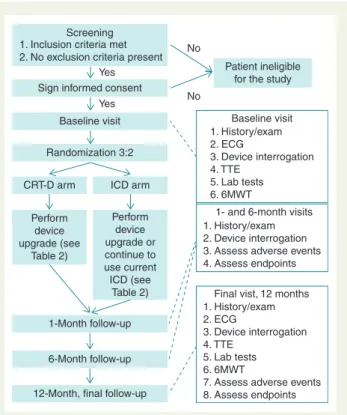
This is a prospective, international, multicentre, randomized con- trolled clinical trial conducted in 30 European and Israeli investiga- tional centres. The study will be conducted in accordance with the Helsinki Declaration, the good clinical practice, and the applicable regulatory requirements.7All patients will provide their written in- formed consent prior to enrolment (Figure1).
Patient population
Patients with chronic cardiomyopathy (regardless of aetiology), re- duced LVEF (≤35%), and symptomatic HF (NYHA functional classes II – IVa) despite optimized medical treatment, who have a single- or dual-chamber PM or ICD implanted at least 6 months be- fore enrolment (with≥20% RV pacing in the time period of ≥90 days prior to enrolment, paced QRS duration≥150 ms).
The intrinsic rhythm is required to assess at enrolment in order to exclude patients with typical Left Bundle Branch Block (LBBB) morphology. Patients with atrial fibrillation/flutter or atrial tachycar- dia can also be enrolled. The rate or frequency control management
What’s new?
† Cardiac resynchronization therapy upgrade from a conven- tional pacemaker (PM) or implantable cardioverter defibrilla- tor (ICD) takes a relatively high part of interventions. The patient selection and the optimal time of the procedures with measuring the risk – benefit ratio are according to the physician’s discretion.
† While the current European Society of Cardiology guideline does not contain conclusive data (with more multicentre, randomized trials) on the superiority of cardiac resynchroni- zation therapy with defibrillator (CRT-D) over ICD in pa- tients with previously implanted PM or ICD devices with reduced left ventricular (LV) function and symptomatic heart failure (HF) who require intermittent or permanent ventricu- lar pacing.
† The results of our prospective, randomized, multicentre clin- ical trial will provide important information on the role of CRT-D upgrade in patients with symptomatic HF (NYHA II – IVa), reduced LV ejection fraction (≤35%), and wide- paced QRS (≥150 ms) with intermittent or permanent right ventricular pacing (≥20%).
Screening 1. Inclusion criteria met 2. No exclusion criteria present
Sign informed consent
Baseline visit
Randomization 3:2
CRT-D arm
Perform device upgrade (see
Table 2)
Perform device upgrade or continue to use current ICD (see
Table 2)
1-Month follow-up
1- and 6-month visits 1. History/exam 2. Device interrogation 3. Assess adverse events 4. Assess endpoints
6-Month follow-up
12-Month, final follow-up ICD arm Yes
Yes
No
Patient ineligible for the study No
Final vist,12 months 1. History/exam 2. ECG
3. Device interrogation 4. TTE
5. Lab tests 6. 6MWT
7. Assess adverse events 8. Assess endpoints
1. History/exam 2. ECG
3. Device interrogation 4. TTE
5. Lab tests 6. 6MWT
Baseline visit
Figure 1 Study flowchart.
is based on the physician’s discretion before the enrolment and dur- ing the follow-up.
Detailed inclusion and exclusion criteria are listed inTable1.
Patient enrolment and randomization
After signing the written informed consent, eligible patients will undergo a baseline evaluation including clinical history, physical examination, assessment of NYHA class, 12-lead ECG with paced and non-paced QRS complexes using VVI 40 bpm settings, echocar- diography, device interrogation for assessment of RV pacing per- centage, assessment of quality of life, 6 min walk test, and optional blood test for assessment of NT-pro-BNP (Figure1).
Subjects meeting all inclusion criteria, without having any of the exclusion criteria, will be randomized to the CRT-D group (upgrade of the existing device into CRT-D) or the ICD group (either contin- ued ICD therapy in patients currently implanted with an ICD or up- grade to an ICD from an existing PM).
Among patients randomized to the control group who require ICD implantation, it is possible to implant a CRT-D device with or without the LV lead implanted at the same time, but LV pacing has to be turned off and the device has to be programmed to VVI/DDD ICD. The de- cision of implanting a CRT-D device with the LV lead deactivated for the ICD arm is left to the discretion of the principal investigator at each site and will be recorded in the electronic CRF system. The random- ization will be performed in a 3:2 ratio (CRT-D:ICD).
A total of 360 patients will be enrolled in the study.
Trial intervention
Device upgrades will be performed within 14 business days from randomization. Patients assigned to the CRT-D group and those with a PM, assigned to the ICD group, will undergo the device up- grade procedure (Table2). Patient with an existing ICD, assigned to the ICD group, may not need a procedure unless a generator re- placement, system revision, or upgrade to a dual-chamber system is indicated by the enrolling physician. Decisions about lead . . . . Table 1 Inclusion and exclusion criteria in the study
Inclusion criteria Exclusion criteria
(1) Age: over 18 years
(2) Cardiomyopathy with LVEF≤35%, ischaemic or non-ischaemic (3) Single- or dual-chamber PM or ICD implanted≥6 months prior to
enrolment (battery depletion or another indication for upgrade is not required)
(4) RV pacing≥20% in the prior≥90 days (use of algorithms to avoid ventricular pacing is recommended, per discretion of the clinician) (5) Paced QRS duration≥150 ms
(6) Symptomatic HF with NYHA functional classes II – IVa≥3 months prior to enrolment, despite optimized medical therapy
(7) Informed consent
(1) CABG or PCI≤3 months ago or planned (2) AMI≤3 months ago
(3) Unstable angina (4) Planned cardiac transplant (5) Acute myocarditis (6) Infiltrative cardiomyopathy (7) Hypertrophic cardiomyopathy
(8) Severe primary mitral, aortic, or tricuspid valve stenosis or insufficiency (9) Tricuspid valve prosthesis
(10) Severe RV dysfunction (RV basal diameter.50 mm) (11) Chronic severe renal dysfunction (creatinine.200mmol/L) (12) Pregnant women of planned pregnancy
(13) Subjects who are unable or unwilling to cooperate with the study protocol
(14) Any comorbidity that is likely to interfere with the conduct of the study (15) Participation in another trial
(16) Patients geographically not stable or unavailable for follow-up (17) Intrinsic QRS with typical LBBB morphology
. . . . Table 2 Study interventions
CRT-D group ICD group
1. Existing PM
Addition of RV defibrillator lead
Addition of RA pacing lead (unless already has one or has permanent AF) Addition of LV pacing lead
Extraction of old RV PM lead optional (physician’s judgement) Any revision of the old lead(s) and device pocket, as necessary Generator change to CRT-D
2. Existing ICD
Addition of RA pacing lead (unless already has one or has permanent AF) Addition of LV pacing lead
Any revision of the old lead(s) and device pocket, as necessary Generator change to CRT-D
1. Existing PM
Addition of RV defibrillator lead
Addition of RA pacing lead optional (physician’s judgement, unless already has one or has permanent AF)
Extraction of old RV PM lead optional (physician’s judgement) Any revision of the old lead(s) and device pocket, as necessary Generator change to VVI or DDD ICD,
2. Existing ICD
Continue with existing device
Addition of RA pacing lead and upgrading to a DDD ICD is optional (physician’s judgement, unless already has one or has permanent AF)
extraction are left to the individual judgement of each physicians based on the actual recommendations.8
Use of Boston Scientific Corporation (Marlborough, MA, USA) ICDs or CRT-D is encouraged in the study; however, it is left to the physician’s discretion to even use device manufactured by other companies. All devices implanted in the study have a CE certificate and will be implanted according to their instruction for use and cur- rent guidelines.9In the ICD arm, choosing single- or dual-chamber device is left to the physician’s decision. In the CRT-D arm, the LV lead is recommended to be implanted in the lateral or postero- lateral side branch of the coronary sinus. Transvenous implantation is strongly preferred; however, alternative methods are also ac- cepted if the transvenous attempt fails.
Follow-up
Patients will be followed up for 12 months after randomization (Figure1). The estimated duration of enrolment period is 36 months, which may be extended depending on the recruitment rate.
Regular, in-office follow-ups will be performed at 1, 6, and 12 months. Clinical parameters, ECG, device, echocardiographic, and biochemical parameters will be collected at each follow-up visit and stored in a central electronic database (Table3).
Echocardiography assessment
After enrolment, a detailed echocardiographic report will be sub- mitted to the Echocardiography Core Laboratory for central assess- ment (Semmelweis University, Heart and Vascular Center, Budapest, Hungary). Left ventricular volumes and ejection fraction will be calculated using the biplane Simpson method. Echocardio- graphic images will be obtained at baseline visit (after randomization
and before implantation) and at the 12-month follow-up visit. A de- tailed echocardiographic protocol has been prepared and will be sent to the enrolling centres for standard acquisition of the echocar- diographic images. All sonographers in the study will be certified by the Echocardiography Core Laboratory according to the inter- national standards.
Device interrogation and programming
Lead impedance, sensing, and pacing parameters will be determined for each lead. Device settings, Holter recording data including RV pacing percentage, and all available tachyarrhythmia episodes with EGMs will be interrogated. All retrieved data will be printed, saved to a disk or a USB, and uploaded to a digital archive maintained by the Coordination and Data Centre.
A DDD(R) or VVI(R) mode is required with base rate setting be- tween 40 and 70 bpm. Atrio-ventricular (AV) delay should be pro- grammed in DDD(R) devices to minimize RV pacing by using SMART AV delay10or echocardiographic optimization or fixed va- lues (sensed AV delay 120 – 140 ms/paced AV delay 140 – 160 ms).
Recommended tachycardia therapy settings are as follows: VT zone between 170 and 200 bpm monitor zone without therapy, or VT therapy in case of secondary prevention with a duration delay of 2.5 s. Ventricular fibrillation (VF) zone is over 200 bpm with a 2.5 s delay, ATP during charging (8 pulses at 88% of the tachycardia cycle length) and subsequent shocks (first shock DFT+10 or 30 J, subsequent shocks should be maximum energy shocks).
Assessment of endpoints
Ascertainment of the endpoints of death and HF events will be per- formed by the site investigators. The assessment of HF events are . . . . Table 3 Study procedures
Visit/evaluation Patient
enrolment visit Day 0
Device implantation and programming Within 14 days
1 month FU visit Day 30
6 months FU visit Day 180
12 months FU visit Day 365
Inclusion criteria x
Exclusion criteria x
Signed informed consent x
Clinical history x x x x
Physical examination x x x x
Assessment of NYHA class x x x x
12-Lead ECG (paced) x x
12-Lead ECG (at VVI 40 bpm) x x
Echocardiography x x
Device interrogation (print, save, upload) x x x x
Blood test (NP-pro-BNP) x1 x1
6 Min walk test x2 x
Randomization x
Assessment of clinical endpoints x3 x3 x3
Assessment of post-implantation complications x
SAE, AE, UADE, USADE x x x x x
Quality-of-life assessment using EQ-5D x2 x
1, Optional; 2, after the randomization but before implantation; 3, clinical endpoints; SAE, serious adverse event; AE, adverse event; UADE, unanticipated adverse device effect;
USADE, unanticipated serious adverse device effect.
based on the physicians’ discretion. Clinical signs and symptoms are required that are responsive to intravenous decongestive therapy or an augmented decongestive regimen with oral or parenteral medi- cations during hospitalization.
Death should be reported immediately. An independent HF and Death committee blinded to treatment group or clinical characteristics of the patients will review HF and death events. An event will be clas- sified as a HF event (i) if the patient had symptoms and/or signs con- sistent with congestive HF and (ii) received intravenous diuretic and/or positive inotropic therapy for longer than 2 h or (iii) received an aug- mented oral or intravenous HF therapy during an in-hospital stay due to worsening of HF. An independent, blinded Arrhythmia Adjudication Committee will review atrial and ventricular arrhythmia episodes.
Safety plan/study termination
A Data Safety Monitoring Board (DSMB) will perform pre-specified scheduled interim safety analysis following the enrolment of 30 and 60% of study population. The statistical design will permit early ter- mination of the trial if CRT efficacy is meaningfully greater than that hypothesized for ICD only, or ICD-only efficacy is meaningfully greater than that hypothesized for CRT. The study will be termi- nated if the DSMB identifies a significant harm with an implanted CRT-D over an ICD during the interim safety analysis.
Statistical analysis
The main objective of this study is to demonstrate a reduction in the primary composite clinical and echocardiographic endpoint after CRT upgrade (superiority of CRT-D upgrade vs. ICD only). Ana- lyses will be performed (i) on an intention-to-treat basis (without regard to device actually implanted/revised) and (ii) on efficacy basis, censoring follow-up when a patient crosses over to a different de- vice. The primary analyses will be stratified by the percentage of baseline RV pacing as pre-specified in the study.
Sample size and statistical methods
A total of 360 patients will be enrolled and randomized to CRT-D vs. ICD in a 3:2 ratio. The null hypothesis for the primary endpoint is
that the hazard rate, which is assumed to be constant across all study intervals, is identical in the two groups (CRT-D v. ICD). The hypoth- esis will be tested in a study in which subjects are entered and fol- lowed up until (i) the primary composite endpoint occurs, (ii) the patient drops out of the study, or (iii) the study ends while the pa- tient is still being followed, in which case the patient is censored. All subjects will be followed up for 12 months.
Power was calculateda prioribased on a hazard ratio of 0.7 and a primary composite endpoint event rate of 80% in the ICD group over 12 months. The power calculation was based on higher RV pa- cing rates, while no data is available,40%. Although the risk seems to correlate with RV pacing, the exact correlation is unclear. The at- trition (dropout) rate was assumed at 0.01/interval. An instantan- eous hazard rate of 0.134 for the ICD group and 0.094 for the CRT-D group was assumed—this equals to a median survival time of 5.17 intervals in the ICD group and 7.38 intervals in the CRT-D group, a cumulative event-free survival at 12 intervals of 0.2 for the ICD group and 0.32 for the CRT-D group. The two-tailed alpha was set at 0.05. A total of 144 patients will be entered into the ICD group and 216 into the CRT-D group to achieve a power of 80.1% to yield a statistically significant result.
Discussion
Several guidelines have been published on optimal patient selection for CRT implantation; however, recommendations on CRT upgrades are still ambiguous without any details for the different causes for chronic RV pacing (Table4). The 2013 European Society of Cardi- ology (ESC)/European Heart Rhythm Association (EHRA) guidelines recommend CRT upgrade in patients with LVEF,35%, NYHA III and IVa, and high percentage of ventricular pacing—although the cited evidence stands forde novoCRT implantations and crossover trials as opposed to upgrades from existing devices, with level of evidence ‘B’
and class I indication.9The 2012 ACCF/AHA/HRS guidelines are list- ing CRT upgrade with IIa indication, level of evidence ‘C’ for patients with LVEF≤35%, and a need for at least 40% ventricular pacing, for both new implants and device replacements.11The 2012 ESC/HFA Table 4 Indication for upgrade to CRT in patients with existing PM or ICD
ESC/EHRA 20139 CRT is indicated in HF patients with LVEF,35% and high percentage of ventricular pacing who remain in NYHA class III and ambulatory IV despite adequate medical treatment.
Remark: Patients should generally not be implanted during admission for acute decompensated HF. In such patients, guideline-indicated medical treatment should be optimized and the patient reviewed as an out-patient after stabilization.
It is recognized that this may not always be possible.
Class I LOE B
ACCF/AHA/HRS 201211 CRT can be useful for patients on GDMT who have LVEF≤35% and are undergoing new or replacement device placement with anticipated requirement for significant (.40%) ventricular pacing.
Class IIa LOE C
ESC/HFA 201212 CRT is recommended as an alternative to conventional RV pacing in patients with HF-REF who have a standard indication for pacing or who require a generator change or revision of a conventional PM.
No specific recommendations for CRT upgrade
ESC/EHRA, European Society of Cardiology/European Heart Rhythm Association; GDMT, Guideline determined medical therapy; ACCF/AHA/HRS, American College of Cardiology Foundation/American Heart Association/Heart Rhythm Society; ESC/HFA, European Society of Cardiology/Heart Failure Association.
guidelines, the 2013 Appropriate Use Criteria document, endorsed by the ACCF/HRS/AHA,13and the most recent 2015 ESC/EHRA guideline on ventricular arrhythmias and sudden cardiac death do not provide any recommendations on CRT upgrade.14
Effects of chronic right ventricular pacing
Large randomized trials have shown that chronic RV pacing is asso- ciated with an increased risk of HF, atrial fibrillation, and mortality.9,15 The Dual-Chamber and VVI Implantable Defibrillator (DAVID) trial demonstrated worse outcomes in patients with reduced LVEF and dual-chamber ICD programming to DDDR 70 bpm when compared with patients with VVI 40 bpm pacing. Every 10% increase in RV pa- cing increased the risk of death or HF hospitalization by 16%. The most significant separation was observed with 40% RV pacing, strong- ly predicting death or HF hospitalization (HR¼5.2,P¼0.008).16
Another multicentre, randomized clinical trial, the Mode Selec- tion Trial, confirmed the correlation of RV pacing and impaired clin- ical outcome in patients with preserved LVEF and sinus node dysfunction. The risk of HF hospitalization linearly increased with RV pacing up to 40%.15
In contrast, Olshanskyet al.suggested that reducing RV pacing does not necessarily eliminate the risk of an adverse outcome. In the INTRINSIC RV study, patients were categorized into six groups based on increasing RV pacing rates. A significant difference was found between rates concerning patients’ age, history of ventricular tachycardia, atrial fibrillation, atrial flutter, and amiodarone therapy.
Adjusting for these parameters, the best outcome was seen in patients with RV pacing between 10 and 19% (2.8% event rate over a median follow-up of 11.6 months). Increasing RV pacing has been found predictive of death or HF hospitalization (P¼0.003). Other than expected, patients with rare RV pacing (0 – 9%) experienced worse outcome (8.1% event rate,P¼0.016), although a lower RV pacing rate may be advantageous to improve AV dyssynchrony.17
Possible benefits and limitations of cardiac resynchronization therapy in patients with heart failure and intermittent pacing
Small crossover trials have compared RV pacing only to CRT in patients with symptomatic bradycardia and reduced LVEF. They showed that CRT reduced mortality, HF hospitalization, and lead to reverse ventricular remodelling.9,18For the first time, the Biventricular vs. Right Ventricular Pacing in Heart Failure Pa- tients with Atrioventricular Block Trial (BLOCK HF) showed that CRT is superior to RV pacing in patients with AV block, LVEF≤ 50%, and HF class NYHA I – III. After a median follow-up of 37 months, primary endpoints (time to death from any cause, HF visit that required intravenous therapy, or≥15% increase in LVESV in- dex) occurred in 190 of 342 patients (55.6%) in the RV pacing group, compared with 160 of 349 (45.8%) in the CRT group (HR¼0.6 – 0.9, posterior probability of HR,1¼0.9978). The LV lead-related complications occurred in 6.4% of patients in the CRT-treated group.9
The Homburg Biventricular Pacing Evaluation trial compared CRT with RV pacing in patients with bradycardia and LV dysfunction (LV end-diastolic diameter ≥60 mm and LVEF≤40%). Three
months of RV pacing vs. biventricular pacing were studied in 30 pa- tients. Improved echocardiographic parameters, laboratory values, and quality of life scores, as well as improved peak exercise capacity, were found only with biventricular pacing.9
The Conventional vs. Multisite Pacing for BradyArrhythmia Ther- apy (COMBAT) crossover study compared CRT vs. RV pacing in 60 patients with AV block, LVEF,40%, and HF with NYHA classes II–IV.
After a follow-up of 17.5 months, the quality of life, NYHA class, and echocardiographic parameters improved in patients with CRT. Overall mortality was significantly higher in patients with RV pacing alone (86.7% vs. 13.3%,P¼0.012).18Studies performed in patients with preserved LVEF also demonstrated benefit with CRT, showing increased reverse LV remodelling.9
The Long term from the Pacing to Avoid Cardiac Enlargement trial investigated the clinical outcomes of 149 patients with CRT, randomized to 1 year of RV or biventricular pacing after an ex- tended follow-up of 5 years (mean 4.8+1.5 years). In the RV pacing group, LVEF and LVESV worsened progressively during 1-year, 2-year, and long-term follow-ups, whereas both parameters re- mained unchanged in the CRT group (LVEF difference, respectively, P,0.001). However, patients with RV pacing needed significantly more HF hospitalization (23.9%) than CRT patients (14.6%).19In summary, chronic biventricular pacing seems to be superior to RV-only pacing, but the results cannot be extrapolated to patients with intermittent or chronic pacing who developed worsening of HF only recently.
The RD-CHF study upgraded 56 patients from VVI pacing (NYHA III and IV, and LV dyssynchrony) to CRT at the time of gen- erator replacement. The study had a 3-month crossover design with RV pacing only or CRT. Cardiac resynchronization therapy pacing significantly improved NYHA class, 6MWT, and quality of life.9
In the Resynchronization – Defibrillation for Ambulatory Heart Failure Trial (RAFT) study, 644 of 1346 enrolled (48%) patients underwentde novoCRT implantation, 80 patients were upgraded to CRT from a previously implanted ICD device, and 60 patients underwent CRT upgrade 6 months after the end of the initial study.
The success rate was 95.2% forde novo, 96.3% for upgrade, and 90.0% for post-trial CRT upgrade sub-study (P¼0.402). The acute complication rate was 26.2% forde novo, 18.8% for upgrade, and 3.4% for the sub-study CRT upgrade (P,0.001), most commonly due to LV lead dislodgement. The main reasons for not attempting upgrade in the sub-study group were patient preference (31.9%), NYHA class I (17.0%), and QRS,150 ms (13.1%). The authors conclude that the success of CRT upgrade is high and that the com- plication rates are similar tode novoCRT implantation.20However, in the prospective REPLACE Registry with 1750 patients undergoing device replacement, those who required upgrade experienced a high rate of major complications during a 6-month follow-up time (18% vs. only 4%).
There is still limited information concerning the efficacy and safety of CRT upgrade among patients with LV dysfunction and intermittent RV pacing, particularly in patients with moderate symp- toms with lower percentage of RV pacing, or with a narrow QRS (,150 ms). Patients who receive CRT-D upgrade may experience additional benefits, but this needs to be proved in a prospective study. Therefore, we have designed the BUDAPEST-CRT Upgrade Study.
Conclusion
The BUDAPEST-CRT Upgrade Study will evaluate the efficacy and safety of CRT-D upgrade when compared with ICD therapy in pa- tients with previously implanted PM or ICD devices, reduced LVEF≤35%, symptomatic heart failure (NYHA II – IVa), and inter- mittent or permanent RV pacing with wide-paced QRS≥150 ms.
Our study results will provide conclusive data on the effects of CRT-D upgrade procedure in this patient population and confirm the indication of CRT-D upgrade.
Funding
The study is supported by an unrestricted research grant from the Boston Scientific (St. Paul, MN, USA) Investigator Sponsored Research Fund to the Semmelweis University, Budapest, Hungary.
Conflict of interest: none declared.
References
1. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh Eet al.Cardiac resynchronization in chronic heart failure.New Eng J Med2002;346:1845 – 53.
2. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco Tet al.
Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure.New Eng J Med2004;350:2140 – 50.
3. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger Let al.
The effect of cardiac resynchronization on morbidity and mortality in heart failure.
New Eng J Med2005;352:1539 – 49.
4. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP et al.
Cardiac-resynchronization therapy for the prevention of heart-failure events.
New Eng J Med2009;361:1329 – 38.
5. Bogale N, Witte K, Priori S, Cleland J, Auricchio A, Gadler Fet al.The European Cardiac Resynchronization Therapy Survey: comparison of outcomes between de novo cardiac resynchronization therapy implantations and upgrades.Eur J Heart Fail2011;13:974 – 83.
6. Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge Ret al.Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator re- placements and upgrade procedures: results from the REPLACE registry.Circulation 2010;122:1553 – 61.
7. World Medical Association. World Medical Association Declaration of Helsinki:
ethical principles for medical research involving human subjects.JAMA2013;310:
2191–4.
8. Wilkoff BL, Love CJ, Byrd CL, Bongiorni MG, Carrillo RG, Crossley GH 3rdet al.
Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA).Heart Rhythm2009;6:1085 – 104.
9. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA et al.2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy:
the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA).Europace2013;15:1070 – 118.
10. Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD et al.Primary results from the SmartDelay determined AV optimization: a compari- son to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography- guided, and algorithmic atrioventricular delay programming in cardiac resynchroni- zation therapy.Circulation2010;122:2660 – 8.
11. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS et al.2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/
HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Asso- ciation Task Force on Practice Guidelines and the Heart Rhythm Society.J Am Coll Cardiol2013;61:e6 – 75.
12. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein Ket al.
ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC.Eur Heart J2012;33:
1787 – 847.
13. Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup Met al.
ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy:
a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardio- vascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance.Heart Rhythm 2013;10:e11 – 58.
14. Authors/Task Force M, Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm Jet al.2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Pre- vention of Sudden Cardiac Death of the European Society of Cardiology (ESC) En- dorsed by: Association for European Paediatric and Congenital Cardiology (AEPC).
Europace2015;17:1601 – 87.
15. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KLet al.Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction.Circulation2003;107:
2932 – 7.
16. Sharma AD, Rizo-Patron C, Hallstrom AP, O’Neill GP, Rothbart S, Martins JBet al.
Percent right ventricular pacing predicts outcomes in the DAVID trial.Heart Rhythm2005;2:830 – 4.
17. Olshansky B, Day JD, Lerew DR, Brown S, Stolen KQ, Investigators IRS. Eliminating right ventricular pacing may not be best for patients requiring implantable cardioverter-defibrillators.Heart Rhythm2007;4:886 – 91.
18. Martinelli Filho M, de Siqueira SF, Costa R, Greco OT, Moreira LF, D’Avila Aet al.
Conventional versus biventricular pacing in heart failure and bradyarrhythmia: the COMBAT study.J Card Fail2010;16:293 – 300.
19. Yu CM, Fang F, Luo XX, Zhang Q, Azlan H, Razali O. Long-term follow-up results of the pacing to avoid cardiac enlargement (PACE) trial.Eur J Heart Fail2014;16:
1016 – 25.
20. Essebag V, Joza J, Birnie DH, Sapp JL, Sterns LD, Philippon Fet al.Incidence, predic- tors, and procedural results of upgrade to resynchronization therapy: the RAFT up- grade substudy.Circ Arrhythm Electrophysiol2015;8:152 – 8.