Proof Central
Dear Author
Please use this PDF proof to check the layout of your proof. If you would like any changes to be made to the layout, you can leave instructions in the online proofing interface. Making your changes directly in the online proofing interface is the quickest, easiest way to correct and submit your proof. Please note that changes made to the proof in the online proofing interface will be added to the proof before publication, but are not reflected in this PDF proof.
If you would prefer to submit your corrections by annotating the PDF proof, please download and
submit an annotatable PDF proof by clicking here and you'll be redirected to our PDF Proofing
system.
Journal:AVET
Article Number:004.2020.00001
Dear Author,
Please check your proof carefully and mark all corrections at the appropriate place in the proof.
Queries and/or remarks
[Q1] Please confirm that the forename(s) and surname(s) have been identified correctly and are presented in the desired order, and please carefully verify the spelling of all authors’names.
[Q2] References“Nordmann, 1832; von Nordmann, 1832; McCallum, 1921; Ivashkin, 1964; Hanek and Threlfall, 1969; Müller, 1936”are cited in the text but not provided in the reference list. Please provide them in the reference list or alternatively delete the citations from the text.
[Q3] Please provide the volume number and page range for the bibliography in Ref.‘Czeglédi et al., 2019’.
[Q4] Please provide the volume number and page range for the bibliography in the references‘Moshu (2014)’.
[Q5] Please provide a definition for the significance of“bold”values in Table 1 and Table 2.
[Q6] Please note that as per style, the ORCID information is mandatory for the corresponding author.
Hence, provide ORCID for the author“Gábor Cech”.
New record of metacercariae of the North American Posthodiplostomum Centrarchi (digenea, diplostomidae) in pumpkinseed ( Lepomis Gibbosus ) in Hungary
G ABOR CECH
1p, DI ANA S ANDOR
1,2, K ALM AN MOLN AR
1,
Q6PETRA PAULUS
3, MELITTA PAPP
3, B ALINT PREISZNER
4,
ZOLT AN VIT AL
4, AD AM VARGA
1and CSABA SZ EKELY
1 Q11Institute for Veterinary Medical Research, Centre for Agricultural Research, Hungaria krt. 21, H-1143, Budapest, Hungary
2E€otv€os Lorand University, Doctoral School of Biology, Programme of Zootaxonomy, Animal Ecology and Hydrobiology, Budapest, Hungary
3National Food Chain Safety Office, Veterinary Diagnostic Directorate, Budapest, Hungary
4Centre for Ecological Research, Balaton Limnological Institute, Tihany, Hungary
Received: October 18, 2019 • Accepted: November 21, 2019
ABSTRACT
Two species of the genus Posthodiplostomum (Digenea: Diplostomatidae) (Posthodiplostomum brevicaudatum Nordmann, 1832 and Posthodiplostomum cuticola Nordmann, 1832) are known as parasites of Hungarian nativefishes. Metacercariae ofP. cuticolaare widespread in Europe and cause black spot disease. Several species ofPosthodiplostomumwere described also from North America but none of them has been isolated in Hungary up to now.Posthodiplostomum centrarchiHoffman, 1958 has been detected recently in pumpkinseeds (Lepomis gibbosusL., 1758) in several European countries.
Posthodiplostomum centrarchiwas isolated for thefirst time in Hungary from pumpkinseeds caught in the Maconka water reservoir in 2015. Thereafter, several natural waters (e.g. the river Danube, Lake Balaton and the Sio channel) were sampled in order to determine its presence and distribution. Only the native speciesP. cuticolawas detected in Lake Balaton on cyprinids but a relatively high infection rate was observed in the Sio channel close to the lake. Pathological changes were absent, and metacercariae were mostly attached to the surface of the liver, kidney and heart. The phylogenetic analysis of the ITS and COI sequences ofP. centrarchiandP. cuticolaclustered into two distinct branches, which was in agreement with the morphological results.
KEYWORDS
Lepomis gibbosus,Posthodiplostomum, non-native species, first observation in Hungary, histology
INTRODUCTION
PosthodiplostomumDubois, 1936 (Digenea: Diplostomidae) species are distributed worldwide (Lopez-Hernandez et al., 2018;Niewiadomska 2002;Ritossa et al. 2013) and well known asfish and water-bird parasites (Lumsden and Zischke 1963;Miller 1954). The metacercariae of these species, especially P. cuticola (von Nordmann, 1832), cause black spot disease or Post- hodiplostomuminfection that occurs on the body surface,fins, scales, or in the musculature and body cavity offishes (Horak et al. 2014; Ondrackova et al. 2004; Tobler and Schlupp 2008;
Zrncic et al. 2009). These encysted stages often induce developmental disorders of thefish skeleton that may be lethal in the case offingerlings (Lucky 1970). The most common signs are
Acta Veterinaria Hungarica
DOI:
10.1556/004.2020.00001
© 2020 The Author(s)
ORIGINAL ARTICLE
*Corresponding author.
E-mail:cech.gabor@agrar.mta.hu. Tel.:
þ36 (1) 467 4060; fax:þ36 (1) 467 4076
RESEARCH ARTICLEAVET-2020.00001_proof 21 February 2020 9:20 pm
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114
weight loss due to appetite decrease, lesions of the liver and kidney, and digestive dysfunction (Iqbal et al. 2014;Lane and Morris 2000; Lopez-Hernandez et al. 2018; Rolbiecki 2004;
Sch€aperclaus 1990).
More than 25 species of Posthodiplostomum have been described in the world (Lopez-Hernandez et al. 2018;Ritossa et al. 2013) but in Europe only four species of this genus have been reported: Posthodiplostomum cuticola (von Nordmann, 1832) and P. brevicaudatum (von Nordmann, 1832) as native species, as well as P. minimum(McCallum, 1921) and P. centrarchi Hoffman (1958), both originating from North America. The occurrence of P. minimum in Europe was based on only three metacercariae (Grabda- Kazubska et al. 1987) and no further confirmations of this record were published. Posthodiplostomum centrachi had not been reported in the European pumpkinseed population before 2017 (Bykhovskaya-Pavlovskaya and Kulakovskaya 1985; Molnar 1969; Moravec 2001; Moshu 2012, 2014;
Roman-Chiriac 1960). In Hungary, the native species P.
cuticola and P. brevicaudatum were documented by Jaczo (1941)andMolnar (1968), whileSzekely and Molnar (1996) reported only P. cuticola. Recently, the presence ofP. cen- trarchi has been reported published from several European countries: Bulgaria, Slovakia, the Czech Republic, Portugal, Ukraine and Germany (Kvach et al. 2017, 2018a;
Ondrackova et al., 2019;Stoyanov et al. 2017a).
The nomenclature and the fish intermediate host speci- ficity ofP.centrarchiare still under debate.Hoffman (1958, 1999)consideredP. centrarchi a subspecies ofP. minimum and named itP. minimum centrarchibecause he proved that it could infect only centrarchid fishes while P. minimum minimumwas able to infect only cyprinids. The strict host specificity of theP. minimumsubspecies was supported by Locke et al. (2010a,b), Lane et al. (2015), Stoyanov et al.
(2017a)andBoone et al. (2018). Accordingly,Stoyanov et al.
(2017a) elevatedP. minimum centrarchi to species rank as P. centrarchiand this novel name has become general since then (Kvach et al. 2018a;Ondrackova et al. 2019).
Metacercariae of an unknown Posthodiplostomum spe- cies, non-indigenous in Hungary, werefirst recorded in 2015 when pumpkinseeds were sent to the National Food Chain Safety Office (NFCSO) from a North Hungarian water reservoir for a routine survey. These metacercariae were tentatively identified as P. centrarchi, a typical parasite of North American centrarchidfishes. To investigate the extent of this infection, we started a survey on the distribution, development and possible occurrence of these metacercariae in the Hungarian pumpkinseed population and on their pathogenic role in native fishes.
In this study, we report the first finding ofP. centrarchi metacercariae in pumpkinseeds from a small water reservoir in Hungary. We also describe a recent occurrence of this non-native trematode parasite in the Sio channel close to Lake Balaton. In order to obtain adult stages and to study the histological changes induced in the host, we successfully infected chicks with metacercariae under laboratory condi- tions. Both metacercariae and adult stages were studied by morphological and molecular methods.
MATERIALS AND METHODS
Sample collection
Up to 2015, pumpkinseed specimens had been collected randomly from different areas of Hungary and studied for parasitic infections. In 2015, 6 out of 8 pumpkinseeds were found to be parasitised byP. centrarchimetacercariae during a general veterinary survey in the Maconka water reservoir.
After the detection of the first metacercariae in pumpkin- seed, our research group started to study the parasite fauna of the pumpkinseeds in Lake Balaton and in its outflow, the Sio channel (Table 1). In the littoral zone of Lake Balaton, the pumpkinseed is the most abundant non-native fish species (Czegledi et al. 2019). Eighteen pumpkinseed speci- mens derived from the River Danube at Szentendre and a single specimen from the River Ipoly at Ipolyt€olgyes was examined (Table 2). Besides 142 pumpkinseeds, 16 speci- mens of roach (Rutilus rutilus) and 11 white breams (Blicca bjoerkna) were collected from Lake Balaton and preserved to check for Posthodiplostomum infection in cyprinid fishes.
Moreover, 10 pumpkinseed individuals were caught from the Sio channel. Most of the fish were gathered using an electrofishing gear but several individuals were obtained by a 15-metre-long seine net. The fish were carried to the labo- ratory alive in oxygenated plastic bags, kept in aerated water tanks and subjected to complete parasitological dissection within 3 days.
Dissection, artificial digestion and microscopy
Before extermination, the pumpkinseeds were sedated with a drop of clove oil added to their water, then killed by a cer- vical cut. Each fish was investigated under a Zeiss dissecting microscope and the left side of the abdominal wall was cut down. Metacercariae were collected from the surface of the organs, excysted using a fine needle and placed in 0.9% sa- line solution for approximately 10 min. About 300 live metacercariae were kept alive for experimental infections.
The rest of the parasites were counted and fixed in 80%
ethanol for molecular examinations. The infected tissue samples were fixed in Bouin’s solution, embedded in paraffin wax, cut to 4–5
m
m thick sections and stained with hae- matoxylin and eosin. Live metacercariae and histological sections were studied with an Olympus BH2 compound microscope equipped with Nomarski differential interfer- ence contrast optics, then photographed with an Olympus DP 20 digital camera.Experimental infection
Two experimental infections were carried out to obtain adult stages ofPosthodiplostomumspp. for detailed morphological analysis and description. In the first case (09.02.2016–
22.02.2016), each of two one-day-old unfed domestic chicks (Gallus gallus domesticusL., 1758) (‘A’and ‘B’ individuals) was force-fed with 50 freshly collected P. centrarchi meta- cercariae from pumpkinseeds in the Maconka water reser- voir. After the infection, the chicks were marked with green 115
116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171
172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228
colour on their head and wings. Two more individuals were preserved as negative control, which were left unmarked.
Chick ‘A’was killed by a cervical cut on 17.02.2016, while specimen ‘B’ and the negative controls were killed on 22.02.2016.
During the second experiment (03.10.2017–11.10.2017), 3 one-day-old unfed domestic chicks (‘C’, ‘D’, ‘E’) were infected per os with 50 P. cuticola metacercariae collected from white breams, and these birds were marked with red colour on their head. The two negative controls were un- marked. Chick ‘C’ was decapitated on 10.10.2017, then chicks‘D’,‘E’and the negative controls were decapitated on
11.10.2017. All chicks had been purchased from a com- mercial supplier (Hegyhat BR Kft., Szentgotthard-Rabaf€uzes, Hungary). Formal ethical approval was given by the Gov- ernment Office of Pest County (permit PEI/001/1004-4/
2015).
After the dissection, the gut system of each bird was isolated, then divided into three regions, namely duodenum plus jejunum, ileum and colon. These parts of the intestine were cut up and immersed in three different sedimentation cones containing 0.9% saline solution. After stirring for 10 min, they were allowed to subside for 30 min, then the pieces of gut and the intestinal content in the sediment were Table 2.Collection sites and dates of pumpkinseed (Lepomis gibbosus)
Collection site Collection date Number/fish
Size of fish (cm)
Infection with P. centrarchi Szentendre (Danube)
47839050.300N, 1984053.500E
04.30–05.04.2009 5 7.8 (±1.8) No
07.20–08.06.2013 12 7.6 (±2.1) No
09.07.2015 1 14 No
Maconka water-reservoir 4881045.900N, 1984908.900E
12.18.2015 8 10.6 (±0.7) 6
Ipoly, Ipolyt€olgyes 47855051.200N, 18846015.300E
05.12.2017 1 11 No
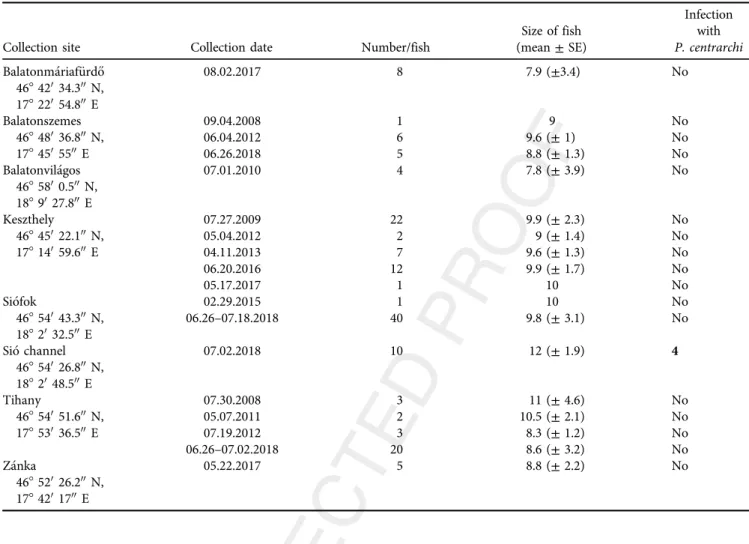
Table 1.Collection sites and dates of pumpkinseed (Lepomis gibbosus) in the Lake Balatonregion Q5
Collection site Collection date Number/fish
Size of fish (mean±SE)
Infection with P. centrarchi Balatonmariaf€urd}o
46842034.300N, 17822054.800E
08.02.2017 8 7.9 (±3.4) No
Balatonszemes 46848036.800N, 1784505500E
09.04.2008 1 9 No
06.04.2012 6 9.6 (±1) No
06.26.2018 5 8.8 (±1.3) No
Balatonvilagos 4685800.500N, 1889027.800E
07.01.2010 4 7.8 (±3.9) No
Keszthely 46845022.100N, 17814059.600E
07.27.2009 22 9.9 (±2.3) No
05.04.2012 2 9 (±1.4) No
04.11.2013 7 9.6 (±1.3) No
06.20.2016 12 9.9 (±1.7) No
05.17.2017 1 10 No
Siofok
46854043.300N, 1882032.500E
02.29.2015 1 10 No
06.26–07.18.2018 40 9.8 (±3.1) No
Sio channel 46854026.800N, 1882048.500E
07.02.2018 10 12 (±1.9) 4
Tihany
46854051.600N, 17853036.500E
07.30.2008 3 11 (±4.6) No
05.07.2011 2 10.5 (±2.1) No
07.19.2012 3 8.3 (±1.2) No
06.26–07.02.2018 20 8.6 (±3.2) No
Zanka
46852026.200N, 1784201700E
05.22.2017 5 8.8 (±2.2) No
Acta Veterinaria Hungarica
3
229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285
286 287 288 289 290 291 292 293 294 295 296 297 298 299 300 301 302 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 324 325 326 327 328 329 330 331 332 333 334 335 336 337 338 339 340 341 342
RESEARCH ARTICLEAVET-2020.00001_proof 21 February 2020 9:20 pm
studied for trematodes under a Zeiss stereo microscope. All detected worms were removed and stored in 80% ethanol.
Molecular methods
Samples preserved in 80% ethanol were centrifuged at 8,000g for 5 min to remove the ethanol. The DNA was extracted using a QIAGEN DNeasyTM tissue kit (animal tissue pro- tocol; Qiagen, Hilden, Germany) and eluted in 100
m
L AEbuffer. The ITS region (part of 18S rDNA, ITS1, 5.8S rDNA, ITS2 and part of 28S rDNA) was amplified by a nested PCR as described by Sandor et al. (2017). The amplification of COI was performed using the primers (Plat-diploCOX1F and Plat-diploCOX1R) and protocol described by Moszc- zynska et al. (2009). PCR products were electrophoresed in 1.0% agarose gels in Tris-Acetate-EDTA (TAE) buffer gel, stained with 1% ethidium bromide. Purification was carried out with EZ-10 Spin Column PCR Purification Kit (Bio Basic Inc., Markham, Canada). Purified PCR products of the ITS region and COI were sequenced with the PCR primers and with two additional inner primers 5.8Sr (50-TGTCGAT- GAAGAGCGCAGC-30) and 5.8S2 (50-TAAGCCGACCCTCGG- ACAGG-30) (Tkach et al. 2000) in the case of the ITS region. ABI BigDye Terminator v3.1 Cycle Sequencing Kit was used for sequencing and the sequences read using an ABI 3100 Genetic Analyser.
Phylogenetic analysis
Assembly of the sequenced fragments was done by MEGA version X (Kumar et al. 2018) and ambiguous bases clarified using corresponding ABI chromatograms. Alignments of the genes ITS and COI were done with the software CLUSTAL W (Thompson et al. 1994). The two alignments (ITS region and COI) were corrected manually using the alignment editor of the software MEGA version X. Sequences were deposited in the GenBank under the accession numbers (MN08027492, MN17928090, Table 3). DNA pairwise distances were calculated with the MEGA X software using the p-distance substitution model. Maximum likelihood (ML) analysis was performed for both alignments. The analysed samples are listed inTable 3.Bolbophorus damni- ficus and Clinostomum marginatum (AF470583 and MK426663) were chosen as the outgroup for ITS and COI genes. The dataset was tested using MEGA X for the nucleotide substitution model of best fit, and the model, shown by the Akaike Information Criterion (AIC) as the best-fitting one, was chosen for each partition. ML analyses were performed in MEGA X under the HKYþG model for the ITS region and GTRþG for the COI. Bootstrap values based on 1,000 resampled datasets were generated. The phylogenetic trees were visualised using the tree explorer of MEGA X.
RESULTS
The first Posthodiplostomum centrarchi infection of 6 pumpkinseed individuals was accidentally recorded from
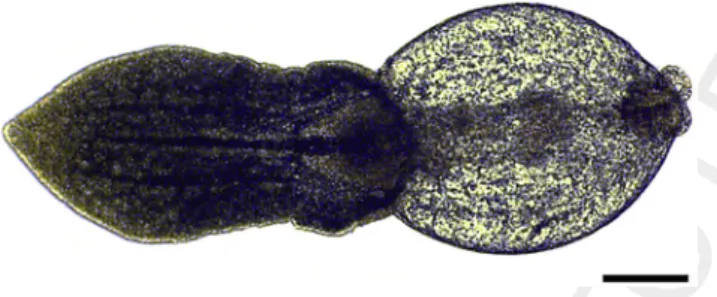
the Maconka water reservoir in 2015. Each fish was par- asitised by 35–150 metacercariae located on the surface of internal organs and the mesentery; furthermore, some individuals were found in excysted condition. The excysted specimens clearly showed the morphological features of the genus Posthodiplostomum and corre- sponded to P. centrarchi (Figs 1 and 2) as characterised and depicted byHoffman (1999)andKvach et al. (2018a).
In 2016 and 2017, large numbers of pumpkinseeds were examined from different regions of Lake Balaton and from the rivers Danube and Ipoly but such infection proved to be absent from these samples. Finally, our research iso- lated metacercariae of P. centrarchi in pumpkinseed specimens from the Sio channel in 2018, at the outflow of the water-gate. No P. centrarchi infection was found in roach and white bream dissected as controls; nevertheless, cysts of P. cuticola were recorded in 3 white bream specimens. In contrast, 4 out of the 10 pumpkinseed specimens collected from the Sio channel showed infec- tion with 20–150 metacercariae.
These twoPosthodiplostomumspecies could be identified and distinguished easily under compound and dissecting microscope based their morphology and predilection site.
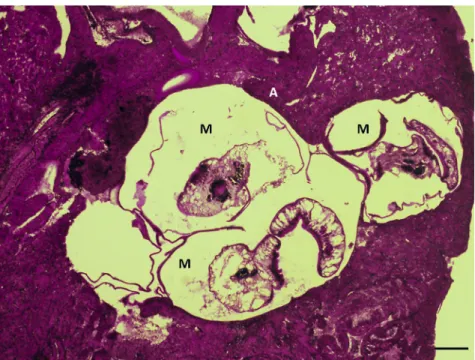
All specimens ofP. centrarchiwere found in the abdominal cavity contrary to metacercariae of P. cuticola, which were located in the skin, muscles andfins. In histological sections, theP. centrarchicysts were mostly found in the mesenteries and inside the interstitium of the kidney and liver (Fig. 3).
Less frequently they were located in the muscle of the heart’s atrium (Fig. 4). They were never found in the skeletal muscle or in the muscle of the ventricle but they readily adhered to this latter. A special observation is that 3 out of the 4 P.
centrarchi infected specimens from the Sio channel had a heavy co-infection with the nematode Schulmanela petrushewskii (Shulman 1948) Ivashkin 1964 in the liver (Fig. 5).
In the first experimental infection, two chicks were fed withP. centrarchimetacercariae per os. After 8 days, 5 and 4 adult trematodes, respectively, were found in their colon at dissection (Fig. 6). During the second experiment, when three chickens were infected withP. cuticolametacercariae, only 2 adultflukes were found in the bursa of Fabricius of a single chick after incubation for 8 days.
NineteenPosthodiplostomumsamples were analysed for the ITS region and COI genes, including metacercarial and adult developmental stages (Table 3). The amplified ITS region of the samples was more than 1,200 bps, with the alignment being 1,067 bps long after removing poorly aligned positions and divergent regions, and containing 807 conservative and 216 variable (116 of them parsimony- informative) sites. The COI fragments exceeded 460 bps and the alignment consisted of 458 bps, including 230 conser- vative and 228 variable (158 of them parsimony-informa- tive) sites. The sequences of the ITS region and COI genes of the samples were in agreement with the results from the morphological and experimental studies. The analysed samples were similarly positioned on both phylogenetic trees (Figs 7A and B), one major group constituted by the samples 343
344 345 346 347 348 349 350 351 352 353 354 355 356 357 358 359 360 361 362 363 364 365 366 367 368 369 370 371 372 373 374 375 376 377 378 379 380 381 382 383 384 385 386 387 388 389 390 391 392 393 394 395 396 397 398 399
400 401 402 403 404 405 406 407 408 409 410 411 412 413 414 415 416 417 418 419 420 421 422 423 424 425 426 427 428 429 430 431 432 433 434 435 436 437 438 439 440 441 442 443 444 445 446 447 448 449 450 451 452 453 454 455 456
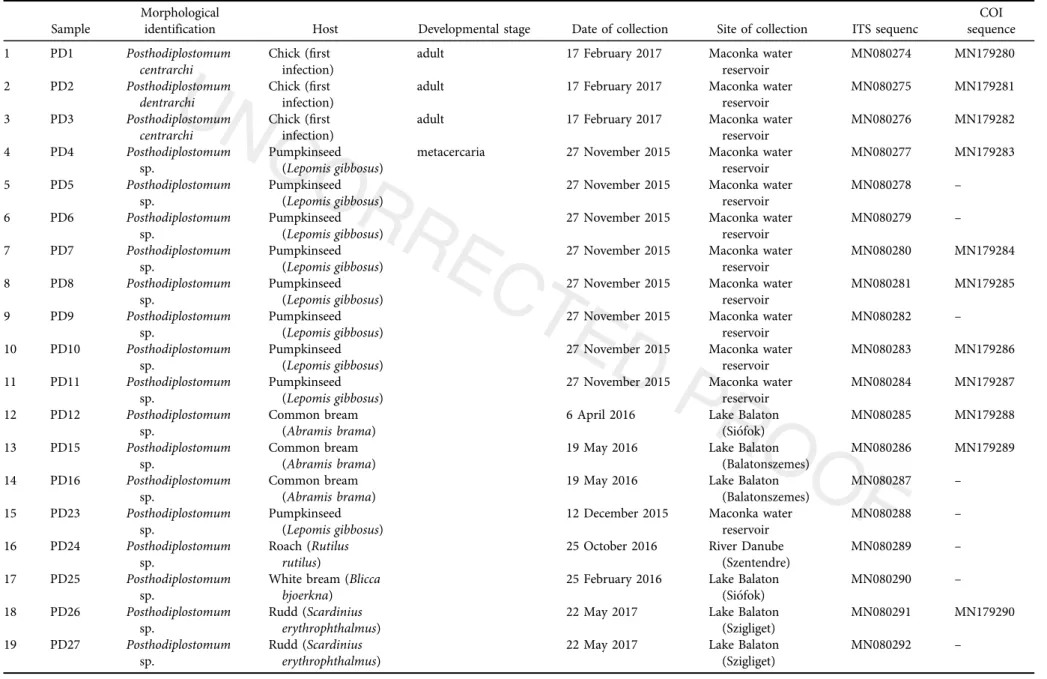
Table 3.List of the sequenced metacercariae and adult samples ofPosthodiplostomumspp.
Sample
Morphological
identification Host Developmental stage Date of collection Site of collection ITS sequenc
COI sequence
1 PD1 Posthodiplostomum
centrarchi
Chick (first infection)
adult 17 February 2017 Maconka water
reservoir
MN080274 MN179280
2 PD2 Posthodiplostomum
dentrarchi
Chick (first infection)
adult 17 February 2017 Maconka water
reservoir
MN080275 MN179281
3 PD3 Posthodiplostomum
centrarchi
Chick (first infection)
adult 17 February 2017 Maconka water
reservoir
MN080276 MN179282
4 PD4 Posthodiplostomum
sp.
Pumpkinseed (Lepomis gibbosus)
metacercaria 27 November 2015 Maconka water reservoir
MN080277 MN179283
5 PD5 Posthodiplostomum
sp.
Pumpkinseed (Lepomis gibbosus)
27 November 2015 Maconka water reservoir
MN080278 –
6 PD6 Posthodiplostomum
sp.
Pumpkinseed (Lepomis gibbosus)
27 November 2015 Maconka water reservoir
MN080279 –
7 PD7 Posthodiplostomum
sp.
Pumpkinseed (Lepomis gibbosus)
27 November 2015 Maconka water reservoir
MN080280 MN179284
8 PD8 Posthodiplostomum
sp.
Pumpkinseed (Lepomis gibbosus)
27 November 2015 Maconka water reservoir
MN080281 MN179285
9 PD9 Posthodiplostomum
sp.
Pumpkinseed (Lepomis gibbosus)
27 November 2015 Maconka water reservoir
MN080282 –
10 PD10 Posthodiplostomum sp.
Pumpkinseed (Lepomis gibbosus)
27 November 2015 Maconka water reservoir
MN080283 MN179286 11 PD11 Posthodiplostomum
sp.
Pumpkinseed (Lepomis gibbosus)
27 November 2015 Maconka water reservoir
MN080284 MN179287 12 PD12 Posthodiplostomum
sp.
Common bream (Abramis brama)
6 April 2016 Lake Balaton (Siofok)
MN080285 MN179288 13 PD15 Posthodiplostomum
sp.
Common bream (Abramis brama)
19 May 2016 Lake Balaton (Balatonszemes)
MN080286 MN179289 14 PD16 Posthodiplostomum
sp.
Common bream (Abramis brama)
19 May 2016 Lake Balaton (Balatonszemes)
MN080287 –
15 PD23 Posthodiplostomum sp.
Pumpkinseed (Lepomis gibbosus)
12 December 2015 Maconka water reservoir
MN080288 –
16 PD24 Posthodiplostomum sp.
Roach (Rutilus rutilus)
25 October 2016 River Danube (Szentendre)
MN080289 –
17 PD25 Posthodiplostomum sp.
White bream (Blicca bjoerkna)
25 February 2016 Lake Balaton (Siofok)
MN080290 –
18 PD26 Posthodiplostomum sp.
Rudd (Scardinius erythrophthalmus)
22 May 2017 Lake Balaton (Szigliget)
MN080291 MN179290 19 PD27 Posthodiplostomum
sp.
Rudd (Scardinius erythrophthalmus)
22 May 2017 Lake Balaton (Szigliget)
MN080292 –
ActaVeterinariaHungarica
5
457458459460461462463464465466467468469470471472473474475476477478479480481482483484485486487488489490491492493494495496497498499500501502503504505506507508509510511512513 514515516517518519520521522523524525526527528529530531532533534535536537538539540541542543544545546547548549550551552553554555556557558559560561562563564565566567568569570
RESEARCHARTICLEAVET-2020.00001_proof21February20209:20pm
belonging toP. centrarchi, and apart from them another one consisting ofP. cuticolasamples, both groups supported by high bootstrap values (above 97). Between them, several other Posthodiplostomum and Ornithodiplostomum species were placed, most of them described and discussed by Locke et al. (2010a,b).
DISCUSSION
In this study, the first finding of Posthodiplostomum cen- trarchimetacercariae was reported from pumpkinseeds in a small Hungarian water reservoir (Maconka) in 2015. In 2018, additional data were presented about the current presence of this non-native trematode parasite from Sio channel, which is the outflow of Lake Balaton. This was the first record of P. centrarchi being in close relation with the waters of Lake Balaton. All samples were examined by morphological, histological and molecular methods;
furthermore, 5 one-day-old chicks were infected with met- acercariae in order to obtain adult stages ofP. centrarchi.
The parasite fauna of pumpkinseed in Europe is rela- tively well studied: for a long time only three monogenean species,Gyrodactylus avalonia Hanek and Threlfall, 1969, Onchocleidus similis M€uller, 1936 and O. dispar M€uller, 1936 were known from Hungary and the neighbouring countries (Gussev et al. 1985;Kvach et al. 2018b;Moravec 2001; Ondrackova et al. 2011; Roman 1953; Roman- Chiriac 1960; Stoyanov et al. 2017b; Vojtek 1958), repre- senting the original American trematode parasite fauna of pumpkinseeds. However, several other protozoan and helminth species common in Europe were also recorded Figure 2.Excysted metacercaria of Posthodiplostomum centrarchi
from the abdominal cavity of a pumpkinseed. Scale bar5100mm
Figure 3.Histological section of the liver of a pumpkinseed infected with two metacercariae ofPosthodiplostomum centrarchi. M: meta- cercaria. Haematoxylin and eosin (HE) staining. Scale bar5100mm
Figure 1.Encysted metacercaria ofPosthodiplostomum centrarchi from the abdominal cavity of a pumpkinseed. Scale bar5100mm 571
572 573 574 575 576 577 578 579 580 581 582 583 584 585 586 587 588 589 590 591 592 593 594 595 596 597 598 599 600 601 602 603 604 605 606 607 608 609 610 611 612 613 614 615 616 617 618 619 620 621 622 623 624 625 626 627
628 629 630 631 632 633 634 635 636 637 638 639 640 641 642 643 644 645 646 647 648 649 650 651 652 653 654 655 656 657 658 659 660 661 662 663 664 665 666 667 668 669 670 671 672 673 674 675 676 677 678 679 680 681 682 683 684
in thisfish (Moshu 2012,2014;Roman-Chiriac 1960). The pumpkinseed has been present in Hungary since 1905 and it became widespread all over the country during the second half of the last century (Takacs et al. 2017).Molnar (1963,1968)was thefirst to study the parasite fauna of the pumpkinseed in Hungary and he reported also O. similis andO. dispar.Recently,Stoyanov et al. (2017a)has proved thatP. centrarchioccurs in several European countries and even in Sturovo (478490 3400 N, 188380 3800 E), Slovakia,
next to the Hungarian border.Kvach et al. (2018a)stated that the infection of the European pumpkinseed popula- tion with P. centrarchi might be caused by the repeated imports of largemouth bass (Micropterus salmoides) to Europe. However, the results ofBoone et al. (2018)seem to contradict this by emphasising that there are differences in the host specificity of Postodiplostomum species towards the fish species belonging to the genera Micropterusand Lepomis.
Figure 4.Histological section of the atrium of the heart of a pumpkinseed infected with metacercariae ofPosthodiplostomum centrarchi.M:
metacercaria. HE staining. Scale bar5100mm
Figure 5.Histological section of the liver of a pumpkinseed containing two metacercariae of Posthodiplostomum centrarchi and several specimens ofSchulmanela petruschewskiinematodes (arrows). M: metacercaria. HE staining. Scale bar5100mm
Acta Veterinaria Hungarica
7
685 686 687 688 689 690 691 692 693 694 695 696 697 698 699 700 701 702 703 704 705 706 707 708 709 710 711 712 713 714 715 716 717 718 719 720 721 722 723 724 725 726 727 728 729 730 731 732 733 734 735 736 737 738 739 740 741
742 743 744 745 746 747 748 749 750 751 752 753 754 755 756 757 758 759 760 761 762 763 764 765 766 767 768 769 770 771 772 773 774 775 776 777 778 779 780 781 782 783 784 785 786 787 788 789 790 791 792 793 794 795 796 797 798
RESEARCH ARTICLEAVET-2020.00001_proof 21 February 2020 9:20 pm
The occurrence of P. centrarchi in Hungary showed a unique pattern. After finding the parasite in a small water reservoir, several efforts were made to recover it from other areas in Hungary where pumpkinseed are abundant. Lake Balaton is the largest natural water in Hungary and the adjacent countries, where the most abundant population of pumpkinseeds is present. Despite dissecting pumpkinseeds of different size from distinct parts of Lake Balaton, no infected specimens were detected. Surprisingly, a relatively heavy infection was recorded in the Sio channel, only a few metres
away from Lake Balaton. In contrast, the native P. cuticola was detectable in cyprinidfishes all over Lake Balaton as well as in the river Danube and its tributaries.
The spread of trematodes by their possible final host is understudied due to the difficulties of following water-birds and their parasites across countries. Up to now, Stoyanov et al. (2017a) has reported an adult Posthodiplostomum centrarchifrom the small intestine of a grey heron (Ardea cinerea L.) at a bird recovery centre in Catalonia (Spain), originating from the Lagoon Bassa de les Olles, Ebro Delta, Spain. Our research group tried to get additional data on the host range of this trematode but wild water-birds were not available for research owing to the strict protection of wildlife. However, the infection experiments were successful, although the adult stages in chicks do not necessarily prove the wide host range of this trematode because these freshly hatched birds can be infected by several unspecific parasites.
Nevertheless, the adult life stages obtained in infection ex- periments might serve as a basis for further research.
Despite heavy infections with P. centrarchi metacercar- iae, clinical changes were not found in the dissected pumpkinseed specimens. Remarkable histological changes with a general host reaction were not diagnosed either in the case when metacercariae were located inside internal organs or in the case when dozens of metacercariae covered the Figure 6.Micrographs of adult of Posthodiplostomum centrarchi
collected post mortem from the guts of experimentally infected chicks. Scale bar5100mm
Figure 7.Maximum likelihood tree of the samples ofPosthodiplostomumspp. from the present study (A: ITS region, B: COI) in relation to other diplostomid sequences deposited in GenBank. Bootstrap values are given at the nodes. Samples from the present study are in bold. The
scale bar indicates the expected number of substitutions per site 799
800 801 802 803 804 805 806 807 808 809 810 811 812 813 814 815 816 817 818 819 820 821 822 823 824 825 826 827 828 829 830 831 832 833 834 835 836 837 838 839 840 841 842 843 844 845 846 847 848 849 850 851 852 853 854 855
856 857 858 859 860 861 862 863 864 865 866 867 868 869 870 871 872 873 874 875 876 877 878 879 880 881 882 883 884 885 886 887 888 889 890 891 892 893 894 895 896 897 898 899 900 901 902 903 904 905 906 907 908 909 910 911 912
serous membranes in the abdominal cavity. Infection of the liver of pumpkinseed with Schulmanela petruschewskii nematodes also seems to be common in the former USSR as Moravec (1994)reported the results ofShulman (1948)who had recorded heavy infections similar to our cases.Stoyanov et al. (2017b) also found this parasite frequently in pump- kinseed in Bulgaria. Nevertheless, changes in the liver due to P. centrarchiinfection, especially in specimens also infected with Schulmanela nematodes, indicate the potential patho- genicity of P. centrarchi.
ACKNOWLEDGEMENTS
This work was supported by the European Regional and Development Fund and the Government of Hungary project GINOP-2.3.2-15-2016-00004: ‘Establishing the sustainable angling-aimed management of Lake Balaton’.
REFERENCES
Bykhovskaya-Pavlovskaya, I. E. and Kulakovskaya, A. P. (1985):
Class Trematoda Rudolphi, 1808. In: Bauer, O. N. (ed.), Key to Parasites of Freshwater Fishes of the Fauna of USSR. Parasitic Metazoans. Nauka Publishing Leningrad Branch. USSR, Leningrad, Russia3(2), 77–198.
Boone, E. C., Laursen, J. R., Colombo, R. E., Meiners, S. J., Romani, M. F. and Keeney, D. B. (2018): Infection patterns and molecular data reveal host and tissue specificity of Posthodiplostomum species in centrarchid hosts. Parasitology145, 1458–1468.
Czegledi,
Q3 I., Preiszner, B., Vital, Z., Kern, B., Boross, N., Specziar, A., Takacs, P. and Er}os, T. (2019): Habitat use of invasive monkey goby (Neogobius fluviatilis) and pumpkinseed (Lep- omis gibbosus) in Lake Balaton (Hungary): a comparison of electrofishing and fyke netting. Hydrobiologia 1–12.
Grabda-Kazubska, B., Baturo-Warszawska, B. and Pojma~nska, T.
(1987): Dynamics of parasite infestation offish in lakes Dgał Wielki and Warniak in connection with introduction of phytophagous species. Acta Parasitol. Pol.32, 1–28.
Gussev, A. V. (1985): Class Monogenea, subclass Polyonchoinea, order Dactylogyridea. 1 Family Dactylogyridae, 2 Family Ancyrocephalidae. In: Gussev A. V. (ed.), Key to Parasites of Freshwater Fishes of the Fauna of USSR. Parasitic Metazoans.
Nauka Publishing, Leningrad Branch. USSR, Leningrad, Russia 2(1), 10–253.
Hoffman, G. L. (1958): Experimental studies on the cercaria and metacercaria of a strigeoid trematode, Posthodiplostomum minimum. Exp. Parasitol.7, 23–50.
Hoffman, G. L. (1999): Parasites of North American freshwater fishes, 2nd edition Cornell University Press, Ithaca & London.
539 pp.
Horak, P., Kolarova, L. and Mikeš, L. (2014): Schistosomatoidea and Diplostomoidea. Adv. Exp. Med. Biol.76, 331–364.
Iqbal, Z., Shukerova, S. A. and Minhas, I. K. (2014): Occurrence of black spot disease inLabeo rohita (Hamilton) fry in carpfish hatchery Lahore, Pakistan. Can. J. Pure. Appl. Sci.8, 2727–2731.
Jaczo, I. (1941): Parasitology lecture notes [in Hungarian]. I.
Magyar. Biol. Kut. Munk.13, 277–289.
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018):
MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol.35, 1547–1549.
Kvach, Y., Jurajda, P., Bryjova, A., Trichkova, T., Ribeiro, F., Prikrylova, I. and Ondrackova, M. (2017): European distri- bution for metacercariae of the North American digenean Posthodiplostomum cf. minimum centrarchi (Strigeiformes:
Diplostomidae). Parasitol. Int.66, 635–642.
Kvach, Y., Matvienko, N., Bryjova, A. and Ondrackova, M. (2018a):
Aquaculture as a possible vector in the spread of Post- hodiplostomum centrarchi (Hoffman, 1958) (Digenea: Diplo- stomidae) in Europe. BioInv. Rec.7, 427–432.
Kvach, Y., Ondrackova, M., Kutsokon, Y. and Dzyziuk, N. (2018b):
New record of monogenean parasites on non-indigenousfishes in the Ukrainian Danube delta. BioInv. Rec.7, 65–72.
Lane, R. and Morris, J. (2000): Biology, prevention and effects of common grubs (digenetic trematodes) in freshwaterfish. Tech.
Bull.115, 1–6.
Lane, B., Spier, T., Wiederholt, J. and Meagher, S. (2015): Host specificity of a parasiticfluke: isPosthodiplostomum minimuma centrarchid-infecting generalist or specialist? J. Parasitol.101, 6–17.
Locke, S. A., McLaughlin D. J. and Marcogliese, D. J. (2010a): DNA barcodes show cryptic diversity and a potential physiological basis for host specificity among Diplostomoidea (Platy- helminthes: Digenea) parasitizing freshwater fishes in the St.
Lawrence River, Canada. Mol. Ecol.19, 2813–2827.
Locke, S. A., McLaughlin D. J., Dayanandan S. and Marcogliese D.
J. (2010b): Diversity and specificity inDiplostomumspp. met- acercariae in freshwaterfishes revealed by cytochrome c oxidase I and internal transcribed spacer sequences. Int. J. Parasitol.40, 333–343.
Lopez-Hernandez, D., Locke, A. S., Lane de Melo, A., Rabelo, L. M.
E. and Pinto, S. H. (2018): Molecular, morphological and experimental assessment of the life cycle ofPosthodiplostomum nanum Dubois, 1937 (Trematoda: Diplostomatidae) from Brazil, with phylogenetic evidence of the paraphyly of the genus PosthodiplostomumDubois, 1936. Infect. Genet. Evol.63, 95–
103.
Lucky, Z. (1970): Pathological changes with posthodiplostomosis of fish fry. Acta. Vet. Brno.1, 51–66.
Lumsden, R. D. and Zischke, J. A. (1963): Studies on the trematodes of Louisiana birds. Z. Parasitenkd.22, 316–366.
Miller, J. H. (1954): Studies on the life history of Post- hodiplostomum minimum(MacCallum 1921). J. Parasitol. 40, 255–270.
Molnar, K. (1963): Mono- and digenetic trematodes fromfishes [in Hungarian].Allattani K€ ozlemenyek50, 103–107.
Molnar, K. (1968): Beitr€age zur Kenntnis der Fischparasiten in Ungarn. 3. Weitere Monogeneidenarten aus Fischen [in German]. Acta. Vet. Acad. Sci. Hung.18, 295–311.
Molnar, K. (1969): Beitr€age zur Kenntnis der Fischparasitenfauna Ungarns IV. Trematoden [in German]. Parasitol. Hung. 2, 119–136.
Moravec, F. (1994): Parasitic nematodes of freshwater fishes in Europe, Academia, Prague,168, 473.
Acta Veterinaria Hungarica
9
913 914 915 916 917 918 919 920 921 922 923 924 925 926 927 928 929 930 931 932 933 934 935 936 937 938 939 940 941 942 943 944 945 946 947 948 949 950 951 952 953 954 955 956 957 958 959 960 961 962 963 964 965 966 967 968 969
970 971 972 973 974 975 976 977 978 979 980 981 982 983 984 985 986 987 988 989 990 991 992 993 994 995 996 997 998 999 1000 1001 1002 1003 1004 1005 1006 1007 1008 1009 1010 1011 1012 1013 1014 1015 1016 1017 1018 1019 1020 1021 1022 1023 1024 1025 1026
RESEARCH ARTICLEAVET-2020.00001_proof 21 February 2020 9:20 pm
Moravec, F. (2001): Checklist of the metazoan parasites offishes of the Czech Republic and the Slovak Republic (1873–2000).
Academia, Prague, 168.
Moshu, A. (2012): Protistian parasites (Protista) of the pumpkin- seed, Lepomis gibbosus (L., 1758) (Perciformes, Centrarchidae), from of Prut-Dniester hydrographic interfluvial space (in Russian, with English summary). Materials of the International Conference‘Modern Problems of General Parasitology’, Mos- cow, October 30–November 1, 2012, pp. 225–229.
Moshu, A. (2014): Helminths offishes from waters of Prut-Dniester interfluve potentially dangerous to human health [in Russian].
Eco-TIRAS,
Q4 Chis¸inau 88.
Moszczynska, A., Locke, S. A., McLaughlin, J. D., Marcogliese, D. J.
and Crease, T. J. (2009): Development of primers for the mitochondrial cytochrome c oxidase I gene in digenetic trem- atodes (Platyhelminthes) illustrates the challenge of barcoding helminths. Mol. Ecol. Resour.9(Suppl. s1), 75–82.
Niewiadomska, K. (2002): Family Diplostomidae Poirier, 1886. In:
Gibson, D. I., Jones, A., Bray, R. A. (eds) Keys to the Trematoda Volume 1. CAB International and the Natural History Museum, London, U.K. pp. 167–196.
Ondrackova, M., Bartosova, S., Valova, Z., Jurajda, P. and Gelnar, M. (2004): Occurrence of black-spot disease caused by meta- cercariae ofPosthodiplostomum cuticolaamong juvenilefishes in water bodies in the Morava River basin. Acta. Parasitol.49, 222–227.
Ondrackova, M., Davidova, M., Prikrylova, I. and Pecínkova, M.
(2011): Monogenean parasites of introduced pumpkinseed Lepomis gibbosus(Centrarchidae) in the Danube River Basin. J.
Helminthol.85, 435–441.
Ondrackova, M., Kvach, Y., Martens, A. and Jurajda, P. (2019):
Limited parasite acquisition by non-native Lepomis gibbosus (Antinopterygii: Centrarchidae) in two ponds at the Upper Rhine in Germany. J. Helminthol.93, 453–460.
Ritossa, L., Flores, V. and Viozzi, G. (2013): Life-cycle stages of a Posthodiplostomum species (Digenea: Diplostomidae) from Patagonia, Argentina. J. Parasitol.99, 777–780.
Rolbiecki, L. (2004): Distribution of Posthodiplostomum cuticola (Nordmann, 1832) (Digenea; Diplostomidae) metacercariae in cyprinids of the Vistula lagoon, Poland. Arch. Pol. Fish.12, 93–98.
Roman, E. (1953): Parasite fauna of sunfishLepomis gibbosus(L.), acclimatized in the Danube. Doklady Akademii Nauk SSSR89, 765–768.
Roman-Chiriac, E. (1960): Clasa Monogenoidea. In: (eds) Fauna Republici Populare Romine. Platyhelminthes2, p. 147.
Sandor, D., Molnar, K., Gibson, D. I., Szekely, C. S., Majoros, G.
and Cech, G. (2017): An investigation of the host-specificity of metacercariae of species of Apophallus (Digenea:
Heterophyidae) in freshwater fishes using morphological, experimental and molecular methods. Parasitol. Res. 116, 3065–3076.
Sch€aperclaus, W. M. (1990): Fischkrankheiten.fifth Auflage. Aca- demie Verlag, Berlin.
Shulman, S. S. (1948): A new species of round worms parasitic in liver of fishes. Izvestiya Vsesoyuznogo Nauchno-Issledova- tel’skogo Instituta Ozernogo i Rechnogo Ribnogo Khozyaystva 37, 235–238.
Stoyanov, B., Georgieva, S., Pankov, P., Kudlai, O., Kostadinova, A.
and Georgiev, B. B., (2017a): Morphology and molecules reveal the alien Posthodiplostomum centrarchi Hoffman, 1958 as the third species of Posthodiplostomum Dubois, 1936 (Digenea:
Diplostomidae) in Europe. Syst. Parasitol.94, 1–20.
Stoyanov, B., Mutafchiev, Y., Pankov, P., and Georgiev, B. B.
(2017b): Helminth parasites in the alienLepomis gibbosus(L.) (Centrarchidae) from the Lake Atanasovsko wetlands, Bulgaria:
survey of species and structure of helminth communities. Acta.
Zool. Bulg.69, 555–574.
Szekely, C. and Molnar, K. (1996): Preliminary survey of the parasite fauna of some important fish species in the Upper- Reservoir of the Kis-Balaton System. Parasit. Hung.29–30, 45–
54.
Takacs, P., Czegledi, I., Ferincz,A., S aly, P., Specziar, A., Vital, Z., Weiperth, A. and Er}os, T. (2017): Non-nativefish species in Hungarian waters: historical overview, potential sources and recent trends in their distribution. Hydrobiologia795, 1–22.
Thompson, J. D., Higgins, D. G. and Gibson, T. J. (1994): CLUS- TAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position- specific gap penalties and weight matrix choice. Nucleic Acids Res.22, 4673–4680.
Tkach, V. V., Pawlowski, J. and Sharpilo, V. P. (2000): Molecular and morphological differentiation between species of thePla- giorchis vespertilionisgroup (Digenea, Plagiorchiidae) occurring in European bats, with a redescription of P. vespertilionis (M€uller, 1780). Syst. Parasitol.47, 9–22.
Tobler, M. and Schlupp, I. (2008): Influence of black spot disease on shoaling behaviour in female western mosquitofish, Gambusia affinis(Poeciliidae, Teleostei). Environ. Biol. Fish81, 29–34.
Vojtek, J. (1958):UrocleidusMueller, 1934, Novy rodzabrohlístu (Trematoda, Monogenea) PRO CSR. Biologia (Bratislava) 13, 612–615.
Zrncic, S., Oraic, D., Mihaljevic,Ž.,Caleta, M., Zanella, D., Jeli c, D., and Jelic, M. (2009): First observation of Posthodiplostomum cuticola (Nordmann, 1832) metacercariae in cypriniformesQ2 from Croatia. Helminthologia46, 112–116.
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited, a link to the CC License is provided, and changes–if any–are indicated. (SID_1)
1027 1028 1029 1030 1031 1032 1033 1034 1035 1036 1037 1038 1039 1040 1041 1042 1043 1044 1045 1046 1047 1048 1049 1050 1051 1052 1053 1054 1055 1056 1057 1058 1059 1060 1061 1062 1063 1064 1065 1066 1067 1068 1069 1070 1071 1072 1073 1074 1075 1076 1077 1078 1079 1080 1081 1082 1083
1084 1085 1086 1087 1088 1089 1090 1091 1092 1093 1094 1095 1096 1097 1098 1099 1100 1101 1102 1103 1104 1105 1106 1107 1108 1109 1110 1111 1112 1113 1114 1115 1116 1117 1118 1119 1120 1121 1122 1123 1124 1125 1126 1127 1128 1129 1130 1131 1132 1133 1134 1135 1136 1137 1138 1139 1140