S T R U C T U R A L A N D F U N C T I O N A L O R G A N I Z A T I O N O F A N E P I T H E L I A L C E L L B R U S H B O R D E R
R O B E R T K . C R A N E
1
Department of Biochemistry, Chicago Medical School, Chicago, Illinois
Studies of sectioned material carried out by numerous workers over a period of years with the light and electron microscopes have revealed the brush border of the epithelial cell of the small intestine to be a complex organelle composed of several identifiable substructures
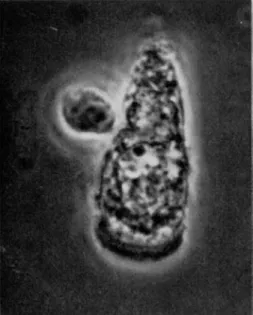
[ 1 0 ] . F r o m these studies, the relationship of the brush border to the rest of the cell can certainly be deduced, but it is perhaps more reveal- ing of the direction of our thoughts to observe the appearance under the phase-contrast microscope of isolated, intact epithelial cells, as shown in F i g s . 1 and 2. The cells in the photographs were prepared from hamster small intestine and are representative of the populations obtained. The cell shown in F i g . 1 was prepared in a medium contain- ing polyvinylpyrolidone to provide appropriate osmotic conditions to prevent swelling; the cell shown in F i g . 2 was prepared in a medium without polyvinylpyrolidone. Both media contained the dye nigrosine to serve as an indicator of the integrity of the cell membrane [ 4 1 ] . The intense staining of the cytoplasmic elements of the cells, which occurred within the first one or two minutes of incubation, suggests that the integrity of the membrane was not preserved during the procedures required for isolation and that cells prepared in this way are not useful for studies on transport. However, the over-all a p p e a r - ance of the cells is noteworthy. In both photographs, the brush border region is easily identified. I t is separated from the main body of the cell by a translucent zone, which the electron microscope has revealed to be bounded by the terminal bar that joins epithelial cells into sheets and to contain a diffuse, difficultly stained material called the terminal web [ 1 0 ] . When the cells swell, as in F i g . 2, the terminal bar and terminal web region retain approximately their original size and appear now as a ring or collar further accentuating the physical
1
Present address: D e p a r t m e n t of Physiology, Rutgers Medical School, New Brunswick, New Jersey.
71
FIG. 1. Isolated epithelial cell of hamster small intestine, normal, under phase-contrast illumination. For explanation see text. Magnification: χ 1050.
FIG. 2 . Isolated epithelial cell of hamster small intestine, swollen, under phase-contrast illumination. For explanation see text. Magnification: χ 1050.
ORGANIZATION OF AN E P I T H E L I A L CELL B R U S H BORDER 73
separation of the brush border region from t h e m a i n body of the cell. T h e degree of this separation strongly suggests t h e possibility t h a t t h e border region m a y a c t u a l l y form a s u b c o m p a r t m e n t of t h e epithelial cell.
T H E F U N C T I O N S O F T H E B R U S H B O R D E R
Several y e a r s ago, M c D o u g a l , L i t t l e , a n d C r a n e obtained d a t a from microdissection experiments which showed t h a t sugar a c c u m u - lated b y in vitro p r e p a r a t i o n s of h a m s t e r small intestine was present a t its highest concentration within t h e epithelial cells [ 4 9 ] . F r o m this result, it was concluded t h a t t h e absorptive process for sugars is located within t h e brush border region. T h i s conclusion h a s been a m p l y corroborated b y t h e work of K i n t e r [42] a n d of K i n t e r a n d Wilson [ 4 3 ] , who have used an a u t o r a d i o g r a p h i c technique, a n d has been extended to t h e absorptive process for amino acids. These studies clearly show t h a t sugar, present in t h e luminal m e d i u m a t a low concentration, moves across t h e brush border m e m b r a n e into t h e cell a t a high c o n c e n t r a t i o n ; t h e y also support t h e assumption t h a t t h e process of sugar active t r a n s p o r t , together with w h a t e v e r accessory reactions or metabolic gears m a y be necessary, is entirely contained within t h e brush border. W h a t e v e r h a p p e n s to sugar after it h a s crossed t h e brush border is t h u s a question of cell m e t a b o l i s m a n d not of specific absorption.
Along other lines, evidence h a s a c c u m u l a t e d for m a n y y e a r s to indicate t h a t the t e r m i n a l stages of t h e c a r b o h y d r a t e digestion are associated with p a r t i c u l a t e elements of t h e cell [74] a n d are not, as h a d been formerly t h o u g h t , secreted into t h e lumen as p a r t of a "succus e n t e r i c u s " [ 7 ] . I n point of fact, L a r n e r a n d Gillespie [44]
suggested on t h e basis of cell fractionation studies t h a t t h e disaccha- ridases of t h e small intestine are associated with a " m i c r o s o m a l " frac- tion of t h e cell. W i t h these suggestions as a guide, Miller a n d C r a n e
[53] began studies on t h e relationship between absorption and utiliza- tion of monosaccharides. T o further this end, it was desirable to b y - pass t h e absorptive process, t h a t is, to introduce monosaccharide di- rectly into t h e m a i n body of the cell. On the basis of the above, sucrose a n d other disaccharides, which were t r i e d as s u b s t r a t e s , should h a v e been hydrolyzed after crossing t h e brush border. C o n t r a r y to this a n t i c i p a t e d result t h e disaccharides were hydrolyzed in a super- ficial location t h a t a p p e a r e d to coincide morphologically with t h e brush border. This result, when t a k e n together with the a p p e a r a n c e
of isolated cells, as shown in Figs. 1 and 2, suggested the possibility t h a t t h e disaccharidases are a n integral p a r t of t h e brush border and t h a t this region of t h e cell might be separable from t h e m a i n body if a p p r o p r i a t e m e a n s could be found. T h e means were found in a controlled homogenization of mucosal scrapings in dilute solutions of a m e t a l - b i n d i n g agent [ 5 2 ] . Under such conditions, t h e cells of
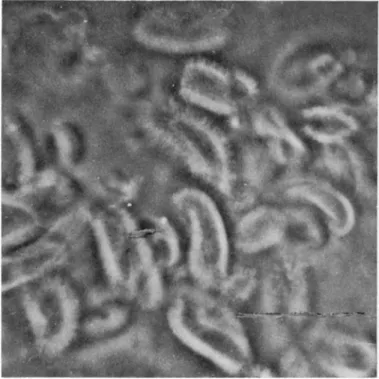
FIG. 3. Isolated brush borders of hamster intestinal epithelial cells, under phase-contrast illumination. Magnification: χ 1050. (From Miller and Crane
[1961].)
h a m s t e r small intestine disrupt to release t h e brush border portion of t h e epithelial cell (Fig. 3) as t h e only intact, large subcellular element which can be recovered by low-speed centrifugation. W i t h cells from other species, t h e result is much t h e same except t h a t the nuclei frequently do n o t disrupt a n d m u s t be s e p a r a t e l y removed.
Recently, a s o m e w h a t more sophisticated a n d possibly more generally applicable m e t h o d for t h e p r e p a r a t i o n of isolated i n t a c t brush borders has been devised [ 3 6 ] . As anticipated from the studies of disaccharide
ORGANIZATION OF AN E P I T H E L I A L CELL B R U S H BORDER 75
hydrolysis by i n t a c t tissue, enzyme assays of isolated brush borders h a v e shown t h e presence of a n u m b e r of h y d r o l y t i c enzymes [31, 37, 5 2 ] , as listed in T a b l e I.
T w o of these enzymes, alkaline p h o s p h a t a s e [28] a n d leucyl n a p h t h y l a m i d a s e [ 5 4 ] , h a d previously been localized to t h e brush border region by histochemical methods, a n d b o t h are found in isolated brush borders in t h e q u a n t i t y t h a t would be expected. T h e recovery of disaccharidase a c t i v i t y with t h e brush borders is in a b o u t t h e same proportion a n d is t h u s great enough to lead one to conclude t h a t t h e brush border is t h e p r e d o m i n a n t , if n o t exclusive, location of these enzymes. I n addition, isolated brush borders possess some small activity for acid p h o s p h a t a s e , which is n o t removed on r e p e a t e d w a s h - ing of t h e brush borders, a n d for two distinct adenosinetriphosphatases
( A T P a s e s ) as will be described in somewhat greater detail below.
Specifically, with regard to t h e disaccharidases, this location in t h e brush border has now been a m p l y corroborated by t h e work of others, n o t a b l y t h a t of S m y t h a n d his colleagues in Sheffield [ 5 6 ] , a n d of Rosen a n d K r e t c h m e r [62] a t Stanford, who h a v e used entirely i n d e - p e n d e n t methods. W e t h u s feel completely justified in representing the m e m b r a n e of t h e brush border as a digestive-absorptive surface in which t h e disaccharides are hydrolyzed as t h e y enter a n d t h e m o n o - saccharides formed are caused to move t h r o u g h t h e m e m b r a n e against their concentration gradients.
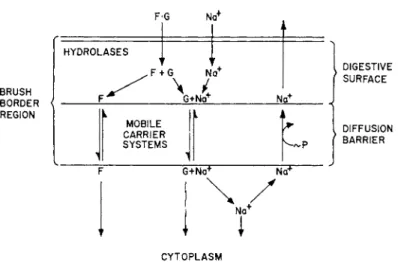
R e a s o n i n g along these lines, we formulated several y e a r s ago [16, 19] a hypothesis for t h e functional organization of t h e brush border as depicted in Fig. 4. This figure is slightly simplified from t h e original in order to m a k e it more suitable for t h e present discussion. T h e hydrolases of t h e brush border are represented as being external to the diffusion barrier for monosaccharides because it is known t h a t phlorizin prevents t h e e n t r y of monosaccharides into t h e cell, irrespec- tive of w h e t h e r t h e monosaccharide is added to t h e m e d i u m in t h e free form or is produced in t h e brush border as t h e result of disaccha- ridase a c t i v i t y [ 5 3 ] . Similarly, t h e absence of N a
+
, which is required for the e n t r y of monosaccharides into t h e cell a t a m e a s u r a b l e r a t e when t h e y are present in low concentration, does not stop disaccha- ridase action b u t does p r e v e n t accumulation of t h e monosaccharide formed [ 1 6 ] . T h e representation in Fig. 1 m a y t h u s be seen to be based on current concepts of cell m e m b r a n e structure and function which assume t h a t the barrier to the free e n t r y of water-soluble com- pounds is the lipid leaflet of the t r i l a m i n a r m e m b r a n e [ 2 7 ] .
T h e characteristics of N a +
- d e p e n d e n t m o v e m e n t of glucose and its analogs across this diffusion barrier are described in detailed r e - p o r t s presented elsewhere [17, 2 0 ] . I n brief, these reports contain t h e following information a n d conclusions. Bihler, H a w k i n s , a n d C r a n e [9] obtained d a t a which identified t h e e n t r y of sugars across t h e brush border m e m b r a n e as t h e p r i m a r y site of N a
+
involvement in t h e over-all process of sugar t r a n s p o r t . W e interpreted our experi- m e n t s to have "established t h e existence of a substrate-specific, N a
+ - dependent a n d energy-independent process m e d i a t i n g t h e r a p i d equili-
MEDIUM
F G
BRUSH BORDER REGION
HYDROLASES
F H
F ^
hG Ν
^ i
G+Na
Ν a
+
MOBILE CARRIER SYSTEMS
G+Na
+
N<f
i
Na
+
DIGESTIVE SURFACE
DIFFUSION BARRIER
CYTOPLASM
FIG. 4 Model suggested by the characteristics of disaccharide and phosphate ester hydrolysis and of sugar active transport by in vitro preparations of hamster small intestine. (Modified from Crane et al. [1961].)
b r a t i o n of sugars between t h e cells a n d t h e m e d i u m " ; it is this process which is depicted in Fig. 4. Along the lines of current mobile carrier concepts [ 6 3 ] , interaction of sugar w i t h a specific binding site on a mobile carrier was postulated to account for t h e specificity of t h e over-all process [15, 73] a n d for t h e competitive characteristics of phlorizin inhibition of sugar t r a n s p o r t [ 2 ] . I t was also p o s t u l a t e d t h a t t h e carrier possessed a second binding site, specific for N a
+ , a n d t h a t binding of N a
+
to this site was essential to t h e ability of t h e carrier to equilibrate sugar across t h e brush border m e m b r a n e . Simul-
ORGANIZATION OF AN E P I T H E L I A L CELL BRUSH BORDER 77 taneous m o v e m e n t s of N a
+
and sugar were t h e n assumed to occur as a direct consequence of the p o s t u l a t e d interactions.
As we visualized it, and as it is more elaborately r e d r a w n in Fig.
5, the carrier system is, of itself, capable only of equilibration; t h e a s y m m e t r y required to achieve " u p h i l l " accumulation of sugar being ascribed to a gradient of N a
+
concentration, "downhill," into the cells, m a i n t a i n e d by the operation of the o u t w a r d l y directed, energy-depen- dent N a
+
- p u m p depicted in Fig. 4 to be present a t a different locus in t h e same m e m b r a n e [ 1 9 ] . T h e presence of this N a
+
- p u m p in t h e brush border m e m b r a n e was assumed largely because of t h e p r o b a b i l - i t y t h a t t h e brush border region, as suggested by its a p p e a r a n c e in
C E L L M E M B R A N E
FIG. 5 T h e postulated mobile carrier and its interactions with substrate and N a
+
. ( F r o m Crane, Forstner, and Eichholz [ 1 9 6 5 ] . )
Figures 1 a n d 2 a n d by t h e fact t h a t it can be isolated as a discrete entity, a c t u a l l y forms a s u b c o m p a r t m e n t of t h e cell. Although this assumption is to some extent further justified by t h e fact t h a t a N a
+ , K
+
- d e p e n d e n t A T P a s e is present in the m e m b r a n e fraction of t h e microvillus (see below), it should be emphasized t h a t t h e position of t h e p u m p is not of p r i m e importance. I t is essential to t h e h y p o t h e - sis only t h a t t h e local i n t e r n a l N a
+
concentration in t h e region of t h e equilibrating carrier be m a i n t a i n e d low relative to t h e concentra- tion in t h e m e d i u m . I n t h e i n t a c t epithelial cell, this could as well be accomplished by t h e p u m p in t h e basal m e m b r a n e , which is a s - sumed t o be present a n d to be responsible for t h e absorption of salt a n d water [ 2 2 ] , or by p u m p s in both m e m b r a n e s . I t should also be
emphasized t h a t the diagram of t h e carrier system in Fig. 5 is a kinetic model only and is not intended to represent a n y p a r t i c u l a r molecular characteristics of t h e carrier other t h a n t h e interactions of N a
+
and s u b s t r a t e with spécifie binding sites. However, the model is intended to illustrate the usual assumption t h a t the carrier migrates freely between the interfaces of the cell membrane in either free or the combined forms and our special assumption t h a t substrate and N a
+
interact with the specific binding sites on the carrier in approxi- mately the same way, in response to their respective local concentra- tions, on both sides of the diffusion barrier.
The theoretical basis for viewing translocation of molecules through cell membranes against concentration or electrochemical gra- dients as the consequence of metabolism-dependent asymmetries has been thoroughly reviewed by Rosenberg and Wilbrandt [63]. Those asymmetries t h a t affect Na
+
-dependent transport in hamster small intestine appear, on the basis of numerous experiments, to be at least the following: (1) The inward "downhill" N a
+
gradient, which may be assumed to be effective because the rate of carrier movement ap- pears to depend upon its interaction with N a
+
; (2) the inward "uphill"
K +
gradient, which m a y be assumed to be effective because the rela- tively high K
+
concentration within the cell would be expected to interfere with Na
+
-carrier interaction for outward movement; and (3) a gradient of substrate-carrier affinity, which m a y be assumed to result from the fact t h a t this affinity is directly influenced by the N a
+ and K
+
concentrations. Because of this last asymmetry, a tissue/medium substrate ratio greater than unity is required to estab- lish a stationary state in which the rates of substrate efflux and influx are equal. All three of these asymmetries, it should be empha- sized, depend ultimately upon the energy-dependent translocation of N a
+
out of the cell, which should possibly be classified as a vectorial biochemical activity, but it is important to recognize t h a t N a
+ - d e - pendent transport, per se, is depicted here as an equilibrating system and not as a vectorial biochemical activity.
On the basis of such kinetic studies as have been done, there thus appears to be acceptable evidence for assuming t h a t the disacchari- dases responsible for the terminal stages of carbohydrate digestion and the Na
+
-dependent transport system for monosaccharides are con- tained within the brush border in the approximate relationship de- picted in Fig. 4. I t is our desire now to identify these functions of the brush border with morphological elements so as to decide their
ORGANIZATION OF AN E P I T H E L I A L CELL B R U S H BORDER 79
location more precisely a n d to p e r m i t elucidation of their chemical n a t u r e .
F I N E S T R U C T U R E O F T H E B R U S H B O R D E R
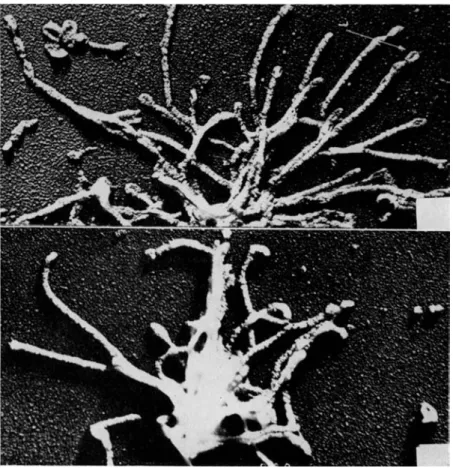
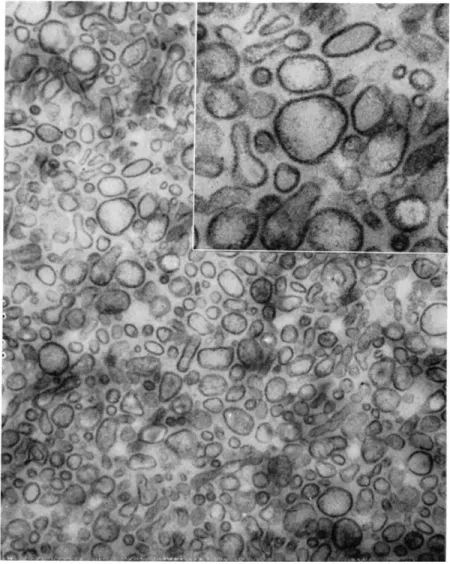
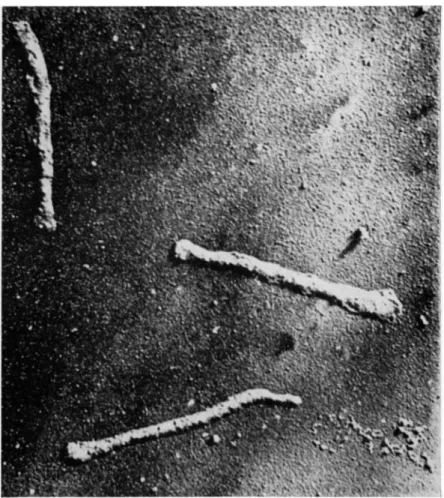
T h e structure of brush borders as p r e p a r e d by t h e method of Miller a n d C r a n e is revealed b y t h e electron microscope to be well preserved, as shown in Fig. 6, and to contain those elements t h a t h a v e been described in studies of sections of i n t a c t epithelium. Identifiable in Fig. 6 are t h e t e r m i n a l bars, t h e p l a s m a m e m b r a n e of t h e microvilli, and t h e t e r m i n a l web area into which t h e rootlets of t h e microvilli cores are seen to project and with which t h e y p r o b a b l y connect. R e - cently, Eichholz and C r a n e [30] h a v e achieved a separation of iso- lated brush borders into their principal subelements. W h e n incubated with 1 M tris ( h y d r o x y m e t h y l ) a m i n o m e t h a n e ( T r i s ) , a t n e u t r a l p H , isolated brush borders are seen under the p h a s e - c o n t r a s t microscope to disintegrate into a v a r i e t y of smaller elements which c a n n o t be identified a t t h e highest power. T h e y can, however, be s e p a r a t e d by centrifugation on glycerol density gradients into five b a n d s which can be recovered by a p p r o p r i a t e m e a n s a n d viewed under t h e electron microscope. I n collaborative studies with D r . J a n e Overton of t h e University of Chicago [ 5 8 ] , the elements in t h r e e of these b a n d s have been identified with structures seen in Fig. 6. T h e a p p e a r a n c e of the sections in Fig. 6 suggests t h a t the brush border m a y most easily be interpreted, in three dimensions, as being composed of a m e m b r a n e , or " s k i n , " covering a " s k e l e t o n " of rod-like elements em- bedded in an u n d e r l y i n g meshwork. On this basis, t h e elements in one of the density gradient fractions (Fig. 7) would a p p e a r to be identified as t h e skeleton of rod-like elements, n a m e l y , t h e cores of the microvilli, still a t t a c h e d to t h e t e r m i n a l web. T h i s i n t e r p r e t a t i o n is supported by t h e fact t h a t t h e p l a s m a m e m b r a n e s , which cover t h e microvilli, are found in an entirely different density g r a d i e n t frac- tion, shown as a section of pelleted m a t e r i a l in Fig. 8 a n d as a shadowed, i n t a c t p r e p a r a t i o n in Fig. 9. T h e size a n d shape of t h e cores and of t h e m e m b r a n e s are a p p r o p r i a t e for t h e m to h a v e the identity ascribed to t h e m . A t h i r d fraction from t h e density gradient, situated v e r y close t o t h e principal m e m b r a n e fraction, can also be identified as m e m b r a n o u s in origin, b u t it h a s slightly different char- acteristics and possibly originates from a population of cells t h a t is a relatively small proportion of t h e t o t a l . T h e two r e m a i n i n g frac- tions obtained from t h e density gradient cannot be identified, a t t h e
FIG. 6. Sectioned pellet of a brush border preparation. Arrows indicate inden- tations at the terminal bar region. Magnification: X9000. (From Overton, Eichholz, and Crane [1965].)
ORGANIZATION OF AN E P I T H E L I A L CELL BRUSH BORDER 81
FIG. 7. Shadowed preparations of compact rods attached in clusters. Mag- nification: X32,000. (From Overton, Eichholz, and Crane [1965].)
n o t comprise t h e e n t i r e t y of t h e substance of the microvilli, a n d it is t h u s possible t h a t these fibrils represent a different physical s t a t e of t h e m a t e r i a l which is ordinarily contained between t h e cores a n d t h e coats in i n t a c t microvilli.
T h e distribution of enzymes along t h e density g r a d i e n t a n d espe- cially in t h e fractions identified u n d e r t h e electron microscope has present time, with k n o w n s t r u c t u r a l elements of t h e brush border, but are nonetheless of interest. W h e n a p p r o p r i a t e l y diluted these frac- tions form fibrils, possibly identical with those reported several y e a r s ago [ 2 9 ] . Such fibrous m a t e r i a l is n o t seen in sections of i n t a c t cells.
However, it should be noted t h a t t h e cores a n d t h e m e m b r a n e s do
FIG. 8. Sectioned pellet of membrane fraction with osmium tetroxide fixa- tion. Magnification: X32,000; insert X80,000. (From Overton, Eichholz, and Crane [1965].)
been studied. As a result of the elegant studies of such workers as C l a r k [14] a n d Goldfîscher et al. [33], who have localized the enzyme alkaline phosphatase not only to the brush border region of the epi- thelial cell but also, at the level of the electron microscope, to the membrane covering the microvilli, alkaline phosphatase can be used
ORGANIZATION OF AN E P I T H E L I A L CELL BRUSH BORDER 83
as a label for the microvillus m e m b r a n e . T h u s , as expected, akaline phosphatase is found to be localized in t h e principal m e m b r a n e frac- tion from t h e density gradient together with l e u c y l - n a p h t h y l a m i d a s e a n d all of t h e disaccharidases listed in T a b l e I except lactase. L a c t a s e
FIG. 9. Shadowed preparation of membrane fraction. Magnification: X 40,000.
(From Overton, Eichholz, and Crane [1965].)
has a slightly different distribution on t h e density gradient, suggesting t h a t it is distributed nonuniformly in t h e t o t a l population of m e m b r a n e s .
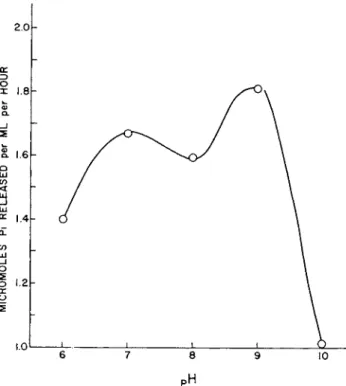
As noted in T a b l e I, isolated brush borders contain two A T P a s e activities [31, 3 5 ] . This is documented by studies of t h e effect of
p H on t h e A T P a s e a c t i v i t y of i n t a c t isolated brush borders in which two m a x i m a h a v e been found (see Fig. 10). Following separation of Tris-disrupted brush borders on t h e density gradient, t h e A T P a s e with an o p t i m u m a t a b o u t p H 9 is found in t h e m e m b r a n e fraction, whereas t h e A T P a s e with t h e o p t i m u m a t a b o u t p H 7 is associated with t h e fibrils formed by dilution of the two morphologically uniden- tified fractions. These A T P a s e s h a v e been shown, principally by studies of p h e n y l a l a n i n e inhibition, s u b s t r a t e specificity, a n d m e t a l ion requirement, to be distinct from alkaline p h o s p h a t a s e . B o t h en- zymes a p p e a r to be Na% K
+
- d e p e n d e n t A T P a s e s of t h e k i n d which
TABLE I . Hydrolytic Enzymes Present in Isolated Brush Borders P r e d o m i n a n t l y or exclusively
Alkaline phosphatase Leucyl n a p h t h y l a m i d a s e
Maltase Lactase Invertase Isomaltase Trehalase Significant activity
Acid phosphatase
ATPase, p H o p t i m u m a t 7 ATPase, p H o p t i m u m at 9
Skou [68] a n d others [40] h a v e described a n d which are currently assumed to be expressions of m e m b r a n e N a
+
- p u m p s . T h e A T P a s e of t h e fibrillar m a t e r i a l is, in addition, strongly a c t i v a t e d by magnesium a n d calcium. B o t h enzymes are inhibited by o u a b a i n [ 3 1 ] .
L O C A L I Z A T I O N O F T H E F U N C T I O N S O F T H E B R U S H B O R D E R
Although our ability to discriminate among t h e several p a r t s of t h e p l a s m a m e m b r a n e of t h e microvilli is not y e t a d v a n c e d enough to p e r m i t incontrovertible assignment of function to specific regions, there are, nonetheless, a n u m b e r of w h a t seem to be r a t h e r compelling reasons to believe t h a t terminal c a r b o h y d r a t e digestion and monosac- charide absorption are functions associated with proteins which are integral elements of t h e outer coat.
I n t h e first place, when t h e m e m b r a n e fraction of Tris-disrupted brush borders is isolated on a n d recovered from a glycerol density
ORGANIZATION OF AN E P I T H E L I A L CELL BRUSH BORDER 85 gradient t h e disaccharidases are recovered q u a n t i t a t i v e l y with it [ 3 0 ] . T h u s , these enzymes are surely contained either within t h e protein portion of the m e m b r a n e , or t h e y are adsorbed to it. Moreover, as mentioned above, current concepts of t h e n a t u r e of cell m e m b r a n e s assume t h e barrier to free e n t r y of water-soluble compounds, such as monosaccharides, to be t h e lipid leaflet of t h e m e m b r a n e a n d t h e
2.oh
3
PH
FIG. 10. T h e p H optima of the ATPase activity of isolated brush borders.
(From Eichholz and Crane [1966].)
function of carrier systems to act as a specific m e a n s for e n t r y of a selected group of compounds. Since t h e disaccharidases of i n t a c t tissue are n o t only superficial in their location b u t also external to t h e phlorizin- a n d N a
+
- s e n s i t i v e sugar carrier system, it would t h e n follow t h a t t h e y form a p a r t of t h e outer of t h e two surfaces. I t is a t this point t h a t serious controversy enters.
F r o m t h e results of early a t t e m p t s a t histochemical localization of disaccharidase activity, D a h l q v i s t a n d B r u n [25] concluded t h a t
these enzymes are n o t a t all a p a r t of t h e brush border b u t are present in a v a r i e t y of unidentified cytoplasmic granules. M o r e recently, D a h l q v i s t [23] h a s r e p e a t e d these studies in our l a b o r a t o r y , using a somewhat modified histochemical technique, a n d h a s obtained evi- dence causing h i m t o change his original conclusions t o t h e extent t h a t he now believes the disaccharidases to be present in t h e brush border as well as in inferior locations within t h e cell. I n our view, t h e p h o t o g r a p h s of sections to which this histochemical procedure has been applied are not convincing.
2
However, these results do exist and although t h e y are a t v a r i a n c e with t h e information provided by m e m b r a n e isolation as well as the results of S m y t h and K r e t c h m e r quoted above, t h e y should be accepted for discussion, as representing one view on t h e precise location of the disaccharidases which is diver- gent from our own. T h e r e is, also, a second divergent view. Ugolev and his co-workers have recently published studies [39, 71] which suggest, as did earlier studies of Miller a n d C r a n e [ 5 3 ] , a superficial location for t h e disaccharidases—one by which these enzymes can be identified with t h e brush border region. However, these workers have concluded t h a t the disaccharidases are not deep within t h e p r o - tein outer coat but are more like amylase, which is merely adsorbed to its surface.
Figure 11 has been devised in order to represent these divergent views a n d to clarify t h e issue. F r o m this diagram, it should be clear t h a t we believe t h e disaccharidases to be fixed elements of t h e protein outer coat of t h e microvillus m e m b r a n e formed during t h e m a t u r a t i o n and differentiation of t h e brush border as the epithelial cell migrates u p w a r d on the side of t h e villus [59] a n d n o t merely adsorbed or h a v i n g an inferior location of q u a n t i t a t i v e consequence.
I n support of this position, we m a y again cite the evidence from the kinetics of disaccharide hydrolysis, from m e m b r a n e isolation and from m e m b r a n e structure. However, in addition, we m a y now add w h a t seems to be a p a r t i c u l a r l y r e l e v a n t line of evidence, lately ob- tained from studies of some diseases of digestion and absorption. Over the p a s t few y e a r s , a n u m b e r of individuals h a v e been found in whom oral ingestion of various mono- and disaccharides elicits a w a t e r y , acid diarrhea. M a n y of these, upon further study, h a v e been found to be suffering from diseases in which t h e structure of t h e villi and t h e cells lining t h e m is grossly altered [13, 5 9 ] . I n such individuals, i m p r o v e m e n t of the Dahlqvist and Brun procedure has resulted in histo- chemical localization of disaccharidases only to the brush border [39a].
ORGANIZATION O F A N EPITHELIA L CEL L BRUS H BORDE R 87 a reductio n i n th e digestiv e an d absorptiv e capacit y o f th e intestin e is t o b e expected . I n others , however , gros s structura l abnormalit y is no t seen , ye t the y develo p simila r symptom s upo n ingestio n o f sugar [24 , 46 , 47 , 6 0 ] . Th e principa l differenc e betwee n thes e tw o groups o f sugar-intoleran t individual s i s t h a t th e forme r develo p symptoms i n respons e t o al l o r almos t al l dietar y sugar s an d hav e als o
PLASMA MEMBRAN E MEDIUM
U G O L E V
C E L L INTERIO R
CRANE
à Ù '.6 Q
à Ό
D A H L Q V I S T
FIG. 11. Representation of three possibilities for the localization of epithelial cell disaccharidases.
a reduced capacity to digest and absorb fat, whereas t h e l a t t e r develop s y m p t o m s in response to oral a d m i n i s t r a t i o n of only one or two specific compounds o u t of t h e whole v a r i e t y of disaccharides which are split b y enzymes of t h e intestine or of monosaccharides which can be a b - sorbed. T h e l a t t e r are clearly specific intolerances and, consistent with the known p a t t e r n of h u m a n intestinal disaccharidase activity [ 6 ] , only two varieties of disaccharide intolerance h a v e so far been de- scribed, n a m e l y , intolerance t o w a r d sucrose and isomaltose or t o w a r d
lactose [ 6 0 ] . Sucrose a n d isomaltose intolerance h a v e a l w a y s been found together and almost exclusively in y o u n g children. Lactose in- tolerance has been observed in some children a n d in a s u b s t a n t i a l proportion of t h e a d u l t population. A t h i r d v a r i e t y of specific sugar intolerance, to t h e monosaccharides glucose and galactose, has been observed in a few children.
Studies of the enzyme p a t t e r n of mucosal biopsies from individuals with specific disaccharide intolerance h a v e related t h e disease t o t h e v i r t u a l l y complete absence of t h e specific disaccharidase(s) of t h e brush border. Moreover, this absence, a t least in sucrose-isomaltose intolerance and t h e lactose intolerance of children, has been found to h a v e a familial basis. I t has been concluded t h a t t h e disaccharide intolerances of childhood represent another k i n d of genetically linked enzyme-deficiency disease of which there are now m a n y examples in t h e literature [ 6 9 ] .
W h a t is p e r h a p s more tenuous, b u t potentially more fundamental to concepts of m e m b r a n e functions, is t h a t t h e same reasoning, when applied to monosaccharide intolerance, brings us to an identical con- clusion concerning t h e n a t u r e of t h e sugar t r a n s p o r t process. I t is u n f o r t u n a t e , b u t unavoidable, t h a t when brush border m e m b r a n e s are isolated, t h e activity of interest, namely, t r a n s p o r t , can no longer be measured as a function; one m u s t be content with the theoretical implications of t h e fact t h a t t r a n s p o r t activity is a kinetic link be- tween t h e two faces of t h e p l a s m a m e m b r a n e . I t is, functionally, a specific binding site which provides access for t h e s u b s t r a t e to both t h e inner a n d outer fluid c o m p a r t m e n t s . T h e questions to be asked a r e : W h a t is its chemical n a t u r e and where precisely is it located?
T h e occurrence of ^specific monosaccharide malabsorption a p p e a r s to provide useful information.
T a b l e I I shows t h e specificity for absorption of sugar as delineated by studies of t h e intestine of small animals in vitro [ 1 , 15, 2 1 , 7 3 ] . T a b l e I I also indicates, as does Fig. 4, t h a t there are two different specific w a y s for sugar to enter t h e epithelial cell. One is a N a
+ - d e - pendent process which has t h e general specificity indicated by the pyranose ring s t r u c t u r e ; the other is a N a
+
- i n d e p e n d e n t process or group of processes of undetermined specificity. All of the d a t a so far available—including t h e existence of monosaccharide m a l a b s o r p - tion—show t h a t t h e N a
+
- d e p e n d e n t a n d N a +
- i n d e p e n d e n t processes are entirely separate from one another a n d t h a t the substrates of t h e one c a n n o t enter b y t h e other. T h e s u b s t r a t e s of t h e N a
+
- d e p e n d e n t
ORGANIZATION OF AN EPITHELIAL CELL BRUSH BORDER 89 process are k n o w n to include glucose, galactose, 3-methylglucose, xylose, and certain related compounds, a n d this process is known to result in t h e accumulation of sugar against a concentration gradient.
N a +
- i n d e p e n d e n t processes, on the c o n t r a r y , do n o t lead to a c c u m u l a - tion against a concentration gradient, b u t t h e y do p e r m i t e n t r y . F r u c - tose, 2-deoxyglucose, mannose, sorbitol, and the like, if t h e y enter a t all, do so b y N a
+
- i n d e p e n d e n t processes. Now, r e l e v a n t t o t h e present discussion, it is to be noted t h a t those studies which have been completed on children with monosaccharide m a l a b s o r p t i o n show t h a t all of t h e sugars listed under t h e N a
+
- d e p e n d e n t absorption col-
TABLE I I . The Specificity of Absorption of Sugar from the Intestine
O H Na
+
-dependent Na -independent 4-
Glucose Fructose
Galactose Mannose
3-Methylglucose 2-Deoxy glucose
Xylose Sorbitol
u m n in T a b l e I I elicit t h e s y m p t o m s of m a l a b s o r p t i o n [ 5 1 ] , whereas these same children are in complete remission, a t least so far as d i a r - rhea is concerned, a n d thrive when fed fructose [4, 46, 4 8 ] . This result can be interpreted as showing t h e absence of t h e N a
+
- d e p e n d e n t process for glucose b u t t h e continued presence of a specific, b u t N a
+ - independent, mechanism for the absorption of fructose. Consistent with current concepts of inherited enzyme-deficiency diseases, m o n o - saccharide m a l a b s o r p t i o n m a y t h u s be a t t r i b u t e d to t h e genetically linked absence of a specific p r o t e i n ; a unifying concept for monosac- charide m a l a b s o r p t i o n and disaccharidase deficiency would be to view t h e m both as representatives of a k i n d of cell m e m b r a n e disorder or " b r u s h border disease" in which t h e r e h a s been a failure of m a t u r a - tion or differentiation of a specific protein element of the outer coat of t h e m e m b r a n e [ 1 8 ] .
A further element of t h e structure of t h e brush border m e m b r a n e which m u s t be given serious consideration, b u t a b o u t which t h e r e is too little information to do so a t t h e present time, is t h e mucopoly- saccharide coat revealed b y periodic aeid-Schiff ( P A S ) staining proce- dures (ref. [ 1 0 ] , p . 16). I n some animals this coat is a b u n d a n t a n d extends into a n d fills t h e lumen of t h e intestine [ 3 8 ] . F o r this reason we m a y assume t h a t it forms t h e t r u e , outer surface of t h e p l a s m a m e m b r a n e ; i.e., t h a t it is entirely external to t h e outer protein coat and forms a layer over it—it is depicted in this w a y in discussions below. However, it is also possible t h a t mucopolysaccharide is inter- spersed with protein to form a coat of mixed properties. I n a n y case, w h a t role this m a t e r i a l m a y p l a y in t h e s t r u c t u r a l a n d functional organization of t h e p l a s m a m e m b r a n e is n o t y e t clear.
F U N C T I O N A L O R G A N I Z A T I O N O F T H E E L E M E N T S O F T H E P R O T E I N O U T E R C O A T
I n our early studies on t h e functional relationship between t h e hydrolytic enzymes of the brush border a n d the monosaccharide t r a n s - p o r t processes, we viewed t h e situation as one of c o m p a r t m e n t a t i o n in which t h e assumption w a s n a t u r a l l y implicit t h a t these functions were p r o b a b l y s e p a r a t e d into regions t h a t would eventually be m o r - phologically distinguishable. As our ideas h a v e developed, however, it has become evident t h a t c o m p a r t m e n t a t i o n , as a phenomenon, need not h a v e a gross s t r u c t u r a l aspect b u t m a y be one experimental aspect of a spatial ordering a t t h e molecular level, if such ordering were to confer a kinetic advantage for t h e occurrence of some events rela- tive to others.
T h e r e is evidence to suggest t h a t t h e disaccharidases are function- ally separated from t h e medium as though t h e aqueous phase in t h e protein coat of t h e surface were n o t in complete a n d i n s t a n t a n e o u s equilibrium with t h e aqueous phase of t h e medium. Miller a n d C r a n e [53] were led t o this conclusion from their studies of t h e effect of dilution or of t h e addition of glucose oxidase to t h e m e d i u m on t h e relative degree to which t h e p r o d u c t s of brush border disaccharidases a c t i v i t y either were t r a n s p o r t e d into t h e cell or diffused into medium.
Their d a t a suggested t h a t t h e active sites of t h e h y d r o l y t i c enzymes are so situated t h a t t h e former is favored over t h e l a t t e r ; t h a t there is, indeed, a n i m p o r t a n t kinetic a d v a n t a g e in a sense t h a t is n o r m a l to t h e surface of the cell. D a h l q v i s t a n d Thompson [26] h a v e recently found t h a t t h e kinetic constant, Km, for invertase is considerably
ORGANIZATION OF AN E P I T H E L I A L CELL B R U S H BORDER 91
increased when measured on i n t a c t tissue t h a n w r
hen measured on t h e isolated enzyme. T h i s result m a y also be t a k e n as evidence for a functional separation of disaccharidases from t h e m e d i u m since it indicates a restriction in t h e free access of s u b s t r a t e to t h e enzyme active site. However, in view of t h e limited information available, it is also possible t h a t t h e shift in kinetic c o n s t a n t s is a p r o p e r t y conferred by association of t h e disaccharidase with a surface [50]
or by t h e accumulation within t h e surface of t h e m e m b r a n e of a local high concentration of glucose, which is an inhibitor of invertase.
Stronger s u p p o r t for t h e notion of a functional separation of t h e hydrolases of the brush border m e m b r a n e from t h e medium is to be found in t h e evidence t h a t t h e p r o d u c t s of different hydrolytic activities h a v e different degrees of kinetic a d v a n t a g e with respect to the sugar t r a n s p o r t process—a result t h a t m a y be interpreted as showing s p a t i a l relationships within t h e outer protein coat which are parallel to t h e surface as well as n o r m a l . Miller a n d C r a n e [53]
found t h a t t h e effect of dilution of t h e m e d i u m on t h e a p p e a r a n c e in the medium of monosaccharide formed by the action of either sucrase or alkaline p h o s p h a t a s e was m i n i m a l a n d m u c h t h e same for both enzymes. However, when glucose oxidase was added to t h e m e d i u m in high concentrations the result was considerably different.
T h e proportion of glucose formed by alkaline p h o s p h a t a s e action on glucose-1-phosphate t h a t entered t h e cell was greatly reduced; t h e proportion of glucose formed as t h e result of t h e action of sucrase on sucrose was not appreciably affected. Goodridge h a s repeated these studies with t h e same result [ 3 4 ] . F i g u r e 12 is a representation of w h a t we t a k e to be the meaning of this kinetic a d v a n t a g e in t e r m s of one possible spatial a r r a n g e m e n t of t h e t h r e e functional elements of t h e m e m b r a n e which contribute to t h e results of this p a r t i c u l a r experimental procedure.
T h e results of recent studies on isolated m e m b r a n e s of T r i s - d i s - r u p t e d brush borders are entirely consistent with such notions of spatial ordering. Alkaline p h o s p h a t a s e , t h e disaccharidases, a n d leucyl n a p h t h y l a m i d a s e are bound to t h e m e m b r a n e of t h e brush border in d e m o n s t r a b l y different w a y s . As an illustration of this fact, t h e d a t a in Fig. 13 show one k i n d of result t h a t h a s been obtained. I n t h e experiments depicted, isolated m e m b r a n e s were t r e a t e d with p a p a i n for v a r y i n g lengths of t i m e . P a p a i n a c t i v i t y was stopped a n d the mixture was s e p a r a t e d by centrifugation. T h e proportions of en- zyme a c t i v i t y remaining with sedimentable m a t e r i a l and present jn
t h e s u p e r n a t a n t fluid were t h e n measured. T h e curves in Fig. 13 show t h a t invertase, here representative of all t h e disaccharidases, is r e - leased almost immediately, whereas alkaline p h o s p h a t a s e is released relatively very slowly. Along the same lines, it is of interest t h a t t h e A T P a s e a c t i v i t y of t h e m e m b r a n e is also released very slowly under these conditions. This enzyme has been found b y Oda and Sato to be localized to t h e inner of t h e two protein coats [ 5 7 ] , whereas alkaline p h o s p h a t a s e is associated with the outer coat (see a b o v e ) .
C E L L FLUID T H E LAYERS OF T H E PLASMA MEMBRANE MEDIUM
®
Θ Θ
PROTEIN LIPID
FIG. 12. Model of the spatial ordering of the outer protein coat of the microvillus membrane. P S = mucopolysaccharide, G = glucose, F = fructose Pi = phosphate. For discussion, see text.
F r o m another direction, Semenza and his colleagues have obtained results which also suggest a close relationship between t h e disaccha- ridases and t h e sugar t r a n s p o r t process. These workers h a v e described
[67] an activation of sucrase by N a +
which is, in m a n y respects, entirely analogous to t h e N a
+
activation of the N a +
- d e p e n d e n t sugar t r a n s p o r t process. T h e activation constants t h a t Semenza calculates from d a t a on h a m s t e r intestinal sucrase (see Fig. 14) [66] are, in fact, very close to t h e similar constants which can be calculated from
ORGANIZATION OF AN EPITHELIAL CELL BRUSH BORDER 93 the t r a n s p o r t d a t a of C r a n e , Forstner, and Eichholz [ 2 0 ] . F r o m such observations, Semenza has concluded t h a t t h e disaccharidases a n d the t r a n s p o r t system are, if not identical, so closely related t h a t t h e y are influenced by t h e binding of N a
+
to t h e same specific site. This i n t e r p r e t a t i o n is supported by Semenza's further finding t h a t a c t i v a - tion of r a b b i t sucrase by N a
+
involves an a p p a r e n t shift in t h e m a x i -
10 2 0 30 MINUTES O F INCUBATION
FIG. 13. Effect of papain on the distribution of the enzymes of the membrane.
(From Eichholz and Crane [1966].)
mal r a t e [ 6 6 ] , as does t h e activation of t r a n s p o r t by N a +
[ 6 5 ] . T h e r e is no shift in m a x i m a l r a t e in t h e h a m s t e r . These results are not, however, interpreted to m e a n t h a t t h e disaccharidase active site is, per se, t h e t r a n s p o r t system, because there is ample evidence to suggest t h a t it is n o t [ 1 2 ] .
A l v a r a d o , who is studying t h e relationship between the N a +
- d e - pendent t r a n s p o r t of amino acids a n d sugars, has interpreted [3]
the interactions between these t r a n s p o r t processes [55, 6 4 ] , as also
suggesting a close spatial relationship between t h e individual protein elements which possess t h e specific binding sites of t h e several t r a n s - p o r t processes.
I n other studies, we h a v e a t t e m p t e d to isolate soluble enzymes of t h e m e m b r a n e fraction of t h e brush borders by procedures less drastic t h a n p a p a i n digestion [ 5 ] . W e find t h a t t h e disaccharidases
8 0 0 -
100 2 0 0 3 0 0 ι
S U C R O S E FIG. 14. N A
+
activation of hamster intestinal sucrase (invertase). (From Semenza [1965].)
of T r i t o n - X - 1 0 0 extracts of m e m b r a n e s do not separate on Sephadex columns as discrete p e a k s b u t t r a v e l together until t h e y h a v e been t r e a t e d with p a p a i n [ 6 1 ] .
T h e single suggestion which all of these varied observations a p p e a r to support is t h a t t h e hydrolytic digestive enzymes a n d t h e t r a n s p o r t proteins form some sort of a macromolecular complex on t h e surface of t h e microvillus m e m b r a n e . If t h e d a t a are interpreted in this w a y , t h e complexities of organization suggested by t h e studies on relative
ORGANIZATION OF AN EPITHELIAL CELL BRUSH BORDER 95
kinetic advantage and by interaction of transport systems with N a + and with each other would appear to be simplified.
In this connection, it should be noted t h a t negatively stained prep- arations of isolated membranes [58] or sections of brush borders [57]
show the surface of the microvillus membrane to be covered by spheri- cal particles of about 40 to 60 Â in diameter. There is, as yet, no reliable evidence to identify these particles with functional protein elements of the brush border, but it is tempting to speculate t h a t these particles are a visualization of units of structure and function in which the hydrolytic digestive enzymes and the transport processes are arranged in such a way t h a t absorption of the products of hydroly- sis is greatly favored over their release into the lumen of the intestine.
S P E C U L A T I O N S O N T H E N A T U R E O F C A R R I E R S Y S T E M S
In the preceding sections the available observations have been so arranged t h a t they become, in sum, highly suggestive of intimate molecular association between the various functional elements within the membrane surface. However, it should be clear t h a t this suggestion is a product of the structure of the argument; few of the observations are yet at the level of proof. Nonetheless, having had this much reason to review the structure of the membrane, we have been led to recon- sider current concepts of the meaning of membrane transport kinetics with particular regard to the permissible nature of "mobile carrier"
systems.
A salient feature of membrane transport kinetics is t h a t they are usually calculated with the implicit assumption t h a t the binding site of the carrier, with or without attached substrate, is exposed alter- nately at the two interfaces of the lipid matrix of the membrane in such a way as to be in intimate and rapidly equilibrating contact with the medium and the cell contents, respectively. Several observa- tions discussed above suggest t h a t this is not, or at least not always, the case. Studies on hamster intestinal epithelial cells appear to have documented a kinetic advantage for the transport of monosaccharides formed by disaccharidase action which can be interpreted to mean t h a t the monosaccharides are released within the protein matrix of the membrane rather t h a n in the medium. Such a kinetic advantage would suggest t h a t the transport binding site is buried at some dis- tance, measured in molecular diameters, below the free cell surface.
Other observations suggest t h a t the hydrolases and possibly also the transport proteins are associated into macromolecular complexes. T a k -
ing these observations all together, it is possible to construct a n u m b e r of models which would provide t h e k i n d of a p p a r e n t c o m p a r t m e n t in the outer protein coat of the m e m b r a n e which has been kinetically visualized. F o r example, it m a y be imagined t h a t t h e hydrolases are associated w i t h one another in such a w a y as to form a crevasse or, perhaps, even a hollow cylinder, of which one end is open to t h e m e d i u m a n d t h e other is occupied by t h e t r a n s p o r t protein, form- ing a "well." T h e specific binding site of t h e t r a n s p o r t protein for s u b s t r a t e m a y be assumed to lie a t t h e b o t t o m of this well a n d t h e reactive sites of t h e hydrolases to occupy positions in t h e wall close to the bottom. Such a well would act as a region in which local high concentrations of monosaccharides for inward m o v e m e n t would be provided by disaccharidase action and similar local high concentra- tions would result from t h e o u t w a r d t r a n s p o r t of previously equili- b r a t e d sugar. T h e concentration difference of s u b s t r a t e between the well and t h e m e d i u m would then depend upon t h e r a t e s of disaccha- ridase action and of t r a n s p o r t relative to the r a t e of diffusion of s u b s t r a t e out of the well, b u t t h e concentration in t h e well would always be the greater. T h e existence of a local high concentration of s u b s t r a t e would provide, in experiments on s u b s t r a t e efflux into substrate-free media, a significant inward r a t e of t r a n s p o r t ("back r e a c t i o n " ) . N e t efflux would reflect a steady state and its r a t e would be expected to increase following t h e addition of a competing s u b s t r a t e to t h e medium. W h e n viewed in this w a y , this aspect of "mobile c a r r i e r " kinetics would a p p e a r to be satisfied by assumptions concern- ing only t h e a r r a n g e m e n t of elements in t h e protein outer coat and not their n a t u r e .
T h e second aspect of t r a n s p o r t function which m u s t be considered is t h e w a y in which t h e binding site of t h e t r a n s p o r t protein can be assumed to move across t h e lipid m a t r i x of the m e m b r a n e . T h e usual assumptions of t h e n a t u r e of "mobile c a r r i e r s " are illustrated in Fig. 5, above, and described briefly in the accompanying text. If the t r a n s p o r t protein were, indeed, bound to a macromolecular com- plex in the outer protein surface, as we have surmised, this description would not readily serve w i t h o u t t h e additional elaboration of a dis- tensible connecting link between t h e t r a n s p o r t protein a n d t h e complex of sufficient length a n d flexibility to p e r m i t t h e portion containing t h e binding site to t r a v e r s e t h e lipid m a t r i x w i t h o u t restriction. Such notions of t h e n a t u r e of carriers have lately become r a t h e r common, in keeping with the unifying assumption t h a t their kinetics require
ORGANIZATION OF AN E P I T H E L I A L CELL BRUSH BORDER 97 t h e specific s u b s t r a t e binding site to move across t h e lipid m a t r i x , individually a n d independently of other protein components. T h e r e seem to be other possibilities.
Some y e a r s ago, B e n n e t t [8] suggested a m e c h a n i s m for p i n o c y t o - sis in which t h e assumptions were limited s u b s t a n t i a l l y to two in n u m b e r ; n a m e l y , (1) t h a t specific binding sites exist on the surface of the m e m b r a n e , a n d (2) t h a t portions of t h e m e m b r a n e with a t - t a c h e d s u b s t r a t e become i n v a g i n a t e d a n d engulfed as pinocytotic vacuoles. A representation of this well-accepted concept is given in
FIG. 15. Model of pinocytosis. (Based on Bennett [1956].)
Fig. 15. Recently, it has occurred to us t h a t it m a y be possible to account for "mobile carrier" t r a n s p o r t kinetics within the same con- ceptual framework, i.e., with a similar kind of binding site b u t w i t h - out, of course, the invagination and engulfment of pinocytosis. One additional p r o p e r t y of the m e m b r a n e m u s t be p o s t u l a t e d .
If it m a y be assumed t h a t cell m e m b r a n e s , as t h e y are ordinarily viewed under the electron microscope, in a fixed, d r y state, h a v e u n d e r - gone shrinkage, even of a slight degree, it m a y be imagined t h a t t h e y have, in t h e n a t i v e state, a certain degree of flexibility—enough flexibility, p e r h a p s , to respond to t h e forces of t h e local environment with movements of sufficient m a g n i t u d e to produce a t r a n s i e n t , local
engagement of t h e two protein surfaces of t h e m e m b r a n e , as depicted in Fig. 16. If this engagement were of such a degree t h a t t h e specific binding site of t h e t r a n s p o r t protein would be exposed briefly to t h e cell contents, reversible, equilibrating t r a n s p o r t could be explained.
I t need not be expected, even if one could observe t h e surface of the living m e m b r a n e , t h a t such an engagement would a p p e a r as a n y - thing more t h a n one, or a t t h e most a few, small " d i m p l e s " moving with great r a p i d i t y over the surface of each microvillus of t h a t present techniques with the electron microscope should bring t h e m to light.
M
FIG. 16. Model of the concept of the mobile membrane. See text for description.
owing p r i m a r i l y to the anticipated negative influence of chemical fixa- tion on the flexibility of the m e m b r a n e , but also to shrinkage, as r e m a r k e d above.
If an engagement of this sort is assumed to occur, it is then neces- s a r y only to suppose t h a t its extent is minimal so as to eliminate t r a n s m e m b r a n e movements of extraneous, large substrates in a non- specific w a y and t h a t t h e r a t h e r free movements of small molecules and of water, which occur through m e m b r a n e s and which are measured as an " a p p a r e n t pore r a d i u s " [ 4 5 ] , are one measure of its extent.
W i t h regard to the kinetics of m e m b r a n e transport, the phenome- non of counterflow a p p e a r s to be most critical and most widely a c - cepted as evidence on which to base the assumption of a "mobile carrier" system [ 6 3 ] . However, t h e same phenomenon a p p e a r s to be explainable on the basis of t h e "mobile m e m b r a n e " — s o long as the increased r a t e of efflux of previously equilibrated s u b s t r a t e by the
ORGANIZATION OF AN EPITHELIAL CELL BRUSH BORDER 99 addition to the medium of a competing substrate is explainable as a result of an apparent compartment in the membrane surface (see above). The major difficulty is to explain alterations in maximal t r a n s - port rate. For this purpose it m a y be assumed t h a t interactions which produce a reduction in maximal rate reflect configurational or con- formational shifts in the macromolecular complex which, at a given temperature, reduce the rate of successful engagement of the substrate spécifie areas of the two protein surfaces. Conformational shifts have previously been invoked to explain p a r t of the effect of N a
+
on trans- port [20].
In conclusion, it m a y be noted t h a t this view of the membrane is, at a more microscopic level, very similar to the concept of an undulating membrane recently described by P a u l Weiss [72] and t h a t it also bears some resemblance to, but is mechanistically less complex than, the concepts presented by Booij in this volume [11]. We present these concepts, at the present time, primarily for consideration and, if possible, testing. The increasing complexity of interaction between N a
+
and various transport processes, N a +
and the hydrolases, and the substrates of one transport process with another, which has evolved from our work and t h a t of others, would seem, otherwise, to require a conception of molecular architecture for the membrane that would have to be highly specific throughout the entire thickness.
Though this degree of order is not impossible to imagine, it is not irrelevant to note t h a t what appear to be "unit" membranes have been produced by the simple expedient of mixing the appropriate ingredients of lipid and protein [70], thus suggesting t h a t membranes are formed by ordinary chemical interactions which it is difficult to expect to be discriminating at the level of a protein "active center."
The alternative t h a t we propose to complete and over-all substrate- specific order in the membrane is to postulate t h a t the specific t r a n s - port properties of membranes are conferred by the proteins of the surfaces which are assembled into macromolecular complexes, perhaps of a kind t h a t have already been visualized for enzymes [32]. Our view assumes these complexes to be attached to the lipid matrix by ordinary bonds and to operate as a result of substantially random deformations of the membrane.
ACKNOWLEDGMENT
I should like to record the fact that the discussions in this paper are a distilled result of conversations held over a period of many months with my colleagues Alvarado, Eichholz, Lyon, and Semenza; this is reason enough to
require them to share the credit, or blame, as the case may be. I should like also to thank the National Science Foundation and the National Institutes of Health for their generous support of those of our experiments which have been referred to.
REFERENCES
1. Alvarado, F., Experientia 2 0 , 302 (1964).
2. Alvarado, F., and Crane, R. K., Biochim. Biophys. Acta. 5 6 , 170 (1962).
3. Alvarado, F., Science 1 5 1 , 1010 (1966).
4. Anderson, C. M., Kerry, K. R., and Townley, R. R. W., Arch. Disease Childhood 4 0 , 1 (1965).
5. Auricchio, S., Dahlqvist, Α., and Semenza, G., Biochim. Biophys. Acta 7 3 , 582 (1963).
6. Auricchio, S., Semenza, G., and Rubino, Α., Biochim. Biophys. Acta 9 6 , 498 (1965).
7. Baldwin, E., "Dynamic Aspects of Biochemistry," p. 237. Cambridge Univ.
Press, London and New York, 1957.
8. Bennett, H . S., / . Biophys. Biochem. Cytol. 2 (Suppl.), 99 (1956).
9. Bihler, I., Hawkins, Κ. Α., and Crane, R. K., Biochim. Biophys. Acta 5 9 , 94 (1962).
10. Bloom, W., and Fawcett, D . W., "A Textbook of Histology," 8th ed., p.
446. Saunders, Philadelphia, Pennsylvania, 1962.
11. Booij, H . L., This volume.
12. Bosackova, J., and Crane, R. K., Biochim. Biophys. Acta 1 0 2 , 423 (1965).
13. Brackenbury, W., and Stewart, J. S., Med. Biol. Illustration, 13, 220 (1963).
14. Clark, S. L., Jr., Am. J. Anat. 1 0 9 , 57 (1961).
15. Crane, R. K., Physiol. Rev. 4 0 , 789 (1960).
16. Crane, R. K , Federation Proc. 2 1 , 891 (1962).
17. Crane, R. K , Federation Proc. 2 4 , 1000 (1965).
18. Crane, R. K , Gastroenterology 5 0 , 254 (1966).
19. Crane, R. K., Miller, D., and Bihler, I., In " M e m b r a n e Transport and M e - tabolism" (A. Kleinzeller and A. Kotyk, E d . ) , p . 439. Academic Press, New York, 1961.
20. Crane, R. K., Forstner, G., and Eichholz, Α., Biochim. Biophys. Acta 1 0 9 , 467 (1965).
21. Csaky, T . Z., and Lassen, U. V., Biochim. Biophys. Acta 8 2 , 215 (1964).
22. Curran, P., Federation Proc. 2 4 , 993 (1965).
23. Dahlqvist, Α., in "Ciba Foundation Symposium on Control of Glycogen Metabolism" (W. J. Whelan and M. P . Cameron, ed.), p. 57. Little, Brown, Boston, Massachusetts, 1964.
24. Dahlqvist, Α., in "Disorders due to Intestinal Defective Carbohydrate Diges- tion and Absorption" ( P . Durand, éd.), p. 9. 11 Pensiero Scientifico, Rome, 1964.
25. Dahlqvist, Α., and Brun, Α., J. Histochem. Cytochem. 1 0 , 294 (1962).
26. Dahlqvist, Α., and Thomson, D . L., Biochim. Biophys. Acta 9 2 , 99 (1964).
27. Davson, H., and Danielli, J. F., " T h e Permeability of N a t u r a l Membranes,"
p. 321. Cambridge Univ. Press, London and New York, 1943.
28. Deane, H . W., and Dempsey, E . W., Anat. Record 9 3 , 401 (1945).
ORGANIZATION OF AN E P I T H E L I A L CELL B R U S H BORDER 101
29. Eichholz, Α., and Crane, R. K. Federation Proc. 2 2 , 416 (1963).
30. Eichholz, Α., and Crane, R. K., J. Cell Biol. 2 6 , 687 (1965).
31. Eichholz, Α., and Crane, R. K., Federation Proc. 2 5 , 656 (1966).
32. Fernandez-Moran, H., Reed, L. J., Koike, M., and Willms, C. R., Science, 1 4 5 , 930 (1964).
33. Goldfischer, S., Essner, E., and Novikoff, A. B., J. Histochem. Cytochem.
1 2 , 72 (1964).
34. Goodridge, G., and Crane, R. K., unpublished observations.
35. Harrison, D . D., Personal communication (1965).
36. Harrison, D . D., and Webster, H . L., Biochim. Biophys. Acta 9 3 , 662 (1964).
37. Holt, J. H., and Miller, D., Biochim. Biophys. Acta 5 8 , 239 (1962).
38. I t o , S., Anat. Record 1 4 8 , 295 (1964).
39. Jesuitova, Ν . N., DeLaey, P., and Ugolev, A. M., Biochim. Biophys. Acta 8 6 , 205 (1964).
39a. Jos, J., Frezal, J., Ray, J., and Lamy, M., Nature, in press.
40. J u d a h , J. D., and Ahmed, K., Biol. Rev. Cambridge Phil. Soc. 39, 160 (1964).
41. Kaltenbach, J. P., Kaltenbach, M. H., and Lyons, W. B., Exptl. Cell. Res.
1 5 , 112 (1958).
42. Kinter, W. B., in "Proceedings 12th Ann. Conf. Nephrotic Syndrome" (J.
Metcoff, ed.), p . 59. N a t i o n a l Kidney Disease Foundation, 1961.
43. Kinter, W. B., and Wilson, T . H., J. Cell. Biol. 2 5 , 1 9 , (1965).
44. Larner, J., and Gillespie, R. E., J. Biol. Chem. 2 2 3 , 709 (1956).
45. Lindeman, B., and Solomon, A. K., J. Gen. Physiol. 4 5 , 801 (1962).
46. Lindquist, B., and Meeuwisse, G. W., Acta Paediat. 5 1 , 674 (1962).
47. Littmann, Α., and H a m m o n d , J. B., Gastroenterology 4 8 , 237 (1965).
48. Liu, Η . Y., Personal communication (1965).
49. McDougal, D . B., Jr., Little, K. D., and Crane, R. K., Biochim. Biophys.
Acta 4 5 , 483 (1960).
50. McLaren, A. D., Science 1 2 5 , 697 (1957).
51. Marks, J. F., Fordtran, J., and Norton, J. B., Personal communication (1965).
52. Miller, D., and Crane, R. K., Biochim. Biophys. Acta 5 2 , 293 (1961).
53. Miller, D., and Crane, R. K., Biochim. Biophys. Acta 5 2 , 281 (1961).
54. Nachlas, M . M., Monis, B., Rosenblatt, D., and Seligman, A. M., J. Biophys.
Biochem. Cytol. 7, 261 (1960).
55. Newey, H., and Smyth, D . H., Nature 2 0 2 , 400 (1964).
56. Newey, H., Sanford, P . Α., and Smyth, D . H., J. Physiol {London) 1 6 8 , 423 (1963).
57. Oda, T., and Sato, R., Symposium, Amer. Soc. Cell Biol., November, 1964, unpublished.
58. Overton, J., Eichholz, Α., and Crane, R. K., J. Cell Biol 2 6 , 693 (1965).
59. Padykula, Η. Α., Strauss, E . W., Ladman, A. J, and Gardner, F . H., Gastro- enterology 4 0 , 735 (1961).
60. Prader, Α., Semenza, G., and Auricchio, S., Schweiz. Med. Wochschr. 9 3 , 1272 (1963).
61. Rhodes, J. B., Eichholz, Α., and Crane, R. K., observations to be published.
62. Rosen, D., and Kretchmer, N., Personal communication (1964); now p u b - lished as: Doell, R. G., Rosen, G., and Kretchmer, N., Proc. Nat. Acad. Sci.
5 4 , 1268 (1965).
63. Rosenberg, T., and Wilbrandt, W., J. Theoret. Biol. 5 , 288 (1963).
64. Saunders, S. J., and Isselbacher, K. J., Nature 2 0 5 , 700 (1965).
65. Schultz, S. G., and Zalusky, R., Gen. Physiol. 47, 1043 (1964).
66. Semenza, G., Personal communication (1965).
67. Semenza, G., Tosi, R., Vallotton-Delachaux, M . C., and Mulhaupt, E., Bio- chim. Biophys. Acta 8 9 , 109 (1964).
68. Skou, J. C., Biochim. Biophys. Acta 2 3 , 394 (1957).
69. Stanbury, J. B., Wyngaarden, J. B., and Fredrickson, D . S., (eds.), T h e Metabolic Basis of Inherited Disease." McGraw-Hill, New York, 1960.
70. Stoeckenius, W., in " T h e Interpretation of Ultrastructure" (R. J. G. Harris, ed.), p. 349 Academic Press, New York, 1962.
71. Ugolev, A. M., Jesuitova, Ν . N., and DeLaey, P., Nature 2 0 3 , 879 (1964).
72. Weiss, P., Proc. Natl. Acad. Sci. U.S. 5 2 , 1024 (1964).
73. Wilson, T. H., "Intestinal Absorption," p. 86. Saunders, Philadelphia, Pennsyl- vania, 1962.
74. Wright, R. D., Jennings, Μ . Α., Florey, H . W., and Lium, R., Quart. J.
Exptl. Physiol. 3 0 , 73 (1940).