Genetic Recombination in Streptomyces
G . SERMONTI AND D . A . HOPWOOD
I. I n t r o d u c t i o n 223 I I . G e n e R e c o m b i n a t i o n in Streptomyces coelicolor A 3 ( 2 ) 224
A . T h e O r g a n i s m 224 B . F o r m a l G e n e t i c s 226 C. T h e G e n e t i c S y s t e m 237 I I I . G e n e t i c P h e n o m e n a in O t h e r S t r e p t o m y c e t e s 242
A . B a l a n c e d H e t e r o k a r y o s i s 242 B . G e n e R e c o m b i n a t i o n 243 C. C y t o p l a s m i c I n h e r i t a n c e — E p i s o m i c E l e m e n t s 246
I V . Streptomyces G e n e t i c s a n d A n t i b i o t i c P r o d u c t i o n 246 V. G e n e t i c S y s t e m s of S t r e p t o m y c e t e s a n d E u b a c t e r i a 248
R e f e r e n c e s 250 I. Introduction
T h e streptomycetes have the most complex colonial organization to be found among the bacteria. Their colonies have a superficial resemblance to mold colonies, but are on a much smaller scale. T h e y have even been considered to be intermediate between bacteria and fungi,1 but their cellu
lar dimensions, their cytology, and, as we shall see, their genetics, place them without doubt among the bacteria.
The streptomycetes m a y be regarded as one of the most advanced groups of bacteria, and for this reason m a n y people were stimulated to investigate their genetic system during the time when the process of conjugation in Escherichia coli was beginning to reveal some of its peculiar features (see Chapter 1). At the same time, the discovery of the parasexual cycle in imperfect fungi2 had made accessible to genetic analysis and breeding a large group of industrial microorganisms. T h e streptomycetes, which pro
duce all widespread antibiotics except the penicillins, appeared to be the next group to which to extend genetic investigations.
A search for gene recombination in the streptomycetes was begun in m a n y laboratories in about 1954, and the following year the first successful result was published by Sermonti and Spada-Sermonti.3 This report was soon followed by o t h e r s ,4 - 1 0 and genetic recombination was obtained in m a n y species of the genus Streptomyces; there were very few negative reports.
Attempts to obtain practical results in the industrial field are still in progress, and most of them are unpublished, while investigation of the
2 2 3
2 2 4 G. SERMONTI AND D. A. HOPWOOD
genetic system of Streptomyces has been virtually confined to the strain A 3 ( 2 ) of Streptomyces coelicolor, studies on other strains having been
abandoned or limited to particular problems. I n this chapter we shall de
scribe the present state of knowledge of the genetic system of the strepto
mycetes, with particular reference to S. coelicolor A 3 ( 2 ) . This topic has been the subject of a recent m o n o g r a p h ,1 1 and the reader is referred to it for more detailed information. T h e genetics of this strain must serve as a model for the genus, and the studies with other streptomycetes, which are reviewed in a later section of the chapter, m a y be compared with it. A few preliminary observations on the industrial applications of streptomyces genetics are described near the end of t h e chapter.
II. G e n e R e c o m b i n a t i o n in Streptomyces coe//co/or A 3 ( 2 )
A . T H E ORGANISM 1. CULTURAL CHARACTERISTICS
Streptomyces coelicolor (S. violaceoruber, according to Kutzner and W a k s m a n1 2) grows vigorously on a variety of agar media, both complex and synthetic. T h e minimal growth requirements are satisfied by glucose, nitrate, or other simple source of nitrogen, and small quantities of inor
ganic ions. This makes t h e isolation and characterization of biochemical mutants a simple operation. This species is distinguished by producing a pigment which is blue and diffusible a t alkaline p H , and red and bound to the hyphae a t acid p H .
2 . MORPHOLOGY
T h e characteristic feature of t h e streptomycetes, which distinguishes them from t h e other actinomycetes, is the production of regular chains of aerial spores. Colonies t h a t arise on agar media from an inoculum of iso
lated spores reach a diameter of 2 - 3 mm. after about 4 days incubation a t 2 8 - 3 0 ° C . Several hundred well-isolated colonies m a y be grown on a Petri dish. T h e young colonies are slightly shiny, and very coherent since they consist of an interconnected and intertwined system of hyphae. When they are 3 - 4 days old, the colonies assume a dry powdery appearance because of the production of aerial hyphae, followed by sporulation. T h e colonies then consist of a lower layer of mycelium and an upper layer of spores and sporulating hyphae.
T h e spores of Streptomyces coelicolor are roughly spherical, about 1 - 1 . 5 μ in diameter, and are surrounded by a wall about 3 0 m/* thick; this wall is frequently overlain by remnants of the wall of the p a r e n t hypha in which t h e spores are produced in chains.1 3 Each spore usually contains a
single chromatinic b o d y ,1 4 and a single corresponding nuclear region is visi
ble in electron micrographs of thin sections of spores.1 5 N o visible mem
brane separates the nuclear region from the cytoplasm. Studies of genetic segregation, which are described below, indicate t h a t each spore usually contains a single haploid genome, and the results of mutational and radio- kinetic studies are in agreement with this.
T h e spores germinate by producing one or more fine germ tubes whose walls are continuous with t h a t of the parent spore. The germ tubes elongate and branch repeatedly to produce a system of interconnected hyphae, t h e substrate or vegetative mycelium. T h e colony increases in size b y t h e radial growth of the hyphae a t the margin of the colony, and other hyphae penetrate into t h e agar. The diameter of the hyphae of the substrate m y celium varies from about 0.3 t o 1.0 μ. Cytological evidence of hyphal anas
tomosis in t h e substrate mycelium of t h e streptomycetes is inconclusive owing to t h e small size of the hyphae. There have been several reports of hyphal fusion; probably the most convincing pictures are those of Greg
o r y ,1 0 who studied Streptomyces scabies. T h e cytoplasm of t h e hyphae is divided into compartments b y septa; each compartment contains several nuclear regions, which vary from small spherical structures to complex lobed bodies.
Certain branches of the substrate hyphae grow upward and give rise to a system of hyphae of rather larger diameter.1 7 The hyphae of this aerial mycelium are eventually transformed into chains of spores b y septation of the h y p h a e .1 3 T h e process of spore formation is preceded b y a series of characteristic changes in t h e configuration of t h e nuclear material in t h e aerial h y p h a e .1 4 I n the young hyphae, the nuclear material is in the form of long rods which fill a large p a r t of t h e space between adjacent septa.
These nuclear rods subdivide, in a manner whose details are still unclear, to produce progressively shorter rods and eventually spherical chroma
tinic bodies, one of which is included in each spore.
M a n y features of t h e cytology of Streptomyces coelicolor are identical with those of typical eubacteria. The nuclear bodies stain with basic dyes in the same way as those of eubacteria and like them remain stainable a t all stages of their division, and divide without the formation of recognizable spindles. Electron micrographs of thin sections of S. coelicolor are strik
ingly similar to those of most eubacteria; the nuclear bodies are recogni
zable as regions of lower average density t h a n t h e cytoplasm, containing fine fibrils,15 and there is no nuclear membrane. The cytoplasm is extremely electron dense owing to its high content of ribonucleoprotein particles and it differs from t h a t of eubacteria only in containing a larger membranous component.1 8 , 1 9 The walls of the hyphae resemble those of Gram-positive eubacteria in their thickness and appearance in thin sections,1 3 and also
226 G. SERMONTI AND D. A. HOPWOOD
in their chemical composition,2 0 being composed of amino sugars and a small number of characteristic amino acids, including diaminopimelic acid.
B. FORMAL GENETICS 1. TECHNIQUES
T h e techniques for the harvesting and plating of spores, for the isolation of biochemical and resistance m u t a n t s for use as genetic markers, and for the characterization of segregants are essentially those used in fungal genetics.2 Special methods for making crosses, and for the detection and analysis of heterogenotes will be mentioned briefly in the appropriate sections of this chapter, while full details will be found in the monograph by Hopwood and Sermonti.1 1
2. SELECTIVE ANALYSIS OF M I X E D CULTURES
The first attempt to analyze the genetic system of Streptomyces coeli
color A3 (2) was made by H o p w o o d2 1 using a selective analysis of the recombinants obtained from mixed cultures of genetically marked strains.
When two strains differing by a number of nutritional and resistance m a r k ers are inoculated together on a slope of complete medium, they grow to give a mixed culture, which becomes covered with spores after 3 to 4 days.
If these spores are collected in water and sown on an appropriate selective medium which does not allow the growth of the parental strains, a small proportion of them (about 1 0- 3 to 1 0 ~5) give rise to colonies; these turn out to be new, stable strains carrying the parental markers in new combi
nations.3 , 4' 2 1 '2 2
I n a four-point cross, it is possible to select nine different genotypes of re
combinants, the other seven genotypes, including the two parental ones, not being recoverable on a medium on which neither parent can grow. The various recombinant genotypes appear in repeated crosses with reproduc
ible relative frequencies ; certain pairs of markers show a regular tendency to segregate in the parental combinations, while others tend to segregate independently (Table I ) . B y studying crosses in which the same markers were in different coupling arrangements, it was possible to establish link
age between certain loci and two linkage groups were identified.2 1 The distances between the loci in the same linkage group showed reasonable additivity. Thus a preliminary linkage m a p was constructed which allowed the subsequent investigation of the genetic system of S. coelicolor.
With the discovery of heterogenotes (see next section), a more reliable method for the location of new markers in S. coelicolor became available which does not require the selection of rare recombinants within a huge
T A B L E I
SELECTIVE ANALYSIS OF A CROSS IN 8. coelicolor A3(2)*«f Cross{ :
Regions:
met-2 + + his-1
1
str-1 phe-1
~ + + ~ 2
Selective media (minimal medium + )
— Average num- Crossover Selectable phenyl- Methio- ber of recom- in regions recombinant M e t h i o_ alanine, ph μ nine, binants for
6 g e n o t y p e s ; ni ne histidine, a]anine histidine, each crossover strepto- strepto- pattern
mycin mycin
— ftw + + +
1+
h s ρ64
42
53
1
(+ + s ρ
1+ + + +
15 7
4
4
2
ira + s +
1+
h s +26
12
27 13
20
1,2
( + + s + + + + P [h m s +
1 0 1
0
1 1
1
T o t a l r e c o m b i n a n t s per m e d i u m
92 59 12 42
R e c o m b i n a t i o n R e c o m b i n a t i o n
in r e g i o n 1 i n r e g i o n 2
= 4 + 1/53 + 4 + 20 + 1 =
= 20 + 1/53 + 4 + 20 + 1
= 7%
= 2 8 %
* U n p u b l i s h e d d a t a of H o p w o o d .
t R e c o m b i n a n t s r e c o v e r e d on four s e l e c t i v e m e d i a .
t met-2 (ra), his-1 (h), phe-1 (p) = r e q u i r e m e n t for m e t h i o n i n e , h i s t i d i n e , or p h e n y l a l a n i n e , r e s p e c t i v e l y ; str-1 (s) = r e s i s t a n c e t o s t r e p t o m y c i n . E q u a l v o l u m e s of spore s u s p e n s i o n f r o m t h e m i x e d c u l t u r e w e r e p l a t e d o n t h e four m e d i a , a n d all r e c o m b i n a n t s w e r e classified for t h e u n s e l e c t e d m a r k e r s . T h e f r e q u e n c i e s of g e n o t y p e s p r o d u c e d b y t h e s a m e c r o s s o v e r p a t t e r n are a s s u m e d t o b e e q u a l , a n d t h e y h a v e b e e n a v e r a g e d .
population of parental spores. However, the analysis of recombinants ob
tained directly from mixed cultures of marked strains is still an efficient method for detecting very close linkages, like those between mutations of identical phenotype. Strains and media for such an analysis are chosen in such a way t h a t on one medium recombinants between two unlinked or
228 G. S E R M O N T I AND D. A. HOPWOOD
loosely linked loci m a y be selected, and on another recombinants between the two markers being studied.2 1 If, for the same number of spores plated, the colony counts on the second medium are much lower t h a n on the first, a close linkage is indicated. Selective analysis is also useful for estab
lishing the order of two closely linked mutational sites with respect to outside markers (Table I I ) .
3. ANALYSIS OF HETEROZYGOUS CLONES (HETEROCLONES)
a. Detection of Heteroclones. Among the colonies t h a t grow on a selective medium when spores from a mixed culture are sown on it, there are a few
T A B L E I I
DETERMINATION OF THE ORDER OF T W O CLOSELY L I N K E D LOCI IN LINKAGE GROUP I OF S. coelicolor A 3 (2) WITH RESPECT TO OUTSIDE M A R K E R S *
Arrangement of markers f:
Regions:
Cross met-2 his-1
a
+ +
Cross b
met-2 + his-9 +
Arrangement of markers f:
Regions: + +
1 2
his-9 arg-1 3
+ his-1
1 2 + arg-1
3 Crossover in
regions
Genotypes of recombinants:):
Observed numbers
Genotypes of
recombinants J Observed numbers
2 + + + + 29 m -\—(- a 26
1,2 m + + + 8 + + + a 10
2,3 + + + a 7 m + + + 10
1,2,3 m -\—h a 0 + + + + 4
* D a t a f r o m H o p w o o d a n d S e r m o n t i .1 1
f met-2 (m) = r e q u i r e m e n t for m e t h i o n i n e ; his-1, his-9 = r e q u i r e m e n t for h i s t i - dine; arg-1 (a) = r e q u i r e m e n t for a r g i n i n e .
Î R e c o m b i n a n t s w e r e s e l e c t e d o n m i n i m a l m e d i u m p l u s a r g i n i n e a n d m e t h i o n i n e ( s e l e c t i n g for c r o s s i n g o v e r in r e g i o n 2, w h i c h is l e s s t h a n 1 u n i t l o n g ) .
t h a t turn out to contain a mixture of different genotypes. These mixed col
onies are particularly common when selection is for two closely linked nu
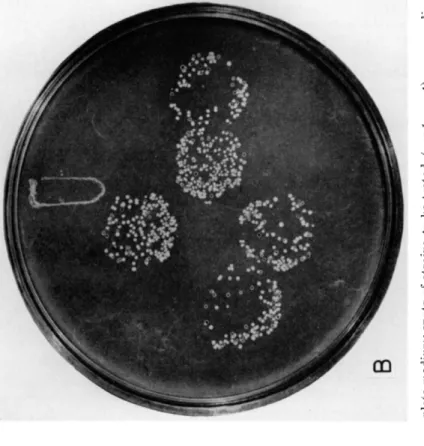
tritional markers. They are usually smaller t h a n the true recombinants, and their characteristic property is t h a t they are not transferred by replica plating on to a medium of the same composition as t h a t on which they were first selected2 3 (Fig. 1). The great majority of their spores have one or the other of the two nutritional requirements against which selection was made, indicating t h a t the colonies must have been able to grow on the original selective medium by virtue of being heterozygous for the two loci in ques
tion.
The nature of the mixed colonies becomes clear when we consider the genotypes of their spores with respect to the whole set of markers employed
FIG. 1. D e t e c t i o n of heteroclones. A . Colonies arising on a m e d i u m selective for closely linked markers in repulsion. B . A replica plate o n t h e same m e d i u m . N o t e that t h e large colonies (haploid recombinants) give rise t o growth o n t h e replica plate, while t h e small colonies (heteroclones) d o n o t .
in the cross. These genotypes are determined by harvesting spores from a mixed colony in a drop of water and sowing them on complete medium ; a sample of the resulting colonies is then classified for t h e markers intro
duced into t h e cross. T h e markers appear in all possible combinations,
230 G. SERMONTI AND D. A. HOPWOOD
with frequencies which are explicable in terms of the linkage relations of the loci (Table I I I ) . There is no excess of parental genotypes, so t h a t the two parental genomes must be associated in a state formally equivalent to diploidy. The nucleus at the origin of such colonies will be referred to as a heterogenote,2 4 and the colonies as heteroclones.2 3
T h e number of different genotypes of segregants recoverable from a
T A B L E I I I
SEGREGANTS RECOVERED FROM A HETEROCLONE OF S. coelicolor A3(2)*-f Linkage groups:
Arrangement of markers! :
Regions :
I his-1
+
II gua-1 +
+
met-2 -\- arg-1 1 2
+ str-1 p, 3 4
Combinations of markers of group IÎ Cross
over in regions + h + m + a m + + + h a mh + + + a + + + m h a Total
Cross
over in regions
+ s V 64 18 10 4 1 1 1 0 99
g + + 19 9 3 1 2 0 0 0 34
+ s + 21 5 1 5 0 0 0 0 32 4
g + V 3 3 1 1 0 0 0 0 8
Combinations
of markers of + + + 15 2 1 0 0 0 0 0 18 3
group II Î g s V 0 0 1 0 0 0 0 0 1
+ + V 0 0 0 0 0 0 0 0 0 3 4
g s + 0 0 0 0 0 0 0 0 0
Total 122 37 17 11 3 1 1 0 192
Crossover in regions 2 1 1 2
* Unpublished data of Hopwood.
f Each figure is the frequency of a segregant genotype: markers of group I are given at the head of the column, and those of group II at the left of the row. The table shows the independent segregation of the markers in the two linkage groups: χ2 of independence (calculated from figures in the first 4 columns and the first 5 rows: 12 d.f.) = 14.64; Ρ = 0.27.
X met-2 (m), his-1 (Λ), arg-1 (a), gua-1 (g), phe-1 (p) = requirement for methionine, histidine, arginine guanine, or phenylalanine, respectively; str-1 (s) = resistance to streptomycin.
heteroclone appears to be limited only by the size of the sample of segre
gants examined. We must therefore postulate a large number of segrega- tional events (méioses) occurring within the colony; this means t h a t the original heterogenote must have undergone m a n y equational divisions
(mitoses) before segregating. Segregation is virtually complete by the time the spores are formed. Thus, in a genetic analysis we can assume t h a t all the colonies obtained by sowing spores from a heteroclone on to complete medium are the products of segregation, and the main disadvantage of a selective analysis is overcome.
Various irregularities are found in t h e pattern of segregation of every heteroclone, usually showing themselves as the absence or deficiency of one or more alleles. These disturbances reduce the amount of information avail
able for genetic analysis but, on the other hand, they throw some light on the nature of t h e heterogenotes themselves. Since t h e irregularities vary from colony to colony, each heteroclone h a s t o be considered separately.
I n the selection of the heteroclones, we have to choose between two a l ternatives. W e can select under conditions which necessitate t h e presence of the maximum number of markers, and also hinder as far as possible t h e formation of sectors capable of growth on t h e selective medium; these conditions occur on minimal medium with few or no growth factor supple
ments, and are those required for formal genetic analysis and for the study of smaller disturbances in t h e segregations. Alternatively, if we wish to study t h e range of possible types of heteroclone, we need to select for a pair of closely linked nutritional markers in repulsion, on a medium supple
mented with all the other growth requirements of the parents. Under these conditions, few loci usually show segregation, the test of independence be
tween linkage groups (see later) can rarely be applied, and the reliability of the segregation data is difficult to verify; furthermore, the formation of sectors cannot be completely prevented. However, a larger number a n d a much greater variety of heteroclones are recoverable, the majority of them lacking m a n y markers, and often all t h e markers on one member of t h e pair of chromosomes not bearing t h e selected loci are absent.
I n the following paragraphs we shall describe the formal genetic analysis of heteroclones selected on minimal medium supplemented with few or no growth factors. Although the presence of growth factors m a y result in t h e emergence of easily recognized sectors, t h e segregation of t h e markers in the heteroclone seems otherwise to be unaffected by the supplements pres
ent in the selective medium.
6. Independent Segregation of Two Linkage Groups. I n most of the het
eroclones, the markers in the two linkage groups segregate independently ; t h a t is, no preferential association of various combinations of markers in the first linkage group with those in t h e second is observed. I n nearly all heteroclones failing to show independent segregation of markers in the two linkage groups, this is due to a n excess of only one, or rarely of two, geno
types, which could have emerged as sectors during the development of the colony. A test of independence between the two linkage groups (see Table I I I ) is therefore used routinely to judge t h e reliability of t h e segregation and so to eliminate colonies in which haploid subclones have emerged.
A special kind of mixed colony which is recognized b y the test of inde
pendence is one in which the two parental genotypes make u p t h e bulk of the segregants, usually together with one or more rare recombinant classes.
232 G. SERMONTI AND D. A. HOPWOOD
These colonies have been interpreted as heterokaryons, in which the two parental genomes must be isolated from one another in such a way t h a t op
portunities for recombination rarely occur.
Once independence has been demonstrated, the two linkage groups can be considered separately for the purposes of elaborating the data. Both link
age groups have shown the same type of behavior with regard to the dis
turbance of the segregations, and there is no obvious correlation between the disturbances in the two groups. The two linkage groups will therefore be considered formally as two different chromosomes in the two following sections, in which we discuss models to account for the observed segre
gations.
c. Models for the Treatment of Segregation Data. Complementary geno
types hardly ever segregate from the heterogenotes in equal numbers, even if the markers of a single linkage group are considered. T h e great majority of the segregation patterns can be interpreted, and the data elaborated, on the basis of two general models, which apply to single linkage groups.
1. I n some segregations, all the deviations from equality of the frequen
cies of complementary genotypes can be attributed to a single disturbance, which influences directly only the locus whose allele ratio deviates most from 1:1. This locus is always one of the terminal loci in the linkage group.
The allele ratios at the other loci are progressively less unbalanced, and at all of them the less frequent allele is contributed by the same parent. T o describe this situation, a model has been adopted in which the chromosome carrying the less frequent alleles is truncated at a point distal to the most unbalanced locus:
a b c
1 2 y
A B C
The deletion of the chromosome segment distal to the point of truncation functions as a haplolethal, and the reduction in the frequency of c is due to the necessity for a crossover between the locus of c and the breakage point (that is, in region y) in order to form a complete chromosome carrying the allele c. On this model, the frequencies of all the segregant classes car
rying c must be reduced in the same proportion relative to the complemen
tary classes carrying C (Table I V ) . This proportion, which corresponds to the allele ratio c/C, gives an estimate of the distance y.
From the point of view of the formal genetic analysis, the most impor
t a n t consequence of a single disturbance, whatever its cause, is t h a t it does
not invalidate the estimation of recombination frequencies between pairs of loci simply by expressing the numbers of segregants with recombinant genotypes as a percentage of the total segregants, since the frequencies of
T A B L E I V
S E G R E G A T I O N I N A H E T E R O C L O N E O F S. coelicolor A 3 ( 2 ) O F M A R K E R S I N L I N K A G E G R O U P I I S H O W I N G A S I N G L E D I S T U R B A N C E *
Arrangement of markersfj:
str-1
1
ade-3 \+
Arrangement of
1
markersfj:
Arrangement of markersfj:
1
+ + 1
ura-11
Regions : 1 2 y
Allele ratios: 43
88
33 98
28 103 Crossover
in regions
Genotypes of
segregants î Observed
numbers
.
+ +
ura 771 str + ura 16
2 str ade ura 8
1,2 + ade ID a 2
y str ade
+
19i , y + ade
+
42,y
+ + +
5l , 2 , y str +
+
0131 R e c o m b i n a t i o n in r e g i o n 1 = 22/131 = 17%
R e c o m b i n a t i o n in region 2 = 15/131 = 11%
R e c o m b i n a t i o n in region y = 28/131 = 2 1 % x2 (3 d.f.)§ = 0.785; Ρ = 0.85
* D a t a f r o m H o p w o o d et αΖ.2 5
f A t e r m i n a l d e l e t i o n d i s t a l to ura-l+ ( i n d i c a t e d b y d o t t e d line) has b e e n p o s t u l a t e d to a c c o u n t for t h e d i s t u r b a n c e in t h e s e g r e g a t i o n (see p . 232).
I str-1 = r e s i s t a n c e to s t r e p t o m y c i n ; ade-3, ura-1 = r e q u i r e m e n t for a d e n i n e or uracil.
§ T e s t i n g d e p a r t u r e from e q u a l i t y of r a t i o s b e t w e e n t h e f r e q u e n c i e s of c o m p l e m e n t a r y c l a s s e s .
the parental and recombinant classes are altered proportionately. Recom
bination percentages between m a n y pairs of loci have been determined from data of heteroclones showing a single disturbance in their segregation for one linkage group, and the results are consistent.1 1 They also agree
234 G . S E R M O N T I A N D D . A . H O P W O O D
with those obtained by selective analysis, when available, except t h a t the distances calculated from the results of selective analysis are syste
matically larger. The order of groups of loci can be determined by examin
ing the segregation of trios of loci, and identifying the double crossover classes by their low frequencies.
2. A single disturbance in one linkage group is not sufficient to explain the anomalies in the segregation in many of the heteroclones. Two lethal points in a single linkage group have often to be postulated, and almost invariably they turn out to be at opposite ends of the two homologous chromosomes, t h a t is, in the trans configuration. The most obvious evi
dence of such double disturbances is the deviation from 1:1 of the allele ratios at both the terminal loci, but in opposite directions (see the first example in Table V ) . This m a y not be evident if one of the lethal points is relatively distant from the group of loci under consideration (see the second example in Table V ) , but the presence of two disturbances is re
vealed when the frequencies of the various segregant classes are examined.
When two disturbances in trans are implicated, the ratios between the frequencies of complementary classes are not constant, and a significant deviation allows the distinction of segregations of this kind from those with a single disturbance (Table V ) . A consequence of this situation is that the recombination frequencies are overestimated.1 1
The model for the elaboration of the data is as follows:
a b c
! j 1 .
χ 1 2 y
A B C
Two haplolethal deletions are postulated, external to the two terminal loci, and in the trans configuration. Clearly only odd numbers of crossovers
(single or triple) can give rise to viable segregants in which both ends of the chromosome are complete.
If we consider three loci, four of the eight classes correspond to single crossovers (ABC, aBC, abC, abc) and the other four to triples (Abe, ABc, AbC, aBc). Obviously the first four classes are relatively common, and the last four very much rarer (Table V ) . The observed numbers of segregants of the four classes t h a t correspond to single crossovers provide estimates of the relative frequencies of crossing over in the four regions (χ, 1, 2, and y ) . If the length of one region is already known (from data of segregations with a single disturbance), the others can be calculated from it. I n this way a new locus can be mapped from segregation data showing two dis-
turbances in the same linkage group, so long as at least two known loci are segregating in the same linkage group as the unknown marker.
d. Anomalous Segregations. The great majority of the data collected so far can be interpreted in terms of the two models proposed. However, in
T A B L E V
S E G R E G A T I O N I N H E T E R O C L O N E S O F S. coelicolor A3(2) W I T H D O U B L E D I S T U R B A N C E S *
Linkage groupf: I II
+ 1 +
1
arg-1
\
+
1
+ 1
ade-31
Arrangements of markersî§:
Arrangements of markersî§:
1
acr-3
1
his-1
1 +
1
his-3
1
str-1
1 +
Regions: X 1 2 y X 1 2 y
Allele ratios: 74 24
51 47
26 72
142 24
127 39
84 82 Crossover
in regions Genotypes of
segregants J Observed
numbers Genotypes of
segregants i Observed numbers
X acr his
+
20 his str+
201
+
his+
27 + str 182
+ + +
22+ + +
43y
+ +
arg 25+ +
ade 80χ , 1 , 2 acr
+ +
3 his ++
1χ , Ι , ν acr
+
arg 1 his + ade 3x , 2 , y acr his arg 0 his str ade 0
l , 2 , y
+
his arg 098
+ str ade 1
166 x2 (3 d.f.)
II =
42.94; Ρ « 0.01 X2 (3 d.f.)II
= 110.02; Ρ « 0.01* U n p u b l i s h e d d a t a of H o p w o o d a n d of S p a d a - S e r m o n t i .
f D a t a for t h e t w o l i n k a g e g r o u p s c o m e from different h e t e r o c l o n e s .
I acr, str = r e s i s t a n c e t o acriflavin or s t r e p t o m y c i n ; his, arg, ade = r e q u i r e m e n t for h i s t i d i n e , a r g i n i n e or a d e n i n e .
§ T w o t e r m i n a l d e l e t i o n s in trans ( i n d i c a t e d b y d o t t e d lines) h a v e b e e n p o s t u l a t e d t o a c c o u n t for d i s t u r b a n c e s in t h e s e g r e g a t i o n s (see p . 234)
II See f o o t n o t e § t o T a b l e I V .
exceptional cases it is necessary to postulate a rearrangement of the markers with respect to their coupling in the parent strains in order to adapt them to one of the models.
Two kinds of rearrangement have been found. The first is homozygosity of one or more terminal markers. I n segregations of this kind, one allele at a locus is missing altogether, but its absence is not reflected in a dis-
2 3 6 G . S E R M O N T I A N D D . A . H O P W O O D
turbance in segregations at linked loci of the kind t h a t would be expected if a lethal point coincided with the missing allele, or a deletion included it. The simplest explanation is t h a t the missing marker has been replaced by its allele, which is now in the homozygous condition. The second kind of rearrangement, of which only a few examples have been found so far, is a change in the coupling arrangement of the markers in a linkage group with respect to the parental configuration, but all the loci remain hetero
zygous.
Some other anomalous segregations can be accounted for by trivial effects, such as sectors in the heteroclones, but certain rarer anomalies so far remain unexplained.
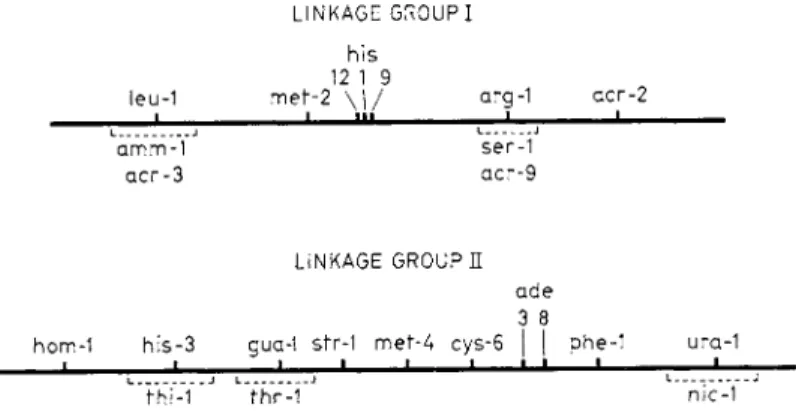
L I N K A G E G R O U P I h i s
12 1 9
leu-1 m e f - 2 \ | / arg-1 a c r - 2
ι I i n i l
amm-1 ser-1 a c r - 3 a c r - 9
L I N K A G E G R O U P E a d e 3 8
hom-1 h i s - 3 gua-1 str-1 m e f - 4 c y s - 6 | | phe-1 ura-1
• • — fhi-1 fhr-1 nic-1
FIG. 2 . L i n k a g e m a p of Streptomyces coelicolor s t r a i n A 3 ( 2 ) . L o c a t i o n s of m a r k e r s n o t a l r e a d y p u b l i s h e d are b a s e d on u n p u b l i s h e d d a t a of H o p w o o d , w i t h t h e e x c e p t i o n of acr, w h i c h w e r e l o c a t e d b y S e r m o n t i a n d S p a d a - S e r m o n t i .
4. T h e L i n k a g e M a p
Figure 2 shows the genetic m a p of Streptomyces coelicolor strain A3 (2).
All the markers so far studied are located in two linkage groups. All at
tempts to detect linkage between the terminal markers of different linkage groups have failed. We cannot assert t h a t the two linkage groups corre
spond to two independent chromosomes, although this is at the moment a simpler hypothesis than a nonrandom distribution of marked loci or of crossovers along a single chromosome. The length of each linkage group, as estimated by the analysis of the segregants from heterogenotes, is of the order of 50 recombination units. Thus the total known m a p barely exceeds 100 units in length; it is somewhat longer if we use data from selective analysis.
In eubacteria, several examples have been found in which loci controlling different steps in the same biosynthetic pathway are located next to one another on the linkage m a p2 6; the equivalent loci in fungi, although not
always randomly distributed, do not usually occur in close proximity. The most fully studied example of a cluster of related loci in a bacterium is the series of histidine loci of Salmonella, all of which occupy a short unin
terrupted segment of the chromosome.2 7 I n Streptomyces coelicolor, the loci his-1, his-9, and his-12, which control different reactions in histidine synthesis, are located in a short region in linkage group I, while a fourth locus, his-3, lies in linkage group I I . The few data t h a t are available there
fore suggest t h a t S. coelicolor resembles eubacteria in the close linkage of some groups of related loci, although this tendency m a y not be so pro
nounced.
C . TH E GE N E T I C SY S T E M
1. LI N E S O F EV I D E N C E
The d a t a collected and analyzed by the methods t h a t have just been described allow us to build up a preliminary picture of the genetic system in which recombination occurs. Separation of the cells during nuclear t r a n s fer, and study of the delay in phenotypic expression of the recombinant genotypes, which have been so useful in building up a picture of the genetic system of Escherichia coli K 1 2 ,2 8 have so far not been possible in S. coeli
color owing to its filamentous growth habit. However, a glimpse of the events leading to the emergence of spores containing recombinant genomes is provided by the fortunate discovery of exceptional heterogenotes, in which the segregation process is not yet complete. Most of our information is derived from the interpretation of the final results of the process of segregation in the heterogenotic colonies (heteroclones).
2. CO N J U G A T I O N
Direct proof t h a t the transfer of genes from one hypha to another in the mixed culture occurs by a process of cell conjugation has not been obtained. Claims of hyphal anastomosis in other streptomycetes have been made by several authors, 1G> 2 9 ; 3 0 but they must be regarded as inde
cisive. Moreover, even if hyphal anastomosis is proven, there is no evidence t h a t it is the process responsible for gene transfer. If gene transfer oc
curred through a narrow bridge, as in E. coli K 1 2 ,3 1 this would not be seen in the light microscope.
T h e occurrence of conjugation processes is strongly suggested by the facts t h a t heterokaryotic hyphae can be isolated from mixed cultures of nutritionally marked strains of m a n y streptomycetes (Section I I I , A ) , and t h a t in S. coelicolor A3 (2) the existence of heterogenotes containing two nearly complete parental genomes can be demonstrated. T h e transfer of large pieces of genetic material from strain to strain was in fact indicated
238 G. SERMONTI AND D. A. HOPWOOD
even by the first data on gene recombination in this organism.4 I t is diffi
cult to imagine the transfer of complete or nearly complete genomes from one hypha to another by a mechanism t h a t does not involve some kind of conjugation.
Mixtures of spores do not give rise to recombinants if they are not allowed to germinate, while recombinants can be isolated from mixed cultures before the aerial mycelium has begun to form, so t h a t it is very probable t h a t conjugation occurs among the hyphae of the substrate mycelium.
I n certain streptomycetes, such as S. griseus?2 the only result of conjuga
tion seems to be the formation of heterokaryotic hyphae, while in S.
coelicolor A3 (2) we find almost exclusively zygotes. R a r e heterokaryons seem to be formed in S. coelicolor A3 (2), especially in the least fertile com
binations of strains, but proof t h a t they are true heterokaryons and not syntrophic growths of mycelium has not been obtained for this strain.
The formation of heterokaryons is particularly significant since it implies the passage of whole nuclei from cell to cell, which is hardly ever found in the eubacteria. However, we cannot y e t say whether heterokaryosis is related to zygote formation, or whether the two are distinct phenomena.
3. SE X U A L I T Y
Two groups of strains of Streptomyces coelicolor A3 (2) have been recognized. Strains of the first group give no or very few recombinants when crossed among themselves, while those of the second group are fertile when crossed with each other or with strains of the first group (Fig. 3 ) . Some crosses are fertile when the selection is for certain markers, b u t sterile when other markers are selected. The situation is comparable with t h a t in Escherichia coli K12. T h e two groups of strains would correspond to F ~ and F + or Hfr strains of E. coli, respectively.3 3 We shall call the strains of S. coelicolor R ~ and R + (R = recombination) until such time as the basis of the difference is better understood.
F r o m a cross between an R + and an R ~ strain, a sample of each of the parental genotypes was isolated. All 12 of those having the genotype of the R + parent were fertile when crossed with the R ~ parent, while about half of those carrying the markers of the R ~ parent were fertile with an R ~ tester strain. The fertility was, however, relatively low. This indicates t h a t the transfer of a factor conferring fertility occurred with a very high efficiency, while the frequency of transfer of chromosomal markers, as judged by the recovery of recombinant spores, was of the order of 1 0- 4. Nearly all the recombinants turned out to be R + . The same behavior was found in another cross involving different strains. These observations indi
cate the presence of a contagious factor, not integrated in the genome, which promotes fertility in S. coelicolor A3 (2).
FIG. 3. Spot testing for fertility. A. Three day-old mixed cultures on complete medium; spots of strains to be tested (arg-1 ura-1) were replic plated on a background of a sterile tester strain (his-1). B. Replica plate of the mixed cultures on minimal medium plus arginine (selective fo recombinants between his-1 and ura-1). Five of the tested strains are fertile, while four are sterile.
239
2 4 0 G . S E R M O N T I A N D D . A . H O P W O O D
In crosses between R + and R ~ strains, the R + parent appears to con
tribute fewer markers to the progeny t h a n the R ~ s t r a i n .3 3 a
4 . NA T U R E O F T H E HE T E R O G E N O T I C NU C L E I
Following conjugation, heterogenotic nuclei are formed. Such nuclei re
veal themselves in the cells t h a t give rise to the heteroclones, and their constitution is analyzed by studying the segregants in the heteroclones.
However, these nuclei probably do not represent the primary zygote nuclei, but are derived from them by subsequent multiplication.
The nuclei t h a t give rise to the heteroclones are often very incomplete, many markers from one or other or from both parents being absent.
Usually the markers of one member of one of the pairs of chromosomes are absent when they are not necessary for growth on the selective medium (Table V I ) . Even when heteroclones are selected on minimal medium, where the wild-type alleles of all the nutritional markers are indispensable, the segregation of the markers shows irregularities which reveal chromo
somal deficiencies in the regions outside the marked loci. These irregulari
ties in segregation could also be explained by postulating lethal genes at the points t h a t have been represented formally as the ends of truncated chromosomes, but such a hypothesis is improbable in view of the extreme variability of the types of disturbances from heteroclone to heteroclone.
The nuclei t h a t give rise to the heteroclones can therefore be considered as heterogenotes with more or less extensive chromosomal deficiencies.
W h a t we cannot yet say is whether these deficiencies are already present in the primary zygotes. Although this is likely, postzygotic elimination of chromosome segments must also be postulated to explain the fact t h a t there are often deficiencies in the contributions of both parents to the genetic make-up of the heteroclones.
The association of the two genomes (or parts of them) in the hetero
genotic nuclei is comparable with t h a t in the diploid nucleus of a higher organism, since the two genomes divide synchronously for a number of nuclear generations before eventually interacting to give rise to recombi
nants. However, the association between the two genomes is probably different from t h a t in higher organisms because of the absence of a nuclear membrane in Streptomyces.
5 . MU L T I P L I C A T I O N O F T H E HE T E R O G E N O T I C NU C L E I
T h e large number of different recombinant genotypes recoverable from each heteroclone t h a t originates from a heterogenotic nucleus indicates t h a t the original nucleus must have undergone a number of divisions before segregating. Since the frequencies of the rarest genotypes are less than 1 0- 4,2 3 at least m a n y thousands of segregants must arise indepen
dently within the colonies, and therefore at least several thousand hetero-
T A B L E V I
SEGREGATION IN H I G H E R ORDER HETEROCLONES ISOLATED ON A M E D I U M SELECTIVE FOR W I L D - T Y P E ALLELES AT T W O CLOSELY L I N K E D LOCI (Met-2 AND His-1)*-f
Linkage
group: I I I
Arrangement of markers:
met-2 + arg-1 + his-1 +
str-1 ade-3 + + + phe-1 Allele ratios
Linkage
group Markers X Allele ratios in heteroclone
Linkage group
1 2 3 4
I met/-\-
+/his arg/+
3 5 / 1 5 3 6 / 1 4 3 1 / 1 9
14/36 1 7 / 3 3 2 9 / 2 1
2 2 / 7 7 6 2 / 3 7 7 9 / 2 0
7 / 4 0 1 2 / 3 5 0 / 4 7
I I str/+
ade/-\- +/phe
5 0 / 0 5 0 / 0 5 0 / 0
5 0 / 0 5 0 / 0 5 0 / 0
9 9 / 0 9 9 / 0 9 9 / 0
0/47 4 7 / 0 4 7 / 0 Segregation in linkage group I
Genotypes % Observed numbers in heteroclone
1 2 3 4
met
+
arg 22 10 17 0+
his+
6 16 12 35met
+ +
12 4 3 7+
his arg 7 17 33 0+ +
arg§ 2 2 29 0met his
+
1 0 2 0+
+ + § 0 1 3 5met his arg 0 0 0 0
50 50 99 47
* U n p u b l i s h e d d a t a of H o p w o o d a n d S p a d a - S e r m o n t i . t T h e p r i m a r y h e t e r o c l o n e s h o w e d s e g r e g a t i o n a t all 6 l o c i .
t met, his, arg, ade, phe = r e q u i r e m e n t for m e t h i o n i n e , h i s t i d i n e , a r g i n i n e , a d e n i n e , p h e n y l a l a n i n e , r e s p e c t i v e l y ; str = r e s i s t a n c e t o s t r e p t o m y c i n .
§ G e n o t y p e s t h a t c o u l d g r o w o n t h e s e l e c t i v e m e d i u m a n d e m e r g e as s e c t o r s .
genotic nuclei must have been formed from the original nucleus and un
dergone segregation. I n fact, if a heteroclone colony is broken u p into small fragments and the resulting suspension of spores and small pieces of hypha is plated on a suitable selective medium, more t h a n a thousand new heterogenotic colonies can be obtained.
Examination of some of these colonies shows t h a t a large proportion of the secondary heterogenotic nuclei resemble the primary one, b u t some
242 G . S E R M O N T I A N D D . A . H O P W O O D
of them differ, indicating the occurrence of chromosomal changes during the multiplication of the heterogenotic nuclei. The following changes have been found: loss of a chromosome (indistinguishable from homozy
gosity of all the markers of one linkage group), accentuation of terminal deletions, establishment of homozygosity of one or more terminal markers, and rare changes in the coupling arrangements of pairs of alleles. At the moment it is difficult to calculate the frequencies of such processes, since the mere identification of each situation requires a complex analysis.
6. FO R M A T I O N O F RE C O M B I N A N T S
Stable haploid recombinants can be obtained directly by plating spores from the mixed culture, or by plating spores from the heteroclones. The segregation patterns obtained are substantially similar, except for the obvious difference t h a t the recombinant spores in the mixed culture are accompanied by a great majority of spores of the parental genotypes. A simplifying hypothesis is t h a t the recombinants in the mixed culture arise from heteroclones growing in the culture; thus all recombinants m a y be produced from a transient heterozygous stage. The occurrence of other processes of recombination in the mixed culture cannot, however, be ex
cluded.
The spores produced by a heteroclone are almost exclusively haploid, and the markers of the two linkage groups segregate completely indepen
dently. I t is difficult to say whether reduction of the two linkage groups is always contemporaneous and whether crossing over is synchronized with reduction. Sometimes crossing over appears to occur independently of reduction (mitotic crossing over), to give rise to heterogenotes with new arrangements of markers, but we cannot rule out the possibility t h a t such rearrangements, which have so far been observed rarely, arise by a reas- sociation of haploid products (recycling of meiosis3 4).
We m a y summarize our tentative picture of the process of recombina
tion in S. coelicolor A3(2) as follows: Heterozygous nuclei are produced following transfer of probably incomplete nuclei from hypha to hypha of the substrate mycelium by a process of conjugation; the heterozygous nuclei multiply, and during their multiplication chromosomal losses and chromosomal terminal deletions occur, and possibly some mitotic cross
ing over; finally haploid recombinants arise by crossing over and reduc
tion.
III. G e n e t i c Phenomena in O t h e r S t r e p t o m y c e t e s
A . BA L A N C E D HE T E R O K A R Y O S I S
Heterokaryosis in Streptomyces was first found by Bradley and Leder
b e r g3 2 in three different species. I t was recognized by the production of
colonies capable of growth on minimal medium by mixtures of two auxo
trophic strains. Prototrophy could not be perpetuated through the spores, which had exclusively parental genotypes, while colonies of both parental genotypes arose from fragments of mycelium of the heterokaryotic colo
nies isolated by means of a micromanipulator. Similar results were ob
tained by Braendle and S z y b a l s k i5'6 with various other species of Strep
tomyces, and by S a i t o8 with S. gnseoflavus.
Braendle et al.35 found t h a t strains of the two parental phenotypes re
covered from a heterokaryon gave rise to new heterokaryons with a much higher frequency t h a n the original strains. T h e ability to give high yields of heterokaryons was preserved for several subcultures, but eventually disappeared.
Heterokaryosis in S. scabies has been used to study the apparent cyto
plasmic inheritance of tyrosinase production ; this is discussed on page 246 while the occurrence of heterokaryosis in S. coelicolor A3 (2) has been mentioned on page 231.
T h e term "heterokaryons" has been applied by B r a d l e y3 6 to almost stable prototrophic strains of S. coelicolor producing rare parental segre
gants (see later, p. 244). Whatever the nature of these strains, they are not heterokaryons in the sense in which the term has been applied to m o l d s3 7 and adopted to describe formally comparable phenomena in streptomycetes.
B. GE N E RE C O M B I N A T I O N
1. GE N E T I C SY S T E M S RE S E M B L I N G TH A T O F S. coelicolor A3 (2)
Genetic recombination has been reported in several strains belonging to various species of the genus Streptomyces, and only a few attempts to de
tect it have been unsuccessful. Only in a few strains have investigations been carried far enough to allow comparison with the results obtained with S. coelicolor A3 (2). All the authors who have used multiply marked strains and plated spores from mixed cultures on suitably supplemented selective media have found a great variety of recombinant phenotypes among these spores, and the great majority of the recombinants have proved to be stable on repeated subculture. Such results have been ob
tained with S. coelicolor strain I . S . S . ,3 , 2 2>3 8 in S. fradiae,6 and in S. griseo- flavus,32 and have generally been taken as demonstrating the haploid n a ture of the recombinants, and of the parental strains.
I n S. griseoflavus, however, certain observations contradicted the con
clusion of h a p l o i d y3 9; these were the cytological observation of bipartite spore nuclei, the finding of a two-hit survival curve after t r e a t m e n t with X - r a y s (in contrast to the one-hit curve of S. coelicolor A3 ( 2 )1 1 and S.
coelicolor I.S.S.3 9), and the observation t h a t m a n y reversions of biochemi-