Válasz Dr. Hideg Éva, az MTA Doktora bírálatára
Megköszönöm alapos munkáját a dolgozat gondos átvizsgálásában. Kritikai megjegyzéseire és kérdéseire az alábbiakban válaszolok:
Megjegyzések:
1) Az anyag és módszer részb ı l kimaradt, hogy a klorofill-a fluoreszcencia indukció és fotoszintetikus aktivitás mérése során milyen volt a fényintenzitás.
A következ ı mér ı fényeket alkalmaztuk:
Klorofill-a fluoreszcencia mérések: PAM-2000 készüléket használtunk. A 2. fotokémiai rendszer kvantumhatásfoka paraméternél a mér ı fény << 1 µmol m
-2s
-1, a telítési fény megközelít ı leg 8000 µmol m
-2s
-1, az aktinikus fény pedig a kamrában lév ı fény volt (200- 250 µmol m
-2s
-1).
A LI-6400 készülékkel mért fotoszintézis paramétereknél 200 µmol m
-2s
-1intenzitású fényt használtunk.
2) Az értekezésben benne maradt néhány laboratóriumi zsargon.
A jöv ı ben jobban fogok figyelni a helyes nyelvhasználatra.
3) Az eredmények megvitatása rész végére hasznos lett volna beilleszteni egy összefoglaló ábrát.
Az ábra az el ı adásba már be fog kerülni.
Kérdések:
1) A szalicilsav hatásvizsgálataira létrehozott, biokémiailag komplex, növényfiziológiai mérésekkel is kell ı képpen kiegészített kísérleti rendszerb ı l már csak a génaktivációs ill. - expressziós kutatási megközelítés hiányzik. Érdekes lenne például annak nyomonkövetése, milyen szabályozási szinteken befolyásolja a szalicilsav az antioxidánsok megfigyelt változásait. Vannak-e ilyen kísérletek tervben, esetleg folyamatban?
Az antioxidánsokkal kapcsolatban nem végeztünk ilyen vizsgálatokat és egyel ı re
folyamatban sincsenek, de a magáztatásos kísérletek kapcsán végeztünk a jelöléses kísérletek
mellett génexpressziós kísérleteket is. Az alábbi ábrán az izokorizmát-szintáz (a) és az
korizmát-szintáz (b) enzimek génexpressziójában bekövetkezett változások láthatóak SA
kezelés hatására borsóban a különböz ı növényi részekben.
Mind a korizmát-szintáz, mind az izokorizmát-szintáz génexpressziója megn ı tt az epikotilban, ami összhangban volt az epikotil megemelkedett SA szintjével, míg a jelölt SA nem volt kimutatható a szabad SA frakcióban és a kötöttben is csak jóval kisebb mennyiségben, mint azt az egyébként mért szint indokolta volna. Ezzel még jobban alátámasztottuk, hogy a megnövekedett SA-szint de novo szintézis eredménye. Az eredmények a Journal of Plant Physiologyban jelentek meg 2011-ben, a különlenyomatot a válaszhoz csatolom.
2) A tápoldathoz adott kadmium hatásaival kapcsolatos kérdésem: A szalicilsav levélkárosodás mérsékl ı hatása a levelek magasabb kadmium tartalma és a gyökerek életképesség csökkenése mellett volt megfigyelhet ı . Hogyan magyarázható a levél membránok kisebb mérték ő károsodása a nagyobb mennyiség ő kadmium ellenére?
Korábban kimutattuk, hogy a SA kezelés hatására megn ı a poliaminok mennyisége kukoricanövényekben. A putreszcin szintje már a SA kezelés során megn ı tt normál h ı mérsékleten, míg a spermidin szint csak a hideg hatására a SA-kezelt növényekben. Mivel a poliaminok polikationok, ezért er ı sen tudnak köt ı dni negatív töltés ő makromolekulákhoz, így pl. a nukleinsavakhoz, fehérjékhez, lipidekhez. A poliaminok gátolják a lipid-peroxidációt és a membránokhoz köt ı dve stabilizálják a foszfolipid-kett ı sréteget, valamint a tilakoidok molekuláris komplexeit.
Cd-stressz esetében ugyan nem végeztünk ilyen vizsgálatokat, de feltételezhetjük, hogy a SA-
el ı kezelés ill. kezelés itt is poliaminok szintézisét indukálhatja, s ez megnövelheti a
membránok stabilitását a megnövekedett Cd szint ellenére is.
Mennyiben tulajdonítható ez a megfigyelt (korlátozott és kis mérték ő ) enzim aktivációnak és mennyiben feltételezhet ı , hogy a szalicilsav más úton változtatja meg a kadmium levélszöveteken belüli hatását?
Mivel a gyökér van kitéve els ı dlegesen a nagyobb fémion-terhelésnek ott egy aránylag nagyobb állandó fitokelatin-szintáz aktivitást mérhetünk. A gyökerekkel ellentétben a levelekben ez az aktivitás alacsony és inkább csak nehézfémstressz hatására, ezen belül is f ı leg Cd-ra n ı meg. Ugyan a fitokelatinok szintje nem n ı tt meg SA kezelés hatására, de mivel a levélbe a gyökérhez képest azért relatíve kevés Cd jut fel, ez az alacsonyabb szint is képes lehet egy részét kelátolni. Ezen kívül a SA kezelés emelte a glutation-reduktáz enzim aktivitását. Korábbi kísérletekben kimutattuk, hogy SA kezelés hatására a redukált glutation mennyisége megemelkedik a növényekben, a megemelkedett glutation-reduktáz aktivitás is hozzájárulhat ehhez. Mivel a Cd oxidatív stresszt okoz a növényekben a megemelkedett redukált glutation szintnek kett ı s szerepe lehet: egyrészt mint antioxidáns csökkentheti a reaktív oxigénformák mennyiségét, ezzel is mérsékelve a stresszhatást, másrészt a glutation SH csoportjaihoz is köt ı dhet a kadmium, s így is csökken a sejtet károsító szabad Cd-ionok mennyisége.
Milyen más, a fotoszintetikus funkciót véd ı szalicilsav indukált mechanizmusokat tart lehetségesnek?
Folyamatban lév ı kísérletek szerint a SA kezelés megnövelte a nem-fotokémiai kioltást dohány növényekben, de hogy pontosan milyen mechanizmus alapján, az még nem tisztázott.
A nem-fotokémiai kioltás egy védekez ı mechanizmusnak tekinthet ı , melynek során az extra gerjesztési energia h ı formájában távozhat. Ezzel kapcsolatban tervbe van véve a xantofill- ciklus komponenseinek vizsgálata SA kezelés során.
Másrészt a fotoszintézis során reaktív oxigénformák keletkeznek, s korábbi munkáinkban kimutattuk, hogy a SA megnöveli egyes antioxidáns enzimek aktivitását, s ezzel is véd ı hatást fejthet ki a fotoszintetikus folyamatokra.
3) Az értekezésben bemutatott kísérletek alátámasztják az endogén ortoortohidroxi-fahéjsav stressz adaptációban játszott kulcs szerepét. Az eredmények arra utalnak, hogy ez a vegyület antioxidáns hatásának tulajdonítható és nem az endogén szalicilsav perkurzor voltának.
Levonható-e az a következtetés is, hogy a vizsgált stressz körülmények között a szalicilsav szint emelkedésnek az orto-ortohidroxi-fahéjsav perkurzor elérhet ı sége nem meghatározó, regulatív lépése?
A SA bioszintézise a sikiminsav-fenilpropanoid útvonalból indul ki. A fenilalanin el ı ször
fahéjsavvá alakul a fenilalanin-ammonia-liáz katalizálta reakcióban. Majd a fahéjsavból két
úton szintetizálódhat a SA. A két út az aromás gy ő r ő hidroxilációs és az oldallánc oxidációs
reakciójának sorrendjében különbözik. Az egyik útvonalon a fahéjsav orto-hidroxi-fahéjsavvá
(oHCA, o-kumársav) alakul egy hidroxilációs lépés során, majd az oHCA az oldalláncon
tovább oxidálódik. A másik lehet ı ség, hogy a fahéjsav el ı ször benzoesavvá (BA) alakul az
oldallánc oxidációja során és a hidroxiláció ezután következik. A szalicilsav bioszintézisének
vázlata az alábbi ábrán található.
Dohány mozaik vírussal fert ı zött dohány levelében azt tapasztalták, hogy 14C-fahéjsavas kezelést követ ı en 14C-jelölt oHCA nem jelent meg, azaz a SA a fenilalaninból a benzoesavon keresztül szintetizálódik. Szintén dohányban mutatták ki, hogy a SA a benzoesavból szintetizálódik, de közvetlen prekurzora nem a szabad benzoesav, sokkal inkább annak konjugált, glikozidos formája. Uborkában, paradicsomban és rizsben is igazolást nyert, hogy a SA a fahéjsavból a benzoesavon keresztül. Nem szabad figyelmen kívül hagynunk azonban azt a tényt, hogy e tanulmányok a patogén-indukált SA-szintézist vizsgálták.
A legújabb kutatási eredmények szerint a SA abiotikus stresszek során is a benzoesavból szintetizálódik a benzoesav-2-hidroxiláz enzim katalizálta reakcióban. Így borsóban h ı akklimatizáció során a szabad SA-szint pozitív korrelációban van a megnövekedett benzoesav-2-hidroxiláz aktivitással. Kukoricában Cd-stressz során a kötött benzoesav akkumulációját mutattuk ki, melyet SA-növekedés követett. Rizsben sóstressz, alacsony h ı mérséklet és H2O2 kezelés hatására is indukálódott a benzoesav-2-hidroxiláz és megemelkedett a SA-tartalom. Ugyanakkor a NaCl-dal nem kezelt rizsnövényekben a SA- tartalmat nem befolyásolta a benzoesav-2-hidroxiláz gátló unikonazol, ami azt feltételezi, hogy stresszmentes állapotban a SA nem a benzoesav-2-hidroxiláz segítségével szintetizálódik.
Baktériumokban mutatták ki el ı ször a SA-szintézis egy harmadik módját, majd Arabidopsis
kloroplasztiszban is azonosították a szintézisért felel ı s izokorizmát-szintázt kódoló gént
(ICS1). Az is bizonyítást nyert, hogy az ICS1 patogén fert ı zés után lokálisan és szisztémásan
is aktiválódik, és az ICS által szintetizált SA szükséges a SAR kialakulásához. Felmerül a
kérdés, ha a fahéjsavból, illetve az izokorizmátból kiinduló SA-szintézis egy adott
növényfajban fordul el ı , mindkét útvonal specifikusan stimulálódik, vagy a fahéjsavas
útvonal csak egy alap SA-szintet produkál a nem fert ı zött növényben. Ez utóbbi feltevést
támasztja alá, hogy Nicotiana benthamiana növényekben az ICS gén expressziójának gátlása
er ı teljesen csökkentette a SA-akkumulációt ózon, illetve patogén stresszt követ ı en. A
fennmaradó SA-szint valószín ő leg a fenilalanin-benzoesav útvonalon keresztül szintetizálódik
és nem képes kompenzálni a génszupresszióból adódó SA-hiányt.
A fenti adatok alapján, mivel a SA-nak akár három prekurzora is lehet a növényekben, s gyakran nem is az oHCA-út m ő ködik, így az oHCA-szint elérhet ı sége nem meghatározó reguláló lépés az SA bioszintézis során. Mint már az 1. kérdésre adandó válaszomban is említettem, génexpresszós vizsgálatoknál mi is azt kaptuk, hogy a korizmát-szintáz és az izokorizmát-szintáz génjeinek expressziója megn ı tt SA kezelésre, ami szintén azt támasztja alá, hogy nem csak az oHCA-n át történ ı szintézis m ő ködik a növényekben. Másrészt irodalmi adatokból az is ismert, hogy a SID2 hiányos Arabidopsis mutánsokban (a SID2 /vagy ICS1/gén kódolja az izokorizmát-szintázt) nagyon lecsökkent a SA szintje, amib ı l arra következtethetünk, hogy Arabidopsisban f ı leg a korizmasav-út m ő ködik és nem a fenilalanin- út, ahogy azt korábban gondolták.
Ismert-e olyan mechanizmus, ami megakadályozza hogy a megnövekedett mennyiség ő orto- ortohidroxi-fahéjsavból ne keletkezzen túl sok, toxikus mennyiség ő szalicilsav?
Mint már említettem, nem az oHCA az egyetlen prekurzora a SA-nak a növényekben, így a különböz ı növényfajokban különböz ı bioszitézisutak kerülhetnek el ı térbe normál körülmények között illetve biotikus vagy abiotikus stresszek során. A szabad oHCA-szint egyik lehetséges szabályozási mechanizmusa lehet a kötött formává alakítás. Búzában hidegedzés során fényben nagymérték ő kötött oHCA felhalmozódást tapasztaltunk. Arra viszont nincsenek adatok, hogy ez a kötött forma visszalakulhat-e szabad oHCA-vá (raktározott forma) vagy pedig végleges inaktiváció.
Vannak-e arra vonatkozó adatok, hogy miben áll az orto-ortohidroxi-fahéjsav feltételezett antioxidáns m ő ködése? Közvetlen (esetleg specifikus) gyök vagy más reaktív oxigén származék semlegesítésében vagy más antioxidánsok aktívációjában?
A fenolsavak, melyek a növényekben széleskör ő en el ı fordulnak, er ı teljes atioxidáns tulajdonsággal rendelkeznek. A fenolsavak egyik csoportja, a hidroxifahéjsavak, melyek gabonanövényekben gyakran el ı fordulnak, szintén jó antioxidáns tulajdonságokat mutatnak.
Ezek közül az egyik leggyakoribb a ferulsav és oxidatív terméke a diferulsav, de ezenkívül még számos fahéjsav, mint pl. a kávésav és az általunk tanulmányozott oHCA is megtalálható a gabonanövényekben. A hidroxifahéjsavak, mint pl. az oHCA, leírták, hogy jól közömbösítik a singlet oxigént, tehát maguk a fahéjsavak antioxidáns tulajdonságúak és nem más védekez ı mechanizmusok aktiválásával vesznek részt az oxidatív stressz elleni védelemben. Így elmondhatjuk, hogy az oHCA felhalmozódás növényekben stressz során függetlenül a szalicilsav bioszintézist ı l a védekez ı mechanizmus része lehet.
És végül megköszönöm Hideg Éva elismer ı szavait, megjegyzéseit, érdekes szakmai kérdéseit és eredményeim új eredményként való elfogadását az általa felsorolt, illetve megjelölt területekben és témákban.
Martonvásár, 2011-04-27
/Szalai Gabriella/
Journal of Plant Physiology168 (2011) 213–219
Contents lists available atScienceDirect
Journal of Plant Physiology
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . d e / j p l p h
Salicylic acid treatment of pea seeds induces its de novo synthesis
Gabriella Szalai
∗, Szabina Horgosi, Vilmos Soós, Imre Majláth, Ervin Balázs, Tibor Janda
Agricultural Research Institute of the Hungarian Academy of Sciences, H-2462 Martonvásár, Brunszvik u. 2., Hungary
a r t i c l e i n f o
Article history:
Received 4 May 2010
Received in revised form 25 June 2010 Accepted 2 July 2010
Keywords:
Antioxidant enzyme Gene expression o-Coumaric acid Polyamines Salicylic acid
a b s t r a c t
Salicylic acid (SA), which is known as a signal molecule in the induction of defense mechanisms in plants, could be a promising compound for the reduction of stress sensitivity. The aim of the present work was to investigate the distribution of SA in young pea (Pisum sativumL.) seedlings grown from seeds soaked in3H-labeled SA solution before sowing, and to study the physiological changes induced by this seed treatment. The most pronounced changes in SA levels occurred in the epicotyl and the seeds. Radioactivity was detected only in the bound form of SA, the majority of which was localized in the seeds, and only a very low level of radioactivity was detected in the epicotyl. SA pre-treatment increased the expression of the chorismate synthase and isochorismate synthase genes in the epicotyl. Pre-soaking the seeds in SA increased the activities of some antioxidant enzymes, namely ascorbate peroxidase (EC 1.11.1.11) and guaiacol peroxidase (EC 1.11.1.7) and the level of ortho-hydroxycinnamic acid, but decreased the level of polyamines. These results suggest that the increased level of free and bound SA detected in plants growing from seeds soaked in SA solution before sowing is the product ofde novosynthesis, rather than having been taken up and mobilized by the plants.
© 2010 Elsevier GmbH. All rights reserved.
Introduction
Plant growth and development are hampered by various biotic and abiotic stress factors. Investigations of compounds capable of reducing the stress sensitivity of crops are of great importance from both theoretical and practical points of view. Plant hormones play an important role in developmental processes, and some of them have key roles in mechanisms leading to acclimation to changing environments. Salicylic acid (SA) has long been known as a sig- nal molecule in the induction of defense mechanisms in plants (Raskin, 1992; Shah, 2003). Recent studies suggest that it also par- ticipates in signaling during abiotic stresses (Horváth et al., 2007).
There are two main routes for SA biosynthesis in plants (Shah, 2003). Earlier studies suggested that SA is synthesized from pheny- lalanine via cinnamic acid, an intermediate in the shikimic acid pathway. The decarboxylation of the side chain of cinnamic acid may generate benzoic acid, which may then undergo hydroxyla- tion at the C-2 position forming SA, as proposed in tobacco (Yalpani et al., 1993; Ribnicky et al., 1998) and rice plants (Silverman et al., 1995). Cinnamic acid can also be hydroxylated by trans-cinnamate-
Abbreviations:cad, cadaverine; CS, chorismate synthase; ICS, isochorismate syn- thase; oHCA, ortho-hydroxycinnamic acid; put, putrescine; SA, salicylic acid; spd, spermidine; spn, spermine.
∗Corresponding author at: Agricultural Research Institute of the Hungarian Academy of Sciences, H-2462 Martonvásár, POB 19, Hungary. Tel.: +36 22569502;
fax: +36 22569576.
E-mail address:szalaig@mail.mgki.hu(G. Szalai).
4-hydroxylase, which was first detected in pea seedlings (Russell and Conn, 1967), forming o-hydroxycinnamic acid (oHCA, or o- coumaric acid). oHCA may also form SA through decarboxylation;
however, the enzyme that activates the conversion of oHCA to SA has not yet been identified (Hayat et al., 2010). It has been suggested that the benzoic acid pathway operated in non-infected tomato plants, whereas seedlings infected withAgrobacterium tumefaciens synthesized SA via oHCA (Chadha and Brown, 1974). Recent stud- ies inArabidopsisplants showed that the phenylalanine pathway cannot account for all the SA in plant cells, suggesting that there is another main route for SA biosynthesis taking place in the chloro- plasts, where SA is synthesized from chorismate via isochorismate in processes catalyzed by the isochorismate synthase (ICS) and iso- chorismate pyruvate lyase enzymes, respectively (Wildermuth et al., 2001; Métraux, 2002; Wildermuth, 2006; Mustafa et al., 2009).
Previous results suggest that SA could be a promising com- pound for the reduction of abiotic stress sensitivity in plants, since under certain conditions it has been found to mitigate the damag- ing effects of various stress factors in plants (Horváth et al., 2007).
Several methods of application (soaking seeds in SA prior to sow- ing, adding SA to the hydroponic solution, irrigating or spraying with SA solution) have been shown to protect various plant species against abiotic stress factors, such as heavy metals (Krantev et al., 2008), high temperature (Dat et al., 1998), chilling (Janda et al., 1999; Szalai et al., 2000), or salinity (El-Tayeb, 2005; Szepesi et al., 2009) by inducing a wide range of processes involved in stress tolerance mechanisms. SA was also shown to influence a number of physiological processes, including seed germination, fruit yield, 0176-1617/$ – see front matter© 2010 Elsevier GmbH. All rights reserved.
doi:10.1016/j.jplph.2010.07.029
214 G. Szalai et al. / Journal of Plant Physiology168 (2011) 213–219
flowering, ion uptake and transport, photosynthetic rate, stomatal conductance, etc. (Raskin 1992). It may induce heat production due to the enhanced activity of the cyanide-resistant or alternative respiration chains (Raskin et al., 1987).
SA is part of an extremely complex signal transduction network linked with pathways related to other stress hormones, such as jas- monic acid (Navarro et al., 2008), gibberellic acids (Alonso-Ramírez et al., 2009) and abscisic acid (Mosher et al., 2010), and several questions about the mode of action of SA are still unanswered. It is assumed that there may be at least two translocated signals: a lipid-derived molecule and MeSA (Park et al., 2007). However, it is not clear whether the effects of exogenous SA are direct or whether they are connected with that of endogenous SA. Furthermore, many related compounds, especially its putative precursors, exert similar effects. It has also been shown that pre-soaking seeds in SA can pro- tect the plants against abiotic stresses (El-Tayeb, 2005; Szepesi et al., 2008; Moussa and El-Gamal, 2009; Popova et al., 2009), but the background of this effect is not known. The aim of the present work was to investigate the distribution of SA in young pea seedlings fol- lowing SA pre-treatment of the seeds, and the changes induced by SA pre-soaking in certain metabolic pathways (polyamines, antiox- idant enzymes), which could be partly responsible for increased stress tolerance.
Materials and methods Plant material
Seeds of pea (Pisum sativumL. ‘Kelvedon’) were soaked in dis- tilled water, or in 0.1 or 0.5 mM salicylic acid (SA) (100 seeds in 100 mL) for 24 h, then sown in trays containing 3:1 (v/v) loamy soil and sand. For the labeled experiments, radioactive SA (ring3H;
296 kBq/100 mL; specific activity: 1850 GBq/mmol; final concen- tration of labeled and carrier/non-labeled/SA: 0.5 mM) was used for soaking. The seedlings were grown at 22/20◦C with 16/8-h light/dark periodicity in a Conviron PGR-15 plant growth cham- ber for seven days. The photosynthetic photon flux density was 340mmol m−2s−1, provided by metal halide lamps, with a relative humidity of 75%. Shoots, epicotyls, seeds and roots were collected for analysis.
Enzyme assays
For the analysis of antioxidant enzyme activity, 0.5 g tissue was homogenized in 2.5 mL ice-cold Tris buffer (0.5 M, pH 7.5) contain- ing 3 mM MgCl2and 1 mM EDTA. The catalase (EC 1.11.1.6.) activity of the extract was measured spectrophotometrically by monitor- ing the decrease in absorbance at 240 nm, as described byJanda et al. (1999). The reaction mixture contained 0.44 M Tris buffer (pH 7.4), 0.0375% H2O2and enzyme extract. The ascorbate peroxidase (EC 1.11.1.11.) activity was determined in the presence of 0.2 M Tris buffer (pH 7.8) and 5.625 mM ascorbic acid, as described by Janda et al. (1999). The reaction was started with 0.042% H2O2. The decrease in absorbance at 290 nm was monitored. The guaia- col peroxidase (EC 1.11.1.7.) activity was measured at 470 nm as described byÁdám et al. (1995). The reaction mixture consisted of 88 mM Na-acetate buffer (pH 5.5), 0.88 mM guaiacol, 0.0375%
H2O2and enzyme extract. The glutathione reductase (EC 1.6.4.2.) activity was determined at 412 nm according toSmith et al. (1988).
The reaction mixture contained 75 mM Na-phosphate buffer (pH 7.5), 0.15 mM diethylenetriamine-pentaacetic acid, 0.75 mM 5,5′- dithiobis(2-nitrobenzoic acid), 0.1 mM NADPH, 0.5 mM oxidized glutathione and 50mL plant extract in a total volume of 1 mL.
The glutathione-S-transferase (EC 2.5.1.18) activity was detected at 340 nm as previously described (Kocsy et al., 2005).
Salicylic acid and o-hydroxycinnamic acid extraction; analytical procedure
SA and o-hydroxycinnamic acid (oHCA) were measured accord- ing toMeuwly and Métraux (1993)andPál et al. (2005)by grinding 1.5 g of plant tissue (approx. 4–5 plants/sample) in liquid nitrogen in a mortar and pestle, in the presence of 0.75 g quartz sand. The tissue powder was transferred to a centrifugation tube and mixed with 2 mL of 70% methanol containing 250 ng ortho-anisic acid (oANI) (used as internal standard) and 25mg para-hydroxybenzoic acid (pHBA) (used as extraction carrier). The extract was cen- trifuged at 10,000×g for 20 min. The pellet was resuspended in 2 mL 90% methanol, vortexed and centrifuged as above. The methanol content was evaporated from 2 mL of the mixed super- natants at room temperature under a vacuum. After adding 1 mL of 5% (w/v) TCA to the residual aqueous phase, the mixture was cen- trifuged in an Eppendorf centrifuge at 15,000×gfor 10 min. The supernatant was gently partitioned twice against 2 mL of a 1:1 (v/v) mixture of ethyl acetate/cyclohexane. The upper organic layers contained the free phenolic portion. The aqueous phases contain- ing the methanol-soluble bound phenolics were acid hydrolyzed by adding 250 ng oANI, 25mg pHBA and 1.3 mL 8 N HCl to the aque- ous phase and incubating for 60 min at 80◦C before partitioning twice as above. Just prior to the HPLC analysis, the samples were resuspended in 1000mL of the HPLC starting mobile phase. After separation on a reverse phase column (ABZ+, 150 mm×4.5 mm, 5mm, Supelco, Bellefonte, USA) SA and oHCA were quantified fluorimetrically (W474 scanning fluorescence detector, Waters, Milford, USA), with excitation at 317 nm and emission at 436 nm for oHCA, followed by excitation at 305 nm and emission at 407 nm for SA. Radioactive peaks were determined using a 150TR flow scin- tillation analyzer (Canberra Packard, Meriden, CT, USA) connected to the HPLC.
Polyamine analysis
Two hundred mg plant tissues were homogenized with 1 mL 0.2 M ice-cold perchloric acid and were allowed to stand on ice for 20 min. The extract was centrifuged at 10,000×gfor 20 min and the supernatant was used. Polyamines were analyzed as dansy- lated derivatives via HPLC using a W2690 separation module and a W474 scanning fluorescence detector (Waters, Milford, MA, USA) as described bySmith and Davies (1985). The results are the means of 5 measurements.
Gene expression analysis
To analyze the expression of the chorismate synthase (CS) and isochorismate synthase (ICS) genes, samples, each derived from five plants, were harvested from epicotyls, leaves, seeds and roots of pea plants exposed to 0.1 and 0.5 mM SA (four technical repli- cates of each of three independent biological replicates). RNA was isolated using TRIzol reagent (Nitrogen, Carlsbad, CA, USA) and the samples were treated with DNase I and cleaned with a MinElute Kit (Qiagen, Hilden, Germany) according to the manu- facturer’s instructions. 150 ng RNA was reverse transcribed with SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA).
Real-time PCR was performed with Applied Biosystems 7500 using SYBR Green detection chemistry (Applied Biosystems, Foster City, CA, USA) and gene-specific primers. For the endogenous control, the pea actin (X90378.1) gene was used (forward: ATGCTAGTG- GCCGTACAACTGGT; reverse: ACGTCCTGCCAGATCCAACCGA). CS (soybean CS DQ075204.1; forward: ATGACATGAAATATGGTGT;
reverse: GACATAGGCAAGAATCTCAGTTC) and ICS (soybean ICS AW596452.1; forward: TTTGATCGAGGGATGTATGCT; reverse:
ACTATCCCTGTCCCAGC) primers were designed to be specific to the highly conserved domain of knownFabaceaeESTs and gene sequences. The corresponding sequences were amplified, cloned
G. Szalai et al. / Journal of Plant Physiology168 (2011) 213–219 215
into pGEM-T Easy (Promega, Madison, Wisconsin, USA) vector and transformed intoEscherichia coliJM-109 cells (Promega, Madison, Wisconsin, USA). Single colonies were isolated and the plasmid DNA was sequenced (ABI 3100 Genetic Analyzer; Applied Biosys- tems, Foster City, California, USA). The resulting sequences proved to be homologous to the ICS and CS genes. Confirmation of spe- cific product amplification was done by PCR end-point analysis andTmanalysis using the dissociation curve option. PCR efficiency (derived from the log slope of the fluorescence versus the cycle number in the exponential phase of each amplification plot) for the three primer pairs ranged from 96% to 98.0%. The relative ratio of threshold cycle (Ct) values between the endogenous control and the specific gene and their standard deviations were calculated for each sample.
Statistical analysis
The experiments were repeated three times, and a representa- tive set of experiments was selected. The results are the means of 5 measurements and were statistically evaluated using the standard deviation andt-test methods.
Results
Endogenous level of free and bound SA after SA treatment
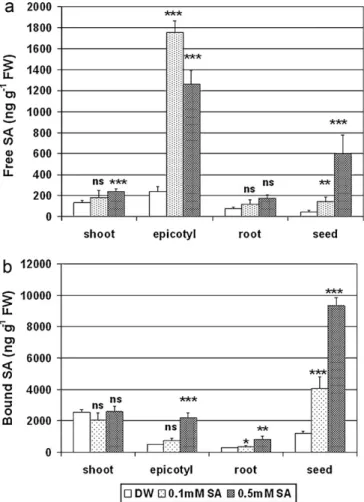
To detect the effect of pre-soaking pea seeds in SA on the dis- tribution of SA within the plants, the levels of free and bound SA and of its putative precursor, oHCA, were measured in the plant organs (seed, epicotyl, shoot, and root) of young, 1-week-old pea seedlings (Fig. 1). Both the free and bound SA content of the seeds showed a marked, concentration-dependent increase. While the free SA content did not change significantly in the roots and only a slight increase was seen in the shoots at a concentration of 0.5 mM, it showed a substantial increase in the epicotyl even when 0.1 mM was used for seed soaking. Similarly, the most pronounced changes in bound SA occurred in the epicotyl and the seeds, while it did not change in the shoot, and only a slight, though statistically signifi- cant increase occurred in the root. To follow the fate of exogenous SA, radioactive SA was added to the solution used for pre-soaking, and the total (labeled + non-labeled) SA, the level of radioactive SA, was also determined. Interestingly, radioactivity was detected only in the bound form of SA. The quantity of bound SA in the seed was 4.5 times that in the epicotyl, while the level of radioactivity was 16 times as great (Figs. 1 and 2). The majority of the SA absorbed during seed soaking thus appears to have remained in the seed, and only traces were transported to the epicotyl. The shoots and roots only contained non-labeled SA.
It has been shown that not only SA, but also related compounds, such as o-HCA, could induce protection against abiotic stress (Janda et al., 2000; Horváth et al., 2002). The level of free oHCA was below the detection limit in the seeds, root and shoots, while it was approx. 1.4mg g−1FW in the epicotyl, where it showed no change after SA treatment (data not shown). The level of the bound form of oHCA was approx. 4–5 times higher in the seeds and shoot than in the epicotyl and root (Fig. 3). Pre-soaking the seeds in SA solution before sowing caused a further increase in the bound oHCA level in all the organs in 1-week-old pea plants.
Expression of the chorismate synthase and isochorismate synthase genes
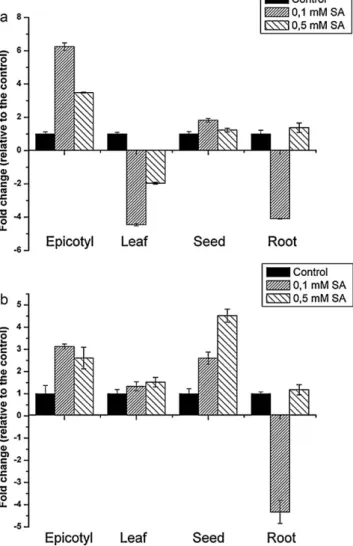
The application of 0.1 and 0.5 mM exogenous SA to pea plants resulted in alterations in the expression patterns of known SA biosynthesis-related genes. The transcript abundance of the ICS
Fig. 1.Endogenous free (a) or bound (b) SA levels in the shoots, epicotyls, roots and seeds of 1-week-old pea plants pre-soaked in distilled water (control), or in 0.1 or 0.5 mM SA solution. *, ** and *** significantly different from the control at the p≤0.05, 0.01 and 0.001 levels, respectively.
gene relative to the control samples was the highest in the epi- cotyl, and a slight upregulation was observed in the seeds (Fig. 4a).
The gene was downregulated in the young leaflets and in the roots, while the application of 0.5 mM SA was less effective and did not affect the ICS expression in roots.
The relative amount of CS transcript exceeded that in the control treatments in epicotyls and seeds, and remained unaffected in the leaflets, while the gene was downregulated in roots treated with 0.1 mM SA (Fig. 4b).
Fig. 2.Radioactively labeled bound SA levels in the epicotyls and seeds of 1-week- old pea plants pre-soaked in 0.5 mM SA solution.
216 G. Szalai et al. / Journal of Plant Physiology168 (2011) 213–219
Fig. 3.Endogenous bound oHCA levels in the shoots, epicotyls, roots and seeds of 1-week-old pea plants pre-soaked in distilled water (control), or in 0.1 or 0.5 mM SA solution. *, ** and *** significantly different from the control at thep≤0.05, 0.01 and 0.001 levels, respectively.
Fig. 4.Expression analysis of isochorismate synthase (a) and chorismate synthase (b) genes in response to SA treatment using the RT-PCR technique. Relative transcript abundance was calculated and normalized with respect to the pea actin (X90378.1) transcript level. The water-treated control samples were set as calibrators. Data represent mean values obtained from four technical replicates. The experiment was repeated three times (three independent biological replicates) with similar results.
Fig. 5.Activities of the ascorbate peroxidase (a) and guaiacol peroxidase (b) enzymes isolated from the shoots, roots or epicotyls of 1-week-old pea plants pre- soaked in distilled water (control; white bars), or in 0.1 mM (striped bars) or 0.5 mM SA (black bars) solutions. *, ** and *** significantly different from the control at the p≤0.05, 0.01 and 0.001 levels, respectively.
Antioxidant enzyme activities
Pre-soaking pea seeds in 0.1 or 0.5 mM SA solution caused no changes in the catalase, glutathione reductase or glutathione S- transferase activities in any of the plant organs (shoot, root, and epicotyl) tested in 7-day-old pea plants (data not shown). There was a slight increase in the ascorbate peroxidase activity in the shoots of plants pre-treated with 0.1 mM SA, while an increase in the epicotyls and a decrease in the roots were detected when the plants were treated with 0.5 mM SA (Fig. 5a). The guaiacol peroxi- dase activity showed a 2.5-fold increase at both SA concentrations in the shoots (Fig. 5b) and a slight increase was seen in the roots after 0.1 mM SA treatment. There were no changes in the guaiacol peroxidase activity in the epicotyl.
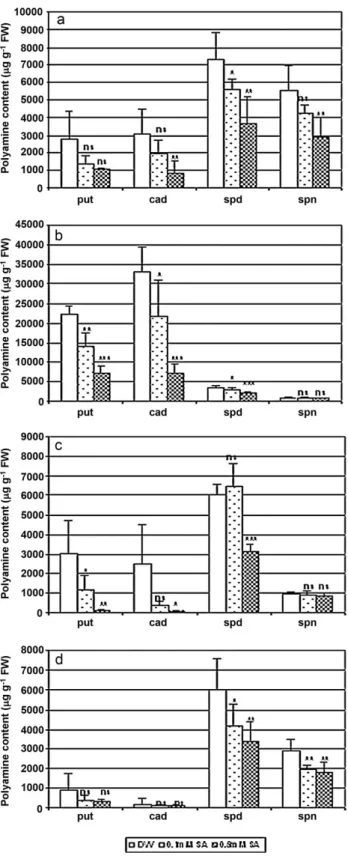
Polyamines
The levels of free soluble polyamines were traced in several plant parts after SA treatment. Among the plant organs tested, the highest absolute cadaverine (cad) and putrescine (put) levels were detected in the epicotyls of young pea plants (Fig. 6b), while this organ con- tained the lowest amounts of spermidine (spd) and spermine (spn).
The spn content was 2–4 times higher in the shoot than in the other organs tested (Fig. 6a). The lowest levels of put and cad were found in the seeds (Fig. 6d). Pre-soaking the seeds in SA solutions before sowing generally led to lower polyamine levels in all the organs of pea plants (Fig. 6).
Discussion
Several reports have been published in the last decade demon- strating the role of SA in various physiological processes, especially
G. Szalai et al. / Journal of Plant Physiology168 (2011) 213–219 217
Fig. 6.Polyamine contents in the shoots (a), epicotyls (b), roots (c) and seeds (d) of 1-week-old pea plants pre-soaked in distilled water (control), or in 0.1 or 0.5 mM SA solutions. *, ** and *** significantly different from the control at thep≤0.05, 0.01 and 0.001 levels, respectively. (put, putrescine; cad, cadaverine; spd, spermidine;
spn, spermin).
in stress responses. Some of these papers demonstrated the pro- tective or damaging effect of exogenous SA; others investigated the role and possible action of endogenous SA in the signal trans- duction processes. Priming pepper seeds in acetyl-SA improved the final germination percentage, and led to higher shoot and root dry
weights (Korkmaz, 2005). Other positive effects of grain soaking with SA have been reported under Cd stress conditions (Krantev et al., 2008). It was also shown that pre-soaking pea seeds in SA had a beneficial effect on growth and photosynthesis, and led to a decrease in the oxidative injuries caused by Cd (Popova et al., 2009).
However, it should also be noted that a dramatic inhibition of the germination process was reported above a concentration of 1 mM in maize (Guan and Scandalios, 1995) andArabidopsis(Rajjou et al., 2006) plants.
Soaking seeds in SA solution before sowing caused a concentration-dependent increase in both the free and bound SA contents of the seeds. It can be assumed that the increase in the free SA form in the seeds originates at least in part from the SA used for the seed soaking procedure. However, radioactivity could only be detected in the bound form of SA, not only in the seeds, but in the epicotyl, indicating that the absorbed SA was converted to the methanol-soluble bound forms. The level of radioactivity detected from bound SA in the epicotyl, on the other hand, was far lower than expected based on the increase in the SA quantity.
So what is the source of the increased SA level in organs other than the seed, especially in the epicotyl, where the highest level of changes was detected? Large quantities of both free and bound SA accumulated in the epicotyl, but radioactivity was only detected at a low level in the bound form, suggesting that the free SA in the epicotyl did not originate from that absorbed by the seeds. To con- firm this, the expression of the ICS and CS genes was analyzed. The SID2 gene encodes ICS (ICS1), an enzyme in the SA biosynthesis pathway. Mutants with a defect in SID2 contain strongly reduced levels of SA, suggesting that, inArabidopsis, SA is predominantly produced from isochorismate and not phenylalanine, as previously thought (Mukherjee et al., 2010). Pre-soaking the seeds in SA solu- tion induced the expression of CS and ICS genes in the epicotyl (Fig. 4). This gene expression pattern was similar to that observed for SA accumulation (Fig. 1). The results suggest that the increased level of free and bound SA detected in plants growing from seeds soaked in SA solution before sowing is the product ofde novosyn- thesis, rather than having been taken up and mobilized by plants.
Imbalances in the cellular machinery may lead to the accumu- lation of harmful reactive oxygen species. The elevated level of SA may be directly involved in scavenging as an antioxidant or may indirectly modify the redox balance through the activation of antioxidant responses (Guo et al., 2007 Popova et al., 2009). Pre- vious studies demonstrated that SA or related compounds could be used as effective preventive compounds against oxidative damage in plants. oHCA is also able to quench singlet molecular oxygen (Foley et al., 1999), and although there is as yet no proof that it serves as a direct precursor of SA in pea plants, its increased level in SA-treated plants indicates that the pathway leading to SA synthesis is activated in SA-treated (i.e. seed-soaked) pea plants.
Pre-treatment of cucumber and tobacco leaves by spraying them with Na-SA can protect them from paraquat-induced oxidative stress (Strobel and Kuc, 1995). Similarly, the pre-treatment of bar- ley seedlings with SA decreased the paraquat-induced production of H2O2 (Ananieva et al., 2002), and led to increased activity of certain antioxidant enzymes (Ananieva et al., 2004). Several exper- iments have shown the direct role of SA in the regulation of the redox balance and in protecting rice plants from oxidative dam- age. SA may serve as a scavenger of hydroxyl radicals and as an iron-chelating compound, thereby inhibiting both the direct impact of hydroxyl radicals and their generation via the Fenton reaction (Dinis et al., 1994). At relatively low concentrations (0.05–0.5 mM) exogenous SA acts as a moderate stress, having an effect on the oxidative status of the plant similar to that of stress acclimation pro- cesses (Horváth et al., 2007). Higher concentrations of SA may cause a level of oxidative stress that the plant is unable to overcome, and which may result in the death of the plant. SA treatment may cause
218 G. Szalai et al. / Journal of Plant Physiology168 (2011) 213–219
an increase in the H2O2concentrationin vivo, which is thought to be a signal for the expression of pathogenesis-related genes (Chen et al., 1993) and is assumed to lead to the induction of protective mechanisms, including antioxidant enzymes (Prasad et al., 1994;
Janda et al., 1999). In the present work, pre-soaking pea seeds in 0.1 or 0.5 mM SA solution caused no change in the catalase, glutathione reductase or glutathione S-transferase activities in pea plants, and only a slight increase was detected in the ascorbate peroxidase activity in the epicotyl and the shoot, and in the guaiacol peroxidase activity in the shoots. The ascorbate peroxidase activity showed a slight decrease in the root. It is, of course, impossible to say whether these changes have a direct or indirect role in the positive effect of SA pre-soaking on germination, and especially in the increased plant growth and yield (Elwan and El-Hamahmy, 2009; Hayat et al., 2010). In the present study, the pea plants were, in principle, grown under normal control conditions, where no stress treatment was directly applied. However, even when plants are grown under “con- trol” conditions, it is impossible to be sure that they are not stressed.
The slight differences observed in antioxidant activities indicate that the pea plants suffered no severe oxidative damage under the given conditions; however, these slight changes in the antiox- idative activity may pre-harden the plants for a subsequent stress factor, as was recently shown in the case of Cd (Popova et al., 2009).
To obtain additional information about the stress responses of plants developing from seeds soaked in SA solution, another group of stress-related compounds, the polyamines, were examined. Sev- eral kinds of stressors, for example drought or low temperature or any kind of oxidative stress, have been reported to induce the genes responsible for polyamine synthesis in various plant species (Usadel et al., 2008). As cationic compounds, polyamines have been reported to bind to nucleic acids, phospholipids, proteins and other anionic cellular compounds. Polyamine accumulation, especially that of put and agmatine, has also been reported in wheat plants during both short- and long-term cold-hardening periods (Rácz et al., 1996), suggesting their role in acclimation processes. In plants, put, spd and spn are synthesized from ornithine or arginine. cad is synthesized independently of the put–spd–spn pathway, and is thought to act as a free radical scavenger (Kuznetsov et al., 2007).
The signaling pathways leading to polyamine accumulation are still very poorly understood. In an earlier study the addition of 0.5 mM SA to the hydroponic solution for 1 day increased the content of put and spd and reduced the spn level in young maize plants exposed to low temperature stress (Németh et al., 2002). Similarly, an increase in the expression of arginine decarboxylase was reported in tobacco plants after SA treatment (Jang et al., 2009). In the present study, a reduction in the polyamine levels could be detected in plants grown from seeds pre-soaked in SA solution, in spite of the fact that the endogenous SA level also increased in these plants. Protective mechanisms induced by SA, for example the induction of dehydrin (Sun et al., 2009), antioxidant enzyme activity, etc., might decrease the severity of the oxidative stress, thus reducing the polyamine level required to protect the membranes and macromolecules in pea plants. This suggests that polyamine accumulation is not a direct consequence of SA treatment, or rather of the SA-mediated pathway, but rather a secondary symptom occurring due to certain stress conditions.
In conclusion, soaking pea seeds in SA solution before sowing may increase the endogenous SA level; however, this increased endogenous SA level in various tissues originates not from the exogenous SA, but mainly fromde novosynthesis.
Acknowledgements
Thanks are due to Edit Kövesdi and Zsuzsa Kóti for their technical assistance and Barbara Harasztos for revising the English. This work
was supported by a grant from the Hungarian National Scientific Fund (OTKA/NKTH K68158).
References
Ádám A, Bestwick CS, Barna B, Mansfield JW. Enzymes regulating the accumula- tion of active oxygen species during the hypersensitive reaction of bean to Pseudomonas syringaepv. Phaseolica. Planta 1995;197:2410–9.
Alonso-Ramírez A, Rodríguez D, Reyes D, Jiménez JA, Nicolás G, López-Climent M, Gómez-Cadenas A, Nicolás C. Cross-talk between gibberellins and salicylic acid in early stress responses in Arabidopsis thaliana seeds. Plant Signal Behav 2009;4:750–1.
Ananieva EA, Alexieva VS, Popova LP. Treatment with salicylic acid decreases the effects of paraquat on photosynthesis. J Plant Physiol 2002;159:685–93.
Ananieva EA, Christov KN, Popova LP. Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to paraquat.
J Plant Physiol 2004;161:319–28.
Chadha KC, Brown SA. Biosynthesis of phenolic acids in tomato plants infected with Agrobacterium tumefaciens. Can J Bot 1974;52:2041–6.
Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 1993;262:1883–6.
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM. Parallel changes in H2O2and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 1998;116:1351–7.
Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivates (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994;315:161–9.
El-Tayeb MA. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul 2005;45:215–24.
Elwan MWM, El-Hamahmy MAM. Improved productivity and quality associ- ated with salicylic acid application in greenhouse pepper. Sci Horticult 2009;122:521–6.
Foley S, Navaratnam S, McGarvey DJ, Land EJ, Truscott G, Rice-Evans CA. Singlet oxygen quenching and redox properties of hydroxycinnamic acids. Free Radic Biol Med 1999;26:1202–8.
Guan L, Scandalios JG. Developmentally related responses of maize catalase genes to salicylic acid. Proc Natl Acad Sci USA 1995;92:5930–4.
Guo B, Liang YC, Zhu YG, Zhao FJ. Role of salicylic acid in alleviating oxidative dam- age in rice roots (Oryza sativa) subjected to cadmium stress. Environ Pollut 2007;147:743–9.
Hayat Q, Hayat S, Irfan M, Ahmad A. Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 2010;68:14–25.
Horváth E, Janda T, Szalai G, Páldi E. In vitro salicylic acid inhibition of catalase activity in maize: differences between the isoenzymes and a possible role in the induction of chilling tolerance. Plant Sci 2002;163:1129–35.
Horváth E, Szalai G, Janda T. Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul 2007;26:290–300.
Janda T, Szalai G, Tari I, Páldi E. Hydroponic treatment with salicylic acid decreases the effect of chilling injury in maize (Zea maysL.) plants. Planta 1999;208:175–80.
Janda T, Szalai G, Antunovics Z, Horváth E, Páldi E. Effect of benzoic acid and aspirin on chilling tolerance and photosynthesis in young maize plants. Maydica 2000;45:29–33.
Jang E-K, Min K-H, Kim S-H, Nam S-H, Zhang S, Kim YC, Cho BH, Yang K-Y. Mitogen- activated protein kinase cascade in the signalling for polyamine biosynthesis in tobacco. Plant Cell Physiol 2009;50:658–64.
Kocsy G, Laurie R, Szalai G, Szilágyi V, Simon-Sarkadi L, Galiba G, de Ronde JA.
Genetic manipulation of proline levels affects antioxidants in soybean subjected to simultaneous drought and heat stresses. Physiol Plant 2005;124:227–35.
Korkmaz A. Inclusion of acetyl salicylic acid and methyl jasmonate into priming solu- tion improves low-temperature germination and emergence of sweet pepper.
Horticult Sci 2005;40:197–200.
Krantev A, Yordanova R, Janda T, Szalai G, Popova L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 2008;165:920–31.
Kuznetsov V, Shorina M, Aronova E, Stetsenko L, Rakitin V, Shevyakova N. NaCl- and ethylene-dependent cadaverine accumulation and its possible protective role in the adaptation of the common ice plant to salt stress. Plant Sci 2007;172:363–70.
Métraux JP. Recent breakthroughs in the study of salicylic acid biosynthesis. Trends Plant Sci 2002;7:332–4.
Meuwly P, Métraux JP. Ortho-anisic acid as internal standard for the simultane- ous quantitation of salicylic acid and its putative biosynthetic precursors in cucumber leaves. Anal Biochem 1993;214:500–5.
Mosher S, Moeder W, Nishimura N, Jikumaru Y, Joo S-H, Urquhart W, Klessig DF, Kim S-K, Nambara E, Yoshioka K. The lesion mimic mutant cpr22 shows alter- ations in abscisic acid signaling and abscisic acid insensitivity in a salicylic acid-dependent manner. Plant Physiol 2010., doi:10.1104/pp.109.152603.
Moussa HR, El-Gamal SM. Role of salicylic acid in regulation of cadmium toxicity in wheat (Triticum aestivumL.). Acta Agron Hung 2009;57:321–33.
Mukherjee M, Larrimore KE, Ahmed NJ, Bedick TS, Barghouthi NT, Traw MB, Barth C. Ascorbic acid deficiency inArabidopsisinduces constitutive priming that is dependent on hydrogen peroxide, salicylic acid, and the NPR1 gene. Mol Plant Microbe Interact 2010;23:340–51.
G. Szalai et al. / Journal of Plant Physiology168 (2011) 213–219 219
Mustafa NR, Kim HK, Choi YH, Erkelens C, Lefeber AWM, Spijksma G, van der Heij- den R, Verpoorte R. Biosynthesis of salicylic acid in fungus elicitedCatharanthus roseuscells. Phytochemistry 2009;70:532–9.
Navarro L, Bari R, Achard P, Lison P, Nemri A, Harberd NP, Jones JDG. DELLAs con- trol plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol 2008;18:650–5.
Németh M, Janda T, Horváth E, Páldi E, Szalai G. Exogenous salicylic acid increases polyamine content but may decrease drought tolerance in maize. Plant Sci 2002;162:569–74.
Pál M, Horváth E, Janda T, Páldi E, Szalai G. Cadmium stimulates the accumulation of salicylic acid and its putative precursors in maize (Zea maysL.) plants. Physiol Plant 2005;125:356–64.
Park S-W, Kaimoyo E, Kumar D, Mosher S, Klessig DF. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 2007;318:113–6.
Popova LP, Maslenkova LT, Yordanova RY, Ivanova AP, Krantev AP, Szalai G, Janda T. Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol Biochem 2009;47:224–31.
Prasad TK, Anderson MD, Martin BA, Stewart CR. Evidence for chilling-induced oxidative stress in maize seedlings and regulatory role for hydrogen peroxide.
Plant Cell 1994;6:65–74.
Rácz I, Kovács M, Lasztity D, Veisz O, Szalai G, Páldi E. Effect of short-term and long- term low temperature stress on polyamine biosynthesis in wheat genotypes with varying degrees of frost tolerance. J Plant Physiol 1996;148:368–73.
Rajjou L, Belghazi M, Huguet R, Robin C, Moreau A, Job C, Job D. Proteomic investigation of the effect of salicylic acid onArabidopsisseed germination and establishment of early defense mechanisms. Plant Physiol 2006;141:
910–23.
Raskin I, Ehmann A, Melander WR, Meeuse BJD. Salicylic acid: a natural inducer of heat production inArumlilies. Science 1987;237:1601–2.
Raskin I. Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 1992;43:439–63.
Ribnicky DM, Shulaev V, Raskin I. Intermediates of salicylic acid biosynthesis in tobacco. Plant Physiol 1998;118:565–72.
Russell DW, Conn E. The cinnamic acid 4-hydroxylase of pea seedlings. Arch Biochem Biophys 1967;122:256–8.
Shah J. The salicylic acid loop in plant defense. Curr Opin Plant Biol 2003;6:
365–71.
Silverman P, Seskar M, Kanter D, Schweizer P, Métraux J-P, Raskin I. Salicylic acid in rice: biosynthesis, conjugation, and possible role. Plant Physiol 1995;108:633–9.
Smith MA, Davies PJ. Separation and quantitation of polyamines in plant tissue by high performance liquid chromatography of their dansyl derivatives. Plant Physiol 1985;78:89–91.
Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5,5-dithiobis(2-nitrobenzoic acid). Anal Biochem 1988;175:408–13.
Strobel NE, Kuc A. Chemical and biological inducers of systemic acquired resistance to pathogens protect cucumber and tobacco from damage caused by paraquat and cupric chloride. Phytopathology 1995;85:1306–10.
Sun X, Xi DH, Feng H, Du JB, Lei T, Liang HG, Lin HH. The dual effects of salicylic acid on dehydrin accumulation in water-stressed barley seedlings. Russ J Plant Physiol 2009;56:348–54.
Szalai G, Tari I, Janda T, Pestenácz A, Páldi E. Effects of cold acclimation and salicylic acid on changes in ACC and MACC contents in maize during chilling. Biol Plant 2000;43:637–40.
Szepesi Á, Csiszár J, Gallé Á, Gémes K, Poór P, Tari I. Effects of long-term salicylic acid pre-treatment on tomato (Lycopersicon esculentumMill. L.) salt stress tolerance:
changes in glutathione S-transferase activities and anthocyanine contents. Acta Agron Hung 2008;56:129–38.
Szepesi Á, Csiszár J, Gémes K, Horváth E, Horváth F, Simon ML, Tari I. Salicylic acid improves acclimation to salt stress by stimulating abscisic aldehyde oxi- dase activity and abscisic acid accumulation, and increases Na+content in leaves without toxicity symptoms inSolanum lycopersicumL. J Plant Physiol 2009;166:914–25.
Usadel B, Blaesing OE, Gibon Y, Poree F, Hoehne M, Guenter M, Trethewey R, Kamlage B, Poorter H, Stitt M. Multilevel genomic analysis of the response of transcripts, enzyme activities and metabolites inArabidopsisrosettes to a pro- gressive decrease of temperature in the non-freezing range. Plant Cell Environ 2008;31:518–47.
Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001;414:562–5.
Wildermuth MC. Variations on a theme: synthesis and modification of plant benzoic acids. Curr Opin Plant Biol 2006;9:288–96.
Yalpani N, Leon J, Lawton MA, Raskin I. Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol 1993;103:315–21.