Carp Cultivation in Japan
TADASHI TAMURA
Department of Biology a n d Agriculture,
Faculty of Fisheries, Hokkaido University, Hakodate, Japan
I. Introduction 103 II. Production of Carp 104
III. Species 105 IV. Life History 106
V. Selection of a Location for Fish Farms 107
VI. Propagation Methods 107 A. Pond Culture 107 B. Paddy Field Culture 112 C. Cultivation in Water Reservoirs 113
D. Culture in Lakes 113
VII. Feeds 114 VIII. Fertilizing 117
IX. Agricultural Chemicals and Fish Culture 118
X. Transportation 119 XI. Enemies, Parasites, and Diseases 119
References 119
I. Introduction
Geographically, carp are distributed widely in various lakes and rivers in the world. The original home of carp is believed to be in the Persian part of Asia, but little is really known in this respect. According to Fujita (1933), they were introduced to other parts of the world as follows: to Europe, after the Crusades around 1150; from Austria to England, in 1496; from Prussia to Denmark, in 1560; from Prussia to Russia, in 1729; from France to North America, in 1830; from Japan to North America, in 1877. European cultivation of carp is first reported from Wittingen in Hungary in 1367. As for further historical data, ref
erence is made to Schäperclaus (1933) and Steffens (1956).
The earliest cultivation of carp known in history seems to refer to China. During a war period (500 B.C.), Tao Chukung (Tan-li) wrote a book called Yang Yu Ching (Handbook on Rearing Fish) and advocated that cultivation of carp is an extremely profitable business. At the present time cultivation of carp is extensively carried out not only in Japan,
103
China, Formosa, and in many areas of Southeast Asia, but also in Central Europe, as well as Germany, Austria, Czechoslovakia, and Poland. The Soviet Union has lately become a major producer.
II. Production of Carp
At its peak, production of carp in Japan reached 110,000 metric tons a year (in 1936), but recently production has dropped considerably, owing to the shortage of feed and other reasons. According to the Food and Agricultural Organization ( F A O ) statistics, the Soviet Union is the leading carp producer, followed by Japan (see Table I ) . Recent figures from China are not available but it is to be assumed that it raises more carp than any other single country. To what degree these FAO figures represent artificially cultivated carp is not known. Only for Japan is a
TABLE I
CARP CATCH IN SELECTED COUNTRIES ( 1 , 0 0 0 METRIC TONS)®
1 9 4 8 1 9 5 3 1 9 5 4 1 9 5 5 1 9 5 6 1 9 5 7
United States
Carp 3 . 2 1 5 . 6 1 2 . 5 1 6 . 5 1 4 . 8
—
China0
Cultivated carp
—
6 3 0 . 0 7 6 0 . 0 8 4 0 . 0 8 5 0 . 0—
Formosa
Common carp
—
1.1 1 . 0 1.0 0 . 9 1.0Grass and silver carp
—
2 . 1 1.9 1.7 2 . 0 2 . 3—
3ΪΓ 2^9 2 7Japan
Common carp
(Japanese koi)
—
1.7 2 . 1 2 . 4 2 . 4 2 . 2Crucian carp
(Japanese funa)
—
4 . 8 6 . 5 7 . 7 9 . 5 9 . 2Cultivated common carp
(Japanese koi)
—
4 . 2 4 . 5 6 . 2 6 . 3 5 . 7—
1 0 . 7 1 2 . 1 1 6 . 3 1 8 . 2 1 7 . 1Thailand
Carp 3 . 0 7 . 0 9 . 0 9 . 3 9 . 8 1 0 . 1
Czechoslovakia
Carp
—
6 . 1 5 . 3 5 . 5— —
Soviet Union
Common carp
—
3 6 . 3 5 2 . 0 5 0 . 5 5 1 . 0 4 9 . 9a FAO statistics.
0 The fishes of Cyprinidae are abundant in fresh-water areas in China.
2.9 3.3
distinction made in the statistics. Undoubtedly, some of the catch from natural waters could be attributed to man-controlled stocking, special management of waters, or even some feeding. India, China, and other Southeast Asian nations must have produced considerable amounts of carp, but there is a lack of statistical material concerning those areas.
Recent figures from the Soviet Union give its 1957 carp production as no less than 113,200 metric tons (see Chapter 3, Section V ) .
In Germany, 5,950 tons of carp were produced for food around 1931.
Together with 1,350 tons of trout, it is believed that the total of the two amounted to 12% of the entire catch of fish in Germany that year (Schä
perclaus, 1933). For further information on German conditions, see Runger (1940) and the survey by Mann in Chapter 3 of this volume.
III. Species
The common carp is known as Cyprinus carpio L. and is found all over the world, but locally it splits up into several different strains (sub
species ) .
Leather carp (C. carpio nudus), which has no scales, and mirror carp (C. carpio speculario), which has scales only on parts of the body sur
face, as well as scale carp (Cyprinus carpio) are found in Germany.
Along the Aisch River region in Bavaria, the Aischgrund strain is reared;
this is a very excellent kind of carp with much meat. The ratio between height and body length is 1:2. In Germany, besides the types already mentioned, good strains of carp are being cultivated, such as that of a French lineage with the ratio of 1:2.3, and the Galizier carp with 1:2.5 ratio.
Used in Japan for cultivation are: yamato-goi (Yamato carp) and shinshu-goi (Shinshu carp); the former carries the ratio of body height to body length of 1:2.6, while the latter has the ratio of 1:3.0. Incidentally, the native kind of carp found naturally in Japanese rivers has a slender body and the ratio is 1:3.6. For cultivation, it is necessary to select su
perior strains of carp. According to Menzel and Steffens (1957), their comparative experiments between mirror carp and leather carp as to growth proved the rate of growth was faster for mirror carp than for the latter type.
Among the carp being cultivated in Japan outside of the carp for food, there are higoi (red carp) and iro-goi (multicolor carp) for orna
mental uses. The iro-goi has beautiful shades of colors, and has been cul
tivated for a long time. Related to goi is the crucian carp called "funa"
and listed under this name in the statistics. It is raised for food purposes.
It grows more slowly and evenly than the common carp. It also shows a higher survival ratio (Nakamura, 1955). Its flesh is not as highly esteemed as that of the common carp.
IV. Life History
Although carp are distributed very widely between the tropical and temperate zone, in general, they prefer a warm mild climate. When the water temperature is between 15°C. and 3 0 ° C , the appetite of the carp increases.
When the water temperature is lower than 1 0 ° C , the appetite de
clines, and finally the fish enters a state of hibernation as temperatures get lower. The author did, however, observe an individual carp which, in water of the temperature of 1°C. in Lake Dahlai of North China, was eating sufficient Zooplankton to survive. It can be assumed that the carp may maintain its appetite in a considerably lower temperature than the above-stated minimum, possibly by becoming adjusted to the environ
ment.
The spawning season for the carp is in the spring. When the water temperature exceeds 1 7 ° C , male and female carp gather in shoals where water plants grow. They discharge eggs and milt while rubbing against each other's bodies. At this moment the eggs are fertilized and firmly attached to the surface of adjacent water plants. The number of eggs that a carp deposits depends on the age of the carp, but is between 10,000 and 800,000 averaging 400,000. The length of time needed for hatching differs, depending on the temperature of the water. According to Higurashi and Nakai (1924), if the water is 20 ° C , fry emerge after 5 days. These young, just hatched, are about 5 mm. long and have a yolk sac in the abdominal area. Carp grow the first three to four days by utilizing the yolk sac, but afterward devour small Zooplankton. According to Nakamura (1947), Moina larvae of Cladocera is suitable as carp food.
During the early period of growth, carp start taking animal food and eating plankton, but when they grow up they become polyphagous and eat Chironomus larvae from the silt, and other vegetable matter as well.
The growth of carp is shown in Table II.
The feeding habits of the crucian carp differ slightly from those of common carp. They depend less on the comparatively large bottom or
ganisms but more on minute adhering organisms, algae, and organic detritus (Nakamura, 1955). As these are more ubiquitously present than
TABLE I I
T H E GROWTH OF CARP IN J A P A N0
Age (years)
1 2 3 4 5
Natural 38 225 600 1,130 1,850
Cultured 375 927 1,580 1,980 2,300
a Weight is given in grams.
V. Selection of α Location for Fish Farms
Warm regions are preferable for fish farms. In selecting a place for fish farms, many conditions—the origin and quality of the water that will be used, a source of supply and the price of carp food, selling conditions and the situation of demand, and transportation problems—should be considered before making a decision.
For carp culture, water that is warm, rich in oxygen, and free from poisonous substances is required. Furthermore, it is desirable that it be available abundantly all year round. The longer the period in which the water temperature remains between 2 0 ° C . and 3 0 ° C , the better, because of the above-mentioned prerequisites for the good appetite of the carp.
The salt content of the water has to be below 7 parts per thousand.
According to Brockway ( 1 9 5 0 ) and Kawamoto ( 1 9 5 7 ) , the rate of growth of carp in a pond is in inverse proportion to the amount of am
monium nitrogen excreted.
VI. Propagation Methods
Like any other livestock, carp can be raised from eggs up to the size ready to be used as food by going through the process of acquiring eggs from fertile carp, an artificial incubation and fry-rearing, to the final edible carp. Propagation methods can be generally categorized as the following four:
A. P O N D C U L T U R E
Ponds are constructed especially for the cultivation of carp. They are stocked with larvae in order to obtain rapid growth: the yield per unit area is, in this case, the highest as compared with the three other methods.
There are two types of carp ponds: single cultivation, and mixed cultiva
tion together with such fish as goldfish and striped mullet.
the food of the carp, this might explain their more rapid development.
See Section I I I of this chapter.
1. Necessary Types of Cultivation Fonds
a. A P A R E N T F I S H P O N D
This is a pond to rear parent fishes. The depth of water should be 1.5-2 m. with a deeper spot of an additional 0.5 m. in the central part of the pond. For each 5 parent fishes, an area of one tsubo (3.3m.2) is required.
b. A S P A W N I N G P O N D
When a spawning season approaches, the spawning day can be pre
dicted by a close observation of the carp's activities. At this point, parent carp are to be moved to a spawning pond. By having the water 0.5-0.7 m.
deep, temperature of the water goes up, and then parent carp deposit spawn in the water.
c. A N I N C U B A T I O N P O N D
Remove the fish nests, to which carp eggs are attached, to an incuba
tion pond and hatch them there. The depth of the water is shallow, about 15 to 20 cm. It is more convenient to have many small ponds for the hatching. From 20,000 to 30,000 eggs can be hatched in the area of one tsubo (3.3 m .2) .
d. A F R Y - R E A R I N G P O N D
This is a pond to nurse the fry that are few days old. There are two occasions for the use of this type of pond: one is for the raising of larvae up to a body length of 3 cm. and the other is for cultivating carp until fall, as edible carp.
e. A N U R S E R Y P O N D
This is principally for the rearing of edible carp. The depth of the water is to be between about 1.0 m. and 1.5 m. The most common size is between 1,000 and 10,000 m.2 When the supply of water for the pond is limited, a closed water system is used, in which case the size of a pond usually is larger. When the water supply is plentiful, the running water system always is used: the size of a pond, in this case, usually is smaller, perhaps 30 m.2
f. A " W I N T E R I Z I N G " P O N D
This type of pond is used in a region where the water surface of nursery ponds freezes during the winter months. The depth of the water
should be deep (2.0 m.), and should have continuously running water.
All the carp are kept in this pond for hibernation. During the winter months, a wooden cover should be placed over the pond.
2. Selection of Parent Carp
The carp for stocking must be the offspring of carp that are excellent in quality. A superior strain implies that the growth is fast, that they are physically strong and healthy, and, besides, that the height of body is high so that the amount of edible meat is large. With regard to parent fish, the female carp that are 10 years of age or older are most desirable as parent carp, while the carp of 4 to 5 years of age are most desirable as male parent carp. Feed that contains much fat is not suitable for carp being reared as parent fish.
3. Methods of Procuring Eggs
Carp start to lay eggs when the temperature of the water in a pond reaches 20°C. in the spring. Female and male carp are kept separate, prior to the spawning season, and then, when the pond is ready and the water is sufficiently warm, they are brought together in the pond. Most often they deposit spawn on the following morning. Some water plants, such as goldfish-duckweed and tuft-duckweed, or cryptomeria leaves and willow roots, are required, floating on the surface of the water in the spawning pond for the depositing of eggs. In order to obtain eggs arti
ficially, according to Wielenbach (1938), mature eggs are procured by pressing the abdominal part of the female carp when the spawning act has begun. By transferring eggs obtained in this way into water in which male carp's milt is present, fertilization of the eggs is accomplished.
4. Incubation
The percentage of incubation in a hatching or incubation pond lies between 0 . 1 % and 48.8%, averaging about 1 4 % , depending on the weather.
It is normal if 10,000 fry, whose size is about 1.5 cm. to 2.0 cm., can be obtained from each female carp. For feeding the fry right after hatch
ing, small Zooplankton of the Cladocera family, such as Moina and Daph- nia, are propagated in separate ponds. This is an old-time procedure in Japan. Sometimes, boiled egg yolks, which are made powder-like by placing them in a cloth bag, are used as a substitute.
5. Rearing of Fry
Even if all the conditions are identical, the growth of each carp varies greatly. According to Yamada (1938), the biggest specimen was 22.5 cm.
long, the smallest one 2.25 cm. long. Carp 4 - 5 cm. long constituted the majority, namely 87.1% of the total crop. Only 4.4% was more than 12 cm. long, out of the 889 carp at the time of cropping in the fall reared from 40,000 carp originally planted in the spring. Pisciculturists call these fast-growing carp tobi and use them for further cultivation or as parent carp. If 100,000 fry about 3.0 cm. in length are obtained from 300,000 fry of 1.0 cm. when planted in each tank (9.9 ares), it should be con
sidered very successful. For such a result, 10 female and 40 male carp are required as parents.
Incidentally, it is necessary to decrease the number of fry for each unit of area as they grow in size. Also, it is necessary to keep the fry of the same size in a separate pond. Carp of different sizes should not be mixed together. In order to keep the size of carp uniform in ponds it is convenient to use a selecting tool made out of wire screens for picking out those of a certain size. The standard numbers of carp to be released in each unit of area are shown in Table III.
TABLE I I I
NUMBER OF CARP PER tsubo ( 3 . 3 m.2)
Number of months after incubation
1 2 3 4
Number of carp 5 , 0 0 0 - 1 , 0 0 0 3 0 0 - 1 0 0 1 0 0 - 3 0 1 0 - 5
6. Cultivation of Carp for Food
a. C L O S E D W A T E R P O N D S
It is most common to release the fry that were incubated the previous year into a nursing pond in the spring. One to 2 fry of 10 cm. in size per tsubo (3.3 m.2) are planted. The number is reduced to half one month later, and then they are cultivated up to the fall period. Then, a carp 30 to 40 cm. long, weighing 700 to 1,000 g., can be obtained and sold as food. The limit for rearing carp per tsubo (or 3.3 m.2) is about 1.2 to 1.5 kg.
The various feeds employed are listed in Table VII. For the first artificial feeding, when they are just released in a pond in the spring, cooked oats (or wheat) is recommended. It is readily digested, and the
carp is easily adjusted to this kind of feed. After a while, various other feeds can be mixed with this kind of feed.
The amount of feed to be administered is to be adjusted to the ap
petite of the carp. This is influenced by the temperature of the water in the pond, the amount of oxygen dissolved in the water, the pH, and also by the weather. Consequently, some caution is necessary, as these environmental conditions should favor a good appetite and not be detri
mental to the feeding. One should always be very careful not to allow food to be left in the ponds. Special attention has to be given to the risk of an accumulation of excretory substances, impeding production (Iver, 1935; Brockway, 1950). The degree of water renewal and the oxygen balance have to be followed closely.
In the Tokyo area, the ratio of feeding referring to each month is standardized as indicated in Table IV.
TABLE I V
PERCENTAGE OF FEEDING IN EACH MONTH
Month
4 5 6 7 8 9 10 11 Water temp. C.° 14 16 20 25 26 22 15 13 Feeding percentage 1 4 15 20 30 20 9 1 No. of feedings/day 0.5-1.0 1-3 3-6 4-7 5-9 4-7 2 - 5 2-0.5
Supposing fry " W0" in weight is planted in a certain pond in the spring, and a crop of "Wi" is expected at the time of harvesting in the fall, the amount of feed can be calculated under the supposition that the natural productivity of the pond and the depletion of the pond during the raising are zero, the amount of feed needed, "A," up to the fall, when the coefficient of meat increase is "a," can be estimated very easily as follows:
A = ( W i — Wo) a
b. R U N N I N G W A T E R P O N D S
This method was introduced nearly 50 years ago by Tomoichi Tanaka.
This is a method of cultivating large quantities of carp in a small pond by using substantial amounts of water. An example: 7,500 second-year carp, weighing 630 kg., were released in a pond of 40 m.2, and 7,418 carp totaling 8,850 kg. were obtained in the fall. The depth of the water was 2 m., the amount of running water supplied to the pond was 360 liter/sec. The water kept flowing and changing more or less as in a river
stream. Fresh pupae of silkworms were used for feeding: they were pro
vided to the fish every one to half hour in this experiment.
B . P A D D Y F I E L D C U L T U R E
The method of raising carp in paddy fields was introduced about 1,500 years ago into Southeast Asia from India, together with the Hindu culture. It is believed that this method was applied in Japan for the first time around 1844 in the village of Sakurai in the Nagano Prefecture. Ac
cording to Kasamura (1950), in the year of 1945, 300 million fry carp were released into 60,000 hectares of paddy fields. The final crop amounted to 7,500 metric tons.
Since there are vast areas of paddy fields in Japan, China, and the Southeast Asian countries, it is extremely beneficial to utilize these waters for fish cultivation. It has been confirmed by tests carried out by agri
cultural experimental stations or fisheries experimental stations that the rearing of carp in the rice fields never results in a reduction of the rice crop.
There are two kinds of carp culture in rice fields: one is to rear the young carp, which have just been hatched, through the fall artificially to make them ready to be planted next spring as so-called larvae; the other method is to stock the fields with second-year carp, making them ready for eating by the fall.
The following precautions are observed: the fields should be so lo
cated as to allow the supplying of warm water; there should be no risk of any flooding; the ditch for the incoming water supply and the drainage from the field should be covered by a bamboo mesh so that carp cannot escape from the fields.
When the rearing is accomplished only by natural foods available in the irrigated fields, the number of carp released to the fields actually should be lower. If the size of the fry is about 3 cm., the most suitable number to release is approximately 300 to 1,000 to each 10 ares. In case of supplying feed regularly from the outside, 1,000 to 3,600 carp can be planted in each 10 ares. Thus, by the fall, carp will grow in size to 6-24 cm., the yield would be 11.2-26.2 kg., when no additional feed is given, and 26.2-75.0 kg. when supplemented.
Since Zooplankton and other natural foods that grow in irrigated fields are very good for the growth of carp, manuring to promote the increase of natural foods is also practiced. In Japan, around April in the spring, for each 10 ares of water fields, a total of 300 kan (each kan is
3.75 kg.) of compost and manure (produced in stable), and 108-180 liters of human manure are spread, or 75-112 kg. of lime is scattered for each 10 ares (of fields); afterward 75 kg. of "meal of pupa of silk worm,"
and about 37 kg. in all of fish meal, rice bran, and similar products, are given. It is known to be effective for the development of Zooplankton as well as for the growth of rice plants.
C . C U L T I V A T I O N I N W A T E R R E S E R V O I R S
Irrigation ponds and water reservoirs for livestock are found in many places of the world. In addition to these, many hydroelectric dams have been built in recent years. All these water resources can be utilized for carp cultivation.
An example of utilizing reservoirs in Japan is the Shimizu Reservoir, which is located in the town of Takahatake-cho, in the Yamagata Pre
fecture. This is 9.7 hectares in size and the depth of the water is 3 m.
It was stocked in November with 11,410 kg. of carp, each weighing 150- 170 g. They were fed with chrysalises and mud snails, starting in the fol
lowing spring. By the next fall, a total of 207,000 kg. of chrysalises and 16,900 kg. of dried mud snails, 3,680 kg. of raw mud snails, and 75,000 kg. of green vegetable leaves had been fed. The result was a crop of 82,000 kg. of carp. More than 9 0 % of the carp weighed 750 g. or more, and were sold as food (Akashi, 1936).
According to Nakamura (1954) and others, it is proved that when carp are reared in a reservoir, it is advantageous to mix them with crucian carp (Carassius auratus). They have different eating habits. It is also effective to increase the phyto-plankton content through fertilizing, thus providing the goldfish with increased food supplies.
D . C U L T U R E I N L A K E S
Utilizing lakes and rivers for carp culture is also practiced in many places. In this case, there are two procedures: one is to stock every year;
the other is to transplant carp into lakes where previously no carp were found, and recover them after a lapse of some years, in which period measures are taken to prohibit fishing, so the carp can grow naturally and propagate. Lake Biwa is stocked with young carp annually. Some of these results are listed in Table V.
Tauchi and Miyoshi (1936) carefully analyzed the statistics concern
ing the planting of carp in Lake Biwa, Lake Suwa, and Lake Kasumi- gaura. They pointed out that in the two lakes besides Lake Biwa the
effect of releasing carp is clearer. The annual production of carp in these selected lakes is shown in Table VI.
TABLE V
CARP YIELDS IN LAKE BIWA
Amount of carp produced per Amount of carp each 1 0 , 0 0 0 Number of carp produced released
released yearly annually carp
Year (average) (kg.) (kg.)
1 9 1 9 - 2 3 5 , 9 4 4 , 0 0 0 3 7 , 7 9 6 , 6 0 0 6 3 , 6 0 0 1 9 2 4 - 2 8 6 , 4 1 7 , 0 0 0 5 0 , 9 2 9 , 8 7 5 8 3 , 5 1 3 1 9 2 9 - 3 3 6 , 8 2 9 , 0 0 0 5 5 , 8 0 3 , 7 5 0 8 5 , 8 6 3
TABLE V I
AMOUNT OF CARP PRODUCED IN SELECTED JAPANESE LAKES
Area Yield in
Names of lakes (hectares) kg./hectare
Kasumigaura 1 9 , 0 4 7 1 5 . 6
Kitaura 4 , 0 1 8 3 . 2
Suwa 1 , 4 4 8 7 . 9 - 1 6 . 9
Biwa, 1 9 1 8 - 2 2 6 8 , 0 4 0 5 . 0
Biwa, 1 9 3 4 - 3 7 6 8 , 0 4 0 9 . 1 - 1 0 . 3
VII. Feeds
When they are in the fry stage, carp eat Zooplankton and thus depend on an animal diet. But as they grow, their eating habits change. They become polyphagous and are able to digest and absorb vegetable, as well as animal, matter. What may then be used as carp feed? That de
pends on each country's specific conditions and productive capabilities.
At any rate, certain general qualifications of a good carp feed are evident.
It should be such that ( 1 ) carp like to eat it, ( 2 ) it is inexpensive and plentiful, ( 3 ) it is easy to digest and provides a high yield in flesh weight, and ( 4 ) it has no detrimental or harmful effect on the carp. Besides this, it should be easy to keep and to transport.
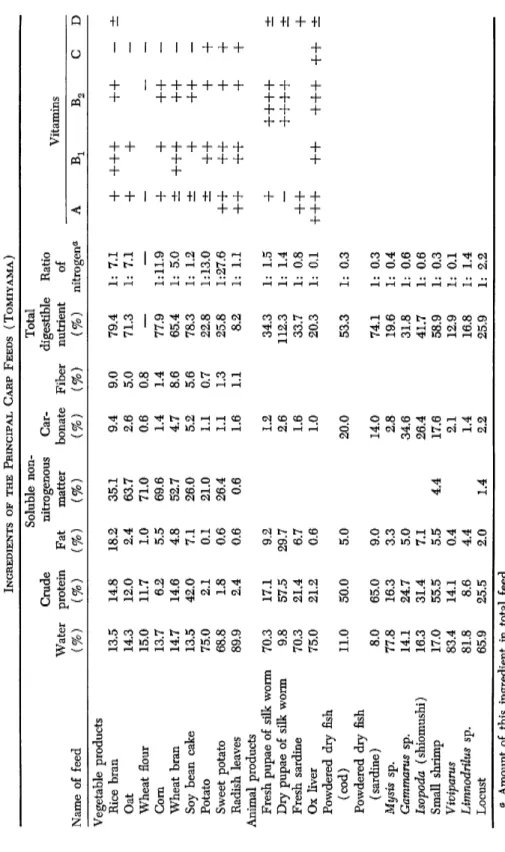
The principal types of feed used in Japan at the present time and their ingredients are indicated in Table VII.
Concerning artificial feeding, it is most important to pay great atten
tion to the effect on the growth and health of fish. At the same time, con
sideration should be given to costs, naturally favoring less expensive feeds. To raise carp on one single kind of feed often tends to create nu-
TABLE VII INGREDIENTS OF THE PRINCIPAL CARP FEEDS (TOMIYAMA) Soluble non-Total Crude nitrogenous Cardigestible Ratio Water protein Fat matter bonate Fiber nutrient of Vitamins Name of feed (%) (%) (%) (%) (%) (%) (%) nitrogena A Bi C Vegetable products Rice bran 13.5 14.8 18.2 35.1 9.4 9.0 79.4 1: 7.1
+ ++ + ++
— Oat 14.3 12.0 2.4 63.7 2.6 5.0 71.3 1: 7.1+ +
— Wheat flour 15.0 11.7 1.0 71.0 0.6 0.8— —
— — — Corn 13.7 6.2 5.5 69.6 1.4 1.4 77.9 1 :11.9+ + ++
— Wheat bran 14.7 14.6 4.8 52.7 4.7 8.6 65.4 1 : 5.0++ + ++
— Soy bean cake 13.5 42.0 7.1 26.0 5.2 5.6 78.3 1 : 1.2+ ++
— Potato 75.0 2.1 0.1 21.0 1.1 0.7 22.8 1 :13.0++ + +
Sweet potato 68.8 1.8 0.6 26.4 1.1 1.3 25.8 1 :27.6+ + ++ + +
Radish leaves 89.9 2.4 0.6 0.6 1.6 1.1 8.2 1 : 1.1++ + +
Animal products Fresh pupae of silk worm 70.3 17.1 9.2 1.2 34.3 1 : 1.5+ +++ +
Dry pupae of silk worm 9.8 57.5 29.7 2.6 112.3 1 • 1.4 —+++ +
Fresh sardine 70.3 21.4 6.7 1.6 33.7 1 0.8+ + +
Ox liver 75.0 21.2 0.6 1.0 20.3 1 0.1+ + + ++ ++ + ++
Powdered dry fish++
(cod) 11.0 50.0 5.0 20.0 53.3 1 0.3 Powdered dry fish (sardine) 8.0 65.0 9.0 14.0 74.1 1 0.3 Mysis sp. 77.8 16.3 3.3 2.8 19.6 1 0.4 Gammarus sp. 14.1 24.7 5.0 34.6 31.8 1 0.6 Isopoda (shiomushi) 16.3 31.4 7.1 26.4 41.7 1 0.6 Small shrimp 17.0 55.5 5.5 4.4 17.6 58.9 1 0.3 Viviparus 83.4 14.1 0.4 2.1 12.9 1 0.1 Limnodrilus sp. 81.8 8.6 4.4 1.4 16.8 1 1.4 Locust 65.9 25.5 2.0 1.4 2.2 25.9 1: 2.2 a Amount of this ingredient in total feed.tritional imbalance, so it is more common to have mixed feeds. Vitamins are essential nutritive elements for the growth of carp, but it is generally not necessary to supply them by adding to the feed, because most vita
mins are present in the plankton of the lake water as well as normally in several feeds. Since the "Η-factor" also is essential to fish, attention should be given to this nutrient.
When protein is short in the feed, carp will suffer, and, even when there is enough of other nutrients, will lose weight and finally succumb.
The body protein is provided by the feed, so a minimum amount must be included. The maintenance protein of carp, i.e., the minimum amount required for sustenance, is 30 mg. per 100 g. of carp for a day, when the temperature of the water is 22-24°C. (Migita et al, 1937). In other words, this indicates that approximately 1/1000 of the total protein content of a carp is sufficient for maintenance.
The ratio between the amount of digestible protein and that of other digestible nutrients in a feed is called caloric percentage, and is com
puted as follows:
digestible protein digestible and soluble non-
fat χ 2.44 + nitrogenous matter
Caloric percentage = . other digestible matter
Feed serves two functions after being taken up by the body: one part of it contributes "weight-maintaining feed" (subsistence) while the re
mainder is used to increase the weight. The former sustains life, while the latter is reserved for growth. How large a portion of the feed goes to each of these major functions depends on the sex and the age of fish. A feed high in caloric percentage, i.e., containing less protein, will primarily serve as maintenance feed. When carp are in their infancy and in rapid growth, they require feed with a low caloric percentage, i.e., high protein content (Knauthe, 1901). The higher the caloric percentage of the feed, the greater the protein reserve of the body (Knauthe and Zuntz, 1898;
see also Table V I I I ) .
TABLE V I I I
T H E RELATIONSHIP BETWEEN CARP AGES AND CALORIC PERCENTAGES
Age
Caloric percentages
Age Summer Fall
Fry 1:0.4-0.5 1:0.8
One-year fish 1:0.7-0.8 1:1.0-1.22
Two-year (or 2nd-year) fish 1:1.0-1.25 1:1.4-1.8 Adult fish (parents) 1:3.0-4.0 1:3.0-4.0
It is further essential to know the weight increase of carp as related to the amount of feed. This differs, depending on age, season, and feeding methods, previously mentioned; the balance between sustenance feed and feed for growth plays a role. Pisciculturists have several empirical data in this respect.
The amount of feed required to bring about a weight increase of one unit is called the growth coefficient of the feed:
. ^ quantity of feed Growth coefficient = -
weight increase (flesh)
The growth coefficients of some principal carp feeds are listed in Table I X .
TABLE I X
GROWTH COEFFICIENTS OF VARIOUS CARP FEEDS
Kind of feed Growth coefficients Established by Dry pupae of silkworms 1.3-2.1 Higurashi
Fresh pupae of silkworms 5.0 Tanaka
Dry Mysis 2.0 Higurashi and Kawamura
Isopoda (Shiomushi) 32.2 Matsui
Clam (Asari) 1.3 Higurashi
Meat powder 2.0 Susta
Dehydrated blood 1.5-1.7 Susta
Oats 2.6 Tokuhisa
Wheat flour 7.2 Matsui
Corn 4.7-5.0 Susta
Beer cake 26.3-26.6 Susta
Peas 2.7-2.8 Susta
Potato 33.9 Schröder
VIII. Fertilizing
In Germany, it was known more than fifty years ago that the propaga
tion of plankton as carp food was promoted by applying inorganic fer
tilizers to the culture ponds. Indirectly, this enhanced the production of carp (Neess, 1949). Six typical fish ponds in Missouri (U.S.A.), each of them less than 10 feet in depth and not exceeding 0.5 acre in size, were fertilized and studied as to production (Zeller, 1953). Phosphate turned out to be the principal factor limiting productivity. Most efficient was the application of fertilizer, 1,000 lb. of fertilizer per acre, in which the ratio between Ν, P, and Κ was 4:12:4. In the United States, a ratio of 8:8:4 between N, P205, and K20 is believed to be the most effective combination. It is, however, evident that such statements relate only to
the normal amount of mineral elements naturally present in the pond water.
Nakamura (1954) recognized the favorable effect of the use of fer
tilizers. In ponds for raising carp and crucian carp, 400-450 kg./hectare was obtained when fertilized, but only 186-375 kg./hectare when no fertilizers were used. Common practice is to apply, in all, 500-1,000 lb.
of inorganic artificial fertilizers annually, divided into several lots.
IX. Agricultural Chemicals and Fish Culture
In recent years, various sprays applied in agriculture and forestry are washed into ponds and irrigated fields used for the rearing of carp. Fre-
TABLE X
DEGREE OF DAMAGE THROUGH AGRICULTURAL SPRAYS ON CARP IN RICE FIELDS
Spray chemical
Fatal amount:
death within 24 hr. (p.p.m.)
Fatal amount (p.p.m.)
Total concen
tration in rice field (p.p.m.)
Causes dam
age in ponds or paddy
fields Endrin
emulsion 0.05-0.005 0.01-0.001 2-0.6
+
Dieldrin
emulsion 0.1-0.01 0.1-0.10 1.4-1
+
Parathion
emulsion 5-1 10-1 1.6-0.2
—
Parathion
powder 300-200 100-10 0.7
—
DDT emulsion 2.5-0.5 1-0.1 2.3-0.4 BHC emulsion 1-2 1.0-0.1 1.2-0.2 EPN emulsion 1 1.8-0.5 1.6-0.3
EPN powder 5 5-0.2 0.7
Diazinon
emulsion 3 3-0.4 2.4-0.2 Malathion
emulsion 25-1 100-10 1.4-0.7 Polidol
emulsion
—
20—
?2,4-D
— —
24Chlorothion
—
1.5 ?quently this results in damage. In testing various such chemicals, their detrimental effects on carp were established (see Table X ) (Kuroda et al, 1956).
X. Transportation
In order to transfer fry or for the purpose of transporting adult carp, different methods of transportation are employed. For great distances,
"live-fish cars" (train or truck) are used. For short-distance transporta
tion, pails or boxes are used. For moving carp fry to remote places, they are frequently carried by air in hermetically sealed transportation con
tainers charged with oxygen. For two or three days prior to transporting, no feeding should take place. This procedure is termed "ikejime" and is an age-old tradition among Japanese pisciculturists.
XI. Enemies, Parasites, and Diseases
As far as animals are concerned, weasels, rats, and others constitute natural enemies of carp. Such birds as snow herons, kingfishers, terns (Sterna hirundo), and Siberian black kites may all feed on carp, so the pond area must be freed of such birds. Aquatic insects and larvae are harmful, too. They eat carp fry. Among detrimental skin parasites, men
tion should be made of the "lice" Lernea and Caligus belonging to the crustacean group Copepoda. Spot disease, caused by the attack of certain Pseudomonas backis bacteria, has to be keenly observed and may cause great losses. These diseases, prevailing in Japan, are well covered by Schäperclaus (1954) in a book on carp diseases.
R E F E R E N C E S
Akashi, H. (1936). An example of carp culture in an irrigated pond in the Yamagata Prefecture. (In Japanese.) Suisan-Kenkushi 31, 447-451.
Brockway, D. R. (1950). Metabolic products (of fish) and their effects. Pro
gressive Fish Culturist 12(3), 127-129.
Davis, H. S. (1953). Culture and disease of game fish.
Fisheries Agency of the Japanese Government. (1952). "Inland Water Fisheries and Aquiculture in Japan," pp. 20-26.
Food and Agr. Organization (1957). U.N. Yearbook of Fishery Statist. 7, C1-C8.
Fujita, T. (1933). "Aquiculture." (In Japanese.), 259 pp.
Higurashi, T., and Nakai, N. (1924). Hatching experiments of carp eggs. (In Japanese.) Suisan Koshujo Hokoku 21(2), 35-41.
Iver, U. S. (1935). Die giftige Wirkung der Stoffwechsel produkte der Fische.
Z. Fischerei 32(4), 661-673.
Kasamura, K. (1950). Carp culture in paddy field. (In Japanese.) Suisankai No. 7.
Kawamoto, Ν. Υ. (1957). Production during intensive carp culture in Japan.
Progressive Fish Culturist 19(1), 26-31.
Knauthe, K. (1901). "Die Karpfenzucht," Neudamm.
Knauthe, K., and Zuntz, N. (1898). Über die Verdauung und den Stoffwechsel der Fische. Arch. Anat. Physiol. Lpz. 1, 149-154.
Kuroda, T., Yamada, T., and Masuda, T. (1956). The toxicity of agricultural chemicals upon the fishes. (In Japanese.) Suisan Shigen 2 ( 1 0 ) .
Menzel, Η. U., and Steffens, W. (1957). Über das Wachstums von Spiegel- und Nachtkarpfen. Ζ. Fischerei [N.F.] 6, 385-392.
Migita, Μ., Hanacka, Τ., and Tuzuki, Κ. (1937). A study of vegetable feedstuffs in fish culture. I. Nutritive value of some polysaccharides. (In Japanese with English summary.) Suisan Shikenjo Hokoku No. 8, 99-178.
Nakamura, C. (1947). The feeding habit of carp-fries. (In Japanese.) Bull.
Japan. Soc. Set. Fisheries 13, 111-112.
Nakamura, K. (1954). Fish production in seven farm ponds in the Shioda plain (Nagano prefecture) with reference to the natural limnological environment and artificial treatment. (In Japanese with English summary.) Tansuiku Suisan Kenkyusho Kenkyü Hökoku 3, 27-80.
Nakamura, Ν. (1955). Comparison of the carp and the crucian from the pisci- cultural point of view. (In Japanese with English summary.) Bull. Japan.
Soc. Set. Fisheries 2 1 ( 2 ) , 77-81.
Neess, J. C. (1949). Development and status of pond fertilization in Central Europe. Trans. Am. Fisheries Soc. 76, 335-358.
Runger, F. (1940). Schlesien als teichwirtschaftliches Erzeugungsgebiet im Jahre 1938. Fischereiztg. Neudamm 43(17).
Schäperclaus, W. (1933). "Lehrbuch der Teichwirtschaft." Parey, Berlin.
Schäperclaus, W. (1954). "Fischkrankheiten." Parey, Berlin.
Shimadate, M. (1957). Effect of fertilization and significance of artificial feeding to fish production in farm pond, Shioda plain (Nagoya prefecture). (In Japan
ese with English summary.) Tansuiku Suisan Kenkyusho Kenkyü Hokoku 7, 1-31.
Steffens, W. (1956). "Der Karpfen." Ziemsen Verlag, Wittenberg.
Tauchi, M., and Miyoshi, S. (1936). On the propagation of fish due to liberating fry in four lakes, Biwako, Kasumigaura, Kitaura, and Suwako. (In Japanese with English summary.) Bull. Japan. Soc. Sei. Fisheries 4, 331-334.
Wielenbach, Ε. P. (1938). Neue Wege der Karpfenzüchtung. Arb. Biol. Versuch.
München No. 150.
Yamada, S. (1938). Studies on the "tobi-goi" superior growing young carp group.
(In Japanese.) Yoshoku-kaishi 8 ( 1 ) .
Zeller, Η. D. (1953). Nitrogen and phosphorus concentrations in fertilized and unfertilized farm ponds in Central Missouri. Trans. Am. Fisheries Soc. 82, 281-288.