ContentslistsavailableatScienceDirect
Journal of Pharmaceutical and Biomedical Analysis
jo u r n al ho me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / j p b a
Polysaccharide-based chiral stationary phases as efficient tools for diastereo- and enantioseparation of natural and synthetic Cinchona alkaloid analogs
Attila Bajtai
a, István Ilisz
a,∗, Róbert Berkecz
a, Ferenc Fülöp
b, Wolfgang Lindner
c,∗, Antal Péter
aaInstituteofPharmaceuticalAnalysis,InterdisciplinaryExcellenceCentre,UniversityofSzeged,H-6720,Szeged,Somogyiutca4,Hungary
bInstituteofPharmaceuticalChemistry,InterdisciplinaryExcellenceCentre,UniversityofSzeged,H-6720,Szeged,Eötvösutca6,Hungary
cDepartmentofAnalyticalChemistry,UniversityofVienna,Währingerstrasse38,1090,Vienna,Austria
a rt i c l e i nf o
Articlehistory:
Received14September2020
Receivedinrevisedform19October2020 Accepted20October2020
Availableonline29October2020
Keywords:
HPLC
Cinchonaalkaloids Diastereoseparation Enantioseparation
Polysaccharide-basedchiralstationary phases
a b s t ra c t
Inthisstudy,wepresentresultsobtainedonthediastereo-andenantioseparationofsomebasicnatural andsyntheticCinchonaalkaloidanalogsbyapplyingliquidchromatographic(LC)andsubcriticalfluid chromatographic(SFC)modalitiesonamyloseandcellulosetris-(phenylcarbamate)-basedstationary phasesusingn-hexane/alcohol/DEAorCO2/alcohol/DEAmobilephasesystems.Sevenchiralstationary phasesintheirimmobilizedformwereemployedtoexploretheirstereoselectivityforaseriesofclosely relatedgroupofanalytes.ThemostimportantcharacteristicsofLCandSFCsystemswereevaluated throughthevariationoftheappliedchromatographicconditions(e.g.,thenatureandcontentofthealco- holmodifier,theconcentrationofadditives,temperature).ThecolumnsChiralpakICandIGturnedoutto bethebestinbothLCandSFCmodalities.Temperature-dependencestudyindicatedenthalpy-controlled separationinmostcases;however,separationcontrolledbyentropywasalsoregistered.
©2020TheAuthor(s).PublishedbyElsevierB.V.ThisisanopenaccessarticleundertheCCBYlicense (http://creativecommons.org/licenses/by/4.0/).
1. Introduction
Both natural and synthetic Cinchonaalkaloids have a rather complexstructuralpatternwithmorethanthirtyrepresentatives, amongthesequinine(QN),quinidine(QD),cinchonidine(CD),and cinchonine(CN)representthemaincomponents[1].Inadditionto thepharmaceuticalrelevanceofthemainalkaloidsandderivatives thereof,theymayalsoserveascatalystsinstereo-directedorganic synthesis[2],andaschiralselectorsinthecourseofthedevelop- mentofchiralstationaryphases(CSPs)forhigh-performanceliquid chromatography(HPLC)[3].Besides,quininehasalongtradition tobeappliedasaflavorcomponentinbitterbeverages.InTable1 thechemicalstructuresofnaturalandsyntheticCinchonaalkaloids appliedinthisstudyaredepicted.Withtheexceptionofracemic quinine,theotheranalytepairsQN/QD,DHQN/DHQD,CD/CN,and epi-QN/epi-QD arediastereomers toeach other,although often termedpseudo-enantiomers(Table1).QNandQDaswellasCDand CNarediastereoisomerictoeachotherwhich,inprinciple,eases
∗Correspondingauthors.
E-mailaddresses:ilisz@pharm.u-szeged.hu(I.Ilisz), wolfgang.lindner@univie.ac.at(W.Lindner).
their separation. Analytically, HPLC methods are often applied forthispurposeusingpreferentiallynon-chiralstationaryphases, assummarized earlierbyMcCalley [4]. Recently, capillaryelec- trophoretic[5]andHPLC-based[1,6]methodshavebeendescribed fortheseparationofthefourmajorCinchonaalkaloidsQN,QD,CD, andCNaswellasdihydroquinine(DHQN)anddihydroquinidine (DHQD).Alongthisline,thequantitativedeterminationofsixmajor alkaloidsimplementingsupercriticalfluidchromatography(SFC) hasrecentlyalsobeenreported[7].
Inprinciple,itisnotnecessarytouseCSPstoresolvethegiven fourpairsofdiastereoisomers(seeTable1).Nevertheless,itisstill ofinteresttoexaminetheirstereoselectivemolecularrecognition patterninthecontextoftheresolution oftheseanalytes.How- ever,fortheresolutionofenantiomericanalytes,theapplication ofaCSPismandatory.Aswehavehadaccesstoracemicquinine (rac.QN)producedthrougha novel,fullysyntheticway[8], we hadtheopportunitytoinvestigatethechromatographicresolu- tionofrac.QNversusthediastereomericpairQN/QDandoftheir epimers(9-epi-QN,9-epi-QD).Therefore,theimpactofthechiral environmentofthediversepolysaccharide-typeCSPsontheoverall stereoselectivityparameterscouldbeexplored.
https://doi.org/10.1016/j.jpba.2020.113724
0731-7085/©2020TheAuthor(s).PublishedbyElsevierB.V.ThisisanopenaccessarticleundertheCCBYlicense(http://creativecommons.org/licenses/by/4.0/).
Table1
StructureofnaturalandsyntheticCinchonaalkaloids.
Name Abbreviation Configuration Structure
(+)Quinine (+)QN (+)(1R,3S,4R,8R,9S)
(-)Quinine (-)QN (-)(1S,3R,4S,8S,9R)
(+)Quinidine (+)QD (+)(1S,3R,4S,8R,9S)
(-)9-epi-Quinine (-)9-epi-QN (-)(1S,3R,4S,8S,9S)
(+)9-epi-Quinidine (+)9-epi-QD (+)(1S,3R,4S,8R,9R)
(-)Dihydroquinine (-)DHQN (-)(1S,3R,4S,8S,9R)
(+)Dihydroquinidine (+)DHQD (+)(1S,3R,4S,8R,9S)
(-)Cinchonidine (-)CD (-)(1S,3R,4S,8S,9R)
(+)Cinchonine (+)CD (+)(1S,3R,4S,8R,9S)
InanearlierpaperHoffmannetal.[9]studiedthestereoselective resolutionofQN/QDandCD/CNonachiralcationexchangerfol- lowedbyanotherrecentinvestigationbyBajtaietal.[10]applying chiralzwitterionicCSPs.Intermsofintermolecularlydriveninter- actionmechanismbetweenthechiralselector(SO)motifsofthe CSPsandthechiralanalytes(selectands,SAs),theionexchanger- type CSPs investigated previously and the polysaccharide-type CSPs examined in the present study (see Fig. S1) belong to structurallyentirelydifferentCSPswithrespecttoretentionand stereoselectivitycharacteristics.Inthisstudy,weaimedtotrace thespecificsofthepolysaccharideCSPsusedinnormal-phaseliquid chromatography(NP LC)andinSFCmodalitiesfortheseparation ofacloselyrelatedgroupofCinchonaalkaloids(seeTable1).
Polysaccharide-based PS-CSPs usually displaya great variety ofenantio-,diastereo-,andchemoselectivity[11–17].Avarietyof robustCSPswithverywidespectraofapplicationswereobtained viatheimmobilizationofsubstitutedamylose-andcellulose-based selectors[14,12–17].However,nodatawerefoundintheliter- aturefor theseparationofsomenaturalandsyntheticCinchona alkaloidsandtheirderivativesonPS-CSPs.Themainobjectiveof thepresentpaperistorevealsomegeneraltendenciesofasetof prominentPS-CSPs (Fig.S1)forthediastereo-andenantiosepa- rationofthesetofchiralanalytes(Table1)underNP-LCandSFC conditions.TheinvestigationofstereoselectivitycriteriaofSOsand SAswasinthefocusofthisstudy.Morespecifically,ofthethree isobaricpairsofstereoisomers,QN/QDand9-epi-QN/9-epi-QDare diastereomerictoeachother,characterizedbythechangeofonly thestereogeniccentersofC-8and C-9,whilethe1S,3R,and4S stereogeniccentersofthequinuclidineringresidueremaincon- stant. Forthetruly racemicQN, allfivestereogeniccenters are oppositetoeachother.ForthetwodiastereomericDHQN/DHQD andCD/CNpairs,thediastereomericbehavioroftheanalytesisthe sameasforQN/QD.SeeTable1formoredetails.
Methodological andexperimentalfactors,suchasthenature and theconcentrationofthedifferentmodifiers(alcohol,water, acidorbase)andmobilephasecompositionsinNP-LCandSFC,as
wellasthestructureofPS-CSPsandSAsandthetemperaturewere evaluatedonretention,selectivity,andresolutionofstereoisomers withspecialattentionwithrespecttoelutionsequences.
2. Materialsandmethods 2.1. Chemicalsandreagents
(–)QN, (+)QD, and (–)-1011-(–)DHQN were purchased from Buchler(Braunschweig, Germany). (+)-1011-DHQD, (+)-CN, and (–)-CDwere fromSigma-Aldrich(Vienna,Austria). RacemicQN [1:1 mixtureof (–)QN and (+)QN]wasa generous giftfrom N.
Maulidesynthesizedasdescribedin[8].C9-Epiquinine(–)epi-QN andC9-epiquinidine(+)epi-QDweresynthesizedasdescribedin [18](Table1).
Methanol(MeOH),ethanol(EtOH),andn-hexaneofHPLCgrade werepurchased fromVWRInternational(Radnor,PA,USA).The alcoholadditives1-propanol(1-PrOH),2-propanol(2-PrOH),the base additivediethylamine (DEA), and the acid additive acetic acid(AcOH)allanalyticalreagentgrades,werefromVWR.Liquid CO2 wasfromMesser(Budapest,Hungary).Ultrapurewaterwas obtainedfromUltrapureWaterSystem,PuranityTUUV/UF(VWR International).
2.2. Apparatusandchromatography
Threechromatographic systems were applied in this study.
ThefirstonewasaWatersBreezeapparatusconsistingofa1525 binary pump, a 487 dual-channel absorbance detector, a 717 plusautosampler,and acolumn thermostat.For datacollection Empower2datamanagersoftware(WatersCorporation,Milford, MA,USA)wasapplied.ALaudaAlphaRA8thermostat(LaudaDr.R.
WobserGmbh,Lauda-Königshofen,Germany)wasusedtoregulate columntemperature.
ThesecondliquidchromatographicsystemwasfromShimadzu, andit containeda low-pressurequaternary pump(LC-20AD), a
photodiodearraydetector(SPD-M20A),aModel7125injectorwith a 20-lloop (Rheodyne,Cotati,CA,USA), and LCSolutiondata acquisitionsystem(ShimadzuCorporation,TokyoJapan).Allexper- imentsinnormal-phasemode(NP)werecarriedoutunderisocratic conditionsataflowrateof0.6mLmin–1andatacolumntemper- atureof25◦C(ifnototherwisestated).
Thethirddevice,aWatersAcquityUltraPerformanceConver- genceChromatographyTM(UPC2,WatersCorporation)systemwas appliedforSFCstudieswithabinarysolventpump,anautosam- pler,abackpressureregulator,acolumnoven,andaphotodiode arraydetector.AnEmpower2softwarewasusedtosystemcontrol anddataacquisition.IneverycaseSFCwasperformedinisocratic modeataflowrateof2.0mLmin–1andacolumntemperatureof 40◦C(ifnototherwisestated).Theoutletpressurewassetat150 bar.ThemobilephasesappliedinSFCconsistedofliquidCO2and MeOH,EtOH,1-PrOHor2-PrOHindifferentratios(v/v)containing differentacidandbaseadditives.
Stocksolutionsoftheanalyteswerepreparedbydissolvingthe solidCinchonasamplesinMeOHor2-PrOHin1.0mgmL–1concen- trationandfurtherdilutedwhennecessary.Aninjectionvolume of20LwasappliedinLCand7LinSFC.InLCthedead-time ofcolumns(t0)wasdeterminedbyinjectionoftri-t-butylbenzene, whileinSFCmodethefirstnegativesignalbyinjectingMeOHwas used.AnalytesweredetectedbytheirUVabsorptionat215–230 nm.
Polysaccharide-based columns amylose tris-(3,5- dimethylphenylcarbamate) (Chiralpak IA), amylose tris-(3-chlorophenylcarbamate) (Chiralpak ID), amylose tris-(3,5-dichlorophenylcarbamate) (Chiralpak IE), amylose tris-(3-chloro-4-methylphenylcarbamate) (Chiralpak IF), and amylosetris-(3-chloro-5-methylphenylcarbamate)(Chiralpak IG) aswellascellulosetris-(3,5-dimethylphenylcarbamate)(Chiralpak IB) and cellulose tris-(3,5-dichlorophenylcarbamate) (Chiralpak IC)allwiththesamesize(250mm×4.6mmI.D.,5-mparticle size)weregenerousgiftsfromChiralTechnologiesEurope(Illkirch, France).AllCSPsemployedinthisstudybelongtotheimmobilized PS-typecolumns.ThestructuresofselectorsarepresentedinFig.
S1.
3. Resultsanddiscussion
3.1. EffectsofmobilephasecompositioninNP-LCandinSFC
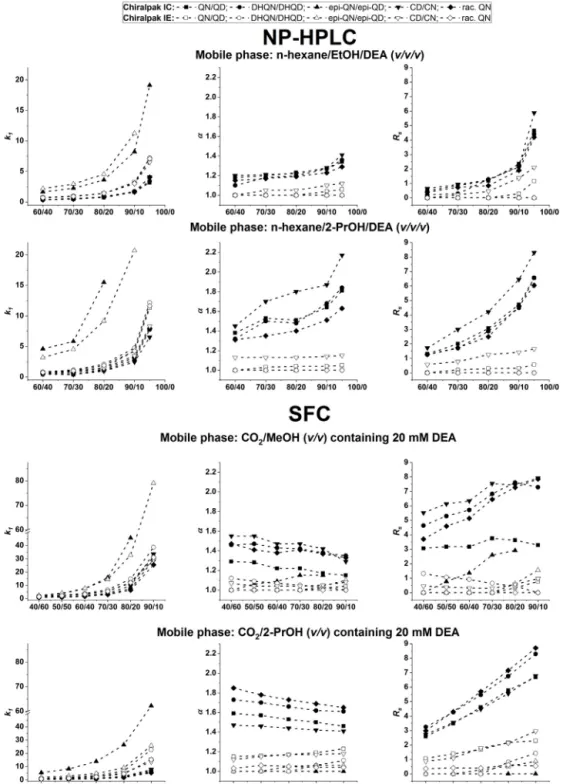
InthecaseofPS-CSPs,thegenerallyacceptedrecognitionmech- anismis basedontheinclusion ofchiral solutesintothechiral cavities ofthepolysaccharide-typeselectordrivenbyadditional attractiveforcessuchasH-bonding,dipole–dipoleand–inter- actions. Inaddition,therole ofsteric“hindrance” forthegiven SAs toenterdeep intothechiral grovesshouldalsobeconsid- ered[11–17].Toregulatetheoverallchromatographicretention, thenatureandconcentrationofanalcoholmodifierinbothnor- mal phase LC (NP LC) [19] and SFC [20] are often varied. To explorethepossibleeffectsofthealcoholmodifierinNP LCand SFC, two columnswere selected: the cellulose-based Chiralpak IC column (as the mosteffective CSP in thescreening process fortheinvestigatedconformationallyrestrictedanalytes)andthe amylose-basedChiralpakIEcolumn,bothpossessingthesamecar- bamatemodification(tris-3,5-dichlorophenylcarbamatemoiety)of thetwodifferentpolysaccharidebackbones.
Withvariationofthenatureofthealcoholforthestudiedana- lytes on the7 CSPsin NP-LC modality, a relatively similarbut slightincreaseink1wasregisteredintheEtOH<1-PrOH<2-PrOH sequencewiththeexceptionofepi-QN/epi-QD,whichwasretained moresignificantly(Fig.S2).Thebeststereoselectivityperformances (higher˛andRS)couldgenerallybeachievedwith2-PrOHonChi-
ralpakIC.InSFCmodalityonthesamecolumns,slightlyhigherk1 valueswereobtainedonmobilephasescontainingMeOHand2- PrOHcomparedtoEtOH(especiallyforepi-QN/epi-QD).For˛and RSthevariationsobservedwerequitesimilartothoseinNP-LC;
namely,ChiralpakIEexhibitedmuchlesseffectivenessthanChi- ralpakIC.Asexpected,underNP-LCandSFCconditionsanincrease oftheapolarcharacterofthealcohol,i.e.,applyingalcoholswith alongerorbranchedchainusuallyresultedinanenhancedana- lyteretention,selectivity,andresolution,especiallyonChiralpak IC(Fig.S2).Itshouldbementioned,thatoppositebehaviorshad alsobeenreportedintheliterature[21,22].Thechangeinenantio- anddiastereoselectivityresultingfromchangingthealcoholmodi- fierwaspreviouslyrationalizedasaresultofalterationofthesteric environmentofthechiralcavitiesbydifferentalcoholmodifiers dueto intra-andinter-molecular solvation effects [22].On the basisoftheseresults,furtherexperimentswerecarriedoutwith theapplicationofEtOHand2-PrOHinNP-LCaswellasMeOHand 2-PrOHinSFCconditions.
Foramorethoroughstudyoftheeffectsofalcoholconcentration onchromatographic parameters in NP LC,n-hexane/EtOH/DEA and n-hexane/2-PrOH/DEA (60/40/0.1–95/5/0.1 v/v/v) mobile phaseswereapplied.UnderSFCconditions,theMeOHand2-PrOH contentinliquidCO2wasvariedfrom10to60v%(with20mM DEAinallexperiments).Theamylose-andcellulose-basedcolumns appliedearlierwiththesameselector(ChiralpakICandChiralpak IE,seeFig.S1)wereselectedforthisstudy.Regardingtheretentive characteristics,atypicalNPbehaviorwasobservedforbothNP LC andSFCmodalities:increasingtheratioofapolarn-hexaneorCO2
resultedinanincreasedk1,especiallyfortheepi-QN/epi-QDpair (Fig.1).Itisnoteworthy,thatwiththeincreaseofthemobilephase polarity,thestrengthoftheH-bondsbetweentheanalytesandthe selectordecreasesandthesolubilityoftheanalytesinthemobile phaseincreases[23].
On Chiralpak IEthe stereoselectivity exhibited only a small enhancementinbothNP-LCandSFC modalitieswithincreasing n-hexaneorCO2 content.However,onChiralpakICinNP-LCin n-hexane/EtOH/DEAmobilephaseaslightincrease,whereasinn- hexane/2-PrOH/DEAmobilephaseamoderateenhancementinthe
˛valuewasregistered.InSFConChiralpakIC˛generallyslightly decreasedordidnotchangesignificantlywithincreasingCO2con- tent.Theepi-QN/epi-QDpair,again,wasanexceptionshowinga slightincreaseonChiralpakICinCO2/MeOH.Itshouldbenoted, thatslightlyhigher˛valueswereregisteredinCO2/2-PrOHthanin CO2/MeOHmobilephases.
RegardingRS values, in both NP-LC and SFC, theyincreased significantly onChiralpak IC and slightly on Chiralpak IEwith increasingofn-hexaneorCO2 content(althoughanunexpected exceptionwasfoundinSFCmodalityforDHQN/DHQDonChiralpak IE,Fig.1).Itisworthmentioningthatthechangeinthechromato- graphic performance caused by the alcohol modifier depended on the structure of the chiral selector as well; the cellulose- basedselectorfortheinvestigatedbasicanalytesoutperformedthe amylose-basedone.
Thealcoholmaybeincorporatedintothepolysaccharidestruc- ture, either into the cavities or between the polymer chains, affectingthetertiarystructureofthechiralpolymeritselfviasol- vationeffects[24].UnderLCconditions,themainadsorbingsites areconsideredtobethepolarcarbamateresidues[25] andthe differentinvolvementoftheNHandCOgroupsintheH-bonding processwerefoundtoberesponsibleforthedifferencesobserved inthestereoselectiveandenantioselectivebindingprocess[26].It isimportanttonote,thatinthisstudy10–60v%ofalcoholicmodi- fierwasemployedunderSFCconditions,i.e.,SFCisoperatedunder subcriticalconditions,wheresignificantdeviationsduetothedif- ferenceofthesetvaluesandactualoperationalconditionscannot beexpected[27].Besidesaffectingthephysicalpropertiesofthe
Fig.1.Effectofmobilephasecompositiononchromatographicparameters,retentionfactor(k),separationfactor(˛)andresolution(RS)fortheseparationofCinchona alkaloidanalogsonChiralpakICandIEcolumnsinNP-LCandSFCmodalities.
Chromatographicconditions:columns,ChiralpakICandChiralpakIE;mobilephase,fornormalphase,n-hexane/EtOH/DEAandn-hexane/2-PrOH/DEA(60/40/0.1–95/5/0.1 v/v/v);forSFC,CO2/MeOH(40/60–90/10v/v)containing20mMDEAandCO2/2-PrOH(40/60–80/20v/v)containing20mMDEA;flowrate,1.0mLmin–1;detectionat220 nm;temperature,25◦C;symbolsforChiralpakIC,䊏,QN/QD, ,DHQN/DHQD,,epi-QN/epi-QD,,CD/CNand ,rac.QN,forChiralpakIE,–,QN/QD;,DHQN/DHQD;, epi-QN/epi-QD;,CD/CNand♦,rac.QN.
eluent (e.g.,polarity,viscosity,anddensity),analterationofthe adsorptionlayershouldalsobeconsideredunderSFCconditions [27].
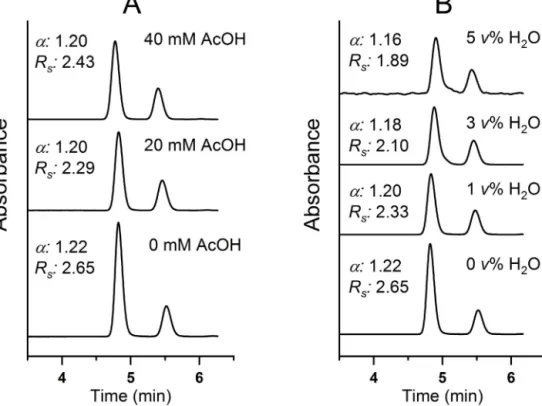
Besides thenatureand concentrationof thealcohol compo- nents,otheradditives(e.g.,acid,base,water)arealsofrequently appliedtomodify“chiral”resolutionondifferenttypesofCSPs.In SFC,beyondimprovementinpeakshape,additivesmayalsohave animpactonretention[28]andonthenumberoftheoreticalplates [29].Fig.2depictstheeffectsofAcOH(A)andH2O(B)additiveson
thechromatographicperformanceofChiralpakICwitheluentsys- temsCO2/MeOH(60/40v/v)containing20mMDEA,and0.0–40 mMAcOH(A)or0.0–5.0v%H2O(B)applyingtheQD/QNpairof diastereomersastestcompounds.Uponincreasingtheconcentra- tionoftheadditiveretentiondecreasedincaseofAcOHandslightly increasedincaseofwaterinparallelwithaminordecreaseof˛ andamarkedreductioninRSvalues(Fig.2),whilethechangein theefficiencywasusuallyintherangeof10–15%(datanotshown).
Theslightchangecausedbytheadditionofwaterwasprobably
Fig.2.EffectofAcOHandH2OcontentonthechromatographicperformanceofChiralpakICforanalyteQD/QNunderSFCconditions.
Chromatographicconditions:mobilephase,CO2/MeOH(60/40v/v)containing20mMDEAandA,0mM,20mMand40mMAcOH;B,0.0v%,1.0v%,3.0v%and5.0v%H2O;
flowrate,1.0mLmin–1;detectionat220nm;temperature,25◦C.
duetothelesspolarcharacteroftheCSPstudiedasobservedby Armstronget.al[29].
3.2. Effectofthestructureofpolysaccharide-typeselectors
The stereoselectivity characteristics of the studied Cinchona alkaloidsonthesevenPS-CSPswerestudiedwithdifferentmobile phase systems, and thecorresponding dataare summarized in Tables2–5.Duetothedifferenceinlinkageofglycopyranosemoi- eties inamylose-andcellulose-based selectors,theinteractions betweenanalyte andselectormaychangeresulting indifferent chromatographic behaviors,which mayeven affectthe elution orderoftheresolveddiastereomersandenantiomers.Thecontri- butionof thepolysaccharidebackbone canbeevaluatedbythe comparison of thechromatographicdata obtainedonthesame carbamateresidue;i.e.,tris-(3,5-dimethylphenylcarbamate)(Chi- ralpakIAvs.ChiralpakIB)andtris-(3,5-dichlorophenylcarbamate) (Chiralpak IE vs. Chiralpak IC), linked to amylose or cellu- lose,respectively.Tofacilitatethecomparisonbetweendifferent columns,chromatographicconditionswerekeptconstant,applying then-hexane/EtOH/DEAandthen-hexane/2-PrOH/DEA(80/20/0.1 v/v/v)mobilephasecompositionsinNP-LC.Inafewcaseswhena partialresolutionoccurred,theseparationwasfurtheroptimized.
DatasummarizedinTable2showthathigherretentioncouldbe obtainedonamylose-thanoncellulose-basedCSPsinmostcases.
Exceptions,however,doexistespeciallyformobilephasescontain- ing2-PrOH.
Itshouldbenoted,thatinNP-LC,despiteshorterretentiontimes, ChiralpakICprovedtobethemosteffectiveCSPintheseparationof thediastereomericpairsofbothCinchonaalkaloidsandofrac.QN.
InSFCmodalitytheeffectofthepolysaccharidebackbonewasalso evaluatedonthesamepairsofCSPsapplyingCO2/MeOHandCO2/2- PrOH(60/40v/v)mobilephasesallcontaining20mMDEA(Table3).
Regardingretention,theresultswithChiralpakIEandICweresimi- lartothedataobtainedinNP-LC;i.e.,theamylosebackboneoffered
higherretentions.ChiralpakIAandIB,inturn,gaveoppositeresults, withthecellulose-basedCSPprovidinghigherretentions.Among theabove-mentionedfourcolumnsunderSFCconditions,thebest separationswereachievedwithChiralpakIC,similartothosefound inNP-LC.
Thefundamentalstructuraldifferencesbetweenamylose-and cellulose-based tris-(3,5-dimethylphenylcarbamate) or tris-(3,5- dichlorophenylcarbamate) were found to be reflected in the stereochemicalrecognitionpatternsevenforsomediastereomeric analytes.Reversalofelutionorderbetweenamylose-andcellulose- basedCSPs containingthe same substituents wasregistered in NP-LCcontainingEtOHasmobilephaseforanalyteCN/CDonChi- ralpakIAvs.IB,andinmobilephasescontainingEtOHand2-PrOH onChiralpakIEvs.IC(Table4).Similarbehaviorswereregistered inSFCmodalityforQN/QDandCN/CDonChiralpakIEvs.IC(Table 2B).Examplesofreversedelutionordersofenantiomericanalytes onamylose-orcellulose-basedcolumnshavebeendescribedpre- viously[19].
Applyingconstantchromatographic conditions inNP-LC and SFCmodality,datalistedinTable2and3canprovideopportunity toevaluatetheeffectofthenatureofthesubstitutedphenylcar- bamatemoiety. BycomparingthedataobtainedwithChiralpak IAvs.IE(bothareamylose-basedCSPs),andIB vs.IC(bothare cellulose-basedCSPs),higherretentionscanclearlybeidentified forallanalytesonCSPswithtris-(3,5-dichlorophenylcarbamate) moiety. The higher ˛ and RS values, observed generally in the case of CSPs with tris-(3,5-dichlorophenylcarbamate), suggest morepronouncedSO–SAinteractionsofthestudiedanalytes.This maybe attributed to a –-typeinteraction increment of the acidicphenylcarbamateandthe-basic-typequinolinemoietyof Cinchonaalkaloids.However,inafewcases,inparticularforepi- QN/epi-QD,lower˛andRS wereregisteredonChiralpakIEand ChiralpakICthanonChiralpakIAandChiralpakIB.Nevertheless, resultsshowedthatthetris-(3,5-dichlorophenylcarbamate)CSPs, inmostcases,outperformotherSOtypes.
Table2
EffectofEtOHand2-PrOHcontentinn-hexaneasbulksolventforthechromatographicdata,k1,˛,RSofCinchonaalkaloidsinnormalphasemodality.
Column Mobilephase k1,˛,RS QN/QD rac.QN DHQN/DHQD epi-QN/epi-QD CD/CN
IA
a
k1 0.59 0.59 0.60 2.47 0.76
˛ 1.18 1.00 1.00 1.00 1.45
RS 1.02 0.00 0.00 0.00 2.47
b
k1 0.60 0.64 0.62 2.45 0.77
˛ 1.19 1.00 1.00 1.00 1.00
RS 0.70 0.00 0.00 0.00 0.00
IB
a
k1 0.51 0.52 0.45 1.15 0.56
˛ 1.00 1.00 1.00 1.28 1.22
RS 0.00 0.00 0.00 1.12 0.47
b
k1 1.02 1.01 0.99 2.72 0.99
˛ 1.00 1.00 1.00 1.46 1.00
RS 0.00 0.00 0.00 1.26 0.00
IE
a
k1 7.12∗∗ 1.45 1.40 4.57 3.00∗
˛ 1.06∗∗ 1.00 1.00 1.00 1.10∗
RS 1.16∗∗ 0.00 0.00 0.00 1.40∗
b
k1 1.48 1.89 2.10 9.15 1.42
˛ 1.00 1.00 1.00 1.00 1.13
RS 0.85 0.00 0.00 0.00 1.25
IC
a
k1 0.73 0.82 0.86 3.61 0.83
˛ 1.23 1.19 1.20 1.00 1.22
RS 1.18 0.84 1.27 0.00 1.22
b
k1 1.05 1.02 1.09 15.48 0.97
˛ 1.52 1.56 1.48 1.00 1.80
RS 3.07 2.67 2.88 0.00 4.20
ID
a
k1 0.68 0.68 0.69 2.68 0.76
˛ 1.21 1.08 1.16 1.14 1.39
RS 1.49 0.22 0.94 1.62 2.55
b
k1 0.80 0.97 1.08 5.37 0.99
˛ 1.13 1.00 1.00 1.14 1.00
RS 0.18 0.00 0.00 1.45 0.00
IF
a
k1 0.99 0.99 0.96 4.17 1.15
˛ 1.00 1.00 1.00 1.00 1.18
RS 0.00 0.00 0.00 0.00 1.22
b
k1 1.37 1.41 1.53 5.73 1.18
˛ 1.00 1.00 1.00 1.03 1.24
RS 0.00 0.00 0.00 0.27 1.00
IG
a
k1 0.98 0.98 0.98 5.02 1.36
˛ 1.27 1.00 1.10 1.08 1.22
RS 2.27 0.00 0.22 1.25 2.14
b
k1 1.06 1.07 1.08 6.64 1.16
˛ 1.10 1.00 1.18 1.09 1.18
RS 0.46 0.00 0.48 1.28 1.64
Chromatographic conditions:columns, ChiralpakIA-IG; mobilephase, a, n-hexane/EtOH/DEA (80/20/0.1v/v/v), ∗ n-hexane/EtOH/DEA (90/10/0.1v/v/v) and∗∗n- hexane/EtOH/DEA(95/5/0.1v/v/v),b,n-hexane/2-PrOH/DEA(80/20/0.1v/v/v);flowrate,1.0mLmin−1;detection,230−250nm;temperature,25◦C.
As mentioned earlier, the structure of SO may affect the sequenceofelutiontoo.Inthisstudy,areversalofelutionsequence was registered in NP-LC modality in EtOH-containing mobile phasesforanalytesQN/QDusingChiralpakIAvs.IE(Table4).The reversalofelutionsequencebychangingthechemicalstructure ofsubstituentsonthetris-(phenylcarbamate)moietywasalready indicatedinearlierpublications[19,30,31].
Theeffectofthepositionofthesubstituentsofthecarbamate moietyofthePS-CSPsonthechromatographicperformanceforthe givenanalyteswasinvestigatedbycomparisonofchromatographic dataobtainedonChiralpakIF,IG,andIDcolumnsinbothNP-LC andSFCmodalities(seeFig.1S).Ingeneral,higherretentionand better˛andRSvalueswereobtainedonChiralpakIGthanonIF.
ChiralpakIDseemstobeasefficientasChiralpakIGinNP-LCmodal- ityinthepresenceofEtOHasbulksolventcomponent,andless efficientinallothercases.Thesecondarystructureofthetris-(3- chloro-5-methylphenylcarbamate)-basedCSP(ChiralpakIG)offers strongerretentiveinteractionswiththeanalytes.Regardingelu- tionsequencesofQN/QDandrac.QN,nochangewasregistered byalteringtheseCSPs.Incontrast,forepi-QN/epi-QDandCD/CNa reversalofelutionsequencewasregisteredinNP-LCinthepres- enceof2-PrOHaseluentconstituent.Thestrongdependence of thesequenceofelutionasafunctionofthemobilephase(eluent compositionandSOstructure),observedinallcases,drawsatten-
tiontotheimportanceofidentificationofeachpeakinthecase ofPS-CSPs.Forchiralion-exchangersinvestigatedpreviously,this scatteredtendencywasnotseen[10].
Intheenantio-anddiastereoseparationoftheinvestigatedCin- chona alkaloids in SFC modality, Chiralpak IC and IG columns performedsignificantlybetter.However,inNP-LC,the3-chloro- substitution in Chiralpak IC and ID promotes an increased stereodiscrimination but with lower effectiveness than in SFC modality.
Theenantio-anddiastereoselectivitiesofthesevenchiralselec- tors for the five pairs of Cinchona alkaloid stereoisomers are summarizedinTables4and5.Fortheseparationofenantiomers, consistencieshavebeennoticednumeroustimeswithanelution sequence(+)QN<(–)QN,butareversedelutionsequencewasreg- isteredonChiralpakIDandIFinSFCmodalityapplying2-PrOHas alcoholmodifier.
Thesituationbecomesevenmorecomplicatedfortheresolu- tionofthediastereomericpairsQN/QD,DHQN/DHQD,CD/CN,and epi-QN/epi-QD,whereacleartrendcannotbeseen.Inboth NP- LCandSFCmodalitiesonthesevencolumns,theelutionsequence QN<QD,DHQN<DHQD,andCD<CNanditsreversalcanalso beobserved, as a clear indicationof thedifficulty to interpret enantioselectivityvsdiastereoselectivity(Tables2and3).Unex- pectedreversalsoftheelutionorderofthediastereomeric(often
Table3
ComparisonoftheeffectofMeOHand2-PrOHcontentinCO2asbulksolventforthechromatographicdata,k1,˛,RSofCinchonaalkaloidsinSFCmodality.
Column Mobilephase k1,˛,RS QN/QD rac.QN DHQN/DHQD epi-QN/epi-QD CD/CN
IA
a
k1 0.38 0.33 0.37 0.83 0.39
˛ 1.21 1.38 1.46 1.11 1.20
RS 1.16 1.91 2.43 0.74 1.03
b
k1 0.30 0.29 0.33 1.16 0.42
˛ 1.41 1.00 1.23 1.00 1.26
RS 1.43 0.00 0.79 0.00 0.65
IB
a
k1 0.41 0.41 0.40 1.47 0.50
˛ 1.00 1.00 1.00 1.00 1.07
RS 0.00 0.00 0.00 0.00 0.21
b
k1 0.52 0.52 0.54 1.55 0.69
˛ 1.00 1.00 1.00 1.18 1.00
RS 0.00 0.00 0.00 0.53 0.00
IE
a
k1 3.20 3.20 3.81 7.57 3.21
˛ 1.00 1.00 1.07 1.00 1.04
RS 0.00 0.00 0.91 0.00 0.32
b
k1 2.28 2.27 3.25 4.83 2.42
˛ 1.17 1.05 1.00 1.05 1.17
RS 1.68 0.40 0.00 0.28 1.79
IC
a
k1 1.87 1.66 1.89 7.52 2.19
˛ 1.22 1.38 1.43 1.09 1.47
RS 3.18 5.14 5.71 1.36 6.34
b
k1 0.90 0.80 0.97 13.86 1.23
˛ 1.53 1.73 1.66 1.00 1.44
RS 4.62 5.69 5.47 0.00 4.48
ID
a
k1 0.95 0.91 1.14 1.74 0.89
˛ 1.08 1.00 1.00 1.00 1.08
RS 0.91 0.00 0.00 0.00 0.73
b
k1 0.71 0.71 1.01 2.63 0.88
˛ 1.31 1.08 1.08 1.00 1.00
RS 2.89 0.00 0.83 0.00 0.00
IF
a
k1 1.14 1.08 1.27 2.95 1.12
˛ 1.00 1.00 1.00 1.00 1.08
RS 0.63 0.00 0.00 0.00 0.76
b
k1 0.70 0.72 0.85 2.62 0.91
˛ 1.34 1.13 1.28 1.00 1.13
RS 1.89 0.68 1.56 0.00 0.74
IG
a
k1 1.26 1.03 1.45 2.08 0.99
˛ 1.12 1.38 1.45 1.33 1.46
RS 1.32 3.84 4.68 3.81 4.37
b
k1 0.71 0.72 1.18 2.92 0.87
˛ 1.87 1.00 1.49 1.18 1.47
RS 5.98 0.00 4.20 2.49 3.82
Chromatographicconditions:columns,ChiralpakIA-IG;mobilephase,a,CO2/MeOH(60/40v/v)containing20mMDEA,b,CO2/2-PrOH(60/40)containing20mMDEA;flow rate,2.0mLmin−1;detection,215−230nm;temperature,40◦C;backpressure,150bar.
termedpseudo-enantiomeric)pairscaneasilyhappenasafunction ofthecompositionofthemobilephase.Inthiscontext,itbecame particularlyinterestingthatonChiralpakIGapplying2-PrOHas eluentcomponentunderLCconditionstheelutionorders(+)DHQD
<(–)DHQNandCN<CDchangedinSFCmodalityto(–)DHQN<
(+)DHQDandCD<CN(Table4and5).Thisisanotherstrongindi- cation abouttherole of solvation oftheselector andselectand moietiesontheoveralldiastereoselectivity.
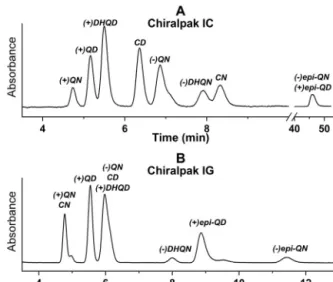
Representative chromatogramsfor theresolution of racemic quinineandfourdiastereomersofCinchonaalkaloidsinSFCmode aredepictedinFig.3andFig.S3onChiralpakICandIGapplying 2-PrOHandMeOHasalcoholmodifiers.Fullseparationandiden- tificationsoffivepairsofenantiomersanddiastereomerscanonly beachievedintwochromatographicruns.
3.3. Effectofthestructureofanalyte
Analytes, except DHQN/DHQD, possess an unsaturated side chain(vinylversusethylgroup)onthequinuclidinemoiety,which mayinfluenceinteractionsbetweenSAandCSP,despitesmalldif- ferencesinsizeandpolarity.SurveyingthedatainTables2and3 revealed, that,in general,at a givenmobile phasecomposition retentionsinNP LCorSFCmodalitiesdifferonlyveryslightly.In somecases,however,thedifferenceismoresignificant.Asaresult,
Fig.3.Chromatogramsforseparationofdiastereomersandenantiomersofnatural andsyntheticCinchonaalkaloidanalogs.
Chromatographicconditions:columns,A,ChiralpakIC;mobilephase,CO2/2-PrOH (70/30v/v)containing20mMDEA,B,ChiralpakIG;mobilephase,CO2/MeOH(70/30 v/v)containing20mMDEA;flowrate,1.0mLmin–1;detectionat220nm;temper- ature,25◦C.
Bajtai,I.Ilisz,R.Berkeczetal.JournalofPharmaceuticalandBiomedicalAnalysis193(2021)113724
Table4
ElutionsequencesofCinchonaalkaloidsinn-hexaneasbulksolventcontainingEtOHor2-PrOHinnormalphasemodality.
Mobilephase:n-hexane/EtOH(80/20v/v)containing0.1%DEA
Column IA IB IE IC ID IF IG
QN/QD (-)QN<(+)QD – (+)QD<(-)QN (+)QD<(-)QN (-)QN<(+)QD – (-)QN<(+)QD
rac.QN – – – (+)QN<(-)QN (+)QN<(-)QN – –
DHQN/DHQD – – – (+)DHQD<(-)DHQN (-)DHQN<(+)DHQD – (-)DHQN<(+)DHQD
epi-QN/epi-QD – (+)epi-QD<(-)epi-QN – – (-)epi-QN<(+)epi-QD – (-)epi-QN<(+)epi-QD
CD/CN CD<CN CN<CD CD<CN CN<CD CD<CN CD<CN CD<CN
Mobilephase:n-hexane/2-PrOH(80/20v/v)containing0.1%DEA
QN/QD (-)QN<(+)QD – (-)QN<(+)QD (+)QD<(-)QN (-)QN<(+)QD – (-)QN<(+)QD
rac.QN – – – (+)QN<(-)QN – – –
DHQN/DHQD – – – (+)DHQD<(-)DHQN – – (+)DHQD<(-)DHQN
epi-QN/epi-QD – (+)epi-QD<(-)epi-QN – – (-)epi-QN<(+)epi-QD (-)epi-QN<(+)epi-QD (+)epi-QD<(-)epi-QN
CD/CN – – CD<CN CN<CD – CD<CN CN<CD
Chromatographicconditions:columns,ChiralpakIA-IG;flowrate,1.0mLmin−1;detection,230−250nm;temperature,25◦C.
Table5
ElutionsequencesofCinchonaalkaloidsinCO2asbulksolventcontainingMeOHor2-PrOHinSFCmodality.
Mobilephase:CO2/MeOH(90/10v/v)containing20mMDEA
Column IA IB IE IC ID IF IG
QN/QD (+)QD<(-)QN – – (+)QD<(-)QN (-)QN<(+)QD – (+)QD<(-)QN
rac.QN (+)QN<(-)QN – – (+)QN<(-)QN – – (+)QN<(-)QN
DHQN/DHQD (+)DHQD<(-)DHQN – (+)DHQD<(-)DHQN (+)DHQD<(-)DHQN – – (+)DHQD<(-)DHQN
epi-QN/epi-QD (-)epi-QN<(+)epi-QD – – (+)epi-QD<(-)epi-QN – – (+)epi-QD<(-)epi-QN
CD/CN CN<CD CN<CD CN<CD CN<CD CN<CD CN<CD CN<CD
Mobilephase:CO2/2-PrOH(80/20)containing20mMDEA
QN/QD (-)QN<(+)QD – (-)QN<(+)QD (+)QD<(-)QN (-)QN<(+)QD (-)QN<(+)QD (-)QN<(+)QD
rac.QN – – – (+)QN<(-)QN (-)QN<(+)QN (-)QN<(+)QN –
DHQN/DHQD (-)DHQN<(+)DHQD – – (+)DHQD<(-)DHQN (-)DHQN<(+)DHQD (-)DHQN<(+)DHQD (-)DHQN<(+)DHQD
epi-QN/epi-QD – (+)epi-QD<(-)epi-QN (+)epi-QD<(-)epi-QN – – – (+)epi-QD<(-)epi-QN
CD/CN CD<CN – CD<CN CN<CD – CD<CN CD<CN
Chromatographicconditions:columns,ChiralpakIA-IG;flowrate,2.0mLmin−1;detection,215−230nm;temperature,40◦C;backpressure,150bar.
8
theslightlylesspolarDHQN/DHQDanalytesaremoreretained.This isnotsurprising,butthemarkedincreaseofretentionofthepair ofepi-QN/epi-QDdiastereoisomersisstriking.Theincreasedreten- tionswereaccompaniedwithhigher˛andRSvaluesonlyinafew cases.Aplausibleexplanationforthisbehaviorismostprobably relatedtotheconfigurationofthefivechiralcentersandtheresult- ingconformationofthestericallyrestrictedanalytes.Namely,for QN/QD,DHQN/DHQD,andCD/CNthechiralcentersofthequinucli- dineringareidentical[(1S),(3R),and(4S)],andonlythetwoother chiralcenters(C-8andC-9carbonatoms)aredifferent[(R)/(S)for QDand(S)/(R)forQN].Thereisasimilarsituationwithrespectto theabsoluteconfigurationofC-9in9-epi-QN[(9S)]and9-epi-QD [(9R)].Thishasastrongimpactontheconformationofmolecules withmultiplechiralcenters.
Inthepresentcase,onlytheoverallretentionbutnotthestere- ochemicaldifferentiationofthediastereomericanalytesisaffected markedlyundertheappliedchromatographicconditions.
3.4. Effectoftemperatureandthermodynamicparameters
The temperature dependence of retention and enantioselec- tivity may provide some valuable information on the chiral recognitionprocess[31–35].Keepinginmindthelimitations of theapproachappliedinthisstudy[33,32–35],thedifferenceinthe changeinstandardenthalpy(H◦)andentropy(S◦)forthe enantiomerswerecalculatedonthebasisofthevan’tHoffequation:
ln˛=−(H◦)
RT +(S◦) R
Table6
Thermodynamicparameters,(Ho),(So),Tx(So),(Go),correlationcoefficients(R2),TisoandQvaluesofCinchona-alkaloidanalogsonChiralpakIA,IB,IC,IE columnsinnormal-phaseandSFCmodalities.
Analyte -(Ho)(kJ/mol) -(So)(J/(mol*K) Correlationcoefficients(R2) -Tx(So)298K(kJ/mol) -(Go)298K(kJ/mol) Tiso(oC) Q NP-LCmodality
ChiralpakIA
QN/QD 1.1 2.3 0.938 0.7 0.4 219 1.6
CD/CN −2.3 −7.9 0.993§ −2.4 0.2 14 0.9
ChiralpakIB
epi-QN/epi-QD 4.5 12.3 0.996 3.7 0.8 95 1.2
ChiralpakIE
QN/QD −0.4 −2.1 0.959 −0.6 0.2 −91 0.7
DHQN/DHQD −0.7 −3.0 0.998♠ −0.9 0.2 −33 0.8
CD/CN −0.2 −1.55 0.931 −0.5 0.3 −170 0.4
ChiralpakIC
QN/QD 1.9 3.1 0.979 0.9 1.0 345 2.1
rac.QN 1.6 1.8 0.926 0.5 1.1 604 3.2
DHQN/DHQD −0.5 −4.9 0.979♣ −1.5 1.0 30* 0.3
DHQN/DHQD 1.9 3.1 0.991♠ 0.9 1.0 2.1
CD/CN −1.0 −8.2 0.963♣ −2.4 1.4 29* 0.4
CD/CN 1.8 0.9 0.973♠ 0.3 1.5 6.0
SFCmodality ChiralpakIA
QN/QD a 3.4 9.0 0.990 2.7 0.7 108 1.3
rac.QN 5.4 14.1 0.990 4.2 1.2 111 1.3
DHQN/DHQD 2.4 2.8 0.996 0.8 1.6 584 3.0
epi-QN/epi-QD −1.4 −5.4 0.997 −1.6 0.2 −15 0.9
ChiralpakIB
epi-QN/epi-QD b −4.2 −14.4 0.999 −4.3 0.1 20 0.9
ChiralpakIE
DHQN/DHQD c 0.5 1.0 0.994 0.3 0.2 217 1.7
epi-QN/epi-QD −1.9 −6.3 0.985 −1.9 4.4 24 0.9
ChiralpakIC
rac.QN c 1.0 0.4 0.997 0.1 0.9 >1000 10.0
DHQN/DHQD 1.0 0.4 0.993 0.1 0.9 >1000 10.0
epi-QN/epi-QD −5.5 −18.8 0.980 −5.6 0.1 18 0.9
Chromatographicconditions:column,ChiralpakIA,IB,IC,IE;mobilephase,inNP-LCmodality,n-hexane/2-PrOH/DEA80/20/0.1(v/v/v),inSFCmodality,a,CO2/MeOH80/20 (v/v)containing20mMDEA,b,CO2/MeOH90/10(v/v)containing20mMDEA,c,CO2/MeOH70/30(v/v)containing20mMDEA;inNP-LCmodality,temperaturerange,
§35−50◦C,♣7.5−30◦C,♠30−50◦Cand∗temperatureofpointofintersection(seeFig.S4);flowrate,inNP-LC,1.0mLmin−1,inSFC,2.0mLmin−1;backpressureinSFC,150 bar;detection,215−230nm;Tiso,temperaturewheretheenantioselectivitycancels;Q=(Ho)/Tx(So)298K.
whereRistheuniversalgasconstant,TistemperatureinKelvin, and˛istheapparentselectivityfactor.
Inordertoinvestigatetheeffectsoftemperatureonthechro- matographicparameters,avariabletemperaturestudywascarried outforfiveanalytesinLC-NPandSFCmodalitiesonfourPS-CSPs (ChiralpakIA,IB,IE,andIC)inthetemperaturerange7.5–50◦C inmobilephasesbasedonn-hexane/2-PrOH/DEA(80/20/0.1v/v/v) andCO2/MeOH(90/10,80/20and70/30v/v)allcontaining20mM DEA.TheexperimentaldataaresummarizedinTablesS1andTable S2.
Inbothmodalitiesonallfourcolumns,theretentioninmost casesdecreasedwithincreasingtemperature.Thetransferofthe analytefromthemobilephase tothestationary phase,in gen- eral,isanexothermicprocess.Asaresult,kand˛aswellasRS decreasewithincreasingtemperature.However,regardingsepa- rationfactorandresolution,severalexceptionswereobserved.On ChiralpakIAforCD/CNinthetemperaturerange30–50◦Candon ChiralpakICforDHQN/DHQDandCD/CNinthetemperaturerange 7.5–30◦C,˛(andRS)increasedwithincreasingtemperature(Table S2).Interestingly,inSFCmodalityforepi-QN/epi-QDonallinves- tigatedCSPs,kdecreased,but˛andRSincreasedwithincreasing temperatureinthestudiedtemperaturerange(TableS2).Apply- ingPS-CSPs,severalexampleswerereportedpreviously forthe increaseofselectivitywithincreasingtemperatureinchromato- graphicsystems[14,19,33,35].Itshouldbenoted,thatseveraltimes inbothmodalities,RSincreasedwithincreasingtemperatureinde- pendentlyofthechangeof˛.Thisphenomenoncanbeattributed totheenhancedkineticeffectuponincreasingtemperature.
From the chromatographic data van’t Hoff plots were con- structed.Asageneraltrend,ln˛vs.1/Tcurvesgaveclearlylinear