Pannon Egyetem
Vegyészmérnöki Tudományok és Anyagtudományok Doktori Iskola
Értéknövelt glicerinszármazékok el ő állítása nyers glicerinb ő l, a biodízelgyártás
melléktermékéb ő l
DOKTORI (PhD) ÉRTEKEZÉS
Készítette:
Szabóné Herseczki Zsanett
okleveles vegyészmérnök
Témevezetők:
Dr. Marton Gyula
†Dr. Dallos András
Pannon Egyetem Mérnöki Kar
2013
DOI: 10.18136/PE.2014.526
ÉRTÉKNÖVELT GLICERINSZÁRMAZÉKOK ELŐÁLLÍTÁSA NYERS GLICERINBŐL, A BIODÍZELGYÁRTÁS MELLÉKTERMÉKÉBŐL
Értekezés doktori (PhD) fokozat elnyerése érdekében Írta:
Szabóné Herseczki Zsanett okleveles vegyészmérnök
Készült a Pannon Egyetem,Vegyészmérnöki Tudományok és Anyagtudományok Doktori Iskolája keretében
Témavezető: Dr. Marton Gyula† Dr. Dallos András
Elfogadásra javaslom (igen / nem) ………...
(aláírás) A jelölt a doktori szigorlaton …... % -ot ért el,
Az értekezést bírálóként elfogadásra javaslom:
Bíráló neve: …... …... igen /nem
………...
(aláírás) Bíráló neve: …... …... igen /nem
………...
(aláírás) A jelölt az értekezés nyilvános vitáján …...% - ot ért el
Veszprém,
………...
a Bíráló Bizottság elnöke
A doktori (PhD) oklevél minősítése…...
………...
Az EDHT elnöke
University of Pannonia
Doctoral School in Chemical Engineering and Material Sciences
Preparation of value-added glycerol derivatives from crude glycerol, the by-product of biodiesel
production
PhD Thesis
Author
Zsanett Szabóné Herseczki
chemical engineer (MSc)
Supervisors
Dr. Gyula Marton
†Dr. András Dallos
University of Pannonia Faculty of Engineering
2013
Kivonat
A szerző célja a biodízelgyártás során melléktermékként keletkező nyers glicerin feldolgozási/hasznosítási lehetőségeinek vizsgálata volt. A gazdasági és környezetvédelmi okokból egyre fontosabb biodízel-gyártás melléktermékeként keletkező glicerin kinyerése és értékes termékké alakítása jelentős mértékben csökkentheti a biodízel előállítási költségeit, ezért a jelölt további feladata volt a glicerin-alapú termékek piacképességi vizsgálata, majd a kiválasztott termékek előállítási technológiájának kidolgozása.
A szerző a kísérleti munka során megvizsgálta egy hazai biodízel üzemből származó nyers glicerin fázis tisztítási lehetőségeit, és különböző tisztaságú, magas glicerin tartalmú elegyeket állított elő, melyek a további kísérletek alapanyagait képezték.
A különböző tisztaságú glicerin elegyekből értéknövelt glicerinszármazékokat:
triacetint, tripropionint, glicerin karbonátot és (2-izobutil-2-metil-1,3-dioxolán-4- il)metanolt állított elő. A kapott termékek minőségét HPLC és GLC mérésekkel követte nyomon.
Kísérleti munkája során meghatározta az optimális reakciókörülményeket, a felhasznált katalizátorok és a glicerin minőségének a reakciókra gyakorolt hatását. Glicerin karbonátot állított elő automata laborreaktorban, és a mérési eredmények alapján elkészítette a reaktor dinamikus modelljét.
Megvizsgálta az előállított tripropionin bekeverésének hatását a Diesel-motor üzemére.
Továbbá megvizsgálta a (2-izobutil-2-metil-1,3-dioxolán-4-yl)metanol termikus stabilitását és oldhatósági tulajdonságait, illetve reakcióikinetikai számításokkal meghatározta a reakció főbb kinetikai paramétereit.
A kísérleti eredmények alapján elkészítette a nyers glicerin tisztítási eljárására, továbbá az értéknövelt glicerinszármazékok előállítására vonatkozó anyagmérlegeket, és összegezte a hagyományos nyers glicerin kezelési és az általa kidolgozott eljárások előnyeit, hátrányait.
Abstract
The aim of the work was to study the purification of crude glycerol and the synthesis process for converting crude glycerol in to different value-added glycerol derivatives (triacetin, tripropionin, glycerol carbonate and (2-isobutyl-2-methyl-1,3- dioxolan-4-yl)methanol).
Influences of raw materials (different glycerol qualities), and various reaction conditions were investigated and optimized. The resulting products were obtained in almost quantitative yields and the most important properties of the products were determined.
For glycerol carbonate a well detailed dynamic model of the reactor where the experiments were performed has been worked out. Effect of tripropionin blending on diesel engine performance characteristics and environmental repercussions were studied.
The kinetical parameters of synthesis of (2-isobutyl-2-methyl-1,3-dioxolan-4-yl)methanol and the properties of the product were determined.
Moreover material balances for crude glycerol purification process and preparations of various value-added glycerol derivatives were calculated. Benefits and disadvantages of elaborated crude glycerol handling processes were compared with the conventional ones.
Auszug
Im Rahmen dieser Arbeit wurden Methoden zur Reinigung von Rohglyzerin studiert und Veredelungsschritte von Rohglyzerin zu verschiedenen Glyzerinderivaten (Triacetin, Tripropionin, Glyzerincarbonat und 2-isobutyl-2-methyl-1,3-dioxolan-4- yl)methanol) wurden entwickelt.
Es wurden die Einflüße der Rohstoffen, die von unterschiedlichen Glyzerinqualitäten ausgehen, sowie die zugehörigen Reaktionsbedingungen untersucht und optimiert. Die entsprechenden Produkte wurden in nahezu quantitativer Ausbeute erhalten.
Die wichtigsten Stoffeigenschaften von den erhaltenen Materialien wurden bestimmt.
Für die Synthese von Glyzerincarbonat wurde ein detailiertes, dynamisches Modell des Reaktors erstellt, in dem die experimentellen Arbeiten ausgeführt wurden.
Der Einfluß der Zumischung von Tripropionin zu konventionellem Dieseltreibstoff auf die Leistungscharakteristik von Dieselmotoren wurde studiert ebenso wie dessen ökologische Wirkungen.
Die kinetischen Parameter für die Synthese von (2-isobutyl-2-methyl-1,3-dioxolan- 4-yl)methanol wurden bestimmt ebenso wie einige Stoffeigenschaften dieses Materials.
Ferner wurden Materialbilanzen für die Reinigung von Rohglyzerin nach einem Raffinierungsverfahren sowie für Synthesen verschiedener Glyzerinderivative erstellt.
Contents
KIVONAT ... 4
ABSTRACT ... 5
AUSZUG ... 6
INTRODUCTION ... 9
AIMS AND SCOPES ... 11
1. LITERATURE ... 12
1.1EXISTING GLYCEROL PURIFICATION TECHNOLOGIES ... 17
1.1.1 Soap Splitting as a Glycerol Pre-treatment Step ... 18
1.1.2 Conventional Processes for Glycerol Purification ... 19
1.1.3 Recent Development in Glycerol Purification Processes ... 22
1.2TRANSFORMATION OF GLYCEROL INTO HIGH-QUALITY PRODUCTS ... 26
1.2.1 Glycerol transforming processes ... 29
1.2.1.1 Aqueous phase reforming ... 29
1.2.1.2 Fischer-Tropsch ... 29
1.2.1.3 Selective reduction ... 29
1.2.1.4 Halogenation ... 30
1.2.1.5 Dehydration ... 30
1.2.1.6 Etherification ... 30
1.2.1.7 Selective oxidation ... 30
1.2.1.8 Pyrolysis ... 31
1.2.1.9 Biotransformation ... 31
1.2.2 Glycerol esters... 32
1.2.2.1 Triacetin ... 32
1.2.2.2 Tripropionin ... 37
1.2.2.3 Glycerol carbonate ... 38
1.2.3. Ketals ... 40
2 MATERIALS AND METHODS ... 43
2.1MATERIALS ... 43
2.2APPARATUS AND PROCEDURE ... 44
2.2.1 Purification of crude glycerol ... 44
2.2.2 Synthesis of triacetin ... 45
2.2.3 Synthesis of tripropionin ... 47
2.2.4 Synthesis of glycerol carbonate... 49
2.2.5 Synthesis of (2-isobutyl-2-methyl -1,3-dioxolan-4-yl)methanol ... 52
2.3ANALYSIS ... 55
3 RESULTS AND DISCUSSION ... 59
3.1PURIFICATION OF CRUDE GLYCEROL ... 59
3.1.1 Dilution by water and acid treatment ... 59
3.1.2 Neutralization by Ca(OH)2 ... 60
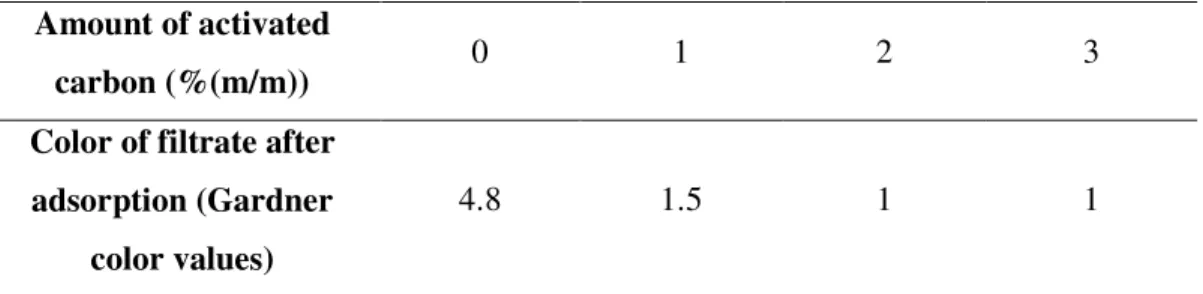
3.1.3 Coloring matter adsorption by activated carbon ... 61
3.1.4 Purification by distillation ... 62
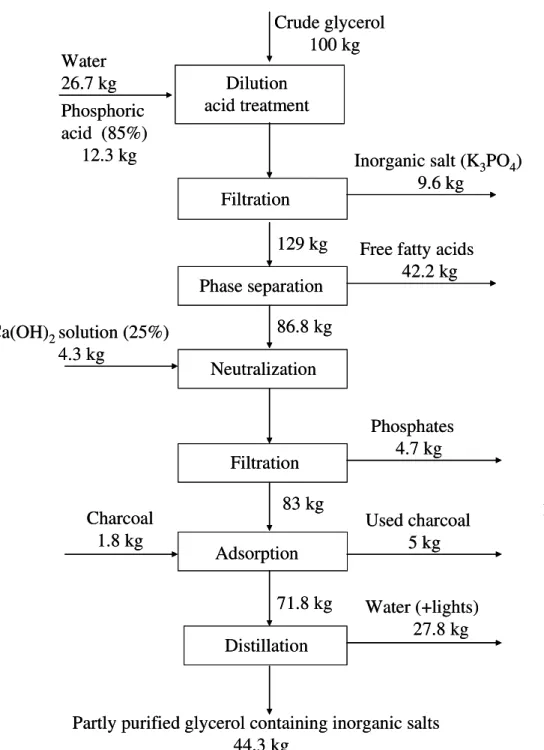
3.1.5 Material balance of crude glycerol purification ... 62
3.2SYNTHESIS OF VALUE-ADDED GLYCEROL DERIVATIVES ... 65
3.2.1 Synthesis of triacetin ... 65
3.2.2 Synthesis of tripropionin ... 77
3.2.3 Synthesis of glycerol carbonate... 83
3.2.4 Synthesis of (2-isobutyl-2-methyl -1,3-dioxolan-4-yl)methanol ... 96
3.3WASTE HANDLING PROPOSALS ... 105
3.4GENERAL WAYS TO UTILIZE CRUDE GLYCEROL ... 106
4 SUMMARY ... 107
5 TÉZISEK ... 110
6 THESES ... 113
7 REFERENCES... 116
8 PUBLICATIONS ... 126
9 ABREVIATIONS ... 129
KÖSZÖNETNYILVÁNÍTÁS ... 130
Introduction
Biodiesel products (gas oil substitutes made from vegetable oils or animal fats) are the leading alternatives to fossil fuels in transportation (Jamróz et al., 2007). There are two primary ways for manufacture and use of biodiesel,
A - direct use of vegetable oils and/or use of blends of native oils with conventionally fuel oils.
B - transesterification of vegetable oils and/or animal fats.
However, the direct use of vegetable oils and/or the use of blends of native oils with conventionally fuel oils have generally been considered to be unsatisfactory and problematic. Conventionally Diesel engines, constructed for use of fossil-oil based fuels afford at least modifications of the fuel injection system for use of biodiesel. But, even than various problems may occur, like carbon deposits in the engine, carbon deposits at the exhaust valves, increased erosion of this valve region and inacceptable contamination of the lubrication oil are often associated with the use of such oils and fats as Diesel fuels (Fangrui et al., 1999).
Transesterification of natural oils and fats by methanol is currently the method of choice. By this procedure triglycerides are converted into fatty acid methyl esters. In contrary to triglycerides such monoesters of fatty acids are highly compatible with conventionally Diesel engines. This approach can be seen as the nucleus of success of the transesterification technology. Unfortunately, there is also a down-side. Usually chemical processes in organic chemistry provide by-products and residues, so as the transesterification technology, too.
The conventional methodology in the production of biodiesel primarily involves the use of NaOH and KOH as homogeneous catalysts and usually methanol as the alcohol component. This transesterification technology is generating always a product mixture consisting of two phases. The upper phase is containing the desired ester products, the crude bio gas oil fraction, representing about 80 to 85 % of the process yield. By using common technologies it appears to be relatively easy to convert such an ester phase into a standardized sales product, the biodiesel.
In contrary to this, the lower phase of such a transesterification process is picking up quite a number of very different chemically individuals beside glycerol, as there are:
water, alkali hydroxides, various semi-saponified triglycerides, alkali salts of fatty acids,
fatty acids, alcohols and last but not least some other remains of the vegetables originated by the oil production.
Glycerol as the main component of the so called “lower phase” (Glycerol Phase, GP) could contribute to a welcomed by-product credit in biodiesel manufacturing, provided a “non-sophisticated” recovery method could be used.
However, as attractive the handling of the ester phase appears under consideration of technical processing, the less attractive is the handling of the lower phase, often called
“crude glycerol” – not to say problematic.
The application of common base technologies like distillation, filtration, extraction, incineration, but even biologically waste treatment of “crude glycerol” is becoming hampered for numerous reasons. Neutralization of the “crude glycerol” is the first step before any further utilization may start. But, even after neutralization any promising utilization of “crude glycerol” is hampering most base technologies.
The exact composition of the raw GP depends on the transesterification methodology and the separation conditions employed in the biodiesel production; in any case, glycerol concentration is usually between 30 and 60 %(m/m). Larger biodiesel plants are often showing high glycerol concentrations of 75-90 %(m/m) (Hájek et al., 2010).
Independently of any further utilization of glycerol it seems to be imperative to employ a “simple” and reliable process to work-up “crude glycerol”. Ideally, such a process should allow the recovery of glycerol, an easy salt removal, elimination of high contaminated waste water and finally the incineration of some organic production residues.
The best approaches to get around the problems in handling of “crude glycerol” can be seen in formation of derivatives of the contained glycerol.
The glycerol derivatives can replace petrochemically derived polyols such as ethylene glycol, propylene glycol, and pentaerythritol used in automobile antifreeze, aircraft deicers and alkyd resins may partially alleviate glycerol surpluses. The development of innovative applications for glycerol appears to be the most constructive approach to utilize crude glycerol. Practically, developing glycerol as a primary chemical building block that may be converted into other value added chemicals becomes more attractive as crude glycerol prices drop.
Aims and scopes
The research line, described in the present work, is focusing on the purification of crude glycerol and the synthesis process for converting crude glycerol in to different value- added glycerol derivatives (triacetin, tripropionin, glycerol carbonate and (2-isobutyl-2- methyl-1,3-dioxolan-4-yl)methanol).
Most of the preparations were carried out using pure glycerol (>99.5%) to get reference results, but our final aim was to use partly purified or crude glycerol for the manufacture of glycerol derivatives.
Aims of the work were to
study the removal of impurities from crude glycerol and optimize the purification steps
investigate the influences of raw materials (different glycerol qualities like pure, partly purified and crude glycerol) on preparation of glycerol- derivatives
optimize the reaction conditions (molar ratio, reaction temperature, catalyst concentration)
examine the purification/decolorization of the products mainly by distillation where it has a chance
determine the important properties of the products where it was necessary collect the benefits and disadvantages of elaborated crude glycerol handling
processes and to compare them with the conventional ones.
1. Literature
Triglycerides (TG) of vegetable oils and fats are becoming increasingly important as alternative fuels for diesel engines due to the diminishing petroleum reserves. However, their high viscosities and low volatilities do not permit their direct use or in oil/petrol blends (McDonnel et al., 1995; Dorado et al., 2002), in any diesel engine type (Monyem et al., 2001). Nowadays, the main process developed to overcome this drawback is the methanolysis reaction to produce biodiesel, a biodegradable, non-toxic diesel fuel substitute that can be used in unmodified diesel engines (Pinto et al., 2005; Fukuda et al., 2001). It has a significant added value compared to petro-diesel because of its higher lubricity, which extends engine life and reduces maintenance costs as well as contributing to fuel economy (Kulkarni et al., 2006).
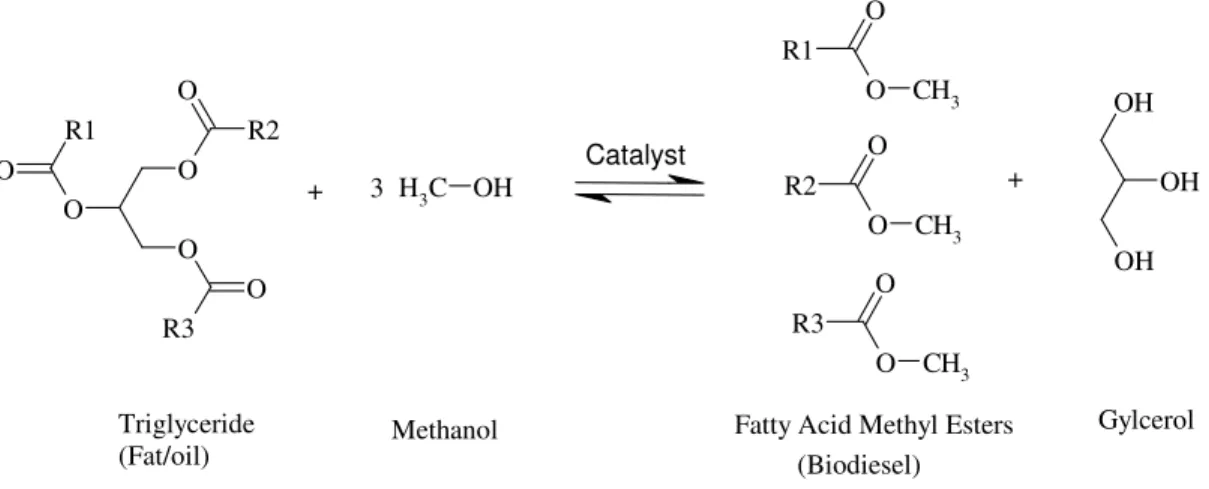
The conventional methodology in the production of biodiesel primarily involves the use of NaOH and KOH as homogeneous catalysts. Ideally, three molecules of fatty acid methyl esters (FAME) and one molecule of glycerol are formed when starting from any molecule of a triglyceride (TG) and three moles of methanol (Ma et al., 1999) (see Figure 1.1).
Figure 1.1 Ideal reaction scheme of transesterification of triglycerides to form biodiesel
However, the process is far from being environmentally friendly as the resulting reaction mixture of transesterification needs to be separated, neutralised and thoroughly washed, generating much waste in terms of inorganic salts, waste water and other residues.
Also, it must be noticed that, the alkalinic catalysts can not be recycled.
R2 O O O
O R1
O R3
O
C
H3 OH R2
CH3 O
O
R3
CH3 O
O R1
CH3 O
O OH
OH OH
Gylcerol (Biodiesel)
Catalyst
+ 3 +
Triglyceride
(Fat/oil) Methanol Fatty Acid Methyl Esters
Inevitably, such additional steps must increase the overall biodiesel production costs, whereas the “crude glycerol” is of poor quality due to the manifold of contained impurities (Verziu et al., 2008).
For various reasons ethanol may appear as a potentially replacement of methanol, but there are no significant benefits in processing when compared with methanol rather than a slower reaction rate. In the case of methanolysis the solubility of oil in methanol is less and reaction is mass transfer limited. On the other hand, methanol makes higher equilibrium conversion due to higher reactive intermediate methoxide (Sridharan et al., 1974). Unlike methanol, ethanol has better solvent properties but the formation of emulsion after the transesterification of oil with ethanol makes the separation of ester very difficult (Issariyakul et al., 2007). Despite of the fact that ethanol is less toxic than methanol (Vale, 2007), biodiesel is commonly manufactured by transesterification of triglycerides with methanol as it is the cheapest alcohol available.
Based on stoichiometrically calculations the transesterification process is resulting approximately 10 %(m/m) of glycerol. However, that part of the biodiesel synthesis containing the glycerol is rather impure, commonly called “crude glycerol”.
“Crude glycerol” as an unrefined residue of the biodiesel synthesis shows usually a content of glycerol varying between 55 %(m/m) and 90 %(m/m). The larger biodiesel plants tend to give the highest “purities”, often around 75 %(m/m) to 80 %(m/m). The rest of the “crude glycerol” consists primarily of remaining, unconverted triglycerides, unreacted methanol, some dissolved FAME, fatty acids, alkali hydroxides, different semi- saponified triglycerides, alkali salts of fatty acids, water, pigments and some other remains of the vegetables originated by the oil production.
Due to this significant contamination of the glycerol fraction the actual amount of formed “crude glycerol” is much larger than by stoichiometrically calculation expected. It varyies between 100/90 and 100/55 parts by mass, which is 1.1 respectively 1.8 times as much. Table 1.1 represents some typically composition data of “crude glycerol”. Most of the contaminants can be traced back to the biodiesel synthesis process, so for example some of the remaining methanol, which was not completely evaporated. Furthermore, the concentrations of Na and K can tell whether caustic soda (sodium hydroxide, NaOH) or potash lye (potassium hydroxide, KOH) were used as catalysts during the transesterification process. Also, it should be mentioned that alkali- and earthalkali metals like Na, K, Ca and Mg are always present in native vegetable oils. Larger quantities of
inorganic sulphates and/or phosphates remain from neutralization of the mixture with sulfuric or phosphoric acid.
Table 1.1 Composition of G-phase after neutralization
Property Value Unit
Glycerol content 77-90 %(m/m) A.R.
Ash content 3.5-7.5 %(m/m) A.R.
Water content 0.1-13.5 %(m/m) A.R.
Lower calorific value 14.9-17.5 MJ/kg A.R.
Kinematic viscosity 120.0 mm2/s
3-monopropylenediol 200-13 500 ppm
Methanol 0.01-3.0 %(m/m)
MONG* 1.6-7.5 %(m/m)
pH 4.5-7.4
Sulphate 0.01-1.04 %(m/m)
Phosphate 0.02-1.45 %(m/m)
Acetate 0.01-6.0 %(m/m)
Na 0.4-20 g/kg
K 0.03-40 g/kg
Ca 0.1-65 mg/kg
Mg 0.02-55 mg/kg
Fe 0.1-30 mg/kg
Mn <0.5 mg/kg
* MONG = matter organic non glycerol
There is no doupt that many down-stream utilizations of glycerol require a refined product. Therefore, it becomes imperative to purify the “crude glycerol”. If so, refined glycerol as a valuable by-product can contribute to reduce the overall-cost, but it would also contribute to a significant reduction of residue- and waste handling.
Independently of the actual glycerol concentration in “crude glycerol” the recovery of glycerol itself appears complicated by most common “straight-forward” technologies:
Biologically waste treatment
Currently most of by-product glycerol is sent to water treatment for digestion but this process is slow, expensive and has a low yield (Gupta et al., 2012).
Incineration
The combustion of glycerol ‘‘as it is’’ would represent a desirable solution (Beatrice et al., 2013). Unfortunately, due to the fact that glycerol polymerizes at higher temperatures (~ 280 °C) and yields toxic acrolein through incomplete combustion (Lin et al., 2013), raw glycerol is hardly usable in conventional energy production plants, such as fuel burners or internal combustion engines (Beatrice et al., 2013).
Distillation
The normal boiling point of glycerol is rather high (290 °C) and glycerol oligomerization starts over 200 °C, so high vacuum (~ 3 mbar or lower) should be used to distil glycerol from heavies (inorganic salt) which makes economic assessment of crude glycerol utilization a challenging task (Lin et al., 2013). Under distillation conditions the salt content causes an enormous viscosity increase of the distillation feedstock, which necessitates so called “salt towers”, an expensive type of equipment, both by investment and by operation cost, too.
Filtration & phase separation
However, it is a common knowledge, that the high content of inorganic salts and water contained in crude glycerol may form severe corrosion problems. Apart from this, just the contained water in crude glycerol may cause some nasty problems, not at all in distillations, only. There are some strong tendencies towards emulsification and foaming of feed-stocks which may cause other severe problems in a number of “normal” handling steps, too.
Fact is that approximately 15-20% of the converted feedstock is released as crude glycerol. The accumulation of crude glycerol not only hampers the development of the biodiesel industry, but it also creates economic and environmental problems (Lin et al., 2013).
It was projected that the world biodiesel market would reach 168 million tons by 2016, which implied that approximately 25 million tons of crude glycerol would be produced (Anand et al., 2012). Too much surplus of crude glycerol from biodiesel
production will impact the refined glycerol market (Yang et al., 2012). For example, in 2007, the refined glycerol's price was rather low, approximately $0.66 per kg (compared to
$1.55 before the expansion of biodiesel production) in the United States. Accordingly, the price of crude glycerol decreased from about $0.55 per kg to $0.11 per kg (Kerr et al., 2007). Therefore, development of sustainable processes for utilizing this raw material is imperative.
Due to the above mentioned problems various approaches have been checked to find processing conditions to eliminate the most critical difficulties in recovery of glycerol.
Independently of any further utilization of glycerol it seems to be imperative to employ a “simple” and reliable process to work-up “crude glycerol”. Ideally, such a process should allow the recovery of glycerol, an easy salt removal, elimination of high contaminated waste water and finally the incineration of some organic production residues.
1.1 Existing glycerol purification technologies
Different processes have been implemented to refine glycerol. However, all of them involve soap splitting (see Figure 1.1.1.1) followed by two main separation steps: salt and methanol removal.
General speaking, the following technologies may be used to process “crude glycerol”, after the soap splitting step:
• fractional distillation
• ion-exchanging
• adsorption
• precipitation
• extraction
• crystallization
• dialysis.
A soap splitting followed by a combination of methanol recovery/drying, fractional distillation, ion-exchange (by using zeolite or resins) and adsorption (using active carbon powder) seems to be the most common purification pathway of “crude glycerol”. Well- known companies involved in crude glycerol purification plants are Desmetballestra and Buss-SMS Canzler (ion exchange equipment). Chemical companies like Rohm & Haas and Lanxess supply ion-exchange granulates while a company such as Norit supplies powder and granulated activated carbon (Brockmann et al., 1987).
However, some of the separation techniques require vacuum due to the heat- sensitivity of glycerol. Its decomposition is known to begin at 180 °C together with remarkable formation of water. There are three possible reactions that may reduce the yield of glycerol during distillation procedures:
(a) Polymerization of the glycerol
At high pH values (by excess of NaOH and elevated temperatures > 200 °C) glycerol is able to form polyglycerol (Garti et al., 1981; Ikuya et al., 1990; Lutz et al., 1998). As a non-distillable polymer the polyglycerol will stay in the bottoms. It may increase the quantities of distillation residues considerably.
(b) Dehydration of the glycerol
During distillation of glycerol at low pH values (by excess of mineralic acids and elevated temperatures > 200 °C) (Hedtke et al, 1996; Monick et al, 1960) a massiv formation of acrolein was reported. Due to the boiling point of acrolein of 52 °C, vacuum problems were obtained, followed by undesired condensation of acrolein into the cold traps.
(c) Oxidation of glycerol
Moderate oxidation of the glycerol to form glycerose, a mixture of glyceraldehyde and dihydroxyacetone was observed (Jungermann et al, 1991; Monick et al, 1960). The partial oxidation products are obtained by oxidizing crude glycerol or other impure, but relatively concentrated glycerol sources. A variety of oxidation processes may leed to glycerose formation, including the reaction of glycerol with an oxidizing agent in the presence of a suitable catalyst; the reaction of glycerol with oxygen or air in presence of water as a solvent; or the reaction of glycerol via enzyme oxidation, bacterial fermentation, catalytic oxidation or the combinations thereof (Gargulat et al., 2009).
1.1.1 Soap Splitting as a Glycerol Pre-treatment Step
Three steps can be distinguished in the purification process. The first step involves a neutralization reaction of the catalyst using a mineralic acid, but also to split the contained soaps. The reaction of an acid with the soaps will give free fatty acids (FFA) and salts (see Figure 1.1.1.1) while its reaction with the base catalyst generates salts and water.
Figure 1.1.1.1 Soap splitting - reaction of hydrochloric acid and soap resulting free fatty acid and inorganic salt
Since FFA’s are insoluble in glycerol and their densities are lower than that of the glycerol, these components will rise to the top of the mixture, so that they can be skimmed off. Some salts, insoluble in glycerol will precipitate out. Ooi et al. (2001) attempted to
NaO R
O
O
H R
O
+ HCl + NaCl
Soap Free fatty acid
palm kernel oil methyl ester plant by using 6 v/v% H2SO4 solution. They reported that such a chemical treatment at low pH had beneficial effects on the recovered “crude glycerol”, such as an increasing glycerol content as well as a reduction of ash forming matter.
However, the content of non-glycerol organic matter (MONG) was slightly higher. The treatment allowed an increased salt removal and reduced the quantity of “crude glycerol”
without affecting the recovery of crude fatty acids.
That procedure was suitable to recover a more concentrated “crude glycerol” from a dilute glycerol source (10-20 %(m/m) glycerol) with a high NaCl (60-70 %(m/m)) content.
The second step concerns the methanol removal. The methanol contained in the glycerol phase can be removed via flash evaporation or using falling film evaporators.
Falling film evaporators show the advantage of a short contact time. Therefore, they are most suitable for these processes owing to the temperature susceptibility of glycerol which can result into its decomposition. The purity of glycerol will be approximately 85 %(m/m) after methanol removal.
In the third step, glycerol can be further purified to 99.5 %(m/m) by using a combination of adsorption, vacuum distillation and ion exchange processes (Knothe et al., 2005). Ooi et al. (2001) reported the recovery of crude glycerol from glycerol residues by a simple distillation at 120-126 °C and 0.04-0.4 mbar to yield around 141.8 g glycerol/kg glycerol residue (~14% yield) at an acceptable purity of 96.6 %(m/m) glycerol, with 0.03
%(m/m) ash, 1 %(m/m) H2O and 2.4 %(m/m) MONG as contaminants.
Hazimah et al. (2003) used the combined process of chemical and physical treatment (acid protonation, ether and ethanolic extractions, filtration and distillation) to recover glycerol and diglycerol from glycerol pitch, recovering a high purity glycerol (~99.1-99.8 %(m/m)) with low content of contaminants (0.11-0.80 %(m/m) H2O, 0.054
%(m/m) ash and 0.56 %(m/m) soap).
1.1.2 Conventional Processes for Glycerol Purification
The conventional process for glycerol purification comprises of various steps including pretreatment, concentration, purification and refining (Hoogendoorn et al., 2007).
The pretreatment step is used to remove color bodies and odor matters as well as any remaining fat components from crude glycerol. In the pretreatment step, sodium
hydroxide is employed to remove fat components via saponification reaction and activated carbon is utilised for bleaching purposes.
The concentration step involves the removal of ionic substances using ion exclusion chromatography. In this process, a bed filled with a strongly acidic exchange resins is charged with a glycerol stream. The principle used for the separation is the Donnan exclusion. Ionic substances are repelled from the resin surface and remain in the liquid volume due to their charge, while the non-ionic ones can be accommodated in the pores of the resins. The column is subsequently rinsed with water, which firstly removes the ionic substances in the liquid and the non-ionic ones later. In some cases when the concentration of ionic substances in the glycerol stream is very high, ion exchangers both cationic and anionic are used.
The following step is the purification of the glycerol phase by means of ion- exchangers, normally used in pairs (cationic and anionic). This purification step is able to remove inorganic salts, fat and soap components as well as color and odor causing matters.
The crude glycerol phase can be purified by ion exchange on Amberlite-252 (strong acid resin) and it has also been suggested that the macroporous Amberlite could be useful for the removal of sodium ions from glycerol/water solutions with high salt concentrations (Carmona et al, 2008). Another technology to purify glycerol with high salt content is by using an ion-exchange-resin Ambersep BD50 (Lancrenon et al., 2008).
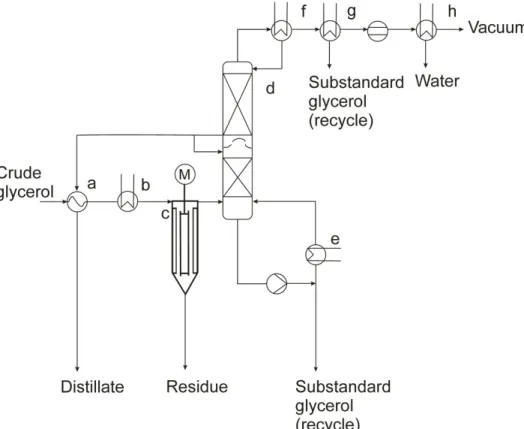
The subsequent step will be based on the treatment of glycerol in multiple vacuum flash evaporators (10-15 kPa), which results in 90-95 %(m/m) glycerol (see Figure 1.1.2.1). An alternative way to do the same job is to use thin film distillation (see Figure 1.1.2.2). In thin film distillation, the glycerol stream is distributed as a thin film on the wall of the evaporator and heated externally. Glycerol will fall down to the bottom of the evaporator as a residue while high volatile components including methanol and water are evaporated and collected at the top. The final concentration of glycerol to 99.5 %(m/m) is carried out under vacuum (0.5-1 kPa) in forced circulation evaporators (Christoph et al., 2006).
21
Figure 1.1.2.1 Continuous glycerol concentration: a) Feed heater, b) Evaporator, c) Separator with demister, d) Water condenser, e) Glycerol heater, f) Glycerol heater/final product cooler, g) Falling film evaporator, h) Glycerol condenser
Figure 1.1.2.2 Continuous glycerol distillation (Cognis Deutschland GmbH, Düsseldorf, Federal Republic of Germany): a) Economizer b) End heater c) Thin-film distillation d) Fractionating Column e) Reboiler f) Reflux Condenser
g) Glycerol condenser h) Water condenser
1.1.3 Recent Development in Glycerol Purification Processes
John E. Aiken (2006) reported various improvements in glycerol purification processes. He proposed five separation steps, which can either be conducted under batch or continuous conditions (Figure 1.1.3.1). This process is claimed to be able to produce glycerol of higher than 99.5 %(m/m) purity from typical crude glycerol containing a mixture of mono-, di- and triglycerides, excess methanol, water, fatty acid alkyl esters, quantities of residual catalyst and salts.
i) First reactor
Crude glycerol, whose purity is typically 86-92 %(m/m), is preheated and then fed to the first reactor, which is generally used to recover triglycerides by reacting entrained methyl esters and glycerol to produce glycerides and methanol (reversed biodiesel
water; thus, the reaction is shifted to glycerides formation. The temperature inside the reactor is maintained at 120-160 °C. A gas effluent stream is then passed through a condenser. Upon separation from condensed methanol and water in a condenser, nitrogen is then recycled to the reactor.
ii) Second reactor
The liquid effluent stream from the first reactor is heated to maintain the second reactor at 120-160 °C. In this reactor, the unreacted methyl esters are reacted to produce methanol and triglycerides. Wash water (containing glycerol) is also added to the second reactor. Similarly, sparging nitrogen is used to stir the mixture inside the reactor and to remove methanol and water. Entrained methanol and water are condensed. After being separated from nitrogen, wash water is recycled. The operating conditions are adjusted in such a way that the glycerol effluent stream contains a maximum of 0.5 %(m/m) of methanol and approximately 5 %(m/m) of water.
iii) Decanter
A decanter is placed after the second reactor. It serves as a feed tank for the flash distillation column and a separator to remove the oil layer of the glycerol stream by lowering the pH below 7 and skimming it from the glycerol layer. The recycle stream from the bottom of the flash distillation column is mixed with the glycerol stream in this tank.
iv) Flash distillation column/stripper
The flash distillation column consists of a packed bed column with a steam-heated reboiler. This column operates at a temperature of 185 °C and a pressure of 0.6-2.6 kPa.
There is no reflux returned to the top of the column. About 80-90% of glycerol in the feed stream is drawn as overhead product, which is then condensed in two condensers in series.
The first condenser is utilised to condense glycerol, while the second condenses water, which will be sent to a waste water stream.
The bottom product of the column, which contains glycerol and heavy compounds, is pumped back to the decanter. Some of it is purged continuously or intermittently to prevent salts and glycerol build-up in the decanter.
Figure1.1.3.1 Simplified flow sheet of the recent development process for glycerol purification, based on the US patent 7,126,032 B1(Aiken, 2006)
v) Adsorption columns
The last step of glycerol refining is the removal of color bodies and trace impurities. Many different materials may be used as adsorbents, including activated carbons, ion exchange resins and molecular sieves. The purified glycerol is then pumped to a storage tank.
1.2 Transformation of Glycerol into High-quality Products
Glycerol has been known since 2800 BC mainly as a by-product of soap production (Hunt et al., 1999). Glycerol production comes mainly from countries with significant oleochemical industries such as United States, Europe, Japan, and South-East Asia. The United States, Japan and more recently, China, are the major importers of glycerol while Europe and South-East Asia region are the dominant exporters of glycerol (Bournay et al., 2005).
Glycerol is most commonly used without modification, or very basic structural modifications, as an additive to materials. Its uses number in the thousands with large amounts being used in the manufacture of food and beverages, tobacco, pharmaceuticals, personal care products, urethane foams, and synthetic resins. Although the personal care industry has seen an increasing demand for glycerol due to consumer desire for eco- friendly “natural” products, this field is relatively mature.
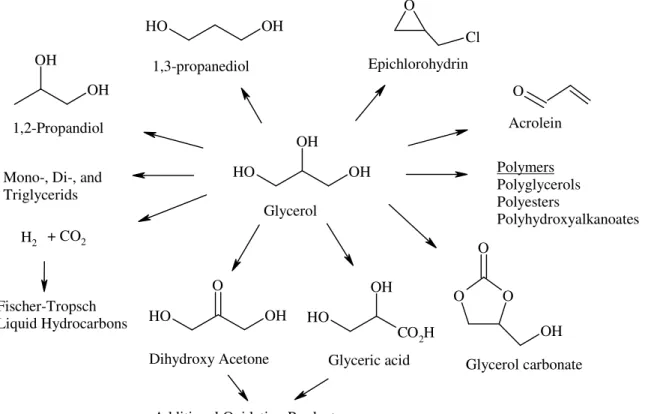
Currently, industry, government and academe are increasing their efforts to develop new and improve existing glycerol chemistry. Figure 1.2.1 depicts some of the platform chemicals that can be derived from glycerol(Kenar, 2007).
As shown, various reactions based on chemical and biochemical oxidation, reduction, bond breaking, and polymerization reactions are available to derive these value added chemicals of commercial interest from the glycerol molecule. Because of the high functionality of glycerol (two primary and one secondary hydroxyl group), reactions can proceed along multiple reaction pathways to give mixtures of products. Therefore, careful development of catalysts and reaction conditions is of paramount importance to selectively obtain the desired products. Continued research should improve these aspects and provide new opportunities to better utilize glycerol and its products as many of these materials have high costs associated with their current preparation methods.
O
H OH
OH O
H OH
O
Cl
O
O O
O
O OH H
OH CO2H O
H OH
O OH
OH 1,3-propanediol Epichlorohydrin
Acrolein Polymers Polyglycerols Polyesters
Polyhydroxyalkanoates
Glycerol carbonate Dihydroxy Acetone Glyceric acid
Additional Oxidation Products H2 + CO2
Fischer-Tropsch Liquid Hydrocarbons
Mono-, Di-, and Triglycerids
1,2-Propandiol
Glycerol
Figure 1.2.1 Platform chemicals derived from glycerol (Kenar, 2007)
Glycerol plays an important role in nearly every industry. Glycerol is used very extensively in the pharmaceutical industry. Because of its valuable emollient and demulcent properties, glycerol is an important ingredient in innumerable pharmaceutical and cosmetic preparations. Glycerol is used as a solvent in the preparation of tinctures. As a humectant, glycerol constitutes an important pharmaceutical ingredient to prevent the drying out of preparations, particularly ointments and creams. Since it is a sweet-tasting liquid it is used as a sweetening agent to impart sweetness to a preparation. It is used as a levigating agent to reduce the particle size of a drug powder.
Due to its preservative qualities, it is used as a stabilizer and an auxiliary solvent in conjunction with water or alcohol. Glycerol is also used in the pharmaceutical industry to extract and prevent inert materials from precipitating upon standing. It is used as a plasticizer to enhance the spread of the coat over tablets, beads and granules.
In the food industry, glycerol is an important moistening agent for baked goods. It is also added to candies and icings to prevent crystallization. Glycerol is used as a solvent
for food colors and carrier for extracts and flavouring agents. The smoothness of lotions, creams and toothpaste is due to the presence of glycerol.
Because of its humectant properties, glycerol is sprayed on pre-processed tobacco to prevent crumbling. With dibasic acids, such as phthalic acid, it reacts to make the important class of products known as alkyd resins, which are used as coatings and in paints.
Glycerol draws water from its surroundings and the heat produced by the absorption makes glycerol feel warm. Due to this property, glycerol is added to adhesives and glues to keep them from drying too fast. Many specialized lubrication problems have been solved by using glycerol or glycerol mixtures. Many millions of pounds of glycerol are used each year to plasticize various materials, like sheets and gaskets. The flexibility and toughness of cellophane, meat casings and special quality papers can be attributed to the presence of glycerol (Pagliaro et al., 2007; Pagliaro et al., 2008).
The chemical industry uses glycerol in the manufacture of sealing compounds and antifreeze. Glycerol is a major starting material for nitroglycerine also called nitroglycerol, which is used in the manufacture of dynamites and propellants. Nitroglycerine is also an active ingredient in pain-relieving drugs for heart patients. A large variety of mono- and diesters of higher fatty acids are commercially manufactured from glycerol. These esters are used as emulsifiers in foods, preparation of baked goods and modification of alkyd resins.
The field of green chemistry is defined by a set of broad principles which focus on reducing environmental impact. A major problem the world currently faces is the disposal of waste, and the green chemistry vision primarily is that waste generation should be prevented and, if not possible, then valorisation methods should be implemented. One method of reducing waste is to incorporate all materials used into a final useable product.
The biodiesel industry currently regards glycerol as a waste by-product; however with novel methods glycerol has the potential to be converted into high value products.
1.2.1 Glycerol transforming processes 1.2.1.1 Aqueous phase reforming
One of the major achievements in glycerol chemistry is the development of aqueous phase reforming process (APR) which involves the conversion of glycerol to hydrogen and carbon monoxide (Synthesis Gas). The process conditions are 250 oC using a Pt-Re catalyst in a single reactor (Soares et al., 2006). This process can also produce high yields of hydrogen from glycerol at low CO concentrations due to favourable water-gas shift (WGS) thermodynamics. This requires significantly lower energy consumption than traditional methane reforming.
1.2.1.2 Fischer-Tropsch
The synthesis gas derived from glycerol can be used as a building block for chemicals and fuels using the Fischer-Tropsch reaction (Gupta et al., 2012). The syngas is converted to useful straight chained alkanes using iron and cobalt catalysts however under certain conditions alkenes and alcohols can be produced. The temperatures used in the process range from 150 °C to 300 oC and pressures of one to a few atmospheres are common. High temperatures lead to small alkanes whereas lower temperatures and high pressures favour longer chain alkanes.
1.2.1.3 Selective reduction
The main processes used to reduce glycerol to glycols are hydrogenolysis, dehydroxylation and biological reduction.
Propylene glycol is commercially produced via hydrogenolysis using a copper chromite catalyst at 200 oC with a pressure below 10 bar. Wang and others (2003) showed that it was possible to produce 1,3-propanediol (PDO) via selective dehydroxylation. The central hydroxyl group of glycerol is selectively converted to a tosyloxyl group which is removed using hydrogenolysis. The biological reduction to 1,3-propanediol involves the use of bacterial strains from groups such as Citrobacter, Enterobacter, Ilyobacter, Klebsiella, Lactobacillus, Pelobacter and Clostridia. In 1881 it was shown by Freund (Biebl et al., 1999) that PDO could be produced using Clostridia, a widely available microorganism found in nature. The process involves a two-step enzyme-catalysed reaction sequence in
which dehydratase catalyses the conversion of glycerol to 3-hydroxypropionaldehyde and this is then reduced to PDO by NAD+-linked oxidoreductase.
1.2.1.4 Halogenation
The chlorination of glycerol yields an important and valuable chemical called epichlorohydrin via 1,3-dichloro-2-propanol. 1,3-dichloro-2-propanol can be produced directly from glycerol using HCl as a catalyst and subsequent dehydrochlorination using NaOH to generate epichlorohydrin and NaCl.
1.2.1.5 Dehydration
The dehydration of glycerol can produce important chemicals such as acrolein, 3- hydroxypropionaldehyde and acrylic acid. When glycerol is protonated it is more susceptible to dehydration because the energy barrier is reduced therefore acrolein can only be produced in acidic conditions. The reaction can be conducted in the liquid or gas phase under high temperatures and/or vacuum to drive the dehydration. In the presence of molecular oxygen acrylic acid can be produced via a one-step oxydehydration step.
1.2.1.6 Etherification
Glycerol alkyl ethers can be synthesized by etherification of alkenes such as isobutylene in the presence of an acid catalyst at temperatures from 50 oC to 150 oC (Klepácová et al., 2007). The typical molar ratios used in the reaction are 1:2 (glycerol:isobutylene) and the yield can be improved by optimising the reaction conditions.
Glycerol can be etherified to form polyglycerol via anionic polymerisation of glycidol through a cation exchange equilibrium initiated by partially deprotonated 1,1,1- tris(hydroxymethyl) propane (Haag et al., 2000). The resulting polymer usually has a polydispersity of below 1.5 and a molecular mass ranging from 1000 to 3000 g⋅mol-1. 1.2.1.7 Selective oxidation
The oxidation of glycerol can be catalysed using highly active aerobic catalysts such as platinum and palladium. Supported gold catalysts are well known for catalytic stability, resistance to oxygen and tolerance against inhibition by aliphatic and aromatic amines. Organocatalysts such as 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) can be
TEMPO has also been used in electrochemical oxidation where glycerol is converted to 1,3 dihydroxyacetone (DHA). The reaction proceeds by applying a small electric potential to a solution containing glycerol, water and 15 %(n/n) TEMPO using a glassy carbon anode (Ciriminna et al., 2006). DHA can also be produced using biological oxidation via microorganisms or enzymes. Other oxidation products include glyceraldehydes, glyceric acid, glycolic acid, hydroxypyruvic acid, oxalic acid and tartronic acid.
1.2.1.8 Pyrolysis
Glycerol was identified as a feedstock for pyrolysis in 1985 (Antal et al., 1985), well before the growth in the biodiesel market. Recent research by Valliyapan and others (2008) has focused on optimising conditions for hydrogen or syn-gas production. Pyrolysis carried out in a continuous down-flow fixed bed microreactor can occur with flow rates of nitrogen from 30-70 ml/min, temperatures of 650-800 oC and at atmospheric pressure. It was shown that the type and size of packing material in the tubular reactor can affect the conversion of glycerol and subsequent product distribution. Typical products include carbon monoxide, hydrogen, carbon dioxide, methane and ethane. At lower temperatures {steam (Antal et al., 1985) or supercritical water (Bühler et al., 2002)} longer molecules such as acrolein, formaldehyde and acetaldehyde are observed.
1.2.1.9 Biotransformation
Glycerol can be converted to a very large number of chemicals using micro- organisms and enzymes. The aerobic conversion of glycerol to 3-hydroxypropionaldehyde (3-HPA) was reported in 1985 by Slininger and Bothast (1985). The cells of Klebsiella pneumonia can be grown on a rich glycerol medium and when added to a buffer containing semicarbazide and glycerol the production of 3-HPA starts. It was shown that a yield of up to 84% could be obtained however the production is sensitive to cell age and cultivation medium. The optimal processing conditions for this experiment are 32 oC, pH 7-8 and glycerol concentrations of 20-50 g/litre.
1.2.2 Glycerol esters
Glycerol molecule is symmetrical and has many rotamers with intramolecular hydrogen bonding, but the OH groups can be esterified into other derivates (Law et al., 2005).
Glycerol can be esterified with carboxylic acids or via carboxylation and nitration (Budarin et al., 2007). Reaction with carboxylic acids may result in monoacylglycerols, diacylglycerols and triacylglycerol. Monoacylglycerols are produced at commercial scale by either continuous chemical glycerolysis of fats and oils (250 oC, alkaline, N2
atmosphere) or by direct esterification with fatty acids (Sonntag, 1992). The reaction of glycerol with dimethyl carbonate produces a high yield of glycerol carbonate in the presence of a catalyst. Glycerol can be converted to glycidyl nitrate by treatment with nitrating and cyclising agents which subsequently can be polymerised to form a valuable polymer.
Oxygen-containing components prepared by esterification of glycerol with organic acids (e.g. acetic acid) or anhydrides (e.g. acetic anhydride) could have suitable properties for use as solvents or as oxygenates in fuels.
Unfortunately, synthesis routes of glycerol esters starting from crude glycerol were not found in the literature.
1.2.2.1 Triacetin
The products of glycerol esterification with acetic acid are monoacetins (1- monoacetin, 2-monoacetin), diacetins (1,3-diacetin, 1,2-diacetin) and triacetin (Figure 1.2.2.1.1), which have great industrial applications.
The monoacetin is used as a food additive and in manufacturing explosives and smokeless powder (Nebel et al., 2008), also is valuable in pharmacochemistry and preparation of a specific antidote (Bernasconi, 1969).
The diacetin has been utilized as a cocoa butter blooming agent or as an intermediate in the synthesis of structural lipids (Watanabe et al., 2005), also is used for plasticizercoating and foodstuffs (Sastry et al., 1998; Baur, 1954).
The mixture of monoacetin and diacetin, have applications in cryogenics and biodegradable polyesters (Taguchi et al., 2000), chemical products in the food (Lal et al., 2006) and cosmetic industries (Baumann et al., 1988).
In terms of triacetin, apart from its use as fuel additives for increasing the octane number in gasoline (Delfort et al., 2005), it is reported to function as a cosmetic biocide, plasticizer, and solvent in cosmetic formulations.
It is a commonly used carrier for flavours and fragrances (Ogawa et al., 1992), and was affirmed as a generally recognized, as safe human food ingredient by the Food and Drug Administration.
Figure 1.2.2.1.1 Products of glycerol esterification by acetic acid
The conversion of glycerine to triacetin is a process that exists but that should increase its performance to be able to treat big quantities of glycerine in the most environmental friendly way (Galan et al., 2009).
Triacetin is commonly prepared by esterification of glycerol with acetic anhydride or acetic acid, or by reacting ketene with glycerol, or by the oxidation of allyl acetate in the presence of acetic acid. Crude triacetin typically contains acetic acid, acetic anhydride and smaller quantities of other impurities. Volatile impurities such as acetic anhydride and acetic acid are usually removed by distillation. The remaining triacetin is then usually
O
H OH
OH
AcO OH
OAc
AcO OH
OH
AcO OAc
OAc
O
H OH
OAc
AcO OAc
OH + AcOH
1-monoacetin
2-monoacetin
1,2-diacetin
1,3-diacetin
triacetin
+ H2O
distilled to remove nonvolatile impurities and to eliminate color and odor. However, distillation generally requires relatively high temperatures that initiate additional reaction products, and thus even after distillation, triacetin typically has an odor and a yellow color, both of which must be eliminated for the triacetin to qualify as pure triacetin (Khramov et al., 1998).
The most common way of triacetin manufacture is the acid-catalyzed reaction of glycerol with acetic acid (Figure 1.2.2.1.2). However the selectivity to triacetin is normally limited.
The normal boiling points of monoacetin, diacetin and triacetin are BpMA=258 °C, BpDA=259 °C, BpTA=258-260 °C respectively (Dunbar et al., 1956), therefore the separation of triacetin from mono- and diacetins by distillation is practically impossible.
That is the reason why it is required to find a triacetin synthesis having the highest selectivity.
Figure 1.2.2.1.2 Reaction scheme for esterification of glycerol with acetic acid The presence of water influences the equilibrium and weakens the acidity of the used catalyst. This may also effect the consecutive acetylation of the hydroxyl groups (Silva et al., 2010).
In the esterification reactions the homogeneous catalysts are very effective but require additional handling steps. It is imperative to remove catalysts before distillation, therefore, higher production costs have to be expected (Lotero et al., 2005). Appropriate fixed-bed catalysts could be incorporated into a packed-bed, continuous flow reactor, simplifying the product separation and purification, but also reducing the waste formation.
In esterification reactions of carboxylic acids with alcohols it is standard to use homogeneous catalysts like sulfuric acid, methane sulfonic acid, hydrofluoric acid or p- toluene-sulfonic acid. Usually, such homogeneous catalysts have to be removed by additional treatment steps during the process.
O
O O
O
O
O O
H OH
OH
H O2
H3C O OH
+
3+
3Therefore, heterogeneous catalysts are becoming more and more in the focus of the industry. The literature is containing numerous publications describing heterogeneous acid catalysts for esterification processes.
So for instance, good results were reported using ion-exchange resins like Amberlyst-15 or Nafion in esterification processes (Chen et al., 1999; Heidekum et al., 1999). Generally speaking, the catalytic activity of organic resins is strongly depending on their swelling properties. Resin swelling capacity is fundamental since it controls substrate accessibility to the acid sites and, therefore, affects its overall reactivity. Once swelled, the resin pores usually become macropores. This means that big molecules with long hydrocarbon chains show no diffusion limitations and can readily access the acid sites in the bulk.
There are some studies in the literature searching for catalysts for the reaction of glycerol and acetic acid, for instance Lu and Ma (1991) get 87% triacetin using acidic ion exchange resin and MgSO4 at room temperature for 72 h. Yang and Lu (1996) use SO42-
/ZrO2-TiO2, referring the best activity of the catalyst at 450 °C and Wu et al. (2007) get a yield of 93.6% at a reaction temperature of 130 °C with the same catalyst. Hou et al.
(1998) use aminosulfonic acid with a yield higher than 90%, Zhang (1999) determines the optimal conditions for the ratio glycerol/acetic acid/catalyst (SnCl4.5H2O/C) obtaining over 96% yield. Zhang and Yuan (2001) use phosphotungstic acid as the catalyst and with the optimum reaction conditions of mass ratio of catalyst to reactant 3.8%, reaction temperature 135–155 °C and reaction time of 7 h, get up to 84.6%.
For Ding et al. (2003), using H3PW12O40 as catalyst, the product yield and purity reached >98% and >99%, respectively. Dong and Guo (2003) use solid sulfated Fe2O3/TiO2, easily recovered and reused. Melero et al. (2007) obtain the best performances (up to 90% of glycerol conversion) using sulfonic acids catalysts within 4 h reaction time, Liu et al. (2007) use p-toluenesulfonic acid/C reaching a product yield of 92%, Li et al.
(2007) use ionic liquids ([HSO3-pmim][PTSA]) and the optimum conditions were obtained at a glycerol/acetic acid ratio of 1:8, catalyst amount 10.5% the total mass of reactants, reaction time 6 h and reaction temperature 120 °C.
Deng et al. (2001) investigated ionic liquid 1-butylpyridinium chloride–
aluminium(III) chloride as green reaction medium for esterification of glycerol with acetic acid. The yields of total monoacetins and triacetin reached a minimum (17.1%) and maximum (24%), respectively, at ca. 75 °C for ionic liquid as catalyst in case of
glycerol/acetic acid ratio of 1:3. The outstanding advantage is that the resultant esters may not dissolved in the ionic liquid and therefore they could be isolated easily.
Liao et al. (2009) carried out esterification of glycerol with acetic acid over resin and zeolites. After 4 hours operation time under optimal reaction condition (at a temperature of 105 °C and an acetic acid to glycerol molar ratio of 9:1), the selectivity of triacetin reaches almost 100% in 15 min by adding thereto acid anhydride.
Several patents propose various approaches for triacetin synthesis in the presence of catalysts, such as Bremus et al. (1981), Gawrikow et al. (1982), Pechenev et al. (1995), Mitsuya and Ogawa (1996), Mhaskar and Kulkarni (2002).
Some of the strategies to favour the formation of triacetin using the Le Chatelier- Braun principle are: to use a large excess of acetic acid, eliminate the water by reaction with acetic anhydride generating acetic acid or eliminate the water by simple distillation (Galan et al., 2009).
Figure 1.2.2.1.3 Preparation of Gliperol
The pursuit of a biofuel that integrates the glycerol is currently a target of high interest. Gliperol is a relatively new biofuel, which consists of a mixture of three molecules of FAME and a molecule of triacetin (Kijeński et al., 2004). It can be obtained after the transesterification of a mole of TG with three moles of methyl acetate (see Figure 1.2.2.1.3) using lipase as a catalyst (Xu et al., 2003; Xu et al., 2005; Du et al, 2004;
Kijeński et al., 2004; Kijeński et al., 2007).
R2 O
O R3 O
R1 O O
O
O
O O
O O
O O
O
R1 O O
R2 O O
R3 O O
+ 3 +
Triglyceride Methyl acetate Triacetin Fatty acid methyl esters
1.2.2.2 Tripropionin
The products of glycerol esterification with propionic acid are monopropionins (1- monopropionin, 2-monopropionin), dipropionins (1,3-dipropionin, 1,2-dipropionin) and tripropionin (see Figure 1.2.2.2.1), which have great industrial applications.
Figure 1.2.2.2.1 Products of glycerol esterification by propionic acid
Di- and tri-propyl esters of glycerol are potential oxygenate-additives to diesel fuels because of their good properties as blending components, but also having excellent solubility in diesel fuel.
Mono-propyl ester of glycerol is more polar and has a low solubility in diesel fuel.
Moreover, the boiling point of monopropionin, dipropionin and tripropionin are BpMP = 266.2 °C, BpDP = 292.0 °C, BpTP = 290.7 °C, respectively (Guidechem), so the separation of tripropionin from mixture containing mono-, di- and tripropionin by simple distillation
OH O O
H OH
OH
O O
O
O O
O
O OH
O
OH
O O
O
OH
O
O OH
O
O O
O
H OH
O O
+
1-monopropionin
2-monopropionin
1,2-dipropionin
1,3-dipropionin
tripropionin
+ H2O
is difficult. Therefore the esterification of glycerol must be directed to the maximum yield of tri-ester.
In the literature only patents were found about the synthesis of tripropionin. The oldest patent from 1935 relates to the manufacture of tripropionin by esterification of glycerol with propionic acid (see Figure 1.2.2.2.2) in presence of catalyst, and more particularly to the manufacture of tripropionin by such esterification, in which the water formed is removed by azeotropic distillation with a water-withdrawing agent (Hull, 1935).
Figure 1.2.2.2.2 Reaction scheme for esterification of glycerol with propionic acid
1.2.2.3 Glycerol carbonate
Glycerol carbonate is a stable and colorless liquid that offers useful applications as a novel component of gas separation membranes, a surfactant component, a new solvent for several types of materials or a nonvolatile solvent in the paint industry, a component in coatings, and a component of detergents. Also glycerol carbonate can be utilized as a source of new polymeric materials (Kim et al., 2007).
As a chemical intermediate it reacts readily with alcohols, phenols and carboxylic acids with loss of CO2 as well as with aliphatic amine with carbon dioxide recovery.
Glycerol carbonate can be obtained according to various methods, using epoxy compounds as well as glycerol as raw materials (Rokicki et al., 2005).
The direct synthesis of glycerol carbonate by the conversion of glycerol with carbon dioxide is an interesting target of scientific research groups (see Figure 1.2.2.3.1).
O
H OH
OH
O O
O O
O O
H O2
OH O
+
3+
3O
H OH
OH
O O
O
O H
Glycerol carbonate Glycerol
+ CO2 + H2O
Carbondioxide Water
Aresta et al. (2006) have investigated this reaction using di(n-butyl)tin dimethoxide, di(n-butyl)tin oxide and tin dimethoxide as catalysts. Under the investigated reaction conditions (50 bar CO2, 180 °C) tin dimethoxide led only to traces of glycerol carbonate.
However, di(n-butyl)tin dimethoxide led to a conversion of glycerol of maximum 7%
depending on the reaction conditions. No solvent was used and molecular sieves were added in order to remove water from the gas phase. Using di(n-butyl)tin oxide as catalyst conversions of glycerol up to 2% were reported.
The glycerolysis of urea (see Figure 1.2.2.3.2) represents an interesting synthetic procedure for glycerol carbonate that may have an industrial application. γ-Zirconium phosphate shows a good activity as catalyst as it affords 80% of conversion of glycerol under mild reaction conditions (Aresta et al., 2009).
Figure 1.2.2.3.2 Synthesis of glycerol carbonate from glycerol and urea
Glycerol carbonate can be synthesized from renewable glycerol and dimethyl carbonate (see Figure 1.2.2.3.3) using lipase in solvent-free reaction system. Lee et al.
(2010) have tested a variety of lipases for their abilities to catalyze transesterification reaction, and Candida antartica lipase B and Novozyme 435 exhibited higher catalytic activities. The glycerol-coated silica gel with a 1:1 ratio (Berger et al., 1992) was supplied to prevent two-phase formation between hydrophobic dimethyl carbonate and hydrophilic glycerol. Glycerol carbonate was successfully synthesized with more than 90% conversion from dimethyl carbonate and glycerol with a molar ratio of 10 using Novozyme 435- catalyzed transesterification at 70 °C. The Novozyme 435 (5 %(m/m) and 20 %(m/m)) and silica gel were more than four times recycled with good stability in a repeated batch operation for the solvent-free synthesis of glycerol carbonate (Lee et al., 2010).
O
H OH
OH
N
H2 NH2 O
O O
O
O H
+ + 2 NH3
Glycerol Urea Glycerol carbonate Ammonia