Clinical Study
Methylchloroisothiazolinone/Methylisothiazolinone and Methylisothiazolinone Sensitivity in Hungary
Györgyi Pónyai, Ilona Németh, and Erzsébet Temesvári
Department of Dermatology, Venereology and Dermatooncology, Semmelweis University, Budapest 1085, Hungary
Correspondence should be addressed to Gy¨orgyi P´onyai; gyorgyi.ponyai@gmail.com Received 3 November 2015; Revised 25 January 2016; Accepted 8 February 2016 Academic Editor: Franz Trautinger
Copyright © 2016 Gy¨orgyi P´onyai et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background. Due to allowing of methylisothiazolinone (MI) in cosmetics, cleaning products, and paints, an epidemic of MI- hypersensitivity emerged. Patch testing Kathon CG(3:1 mixture of methylchloroisothiazolinone and methylisothiazolinone, MCI/MI) does not correctly detect MI contact allergy, due to the low concentration of MI in the test material. Methods. A retrospective survey was performed to estimate the prevalence of MCI/MI hypersensitivity in 14693 patients tested consecutively between 1993 and 2014. Moreover, currently 314 patients were prospectively tested with the allergens MCI/MI and with MI during one year.Results. MCI/MI hypersensitivity increased retrospectively from 0.5% to 6.0%. By current prospective testing we detected 25 patients (8%) with MCI/MI and/or MI positive reactions. Out of the 25 patients 10 were only MCI/MI positive, 9 were only MI positive, and 6 were MCI/MI and MI positive. If MI had not been tested separately, MI contact allergy would have missed in 36% of all detected cases and in 2.8% of the total 314 patients.Conclusions. The frequency of MCI/MI hypersensitivity is increasing also in Hungary. We confirm that, in order to detect MI contact allergy, it needs to be tested separately. A further increase of MI hypersensitivity might be expected in the future as products containing MI are still widely available.
1. Introduction
Due to the introduction of Kathon CG (MCI/MI) in the midseventies a worldwide epidemic of contact allergy to it emerged. This preservative became widely used in cosmetics and household cleaning products because of its efficacy. Con- tact allergy to MCI/MI—mostly provoked by cosmetics—was first reported by de Groot et al. [1]. Frosch and Schulze-Dirks [2] estimated the sensitization rate between 0.4% and 11.1%—
with a mean of 3.0%—in their European multicentre study [3] which was similar to our own data (3.4%) observed in the same period [4].
At the beginning of the new millenium MI was allowed as a separate preservative in industrial products (paints, glues) and initially gained attention as an occupational allergen, and it still is nowadays [5]. Due to its use in cosmetics, a new and unprecedented epidemic arose in Europe, in the USA, and in Asia [6–10].
Unfortunately, MCI/MI tested in routine patch test series does not correctly detect MI hypersensitivity, because of the low concentration of MI in the mixture. So, MCI/MI
testing in itself did not detect a quite high percent of the MI allergy [9]. Uter et al. already suggested in 2012 the routine separate testing of MI and including it into the standard patch test series [11]. Recently, the recommended MI patch test concentration became 2000 ppm (or 0.2% aqua) [12–14]. We here present our experience with MCI/MI and MI contact allergy and the data in Hungary.
2. Material and Methods
(1) We retrospectively reviewed the prevalence of MCI/MI hypersensitivity (European Baseline series Brial Allergen GmbH, Germany, chamber: Curatest) in 14693 patients tested consecutively from 1993 until 2014 at the Allergy Out- patient Unit of the Department of Dermatology, Venereology and Dermatooncology of the Semmelweis University.
(2) Moreover, 314 patients were prospectively tested consecutively between February 1st, 2014, and January 30th, 2015, with the standard allergens MCI/MI 0.01% aqua and MI 0.2% aqua (Chemotechnique Diagnostics, Vellinge, Sweden, chamber:IQ Chambers). We performed parallel testing with
Volume 2016, Article ID 4579071, 5 pages http://dx.doi.org/10.1155/2016/4579071
the European Baseline series (AllergEAZE, Brial Allergen GmbH, Germany, chamber: Curatest including the Brial MCI/MI/0.01% aqua/contact allergen), as well. As we had not tested MI separated in routine patch series before, comparative data concerning the past years have not been available.
The occlusion time by testing was 48 h; the allergens were applied on the back. Evaluation of the test was performed at the 60th minute of the occlusion and then on D2, D3, D4, and D7. Reactions were taken as positive 1+ or more intense.
3. Results
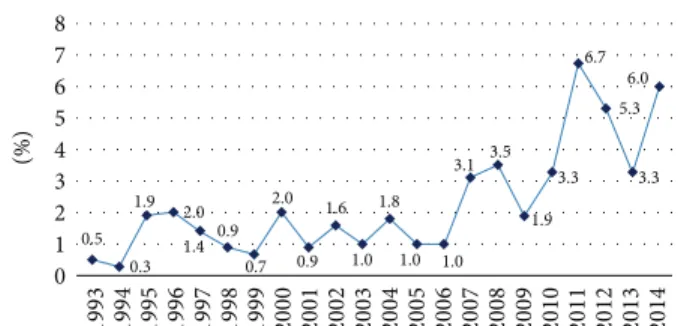
3.1. MCI/MI Hypersensitivity 1993–2014. Assessing the preva- lence of MCI/MI hypersensitivity in our 14693 patients tested consecutively between 1993 and 2014, we detected waver- ing percent rates (MCI/MI hypersensitive patients/tested patients in the year). Starting from a value of 0.5% (1993:
5/1011 patients), it reached 6.7% (2011: 27/401 patients), 5.3%
(2012: 22/413 patients), 3.3% (2013: 13/390 patients), and 6.0%
(2014: 23/383 patients) (Table 1, Figure 1).
3.2. Testing with MCI/MI (0.01%Aqua) and MI (0.2%Aqua) and Parallel Testing with the Standard Brial Baseline Series (Including MCI/MI Allergen) between February 1st, 2014, and January 30th, 2015. The mean age of the 314 tested patients was 48.9 years (range: 13–88 years). There were 79 men with a mean age of 50.6 years (range: 18–88 years) and 235 women with a mean age of 48.4 years (range: 13–88 years).
Regarding the patch test containing MCI/MI, we did not find any differences in the results between theBrialand the Chemotechniqueallergens.
We detected 25 patients (8%) with MCI/MI and/or MI positivity: 17 women and 8 men. MCI/MI hypersensitivity was detected in 16 cases (5.1%) and MI hypersensitivity in 15 cases (4.8%). Regarding parallel positivities: out of the 25 patients 10 were only MCI/MI positive, 9 were only MI positive, and 6 were concurrently MCI/MI and MI positive.
Thus, MI positivity without MCI/MI positivity was found in 36% of these or in 2.8% of the whole tested population of 314 patients. Among the MI sensitized patients the mean age was 39 years (Tables 2(a), 2(b), and 2(c)). Regarding local- isation of contact dermatitis, we observed by MI sensitive patients skin symptoms first of all on the hands, the face, and the scalp. Most typical sources of the allergens were liquid soaps, baths, hair shampoos, hand and face creams, and wet cleansing wipes—mostly rinse-off products.
According to occupational dermatitis, we identified only four patients: two patients (hairdresser and washer-up) with only MI hypersensitivity, one patient (anaesthetist assistant) with both MCI/MI and MI hypersensitivity, and one patient with only MCI/MI hypersensitivity (maid).
Associated contact allergies were detected with, for exam- ple, fragrance mix I, fragrance mix II, propylene glycol, nickel sulfate, and paraphenylenediamine (PPD) in 18 patients.
There were 3 patients in the MCI/MI and MI sensitive group and 4 patient in the MI sensitive group without associated contact hypersensitivities (Tables 2(a), 2(b), and 2(c)).
Table 1: MCI/MI hypersensitivity 1993–2014.
Year Tested patients/year MCI/MI hypersensitive
patients/year %
1993 1011 5 0.5
1994 636 2 0.3
1995 839 16 1.9
1996 1099 22 2.0
1997 938 13 1.4
1998 802 7 0.9
1999 834 6 0.7
2000 797 16 2.0
2001 799 7 0.9
2002 698 11 1.6
2003 701 7 1
2004 670 12 1.8
2005 637 7 1
2006 612 6 1
2007 538 17 3.1
2008 514 18 3.5
2009 512 10 1.9
2010 480 16 3.3
2011 401 27 6.7
2012 413 22 5.3
2013 390 13 3.3
2014 383 23 6.0
8 76 5 4 32 1 0
1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014
(%)
0.3 1.9 2.0
0.9 0.7
2.0
0.9 1.6
1.0 1.0 1.0 1.9
3.3 5.3 6.7 6.0
3.1 3.5
1.8 3.3
0.5 1.4
Figure 1: MCI/MI hypersensitivity data in percent (MCI/MI hypersensitive patients/tested patients in the year) at the Allergy Outpatient Unit of the Department of Dermatology, Venereology and Dermatooncology of the Semmelweis University 1993–2014 (𝑛 = 14693).
4. Discussion
In 1987 methylisothiazolinone was considered to be a weak sensitizer in the animal experiments of Bruze et al. [15].
By allowing much higher concentrations use than before an unprecedented allergy epidemic occurred and still occurs worldwide. Among the problematic MI-containing products, cosmetics have been in a leading position since 2005, as the concentration of MI was authorized in both leave-on and rinse-off products up to 100 ppm [16]. Hair care products even proved to be one of the most problematic ones [6, 7, 9].
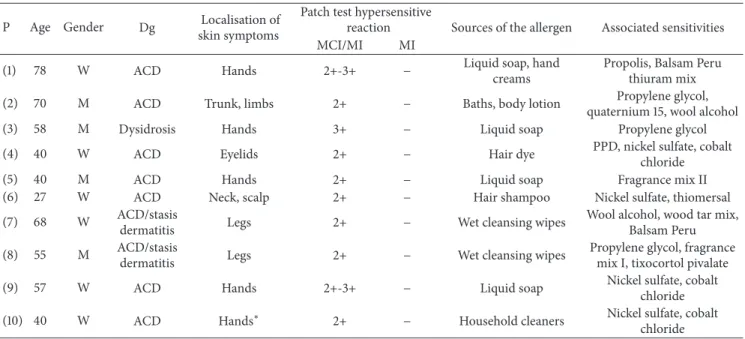
Table 2: Localisation of clinical symptoms and associated sensitivities of MCI/MI and/or MI hypersensitive patients (total tested patients, 𝑛 = 314).
(a) MCI/MI hypersensitive patients: localisation of clinical symptoms and associated sensitivities (𝑛 = 10)
P Age Gender Dg Localisation of
skin symptoms
Patch test hypersensitive
reaction Sources of the allergen Associated sensitivities
MCI/MI MI
(1) 78 W ACD Hands 2+-3+ − Liquid soap, hand
creams
Propolis, Balsam Peru thiuram mix
(2) 70 M ACD Trunk, limbs 2+ − Baths, body lotion Propylene glycol,
quaternium 15, wool alcohol
(3) 58 M Dysidrosis Hands 3+ − Liquid soap Propylene glycol
(4) 40 W ACD Eyelids 2+ − Hair dye PPD, nickel sulfate, cobalt
chloride
(5) 40 M ACD Hands 2+ − Liquid soap Fragrance mix II
(6) 27 W ACD Neck, scalp 2+ − Hair shampoo Nickel sulfate, thiomersal
(7) 68 W ACD/stasis
dermatitis Legs 2+ − Wet cleansing wipes Wool alcohol, wood tar mix,
Balsam Peru
(8) 55 M ACD/stasis
dermatitis Legs 2+ − Wet cleansing wipes Propylene glycol, fragrance
mix I, tixocortol pivalate
(9) 57 W ACD Hands 2+-3+ − Liquid soap Nickel sulfate, cobalt
chloride
(10) 40 W ACD Hands∗ 2+ − Household cleaners Nickel sulfate, cobalt
chloride W: woman, M: man, P: patient, Dg: diagnosis, and ACD: allergic contact dermatitis.
∗Maid,−: negative.
(b) MCI/MI and MI hypersensitive patients: localisation of clinical symptoms and associated sensitivities (𝑛 = 6) P Age Gender Dg Localisation of
skin symptoms
Patch test hypersensitive reaction
Sources of the allergen Associated sensitivities
MCI/MI MI
(1) 25 W ACD Hands 3+ 2+-3+ Wet cleansing wipes, hand creams −
(2) 17 W ACD Hands 2+ 1+-2+ Hand creams −
(3) 56 W ACD Hands, legs 2+ 2+ Baths, dish washing liquids Fragrance mix I,
propylene glycol
(4) 43 W ACD Hands∗ 3+ 2+-3+ Liquid soaps Nickel sulfate
(5) 54 W ACD Eyelids 2+ 2+ Facial cleansing wipes, hair shampoo Nickel sulfate
(6) 16 W ACD Trunk and
limbs 1+-2+ 1+-2+ Baths, body lotion −
W: woman, M: man, P: patient, Dg: diagnosis, and ACD: allergic contact dermatitis.
∗Anaesthetist assistant,−: negative.
(c) MI hypersensitive patients: localisation of clinical symptoms and associated sensitivities (𝑛 = 9) P Age Gender Dg Localisation of
skin symptoms
Patch test hypersensitive
reaction Sources of the allergen Associated sensitivities
MCI/MI MI
(1) 16 W ACD Scalp − 2+ Hair dye −
(2) 61 M ACD Hands − 2+-3+ Liquid soaps −
(3) 66 W ACD Eyelids − 2+ Hair shampoo, face
creams Wood tar mix, tixocortol pivalate
(4) 32 M ACD Hands − 2+ Hair shampoo, baths −
(5) 21 W ACD Scalp − 2+ Hair shampoo, hair dye PPD
(6) 47 M ACD Hands∗ − 3+ Hair shampoo PPD, propylene glycol, Balsam
Peru, budesonide
(7) 50 W ACD Face − 1+-2+ Face creams Fragrance mix I, fragrance mix II
(8) 42 W ACD Hands∗∗ − 2+-3+ Dish washing liquids,
hand creams
Potassium dichromate, nickel sulfate, wood tar mix, thiuram mix, fragrance mix I, fragrance
mix II
(9) 40 M ACD Hands − 2+ Liquid soaps −
W: woman, M: man, P: patient, Dg: diagnosis, and ACD: allergic contact dermatitis.
∗Hairdresser,∗∗washer-up, and−: negative.
Apart from the high concentration of MI used, the increase of MI sensitization can also be explained by the fact that the number of cosmetics containing this preservative (baby care products, baths, make-up, hair, nail, skin care, and sun protection products) has doubled in the USA between 2007 and 2010. Castanedo-Tardana even nominated MI as the “Allergen of the Year 2013” [7]. MI is in the focus of allergology in our days as well, because of further increasing of contact sensitization, caused by leave-on and by rinse- off cosmetic products [12–17]. The widespread use of MI in several products and cumulative exposures to MI may also be responsible for the high percent of sensitization to it.
According to a recent study focusing on contact sensitization in patients with suspected cosmetic intolerance, MI was by far the leading allergen provoking contact sensitization among preservatives [9, 17].
Another recent study examined whether the allowed concentrations of MI in cosmetic rinse-off products have the potential to cause allergic contact dermatitis. According to the results, the rinse-off products with 50 ppm MI or more are not safe for the consumers [18].
In our large study population tested between 1993 and 2014 the prevalence of MCI/MI hypersensitivity gave waver- ing percent rates, but we detected an increasing rate from the beginning to the endpoint. In this process presumably the MI component of the allergen played an important role [14, 19].
Moreover, we started prospective MI patch testing sepa- rately as a routine examination and followed the test results for one year. The 4.8% prevalence of MI hypersensitivity is, though high, may be considered as rather moderate compared to other European data [11, 16–22]. Among the MI sensitized patients the mean age was 39 years. Regarding clinical symptoms, we observed contact dermatitis first of all on the hands, the face, and the scalp [14, 23–25]. The sources of the allergens were mostly rinse-off products (liquid soaps, baths, and hair shampoos).
Interestingly, MI contact allergy without MCI/MI posi- tivity was found in 36% among the patients with positive test reactions to MCI/MI, MCI/MI and MI, and only MI and in 2.8% of the total tested 314 patients. These patients would have been missed if MI had not been tested separately.
In conclusion, MCI/MI and MI contact allergy is a hot topic and an ongoing problem also in Hungary. Despite the restrictions, further increase of MI hypersensitivity may also be expected in the near future as products containing MI are still available widely. The new results worldwide support recommendations for a review of the regulations relating to MCI/MI and/or MI in cosmetics and household products [10, 14, 17, 18].
Ethical Approval
The work has been approved by the ethical committees.
Consent
All subjects gave an informed consent.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors’ Contribution
Gy¨orgyi P´onyai, M.D., Ph.D., was responsible for testing patients and collecting, analysis, and interpretation of data, conception, and design. Ilona N´emeth was responsible for testing patients, collecting and analysis of data, and design.
Erzs´ebet Temesv´ari, M.D., Ph.D., was responsible for testing patients, supervising the survey, and revising the paper critically for intellectual content.
References
[1] A. C. de Groot, D. H. Liem, and J. W. Weyland, “Kathon CG: cosmetic allergy and patch test sensitization,” Contact Dermatitis, vol. 12, no. 2, pp. 76–80, 1985.
[2] P. J. Frosch and A. Schulze-Dirks, “Contact allergy to Kathon CG,”Hautarzt, vol. 38, no. 7, pp. 422–425, 1987.
[3] P. J. Frosch, A. Lahti, M. Hannuksela et al., “Chloromethylisoth- iazolone/methylisothiazolone (CMI/MI) use test with a sham- poo on patch-test-positive subjects. Results of a multicentre double-blind crossover trial,”Contact Dermatitis, vol. 32, no. 4, pp. 210–217, 1995.
[4] ´A. Jurcsik and E. Temesv´ari, “Testing contact sensitization of Kathon CG,”B˝orgy´ogy´aszati ´es Venerol´ogiai Szemle, vol. 66, pp.
9–12, 1990 (Hungarian).
[5] M. Isaksson, B. Gruvberger, and M. Bruze, “Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide,”Dermatitis, vol. 15, no. 4, pp. 201–205, 2004.
[6] M. D. Lundov, T. Krongaard, T. L. Menn´e, and J. D. Johansen,
“Methylisothiazolinone contact allergy: a review,”British Jour- nal of Dermatology, vol. 165, no. 6, pp. 1178–1182, 2011.
[7] M. P. Castanedo-Tardana and K. A. Zug, “Methylisothiazoli- none,”Dermatitis, vol. 24, no. 1, pp. 2–6, 2013.
[8] E. Yim, K. L. Baquerizo Nole, and A. Tosti, “Contact dermatitis caused by preservatives,”Dermatitis, vol. 25, no. 5, pp. 215–231, 2014.
[9] P. Puangpet, A. Chawarung, and J. P. McFadden, “Methylchlo- roisothiazolinone/methylisothiazolinone and methylisothia- zolinone allergy,”Dermatitis, vol. 26, no. 2, pp. 99–102, 2015.
[10] The Scientific Committee on Consumer Safety, “Opinion on Methylisothiazolinone (P94) Submission II. (Sensitisation only),” The SCCS Adopted this Opinion at its 4th Plenary Meeting on 12 December 2013, http://ec.europa.eu/health/scie- ntific committees/consumer safety/docs/sccs o 145.pdf.
[11] W. Uter, W. Aberer, J. C. Armario-Hita et al., “Current patch test results with the European baseline series and extensions to it from the ’European Surveillance System on Contact Allergy’
network, 2007-2008,”Contact Dermatitis, vol. 67, no. 1, pp. 9–19, 2012.
[12] M. Isaksson, K. E. Andersen, M. Gonc¸alo et al., “Multicentre patch testing with methylisothiazolinone by the European Envi- ronmental and Contact Dermatitis Research Group,”Contact dermatitis, vol. 70, no. 5, pp. 317–320, 2014.
[13] K. Ham, C. J. Posso-De Los Rios, and M. Gooderham, “Methyl- isothiazolinone Testing at 2000 ppm,”Dermatitis, vol. 26, no. 4, pp. 166–169, 2015.
[14] F. Latheef and S. M. Wilkinson, “Methylisothiasolinone out- break in the European Union,”Current Opinion in Allergy and Clinical Immunology, vol. 15, pp. 461–466, 2015.
[15] M. Bruze, S. Fregert, B. Gruvberger, and K. Persson, “Contact allergy to the active ingredients of Kathon CG in the guinea pig,”
Acta Dermato-Venereologica, vol. 67, no. 4, pp. 315–320, 1987.
[16] R. Urwin and M. Wilkinson, “Methylchloroisothiazolinone and methylisothiazolinone contact allergy: a new ‘epidemic’,”
Contact Dermatitis, vol. 68, no. 4, pp. 253–255, 2013.
[17] A. Dinkloh, M. Worm, J. Geier, A. Schnuch, and A. Wollenberg,
“Contact sensitization in patients with suspected cosmetic intolerance: results of the IVDK 2006–2011,” Journal of the European Academy of Dermatology and Venereology, vol. 29, pp.
1071–1081, 2015.
[18] K. Yazar, M. Lundov, A. Faurschou et al., “Methylisothiazoli- none in rinse-off products causes allergic contact dermatitis: a repeated open-application study,”British Journal of Dermatol- ogy, vol. 173, no. 1, pp. 115–122, 2015.
[19] J. Geier, H. Lessmann, A. Schnuch, and W. Uter, “Recent increase in allergic reactions to methylchloroisothiazolinone/
methylisothiazolinone: is methylisothiazolinone the culprit?”
Contact Dermatitis, vol. 67, no. 6, pp. 334–341, 2012.
[20] O. Aerts, M. Baeck, L. Constandt et al., “The dramatic increase in the rate of methylisothiazolinone contact allergy in Belgium:
a multicentre study,”Contact Dermatitis, vol. 71, no. 1, pp. 41–48, 2014.
[21] G. A. Johnston, “The rise in prevalence of contact allergy to methylisothiazolinone in the British Isles,”Contact Dermatitis, vol. 70, no. 4, pp. 238–240, 2014.
[22] M. Engfeldt, J. Br˚ared-Christensson, M. Isaksson et al.,
“Swedish experiences from patch testing methylisothiazolinone separately,”Acta Dermato Venereologica, vol. 95, no. 6, pp. 717–
719, 2015.
[23] S. H. Yu, A. Sood, and J. S. Taylor, “Patch testing for meth- ylisothiazolinone and methylchloroisothiazolinone-methyliso- thiazolinone contact allergy,”JAMA Dermatology, vol. 152, no.
1, pp. 67–72, 2016.
[24] O. Aerts, A. Goossens, and F. Giordano-Labadie, “Contact allergy caused by methylisothiazolinone: the Belgian-French experience,”European Journal of Dermatology, vol. 25, pp. 228–
233, 2015.
[25] N. G¨abelein- Wissing, P. Lehmann, and S. C. Hofmann, “Aller- gic contact eczema to long-used cosmetic: methylisothiazoli- non, a type IV- allergen,”Hautarzt, vol. 66, no. 6, pp. 462–464, 2015.
Submit your manuscripts at http://www.hindawi.com
Stem Cells International
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
INFLAMMATION
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Behavioural Neurology
Endocrinology
International Journal ofHindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Disease Markers
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
BioMed
Research International
Oncology
Journal ofHindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Oxidative Medicine and Cellular Longevity
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
PPAR Research The Scientific World Journal
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Immunology Research
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Journal of
Obesity
Journal ofHindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Computational and Mathematical Methods in Medicine
Ophthalmology
Journal ofHindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Diabetes Research
Journal ofHindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Research and Treatment
AIDS
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Gastroenterology Research and Practice
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2014
Parkinson’s Disease
Evidence-Based Complementary and Alternative Medicine
Volume 2014 Hindawi Publishing Corporation
http://www.hindawi.com