factors
Enik ˝o Bárdos
a, Gábor Kovács
a,b,c, Tamás Gyulavári
a, Krisztián Németh
a, Egon Kecsenovity
a, Péter Berki
a, Lucian Baia
b,c, Zsolt Pap
b,c,d, Klára Hernádi
a,∗aDepartmentofAppliedandEnvironmentalChemistryUniversityofSzeged,RerrichBélatér1,HU-6720,Szeged,Hungary
bFacultyofPhysics,Babes¸–BolyaiUniversity,M.Kog˘alniceanu1,RO-400084Cluj–Napoca,Romania
cInstituteforInterdisciplinaryResearchonBio-Nano-Sciences,Babes¸–BolyaiUniversity,TreboniuLaurian42,RO-400271Cluj-Napoca,Romania
dInstituteofEnvironmentalScienceandTechnology,UniversityofSzeged,TiszaLajoskrt.103,HU-6720Szeged,Hungary
a r t i c l e i n f o
Articlehistory:
Received30September2016
Receivedinrevisedform25February2017 Accepted15March2017
Availableonline22March2017
Keywords:
WO3nanocrystallites WO3-TiO2nanocomposites Photocatalyticactivity Carbonnanotubes Photocatalysis
a b s t r a c t
The “build-up” methodology, the importance of the order of the semiconductor layers in WO3- TiO2/MWCNT composite materials was studied in terms of the applied synthesis pathway, morpho-structuralparameters(meancrystallitesize,crystalphasecomposition,morphology)andpho- tocatalyticefficiency(usingoxalicacidasmodelpollutant).Theappearance ofTiWOxphaseinthe compositescontributedtotheenhancementofthephotocatalyticefficiencies,asdifferentsynthesis approachesledtodifferentcrystalphasecompositions.Although,itwasproventhatabeneficialphase’s presencecanbehinderedifanexcessofMWCNTorWO3wasapplied.Astheratioofthementioned materialswasreduced,activecompositeswereobtained,butthepreviouslynoticedTiWOxdisappeared.
Therefore,itwasproven,thatinthecaseofWO3-TiO2/MWCNTnanocompositesystemseveralphoto- catalyticactivityenhancementfactorscanbeintroduced,butnotsimultaneously(thedisappearanceof TiWOxatlowMWCNTandWO3contentsandtheappearanceofhighlycrystallineanatase).
©2017ElsevierB.V.Allrightsreserved.
1. Introduction
Catalysts are of great importance in the synthesis of petro- chemicals, plastics, medical agents or fuelcell materials. Their applicability extends also for environmental applications, for examplethereduction ofemissionsand degradationof various organicpollutants[1].Thecatalyticactivityandselectivitydepend stronglyonseveralparameters, suchascrystal phase and stoi- chiometriccomposition,particlesizeandmorphology,crystallinity gradeofthecatalystortheshapeofthecatalystnanocrystals[2,3].
Amongthecatalyticprocesses,photocatalysisisaveryimportant alternativeinthedegradationoforganicpollutants,anditisbased ontheirradiationofasemiconductorwithphotonsofgreateror equalenergythanitsbandgap.Titaniumdioxide(TiO2)isasemi- conductormetaloxide,whichhasmanyadvantageousproperties thatmakesitalmostidealforphotocatalyticapplications[4,5],itis photostable,biocompatible,non-toxic,relativelycheapandavail- ableinlargequantity[6].UnderUVirradiation,itisanexcellent
∗Correspondingauthor.
E-mailaddress:hernadi@chem.u-szeged.hu(K.Hernádi).
materialforthedegradationofvariousinorganicandorganiccom- pounds[7].However,becauseofitslargebandgapenergy,TiO2 absorbslightonlyintheUV-A,nearvisibleregion.Thisisthemain drawbackofitswiderapplicationsinceonly4–5%ofthesolaremis- sionspectrumfallsinthisrange[8,9].Toovercomethisproblem there aremany differentapproaches inthe scientificliterature.
Themostimportantmethodstoincreasethephotocatalyticactivity containcatalystmodificationbydoping,metaldecoration,surface sensitization,orbydesigningmoreefficientcomposites(e.g.mixed semiconductoroxides)[10],thelatterpossibilityseemstoberather promising.Efficientchargeseparationcanbeobtainedbythecom- binationoftwosemiconductorparticlesthathavedifferentenergy levels[11]. Byproperly tailoringtheproperties ofthe compos- itecomponents,metaloxideheterojunctionscancertainlybean interestingapproachtomodifythephotocatalyticperformancesof individualoxides[12].Everyoxidecomponentcandisplaydifferent chemicalinteractionwiththecontaminanttodecompose.Surpris- ingly,inmanycasesoxidesystemsusedforcatalysisshowmany similaritieswiththeonesinvestigatedforsensing[13].
In the last few years, tungsten trioxide − titanium dioxide (WO3/TiO2) composite photocatalysts have drawn researchers’
attention since they possess interesting and unique photocat- http://dx.doi.org/10.1016/j.cattod.2017.03.019
0920-5861/©2017ElsevierB.V.Allrightsreserved.
alyticfeatures[14–16].WO3showsamoderatelysmallbandgap energy,strongabsorptionwithinthevisiblespectrum,long-term performanceand highchemical stabilityin a widepHrange of aqueoussolutionsunderoxidativecircumstances[17,18].Itiswell- knownin theliteraturethat theconnectionbetweenWO3 and TiO2enablesimprovedchargeseparationandvisiblelightresponse [14–16,18–22]. Furthermore, WO3 based composites possess a strongabsorptioninvisiblelight,buttheirphotocatalyticactivities areverylow.
Comparingbandgapsofthetwocomponents(whilerelatively small−2.4–2.8eV−forWO3andhigh−3.0–3.2eV−forTiO2)itis obviousthatthetransferofelectronsfrombulkTiO2toWO3might furtherminimizetherecombinationlosses.
Besides theabove-mentioned composite formation of semi- conductoroxides,theadditionofcarbonnanotubes(CNT)canbe alsoa feasible methodto elongate thelifetime of photogener- atede−/h+ pairs and preventtheirrecombination [23].Because oftheiruniquechemical,physicalandelectronicproperties,CNTs arewidelyinvestigated[24,25]inordertopromoteapplications inthefieldofcompositematerialsandphotocatalysts[23].Since CNTs canact as anelectron reservoir tostore temporarily and transferthephotogeneratedelectronsfromTiO2[25],withadding CNTstotheoxide,thephotocatalyticactivityofTiO2canbefur- therimproved[26–28]and possiblyconfervisible lightactivity [29].The combinationof thebeneficialproperties ofWO3 with theadvantagesofmultiwalledcarbonnanotubes(MWCNTs)can also result in a synergic effect tocreate materialswith differ- entproperties for targetedapplications.Thus, researchers have studiedWO3/MWCNTcompositesinpreviousworksinorderto investigatetheirgassensing [30,31]andelectrochromic perfor- mances[32].Differentimpregnationmethodswerealsopresented toprepareWO3/MWCNTcomposites:eitherasimpleone-stepfab- ricationmethodorcombinedwithprecipitationbasedsynthesis techniques[33]. Accordingtoourknowledge, just a few publi- cationscanbefoundregarding thephotocatalytic propertiesof ternarycompositescontainingtitaniumandtungstenoxideswith MWCNT. Literaturedata are rather inconsistentconcerningthe build-upmethodology(synthesisapproachesandoxidelayercom- position)oftheabove-discussednanocomposites.
Moresynthesis approaches werealready developedin order toinvestigatetheavailablepossibilitiestoobtaindifferentcon- tactorderofthecompositecomponents.Twopotentialmethods wereidentified:modified/slowhydrolysis(MSH)byRétietal.[26], andimpregnation(I)methodwhichwasinvestigatedbyVassetal.
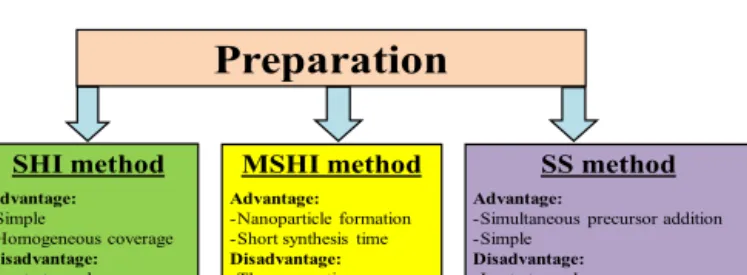
[33].Thesetwoapproaches(combinedaMSHI)providedreliable resultsconcerningtheuniformcoverageofMWCNTswithdifferent semiconductoroxides(In2O3,SnO2,Al2O3,ZnO,SnO2-In2O3).The advantagesanddrawbacksofthesesynthesismethodsaresumma- rizedinFig.1.
Inthispaper,ourinterestistoclarifytheeffectoftheheattreat- mentonthecrystallinityandmorphologyofcompositematerials thatwere obtainedby themethodslistedabove, whilevarying
Fig.1.Thedifferentpreparation methodadvantagesand disadvantages(slow hydrolysisandimpregnation(SHI),amodifiedslowhydrolysisandimpregnation, (MSHI)andasimultaneoussynthesis(SS)).
theorderofthedifferentsemiconductorlayersandattemptingto depositsimultaneouslythetwooxides(simultaneousslowhydrol- ysisandimpregnation−denotedasSSmethod).
2. Materialsandmethods 2.1. Chemicals
Allchemicalsusedwereofanalyticalgrade.TheMWCNTsfrom Nanothinx(NTX1type)titaniumisopropoxideTi(iPrO)4andtung- sten(VI)hexachloride(WCl6)fromSigma-Aldrich,P25fromEvonik Aeroxide(Germany)wereusedwithoutfurtherpurificationforthe preparationofthecompositematerials(secondarycomposites,for detailspleaseconsultSections2.2and2.3)
2.2. Characterizationmethods
FormeasuringtheDRS(DiffuseReflectanceSpectroscopy)spec- tra of the samples, a JASCO-V650 spectrophotometer with an integrationsphere(ILV-724)wasused(=250–800nm).
Theoxidelayerthicknessandcoverageratiooftheoxidesonthe MWCNTwereverifiedwithFEITechnaiG2X-TWINTEM(200kV).
Thesampleswerepreparedasfollows:asmallamountoftheexam- inedcompositewassonicatedin1.25cm3ofethanol.Afewdrops fromthissuspensionweredepositedanddriedontothesurfaceof thegrid(CF200CuTEMgrid).
TheSEMmicrographswererecordedonaHitachiS-4700Type IIFE-SEMscanningelectronmicroscopeequippedwithacoldfield emissionsourceoperatingintherangeof5–15kV.Thesamples weremountedonaconductivecarbontape.
PowderXRDanalysiswascarriedoutonaRigakuMini-flex- IIDiffractometer(angle-range:2=20–80◦,=0,15418nm)using characteristicX-ray(CuK␣)radiation.
TheRamanspectroscopymeasurementswerecarried outon aThermoScientificDXRRamanmicroscopewitha532nmlaser (5mW).
A photoreactor system with 2 LighTech 40W fluorescent lamps (max≈365nm, irradiation distance=5cm, irradiation time=2h) was used to measure the photocatalytic activities.
ThenanocompositesweregrindedtogetherwithP25(secondary composites)in ordertodifferentiatetheirimpactonthephoto- catalyticactivity.Thephotocatalystsuspensioncontainingoxalic- acid (initial concentration of oxalic-acid c0,oxalic-acid=0.5mM;
csuspension=20mg/20cm3=1.0gL−1;total volumeofthesuspen- sion Vsuspension=20cm3) was continuously stirred during the experiments.Theconcentrationdecrease oftheoxalicacidwas followed using a Merck-Hitachi L-4250 system with Merck- Hitachi L-7100 pump. The eluent wasH2SO4 (concentration of H2SO4=19.3mM),theappliedflowratewas0.8cm3min−1andthe detectionwavelengthwas205nm.
2.3. Preparationofnanocomposites
The TiO2-WO3/MWCNT composites were prepared by slow hydrolysisandimpregnation(SHI),amodifiedslowhydrolysisand impregnation,(MSHI)andasimultaneoussynthesis(SS)method.
Thenomenclature ofthecomposites containstheused method abbreviation (listedabove)− firstoxide−calcinationtempera- ture− second oxide− calcination temperatureweight ratioof thecomponents(justforthosesamplesinwhichtheratioofthe componentswaschanged).Ifnoheattreatmentwasappliedfor thespecific oxide,thecalcination temperatureis denoted with 0. Examples:MSHI-TiO2-400-WO3-450, MSHI-TiO2-0-WO3-450.
In thecase ofSSseriesthejointheattreatmenttemperatureis includedinthenomenclature−e.g.SS-TiO2-WO3-700.
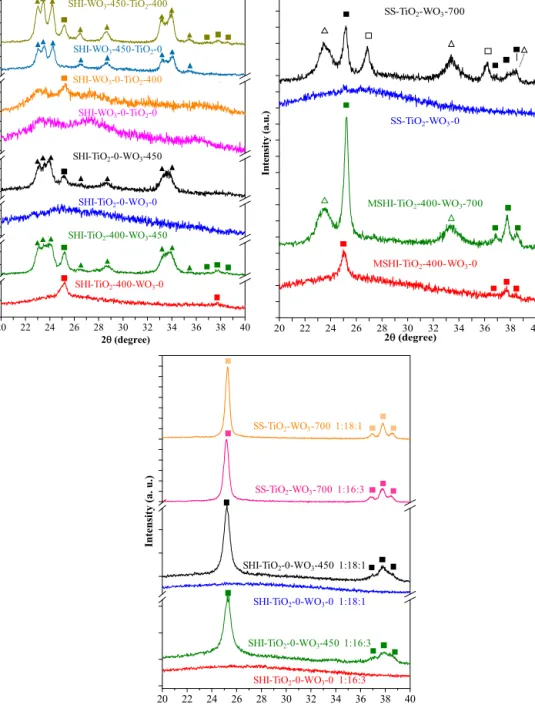
Fig.2.TheXRDpatternsoftheobtainedcompositematerials(−TiWOx,-monoclinicWO3,䊏−AnataseTiO2,䊐−RutileTiO2).
Theadditionofcarbonnanotubeswasdecidedasinthepho- tocatalytic degradation process is presumably responsible for enhancedchargeseparation,ascarbonnanotubesareefficientelec- tronconductingmaterials,whichcanseparatetheelectronsfrom thesemiconductor,minimizingthechanceofrecombination.The compositesmadebySHIwerepreparedusinganalkoxideprecur- sor,titaniumisopropoxideTi(iPrO)4 andethanol assolvent.The weightratioof thereactantswas1:10:15(MWCNT/TiO2/WO3).
The dispersion of 100mg MWCNTs and 150cm3 ethanol was stirredvigorouslyunderinertatmosphere(Ar)whiletheprecur- sor(3.71cm3)wasaddedfollowedbyahomogenizationperiodof 1h.Thesampleswerekeptatroomtemperatureforaweekuntil thesolventevaporatedcompletely.Thesampleswereseparated intotwoparts:thefirstpartwasannealedat400◦Cfor3hinair andgrindedintofinepowderinanagatemortar.Thesecondpart wasnotheattreated.Fortheimpregnationmethod,TiO2/MWCNT wasappliedastherawmaterial(bothinannealedandnotannealed forms)andWCl6astheprecursor.Initially,160mgTiO2/MWCNT
wasaddedin200cm3acetoneandasuspensionwaspreparedvia sonicationfor45min.Duringthistime,thecalculatedamountof precursor(257mg)wasdissolvedinanotherportionofacetone (20cm3).Finally,thesolutionoftheprecursor wasaddeddrop- wiseintotheTiO2/MWCNT-suspensionandthenthemixturewas heatedto55–60◦Ctoevaporatethesolventonaheatedmagnetic stirrer.Whenthesolventwascompletelyremoved,ablackpowder wasobtained.Thispowderwasdriedfurtherat90◦Cfor24h.The sampleswereannealedat450◦Cfor3hinair.Compositeswerealso preparedwhenthedepositionorderoftheoxideswasreversed.
The other TiO2-WO3/MWCNTcomposites were prepared by MSHImethod.Theweightratioforthepreparationwas1:10:15.
2.65cm3Ti(EtO)4 wasaddedinto14.74cm3ethanolandhomog- enized using ultrasonication for 15min to form the precursor solution.ThedispersionofMWCNTswasstirredvigorouslywhile theprecursorsolutionwasaddeddropwise.Themolarratioswere n(TiO2):n(H2O):n(ethanol)=1:3:30.Aftertheadditionofthepre-
cursorsolution,thebeakerwascoveredwithplasticparaffinfilm andwasstirredforanadditional1h. Afterstirring,thesamples werekeptat80◦Cuntilallthesolventevaporated.Sampleswere annealedat400◦Cfor4hinairandgrindedintofinepowderinan agatemortar.Theimpregnationprocesswasthesamelikeinthe caseofSHImethod,butthesampleswereannealedat700◦Cfor 3hininertatmosphere(Ar).
ThecompositeswerepreparedalsobytheSSmethod:100mg MWCNTwasaddedinto150cm3acetonehomogenizedbyultra- sonication for 45min. The dispersion of MWCNTs was stirred vigorouslyunderinertatmosphere(Ar)whiletheTi(iPrO)4precur- sor(3.71cm3)wasadded.WCl6precursor(257mg)wasdissolved inanotherportionofacetone(20cm3)andwasaddeddropwiseto theMWCNTdispersion.Then,thesuspensionwaslefttobestirred for1h.Afterstirring,thesampleswerekeptat23◦Cforaweek untilallthesolventevaporated.Sampleswereannealedat700◦C for3hininertatmosphere.
For the photodegradation of oxalic acid, the previously described MWCNT-based composites and Evonik Aeroxide P25 wereusedforthepreparationofthephotocatalyst(secondarycom- posites).Ineachcase,aspecificratiowasestablishedbetweenthe composite components,30%MWCNT-based composite and 70%
TiO2 (EvonikAeroxideP25).Thenanocompositeswereprepared viamechanicalmixinginanagatemortarfor3×5min.
3. Resultsanddiscussion
3.1. Crystallinestructureofnanocomposites
TheXRDanalysisoftheobtainedcompositematerialsstarted withthesamplesSHI-TiO2-400-WO3-0and SHI-TiO2-400-WO3- 450. Both of them were obtained starting from heat treated TiO2/MWCNT,whichcanbeobservedbythepresenceofthemain anatase(JCPDS21-1272)peakat25(2◦).InthecaseofSHI-TiO2- 400-WO3-0,besidestheanatasepeak,onlyabaselinedistorting wasobserved,behaviorthatindicatesthatWO3isclearlypresent asamorphousphase.Whenasupplementaryheattreatmentwas appliedat450◦CthesignalsofmonoclinicWO3emerged(JCPDS 43-1035,themeancrystallitesizewasof20.2nm).Byapplyingthis supplementaryheattreatment,aslightincreaseofthemeancrys- tallitessizeofanataseTiO2,from25nmto27nm,wasobserved (Fig.2).
Theabove-mentionedobservationschangeddrastically,when theobtained TiO2/MWCNTwas not calcinated. Therefore, after impregnationwithWO3precursornosignofanycrystallinemate- rialwasdetected(thecaseofsampleSHI-TiO2-0-WO3-0).However, ifapostcalcinationwasapplied,bothsignaturesofanataseTiO2and monoclinicWO3appearedintheXRDpattern(SHI-TiO2-0-WO3- 450),althoughasmalleramountofamorphousmaterialremained asthepatternshowsaslightincreaseinthemainbaseline.The meancrystallitesizeofTiO2 andWO3 was29.9nmand74.0nm, respectively.
Reversingthedepositionorder,anotherinterestingaspectof thesematerialsisrevealed.IftheWO3/MWCNTinitialcomposite wasnotcalcined(compositeSHI-WO3-0-TiO2-0)andtheprecur- sorofTiO2wasdeposited,acompletelyamorphousmaterialwas obtained (although there are signs of really small amounts of crystallinematerial).Whenapostheattreatment(compositeSHI- WO3-0-TiO2-400)wasapplied,asmallanatasesignalappeared, whiletherestofthematerialremainedamorphous.Nocrystallite sizecalculationwaspossibleinthecaseofthesetwosamples.
ToinvestigatethebehaviorofTiO2,whichwassubsequently deposited,apriorheat-treatmenttotheWO3/MWCNTcomposite wasapplied.After theimpregnation process,the material con- tainedonlycrystallinemonoclinicWO3,whilenosignofanyTiO2
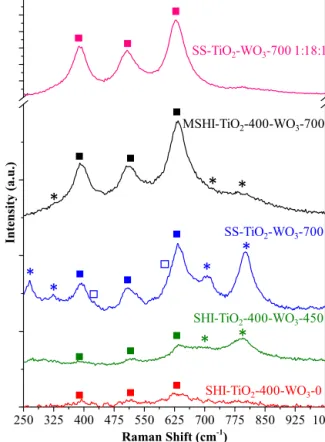
Fig.3.TheRamanspectraofthecompositematerials(*−WO3relatedbands,䊏− AnataseTiO2,䊐−RutileTiO2).
diffractionpeakswasdetected(sampleSHI-WO3-450-TiO2-0).The mean crystal size of WO3 was28.4nm. Whenthe supplemen- taryheattreatmentwasapplied(sampleSHI-WO3-450-TiO2-400), theanatasepeakemerged,andthemeancrystallitesizeofWO3 increasedslightlyto33.4nmandthenewlyformedanatasecrys- tals’sizewas34.6nm.
WhenTiO2wasthefirstoxidedepositedonthesurfaceofMWC- NTs,crystallinematerialswereobtainedinthreecases.WhenWO3 wasthefirstone,thefinalmaterialwascrystallineonlyintwocases.
Itseemslikethepre-depositionofWO3inhibitsthecrystallization ofthesecondoxide.Thisphenomenonwaspreviouslyobservedin literature[34,35].Hence,thefirststrategywasfurtherdeveloped.
Asitwasalreadydetailedinthesectionfocusingonthesynthe- sisofthesematerials,themodifiedsol-gelandthesimultaneous precursorhydrolysisstrategieswereapplied.
ThecompositedenotedasMSHI-TiO2-400-WO3-0possessthe samecrystallineproperties asthesampleSHI-TiO2-400-WO3-0, namely theycontain anatasephase withthe same mean crys- tallite size. However, if a supplementary heat treatment was applied(thistimeat700◦C,inordertofurtherincreasethecrys- tallinityofthesample),newdiffractionpeaksappearedat23.4and 33.1(2◦).Thesepeaksreappearedinthecaseofthecomposite (SS-TiO2-WO3-700,whileSS-TiO2-WO3-0wascompletelyamor- phous),togetherwiththealreadymentionedanatasereflections andthenewonesattributedtorutilephase(themaindiffraction peakat27(2◦),JCPDS21-1276).NeitherTiO2 norWO3 crystal phaseswereidentifiablebythepeaksmentionedearlier.Apossible explanationcouldbefoundintheworkofQureshietal.[36],who shownthepossibleformationofTiWOx,arathersurprisingcom- poundobservedrarelyinthelastdecade.TiWOxappearedonlyin the1:10:15weightratiosamples.Thismeansthatarelativelyhigh amountofWO3andMWCNTisneededtofavortheformationof TiWOx,Wheretheamountofthementionedtwocomponentswas loweredTiWOxwasnotpresentatall.Itshouldbealsonotedthat
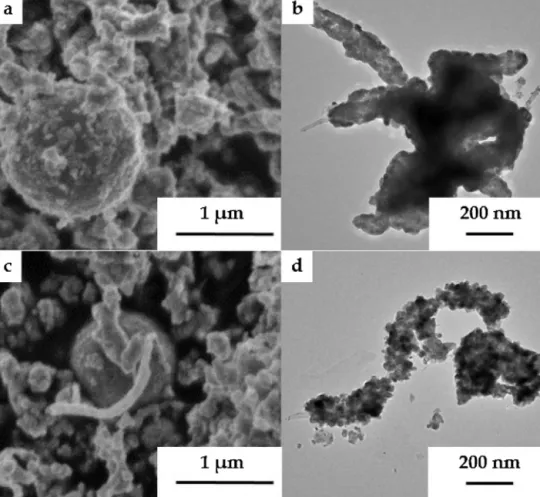
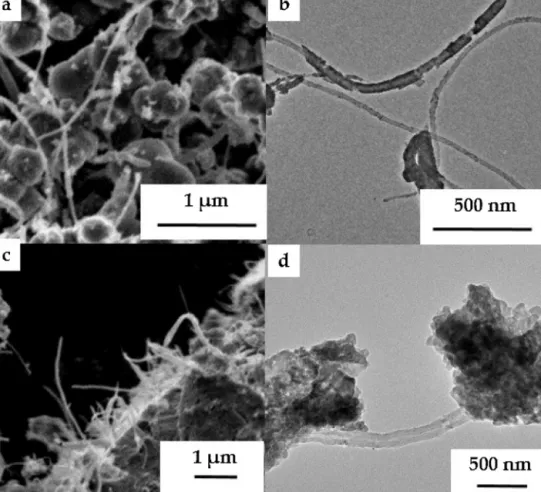
Fig.4. Scanning(a,c)andtransmission(b,d)EMsofTiO2/MWCNT-basedcomposites(SHI-TiO2-0-WO3-450)andSHI-TiO2-400-WO3-450(c,d)).
Fig.5. Scanning(a,c)andtransmission(b,d)EMsofWO3/MWCNT-basedcompositesSHI-WO3-0-TiO2-400(a,b)andSHI-WO3-450-TiO2-400(c,d)).
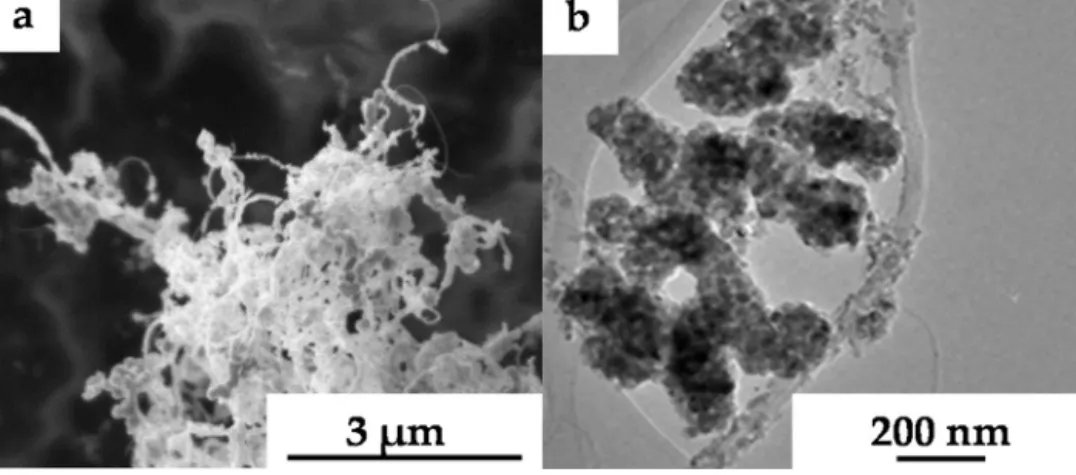
Fig.6.Scanning(a,c)andtransmission(b,d)EMsofcompositesMSHI-TiO2-400-WO3-700(a,b)andSS-TiO2-WO3-700(c,d).
theheattreatmentwasalsocrucialtoobtaintheabovementioned compoundasitwasobtainedonlywhenacalcinationwasapplied at700◦C.
Thephotocatalytic testsdidnotshow anypromising results asitwillbedetailedinSection2.4,althoughtwomethodswere involved(SSandSHImethods),whichafterfurtheroptimization couldleadtophotoactivecomposites.Thestrategyadoptedwas basedonthefact thattheCNTand theWO3 ‘s relativecontent wasratherhighcomparedtoTiO2.Thus,theWO3 andCNTcon- tentswerelowered(newcomponentweightratios1:18:1,1:16:3).
WhentheXRDpatternswereanalyzedforthesecompositesitwas foundthatjustanatasecrystallizedfromthetwooxides,whilethe non-heattreatedsampleswerecompletelyamorphous.
Bycomparingthemeancrystallitesizeofthesecompositestwo importantaspectscanbepointedout.Thus,whentheratioofWO3 decreased(relativetoTiO2)itwasfoundthatthecrystallitesizeof theformedanataseslightlyincreased(from16.3to17.4nm−sam- plesSHI-TiO2-0-WO3-4501:16:3vs.SHI-TiO2-0-WO3-4501:18:1), inthecaseofsampleseriesSHIandmoreintensively(from22.4nm to47.5nm−samplesSS-TiO2-WO3-7001:16:3vs.SS-TiO2-WO3- 7001:18:1) whentheSSmethodwasapplied.Thisobservation emphasizesagainthatthepresenceofWO3inhibitsthecrystalliza- tionofTiO2.Furthermore,atthisratiovalues,WO3doesnotappear atallintheXRDpatternsindicatingitspotentialdopantrole.
ItshouldbementionedthatinallthecaseswhereWO3wasnot observableintheXRD,amorphousW(OH)x ismostprobablythe primaryhydrolysisproduct,thereforenoXRDpeakswereexpected andsubsequentlyobtained.
TheFT-Ramanspectrarevealthepresenceofbandscharacter- isticforTiO2 anataselocatedaround144, 197(these twowere notincludedintheFigure),394,512,638andrutilephaseat445, 610cm−1(bothofthemoverlappedwithanatase),respectively.The
DandGbandsoftheMWCNTdidnotsufferanymodification,and thereforetheywerenotincludedinFig.3.
TheWO3crystallinestructures(includingtungstates)giverise tocharacteristicRamanbands around710and 800cm−1,while O W O bending modes are located at≈260cm−1 and W O stretchingmodesat950–960cm−1 [34,35].Mostoftheidentifi- cationsmadeabovecoincidewiththefindingsdeterminedfrom the XRD results, therefore the observations made for samples SHI-TiO2-400-WO3-0,SHI-TiO2-400-WO3-450andthosewithsim- ilarstructurescanbeconsideredvalid.However,itisinteresting that thebandassociatedwiththepresence ofW O unitsdoes not appear in thespectraof theinvestigated samplesmeaning thatcompletehydration(duringhydrolysis)ornon-stoichiometric specieswereformed.Thebandat330cm−1,whichcorrespondsto W-OH2 stretchingvibrations,suggestedthepreviouslyenounced possibility,asthesespeciesappearwhendefectiveWOxspeciesare present[34,35].ThiswasalsoconfirmedbytheformationofTiWOx
identifiedbyXRD.
Furthermore,iftheratioofthecompositecomponentsisshifted in the favor of titania (while lowering the MWCNT and WO3 content−sampleSS-TiO2-WO3-7001:18:1)thepreviouslymen- tionedRamanbands,specifictoWO3,aremissing,whiletheones attributedtothepresenceofanataseTiO2intensifiedsignificantly.
Thisinformationpointsoutincreasedcrystallinity,highermean crystallitesizeandpossibledopingofanatase/amorphousWO3.
3.2. Morphologicalinvestigationsusingscanningand transmissionelectronmicroscopy
BothSEMandTEMinvestigationsrevealedthatcarbonnano- tubeswerehomogeneouslycoveredbythebinaryoxideandthe thicknessofthelayerwasapproximately20–40nm(Fig.4a,b−
Fig.7. Scanning(a)andtransmission(b)EMsofchangedweightratiocompositesSS-TiO2-WO3-7001:18:1.
sampleSHI-TiO2-0-WO3-450).WhenTiO2washeattreatedprior WO3deposition(Fig.4c,d),theSEMandTEMmicrographsshowed thattitaniumdioxideandtungstenoxideevenlycoveredthesur- faceof MWCNT,however,in manyregions both oxidesformed separateaggregatesaswell.Fromthemicrographsitcanbeseen thatthethicknessofthelayerwasabout20–40nmagainandthe sizeofdetachedoxideparticleswereintherangeof50–200nm.
ChangingthedepositionorderoftheoxidesontheMWCNT, itcanbeclearlyseenfromSEMandTEMmicrographsthatcarbon nanotubeswerenotcoveredhomogeneouslywithtitaniumdioxide andtungstenoxide(Fig.5a,b),asmentionedpreviously,butlarger aggregatesof100–500nmsizewereformedandattachedtothe surfaceofMWCNT.However,whenWO3receivedaheattreatment at450◦CbothSEMandTEMmicrographsconfirmedthatcarbon nanotubeswerehomogeneouslyandthicklycoveredbythebinary oxidelayerofapproximately100nm(Fig.5c,d).
Inthe case ofthe compositessynthesized bymodified slow hydrolysisandimpregnation(MSHI),SEMandTEMmeasurements provedthecoverageonthesurfaceofcarbonnanotubes(Fig.6a,b) Whilethehomogeneousregionofthelayerwascomposedoftung- stenoxide,thetitaniumdioxideformscrystallinenanoparticles insidethecoverage.Theaveragethicknessofthelayerwasofabout 50nm.
Whenthesampleswerepreparedwithsimultaneoussynthesis (SS)methodFig.6(c,d)itcannotbeseenhomogeneouslayeronthe surfaceofMWCNT.SEMandTEMimagesshowlargeroxideparti- clesofapproximately100nm,randomlyattachedontothecarbon nanotubes.Whentheweightratiowaschanged,thesurfaceofcar- bonnanotubewascoveredhomogeneouslywithtitaniumdioxide andtungstentrioxide.Theaveragethicknessofthelayerwasof 50–100nm(Fig.7a,b).
3.3. OpticalpropertiesoftheindividualWO3andcomposites
Inordertoevaluatetheopticalpropertiesofthenanomaterials, diffusereflectancemeasurementswereperformedonthecompos- iteswiththebestphotocatalyticefficiencies.ItcanbeseeninFig.8, thatthematerialsabsorbvisiblelightinrelativelyhighratio(more than80%).ThisbehaviorcanbeattributedtothehigherMWCNT contentinthecomposites(5%).
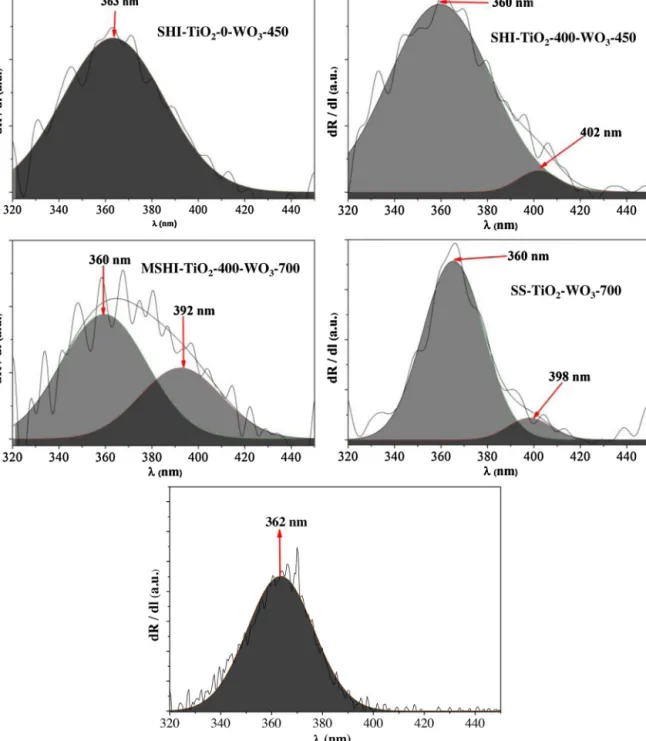
Duetothesmalllight absorptionedge,theband-gapvalues cannotbeevaluatedinthetypicalway,namelybytheKubelka- Munkequation,becausethevalues obtaineddo not reflect the reality(values4.0–5.0eV).Therefore,thefirstorderderivativeof theDRSspectra(Fig.9)wascalculatedinordertogaininformation abouttheelectrontransitionsoccurringduringtheirradiationof thenanomaterials,resultingrealisticband-gapvalues.
Fig.8.ThereflectancespectraofselectedWO3-TiO2/MWCNTcomposites.
Analyzing the first order derivative of the reflectance data, mainlythreeelectrontransitionbandscanbeseen,bothrespon- siblefor theUV light absorptionof thecatalysts, at≈362, 392 and≈400nm,respectively.Thesepeaks,intermsofenergy,arecor- respondingtotheband-gapvaluesofanatase,inthefirstcase,to TiWOxat392nm,andtomonoclinicWO3for400nm.Inadditionto thequalitativepresenceofthesetransitionsitisclearlyvisiblethat thedifferentlysynthetizedcompositeshaveachangeintheratioof thesepeaks.Anotheraspect,whichcanbeobservedwhenWO3is depositedonthesurfaceofthenon-calcinatedTiO2/MWCNTsam- ples,consistsinthatthepeakofWO3cannotbeobservedonthe firstorderderivativeoftheDRSspectra.Thisabsenceofthesignal ismostprobablyduetothepresenceofnon-crystalline/amorphous WO3,which“delays”thecrystallizationprocessofbothsemicon- ductorsinthenanocompositesandmaysuggestapossibledoping.
WhenWO3wasdepositedonthethermallythreatedTiO2-based material,thecharacteristicderivativepeakofWO3appearsaround 400nm,whichmeansthatthecrystallizationprocessissuccess- fulinthiscase,thesemiconductor-oxideis“transformed”intoits monoclinicform(resultswereprovenalsobyXRDandRamanmea- surements,asdiscussedinSection3.1).
Astheratioofthecomponentswaschangedto1:18:1(sam- pleSS-TiO2-WO3-7001:18:1) anatasecrystallized(a clearlight absorptionedgewasvisibleintheDRSspectrum−Fig.8),while nosignsof WO3 wasdetectedintheXRD patternsand Raman spectra.AsitcanbeseeninFig.9thederivativespectrumdoes notshowanyadditionalbands,exceptthecharacteristicbandof
Fig.9.FirstorderderivativespectraoftheWO3-TiO2/MWCNTcomposites.
anataseat365nm.ThisexplicitlypointsoutthattheWO3present inthatsamplemustbeamorphous[35].
3.4. PhotocatalyticactivityofWO3-TiO2/MWCNT-based nanocomposites
Theinitialcompositeswere notactiveunder UVirradiation.
ThisresultcanbeattributedtotherelativelyhighamountofWO3 presentinthecomposite,and,asitisknownfromtheliterature, WO3haslowphotocatalyticactivityunderUVirradiation[37].In ordertogainfurtherinformationaboutthephotocatalyticpotential ofthecompositesawell-knowncommercialphotocatalyst(P25)in aratioof70%P25−30%compositewasprepared.
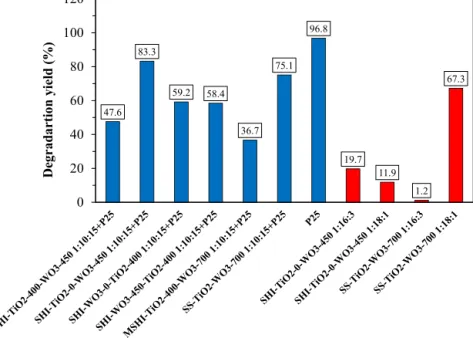
Aftertwohoursof photodegradation(Fig.10)of oxalicacid, itcanbeconcludedthatthebestperformingcompositewasthe
SHI-TiO2-0-WO3–450+P25,withadegradationyieldof83.3%and the composite made bysimultaneous synthesis (+P25) (75.1%).
TheSHI-WO3-450-TiO2-400andSHI-WO3-0-TiO2-400composites had a similarphotocatalytic efficiency toward oxalicacid, both degradinglessthan60%ofthemodelcontaminant(58.4and59.2%, respectively).Thelowestefficiencywasshownbythecomposite madebymodifiedsol-gelsynthesis,havingayieldlessthan40%.
Thecommercialcatalyst(P25)hadthehighestefficiency,decom- posing96.8%oftheoxalicacid.Thisexperimentpointedoutclearly whichoftheinvolvedsynthesismethodscouldleadtothecom- positematerialsthatmaypossessphotocatalyticactivity.Although theobtainedcompositesdeterioratedtheactivityofP25,theper- formedresearchpointedoutwhichofthemethodshaspotentialto yieldphotoactivenanocomposites.Thetwomostpromisingmeth- odswerechosen,SHI-TiO2-0-WO3-450andSS−TiO2−WO3-700
Fig.10.PhotocatalyticactivitiesofvariousTiO2-WO3/MWCNTcomposites(compositesobtainedbythefirstsynthesisapproachandmixedwithP25−bluecolumns;
compositesobtainedinthesecondoptimizationstagewithoutP25–redcolumns).(Forinterpretationofthereferencestocolourinthisfigurelegend,thereaderisreferred tothewebversionofthisarticle.)
andtheircomponent contents furtheroptimized:from1:10:15 (MWCNT:TiO2:WO3)to1:16:3an1:18:1.
ThemostefficientsamplewastheSS-TiO2-WO3–700◦C1:18:1 weight ratio with a degradation yield of 67.3%. The other composites showed low photocatalytic performances, the SHI- TiO2-0-WO3–450◦C 1:16:3 degraded 19.7%, while the sample SHI-TiO2-0-WO3–450◦C1:18:1removedonly11.9%,andfinallythe sampleTiO2-WO3–700◦C1:16:3wasnearlyinefficient(Fig.10).
3.4.1. Searchingfortheoriginofthephotocatalyticactivityof WO3-TiO2/MWCNT-basednanocomposites
Mostofthesamplesshowedverylowphotocatalyticactivity, inwhichacrucialrolewasassignedtothecompositecomponents ratio.Oneofthechosenweightratiosofthecompositecomponents was1:10:15(MWCNT:TiO2:WO3),whichshowedarelativelyhigh amountofWO3andMWCNT.AsWO3hasverylowindividualpho- tocatalyticactivityunderUVirradiation[37],therefore,theactivity deteriorationcouldbeascribedwiththehighestpossibilitytothe relativelyhighcarboncontent.
In order toenlighten the possible reasons for theobtained photocatalyticactivitytrenditwasnecessarytocarryoutaddi- tional investigations. One of the key structural features which might beresponsible for theactivity is thesize of thespecific surfacearea.We expectedthatahighvalueofthespecificsur- face area resultshigh photocatalytic activity, but theobtained resultsdidnotsupportthisstatement.TheSHI-TiO2-0-WO3-450 (1:16:3)samplehadarelativelyhighsurfacearea(112.0m2g−1) however,thementionedsampledegradedonly19.7%oftheoxalic acidcomparedtosampleSS-TiO2-WO3-700(1:18:1)whichpos- sessed88.5m2g−1surfaceareaandshowedasignificantlyhigher degradationefficiencyof67.3%.AlsosampleSHI-WO3-0-TiO2-400 (1:10:15)shouldbepointed out,asit wasthesample withthe highestspecificsurfacearea,butnophotocatalyticactivityatall.
Therefore,weconcludedthat,thephotocatalyticactivitywasinde- pendentfrom thespecific surfaceareainourcase. Thespecific surfaceareavaluesofallsamplescanbefoundinthesupporting information(notallthevalueswereintroducedanddiscussedin detailasmostofthemshowedlowphotocatalyticactivity).
ThefourkeysampleswereexaminedwithXPSinordertoinves- tigatethesourceofthephotocatalyticactivity,asitwasevidentthat
thesurfacequalitywasthekeyfeatureinourcase.TheXPSspectra ofthesamplesrevealedthesamespeciesineachofthecomposites.
Themaindifferencewasintheirconcentration.
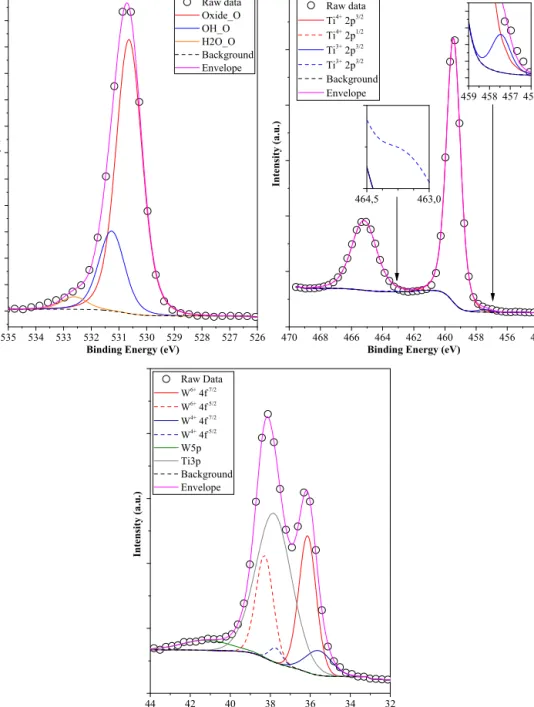
TheX-rayphotoelectronspectra(Ti2p,O1s,W4f)ofsampleSS- TiO2-WO3-7001:18:1arepresented inFig.11.In theTi2pXPS spectrumTi4+andaverysmallamountofTi3+(0.96at.%−peaks at457.3eVand463.4eV)alongwithTi4+(90.04at.%− peaksat 459.1eVand464.8eV)[38]wasdetected,whileintheO1sspec- trum,theusualcomponentsinusualratiowereidentified:oxide oxygenfromTiO2(530.3eV−74.82at.%),surfaceOHgroupoxy- gen(531.3eV− 21.78at.%), and oxygenfromH2O(532.8eV − 3.40at.%).Furthermore,intheO1sspectrumofthematerialsno low binding energy oxygenspecies (528.8eV) appeared. Based onourrecent publications [38,39] this interesting oxygentype couldbeattributedtothepresenceofoxygendefects,ortooxy- genatomswhichareneighbouringtoreducedTisites(i.e.Ti3+).
InthesamplesSHI-TiO2-0-WO3-4501:16:3,SHI-TiO2-0-WO3-450 1:18:1, SS-TiO2-WO3-700 1:16:3 the amount of Ti3+ increased graduallyfrom0.96at.%(SS-TiO2-WO3-7001:18:1)to3.21at.%(SS- TiO2-WO3-7001:16:3),whiletheoxygenformsshowedthesame ratio.ThismeansthattheTi3+actedasrecombinationcenters,con- sumingmostprobablytheavailableholes,inhibitingtheoxalicacid degradationbydirectholeoxidation.
The W4f XPS spectrum of sample SS-TiO2-WO3-700 1:18:1 revealedtwosignificantWspecies(W6+andW4+−36.0/38.2eV and 35.8/37.8eV). W4+ presenceconferred ourmaterialsa pale bluishcolor,asitwaspresentin21.2at.%fromthetotalamountof W.AsWO3wasnotpresentintheXRDpatternsofthesesamples, themostpossibleformofthisoxideisamorphouswithdangling, reducedW4+sites,similarwiththestructurereportedbyAkurati etal.[40].Astheratioofthesespecieswereunchangedintheother samples,emphasizingthatinthecaseofthesecompositesTi3+plays theactivitydefiningrole.
4. Conclusions
Itiswellknownthatduetoitscomplexityphotocatalysiscanbe influencedbymanydifferentparameters.Ourinvestigationwith aternarycompositesystem(WO3-TiO2/MWCNT)describedhere demonstratedthisstatementexemplarily.Itwasprovedthatnot
Fig.11.XPSspectra(Ti2p,O1sandW4f)ofsampleSS-TiO2-WO3-7001:18:1.
onlythesynthesismethodbutalsothesequenceofdepositingthe components(TiO2followedbyWO3),affectsignificantlythefinal propertiesofthenanocomposites.Wehavelearntthattheproper- tiesoftheoxidedepositedinthefirstinstancehasastronginfluence onthecrystallinityofthesecondphase (e.g.differentcrystallite sizeoftheindividualoxideparticles).Atthesametime,thehigher degreeofcrystallinitydoesnotnecessarilycoincideswiththehigh photocatalyticactivity(theexampleofSHI-TiO2-0-WO3-450).The appearanceofTiWOxcrystalphaseathighertemperatureisalso aninterestingandsomewhatunusualphenomenonandmightbe worthconsidering,sincesample SS-TiO2-WO3-700alsoshowed promisingphotocatalyticactivity.However,itcannotbestatedthat thisnewphaseisexclusivelyresponsiblefortheobservedphoto- catalyticperformances(seeMSHIsamples).Thiswasproven,when theratioofthecompositecomponentswaschanged,becausein thosesamplesTiWOxdisappearedandanatasecrystallizedwitha highcrystallitesize,makingitthemaindeterminingfactorforthe
photoactivity.Also,itisimportantthatatlowWO3 andMWCNT contentWO3doesnotappearatallsuggestingapossiblepresence onlyasamorphousWO3.Concerningthesequenceofdepositing theoxidesitcanbeconcludedthattheexistenceofMWCNT-TiO2- WO3 junctionismoreadvantageous.Thephotocatalystshowing thehighestactivityshowedthelowestamountofTi3+,emphasizing theroleofrecombinationcenterofthisspeciesinthesecompos- ites.Basedonliteraturedata[26]itcannotbeexcludedthatthe morphologyofthecompositecomponentsisalsoimportant.
Acknowledgments
ThisworkwassupportedbyagrantfromSwitzerlandthrough Swiss Contribution(SH/7/2/20). G.K.wantsto acknowledgethe financialsupportofHungarianAcademyofSciences,whileZs.P acknowledgesthePremiumPost-Docprojectprovidedalsobythe HungarianAcademyofSciences.Theauthorsarealsoverygrate-
[6]A.Mills,S.J.LeHunte,Photochem.Photobiol.A108(1997)1–35.
[7]M.A.Fox,M.T.Dulay,Chem.Rev.93(1993)341–357.
[8]S.Banerjee,S.K.Mohapatra,P.P.Das,M.Misra,Chem.Mater.20(2008) 6784–6791.
[9]Q.Li,L.Chen,G.J.Lu,Phys.Chem.C111(2007)11494–11499.
[10]N.A.Ramos-Delgado,M.A.Gracia-Pinilla,L.Maya-Trevi ˜no,L.Hinojosa-Reyes, J.L.Guzman-Mar,A.J.Hernández-Ramírez,Hazard.Mater.263(Pt.1)(2013) 36–44.
[11]S.Malato,P.Fernández-Ibá ˜nez,M.I.Maldonado,J.Blanco,W.Gernjak,Catal.
Today147(2009)1–59.
[12]K.Zakrzewska,ThinSolidFilms391(2001)229–238.
[13]M.Epifani,E.Comini,R.Díaz,T.Andreu,A.Genc¸,J.Arbiol,P.Siciliano,G.
Faglia,J.R.Morante,ProcediaEng.87(2014)803–806.
[14]S.Higashimoto,Y.Ushiroda,M.Azuma,Top.Catal.47(2008)148–154.
[15]C.W.Lai,S.Sreekantan,Int.J.HydrogenEnergy38(2013)2156–2166.
[16]A.K.L.Sajjad,S.Shamaila,B.Tian,F.Chen,J.Zhang,J.Hazard.Mater.177 (2010)781–791.
[17]É.Karácsonyi,L.Baia,A.Dombi,V.Danciu,K.Mogyorósi,L.C.Pop,G.Kovács,V.
Cos¸oveanu,A.Vulpoi,S.Simon,ZsPap,Catal.Today208(2013)19–27.
[18]Y.-C.Nah,A.Ghicov,D.Kim,S.Berger,P.Schmuki,J.Am.Chem.Soc.130 (2008)16154–16155.
[29]L.Tian,L.Ye,K.Deng,L.J.Zan,SolidStateChem.184(2011)1465–1471.
[30]C.Balázsi,K.Sedlácková,E.Llobet,R.Ionescu,Sens.ActuatorsB133(2008) 151–155.
[31]C.Bittencourt,A.Felten,E.H.Espinosa,R.Ionescu,E.Llobet,X.Correig,J.J.
Pireaux,Sens.ActuatorsB115(2006)33–41.
[32]P.M.Kadam,N.L.Tarwal,S.S.Mali,H.P.Deshmukh,P.S.Patil,Electrochim.Acta 58(2011)556–561.
[33]A.Vass,P.Berki,Z.Nemeth,B.Reti,K.Hernadi,Phys.StatusSolidiB250 (2013)2554–2558.
[34]G.Kovács,ZsPap,C.Cotet,V.Cos¸oveanu,L.Baia,V.Danciu,Materials8(2015) 1059–1073.
[35]G.Kovács,L.Baia,A.Vulpoi,T.Radu,É.Karácsonyi,A.Dombi,K.Hernádi,V.
Danciu,S.Simon,ZsPap,Appl.Catal.B147(2014)508–517.
[36]M.Qureshi,PrakashGupta,J.Chromatogr.A62(1971)439–448.
[37]I.Székely,G.Kovács,L.Baia,V.Danciu,ZsPap,Materials9(2016)258.
[38]Zs.Pap,É.Karácsonyi,Zs.Cegléd,A.Dombi,V.Danciu,I.C.Popescu,L.Baia,A.
Oszkó,K.Mogyorósi,Appl.Catal.B111(2012)595–604.
[39]Zs.Pap,V.Danciu,Zs.Cegléd,Á.Kukovecz,A.Oszkó,A.Dombi,K.Mogyorósi, Appl.Catal.B101(2011)461–470.
[40]K.K.Akurati,A.Vital,J.-P.Dellemann,K.Michalow,T.Graule,D.Ferri,A.
Baiker,Appl.Catal.B79(2008)53–62.