ContentslistsavailableatScienceDirect
Process Safety and Environmental Protection
jo u r n al ho m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / p s e p
What do we know already about reactor runaway? – A review
Alex Kummer
∗, Tamás Varga
InstituteofChemicalandProcessEngineering,UniversityofPannonia,H-8200Veszprém,Hungary
a rt i c l e i nf o
Articlehistory:
Received6July2020
Receivedinrevisedform28August2020 Accepted25September2020
Availableonline1October2020
Keywords:
Runawayprevention Thermalsafety Earlywarningsystem Processsafety Thermalrisk
Safetyboundarydiagram
a b s t ra c t
Nowadays,reactorrunawayisstillacrucialphenomenfromthesafetyviewpoint.About120scientific journalarticlesarepublishedeveryyearinthelastdecadeinwhichthermalrunawayisakeyword.The possiblecauseandconsequencesofreactorrunawayareadressedwheretheworstcaseistheexplosion ofthereactor.Preventionstepstoavoidthedevelopmentofthermalrunawayincludetheappropriate designofthereactor,theoperationstrategyandanearlywarningdetectionsystem.Theavailableassess- mentmethodsforthermalriskanalysisareaddressedindetail.Reactorrunawaycriteriacanindicate earlythethermalrunaway,whichcriteriaareaddressedinthisreviewindetailunderthreeclasses:
geometry-,sensitivity-,andstability-basedrunawaycriteria.Operationstrategyofsemi-batchreactors canbedesignedbycalculatingWesterterp-diagramwhoseevolutionisclearypresented.Significant worksonthefieldofthereactordesign,operationandreactorsafetyarecollectedandevaluated.Finally possiblefurtherresearchareasaresuggestedtoimproveourknowledgeaboutthermalsafety,suchas investigatingparameteruncertaintyinrunawayindicationoroptimizethesafetyactionstomoderate theconsequencesofrunaway.
©2020TheAuthor(s).PublishedbyElsevierB.V.onbehalfofInstitutionofChemicalEngineers.Thisis anopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/
4.0/).
Contents
1. Introduction...461
2. Causeandconsequenceofthermalrunaway...463
3. Preventionofreactorrunaway...463
4. Methodstoevaluatethermalrisks...464
5. Reactorrunawaycriteria...465
5.1. Mathematicalmodel...465
5.2. Stability-basedcriteria...466
5.2.1. Semenov-criterion...466
5.2.2. VanHeerdenand“practicaldesign”criterion...467
5.2.3. Gilles-Hoffmanncriterion ... 467
5.2.4. Lyapunov-stabilityingeometric-andphase-plane...467
5.2.5. Strozzi-Zaldivarcriterion(Divergencecriterion)...467
5.2.6. Modifieddynamicandslopecondition...468
5.3. Geometry-basedcriteria...468
5.3.1. ThomasandBowescriterion...468
5.3.2. AdlerandEnigcriterion...468
5.3.3. VanWelsenaereandFromentcriterion(orMaxicriterion)...468
5.3.4. Adiabaticcriterion...469
5.4. Sensitivityanalysisofchemicalreactors(Morbidelli-Varmacriterion)...469
5.5. Data-basedpredictionofthermalrunaway...469
∗Correspondingauthor.
E-mailaddress:kummera@fmt.uni-pannon.hu(A.Kummer).
https://doi.org/10.1016/j.psep.2020.09.059
0957-5820/©2020TheAuthor(s).PublishedbyElsevierB.V.onbehalfofInstitutionofChemicalEngineers.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
6. Safetyboundarydiagrams...470
7. Safetyequipment/actionstomoderateseriousconsequences...471
8. Applicationexamplesofrunawaycriteria...472
8.1. Comparisonofreactorrunawaycriteria ... 472
8.2. Reactoroperationdesign...472
8.3. Processcontrol...472
8.4. Runawaypredictionandinhibition ... 473
9. Futuredirections...473
Acknowledgement...473
References...473
Nomenclature
A heattransferarea C concentration cp heatcapacity Da Damköhlernumber E activationenergy Ex exothermicitynumber I penaltyindices
ITHI inherentthermalrunawayhazardindex MF materialfactor
MTSR MaximumTemperatureofSynthesisReaction qgen generatedheat
qrem removedheat
P pressure
Pr probability r reactionrate R gasconstant RI riskindex Ry reactivitynumber
S severity
tdos dosingtime
TMRad TimetoMaximumRateunderadiabaticconditions Tc criticalorcoolingtemperature
Tp;T processtemperature Tta targettemperature Tw walltemperature
U overallheattransfercoefficient
V volume
Wt Westerterpnumber Xac accumulatedreagent Tad adiabatictemperaturerise Hr reactionheat
␣ UA/(Vcp)
 Hr/(cp)
␥ ineticparameter
␦ E/R
Semenovnumber
relativevolumeincrease
density
J Jacobianmatrix I identitymatrix
1. Introduction
Safetyassessmentisalwaysacrucialpointinchemicalplant design and operationdue to thecomplexity of modern, highly integrated plants,anditrequiresdeepknowledgeofallprocess unitsandalltheinteractionsbetweenthem.Itisnecessarytohave informationabouteveryimportantphysicalandphysic-chemical propertiesofeverycomponentwhichoccursorcanoccurinthe
system(Vernières-Hassimietal.,2017).Alsoprocesssafetyreg- ulationshave been gettingstricter in recent decades,and they covereveryprocessunitandsteponeverylevelinmodernchemi- caltechnology.Theseincreasingrequirementsfromprocesssafety systemtriggeredsignificantprogressin processsafety manage- mentthatmakespossibletoavoidunnecessaryeventsinnowadays fullyintegrated technologieswhichoperateina hecticbusiness environment,whichrequiremoreflexibletechnologiesthanever.
However,duetoevolutionarychangesintheindustry,newhaz- ardouseventsoccur,whicharemorerelatedtoorganization,safety culture,and lackof knowledge and awareness(Knegtering and Pasman,2009).
Itiswell-knownthatcertainoperatingconditions,socertain valuesoftheparameterscancausethesystembecomereallysen- sitivetovaluesoftheinitialoroperationparameters.Insensitive regionofthesystem,verysmallchangeininitialconditionleads fullydifferenttrajectorieswithrespecttopressure,temperature, concentrations,etc.Itismoreinterestingifanexothermicreaction iscarriedout,whererunawaycanoccurasaresultofsmallchanges.
Thermalreactorrunawaysarecharacterizedbyarapidincreasein thetemperatureandpressureduetocontinuouslyincreasingrate ofheatgeneration.Therateofheatgenerationincreasesexponen- tiallywiththetemperature,contrarilytheremovedheatincreases onlylinearlywithit.Theriskofthermalrunawayoccursisactu- allytheriskoflosingthecontrolofchemicalreactionswhichtake placeinthesystem(e.g.triggeringarunawayreaction).Areaction runawaymayhavemultipleconsequenceswheretheworstcaseis theexplosionofreactor(Stoessel,2008).
Duringthermalrunawayssomeofthecomponentscanvaporize ordecompositioncanoccurduetotheelevatedtemperature,which increasesthepressureintheprocessunit(Pasmanetal.,1992).In worstcaseitleadstoaBoilingLiquidExpandingVaporExplosion (BLEVE).Ifthepressureincreasingrateishigherthanthedischarge rate,thereactorwillexplodeduetothehighpressure(Liuetal., 2018a).In lesscatastrophic casespreventionofdevelopmentof thermalrunawayshouldbeavoidedbecauseso-calledhot-spots causeearlydeactivationofcatalystand/orqualitydrop.Hence,the determinationofstableoperatingregimesofareactorisacrucial stepinprocessdesignandoperation(Varga,2009).From1995to 200412%ofBLEVEtypeaccidentsoccurredduetorunawayreac- tions,alsofrom1926to20046BLEVEtypeaccidentoccurredled to19deathand171injuredpeople(AbbasiandAbbasi,2007).
Knowledge aboutthe phenomenon of thermal runaway has improvedalotlately,butregretfullythatknowledgeisnotfully integratedintothepractice,anditcausessomeseriousfailuresand processmalfunctionsnowadays.Thermalrunawayisresponsible for26.5%ofthepetrochemicalaccidents(Balasubramanianand Louvar,2002),andreactorrunawaywasresponsiblefor25%ofthe accidentsinFrenchindustry(Dakkouneetal.,2018).Therewere manylethalornon-lethalaccidentsduetothermalrunawayinthe recentpast.TheSeveso-disasterin1976istheprimeexampleof theimportanceofknowingparticularlythephenomenonofther- malrunaway.Inthisdisasteratoxiccloudwasreleasedintothe 461
Fig.1.ExplosionofFu-Kaochemicalplantandthedamagednearbybuildings.Theshockwavedestroyedmanywindowswithinhalf-a-kilometer(KaoandHu,2002).
atmospherethrougharupturediskpoisoningalmost37,000peo- ple(CardilloandGirelli,1981;Fabianoetal.,2017;Jainetal.,2017).
In1990,inStanlowa15m3batchreactoratShellplantproducing 2,4-difluoro-anilinehadarunawayreactionleadingtoanexplo- sion,wheretheentireplantwasdestroyed(Cates,1992),(Mannan, 2014).In1996arunawayreactionoccurredinabatchreactorcre- atinghighpressurethatledtoruptureofthevessel(Partington and Waldram, 2002).In 1997,Ohio,anexplosionoccurredina resinsproductionunit,whereoneworkerdiedandfouremploy- eesinjured(UnitedStates,1999).In1998,inNewJerseyaviolent explosionandfireoccurredduetoareactorrunawayinjuringnine employees(GyenesandCarson,2017).In2001adestructiveexplo- sionoccurredinanacrylicresin manufacturingplantinTaiwan at the Fu-Kao Chemical Plant as a result of runaway reaction.
A batchreactorcarrying outpolymerizationreactionsexploded wheremorethan100peoplewereinjuredandonepersondied.
Thecatastrophicexplosiondestroyedthenearbyplantsanddam- agedthenearbybuildings,whichisshowninFig.1(KaoandHu, 2002).
InJanuary2006anacrylicpolymerbatchreactorexplodeddue tothisphenomenon(GyenesandCarson,2017).In2007areactor explodedanddestroyedinT2LaboratoriesinFloridabecauseofa thermalrunawayreactionleadtothedeathoffouremployees(Hall, 2010).In2008,USA,atBayerCropsciencepesticidemanufacturing unitathermalrunawaycausedanexplosionwhichdemolishedthe processunitleadingtotwolethaldamageandeightpeopleinhaled toxicchemicals(Abbasietal.,2010).Hydrogenperoxideisawidely usedchemical,buttheexothermicdecompositionofthischemical causedsomefireandexplosionaccidentsintherecentpast(Wuand Qian,2018).In2012,anexplosionoccurredinachemicalplantin Japaninjuring36personandkillingonepersonduetotherunaway polymerizationofacrylicacid(Fujitaetal.,2019).
Now it is clearthat wehave todeal withthermal runaway toavoidmoreorlesscatastrophicincidents,andwemust“learn from historyor you’redoomedtorepeatit”(the quoteis from
JesseVentura).Thefirstaspectisalwaysthesafety,whichcanbe realizedthroughstudyingthephenomenonofthermal runaway in detail. Our goalwith this review is toemphasize that engi- neersshouldneverforgetaboutthatthesafetyhasmuchhigher prioritythanincomedespitethefrequencyofaccidentsinchem- icalprocessesare decreasing.Especially onthefield of thermal safety,wheretheignoranceandtheirresponsibilitycanresultin seriousand unfortunately,sometimeslethalconsequences.This articleprovidesthemaincontributionswhichshouldbeknown byeveryprocessengineer.Besidethewell-designedreactor,an appropriate,reliableandearlywarningdetectionsystemshould bedevelopedforsafereactoroperation.Ifitisdone,wehaveto preparethesystemandoperatorsforemergencycases,sowemust designappropriatesafetyactionstomoderatetheconsequences ofthermalrunaway.Basedontheliteraturereviewwehighlight fourfutureresearchdirectionswhichisabouttoinvestigatethe impactofparameteruncertaintyonrunawayindication,handling parameteruncertaintyindetectionofrunawayduringoperation, presentingindetailthedesignphaseswithlaboratoryandpilot- plantexperiments,andperformingcomputationalfluiddynamics (CFD)simulationsforbetterunderstandingofthecausesandcon- sequences.
Thereviewwasmadetogiveacomprehensivepictureaboutthe phenomenonofthermalrunaway,whatthemaincausesandconse- quencesofrunawayare,andhowitcanbeprevented.Theemphasis isclearlyonthedevelopmentandapplicationofthermalrunaway criteriaincludinggeometry-,stability-andsensitivitybasedcrite- ria,andwealsodiscussthetopicofsafetyboundarydiagramsfrom Westerterp.
Theroadmapisasfollows:Section2providestherootcauses andconsequencesofthermalrunaway.Section3informsthereader aboutthebasicrequirementsforthepreventionofreactorrunaway.
InSection4thereadercangetinformationabouthowtoevaluate thethermal risks of a system.Section 5presents thestability- ,geometricandsensitivitybasedrunaway criteria,anda simple
modeltoinvestigaterunawaycriteriaispresented.Section6gives informationabouttheSafetyBoundaryDiagramsandsomeinsight abouthowtoapplythese.Section7providesinformationabout possiblesafetyactionstomoderatetheconsequencesofreactor runaway. InSection8someapplicationexamplesishighlighted fromtheliteraturewithrunawayrelatedresearchesinvestigating themainproblems(reactorandoperationdesign,reactorcontrol, mitigationsystems).Section9providesinsightintothepossible futuredirectionsofreactorrunawayrelatedresearch.
2. Causeandconsequenceofthermalrunaway
Thesafeoperabilityofchemicalreactorsishighlydependenton theappropriatedesignofsafetyaswellascontrolsystemsoftech- nologies.BartonandNolaninvestigatedcasehistories(169cases) from1962to1987.Basedontheirreviewthermalrunawayacci- dentsoccurduetothefollowingcauses(BartonandNolan,1991;
NolanandBarton,1987).
-a basic lack of understanding of the process chemistry and thermochemistry(e.g.noappreciationoftheheat ofreaction, unintendedreactionsandautocatalysisoccurred,productmix- turedecomposed,lowmaterialquality,etc.);
-inadequateengineeringdesignforheattransfer;
-inadequatecontrolsystemsandsafetyback-upsystems(e.g.loss ofcoolingwaterwhichwasnotmonitored,wronglypositioned probeoftemperaturemeasurement,thermocouplescoatedresult inslowresponse,etc.);
-inadequate operationalprocedures and operator training (e.g.
starting the reactorat low process temperature, mischarging ofreactants,inadequatemixing,poorcommunicationbetween operators,etc.).
RimSaadaetal.studiedthirtycasesfrom1988till2013,andthey alsoclassifiedthepossiblecausesthatleadtoarunawaysituation.
Theclassificationconsistsof“TechnicalandPhysicalCauses”and
“HumanandOrganisationalCauses”.Undertechnicalandphysical causesfivecaseswereduetomischargingthereactor.Thisincludes chargingchemicalsorcatalystsininappropriateorderandaddi- tionofincorrectamountofchemicals.Fourcaseshavebeencaused duetoagitatorfailures.Insomecasestracequantitiesofimpuri- tiescausedrunawayphenomena.Fourincidentsoccurreddueto poorplantdesign,andfiveothercaseswerecausedasaresultof wrong processcontrol.Under humanandorganisationalcauses thirteenincidentswereduetooperatorerrors.Operatorsdonot understandthebasicsofchemistryandthermodynamics,andin somecasestheoperatorsdecideontheirownwithoutdiscussing itwiththetechnicaladvisor.Inonecasethereactorwasoperated outsidethesafetylimits.Inadequatetraining,absenceofsupervi- sion,anincreaseinworkload,failuretofollowstandardoperating proceduresandincorrectopening/closingofthevalvesresultedin incidentstoo.Poormanagementinprocessoperationalsoresulted in11incidentsintheinvestigatedtime.Basedontheirsystematic evaluation,twenty-onepeoplediedand393peopleinjureddirectly duetothermalrunaway.Theirresearchindicatedthatlessonshave notbeenlearntfromtheconsequencesofthermalrunaways(Saada etal.,2015).Differentcasestudiesaboutreactorrunawayaccidents withcausesandconsequences isshownin(Gyenesand Carson, 2017;Etchells,1997;Pasquet,2017;Hoetal.,1998).
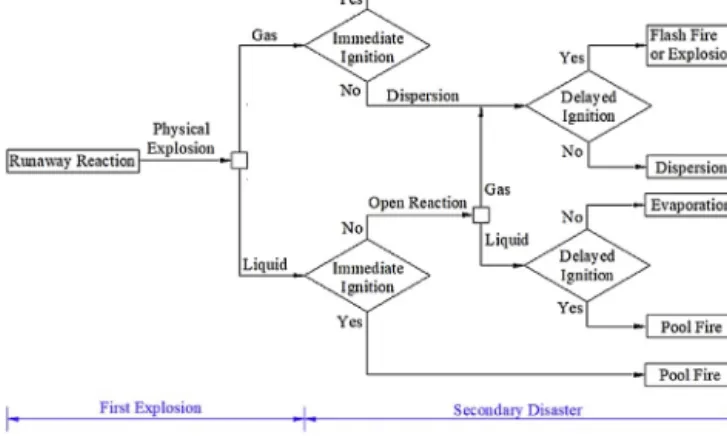
In a betterscenario theconsequenceofa runawayis onlya lowqualityproduct;inaworsecasethereactorphysicallyexplode resultinareleaseoflargequantitiesofflammable,toxicandhaz- ardousmaterials.Liuetal.showedaflowchartofrunawayaccident sequencesshowninFig.2(Liuetal.,2018b).
Fig.2.Flowchartofrunawayaccidentsequences(Liuetal.,2018b).
Ifthegasphasewithhighconcentrationisignitedimmediately afireballoccurs,otherwise,itspreadsaroundthereactor.Thegas phasewilldiffuseanddilutemayresultinavapourcloudexplo- sionorformingapotentialtoxiccloud.Iftheliquidphaseisignited immediatelyapoolfireoccurs,otherwise,thereactantsmaycon- tinuethereaction.Theresidualliquidphasemayignitesandresult inapoolfireoritformsaspirationhazard(Liuetal.,2018b).The sizeofendangeredareacanbeeasilyestimatedbasedonCFDsim- ulations(Liuetal.,2018a),(Tauseefetal.,2011;Chenetal.,2019).
3. Preventionofreactorrunaway
Preventionofreactorrunawaybeginsinthedesignphase.As itisshowninSection2adetailedknowledgeaboutthechemicals anditsthermophysicalpropertiesisnecessaryforsafeoperation.
Detailedkineticinformationaboutthepossiblereactionsisneces- saryfortheappropriatedesignofthereactor.However,wemust calculatewithplant-modelmismatch,wenevercanbeconfident withthatthedevelopedmodelisadequateinnon-runawayand especiallyinrunawaysituations.Firstphaseofpreventionisthe appropriatedesignofthereactorsystemandoperatingconditions.
Engineersmustperforminherentlysaferdesign(ISD),whichis abouttopreventhumanerrorandinvalidationoffacilitytoreduce theriskofaprocessbywaysofminimizing,substituting,moderat- ingandsimplifying.Fourclassesismentionedasstrategestoward ISD(Feietal.,2018):
-Inherent:Eliminatingthehazardbyusingmaterialsandprocess conditionswhicharenon-hazardous.
-Passive:Eliminatingorminimizingthehazardbyprocess and equipmentdesignfeatureswhichreduceeitherthefrequencyor consequenceofthehazardwithouttheactivefunctioningofany device.
-Active:Usingcontrols, safetyinterlocks,emergencyshutdown systems,mitigationdevicestodetectpotentiallyhazardouspro- cessdeviationsandtotakecorrectiveactions.
-Procedural:Usingoperatingprocedures,administrativechecks, emergencyresponse,andothermanagementapproachestopre- ventincidents,ortominimizetheeffectsofanaccident.
Apartfrom theofflineinvestigations, alsoonline prevention measuresarenecessarytodetectanyunexpectedsituationlead- ingtoarunawayscenario.Anearlywarningdetectionsystemis indispensabletodetectunexpecteddangeroussituations.Online applicable thermal runaway criteria are excellent soft-sensors, whichcanpredictthedevelopmentofthermalrunawayandthecri- teriaareabletodistinguishbetweendangerousandnon-dangerous reactorstates.Therefore,arobustsafetycriterionisanessential
elementofanyEarlyWarningDetectionSystem(EWDS).EWDSis necessarytodetectandevaluateunexpecteddangeroussituations.
Wemustprovidesufficienttimeforaprotectionsystemortheplant operatortoperformthenecessarystepstostoportomoderatethe undesiredeffectsofruanwaydevelopment.Thereareseveraltime indiceswhichcanbeappliedtomeasurehowfarthesystemfrom arunawaystateis.Agoodreviewaboutthesetimeindicescanbe foundin(Varga,2009).Theseindicesare:
-Timeofoccurrence:thetimewhenfaultoccurs.
-Reactiontime:theminimumtimerequiredtoexecutearesponse step
-Executiontime:measuredexecutiontimeofthesystem -Responsetime:thetimebetweenthedetectionofinitiatingevent
andtheresponseofthesystem.
-Safetyreactiontime:thetimeneededtosenseaproblemand initiateasafetyshutdowntothecontrolelement.
-Time-in-alarm: the time between timestamps of alarm and return-to-normalevents.
-Irreducible minimum: the minimal time of response, usually approximately100ms.
-ProcessSafetyTime(PST):PSTistheperiodoftimeinwhichthe processcanbeoperatedwithoutprotectionandwithoutunde- siredeventoccurs.VargaandAbonyiintroducedhowPSTcanbe determinedincaseofhighleexothermicreactionsin(Vargaand Abonyi,2010)
-Timeof noReturn: afterthistime itisimpossibletocoolthe reactor(Stoessel,2008).
Thesafetystepstomoderatetheconsequencesofrunawaycan beanopeninga pressurereliefvalve,fullcoolingorquenching (i.e.,additionofinhibitororcoldinertliquidaswellasdumpingof thereactorcontentintoacoldcatchtank)(WesterterpandMolga, 2006).
4. Methodstoevaluatethermalrisks
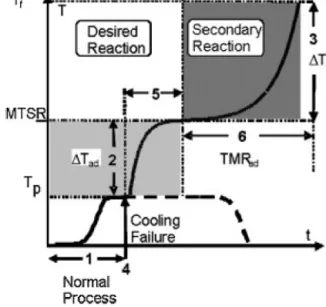
Thegoalisalwaystoreducethermalrisks,forwhichwehave toanswersomequestions.Ifwearepreparedfortheworst-case scenario thenheavyconsequences canbeprevented.Therefore, a systematicassessment procedureisbased onthecoolingfail- urescenarioassumingadiabaticconditions.Inadiabaticcasethe processtemperaturecanrisetothehighest.Basedonthecharacter- istictemperaturelevelsarisingfromthescenario,criticalityclasses weredefinedby(Stoessel(2008).Therepresentationofworst-case scenarioasacoolingfailurewereintroducedbyGygax(1988),and hemadeascenarioforthermalassessment,whichcanbeseenin Fig.3.
In (Nanchenet al.,2009)a gooddescriptionofFig.3canbe found.The processis attemperature Tp when a coolingfailure occurs. Since the reaction is exothermic, in adiabatic case, the presence ofunreactedreagentswillreactincreasingthereactor temperaturewiththeadiabatictemperaturerise(Tad).Themost crucialtimeforacoolingfailureiswhentheaccumulationofunre- actedreagentisatmaximum.MaximumTemperatureofSynthesis Reaction(MTSR)isintroducedfordescribingthepossiblereactor temperaturesduringtheoperation.AtMTSRsecondaryreactions mightbetriggered,andthesecondaryreactionwillincreasefurther toafinaltemperature(Tf).Thedurationofreactionrunawaycan beestimatedbycalculatingtheTimetoMaximumRateadiabatic parameter(TMRad).
MTSRcanbecalculatedbasedonthedegreeofaccumulation ofunconvertedreagentsandtheadiabatictemperatureriseatthe giveninstant.
MTSR=Tp+XacTad,rx (1)
Fig.3. Runawayscenario,wherenumbersrepresentthesixkeyquestions(Stoessel, 2009).
Table1
Assessmentcriteriafortheseverityofarunawayreaction(Stoessel,2009).
Severity Tad P Extension
Catastrophic >400 >Ptest >Site
Critical 200–400 Pmax<P<Ptest Site
Low 50–200 Pset<P<Pmax Plant
Negligible <50 P<Pset Equipment
TMRadcanbecalculatedbasedonthefollowingformulausing theinitialheatreleaserateofthereaction.
TMRad=cpRT2
qgenE (2)
Gygaxformulatedsixkeyquestionswhichhelpsfortheassess- mentofthermalrisk,whichwererefinedforeasierunderstanding (Nanchenetal.,2009).Thekeyquestionsarethefollowing:
1Whatistheheatevolutionrateasafunctionoftimeoftheoper- atingprocesstobecopedwithbytheoperationalequipment?So cantheprocesstemperaturebecontrolledbythecoolingsystem?
2Whattemperaturecanbereachedwhenthedesiredprocessruns away,assumingadiabaticconditionsforacoolingfailure?
3Whattemperature canbeattained after runaway of thesec- ondaryreaction?
4Whichisthemostcriticalinstantforacoolingfailure?Soatwhich timedoesthecoolingfailurehavetheworstconsequences?
5Howfastistherunawayofthedesiredreaction?
6HowfastistherunawayofthedecompositionstartingatMTSR?
ForthermalriskassessmentStoesselproposedaquantitative methodfordescribingtheseverityandprobabilityoftherunaway, whicharedescribedinTable1andinTable2.Fordefiningtheprob- abilityofrunawayanextended Tablecanbefoundin(Stoessel, 2009).
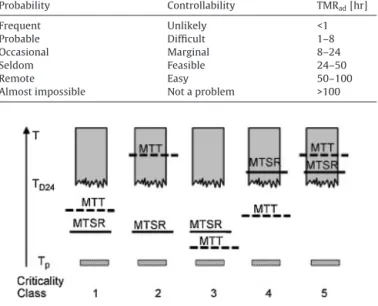
InadditionStoesselformulated5criticalityclassesbasedonthe relativeorderoffourspecifictemperaturelevels,rangingfromthe leastcritical(1–2)tothemostcritical(3–5)presentedin
Fig.4(Stoesseletal.,1997).Thefourspecifictemperaturelevels arethefollowing:
-Theprocesstemperature(Tp):theinitialtemperatureinthecool- ingfailure;
Table2
Assessmentcriteriafortheprobabilityoflossofcontrolofarunawayreaction (Stoessel,2009).
Probability Controllability TMRad[hr]
Frequent Unlikely <1
Probable Difficult 1–8
Occasional Marginal 8–24
Seldom Feasible 24–50
Remote Easy 50–100
Almostimpossible Notaproblem >100
Fig.4. Criticalityclassesofscenario(Stoessel,2009).
-Maximumtemperatureofsynthesisreaction(MTSR):itdepends onthedefreeofaccumulationofunconvertedreactants;
-TemperatureatwhichTMRadis24h(TD24):itisthehighesttem- peratureatwhichthethermalstabilityofthereactionmassis unproblematic;
-Maximumtemperaturefortechnicalreasons(MTT):itcanbea boilingpointinanopensystem,oritcanbeatemperatureatthe maximumpermissiblepressureinaclosedsystem.
Thecriticalityclassificationisausefultoolfortheriskassess- ment and also for the choice and definition of adequate risk reducing measures.InClass1and Class2 thelossofcontrol of themainreactiondoesnottriggersecondary reactionsandalso thetechnicallimitisnotreached.InClass3thetechnicallimitis reachedandmayserveasasafetybarrier,butthesecondaryreac- tionsarenottriggered.InClass4thesecondaryreactionscouldbe triggered,butthetechnicallimitmayserveasabarrier.InClass 5thesecondaryreactionsaretriggeredandthetechnicallimitis reachedastherunawayistoofastforasafetybarriertobeefficient (Stoessel,2009).
Junchengetal.improvedandappliedtheearliermentionedclas- sifications,andtheydevelopedinherentthermalrunawayhazard index(ITHI),whichiscalculatedbymultiplyingthematerialfactor (MF)andriskindex(RI)(Junchengetal.,2020).
ITHI=MF·RI (3)
Riskindexiscalculatedbasedontheseverityofrunawayreac- tionandtheprobabilityoftherunawayreaction.
RI=S·Pr (4)
Materialfactor(MF)iscalculatedbasedontheinitialreaction temperature(Tonset),andMaxpowerdensity(MPD),whereMFis limitedin(Vernières-Hassimietal.,2017;KnegteringandPasman, 2009).MPDisthefunctionofheatofdecompositionandthemax- imumreactionrate.
MF=1+ITonset·IMPD
16 (5)
whereITonset,IMPDarepenaltyindexes.Severityandprobabilityof runawayreactionsaredeterminedbasedonquantitativeintervals basedondifferentpenaltyparameters,whichparameterscanbe foundin(Junchengetal.,2020).
Fig.5. ModifiedStoesselcriticalitydiagram(Jiangetal.,2019).
Jiangetal.developedamodifiedStoesselcriticalitydiagramto considerthefinaltemperature(Tf)oftheprocess.Theirthoughtis basedonthatifthefinaltemperaturedoesnotexceedthetechni- callimit(MTT)thenthetechnicalsafeguardcanreducetheaccident risk.Basedonittheyextendedthecriticalityclasses1and3,crit- icalityclasses2,4and5remainedthesameasStoesselpresented (Jiangetal.,2019).Fig.5presentsthemodifiedStoesselcriticality diagram.
Inthefirstcaseofcriticalityclass1thereactiontemperature willnotreachthetechnicallimitanditwillnotcauseasecondary reaction.MTTcanbereachedonlyifthereactionmixtureisleft intheheataccumulationforalongtime.Inthesecondcaseifthe reactionmixturestaysintheheataccumulationforalongtime,it mayinduceasecondaryreaction,butthefinaltemperaturecannot exceedthetechnicallimit.
Inthefirstcaseofcriticalityclass3thetechnicallimitisreached butasecondaryreactionisnottriggered.Inthesecondcasethe secondaryreactionistriggered,butthefinaltemperaturedoesnot exceedthetechnicallimit.
Nomenetal.developed anopeativetool fortherisk assess- ment(Checkcardsforrunaway(CCR)),whichfollowsafactor-based strategy. Five factors are defined toassess a thermal runaway, whichare:mischargingchemicals,autocatalyticreactions,segre- gation,accumulation,andtemperaturehazard(Nomenetal.,2004).
5. Reactorrunawaycriteria
Reactorrunawaycriteriacanbeappliedtodefinetheboundaries ofsafeandunsaferegimesthroughdistinguishingtherunawayand non-runawaystates. Thisfeatureallowstoapplycriteriain off- linetasks(likeprocessdesign,optimization)andinon-linetasks too(likeearlywarning).Therefore,thermalrunawaycriteriaare applicableindesigningandoperationofchemicalreactors(Jiang etal.,2011).Abriefhistoryaboutthereactorrunawaycriteriauntil 2006canbefoundin(Shouman,2006).
Thermalrunaway criteria can be classifiedinto three types, which are geometry-based criteria, stability-based criteria and sensitivity-based analysis can beperformed to define runaway boundaries,whicharepresentedinthefollowingSections5.2–5.4.
Therunawaycriteriaandtheyearoftheirfirstpublicationarepre- sentedinTable3.Section5.1presentsasimplemathematicalmodel ofatubularreactor(orbatchreactor),onwhichthederivationof runawaycriteriacanbepracticedeasily.
5.1. Mathematicalmodel
Afirstorderreactioncarriedoutinabatchreactorispresentedin thissectionwhichwillprovideasabaseforpresentationofthermal runawaycriteria.Thereactorwasconsideredasperfectlymixedso
Table3
Thermalrunawaycriteriadevelopmentsovertime.
Criterion Yearofpublication Reference
Semenov-criterion 1928 Semenoff(1928),
Semenov(1940)
“PracticalDesign”criterion 1938 Berty(1999)
vanHeerdencriterion 1953 vanHeerden(1953)
Gilless-Hoffmanncriterion 1961 Berty(1999),Gillesand Hofmann(1961) ThomasandBowescriterion 1961 Thomas(1961),Varma
etal.(2005) AdlerandEnigcriterion 1964 AdlerandEnig(1964) vanWelsenaereandFroment
criterion
1970 vanWelsenaereand
Froment(1970) Morbidelli-Varmacriterion 1987 MorbidelliandVarma
(1988)
Adiabaticcriterion 1988 Gygax(1988)
Hopf-bifurcationanalysis 1989 Colantonioetal.(1989)
Vajda-Rabitzcriterion 1992 VajdaandRabitz
(1992)
Strozzi-Zaldivarcriterion 2003 Zaldívaretal.(2003)
Lyapunov-stability 2006 Szeifertetal.(2006)
Adiabaticcriterionbasedon Strozzi-Zaldivarcriterion
2016 Guoetal.(2016)
Kähm-Vassiliadiscriterion 2018 KähmandVassiliadis (2018a)
ModifiedSlopeCondition 2019 KummerandVarga
(2019a)
ModifiedDynamicCondition 2019 KummerandVarga (2019a)
thefollowingdifferentialequationscanbewrittentodescribethe dynamicalbehaviour:
dc
d =−r (6)
dT
d =qgen−qrem (7)
qgen=ˇr (8)
qrem=˛(T−Tw) (9)
Where r=exp
− E RT
c (10)
˛=5l
h,ˇ=180m3K
kmol,=20,E
R =6600K,c0=1kmol
m3 , T0=300K(11) Fig.5showshowthepresentedmodel(Eqs.6–11)issensitiveto
thewalltemperature,anditpresentsthedevelopmentofthermal runaway.
5.2. Stability-basedcriteria
Thestateofthesystemcanbeconsideredstableifafterasmall disturbancethesystemreturnstoinitialstateandduringthetran- sientbehaviourthestateofthereactorstaysclosetothatinitial state.Thistheorycanbeusedtoinvestigatereactorrunawaysince incaseofrunawayreactionssimilarsituationoccurs,wherethe positivefeedbackinthetemperatureandreactionraterelationship canresultinthedevelopmentofrunaway.Thatfirststateofthesys- tem,whenrunawayisoccurredcanbeconsideredasunstablestate, fromwhichthereactorcannotgobacktotheinitialstate.Numer- ousstability-basedrunawaycriteriawereproposedtoindicatethe developmentofthermalrunaway,whicharenowpresentedinthe followingsection.
5.2.1. Semenov-criterion
Firstpioneerworkinthefieldofreactorrunawaywasdoneby Semenov,whichworklaidthegroundworkforfurtherresearches.
Thissectioniswrittenbasedon(Stoessel,2008;Semenoff,1928;
Semenov,1940).Semenovconsideredanexothermalreactionwith zero-orderkinetics.Semenov-diagrampresentstheheat-releasein reactionand theremovedheatbyheattransferasafunctionof temperature.
Fig.7 presents therelationship betweenthe generated and removedheat,wherethegeneratedheatvariesexponentiallywith processtemperature,whiletheremovedheatvarieslinearlywithit.
ThreeessentialpointsdrawattentioninSemenov-diagram,which aremarked asA, Band C, andthe belongingtemperaturesare markedasTw1, Tw2 andTw3.InAwecanrespectastableoperating pointsinceifthecoolingtemperatureislowerthan Tw2,thepro- cesstemperaturewilldecreaseduetothehigherremovedheat untilA,andnoself-ignitionoccurs.Ifthecoolingtemperatureis higherthan Tw2,self-ignitionoccurssince thegenerated heatis continuouslyhigherthantheremovedheat.Cpointrepresentsthe criticalpointincaseofahighercoolingtemperature,wherethe generatedheatcurveistangentatonepointtotheremovedheat line.Thebelongingcoolingtemperatureisconsideredascritical, orasthelowesttemperatureofself-ignition.Inthispointalittle increaseincoolingagenttemperaturethecoolinglinewillhaveno intersectionbetweenthegeneratedheatandremovedheatcurve leadstotherunawayofreaction.
Fortheaimofavoidingthermalrunawayitisnecessarytooper- atethereactor faraway from critical conditions. Based onthe Semenov-diagramandfurtherinvestigationofthecriticalpointa runawaycriterioncanbederived.Inthecriticalpointthegenerated andremovedheat,andalsotheirderivativeswithrespecttotem- peratureequals,thiscanbewrittenasEqs.12–15presents.Since thereagentconsumptionisneglected,thereactionratevariesonly withtemperature,hencethepartialderivativeofthereactionrate canbeconsidered.
qgen=qrem (12)
ˇr=˛(Tc−Tw) (13)
dqgen
dT = dqrem
dT (14)
ˇrT=˛ (15)
Dividingthe13and15.equationsthefollowingcriticalequation istheresult:
r rT =RTc2
E =(Tc−Tw)=Tc (16)
Eq.16 presents that thereis a minimal temperaturedifference betweentheprocessandcoolingtemperaturetokeepthereac- tionoperationstable.Semenov-diagramhelpsustoformulatethe runawaycriterion,becausethecriticaltemperaturedifferenceis alwayssatisfiedwhenthetemperatureisbelowthecriticaltem- peraturevalue.
(T−Tw)≤ RTc2
E (17)
FromEq.17thecriticaltemperaturecanbecalculatedbysolving thequadraticequation.
Tc=1−
1−4RTEw 2R
E
∼= 2
RTwE
+2RTwE
2+4
RTwE
3...
2R E
(18) Ifweconsideronlythefirsttwotermsontherightside,the followingrunawaycriterion(Semenov-criterion)canbederived:
(T−Tw)≤ RTw2
E (19)
WepaytributetotheSemenov-number,whichistheratioof dimensionlessreactionheatparameterand theheattransfer,as follows:
= (−Hr)kcn UA
E
RT2 (20)
Forverylargeactivationenergiesthefollowingcriterioncanbe defined,mentionedintheliteratureasSemenov-criterion(where eisthenaturalnumber):
<1
e= c (21)
Thisequationisdeterminingintheresearchfieldofthermal ignition,becausethefollowingresearchesfocusonhowtodeter- mine thecritical Semenov-number in more realistic cases, like withoutneglectingthereactantconsumption.
However,wearegoingtopresenttherunawaycriteriawith- outinvestigatingtheconcretevalueofSemenov-numbersinthe followingsections,insteadwearegoingtopresentthebasethe- ory.Criticalstates(temperature,concentration,etc.)canbedefined though,andthecriticalSemenov-numberscanbecalculatedfrom thesevariables.
5.2.2. VanHeerdenand“practicaldesign”criterion
BertyclearlypresentedthetheorybehindVanHeerdencrite- rion,whichisoftencalledas“SlopeCondition”(Berty,1999;van Heerden, 1953). In a steady-state operationthe generated and removedheatareequal. Itisevidentalsothattheheatgenera- tionand heatremovalrateincreaseswithtemperature,butthe generatedheatincreasesexponentially.Ifthereisanydisturbance inthereactortemperaturetheheatremovalrateshouldincrease fasterwithtemperaturethanthegeneratedheat,itwouldprevent temperaturerunaways.Mathematicalformofthecriterionisthe following:
dqgen
dT ≤dqrem
dT (22)
TheareaofsensitivedomainwasdefinedbyVanHeerdenin 1953(vanHeerden,1953).Perkinsassumedzeroorderkineticsto defineasafeboundary.ConsideringEqs.22and12thefollowing criterioncanbedefined:
T−Tw≤RT2
E (23)
Bashiretal.derivedthesamecriterioninvestigatingtheinflec- tionpointinageometricplane(Bashiretal.,1992),statingthatthe calculatedmaximumtemperatureinEq.23isthelimitingvaluefor runawayattheinflectionpoint.
5.2.3. Gilles-Hoffmanncriterion
Gilles and Hoffmannin1961 recognizedthe“Dynamic Con- dition”, whichistheconditionthat setsthelimitstoavoidrate oscillation.Criterionisstatedastheincreaseofheatremovalrate withtheincreaseoftemperaturemustbelargerthanthediffer- encebetweenheatgenerationrateincreaseduetotemperature alone andreaction ratedecrease duetotheconcentrationdrop alone(Berty,1999;GillesandHofmann,1961).
∂qgen
∂T
c
+ ∂m
∂c
T
≤dqrem
dT (24)
wheremisthematerialbalancefunction.
5.2.4. Lyapunov-stabilityingeometric-andphase-plane
Szeifertetal.proposedtouseLyapunov’sindirectmethodto forecastreactorrunaway(Szeifertetal.,2006;Sastry,1999).The
stabilityanalysisofasystemdefinedbyasetofnonlineardiffer- entialequationsofthestatevariablesapplyingLyapunov’sindirect methodisreducedtoaneigenvalueanalysisoftheJacobianmatrix.
J= ∂f
∂x (25)
IfrealpartofeacheigenvaluesoftheJacobianmatrixisnegative thenthemodelisstable,butifanyofthesearepositivethensystem isunstableattheinvestigatedoperatingpoint.Lyapunov-stability canbeperformedingeometric-andinphase-planetoo.Thespatial stabilitycriterionisalwaysmoreconservative,becausethestabil- ityinphasespacealwaysfollowsfromthespatialstabilitywhile inverselydoesnot.
In2008López-Garcíaetal.proposedtoinvestigatethesteady- statesolutionswithaperturbationmodel,becausethedynamic studyisessentialtoguaranteethethermallystableoperation.The methodis basedonthelinearization oftheperturbationmodel whichresultintheanalysisoftheeigenvaluesofJacobianmatrix (López-GarcíaandSchweitzer,2008).VajdaandRabitzsimilarly investigatedtheperturbationmodelearlierin1992,buttheyinves- tigatedthesensitivityofmaximumvalues ofeigenvaluesofthe Jacobianmatrix(VajdaandRabitz,1992).
Forinvestigating thedynamicsof asystem,Hopf-bifurcation analysiswassuggested,whichisbasedoninvestigatingtheeigen- values too.Ifthe realpartof a complex-conjugate pairs ofthe Jacobianmatrixbecomespositivethenbifurcationoccurs,andthat meansreactorrunawaymaydevelop(Colantonioetal.,1989;Ball andGray,2013;GómezGarcíaetal.,2016;McAuleyetal.,1995;
Kimetal.,1991;BallandGray,1995;Ball,2011).
5.2.5. Strozzi-Zaldivarcriterion(Divergencecriterion)
StrozziandZaldivarinvestigatedthephase-spacevolumecon- tractionsduringthereactoroperationbasedoninvestigatingthe Lyapunov-exponentsand thedivergence ofthe system(Strozzi etal.,1999).Ithasbeenshownthatthedivergencecriterioncan beappliedfordevelopingsafetyboundarydiagramstodistinguish therunawayandnon-runawaystatesforseveraltypesofreactors (BR,SBR,CSTR)andformultiplereactions,alsowithandwithout ofacontrolsystem(Zaldívaretal.,2003).
StrozziandZaldivarprovidedthefollowingderivationoftheir runawaycriterion(Strozzietal.,1999).AccordingtotheLiouville’s theorem,contractionofastatespacevolumeofad-dimensional dynamicalsystemcanbedefinedbasedonitsdivergence(Arnold, 2006).
dV(t) dt =
divF[x(t)]dx1(t)...dxd(t) (26) wherethedivergenceofthesystemcanbecalculatedas
divF[x(t)]=∂F1[x(t)]
∂x1(t) +∂F2[x(t)]
∂x2(t) +···+∂Fd[x(t)]
∂xd(t) (27) Assumingthatthed-dimensionalvolumeissmallenoughthat thedivergenceofthevectorfieldisconstantoverV(t),then dV(t)
dt =V(t)divF[x(t)] (28)
IntegratingEq.28theinitialphase-spacevolumeV(0)changeswith timeas
V(t)=V(0)exp
t 0
divF[x(t)]d
(29) Hencetherateofchangeofthestate-spacevolumeisgivenby thedivergenceofthesystem,whichislocallyequivalenttothetrace oftheJacobianofF.Theexpansionandcontractionofthestate- spacevolume,sothatthedivergenceoftheinvestigatedsystemis inrelationwithrunawayandnon-runawaysituations.Practically
itmeansthatifthestatevariablesdriftoffforasmallperturba- tionthenthesystemisunstable.Incasethedivergenceisnegative therewillbenorunaway,althoughifthedivergenceispositive, runawaywilldevelop.Therefore,theproposedrunawaycriterion isthefollowing:
divF[x(t)]≤0 (30)
Copelli etal. modified theoriginal divergence criterion,and theyproposedtodisregardallcontributionsarisingfromextent- of-reactions that are not related toheat evolution. Other state variablescangenerateastrongstate-spacevolumecontractionthat isnotrelatedtothedevelopmentofrunawaywhichmayleadsto thefailureofdivergencecriterioninpredictingreactorrunaway.It meansthatforexamplethecomponentswhicharenotreactantare neglectedwhenevaluatingthemodifieddivergenceofthesystem (Copellietal.,2014),(Kahm,2019).
Strozzietal.alsoinvestigatedtheLyapunov-exponentstodefine sensitivity.Lyapunov-exponentcanmonitorthebehaviouroftwo neighbouringpointsofasysteminadirectionofthephasespaceas afunctionoftime:IftheLyapunov-exponentispositive,thenthe pointsdivergefromeachother,iftheexponentbecomesnegative, thenthepointsconverge.Lyapunov-exponentsarerelatedtothe eigenvaluesoftheJacobianmatrix,sinceitaveragestherealparts ofalleigenvaluesalongatrajectory(Strozzietal.,1994;Strozzi andZaldívar,1994).AlthoughtheLyapunov-exponentscanunder- estimatetherunaway boundaryfor likeautocatalyticreactions, becauseitusestheintegralovertimewhich isslowtorespond tofastchange.Therefore,Strozzietal.proposedtoapplydiver- gencecriterion(Strozzietal.,1999).Kähmetal.laterinvestigated theLyapunov-exponentsnotinsensitivitycontext,butinvestigat- ingthevaluesofit.IftheLyapunov-exponentbecomespositive,an unstableprocessispresent(KähmandVassiliadis,2018a;Kähm andVassiliadis,2018b;KähmandVassiliadis,2018c).
We cancalculate thedivergenceonline, withoutneeding to knowthedifferentialequationsofthesystembyusingthetheory ofembedding.Statespacereconstructionisapossibletechnique toaddressthis problemusingtimedelayembeddingvectorsof theoriginalmeasurements(i.e.,temperatureorpressuremeasure- ments)(Boschetal.,2004a;Boschetal.,2004b).Althoughthereis severalmethodsofreconstruction,butthereisnoapriorimethodto decidewhichoneisthebest.In(Zalı ´dvaretal.,2005)Zaldivaretal.
testedseveralmethods:timedelayembeddingvectors;derivative coordinatesandintegralcoordinates,buttheresultsweresimilar andtheyusedderivativecoordinatesbecauseoftheirclearphysical meaning.Therearetworeconstructionparameters:theembedding dimension,andthetimedelay.Theembeddingdimensionisthe dimensionofthestatespacerequiredtounfoldthesystemfrom theobservationofscalarsignals,whereasthetimedelayisthelag betweendatapointsinthestatespacereconstruction(Boschetal., 2004b).
Guoetal.developedanadiabaticcriterionbasedonthediver- genceofanadiabaticmodelofthereactorsystemwithzerofeed rateresultinamorestrictrunawaycriterion(Guoetal.,2016;Guo etal.,2017a).
WalterKähmdevelopedastabilitycriterionbasedontheorigi- naldivergencecriterion,whichisbasedonthedifferencebetween thedivergenceoftheJacobianmatrixoftheinvestigatedreactor systemvariablesandthecorrectionfunction.Thecorrectionfunc- tionisderivedasafunctionofthedivergenceoftheJacobianatthe previoustimestep;Damköhlernumber;Barkelewnumber;Arrhe- niusnumberandtheStantonnumber.Theyintroducedthisstability criterion,becausedivergencecriterionmayoverpredictthether- malrunawaypotentialofthesystem.Thederivationisbasedon a linearapproximationofthedivergence(KähmandVassiliadis, 2018a;Kahm,2019;KähmandVassiliadis,2018d).Theproposed
stabilitycriterionissuccessfullygeneralizedformultiplereactions (KähmandVassiliadis,2019).
5.2.6. Modifieddynamicandslopecondition
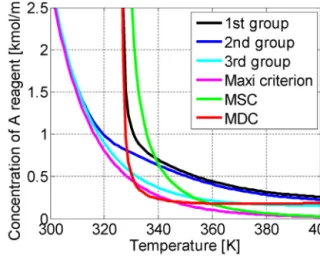
KummerandVargainvestigatedthemostfrequentlyapplied criteriaand derived two newcriteria as a result(Kummer and Varga,2019a).Eq.31presentstheModifiedSlopeCondition(MSC) andEq.32presentstheModifiedDynamicCondition(MDC).We investigatedthreedifferentreactionsystems(singlereactionwith areagent,twoparallelreactions,andanautocatalyticreactionsys- tem)tovalidatetheModifiedDynamicandSlopeConditioncriteria, whichinthereliabilityandthetimeofindicationwerecompared.
MDCdidnotmissanythermalrunawaydevelopment,buttheper- formanceofMSCiscompatiblewiththeinvestigatedones.
∂qgen
∂T
c
≤dqrem
dT
1+qgen
qrem
(31)
∂qgen
∂T
c
+ ∂m
∂c
T
≤ qgen
qrem
dqrem
dT (32)
5.3. Geometry-basedcriteria
Several reactor runaway criteria exist based on a geomet- ric characterization of temperature trajectories, which will be presentedinthis section.Advantagesof inflexion-basedcriteria (ThomasandBowes-,AdlerandEnigcriterion)andadiabaticcri- terionisthatitrequiresonlyatemperatureprofileortrajectory toevaluatethereactionstates,althoughwithoutinvestigatingthe states on a prediction horizon the runaway indications proba- blyoccurslately.Inflection-basedcriteriadonotgiveinformation abouttheintensityofthereactorrunaway.VanWelsenaereand Fromentcriterionisquiteconservativethoughandindicatesreac- torrunaway quite early,but a model of thereactor system is requiredfortheapplication.
5.3.1. ThomasandBowescriterion
ThomasandBowesproposedtoindicatereactorrunawayasthe situationinwhichaninflexionpointappearsbeforethetempera- turemaximuminthegeometricplane(inversustimeorlength).It meansthatthereactoroperationstayscontrollableifthefollowing statementsaresatisfied(Thomas,1961;Varmaetal.,2005).
d2T
dt2 <0whiledT
dt >0 (33)
DenteandCollinain1964independentlyproposedthesame criterion(Varmaetal.,2005).
5.3.2. AdlerandEnigcriterion
AdlerandEnigfounditmoreconvenienttoworkinaphase- plane(intemperature-conversion)thaninthegeometricplane.To indicatereactorrunaway aninflexionpointmustappearbefore thetemperaturemaximuminthephase-plane.Itmeansthatthe reactoroperationstayscontrollableifthefollowingstatementsare satisfied,wherexistheconversion(AdlerandEnig,1964).
d2T
dx2 <0whiledT
dx >0 (34)
5.3.3. VanWelsenaereandFromentcriterion(orMaxicriterion) vanWelsenaere and Fromentdetermined critical conditions basedonthelocusoftemperaturemaximainthetemperature- conversion plane. This criterion can be eliminated based on obtainingtherelationbetweenmaximumprocesstemperatures
Fig.6. Sensitivityofthereactormodelwithrespecttowalltemperature.
Fig.7.Semenov-diagram.
evolvingatdifferentcoolingagenttemperatures(vanWelsenaere andFroment,1970).
dT
dx >0dcm
dTm
>0 (35)
5.3.4. Adiabaticcriterion
Afrequentlyappliedrunawaycriterion(eveninindustrialappli- cation)isthattheprocesstemperatureevolvingunderadiabatic conditions(sotheMTSR)cannotexceedtheMaximumAllowable Temperature(Abeletal.,2000).
Tp+Tad=MTSR≤MAT (36)
5.4. Sensitivityanalysisofchemicalreactors(Morbidelli-Varma criterion)
A.Varmaetal.wroteanexcellentbookabouttheparametric sensitivitiesinchemicalsystems(Varmaetal.,2005).Theanalysis ofhowasystemrespondstochangesintheparametersiscalled parametricsensitivity(Varmaetal.,2005).Inthecontextofchemi- calreactorsBilousandAmundsonperformedapioneerworkonthe fieldofparametricsensitivity,wheretheresearchersshowedhow themaximumtemperaturealongthereactorlengthvarieswith theambient(cooling)temperature(BilousandAmundson,1955;
BilousandAmundson,1956;Grayetal.,1981;Emigetal.,1980;
Grayetal.,1981).Theresultofasimilaranalysiscanbeseenin Fig.6.Sensitiveregionsofoperationsshouldbeavoidedbecauseits
performancebecomesunreliableandchangessharplywithsmall variationsinparameters.Althoughsomeexperimentalstudiesare availableintheliterature(Emigetal.,1980;LewisandVonElbe, 2014),itisdifficulttoperformwholesomeinvestigationsaboutthe reactionsystems(nottomentiontheindustrialsystems),because thesesystemsinvolvemany parametersaffectingthebehaviour ofthereactor.Therefore, modelbasedinvestigations areneces- sary.For theaimofinvestigationthesensitivity ofreactors we shoulddefinevaluableoutputs(dependentvariables),andvalu- ableinputs(independentvariables).Dependentvariablescanbe investigatedingeometric-or/andinphase-plane,whichcanbefor exampleproductivity,processtemperature,processpressureetc.
Inputvariablestypicallyareinitialconditions,operatingconditions andgeometricparametersofthesystem.
MorbidelliandVarmausedthefactthatneartheexplosion(run- away)boundarythesystembehaviourbecomessensitivetosmall changesinsomeoftheinputorinitialparameters,andtheydefined theboundarybetweenrunawayandnon-runawayzonebasedon thissensitivityconcept.Thefirst-orderlocalsensitivityorabsolute sensitivityofthedependentvariable(y)withrespecttotheinput parameters()canbecalculatedbasedonthefollowingform:
sy= ∂y
∂ (37)
Anotherquantityrelatedtolocalsensitivityisthenormalized sensitivity,whichcanbedefinedas:
Sy= y
∂y
∂ = ∂lny
∂ln=
ysy (38) Theadvantageofnormalizedsensitivityisthatitnormalizesthe magnitudesoftheinputparameterandthevariabley.
In Morbidelli-Varma criterion the parametrically sensitive regionofthesystemorcriticalityforthermalrunawaytooccuris definedasthatwheretheabsolutevalueofthenormalizedsensitiv- ityofthetemperaturemaximumreachesitsmaximum(Morbidelli andVarma,1988),(MorbidelliandVarma,1989;Chemburkaretal., 1986).Lacey(1983)andBoddingtonetal.(1983)independently proposedtousethesensitivitymaximumofthetemperaturemax- imum with respect to Semenov number, to define the critical conditionsforthermalexplosion,butMorbidelliandVarmagener- alizedthiscriterionconsideringotherphysicochemicalparameters ofthereactingsysteminthedefinitionofthesensitivity.
Jianget al.proposedtoapplytheabsolutesensitivity inthe followingform:Safeoperatingconditionscanbedefinedbythe temperaturesensitivityvaluewhichislessthanoneinthewhole intervalexceptintheinitialpoint.Theboundarybetweenrunaway andstableconditionisestablishedbythemaximumvalueofthe sensitivityfunctionwhichequalsone,soas:
max
sy
=1(exceptt=0) (39)
Theyexplaineditthrough analysingthemaximumvalues of absolutesensitivities,andnotingthatlowersensitivityvaluesmean lesssensitivesystems.Practicallytheyjustmadeathresholdto makethesystemsaferandthecriterionstricter(Jiangetal.,2011).
5.5. Data-basedpredictionofthermalrunaway
Runaway criteria were developed using data-mining tools, where datawere generated based onthemodel of thereactor system.In(Vargaetal.,2009)adecision-treebasedapproachis developedtodistinguishbetweenrunawayandnon-runawaysit- uation,where thecasestudy isan industrialreactor producing phosgene.A similarapproach is presented in (Dakkouneet al., 2020),wherebinarydecisiondiagramsandlinearclassifierswere appliedtodiagnosethefault.Theydetectedrunawaycriteriabased
on dynamic thresholdsevaluated by investigating temperature characteristics(Amineetal.,2018).Themajordrawbackofthese criteria,thatahugeamountofprocesssimulationsshouldbeper- formed to obtain the necessary amount of data. However, the resulteddecision-treecanbeeasilyunderstoodbyaprocessoper- ator,andthemostappropriatesafetyactionscanbedetermined foranyoftherunawaystates.Kummeretal.developedagenetic programming-basedmethodforconstructingtailoredrunawaycri- teriatoreachamorespecificcriticalequation,thistechniquecanbe usedforanykindofcombinationofreactorandreactionsystems, andtheresultedcriterionismuchmoresuitableforthatsystem thananygeneralcriteriafromtheliterature(Kummeretal.,2019).
6. Safetyboundarydiagrams
Incaseofoperationofbatchandsemi-batchreactorscarrying out exothermicreactionssafetyboundarydiagramscangivean efficientsupportforsafeoperation.Westerterpetal.hadalotof pioneerworkonthisfield,alsoadimensionlessnumberiscalled asWesterterp-number(Wt,earlierCoolingnumber,Co,(Pohorecki and Molga,2010))andthesafetyboundarydiagramoftenmen- tionedasWesterterp-diagram.HugoandSteinbachhaveobserved thatanaccumulationofthenon-convertedcomponentinSBRmay causerunawayevents,andalsoinvestigatedhowthemaximum processtemperaturevariesincaseofabreakdownofcooling(Hugo andSteinbach,1986;Hugoetal.,1988).Westerterpetal.general- izedtheconceptofavoidingreagentaccumulationthroughsafety boundarydiagrams.Theyinvestigatedheterogeneousliquid-liquid andhomogeneousreactionstoo(SteensmaandWesterterp,1988;
SteensmaandWesterterp,1991;SteensmaandWesterterp,1990).
Theproposedsafetyboundarydiagramcanbeappliedgenerally, hencemostoftherecentarticlesusethesamegeneralreactorand homogenousreactionsystemforfurtherinvestigations(Molgaand Lewak,2009).Of course,laboratoryexperimentswerealsoper- formed toinvestigatethe safetyboundary diagrams,a detailed work aboutthethermallysafeoperationofa nitricacidoxida- tioninSBRcanbefoundin(vanWoezik,2000;vanWoezikand Westerterp,2002),
Inidealcasesthereactionrateequalsthefeedrate,meansthat thedosedreagentreactsawayimmediatelyavoidingthereagent accumulation.Inthatcasethereactortemperaturefollowsatra- jectorycalledthetargettemperature,whichcanbeestimatedwith thefollowingequation.Derivationofthisequationcanbeseenin (Westerterpetal.,2014).
Tta=Tc+ 1.05Tad,0 ε
Wt
1+ε
+RH
(40)whereTcisthecoolingtemperature,Tad,0isaninitialadiabatic temperaturerise,εistherelativevolumeincrease,WtisWesterterp number, isdimensionlesstime,RHistheratioofheatcapacities ofthedispersedandthecontinuousphase.
If thedosingiscompleted Eq.40. canbeusedtodefinethe targettemperaturebeside=1.Atthetargettemperaturethereac- tionrateishighenoughforavoidingreagentaccumulation,sothe reactorisoperatedsafely.Therefore,reactorrunawayoccursifthe processtemperatureexceedsthetargettemperature.
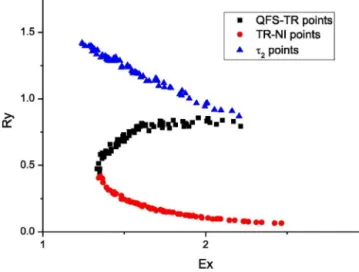
Three zonescan bedistinguishedbased ontheevolution of temperature and concentration trajectoriesin SBRs, which are:
marginalignition(MI,ornoignition),thermalrunaway(TR)and QFS (quick onset, fair conversion, smooth temperature profile) zones,asitcanbeseeninFig.8.Inthemarginalignitionthereactor temperatureisalwaysmuchlowerthanthetargettemperature,the reactiondoesnotignite;hencetheaccumulationistoohighforsafe operation.Inthethermalrunawayzonetheprocesstemperature exceedsthetargettemperature,alsoreachesmuchhighervalues
Fig.8.Safetyboundarydiagram(Westerterpetal.,2014).
thanthetargettemperaturebecauseoftheaccumulatedreagent abruptignitesthereactionbehavingcloselytoabatchoperation.In QFSzonetheprocesstemperaturetrajectoryisveryclosetothetar- gettemperaturetrajectory,becausethefedreagentreactsalmost immediately,whichisthegoalintheoperation.
Thethreezonesarecharacterizedbytwodimensionlessnum- ber,exothermicity(Ex)andreactivity(Ry),whicharedefinedas follows:
Ex= Tad,0(E/R)
Tc2ε(RH+Wt)= ad,0
ˇ2cε(RH+Wt) (41)
Ry=
Da(TR)exp
1−ˇ1c
ε(RH+Wt) (42)
whereTcisthecoolingtemperature,Tad,0isaninitialadiabatic temperaturerise,Eisactivationenergy,Risthegasconstant,εis therelativevolumeincrease,WtisWesterterpnumber, isdimen- sionlesstime,RHistheratioofheatcapacitiesofthedispersedand thecontinuousphase,ad,0isdimensionlessadiabatictempera- turerise,istheArrheniusnumber,ˇcisthedimensionlesscooling temperature,DaistheDamköhlernumber.
Theexothermicitynumberspresentstheratioofthemaximal powergeneratedduetothereactionandthecoolingabilities.The reactivitynumberpresentstheratioofthereactionrateandthe coolingrate.Theboundarylineindicatesthecasewheretheprocess temperaturedoesnotexceedthetargettemperature,onlytouches it(Molgaetal.,2007).Theboundarydiagramsandtheboundary linesdependonthevalueoftheWesterterp-number(Wt)andthe ratioofheatcapacitiesof(RH).
Westerterp-numberpresentsthecoolingabilityrelatedtothe heatcapacityofthereactorcontentatthebeginningoftheprocess.
Dosingtimeisalsoappearsinthisdimensionlessnumberconsider- ingtherateofheatevolution.Westerterp-numbercanbecalculated asfollows:
Wt= (UA)0tdos ε
Vcp
0
(43) whereU0istheinitialheattransfercoefficient,A0istheinitialheat exchangesurface,tdosisthedosingtime,εistherelativevolume increase.
TheWesterterp-numberisthekeyparametertodeterminethe differencebetweenthebehaviourofthelargescale,industrialreac- torandthelaboratoryreactor(WesterterpandMolga,2004).There isaninherentlysaferegion,asitcanbeseeninFig.8.Theydeter- minethemaximumoftheexothermicityvaluesbelowwhichthe heatevolutionisalwaystoolow,hencereactorrunawaydoesnot
Fig.9. SafetyboundarydiagramconsideringMAT(Nietal.,2016).
develop. Thereisalsoa minimumreactivityvalueabovewhich reagentaccumulationdoesnotoccurbecauseofthehighreaction rate,hencereactorrunawaydoesnotdevelopeither(Westerterp and Molga, 2006). These specific values determine unambigu- ouslytheinherentlysaferegion.Boundarydiagramsafetycriterion (BDSC) isbased oncomparingthereactivity and exothermicity numbers to themaximal exothermicityand minimal reactivity numbers,forfurtherinformationsee(Westerterpetal.,2014).The safetyboundarydiagramscanbeeasilyusedforanexistingreactor toidentifythermallysafeoperatingconditionswithoutsolvingthe mathematicalmodelofthereactor.AlsotheWesterterp-diagram canbeeasilyusedtoscalingupreactors(MaestriandRota,2005a;
MaestriandRota,2005b),andalsoakinetics-freeapproachcanbe foundin(Guoetal.,2019).Flowchartfordesigningthermallysafe operatingconditionsbasedonsafetyboundarydiagramscanbe foundin(Molgaetal.,2007;Guoetal.,2017b).
AlthoughtheWesterterp-diagramisunderstandableandeasy touse, thereis nodirectinformation aboutthemaximumpro- cess temperaturesevolvingduring thereactoroperationin the QFS zone, which always should bechecked, because the reac- torsystemmaycannotstandit(maximumprocesstemperature exceeds MAT),or thecoolingcapacity maybenothighenough totransferthedevelopingreactionheat.MaestriandRotaintro- ducedTemperatureDiagrams(TD),whichcanbeappliednextto theWesterterp-diagram. TDs allowfor boundingthemaximum process temperatureas a function of exothermicityor reactiv- itynumbers(MaestriandRota,2006a;MaestriandRota,2006b;
Copellietal.,2010).
Nietal.consideredsecondreactionregiontoothroughinclud- ingtheMATvalueinthedevelopmentofsafetyboundarydiagram, asitcanbeseeninFig.9.EGcurverepresentsthemarginalignition, runawayregionislocatedbetweenEGandEF.QFSregionislocated betweenABCDandEGcurves,andthesecondreactionregionis aboveABCDcurve(Nietal.,2016).Theyalsosuccessfullyapplied thismethodforanautocatalyticreactionsystem,wheretheauto- catalyticbehaviourwasdefinedasparallelreactions,andforthis theyproposedamodifiedExothermicityandreactivitynumber(Ni etal.,2017).
Maximum temperature of synthesis reaction (MTSR) is an importantcriterionforreactordesignandprocesshazardassess- ment, becausein case of a coolingfailure this parametergives information abouttheevolvingprocesstemperatures. Forsafety reasonsitshouldbelowerthantheMAT.Guoetal.investigated thisphenomenonindetail(Guoetal.,2015).Baietal.appliedMTSR valuesinsteadofprocesstemperaturesforcomparingitwiththe
Fig.10.ExtendedBoundaryDiagram(Guoetal.,2018).
targettemperaturevaluestobuildsafetyboundarydiagramsresult inasaferreactoroperation.TheircriterionisdenotedasMaximum temperatureofasynthesisreactioncriterion(MTSRC)(Baietal., 2017a).Flowchartsfordesigningthermallysafeoperationsconsid- eringMTSRvaluescanbefoundin(Baietal.,2017a;Baietal.,2017b;
Zhangetal.,2019).Amoregeneralizedmethodforincludingand investigatingthemaximumprocesstemperaturesdeveloping at givenoperatingparametersareproposedin(Guoetal.,2018).Guo etal.proposedanartificiallydefinedconstanttemperature,which canbecalculatedasfollows:
Tn=Tc+ nTad,0
ε[Wt+RH]n≥1.05 (44)
TngivesinformationabouttheMTSRvaluesevolvingataspecific operationconditions,forexampleatn=2thegivenT2 pointsin SBDcanbeseeninFig.10,whereMTSRvaluesequalsT2(Guoetal., 2018).
Recentlyamulti-featurerecognition(MFR)criterionbasedon patternrecognitionwasproposedtodevelopsafetyboundarydia- grams(Zhangetal.,2020).
Thepresentedmethodsaregreatandeasytouse,butitrequires constantfeedrateofreagents.However,ifwewouldliketomax- imizetheproductivityorotherefficiencymetricsthefeedingrate shouldbevariedintime.Inourhumbleopinionsafetyboundary diagramsshouldbeusedtodefinethesuitableinitialconditions,so todefineinitialprocesstemperature,flowrateofcoolingagentand reagents.ThewholeconceptofSBDsistoavoidtheaccumulation ofreagents,butasthereactortemperatureincreasesthefeedrate ofreagentscanbeincreasedwhereaccumulationwillnothappen.
7. Safetyequipment/actionstomoderateserious consequences
In case we have the most reliable criterion which can be achievedtoforecastrunaway,thenextstepistoprepareoursys- temtodecreasetheeffectofrunawaydevelopment.Whenrunaway occursanditcannotbehandledinnormaloperationsitisneces- sarytostopthereaction,sowecanavoidundesiredscenarios.In suchasituation,shutdownofthereactorisperformedbysome safetyinterlockoremergencyshutdownsystem.Whenpressure increasestoohighacommonlyappliedmitigationsystemisusing apressurereliefvalvewhichdirectstheflowtoaknownlocation,in thiswaythepressurecanbedecreased.However,someconsider- ationalwaysmustbegiventothedirectionandlocationoftheend oftheventline.Duringventing,thedischargemaybepassedto:a