CH A P T E R 6
Genetic Fine Structure in Bacteria
RO Y S T O N C . CL O W E S
I. I n t r o d u c t i o n 253 I I . C o r r e l a t i o n of t h e G e n e w i t h t h e U l t i m a t e G e n e t i c U n i t s of R e c o m b i n a t i o n ,
F u n c t i o n , a n d M u t a t i o n 254 A . T h e C l a s s i c a l G e n e 254 B . T h e C o n c e p t of a C o m p l e x G e n e L o c u s 256
C . I n t e g r a t i o n of G e n e t i c S t r u c t u r e w i t h P h y s i c o c h e m i c a l D a t a 276
D . M u t a t i o n 283 I I I . T r a n s l a t i o n of G e n e t i c I n f o r m a t i o n a n d B i o c h e m i c a l F u n c t i o n 292
A . G e n e s a n d E n z y m e s 293 B . F u n c t i o n a l A n a l y s i s a n d C o m p l e m e n t a t i o n 295
C . M u t a t i o n a n d t h e P h e n o t y p e 300 D . C o l i n e a r i t y , t h e C o d i n g R a t i o , a n d t h e G e n e t i c C o d e 306
I V . G e n e t i c I n t e r a c t i o n s 309 A . S t r u c t u r a l A s s o c i a t i o n s 309 B . R e g u l a t o r y S y s t e m s 314
R e f e r e n c e s 320 I. Introduction
I n cells of higher organisms, chromosomes are clearly visible as thread
like structures appearing within the nucleus a t cell division; they can be seen to be equivalently distributed to the daughter cells. I t is ironical t h a t in bacteria where the hereditary material can be extracted, purified, and manipulated in a variety of physical and chemical ways, chromosomes have only very recently been clearly demonstrated, and appear to be much less complicated structural entities.1* I t seems very likely, however, t h a t this reflects secondary physical arrangements, rather t h a n more fundamental differences. The use of the term "bacterial chromosome" is to this extent justified and in fact, the genetic and molecular details of the hereditary material of bacteria form the basis of m a n y of the modern concepts of genetic structure.
M a n y novel systems of genetic transfer which have been described in
* See H a y e s .1 H o w e v e r , v e r y recently, t w o i n d e p e n d e n t approaches h a v e produced t h e m o s t compelling e v i d e n c e so far published. T h e beautiful electron micrographs of K l e i n s c h m i d t et al.1 s h o w a continuous fiber with a cross-section d i m e n s i o n less t h a n 50 A . T h e autoradiographs of Cairns1 s h o w a c o n t i n u o u s double-stranded structure of length 700-900 μ. F r o m b o t h these v e r y diverse techniques it is rational t o assume that t h e bacterial c h r o m o s o m e consists of a double chain w i t h a cross section of
< 5 ταμ and a length over 105 t i m e s as great (700-900 μ)} and is c o m p a t i b l e w i t h a con
tinuous simple d o u b l e helix of d e o x y n u c l e o t i d e s . 253
detail in previous chapters exist in bacteria. All these systems to a greater or lesser extent transfer fragments of genetic material from one cell into another, and are thus admirably suited to the study of small chromosomal regions. Moreover, since bacteria are haploid organisms,2 their genetic analysis is much simplified, and is uncomplicated by problems of dominance and other perturbations inherent in diploids. I n addition, all bacterial systems so far investigated (with the exception of the Actinomyces]
see Chapter 5 ) appear to possess only one genetic structure or chromosome in each nucleus.3"5 Bacterial genetic systems thus present the ultimate in simplicity of genetic organization; this characteristic, combined with the facility with which one m a y accumulate and handle enormous populations, makes them an ideal tool for genetic studies.
II. C o r r e l a t i o n o f the G e n e w i t h the U l t i m a t e G e n e t i c Units of Re
c o m b i n a t i o n , Function, a n d M u t a t i o n
A. TH E CL A S S I C A L GE N E
1. FO R M A L GE N E T I C AN A L Y S I S
On the Mendel-Morgan concept of heredity,6 the heritable traits of an organism are conceived of as being controlled by a series of independently transmitted units, the genes. F r o m Mendel's early experiments each gene was postulated to exist in one of two alternative forms (alleles), one being dominant to the other when both are present in the same diploid organism on homologous but different chromosomes. An allele was considered to arise by a rare change in one gene resulting in an observable modification of some specific character. T h e recognition of a gene is thus dependent upon the existence of its allele, since its behavior can then be observed in crossing experiments. For example, if A and α, Β and b are alternative forms of two genes, a cross between two diploid parents carrying the combinations A Β and ab will give rise to progeny in which the new combinations A b and aB are observed. The frequency of such recombination is found to be con
stant for each pair of genes. If the numbers of recombinant progeny are less t h a n those carrying the parental combinations, the genes are said to be linked. Linkage is thus indicated if the fraction of recombinants in the total progeny is less t h a n 5 0 % ; the closer the linkage, the smaller the re
combinant fraction becomes. If three genes are closely linked, the recom
bination frequencies between pairs of t h e m show additivity; t h a t is, if the recombinant fraction between A and Β is 7 % and t h a t between Β and C is 3 %, then t h a t between A and C will be either the sum ( 1 0 %) or the differ
ence (4 % ) , from which it can be concluded t h a t a linear relationship exists between these genes, and their order can be fixed as either ABC (AC = 1 0 % ) or AC Β (AC = 4 % ) . Each gene can thus be mapped a t a charac-
6. G E N E T I C F I N E S T R U C T U R E I N B A C T E R I A 255 teristic position, termed its locus, with regard to other genes of the same linkage group, a set of such linkage groups being termed a linkage map.
I n higher organisms, these abstract linkage groups, constructed to rational
ize the results of genetic analysis, are beautifully and intimately correlated with the cytologically observable chromosomes. During meiosis, the paren
tal chromosomes pair, duplicate, and separate in a highly regulated way so as to segregate one complete haploid set of chromosomes to each of the resulting gametes. During this process there m a y be an exchange of parts between homologous chromosomes of the two parents, as a result of an event called "crossing over," so t h a t the chromosomes of the haploid gametes carry new combinations of the parental genes. The frequency with which any two parental genes on the same chromosome are separated by recombination is found to be a measure of the linear physical distance between them. Linked genes can thus be regarded as those genes between which, at meiosis, there is an incidence of less t h a n one such exchange by crossing over as the average of a large number of cells.
2. PS E U D O A L L E L E S
Classical genetic studies are concerned largely with the recombination and interaction of different genes. Genes between which recombination did not occur were considered to be alternatives, or alleles of each other. This assumption t h a t recombination did not take place within the functional unit implied t h a t the units of function and recombination were equivalent.
Early genetic research, however, soon revealed the existence of more t h a n two forms or alleles of the same gene, which were recognized by the fact t h a t when the two recessive alleles were present on different homologous chromosomes, the cell was phenotypically recessive, although the original (wild-type) gene was dominant to each allele. (If A is the wild-type gene, and a and a are two alleles both recessive to A, both A /a and A/a will show the wild phenotype, b u t a/a will show the recessive phenotype.)
I n the early 1950's crosses of such m u t a n t s in Drosophila led to the con
clusion t h a t alleles defined by these functional tests did not behave as true alternatives, since recombination was observed between them. These pseudoalleles, as they were called, form a bridge between the classical studies and those t h a t followed, and which stemmed largely from work with microbial systems.
One such set of pseudoalleles is illustrated by the lozenge (Iz) gene of Green and Green,7 in which three mutations involving eye pigmentation changes were studied. The m u t a n t s were recessive to the wild type and produced a recessive phenotype in combination with each other, and were thus defined as alleles by the functional criterion. In spite of this, recom
bination was found to occur between them, indicating distances of less
R O Y S T O N C . C L O W E S
t h a n one-thousandth the total chromosomal length. Similar studies were reported by Green,8 Ε. B. Lewis,9 and Mackendrick1 0 in Drosophila, and by Pontecorvo and his colleagues1 1'1 3 in Aspergillus. One of the several explanations offered for this finding was t h a t the various mutations might occur within the substructure of the gene controlling the lozenge function, between which recombination could occur.1 3 This particular conclusion shortly received striking confirmation with the almost simultaneous publi
cations in 1955, of Benzer1 4 using T 4 phage, and of Demerec and his col
leagues1 5 , 1 6 in Salmonella typhimurium.
I n general, genetic fine structure has been studied in the closest detail in bacteriophage ("running the m a p into the g r o u n d " )1 7 particularly by the elegant and fine experimental work of Benzer. I n bacteria, the smallest details are similarly demonstrable and show striking analogies with the rather more precise phage data. This contribution will concentrate on those experiments which use bacteria as genetic material and which have the advantage of the greater opportunities of relating genetic structure to function (see Chapter 8).
B. TH E CO N C E P T O F A CO M P L E X GE N E LO C U S
1. AD V A N T A G E S O F MI C R O B I A L SY S T E M S
The investigation of intragenic structure requires not only the isolation of numerous m u t a n t s affecting the same function (pseudoalleles) but also the ability to detect rare recombinants in crosses between pairs of these mutants, which are not likely to occur more frequently t h a n one in a thousand. The short generation time and size of bacteria permit facile accumulation and examination of populations m a n y times the size of the total h u m a n world population—an obvious choice of material for the study of these rare mutational and recombinational events.
Moreover, in bacteria, the genetic characters accessible for study include those involving simple and fundamental biochemical functions. T h u s , although morphological characters are few, mutations which affect a wide range of synthetic and catabolic activities can be investigated. These m u t a n t s have the great advantage of being open to selection, facilitating the isolation of both the rare m u t a n t from the parental, and the recombi
nant from the nonrecombinant progeny of a cross. The use of these bio
chemical m u t a n t s was first employed in the now classical genetic studies of Beadle and T a t u m using the mold Neurospora crassa.ls>19 These ex
periments led to the idea t h a t m a n y genes function by controlling the activity of enzymes. An alteration of a single gene by mutation was en
visaged as resulting in a loss of specificity in the enzyme controlled by t h a t gene, thus leading to a metabolic or biochemical block in a particular
6. G E N E T I C F I N E S T R U C T U R E I N B A C T E R I A 257 synthetic pathway. This one gene-one enzyme relationship constituted a breakthrough in functional genetics, although the use of biochemical m u t a n t s as one of the main tools in the investigation of intermediary metabolism tended at first to overshadow the more fundamental genetic implications.
2. SE L E C T I V E ME T H O D S A N D TE C H N I Q U E S
T h e bacteria which lend themselves most readily to the isolation of biochemical m u t a n t s are those termed "nonexacting," which grow well on a simple medium (minimal m e d i u m )2 0 of inorganic salts together with a carbohydrate and energy source such as glucose. F r o m these parental strains, m u t a n t s can be selected which have lost the ability to grow on this simple medium, most of them being deficient in a single enzymic activity present in the parental (wild-type) strain.2 1 This deficiency can be over
come (in 85-95 % of m u t a n t s )2 2 by the addition to the minimal medium of a single amino acid, vitamin, or purine-pyrimidine base, which is the growth factor whose synthesis is interrupted by the metabolic block brought about by the enzyme deficiency (see Fig. 1).
Such auxotrophic2 3 m u t a n t s m a y arise spontaneously, or m a y be induced by treatment with a mutagenic agent such as irradiation with ultraviolet light,2 4 which increases the over-all mutation rate 100-fold or more. Their frequency in populations is very low, however, and is often no more t h a n 1 in 105 even in irradiated cultures. The introduction of the penicillin screen
ing t e c h n i q u e2 5"2 6 greatly facilitated the selection of these m u t a n t s . This technique makes use of the fact t h a t penicillin is bactericidal only to growing cells. Incubation in minimal medium to which penicillin has been added thus destroys the prototrophic2* wild-type bacteria, but not the mutants, and so effectively enriches the proportion of auxotrophic cells in the culture to more t h a n 1 in 100. [More recent t e c h n i q u e s2 7 - 2 9 involve the use of chemical mutagens as, for example, ethyl methane sulfonate ( E M S ) ,3 0 which yield as high a proportion of auxotrophic m u t a n t s as 1 in 100 and thus obviate the necessity for penicillin enrichment.]
These cultures, containing about 1 % mutants, are then diluted and plated on a complete medium such as nutrient agar, to produce isolated clones, some of which will be auxotrophic. The identification of these clones is much simplified by the replica plating technique introduced by t h e Lederbergs.3 1 A print of the colonies on complete medium is t a k e n by pressing over the surface a sterile pad of material such as velvet, having a
"pile." This print can now be used to inoculate a plate of minimal medium and another of complete medium. Auxotrophic clones are recognized by their failure to grow on the minimal medium. These auxotrophic colonies are picked from the complete medium and inoculated to a template pat-
tern on a similar medium. After growth, a further replication to minimal media plates, supplemented with one of a variety of growth factors, permits the identification of the specific growth factor. As an intermediate step, the use of amino acid p o o l s2 0'3 2 reduces the number of operations and also preserves those m u t a n t s having more complex growth requirements (see next section).
3. TR A N S D U C T I O N I N Salmonella typhimurium
a. Techniques. This system of genetic transfer, first discovered by Zinder and Lederberg3 3 using the temperate phage P22 (see Chapter 2), was put to effective use in fine genetic analysis by Demerec and his collaborators working at the Laboratory of the Carnegie Institution of Washington's Department of Genetics at Cold Spring Harbor.
A large number of independent auxotrophic m u t a n t s responding to a single amino acid or purine were isolated and characterized by the Cold Spring Harbor School. The nomenclature used by Demerec,3 4 which has formed the basis for a suggested uniform notation within microbial genet
ics,3 5 is to designate each m u t a n t with a triletter symbol denoting the growth requirement, e.g., try for tryptophan, pro for proline requirement.
This is followed by a serial number depending merely on the order of isola
tion of the m u t a n t . Some auxotrophs were found to require more than a single supplement, of which most were found to result from a single muta
tion t h a t produced a metabolic block preceding the branching of the syn
thetic pathway into two or more directions.3 6 These mutants, which in general required two growth factors, were designated by a four-letter symbol (e.g., phty denotes a requirement for phenylalanine plus tyrosine) to differentiate them from m u t a n t s with a requirement for two growth factors as a result of two independent mutations (e.g., phe.tyr denotes a m u t a n t with a requirement for phenylalanine plus tyrosine derived by a second mutation from either a phe or a tyr m u t a n t ) .
Each class of m u t a n t was then further examined for its ability to utilise known precursors of the growth supplement, for cross-feeding or syn- t r o p h y ,3 6 and in some instances for the accumulation of such precursors in the culture as could be identified by paper chromatography. By these preliminary, rather crude biochemical tests, the probable metabolic block involved in each m u t a n t could be identified.3 7 - 4 4 The extension of these preliminary studies by more refined techniques involving the isolation and characterization of the associated enzymes has been effected in some i n s t a n c e s .4 5-4 6
By these methods, each group of m u t a n t s responding to a single growth factor was divided into subgroups, within which all m u t a n t s show the same response to various precursors of the growth factor, and the same
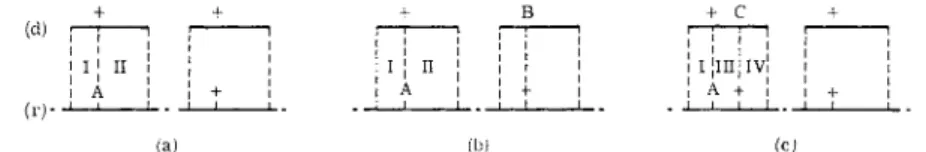
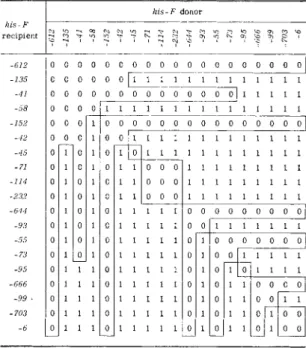
6. G E N E T I C F I N E STRUCTURE I N BACTERIA 259 accumulations and synthrophisms. These phenotypically identical m u t a n t s t h u s have identical biochemical blocks and were considered to arise by mutation in the same functional gene leading to inactivation of the same specific enzyme. To their designation could thus be added a letter denoting the phenotype, e.g., the tryptophan-requiring m u t a n t s were subdivided into four phenotypic groups try A, tryB, tryC, and tryD from the results of biochemical tests shown in Table I, conforming to the metabolic pathway of Fig. 1.
T A B L E I
RESPONSE OF TRYPTOPHANLESS AUXOTROPHS OF Salmonella typhimurium το INTER
MEDIATES OF THE TRYPTOPHAN PATHWAY AND THE R E S U L T S OF CROSS-FEEDING T E S T S "
Growth response to
Cross-feed mutants of group0
Phenotypic group Anthranilic acid&
Indole Tryptophan
Cross-feed mutants of group0
A
+ + +
N o n eΒ
- +
AC
- + +
Α βD
- - +
A,B,C° D a t a of B r e n n e r .3 7
b S u p p l e m e n t s a d d e d t o m i n i m a l agar a t 20 μ£./ιη1.; g r o w t h after 24 hr. a t 3 7 ° C .
c F r o m parallel s t r e a k i n g on m i n i m a l agar after 48 hr. a t 3 7 ° C .
Anthranilic acid
Indole - g l y c e r o l
phosphate
Indole - T R Y P T O P H A N
G r o u p : A M u t a n t s : t r y - 8
Β - 2 , - 4
C - 3
D - 1, - 6 , - 7 , - 9, - 1 0 , - 1 1
FIG. 1. D i a g r a m showing biochemical sequence in t r y p t o p h a n biosynthesis. A, B , C, and D indicate t h e positions of the biochemical blocks w i t h the associated m u t a n t s . After Brenner.3 7
The wild-type Salmonella and each of its auxotrophs are sensitive to the phage P22. Phage lysates containing about 101 1 particles per milliliter were prepared from cultures of each m u t a n t , which could then be concentrated by high-speed centrifugation and suspended in buffer. A cross of two strains
is effected by the use of one strain in the form of a bacterial culture (recipi
ent), which is infected with a bacteria-free phage preparation of the other strain (donor). By this means, the genetic specificity of the donor is intro
duced by the phage vector into the recipient cells. The infected cells are plated on minimal medium on which neither donor nor recipient, being auxotrophic, can grow, b u t on which cells arising from recombination to reconstitute the wild-type genome can be selected from the background growth of nonrecombinant cells.
When one considered the disparity in the amount of genetic material as D N A (see below) in a bacterial cell5 and in a P22 phage particle,4 7 it is obvious t h a t the bacterial D N A must be broken down into fragments less t h a n one-hundredth the size of the total chromosome in order to be accom
modated within a normal-sized phage particle. Thus, unless the mutations of donor and recipient are separated by less t h a n one-hundredth total m a p length, it is unlikely t h a t those fragments carrying the wild-type marker,
+ + + Β + c
! ! »
ι I 1 π Î
; \
! ι ; π j î ! ·
' i ΐ 1 !
î i j m j i v ! !
! Â !
I I 1 . ! + !
I I , . 1 .
! Α ι ! + ι
- I l ! .
! a + ! ! 1 1 1 I 1
(a) (b) (c)
FIG. 2 . D i a g r a m m a t i c representation of transduction of a recipient strain, A, b y phage grown o n ( a ) wild-type d o n o r ; ( b ) donor with unlinked marker B; and ( c ) donor with linked marker C; (r) represents parts of the recipient c h r o m o s o m e ; and ( d ) represents fragments of t h e donor c h r o m o s o m e .
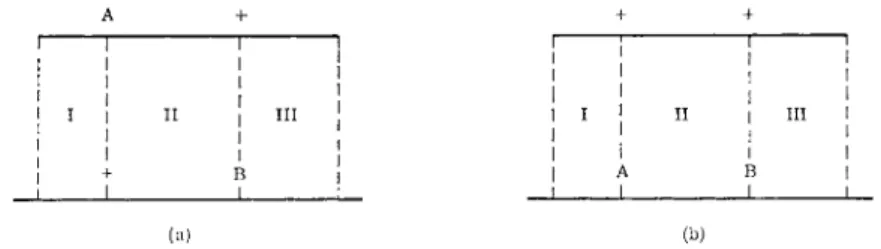
corresponding to the recipient mutation, will also carry the mutation of the donor (see Fig. 2b). Thus, the frequency of wild-type recombinants will be the same as when a wild-type donor is used (crossing over in regions I and I I , Fig. 2a and b). However, when the donor and recipient mutations are very closely linked so as to be carried on the same small fragment (Fig.
2c), the frequency of recombination will be reduced and will be proportional to the distance between the markers, since recombination is now propor
tional to the crossing over in regions I and I I I . The nearer the mutational sites (region I I I ) the smaller the probability of recombination. When the two sites are identical, or separated by a region within which exchange does not occur, there will be no recombination.
b. Preliminary Results. As was found by Zinder and Lederberg,3 3 auxo
trophic recipients crossed with a wild-type donor, or with donors of differ
ent phenotype, gave several hundred prototrophic clones per plate spread with about 108 infected cells. However, in nearly all crosses between strains of identical phenotype some prototrophic clones were produced, in numbers significantly greater t h a n those obtaining when uninfected re-
6 . G E N E T I C F I N E S T R U C T U R E I N BACTERIA 261 cipients, or recipients infected with phage grown on the same strain (ho
mologous crosses), were plated. Table I I shows typical early results1 5 ob
tained from crosses between seven cysB m u t a n t s and two m u t a n t s of a phenotypically similar, b u t nonidentical, group, cysD. T h e ability to produce prototrophic recombinants in a cross is taken to indicate genetic nonidentity of the mutations. I n cysB, the demonstration of a t least seven nonidentical mutations, all separable by recombination, makes unlikely one of the alternative hypotheses to account for similar pseudoalleles,1 3
T A B L E I I
N U M B E R S OF PROTOTROPHIC RECOMBINANT CLONES ARISING FROM PLATING VARIOUS CYSTEINELESS AUXOTROPHS OF Salmonella typhimurium AFTER INFECTION WITH
P 2 2 PHAGE PROPAGATED ON OTHER CYSTEINELESS AUXOTROPHS"
Donor (source of phage) Recipient6 cysB-
10 cysB-
12 cysB-
14
cysB- 15
cysB- 16
cysB- 18
cvsB- '24
cysD- 11
Wild- type (control)
cysB-10 0 4 7 4 2 6 5 2 6 2 9 2 9 1 1 5 7 1 1 1 5
cysB-12 2 8C 0 4 3 8 3 3 3 3 6 2 2 3 5 1 2 1 0 4
cysB-14 2 9 3 8 0 3 3 2 4 6 2 2 2 0 1 7 9 2
cysB-15 7 6 9 5 1 0 7 0 5 4 8 8 6 1 1 0 1 9 9 5 4
cysB-16 1 5 1 9 2 3 4 0 2 3 1 2 2 3 9 7 9
cysB-18 5 2 0 1 2 3 3 0 3 8 5 6 7 7 1
cysB-24 2 5 4 7 1 4 8 4 1 0 8 0 5 2 8 1 6 1 7
cysD-23 1 8 7 9 7 9 1 2 2 9 6 1 3 9 9 9 5 1 8 6 0 1 6 1 6 7 4 2 1 5 9 2
° D a t a of D e m e r e c et al.16
h O n e m i l l i l i t e r of a n o v e r n i g h t c u l t u r e of t h e r e c i p i e n t (ca. 1 09 c e l l s / m l . ) is i n f e c t e d w i t h P 2 2 p h a g e ( g r o w n on d o n o r ) a t a m u l t i p l i c i t y of 8 , a n d 0 . 1 - m l . s a m p l e s s p r e a d o v e r m i n i m a l a g a r .
c E a c h figure r e p r e s e n t s t h e s u m of t h e p r o t o t r o p h i c c l o n e s a p p e a r i n g o n a t o t a l of t h r e e p l a t e s a f t e r i n c u b a t i o n of 4 8 hr. a t 3 7 ° C .
namely, t h a t there are at least seven distinct genes within this region all concerned with cysteine biosynthesis. I n addition, the table shows t h a t the numbers of prototrophs produced by these cysB X cysB crosses are always less t h a n when a cysB m u t a n t is crossed with a cysD m u t a n t or with a wild-type donor. This reduction in recombination suggested t h a t these seven distinct mutations occurred very close to each other and were clustered within a very small region of the chromosome. The more likely interpretation made by Demerec et al.,15 therefore, was t h a t the functional unit of cysB extended over a segment of chromosome, rather t h a n having a point location, and t h a t the integrity of m a n y points on this segment
was necessary for cysB enzyme production and wild-type activity. At many sites on this segment, therefore, mutations could occur, each leading to loss of this integrity and loss of cysB enzyme activity, and thus the production of m u t a n t s with identical phenotype. Between these sites, however, recombination to reconstitute the genome as found in the wild- type strain was possible. For chromosomal regions such as t h a t responsible for the determination of the cysB enzyme, Demerec retained the term
"gene locus," the presence of m a n y sites of mutation within this structure leading to the term "complex gene locus," the various m u t a n t s being termed "nonidentical alleles" (cf. pseudoalleles, heteroalleles) ,4 8
c. Complex Gene Loci in Salmonella. This early work has been extended by analysis of well over a thousand independent m u t a n t s3 8 , 3 9•4 1 - 4 4 , 4 9"5 2 , 5 4 - 5 7
which have been allocated to over fifty phenotypic groups each containing at least two m u t a n t s (up to a maximum of over one hundred), as summa
rized in Table I I I . Crosses have been carried out between most members of each group, resulting in the majority of instances in the production of prototrophs, with a yield considerably less t h a n when m u t a n t s of non- identical phenotypic groups are crossed. The conclusion reached was t h a t
"non-identical allelism (complex structure of loci) is not a special feature of certain gene loci but a general property of a l l . "5 6
4. CO M P L E X GE N E LO C I I N Escherichia coli
Subsequent to the work of Demerec and his colleagues, many complex loci have been demonstrated in Escherichia coli. Study of these loci has in most instances been undertaken because the enzymes involved are readily isolated and manipulated, so t h a t correlated biochemical and genetic studies can be carried out. The details of some of the more characteristic of these systems are summarized in Table I I I and below.
a. Lac Loci and β-Galactosidase. Wild-type E. coli strain K12 can utilize a series of carbohydrates in addition to glucose, as a sole energy and carbon source, among these being the disaccharide lactose. Strains of K12 can be selected t h a t have lost the ability due to the mutation lac+ —* lac~. A series of these lac markers were found to be located in the same chromo
somal region.5 8
The transfer of genetic material from an Hfr donor to a recipient (F~) strain of E. coli, by means of conjugation (see Chapter 1 ) is a highly efficient process, so t h a t if an Hfr strain is chosen which transfers the lac region as an early marker, a large proportion of the recipient cells will receive this marker. Pardee et al.59' 6 0 have isolated large numbers of lac m u t a n t s in F ~ strains, as well as in Hfr strains. The lac loci can be shown as the result of transfers using HfrH to be located as in Fig. 3 , * the order of genes being thr-leu-pro-lac-ade-gal. Among these lac mutants, a group can
* See page 2 6 6 .
6. G E N E T I C F I N E STRUCTURE IN BACTERIA 263 be recognized in which the mutation has led to the loss of the enzyme β-galactosidase, responsible for the hydrolysis of lactose into the two hexoses glucose and galactose. These phenotypically identical lac m u t a n t s have been termed ζ m u t a n t s .5 9 6 1
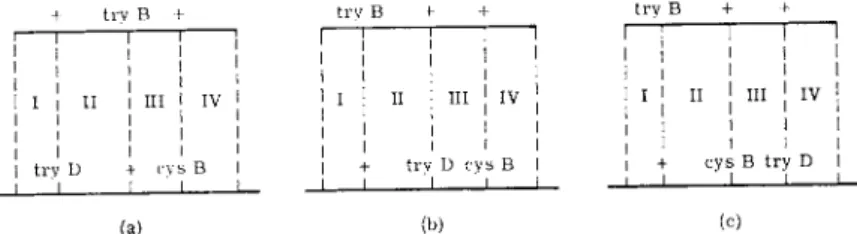
Crosses in which the Hfr and F~~ strains carry independent z~ mutations can be made, of the type Hfr z~l.ade+ .str-s. X F ~ ZB·ade~.str-r (Fig. 4a) and the reciprocal cross in which the ζ markers are reversed (Fig. 4b). I n both crosses, selection is made for ade+. str-r recombinants which are then scored for z+ and z~~ phenotype. The relative order of zA and zB can be concluded from frequencies of z+ recombinants in the reciprocal crosses;
if it is greater in the first cross (a) t h a n in the second cross (b) the order of the markers is zA-ZB-ade since, as shown in Fig. 4,* for z+. ade+. str-r re
combinants, crossovers would be necessary in regions I and I I I in the first cross (a), and in regions I, I I , and I I I in the second cross (b), t h u s requiring an additional crossover in region I I . As indicated in Fig. 3, the crossover frequency in this lac-ade region is 22 %, and so in general the frequency of z+ recombinants in one cross will be four to five times higher t h a n in the reciprocal. I n crosses of both types, the ratio of z+. ade+. str-r recombi
nants to ade+. str-r recombinants is a measure of crossing over within region I and thus a measure of the distance between two ζ mutations.
T h u s the relative order and the distances between a series of ζ m u t a n t sites can be made with accuracy. As can be seen from Fig. 3, a t least 38 independ
ent mutational sites have been recognized and mapped within the ζ locus as shown.6 2' 6 3
b. Gal Loci. The transduction of these loci by defective phage particles,
\dg (see Chapter 2), is yet another highly efficient process of genetic trans
fer. This transduction is restricted to a cluster of bacterial markers con
cerned solely with galactose fermentation, which can be located by standard Hfr X F ~ crosses a t a region very near to the site of the λ prophage locus on the K12 chromosome.5 8' 6 4· 6 5 F r o m the wild K12 strain, which is able to break down D-galactose to provide a source both of energy and of carbon, m u t a n t s which have lost this ability (gal~) can be recognized by plating on a nonselective medium, such as eosin methylene blue or tetrazolium medium, on which gal+ and gal~ clones are differentiated by color.2 0- 5 8
A recipient K12 strain transduced with \dg becomes diploid for this small region on which the gal markers are located, and is termed a synge- note (which m a y be either a heterogenote if the gal markers differ, or a homogenote if the gal marker of the fragment is genetically identical to t h a t of the recipient s t r a i n ) .6 6' 6 7
When a galZ recipient is infected with a high-frequency transducing ( H F T ) lysate from a gal+ strain, the heterogenote gal*/\.gal+ results (see Fig. 5). These heterogenotes (A) which are phenotypically gal+, segregate
* See page 266.
TABLE III COMPLEX GENE LOCI IN BACTERIA Biochemical pathway Sequence of biochemical steps τ Clustered loci, with gene order and numbers of mutants
Interallelic com plementation groups References* Histidine (his)^ Salmonella i G-E-A-H-F-B-C-B-D-D yphimurium E-F-A-H-B-C-D-G 13 37 25 2 34 35 61 11 E(-a-b-c-d) B(-a-b-c-d) D(a-b)
38, 46, 52, 53, 5 54a, 100, 113, 119 152 , 207 Tryptophan (try)t A-B-(C,D) A - Β - D - C 1 4 6 17 None 29 , 37 , 45 , 50 , 49, 5 Threonine (thr) (D,C)-A-B-E D - C - A - Β 6 5 14 16 None 43 Threonine (thr) (D,C)-A-B-E E(l) Isoleucine (He) and isoleucine plus valine (ilva) ileA-ilva (A ,D)-ilvaB-ilvaC He A -ilva A -UvaD-ilvaB-ilvaC 12 6 1 7 2 None 43 Leucine (leu) A A (107) 4 43 , 111 Cysteine (cys)§ C - D - Α - Β -E (?) C-D 107 84
C - D a-b-e-d-c 34 30 73 38 46
28, 40, 51 57 , 9Cysteine (cys)§ C - D - Α - Β -E (?) A (62) a - b - c 43 8 11
118
Cysteine (cys)§ C - D - Α - Β -E (?) cysB(26) - try(A-B-C-D)% c - b - a—(try) 4 18 4
15, 16
Cysteine (cys)§ C - D - Α - Β -E (?) Ε (7) a - d 4 3 Methionine (met) A - Β - C - (E,F) (?) A (15) Β (12)—F (A) C(10) Ε (fi)
44
264
Adenine (ade) and adenine plus thiamine (adth) adth (A ,C,D) -ade (C,E) -adeB adthA adthD adthC-adeC adeB adeE
39, 117 Proline (pro) D - (A,B) - C (A,B) - C D
42 Lactose (lac)\\ Escheri zhia coli i - ο - ζ - y ζ (2 Groups) 58, 58a, 59, 60, 62, 63, 112, 174, 175, 199 Galactose (gal)# k - t - e k-o-t or k-t-o-e or k-t-e-o None 64-69 , 154 , 173, 216- 218 , 219 Arabinose (ara)** Α - Β - D D- A - Β -0 78-87, 215 Alkaline phosphatase (P)tt Ρ 2 Groups 70, 71, 150, 169 , 167 , 178 , 178a , 181 , 182 , 209-211 Tryptophan (in/)§§ D - C - (A,B) D - C-A - Β 72-77, 110, 183 , 2 0 6 * General references 34' 35· 66>61 · 92> 115> 171 · 172> 195· 207 # Figure 5. + Tables IV and VII, Figs. 9 and 10. ** Figure 8. t Table I and Fig. 1. ft Table VII and Fig. 6. § Table II. §§ Table VII, Figs. 1 and 7. II Table VII, Fig. 3.
265
rare gal~ cells which can be shown to be of two genotypes; the haploid
gaiZ
(C) and homogenoticgalZ/\.gal~Z
(B). These homogenotic clones are recognized by their ability to producegal
+ papillae when replicated to alawn of
galy
bacteria, after irradiation and incubation, and are capable of transducing gaQ at high frequency.6 7 , 6 8 Thus when a phage lysate of agalZ/\.galZ
homogenote is applied to agaly
recipient (D), heterogenoticgaly/\.galZ
clones result. These clones m a y be initially eithergal
+ orgal".
l e u
_ J pro
I
t*-20 *J L -22 -^-Γ
Y
I I I I I I 1 I I I I I I I I I I I I I I 1 I I I I I I I I ο
I I I I I I I I I 0.70 -
Ï U U of
m
FIG. 3. G e n e t i c m a p of the lac region in E. coli K 1 2 . T h e upper line represents the location of the lac region w i t h respect t o other linked markers. T h e lower line repre- sents an e n l a r g e m e n t of t h e linked lac loci showing t h e t w o structural g e n e s ζ and y, the operator region o, and the regulator g e n e i. R e c o m b i n a t i o n frequencies are s h o w n b e l o w each line. After J a c o b and M o n o d .6 2 , 6 3
H f r H f r
(a) (b)
F I G . 4. D i a g r a m m a t i c r e p r e s e n t a t i o n of crosses b e t w e e n t w o lac s t r a i n s of E. coli K 1 2 . (a) s h o w s t h e cross of H f r z~.ade^.str-s X F~~ z~.ade~.str-r; (b) s h o w s t h e re
ciprocal cross H f r z~.ade+.str-s X F ~ z~K.ade~.str-r. T h e u p p e r line r e p r e s e n t s a p a r t of t h e H f r c h r o m o s o m e a n d t h e l o w e r line t h e c o r r e s p o n d i n g p a r t of t h e F- c h r o m o s o m e . T h e r e l a t i v e order of m a r k e r s is a s s u m e d to b e ZA-ZB-ade-str.
The gal+ clones result from complementation (see p . 298) when the two gal~ mutations involve different functions, so t h a t the heterogenote pro
duced (galy .gait/\.galy .gal* o r —h / H— ) has a wild (gal+) phenotype (E). Gal~ clones are found when the two gal~ mutations involve the same function since here there is no complementation (F).6 8 , 6 9 When these gai~
heterogenotes are further incubated, gaV" papillae arise from recombination events leading to the production of the two m-heterogenotes, G ( / + + ) and H ( + + / ) (both of which are phenotypically gal+) from the original frans-heterogenote —h/H—· The process of crossing over is assumed to be due to mitotic recombination within the small diploid
6 . G E N E T I C F I N E STRUCTURE I N BACTERIA 267 region. Morse has used this recombination as a measure of the distance between the two gal~~ mutations, and by this means has mapped 17 muta
tions arising in three adjacent gal loci.6 9
galx/λ galx Horn oge note
cis Heterogenote F I G . 5. D i a g r a m m a t i c r e p r e s e n t a t i o n of c o m p l e m e n t a t i o n a n d r e c o m b i n a t i o n w i t h i n t h e gal region of E. coli K 1 2 s t r a i n s i n f e c t e d w i t h d e f e c t i v e \dg p h a g e . P h e n o t y p i c a l l y gal+ cells are c r o s s h a t c h e d , p h e n o t y p i c a l l y gal~ cells are u n h a t c h e d . On t h e l e f t , t h e h e t e r o g e n o t e ( A ) , formed b y i n f e c t i o n of a gaïZ r e c i p i e n t w i t h p h a g e f r o m t h e U V i r r a d i a t i o n of a gal+ cell l y s o g e n i c for λ, is a l l o w e d t o s e g r e g a t e . A m o n g t h e s e g r e g a n t s , t h e rare^aZ" h o m o g e n o t e s (B) are i s o l a t e d and HFTgal* l y s a t e s p r e p a r e d b y U V i n d u c t i o n , p r o d u c i n g equal n u m b e r s of a c t i v e n o n t r a n s d u c i n g p h a g e ( w a v y line w i t h O ) a n d d e f e c t i v e t r a n s d u c i n g p h a g e ( w a v y l i n e w i t h # ) . C o m p l e m e n t a t i o n is i n v e s t i g a t e d b y i n f e c t i n g n o n i d e n t i c a l galy r e c i p i e n t s ( D ) w i t h t h i s H F T X . If t h e t w o gal~ m u t a t i o n s (x and y) i n v o l v e different f u n c t i o n a l u n i t s , t h e n t h e ^rtms-hetero- g e n o t e s f o r m e d will be p h e n o t y p i c a l l y gal+ ( E ) . If, h o w e v e r , different f u n c t i o n a l u n i t s are i n v o l v e d , t h e i r a n s - h e t e r o g e n o t e s will be gal~ ( F ) . T h e s e h e t e r o g e n o t e s will u n d e r g o m i t o t i c c r o s s i n g o v e r , s o m e of t h e p r o d u c t s b e i n g t h e p h e n o t y p i c a l l y gal+
c i s - h e t e r o g e n o t e s (G a n d H ) w h i c h s h o w u p as gal+ p a p i l l a e o n further i n c u b a t i o n . T h e f r e q u e n c y w i t h w h i c h t h e s e arise is a m e a s u r e of t h e d i s t a n c e b e t w e e n t h e t w o gal~ m u t a t i o n a l s i t e s (see L e d e r b e r g6 8 a n d M o r s e6 9) .
c. Alkaline Phosphatase (P) Locus. T h e enzyme, alkaline phosphatase, present in E. coli K12, can be detected by plating bacterial cultures for single colonies on a medium of low phosphate concentration and spraying with the substrate p-nitrophenyl phosphate ( N P P ) which turns yellow
ROYSTON C. CLOWES
when dephosphorylated by this enzyme.7 0 Wild-type ( P+) colonies t u r n yellow within a few seconds of spraying, whereas m u t a n t s having lost this activity (P~~ mutants) remain white. Garen et αϊ.70·71 have isolated P~
m u t a n t s by this method after UV irradiation and have subjected t h e m to an intensive biochemical and genetic scrutiny.
Most m u t a n t s were stable and were crossed by conjugation in all com
binations. A cross of each pair of m u t a n t s (e.g., Hfr Cavalli thr+ .leu+ .PJ str-s X F~" thr". leu~~.PT.str-r) was compared in each case with the recipro
cal cross (Hfr Ρ2 X F ~ P 7 ] and t h e two control crosses in which b o t h Hfr and F - strains carried either P 7 or P 7 (Hfr P 7 X F ~ P 7 ; Hfr PI X F ~ P 7 ) , to evaluate the level of protrophic P+ colonies due to reversion rather t h a n
thr leu
-J L_ Ρ LAC
I
• 9 MIN 20 MIN
X1JL3 U19
» X"TJ18 I ULL |E1 U17 U7 U25 E2 ^ U12 U3 E3 U24\
—I 1 L
+
0.2
FIG. 6. G e n e t i c m a p of t h e alkaline phosphatase ( P ) g e n e . T h e upper line represents t h e part of t h e c h r o m o s o m e transferred in t h e first 20 m i n u t e s at 37°C. b y Hfr Cavalli starting at t h e origin, 0 , w i t h t h e relative order of markers measured b y t i m i n g of interrupted m a t i n g s as indicated i m m e d i a t e l y b e l o w . T h e lower line repre
sents an enlargement of t h e Ρ region showing the relative order of P" m u t a t i o n s fixed b y frequency of P+ recombinants in crosses of t w o P" m u t a n t s . T h e b o t t o m line shows recombination frequency b e t w e e n the t w o extreme P" markers (see A. G a r e n7 0 for d e t a i l s ) .
recombination. T h e parental cultures were mixed, and plated after 90 minutes on minimal medium containing streptomycin in which glycero
phosphate was the sole source of phosphorus, the only cells capable of growth on this medium being those of genotype thr+ .leu+.P+ .str-r. As a control, the same parental mixtures were plated on standard minimal medium containing streptomycin on which thr+.leu+.P~ .str-r cells are selected, so t h a t the percentage of P+ recombinants can be expressed as a fraction of the thr+. leu+ recombinants.
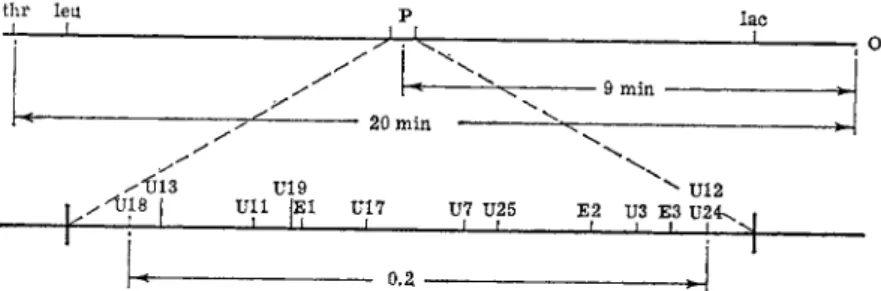
The location and order of the various P~ mutations can therefore be made in a way entirely analogous to the mapping of lac.z" m u t a n t s . A hundred different P~ m u t a n t s tested in this way were in most instances shown to result from mutations a t distinct sites, closely linked in a linear array. A preliminary m a p of some 13 mutational sites is shown in Fig. 6 .7 0·7 1
d. Transduction of the Tryptophan Synthetase Locus. A large number of
6 . G E N E T I C F I N E S T R U C T U R E I N BACTERIA 269 tryptophanless auxotrophs of E. coli K12 have been studied genetically using transduction with P I phage.7 2 The final step in tryptophan biosynthe
sis in E. coli has been shown to be controlled by the enzyme, t r y p t o p h a n synthetase. T h e step is complex and appears to involve the three reactions:
( 1 ) I n d o l e 4- L-serine L- t r y p t o p h a n
(2) I n d o l e glycerol p h o s p h a t e ^± indole + triose p h o s p h a t e
( 3 ) I n d o l e glycerol p h o s p h a t e + L-serine -> L- t r y p t o p h a n + triose p h o s p h a t e
of which the latter reaction appears to be the most important physiologi
cally.7 2 Among the tryptophanless auxotrophs studied, a group of pheno- typically similar m u t a n t s can be isolated which are unable to perform any one of these three reactions and are thus deficient in tryptophan synthetase.
On isolation, this enzyme was found to separate on chromatographic columns into two stable protein components, A and B, neither of which independently had more t h a n 2 to 3 % normal activity in any of the three component reactions, b u t when combined were found to reconstitute the normal level of activity. A large proportion of the m u t a n t s were found to have only one of these protein components inactivated.7 2 A genetic analysis of m u t a n t s has been m a d e7 3 - 7 7 using P I transduction (Chapter 2). This system of "general" transduction is essentially similar to the Salmonella- P22 system, the crosses being carried out by infecting one m u t a n t with P I phage grown on others, and selecting for tryptophan-independent recombi
nants on minimal medium. The more recent crosses have concentrated on m u t a n t s within the A region. Most of the crosses involve doubly auxo- tropic try χ . his~ recipients and tryptophanless donors £n/y .7 2>7 3 Since the try and his markers are not linked closely enough to be carried on the same transducing fragment,7 6 the measurement of the ratio try+/his+ trans
ductants gives a measure of recombination within the try region, with an internal correction for such various experimental variations as the efficien
cies of the donors and recipients, which can normally produce extensive day-to-day experimental fluctuations. T h e frequencies of recombination between two try markers are thus expressed as a proportion of the trans
duction for the his marker, and a value of 5 % was found as the maximum between two A m u t a n t s of the try region.7 2-7 3 However, since the wild-type his+ marker is found to be transduced with only half the efficiency of the try+ marker, a more accurate value for recombination within the try region was obtained by the ratio ^ try+/his+, the maximum value found between two try markers in the A region being now corrected to 2.5 % .7 4 , 7 7 The minimum values were found to be limited by reversions of try to try+. Thus, for mapping closely linked clusters of markers, triple auxotrophs tryZ · cys~~. his"74 were used (the cys and try markers being linked and carried on the same transducing fragment). Using a try y strain as donor, the re-
cipients were plated on minimal media containing histidine (selecting for try+. cys+ transductions) and on minimal medium plus histidine and trypto
phan (selecting cys+ transductants). The recombination between try^ and tryy is now found from the ratio try+. cys+/ cys+. All reversions tryZ to try+ will still be cys~ and thus not interfere with the ratio, while contaminants are not likely to require histidine. A sensitive selection method is thus en
sured.7 4
(69,77,88) (83,91) j
3 51
I I
23,24,27,28,35,36 53
Π Γ
13 14 22 44 64
89 85 75 34
JL
90 94 78 58
76 61 46
1
Γ 10 30 32 49 52 1555 16 82 17 56 44
87 97 1 104
0.004%
-2.5% -
FIG. 7. G e n e t i c m a p of the t r y p t o p h a n s y n t h e t a s e A and Β regions in Escherichia coli. T h e h e a v y horizontal line represents a part of c h r o m o s o m e in t h e try region. M u tants in the Β region form normal A protein, m u t a n t s in t h e A region form normal Β protein. M u t a n t s listed a b o v e t h e m a p form an altered Α - C R M (or B - C R M ) (cross- reacting material) protein, whereas those b e l o w are unable t o form either an A or a Β protein. T h e m u t a n t s are clustered in groups b e t w e e n which there is less t h a n 0.1%
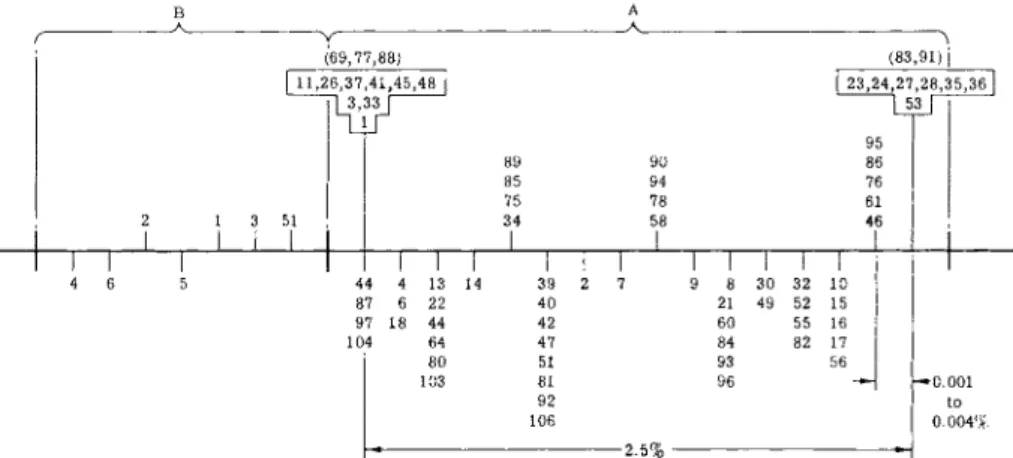
recombination ( b e t w e e n 25 and 100 n u c l e o t i d e s ) . Other m u t a n t s s h o w n in b o x e s do n o t show a n y recombination (less t h a n 0.0002%) consistent with a location less than one or a few n u c l e o t i d e s apart. M u t a n t s s h o w n at the same levels w i t h i n t h e b o x e s form proteins identical in stability t o heat or t o acid precipitation. T h e recombination frequencies are s h o w n on t h e lower lines. F r o m C. Y a n o f s k y et al.72'75'77
I t has been shown t h a t most of the crosses yield try~ cells in low yields, consistent with a clustering of the mutational sites of the auxotrophs.
Crosses of two m u t a n t s in which the A function is affected give rise to less recombinants t h a n A Χ Β crosses, as do Β Χ Β crosses. The values for recombination are roughly additive, and led to the location of Β m u t a n t s to one side and A m u t a n t s to the other side of the locus as shown in Fig.
7/72-74, 77
e. Ara Loci in Escherichia coli B. A series of L-arabinose nonfermenting m u t a n t s (arar), obtained from the wild (ara+) strain of E. coli Β have been isolated on eosin-methylene blue medium in an analogous way to the