Inhibitors of Immune Reactions
Alan C. Aisenberg
I. Introduction 109 II. Immunosuppressants 110 III. Variables That Determine the Success of an Immunosuppressive Regimen 115
A. Drug and Dosage 115 B. Amount, Form, and Route of Antigen Administration 115
C. Timing of Drug and Antigen 115 D. Strength of the Immunological Stimulus 116
E. Nature of the Immune Response 118
F. Species 118 G. Innate Immunological Reactivity 119
H. Resistance to Immunological Injury 119 I. Immunological Nature of the Phenomenon to Be Suppressed 119
IV. Immunosuppressive Mechanisms 119
A. Biochemical 119 B. Immunological Parameters 120
C. Cellular Immunology I2 4
V. Human Applications 126 A. Organ Transplantation 126 B. Autoimmune Disease 128
C. Toxicity 129 References 130
I. INTRODUCTION
Immunological responsiveness is among the fundamental ways in which higher organisms react to their environment. Two quite distinct classes of immune reactions are recognized: antibody responses which are mediated by soluble proteins, and cellular immune responses in which activity cannot be separated from small lymphoid cells. Central to all immune reactivity is a specificity that is directed to the foreign material (antigen). Immunological responsiveness undoubtedly developed to pro-
109
110 ALAN C. AISENBERG
tect higher forms from infection by microorganisms (and perhaps also from neoplastic transformation from within) and much of the direction of classic immunology has been toward increasing immune reactivity.
However, in the past several decades there has arisen in clinical medi- cine a need to suppress immune reactions for two ends, namely, to facili- tate organ transplantation and to control autoimmune disease. Since this need is of considerable importance and since immunosuppressive drugs are the most effective means of diminishing immune responsiveness, a vast literature on these agents has appeared, concerned for the most part with minor differences in species, antigen, immune response mea- sured, or drug employed. In actual fact only a few immunosuppressants were custom-made; the great majority were lifted intact from the field of cancer chemotherapy and applied to their new purpose. Thus, the biochemistry of the immunosuppressants is essentially the biochemistry of the cytotoxic antineoplastic drugs.
The intention of this review is to establish principles of immunosup- pressive drug action rather than to catalog encyclopedic detail; other, more extensive recent reviews are available (1-7). Even so, the biochem- ist may find this chapter both too biological and too pragmatic. This unfortunately is the nature of the field.
A few preliminary points should be made at the outset. This review is restricted to extrinsic agents that inhibit the immune response; inhibi- tion by manipulations of antigen alone (specific immunological unrespon- siveness or tolerance) are excluded from consideration. However, it should be stressed that effective immunosuppression requires that, in some measure, there be specificity toward the antigen, organ, or tissue to which reactivity is to be suppressed, i.e., drug-induced immunological tolerance. Without this specificity, insufficient residual immunological reactivity would remain to cope with the variety of infectious organisms to which every species is susceptible.
Successful immunosuppression also requires that the agent not irrevers- ibly damage any other body function. Since the immunosuppressants are, for the most part, cytotoxic compounds that attack all rapidly divid- ing cells, the principal life-threatening toxicity usually involves depres- sion of bone marrow production of the formed blood elements (polymor- phonuclear leukocytes and blood platelets).
II. IMMUNOSUPPRESSANTS
Tables I and II contain, respectively, a listing of the principal immuno- suppressive agents and their structural formulas. Table I is not meant
T A B L E I
T H E PRINCIPAL IMMUNOSUPPRESSIVE AGENTS
Alkylating agents Nitrogen mustard
Cyclophosphamide (Cytoxan) Chlorambucil (Leukeran) Antimetabolites
Antipurines—6-mercaptopurine, azathioprine (Imuran), 6-thioguanine Folic acid antagonists—methotrexate
Antipyrimidines—5-fluorouracil°, 5-bromodeoxyuridine°
Antibiotics
Actinomycins (C and D) Chloramphenicol Azaserine Mitomycin C°
Puromycin"
Adrenal corticoids—prednisone, etc.
Miscellaneous compounds
Periwinkle alkaloids—vincristine and vinblastine Methylhydrazine derivative—procarbazine Antilymphocyte serum
Thoracic duct drainage X irradiation
α Predominant action in vitro.
to be exhaustive, but contains only those compounds with major immuno
suppressive activity or those of historical or theoretical interest. Agents with a minor effect in a single immunological system have been omitted.
It is immediately apparent that Table I could serve equally well as a classification for cancer chemotherapeutic compounds. The reason for this will be clarified in a later section, but for the present it is obvious that a detailed consideration of the pharmacological or biochemical prop
erties of this diverse group of complex compounds is beyond the scope of this review. Further, little would be gained by such a consideration since a number of excellent monographs in the field of cancer chemo
therapy are available (8-12).
Of the alkylating agents listed in Table I, nitrogen mustard is included largely for historical purposes. The progenitor of nitrogen mustard, sulfur mustard, was among the first materials to demonstrate significant im
munosuppression (13), and nitrogen mustard itself was the first immuno
suppressive alkylating agent with controlled toxicity to receive careful investigation in experimental animals (14, 15) and in man (16). Cyclo
phosphamide is a recently developed alkylating compound which is a
112 A L A N C. A I S E N B E R G
T A B L E I I
STRUCTURAL FORMULAS OF THE PRINCIPAL IMMUNOSUPPRESSIVE D R U G S
N I T R O G E N M U S T A R D C Y C L O P H O S P H A M I D E ( C Y T O X A N )
CH
<
SC HCH22C HCH22C I CIm e t h y l - b i s ( £ - c h l o r o e t h y l ) amine
C H L O R A M B U C I L ( L E U K E R A N )
^CH-CHXI H 0 O C H F C H T C H L - Q - < CP « CH « TH | C p-di (/9-chloroethyl) aminophenylbutyric acid
A Z A T H I O P R I N E ( I M U R A N )
H2 Η H.C 0=P-N
^CHGCHGCI
c - o
H2
XH2CH2CI N, /V-bis(/9-chloroethyl )-Ai<9-propylene phosphoric acid
N*
6- M P SH
N.
>
Ν ι*,
N-CH3
Ν Ν
6 - ( I -methyl - 4 - n i t r o - 5-imidazoyl) thiopurine
A M E T H O P T E R I N ( M E T H O T R E X A T E ) HOOC Η Ο CH3 ^ Ν γ Μ ν , Ν Η
I I 11 I Γ μ Γ 2 H - C - N - C - Γ VN -C H2- ^nA ^ N
CH2 ~ ~ NH2
CH2 COOH
4-Amino-N, Q-methylpteroylglutomic acid
P R E D N I S O N E
ή
6 - M e r c a p t o p u r i n e
6- T G SH
6 - T h i o g u a n i n e
A C T I N O M Y C I N D
H , C X CH
5? I
- C — C H HC — C — 1 I N - C H3 S A R C O S I NE S A R C O S I N E
Ο I I
L - P R O L I NE L - P R O L I N E D - V A L I NE Ο - V A L I N E I— g —C M
H . C - N I 3 I S A R CO
17,21 - Dihydroxypregna- 1,4-diene -3,11,20-trione
remarkably effective immunosuppressant in guinea pigs (17), mice (18), and other rodents. Because of their safety and ease of administration, cyclophosphamide and chlorambucil are logical choices among the al
kylating agents for trial in man.
Modern immunosuppression began slightly over a decade ago with
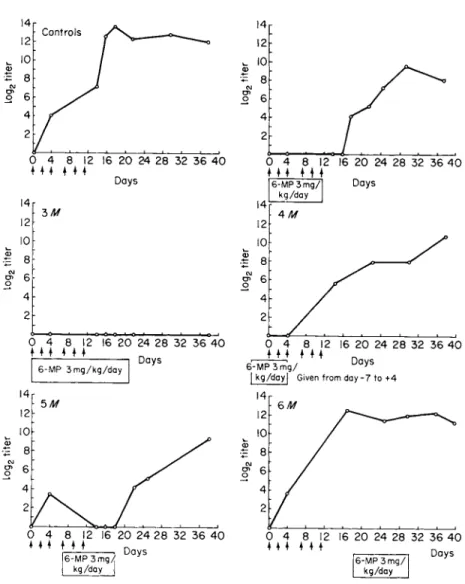
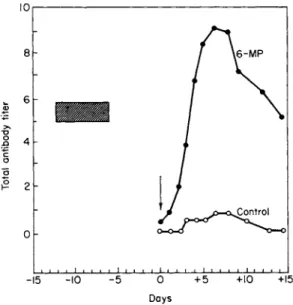
the successful inhibition of immune responsiveness by the antimetabolite 6-mercaptopurine. Schwartz et al. (19) suppressed the antibody response of rabbits to bovine serum albumin after earlier workers (20) had sug- gested the use of the compound but had been unfortunate in their choice of an immune test system. Figure 1, reproduced from the original paper of Schwartz et al. (19), illustrates complete inhibition of antibody forma- tion by nontoxic levels of 6-mercaptopurine. While 6-mercaptopurine is an effective immunosuppressant in the rabbit, a derivative, azathio- prine (21), enjoys a somewhat more favorable ratio of therapeutic to toxic effect in man and has become the standard human immunosuppres- sant. Thioguanine, like the two preceding compounds, appears to be an effective antipurine immune inhibitor and has been employed in con- trolling several human diseases (22, 23).
The immunosuppressant folic acid antagonist methotrexate was intro- duced before the thiopurines (24-29), and this compound has proven to be a potent inhibitor of immune responses in the guinea pig. Unfortu- nately, because of its delayed toxicity and renal route of excretion, methotrexate is a difficult compound to use in man, particularly for treatment of renal homograft rejection. The antipyrimidines (5-fluo- rouracil and 5-bromodeoxyuridine), in contrast to the antipurines and folic acid antagonists, are quite ineffective in vivo (5); they are included because of their in vitro inhibition of immune responses (30). It is of interest that the antipyrimidines, which in cancer chemotherapy act against neoplasms of glandular epithelium, are ineffective immunosup- pressants, whereas the folic acid antagonists and antipurines, which act against lymphoid leukemia and squamous cell neoplasms, are among the most effective.
Of the remaining compounds in Table I, the actinomycins and the adrenal corticoid prednisone (31) have been employed extensively in human immunosuppressive regimens, while azaserine has minor activity when used in combination with other agents (32). All the antibiotics listed in Table I have displayed their most prominent inhibition on in vitro systems, and some like puromycin (83) and mitomycin (34) are active only in vitro. The periwinkle alkaloids (35) and the methyl- hydrazine derivative procarbazine (36) are compounds that combine demonstrated immunosuppressive activity in animal systems with modest and acceptable toxicity in man, and they deserve further human trial.
Antilymphocyte serum (ALS), a recent development in the field of immunosuppressants, has proven effective both in experimental animals and in man. It has an extensive literature of its own (37-89) and will not be considered in detail in this review. Thoracic duct drainage is
14 12
^ 10
•Ξ 8
CM ο 6
4 2
Control s
4 8 12 16 20 24 28 32 36 40 4 4 4 4 4
Day s
3Λ/
0 4 8 12
M l 4 4 4 16 20 24 28 32 36 40
Day s
4 8 12 16 20 24 28 32 36 40 Η 4 4 4
Day s
0 4 4 I 4 8 12
• Μ
6-MP3mg/
kg/day I Given from day-7 to+ 4
16 20 24 28 32 36 40
Day s
0 4 4 4 4 8 12 16 20 24 28 32 36 40
• 4 * Day s
0 4 4 4 4
6-MP 3mg/
kg/day |
8 12 16 20 24 28 32 36 40
• • •
Day s 6-MP3mg/
kg/day
FIG. 1. Course of the immune response in control and 6-mercaptopurine-treated (6-MP) rabbits. Each vertical arrow represents an injection of antigen (bovine serum albumin). The time of administration of 6-MP is represented by the clear block, and each value depicted in the graph represents the average titer of five animals. [From Schwartz et al. (19).]
another new modality that is receiving initial evaluation in man (40).
X irradiation is included in the table because of the many parallels between this form of ionizing irradiation and immunosuppressive drugs
(41,42).
III. VARIABLES THAT DETERMINE THE SUCCESS OF AN IMMUNOSUPPRESSIVE REGIMEN
The following paragraphs consider a number of factors that in large measure determine the effectiveness of an immunosuppressive program.
In essence, these variables establish the relative strengths of the antigenic stimulus and the immunosuppressive counterforce.
A. Drug and Dosage
The choice of drug, route of administration, and dosage is of the same obvious importance in immunosuppression as in any other branch of pharmacology. It should be stressed that, in comparing results in man and experimental animals, it is important to contrast the lower human drug dosage (7, 43, 44, 44&) with the near-lethal levels employed in most animal systems.
B. Amount, Form, and Route of Antigen Administration
In all immunosuppressive regimens, antigen must be administered to- gether with the immunosuppressant. The antigenic requirements are the same as those for the induction of specific immunological tolerance (45, 46); namely, it is preferable that antigen be present in large amounts, in soluble form, and be administered via the intravenous route.
C. Timing of Drug and Antigen
The success or failure of an immunosuppressive program rests in con- siderable measure on the appropriate timing of drug and antigen, the critical point being that maximum immunosuppression and maximum antigen-stimulated lymphoid proliferation must coincide closely in time.
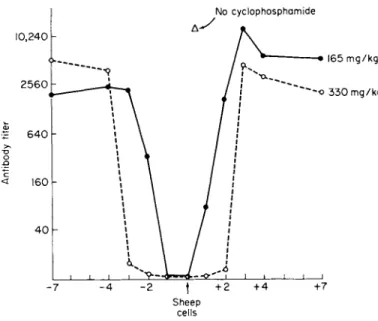
This is illustrated in Fig. 2, which depicts the suppression in mice of the response of a nonreplicating antigen (sheep erythrocytes) with the alkylating agent cyclophosphamide. Cyclophosphamide must be given simultaneously with antigen or within the preceding 24 hours to achieve complete suppression at low drug dosage (165 m g / k g ) , while at higher
No cyclophosphamid e
Shee p cell s
Day cyclophosphamid e injecte d
FIG. 2. Relationship of the times of antigen (sheep cells) and cyclophosphamide injection. The points represent, average hemagglutination titers in mice 5 weeks after sheep cell injection. [From Aisenberg (67).]
drug levels (300 mg/kg) the agent may be given up to 48 hours before or after antigen. With a replicating antigen, the immunosuppressant must be delayed to allow the antigen to build up the necessary level.
Thus, with lymphocytic choriomeningitis virus (4-7), it was found that maximum immunosuppression was achieved when methotrexate was de- layed until 4 days after virus innoculation, the time of peak virus titer.
An elegant example of meticulous timing of immunosuppressant treat- ment is illustrated in the work of Berenbaum and Brown (48). These workers demonstrated that appropriately timed folic acid would "rescue"
guinea pigs from the toxicity of methotrexate with marked improvement in drug mortality and skin graft survival.
D. Strength of the Immunological Stimulus
While it is difficult to quantitate the strength of immunological stimuli, there is little doubt that the ease of achieving immunosuppression is closely related to the strength of the antigen. Thus, the response to weak soluble protein antigens such as serum albumin or gamma globulin
is much more easily repressed than the response to strong particulate antigens (phage, viruses, or heterologous erythrocytes). Similarly, in the field of tissue transplantation, it is far easier to overcome minor histoincompatibilities than major ones. The Η-2 locus is the dominant histocompatibility site (49) in the mouse; suppression of skin homografts bridging this major Η-2 locus is achieved with difficulty, whereas homo- graft reactions involving minor (non-H-2) loci can be controlled with relative ease. In man the HL-A system occupies the same dominant position that the H-2 system occupies in the mouse (50). It now appears that current immunosuppressive regimens are adequate in man for kidney homografts well matched at the HL-A locus but that the long-term survival of homografts mismatched at this locus is less satisfactory (51).
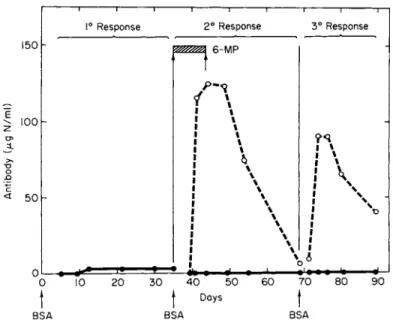
It is also much easier to suppress the primary response (initial expo
sure to antigen) than the secondary (second antigen exposure in the primed animal). Indeed, early workers found it impossible to inhibit the secondary response with 6-mercaptopurine (52), but Fig. 3 indicates
ι ι —ι 1° Respons e
1 ! 1 2 ° Respons e
ι ι • • π—
3 ° Respons e
15 0 6-M P -
10 0
ί *
• 1 1 \ 1 \ 1 1 1 % ι ι! *
! \
• χ| \
I \• %
5 0
0
• χ ι χ
ι χ
• \ ! \
! χ
J \
ι χ
ι \ ι \
• (
1 * • 4 • ι ι
I V
0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 8 0 9 0
J f Day s f
B SA BS A BS A
FIG. 3. Depression of primary, secondary, and tertiary response to bovine serum albumin (BSA) with 6-mercaptopurine (6-MP). The solid lines are drug-treated animals and the broken lines represent controls. The BSA injections are indicated by arrows and the period of 6-MP injection by the cross-hatched block. Results are expressed in antibody binding capacity (micrograms of nitrogen per milliliter).
[Modified from Gabrielsen and Good (5).]
118 ALAN C. AISENBERG
that the secondary, and even the tertiary, response to a weak antigen (bovine serum albumin) can be suppressed in the rabbit with very high doses of this excellent immunosuppressant.
E. Nature of the Immune Response
The spectrum of immunological responses is best divided into those mediated by antibody and those mediated by cells. Antibody is synthe- sized in plasma cells and certain larger lymphocytes and subserves a variety of classic immune responses (precipitin reaction, agglutination, opsinization, immune lysis, complement fixation, and anaphylaxis). In response to many antigens, the antibody sequence is an initial formation of a macroglobulin in the 19 S range, termed IgM, followed by the protracted formation of a smaller 7 S antibody named IgG (53).
Modern immunology has been particularly concerned with the cellular immune reactions mediated by small lymphocytes (54). Responses medi- ated by small lymphocytes include the familiar forms of delayed hyper- sensitivity (bacterial and contact sensitivity), graft-versus-host reac- tions, and, with some reservations, the homograft reaction (55).
There appears to be a difference in the susceptibility of the various forms of immune response to immunosuppressants. For example, with 6-mercaptopurine, delayed hypersensitivity (cellular immunity) is more easily suppressed than the IgG antibody response, which in turn is more easily suppressed than the IgM response (56, 57). Susceptibility to im- munosuppression appears to parallel the thymus dependence of immune reactions; cellular immunity is most thymus dependent, IgG antibody formation intermediate, and IgM formation least (58).
F. Species
There is considerable species variability in the effectiveness of individ- ual immunosuppressants. Examples of successful combinations are cyclo- phosphamide in the mouse, methotrexate in the guinea pig, 6-mercap- topurine in the rabbit, and azathioprine in the dog and man. This differential effectiveness probably reflects only minor differences in drug uptake, transport, and detoxification rather than fundamental difference in the mode of action or the immunological mechanisms in the various species. Nonetheless, the practical importance of properly mating the drug and species should not be underestimated.
G. Innate Immunological Reactivity
Immunological responsiveness is modified by a variety of genetic and environmental factors. 'Among mouse strains, the C57BL animal is par- ticularly reactive to a variety of antigenic stimuli, and it is quite difficult to suppress the immune responses of this strain. Again, the guinea pig displays particular development of delayed hypersensitivity. Further- more, in mice (59) and in guinea pigs (60), it has been clearly shown that reactivity to certain antigens is genetically determined. (The in- herited trait may determine antigen processing.) Man, like the guinea pig, is noteworthy for extreme development of delayed hypersensitivity (61) and exhibits wide variation of responsiveness from one individual to another and a decline of reactivity with age and chronic illness.
H. Resistance to Immunological Injury
There is a wide variation among grafts of different organs and tissues in relation to the extent of immunological insult they can sustain without irreversible damage and functional failure. Thus, the kidney is remark- able in its resistance to immunological injury (62, 68), the skin quite susceptible (64, 65), and the heart in between (66).
I. Immunological Nature of the Phenomenon to Be Suppressed
In assessing the potential benefit of an immunosuppressive program, some attention must be paid to whether the phenomenon to be suppressed is immunological. This applies particularly to autoimmune diseases, where there is often considerable doubt about the immunological nature of the process and of the detailed mechanism involved. The matter is further complicated because immunosuppressants are agents toxic to a variety of cells, and the ability to ameliorate a process is no assurance that the process was of immune genesis.
IV. IMMUNOSUPPRESSIVE MECHANISMS
A. Biochemical
Almost certainly, immunosuppressive drugs act by killing lymphoid cells or preventing their proliferation through interference with the repli-
120 ALAN C. AISENBERG cation of deoxyribonucleic acid ( D N A ) . The evidence for this conclusion, which is in large measure circumstantial, is as follows. First, were this not the case, it seems unlikely that the list of immunosuppressants would include essentially all the cytotoxic agents employed to treat human lymphoid neoplasms (acute and chronic lymphocytic leukemia and the malignant lymphomas). Second, a recent study has shown a close parallel between cyclophosphamide-inhibited spleen D N A synthesis and suppres- sion of the antibody response of mice to heterologous erythrocytes (67).
Finally, in almost every instance, the site of action of the more important immunosuppressants (see below) involves D N A synthesis. For further details, the reader is referred to the specific references below and the reviews in cancer chemotherapy listed in Section II.
The alkylating agents react with a variety of biologically important macromolecules, but the evidence is convincing that their primary action is to cross-link adjacent D N A chains via binding at the guanine residues (68). The mode of action of 6-mercaptopurine is quite complex and remains unsettled. This compound inhibits at least a half-dozen steps in purine and pyrimidine biosynthesis, but it appears most reasonable that its principal action also is to inhibit D N A synthesis (69). Metho- trexate poisons the enzyme dihydrofolate reductase, an enzyme that sup- plies one-carbon fragments for a number of synthetic functions including several essential steps in the synthesis of D N A (70). Actinomycin D is an inhibitor of considerable biochemical interest. This antibiotic binds to D N A and in so doing inhibits DNA-dependent R N A synthesis (71).
With two exceptions, it seems unlikely that the agents listed in Tables I and II have an important primary effect on either protein or ribonucleic acid synthesis. The exceptions are chloramphenicol (72) and puromycin
(73), both of which are inhibitors of protein synthetic pathways.
B. Immunological Parameters
1. NONSPECIFIC SUPPRESSION
Immunosuppressants frequently inhibit immune responsiveness, in part, through nonspecific damage to the lymphoid system. Such suppres- sion is undesirable since it is unrelated to antigen and produces a parallel increase in susceptibility to infectious agents. Figure 4 illustrates this occurrence in mice that have been treated with cyclophosphamide to inhibit their response to sheep erythrocytes. The chart indicates the number of antibody-forming cells (plaque cells) in the spleen of animals 17 days after receiving the immunosuppressant. Note that the animals
200,00 0
No cyclophosphamid e
Cyclophosphamid e (330mg/kg )
6. 4 χ 10 *
FIG. 4. Nonspecific lymphoid injury and specific immunological tolerance in cyclophosphamide-treated mice. Seventeen days before, animals had received either no cyclophosphamide and no sheep cells, or cyclophosphamide with varying amounts of sheep cells. The results are expressed as the average number of antibody-forming cells (hemolytic plaque-forming cells) in the spleen of mice that had, in each case, received antigenic challenge with sheep cells 4 days before plaquing. [From Aisen- berg (67)Λ
that received cyclophosphamide without sheep erythrocytes had but one-sixth the number of antibody-forming cells of those that received no drug, a measure of nonspecific unresponsiveness at this time.
2 . SPECIFIC IMMUNOLOGICAL TOLERANCE
The classic experiments of Billingham et al. (74), in which specific immunological unreactivity was produced by the neonatal injection of
122
antigen, suggested that immaturity of the lymphoid system was essential for the production of tolerance. However, over the past decade an abun- dance of experimental data (45) has indicated that immunological paral- ysis can be produced in the adult when persistent antigen levels can be achieved without provoking an antibody response. The early work of Schwartz and Dameshek (75) established that 6-mercaptopurine was able to induce such specific unreactivity in the adult animal, an observa- tion that has been confirmed with other immunosuppressants (1). The induction of tolerance to sheep erythrocytes (a strong antigen) is illus- trated in Fig. 4, where it will be noted that very large amounts of antigen must be administered together with cyclophosphamide to produce a state of complete unreactivity (67, 76, 77).
3. INHIBITION OF ESTABLISHED IMMUNITY
Potent immunosuppressants are able to inhibit delayed hypersensitiv- ity even when begun after sensitivity has been established. Table I I I illustrates the suppression of established tuberculin hypersensitivity in the guinea pig with methotrexate (28). As is usually the case when immunosuppressants are used in this manner, sensitivity rapidly returns when the drug is discontinued (35).
T A B L E III
SUPPRESSION OF ACTIVELY ESTABLISHED TUBERCULIN HYPERSENSITIVITY IN GUINEA PIGS BY METHOTREXATE"
Experimental group
No. of days after inoculation of BCG6
Experimental
group 14 30 40 50
Control
1 15 X 12 20 X 18 20 X 17 20 X 18
2 15 X 13 20 X 20 20 X 19 20 X 20
3 15 X 14 21 X 19 20 X 20 20 X 19
Methotrexate0
1 15 X 14 0 0 20 X 17
2 17 X 15 0 0 20 X 19
3 18 X 15 0 0 20 X 20
a From Friedman (28).
b The figures indicate cross diameters (in millimeters) of induration of the 24-hour skin reaction. BCG, Bacillus-Calmette-Guerin.
c Each animal received 5 mg of methotrexate day 15 through day 39.
4. INHIBITION OF THE INFLAMMATORY RESPONSE
Some immunosuppressants are able to depress the banal inflammatory response to materials such as egg white and turpentine. This inhibition is an important facet of immunosuppression with adrenal corticoids (8, 81), and it has been well documented with 6-mercaptopurine (78), where it was found that protracted treatment with high levels of antimetabolite was necessary. With other immunosuppressants (methotrexate), drug dosage that produced excellent immune inhibition was without effect on the inflammatory response (28). In instances where immunosuppres- sants do suppress inflammation, it is difficult to assess the extent to which an inhibited delayed skin reaction reflects immune inhibition.
5. GRANULOCYTE SUPPRESSION AND ARTHUS REACTIVITY
The Arthus skin reaction is an immediate response caused by the combination of antigen and antibody in the skin and is to be contrasted with the delayed cell-mediated reaction. The Arthus reaction is fre- quently necrotic and histologically shows polymorphonuclear leukocyte infiltration rather than the mononuclear cells of the delayed response.
Many immunosuppressants, particularly the alkylating agents, pro- foundly depress the level of circulating polymorphonuclear leukocytes and effectively suppress Arthus skin reactivity (79).
6. ADAPTATION OF THE GRAFT
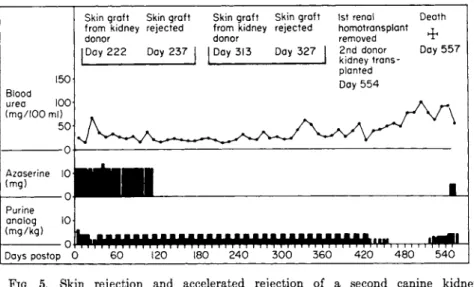
Kidney homografts that have been in place for a protracted period of time achieve a modus Vivendi with the host that permits survival of the graft despite the existence of potentially destructive immunological factors. The protective mechanism presumably involves either a barrier between host and graft, which protects the latter from the destructive elements of the host, or the coating of antigenic sites on the graft, which prevents recognition by host cells. Figure 5, taken from the work of Murray et al. (62), illustrates adaptation of a canine kidney homograft.
In this experiment an initial kidney graft placed under the protection of immunosuppressants survived for 554 days, while skin and a second kidney from the same donor, grafted after the drug dosages had been reduced, were promptly rejected.
7. ENHANCEMENT OF THE I M M U N E RESPONSE
In the course of investigating X-irradiation-induced immunosuppres- sion, Dixon and McConahey (80) observed that sublethal irradiation
124 ALAN C. AISENBERG
15 0 Bloo d ure a 10 0 (mg/10 0 ml )
5 0
Azaserin e 1 0 (mg )
0 Purine analo g (mg/kg ) 10
Skin graf t Ski n graf t fro m kidne y rejecte d dono r
Skin graf t Ski n graf t from kidne y rejecte d dono r
I
Da y 22 2 Da y 23 7 | | Da y 31 3 Da y 32 71s t rena l Deat h homotransplant τ remove d
2n d dono r Da y 55 7 kidne y trans
plante d Day 55 4
Day s posto p 0 h i M P i M P i U P M P i U P M P i M P M P M P i U P i ι
6 0 12 0 18 0 24 0 30 0 36 0 42 0 4 8 0 54 0
FIG. 5. Skin rejection and accelerated rejection of a second canine kidney homograft from the same donor in a dog that had tolerated an initial kidney homograft for 554 days [Modified from Murray et al. (62).]
given 1-4 days before immunization enhanced rather than inhibited anti
body formation. Figure 6 illustrates that very significant enhancement of antibody production can also occur with appropriately timed immuno
suppressant pretreatment (81). This enhancement, which should be dis
tinguished from the enhancement of tumor immunology, has been ex
plained by several unconvincing mechanisms. It has been suggested that the increased response is a result of increased room for proliferation in the depleted but not unsuppressed lymph node or, alternatively, that the drug makes essential nucleic acid precursors available as a result of cell destruction (3). Regardless of the mechanism, this enhancement by immunosuppressants has important implications for the clinical worker. It indicates that potential adverse effects may ensue from unnec
essary modifications of successful immunosuppressive regimens.
C. Cellular Immunology
It is convenient to divide the immune response into an afferent or sensory side which reacts with antigen, a central mechanism which elabo
rates the response, and an efferent or effector side. Immunosuppressants act on the effector limb and the central mechanism; evidence for a significant inhibition of the afferent limb is not convincing (5).
1 I I I I I I I I I I I I I I I I I I I—I I I I I I I I I I 1
-1 5 -1 0 - 5 0 + 5 +1 0 +1 5
Day s
FIG. 6. Enhancement of antibody synthesis by 6-mercaptopurine ( 6 - M P ) . The hatched bar indicates the timing of drug administration, and the vertical arrow the time of antigenic challenge. The amount of antigen employed (20 /*g of bovine gamma globulin) was barely immunogenic in normal rabbits. [From Schwartz (3).]
A variety of immunosuppressants, including alkylating agents, X ir- radiation (82), and corticosteroids (31), are markedly destructive to effector lymphocytes. (The greater sensitivity of small lymphocytes, which mediate cellular immunity, than of plasma cells, which mediate antibody responses, explains in part why cellular reactions are more easily suppressed by these agents.) Antilymphocyte serum is also be- lieved to act by depletion of peripheral lymphocytes (39, 83); the alter- nate explanation that this material "blindfolds" or sterilely inactivates effector cells (38) has received little support.
Quantitating effector lymphocyte depletion is difficult because of our inability to identify the several morphologically similar populations of peripheral lymphocytes (84). At present it is believed that complete immune responsiveness requires the interaction of two populations of small lymphocytes, a long-lived thymus-derived cell and a short-lived marrow-derived cell (58, 85-87). Until the exact function of the two populations is known and until the separate populations can be enumer- ated, it will be impossible to evaluate lymphocyte depletion.
The second important cellular mechanism operating during immuno- suppression is the induction of central inhibition or immunological toler-
126 ALAN C. AISENBERG
ance. It is well known that the immune response is accompanied by- active proliferation of lymphoid cells (88, 89) and equally well estab- lished that rapidly dividing cells are susceptible to the cytotoxic immuno- suppressants (90). Thus, in the induction of drug-induced tolerance some such mechanism as the following takes place. Antigen stimulates the responsive clone of cells to divide rapidly; this rapid proliferation renders the responding clone particularly sensitive to the cytotoxic drug; and the clone responding to the particular antigen is selectively killed or inactivated. As repopulation of the lymphoid system takes place, the new lymphoid cells mature in the presence of antigen and could be expected, by conventional tolerance theory (45), to be tolerant. The result would be a stable state of tolerance.
In practice there is probably a combination of partial tolerance and partial depletion of effector lymphocytes. Neither process is complete, but in successful immunosuppression the result is a satisfactory attenua- tion of the destructive immunological events.
V. HUMAN APPLICATIONS
A. Organ Transplantation
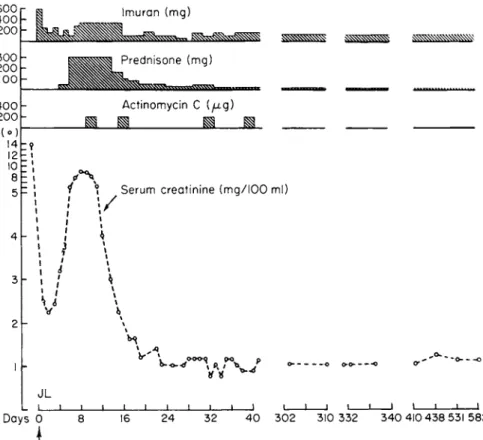
By far the most important human application of immunosuppressants has been in the management of renal homografts. Figure 7 illustrates the results that can be achieved with current techniques in a patient who received a well-matched renal homograft from his brother (91).
This 47-year-old man with terminal polycystic renal disease received an initial dose of azathioprine (8 mg/kg) on the evening prior to surgery and was subsequently maintained at a drug level (1-4 mg/kg/day) that did not significantly depress the daily determined granulocyte or platelet counts. The initial dramatic improvement in renal function was reversed on the third day (rising serum creatinine) because of the onset of a rejection crisis. Rejection was treated with prednisone, first in moderate dosage (60 mg/day) and later in large dosage (300 m g / d a y ) , and then with courses of actinomycin C (200 /xg intravenously 2 successive days of each week). With this program, rejection was reversed and now, more than 5 years after grafting, the man continues to do well, with satisfac- tory renal function.
Azathioprine is the primary immunosuppressant in almost all renal transplantation centers, although there are minor variations of timing
1 /
Serum creatinine (mg/IOO ml)S 6
JL
_ l _
Days Ο 16 2 4 3 2 4 0 3 0 2 31 0 33 2 3 4 0 41 0 43 8 53 1 5 8 2
FIG. 7. Chart of a 47-year-old male with polycystic renal disease who received a kidney homograft from his 43-year-old brother on day 0. Improvement in renal function is indicated by the fall of serum creatinine from 14 to 2.3 mg/100 ml in the first 4 8 hours after transplantation. The rise in serum creatinine on day 3 was caused by a rejection crisis that was successfully treated with prednisone and actinomycin C. [Modified from Austen and Russell (91).]
and dose (7). As would be expected from animal studies, good results are observed when the maximum immunosuppressant effect is produced shortly after grafting, and it is neither necessary nor desirable to cause severe leukopenia. Rejection occurs in many patients despite azathioprine and is best treated with a secondary drug. This is generally prednisone, which many centers now begin to administer at the time of grafting rather than at the time of rejection. As tertiary treatment, antilympho- cyte serum now frequently replaces actinomycin C or D .
The recent statistics of the "Human Kidney Transplant Registry"
indicate a 1-year survival of 78% for kidneys from siblings, 71% for
128 ALAN C. AISENBERG
kidneys from parents, and 45% for grafts from unrelated cadaver donors (92). Present evidence suggests that survival and maintenance of kidney function during the next 4 years is directly related to the histocompatibil
ity match of the grafted kidney (51). One-year survival should be capa
ble of further improvement since many of the first-year deaths are in the immediate postoperative period and are related to technical problems in surgery and organ procurement {93). The results of transplantation of other organs {94) such as lung {95), liver {96), and heart {66) are too preliminary to be evaluated at this time.
B. Autoimmune Disease
There is no doubt that immunosuppressants can decrease the incidence, delay the onset, and ameliorate the course of autoimmune disease in experimental animals. Cyclophosphamide can completely prevent the development of autoimmune allergic encephalomyelitis in both rats and guinea pigs; other drugs also suppress this disorder {97). Therapy with 6-mercaptopurine and methotrexate prevents the development of or com
pletely suppresses established autoimmune thyroiditis in guinea pigs {98). Very interesting results have been obtained in the fascinating and complex autoimmune disease of NZB and NZB χ NZW mice, which closely resembles the human disorder disseminated lupus erythematosus.
To date, immunosuppressive agents have been remarkably effective in influencing the development and course of lupus nephritis in NZB χ NZW hybrids but have not altered the course of hemolytic dis
ease in the NZB strain {99).
When one turns to the control of human autoimmune disease, a number of difficulties arise. Perhaps the greatest problem is the uncertainty that exists as to which disorders are primarily autoimmune in causation and what criteria should be applied to establish an autoimmune pathogenesis (100). Second, if the disease is autoimmune, what is the detailed mecha
nism? Is it an abnormal distribution or structure of antigen or is the primary abnormality in the immunological responsiveness of the individ
ual (7) ? A final complication arises because the remittent and prolonged course of many "autoimmune" disorders makes evaluation of therapy extraordinarily difficult.
The immunosuppressant thiopurines have found a tentative place in the clinical management of idiopathic thrombocytopenic purpura
(101-104) and of autoimmune hemolytic anemia (22, 105) after failure of corticosteroids and splenectomy (7), but it is impossible to comment
on the ultimate place of suppressants in the management of these two diseases. Evaluation of immunosuppressants is equally difficult in a vari- ety of other disorders. Preliminary data suggest that recurrent renal disease (glomerulonephritis) is less frequent in immunosuppressed recipi- ents than in unsuppressed individuals who receive the kidney of an identical twin (92). Variable results have been reported in the treatment of chronic renal disease (nephrosis, glomerulonephritis, and lupus nephri- tis) with immunosuppressants (106, 107). A variety of other disorders
(chronic hepatitis, disseminated lupus erythematosus, ulcerative colitis, regional enteritis, rheumatoid arthritis, scleraderma, dermatomyositis, and periarteritis nodosa) have been treated with immunosuppressants (7). Psoriasis, a disease without immune pathogenesis, also responds to antimetabolite treatment (102, 108). Immunosuppressants are also potentially useful in controlling the adverse immune response to various biologicals of nonhuman origin such as antihemophyllic globulin and insulin. In all of these instances controlled studies with a standardized regimen in a significant number of patients are badly needed.
C. Toxicity
The immunosuppressant drugs are very toxic compounds that affect a variety of tissues and organs. Certain toxic manifestations, such as the suppression of leukocyte and platelet production, are obvious and can be minimized by careful and conservative management. Other toxic- ity, such as the many adverse effects of adrenal corticoids, is the inevi- table but recognized result of long-term treatment. Still other toxic reac- tions are just coming to light, as the immunosuppressants are used for long periods of time in novel clinical situations. These include such occur- rences as insidious hepatic cirrhosis developing in psoriatic patients on long-term methotrexate (109) and malignant lymphomas in renal trans- plant patients receiving antilymphocyte serum (110). [In several animal systems a surprisingly high incidence of lymphomas has followed pro- longed immunosuppression with antimetabolites (111, 112).] The varied and unpredictable toxicity of extended immunosuppression requires that it not be undertaken for trivial clinical indications.
ACKNOWLEDGMENT
The author's research is supported by Research Grant C A - 0 7 1 7 9 of the National Cancer Institute, United States Public Health Service. This is publication 1398 of the Cancer Commission of Harvard University.
130 ALAN C. AISENBERG
REFERENCES
1. R. S. Schwartz, Progr. Allergy 9, 246 (1965).
2. R. S. Schwartz, Fed. Proc, Fed. Amer. Soc Exp. Biol. 26, 879.
3. R. S. Schwartz, in "Human Transplantation" (F. T. Rapaport and J. Dausset, eds.), pp. 440-471. Grune & Stratton, New York, 1968.
4. M. C. Berenbaum, Brit. Med. Bull. 21, 140 (1965).
5. A. E. Gabrielsen and R. A. Good, Advan. Immunol. 6, 91 (1967).
6. Ε. M. Hersh and E. J. Freireich, Methods Cancer Res. 4, 356-454 (1968).
7. C. W. Parker and J. D. Vavra, Progr. Hematol. 6, 1 (1969).
8. J. Burchenal, Cancer Res. 23, 1181 (1963).
9. P. Calabresi and R. E. Parks, in "The Pharmacological Basis of Therapeutics"
(L. S. Goodman and A. Gilman, eds.), 4th ed., pp. 1344-1396. Macmillan, New York, 1970.
10. I. Brodsky and S. B. Kahn, eds., "Cancer Chemotherapy." Grune & Stratton, New York, 1967.
11. R. J. Schnitzer and F. Hawking, eds., "Experimental Chemotherapy," Vol.
4. Academic Press, New York, 1966.
11a. R. J. Schnitzer and F. Hawkins, eds., "Experimental Chemotherapy," Vol.
5. Academic Press, New York, 1967.
12. M. D. Dowling, I. H. Krakoff, and D. A. Karnovsky, in "Chemotherapy of Cancer" (W. H. Cole, ed.), pp. 1-74. Lea & Febiger, Philadelphia, Pennsyl
vania, 1970.
13. L. Hektoen and H. J. Corper, J. Infec. Dis. 28, 279 (1921).
14. S. C. Bukantz, G. J. Dammin, K. S. Wilson, M. C. Johnson, and H. L.
Alexander, Proc Soc Exp. Biol. Med. 72, 21 (1949).
15. F. S. Philips, S. S. Sternberg, L. D. Hamilton, and D. A. Clarke, / . Immunol.
55, 296 (1956).
16. E. L. Dubois, Arch. Intern. Med. 94, 667 (1954).
17. H. C. Maguire, Jr. and Η. I. Maibach, J. Allergy 32, 406 (1961).
18. M. C. Berenbaum and I. N. Brown, Immunology 7, 65 (1964).
19. R. Schwartz, J. Stack, and W. Dameshek, Proc. Soc. Exp. Biol. Med. 99, 163 (1958).
20. J. Sterzl and M. Holub, Cesk. Biol. 6, 75 (1957).
21. G. B. Elion, S. Callanan, S. Bieber, G. H. Hitchings, and R. W. Rundles, Cancer Chemother. Rep. 14, 93 (1961).
22. R. S. Schwartz and W. Dameshek, Blood 19, 483 (1962).
23. H. C. Goodman, S. M. Wolff, R. R. Carpenter, B. R. Anderson, and M. W.
Brandriss, Ann. Intern. Med. 59, 388 (1963).
24. V. H. Haas and S. E. Stewart, Virology 2, 511 (1956).
25. D. E. Uphoff, Proc. Soc Exp. Biol. Med. 99, 651 (1958).
26. R. M. Friedman, C. E. Buckler, and S. Baron, / . Exp. Med. 114, 173 (1961).
27. R. M. Friedman and C. E. Buckler, / . Immunol. 91, 846 (1963).
28. R. M. Friedman, Proc Soc. Exp. Biol. Med. 116, 471 (1964).
29. J. L. Turk, Int. Arch. Allergy Appl. Immunol. 24, 191 (1964).
30. R. W. Dutton and J. D. Pearce, Immunology 5, 414 (1962).
31. J. A. Mannick and R. A. Egdahl, in "Human Transplantation" (F. T. Rapaport and J. Dausset, eds.), pp. 472-481. Grune & Stratton, New York, 1968.
32. J. E. Murray, J. P. Merrill, J. H. Harrison, R. K. Wilson, and G. J. Dammin, N. Engl. J. Med. 268, 1315 (1963).
33. J. O. Smiley, J. G. Heard, and M. Ziff, J. Exp. Med. 119, 881 (1964).
34. B. R. Bloom, L. D. Hamilton, and M. W. Chase, Nature (London) 201, 689 (1964).
35. A. C. Aisenberg and B. Wilkes, / . Clin. Invest. 43, 2394 (1964).
36. J. L. Amiel, C. Brezin, M. Sekiguchi, A. M. Mery, B. Hoerni, S. Garattini, G. Daguet, and G. Mathe, Rev. Fr. Etud. Clin. Biol. 9, 636 (1964).
37. G. E. W. Wolstenholme and M. O'Connor, eds., "Antilymphocyte Serum,"
Ciba Found. Study Group No. 29. Little, Brown, Boston, Massachusetts, (1967).
38. P. B. Medawar, in "Human Transplantation" (F. T. Rapaport and J. Dausset, eds.), pp. 501-509. Grune & Stratton, New York, 1968.
39. P. B. Medawar, Proc. Roy. Soc, Ser. Β 174, 155 (1969).
40. A. F. Dumont, in "Human Transplantation" (F. T. Rapaport and J. Dausset, eds.), pp. 482-488. Grune & Stratton, New York, 1968.
41. W. H. Taliaferro, L. G. Taliaferro, and Β. N. Jaroslow, "Radiation and Immune Mechanisms." Academic Press, New York, 1964.
42. P. Dukor and F. M. Dietrich, Int. Arch. Allergy Appl. Immunol. 34, 32 (1968).
43. R. H. Levin, M. Landy, and E. Frei, N. Engl. J. Med. 271, 16 (1964).
44. Ε. M. Hersh, P. P. Carbone, V. G. Wong, and E. J. Freireich, Cancer Res.
25, 997 (1965).
44a. J. E. Harris and J. G. Sinkovics, "The Immunology of Malignant Disease,"
pp. 176-202. Mosby, St. Louis, Missouri, 1970.
45. D. W. Dresser and N. A. Mitchison, Advan. Immunol. 8, 129 (1968).
46. S. Leskowitz, Annu. Rev. Microbiol. 21, 157 (1967).
47. J. Hotchin, Cold Spring Harbor Symp. Quant. Biol. 27, 479 (1962).
48. M. C. Berenbaum and I. N. Brown, Immunology 8, 351 (1965).
49. G. D. Snell and J. H. Stimpfling, in "Biology of the Laboratory Mouse"
(E. J. Green, ed.), 2nd ed., pp. 457-492. McGraw-Hill, New York, 1966.
50. J. Dausset and F. T. Rapaport, in "Human Transplantation" (F. T. Rapaport and J. Dausset, eds.), pp. 369-382. Grune & Stratton, New York, 1968.
51. R. Patel, M. R. Mickey, and P. I. Terasaki, N. Engl. J. Med. 279, 501 (1968).
52. R. Schwartz, A. Eisner, and W. Dameshek, J. Clin. Invest. 38, 1394 (1959).
53. J. W. Uhr and M. S. Finkelstein, Progr. Allergy 10, 37 (1967).
54. J. L. Gowans and D. D. McGregor, Progr. Allergy 9, 1 (1965).
55. D. B. Wilson and R. E. Billingham, Advan. Immunol 7, 189 (1967).
56. K. Sahier and R. S. Schwartz, Science 145, 395 (1964).
57. Y. Borel, M. Fauconnet, and P. A. Miescher, J. Exp. Med. 122, 263 (1965).
58. R. B. Taylor, Transplant. Rev. 1, 114 (1969).
59. H. O. McDevitt and M. Sela, / . Exp. Med. 122, 517 (1965).
60. Β. B. Levine and B. Benacerraf, Science 147, 517 (1965).
61. A. M. Kligman and W. L. Epstein, in "Mechanism of Hypersensitivity"
(J. H. Shaffer, G. A. LoGrippo, and M. W. Chase, eds.), pp. 713-722. Little, Brown, Boston, Massachusetts, 1959.
62. J. E. Murray, A. G. R. Sheil, R. Moseley, P. Knight, J. D. McGavic, and G. J. Dammin, Ann. Surg. 160, 449 (1964).
63. G. J. Dammin, in "Human Transplantation" (F. T. Rapaport and J. Dausset, eds.), pp. 170-200. Grune & Stratton, New York, 1968.
132 ALAN C. AISENBERG 64. P. B. Medawar, Harvey Lect. 52, 144 (1956-1957).
65. F. T. Rapaport and J. M. Converse, in "Human Transplantation" (F. T.
Rapaport and J. Dausset, eds.), pp. 304-312. Grune & Stratton, New York, 1968.
66. F. C. Spencer, T. Cooper, and S. C. Mitchell, Transplant. Proc. 1, 691 (1969).
67. A. C. Aisenberg, / . Exp. Med. 125, 833 (1967).
68. G. P. Wheeler, Fed. Proc, Fed. Amer. Soc. Exp. Biol. 26, 885 (1967).
69. G. H. Hitchings and G. B. Elion, in "Cancer Chemotherapy" (I. Brodsky and S. B. Kahn, eds.), pp. 26-36. Grune & Stratton, New York, 1967.
70. J. R. Bertino and D. G. Johns, in "Cancer Chemotherapy" (I. Brodsky and 5. B. Kahn, eds.), pp. 14-25. Grune & Stratton, New York, 1967.
71. I. H. Goldberg and M. Rabinowitz, Science 136, 315 (1962).
72. E. F. Gale, Pharmacol. Rev. 15, 481 (1963).
73. D. Nathans and F. Lipmann, Proc. Nat. Acad. Sci. U.S. 47, 497 (1961).
74. R. E. Billingham, L. Brent, and P. B. Medawar, Phil. Trans. Roy. Soc. London, Ser. Β 163, 61 (1956).
75. R. S. Schwartz and W. Dameshek, Nature (London) 183, 1682 (1959).
76. F. M. Dietrich and P. Dukor, Pathol. Microbiol. 30, 909 (1967).
77. A. C. Aisenberg and C. Davis, / . Exp. Med. 128, 35 (1968).
78. A. Page, R. M. Condie, and R. A. Good, Amer. J. Pathol. 40, 469 (1962).
79. J. H. Humphrey, Brit. J. Exp. Pathol. 36, 268 (1955).
80. F. J. Dixon and P. J. McConahey, / . Exp. Med. 117, 833 (1963).
81. D. Chanmougan and R. S. Schwartz, J. Exp. Med. 124, 363 (1966).
82. E. P. Cronkite and A. D. Chanana, in "Human Transplantation" (F. T. Rapa
port and J. Dausset, eds.), pp. 423^439. Grune & Stratton, New York, 1968.
83. P. S. Russell and A. P. Monaco, Transplantation 5, 1086 (1967).
84. W. O. Rieke and M. R. Schwarz, in "The Lymphocyte in Immunology and Haemopoesis" (J. M. Yoffey, ed.), pp. 234-241. Williams & Wilkins, Balti
more, Maryland, 1967.
85. A. J. S. Davies, Transplant. Rev. 1, 43 (1969).
86. J. F. A. P. Miller and G. F. Mitchell, Transplant. Rev. 1, 3 (1969).
87. Η. N. Clamen and E. A. Chaperon, Transplant. Rev. 1, 92 (1969).
88. J. H. Humphrey, in "Immunological Diseases" (M. Samter, ed.), pp. 100-108.
Little, Brown, Boston, Massachusetts, 1965.
89. T. Makinodan and J. F. Albright, Progr. Allergy 10, 1 (1967).
90. J. R. DiPalma, in "Cancer Chemotherapy" (I. Brodsky and S. B. Kahn, eds.), pp. 1-8. Grune & Stratton, New York, 1967.
91. K. F. Austen and P. S. Russell, Ann. N.Y. Acad. Sci. 129, 657 (1966).
92. Advisory Committee of Human Kidney Transplant Registry, Transplantation 6, 944 (1968).
93. Advisory Committee of the Human Kidney Transplant Registry, Transplant.
Proc. 1, 197 (1969).
94. F. T. Rapaport and J. Dausset, eds., "Human Transplantation." Grune &
Stratton, New York, 1968.
95. D. A. Blumenstock, H. P. Otte, Ο. B. Jean, and M. A. Mulder, Transplant.
Proc. 1, 223 (1969).
96. Τ. E. Starzl, L. Brettshneider, F. Penn, P. Bell, C. G. Growth, H. Blanchard, N. Kashiwagi, and C. W. Putnam, Transplant. Proc. 1, 216 (1969).
97. P. Y. Paterson, in "Textbook of Immunopathology" (P. A. Miescher and
H. J. Muller-Eberhard, eds.), Vol. I, pp. 132-149. Grune & Stratton, New York, 1968.
98. H. L. Spielberg and P. A. Miescher, / . Exp. Med. 118, 869 (1963).
99. J. B. Howie and B. J. Helyer, Advan. Immunol. 9, 215 (1968).
100. P. A. Miescher and H. J. Muller-Eberhard, eds., "Textbook of Immuno- pathology," Vol. II. Grune & Stratton, New York, 1969.
101. B. A. Bouroncle and C. A. Doan, J. Amer. Med. Ass. 207, 2049 (1969).
102. C. C. Corley, Jr., Amer. J. Med. 41, 404 (1966).
103. L. N. Sussman, J. Amer. Med. Ass. 202, 259 (1967).
104. D. Ogston and H. W. Fullerton, Postgrad. Med. 42, 469 (1966).
105. J. V. Dacie and S. M. Worlledge, Progr. Hematol. 6, 82 (1969).
106. J. P. Merrill, Blood 20, 119 (1962).
107. A. F. Michael, R. L. Vernier, Κ. N. Drummond, J. I. Levitt, R. C. Herdman, A. J. Fish, and R. A. Good, N. Engl. J. Med. 276, 817 (1967).
108. D. J. Demis, C. S. Brown, and W. H. Crosby, Amer. J. Med. 37, 195 (1964).
109. R. O. Coe and F. E. Bull, J. Amer. Med. Ass. 206, 1515 (1968).
110. I. Penn, W. Hammond, L. Brettschneider, and Τ. E. Starzl, Transplant. Proc.
I, 106 (1969).
111. T. P. Casey, Blood 31, 396 (1968).
112. Μ. Υ. K. Armstrong, J. Andre-Schwartz, and R. S. Schwartz, in "Perspectives in Leukemia" (W. Dameshek and R. M. Dutcher, eds.), pp. 133-155. Grune
& Stratton, New York, 1968.