ENSURING SLAUGHTERHOUSE WASTE FEATHER RECYCLE WITH BIOLOGICAL PRE-TREATMENT
Mézes Lili1– Molnár Szabolcs – Tamás János
Abstract. Feathers are produced in large amounts as a waste in poultry slaughterhouses. Only 60- 70% of the poultry slaughterhouse products are available for human consumption. 15- 20% of the slaughterhouse by-products contain keratin; from this the proportion of feather is 7-9% with 50-70% moisture content. This means more million tons annually worldwide (Papadopoulus et al., 1986; Williams et al., 1991; Hegedűs et al., 1998). The keratin- content of feather can be difficulty digested, so physical, chemical and/or biological pre- treatment are needed in practice, which have to be set according to the utilization method.
The microbiological and enzymatic degradation of feather to soluble protein and amino acids is a very favourable and relatively cheap opportunity to produce valuable products of the resulting feather. Our applied treatments were based on the determination of the most effective method, which is able to follow the biodegradation of poultry feather waste.
The high protein content of poultry feather makes it an excellent raw material for biogas production. The digestion by fermentation of this difficultly disintegrating material produced in large quantities provides an environmentally friendly way of utilization.
Key words: feather, pre-treatment, keratin, biodegradation JEL codes: Q53
INTRODUCTION
The 60-70% of slaughtered broiler chicken product is able to human consumption.
Feather with 50-70% water content is instrumental as by-product in poultry slaughterhouses which is not able to stock utilization or decoration. More million tons of poultry feathers are produced world-wide annually (Williams et al., 1991;
Hegedűs et al., 1998).
The feather protein so-called keratin is insoluble, respectively has high structural stability and capability of resistance for proteolytic microbes because of high degree of cross-linking by cystine disulfide bonds, hydrogen bonding, and hydrophobic interactions (Harrap and Woods, 1964; Kunert, 1973; Kaluzewska et al., 1991). Feather digestion is difficult by proteolytic enzymes like trypsin, pepsin and papain (Letourneau et al., 1998). Specific keratinolytic microorganisms are need to feather degradation (Elődi, 1980; Cohlberg, 1993; Steinert, 1993; Onifade et al., 1998). For this reason the feather waste treatment and utilization are difficult tasks. Physical, chemical and/or biological pre-treatment are needed in practice, which has to be set according to the utilization method (Hegedűs et al., 1998; Perei et al., 2004; Bíró et al., 2008). Previously, feather powder was produced which was used for animal feedstuff in Hungary. The modified constitutional law (1576/2007/EK decree, 1774/2002/EK, European Parlament) disable the feather utilization as feedstuff and depose to the landfill, so innovative developments and methods are need to reach the objectives of alternative poultry
feather utilization.
The microbial or enzymatic feather degradation provides opportunities for cheap products productions from feather (Williams et al., 1990; Shih, 1993). Recently researching results proved that some bacteria from birds’ feather can digest the keratin and degrade the feather quickly in laboratory condition (Lucas et al., 2005).
Keratinolytic bacteria can degrade ß-keratin (Onifade et al., 1998; Lucas et al., 2003; Gunderson et al., 2009). The Bacillus spp. was identified as the most productive feather-degrading bacteria in soil (Kao and Lai (1995). In Fakhfakh et al. (2012) study, keratin hydrolisatum was produced by Bacillus pumilis A1, which was isolated from polluted water of local slaughterhouse. The most study focus on Bacillus, especially on Bacillus licheniformis. Bacillus licheniformis PWD-1 was isolated by Williams et al. (1990) from aerobic extract of poultry slaughter waste.
Wang and Shih (1999) examined the keratinase productivity of Bacillus licheniformis; while other researchers used it for intensify ß-keratin degradation (Lin et al., 1995). In Hungary, Kovács et al. (2002), Perei et al. (2004) and Bálint et al. (2005) examined Bacillus licheniformis. They separated Bacillus from nature (Bacillus licheniformis KK1) which produce extracellular protease and can degrade well the feather and fur because of enzymatic ability. Our applied treatments were based on the determination of the most effective method, which is able to follow the biodegradation of poultry feather waste by Bacillus licheniformis KK1.
MATERIALS AND METHODS
Examination of main quality properties of poultry feather
The sterilization of wet poultry feather from slaughterhouse was completed by a Raypa type autoclave (with 115°C, on 1.5-2.5 atm pressure). Carbon and nitrogen content of poultry feather were analyzed by Elementar VARIO EL universal analyser in Centre Laboratory of Bátortrade Ltd. The feather sample was dried on 80 C until 8 hours in oven. The dry and organic matter content was determined following MSZ 318-3:1979 standard.
Characteristics of applied bacteria
Bacillus licheniformis KK1 bacterial strain was applied in our experiments (Kovács et al., 2002; Perei at al., 2004). Bacillus licheniformis was interpreted as Eubacteria by Pesti (2001), which can be classified into Gram+ (Firmicutes) division Bacilli class. The keratinolytic Bacillus licheniformis KK1 is an aerobic, endo-spore forming bacteria strain with neutral pH and 42°C optimum (Bálint et al., 2005; Bagi, 2006). KK1 produces peptide antibiotic. Storability of KK1 is 1-2 week in liquid medium on +4°C whereas 0.5-1 year in plate. KK1 is tolerant to biogenic environmental conditions (Bagi, 2006).
LB medium was applied to sustain the bacteria which contain: (g/L): peptone, 10;
yeast extract, 5; and NaCl, 5 (Miller, 1972). The pH setting of LB liquid medium
was determined to pH 7.5 with Tris-HCl. Hanna HI2550 multifunctional pH/ORP/temperature/EC/TDS/NaCl device was used to pH setting (Measuring limit: 0-14 pH±0.01; -20-+120°C±0.4). Following the autoclaving, processes of incubation (42°C) and shaking (200 rpm) were completed by Heidolph Unimax 1010 type incubator and shaker device. Cell counting was occurred in Burker chamber with Alpha BIO-3CCD light microscope. Cell number of bacteria was 91x106 cell/ml. 4,55x106 cell/ml was generated for passage with dilution (distilled water).
Measurement of keratin activity in liquid medium
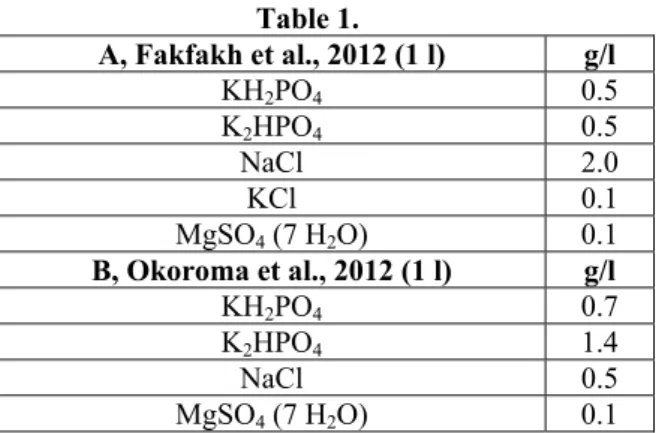
After inoculation of feather: water mixture in liquid medium, determination of hydrolysis efficiency was completed by monitoring of soluble protein content changes according to Bradford (1976). The composition of applied liquid medium is shown in Table 1.
Composition of liquid mediums
Table 1.
A, Fakfakh et al., 2012 (1 l) g/l
KH2PO4 0.5 K2HPO4 0.5 NaCl 2.0
KCl 0.1 MgSO4 (7 H2O) 0.1 B, Okoroma et al., 2012 (1 l) g/l
KH2PO4 0.7 K2HPO4 1.4 NaCl 0.5 MgSO4 (7 H2O) 0.1
Medium filling to 1 litre was occurred with sterilized distilled water, whereas pH 7.5 had been set by Tris-HCl puffer. 200 ml of liquid medium was added into flask with 500 ml capacity. Following this, 2 g whole poultry feather was added into flask and inoculation was occurred with Bacillus licheniformis KK1 strain (4,55x106 cell/ml). Incubation (42°C) and shaking (200 rpm) were completed by Heidolph Unimax 1010 incubator and shaker device. Optical Density (OD) of nutrient solution was examined at 595 nm range by Sacoman Athelie Junior type spectrophotometer.
Determination of protein content from liquid medium with Bradford assay
The Bradford assay relies on the binding of the dye Coomassie Brilliant Blue G250 to protein. The mentioned property could be characterized as the paint binds to arginine and lyzine parts of protein with electrostatic and van der Waals interactions. It follows that during the determination of different proteins, the
replies could be different. Therefore, the applied protein as reference material must be same with the protein which to be examined. In this case, the absorption maximum is changed from 465 to 595 nm. For the creation of calibration curve, dilution series was made from reference solution (Bovine serum albumin: 10 µl, 20 µl, 40 µl, 60 µl, 80 µl, 100 µl,) (Walker et al., 2002). 100 µl distilled water was used as blank sample. The sample was filtered with Wathman no1. filter paper.
After filtration from supernatant, protein content was determined by photometer. 5 ml Coomassie Brilliant Blue reagent was added into examined solutions. After 2 minutes and 1 hour of shaking, absorbance of solutions was measured on 595 nm.
Concentration of protein content of samples could be determined with comparison of absorbance values and calibration curve. Measurement of protein concentration was completed each day until 5 days from liquid medium.
RESULTS AND DISCUSSION
Dry and organic matter content of poultry feather was measured before setting up experiments. The amount of water content was average of 67% of feather waste.
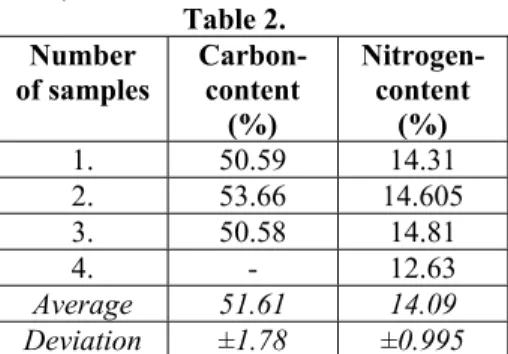
Average of organic matter content of feather is convertible; the value of dry matter content was 66%. The results of carbon and nitrogen content of used feather samples can be seen in Table 2.
Quality parameters of poultry feather
Table 2.
Number
of samples Carbon- content (%)
Nitrogen- content
(%)
1. 50.59 14.31 2. 53.66 14.605 3. 50.58 14.81 4. - 12.63 Average 51.61 14.09 Deviation ±1.78 ±0.995
Keratin mainly contain: carbon (50-55%), oxygen (25-30%), hydrogen (15-18%), nitrogen (7-8%), sulphur (0.5-2%). Keratin contains traces of bore, chlorine, and iron (Ádám, 2001). Comparing the results of our experiments, the values of carbon content were same (51.6%), however nitrogen content was higher (14,1%). The protein content of examined feather sample (nitrogen content*6.25) on average was 88.1±6.22% which was similar to results of Papadopoulos (1985) experiments (85-99%). Therefore, examination of keratin digestion can keep track of the degradation rate of feather. The protein concentration of samples was analyzed in A and B medium (Table 1.) from 4-4 samples per day. In addition, cell counting was repeated every day with Burker chamber. The calibration curve was determined with known absorbance protein (Bovine serum albumin solution with 1 mg/ml protein content) for measurement of protein concentration according to
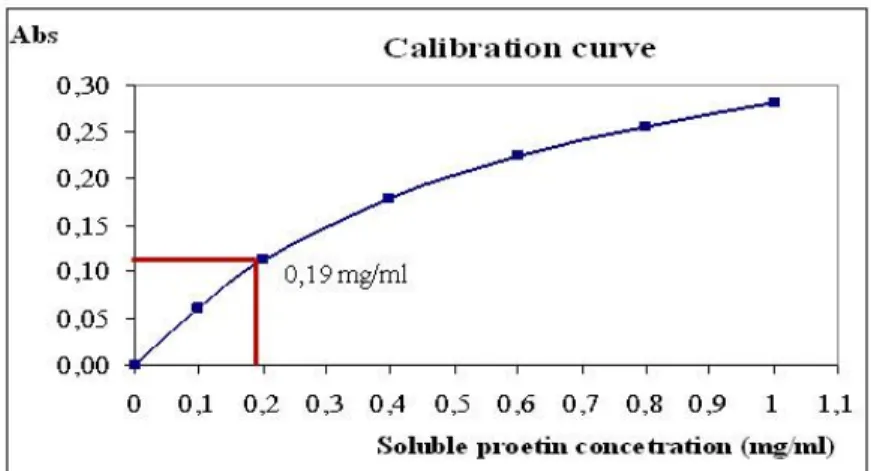
Bradford (Figure 1.).
Figure 1: Determination of protein concentration which is based on calibration curve The dissolved protein concentration of samples could be determined with calibration curve as follow: an absorbance value is needed to select from data of measurements. This value was added to Y-axis of calibration curve. Following this, from this point the curve was sectioned perpendicularly. Perpendicular was added from the resulting intersection to X-axis. The dissolved protein concentration could be known by intersection reading, in our case the value was 0.19 mg/ml. After calculation of total soluble protein concentration, the efficiency of two medium is comparable.
The composition of B liquid medium was more favourable for Bacillus licheniformis KK1 bacteria. The value of dispersion was significant (0.078 mg/ml) in the last results of measurements of B medium, which means 15% difference.
Despite of same mass (2 g) and whole feather samples were used the experiment;
the difference is remained because of different structural features of feathers. More homogeneous poultry feather could be produced if the feather was grinded before.
The rate of keratin degradation could be seen in Table 4.
Keratin degradation rate of different medium
Table 4.
Keratin degradation rate (mg/ml) (%)
Type of media 1. day 2. day 3. day 5. day
A 1.02 2.16 5.56 7.37 B 0.14 2.72 6.47 8.62
Analyzing the rate of keratin digestion, the type of B medium was more effective (+1.25%) in case of same quantity of feather samples.
Monitoring of growth of bacteria cells was also examined by all of the soluble protein concentration measuring with Burker chamber (Table 5.).
Changes of bacterial cell number in applied media
Table 5.
Bacterial cell number (x106 cell/ml)
Type of media 0. day 1. day 2. day 3. day 5. day
A 4.55±0.00 9.0±0.28 16.7±0.42 45.3±1.56 57.6±1.13 B 4.55±0.00 10.2±0.56 21.8±1.13 51.2±1.13 69.6±4.53
Comparing the results the soluble protein concentration and cell number, there is a linear relationship between the protein concentration and bacterial cell number.
CONCLUSIONS
Based on the result of total soluble protein concentration, that B liquid medium composition was favourable for applied bacterial stain. Keratin degradable ratio was more effective (+1.25%) in case of B medium. Results of liquid media experiments indicated that cell number of Bacillus licheniformis KK1 depends on the composition of the culture media and also on the quantity of applied feather. B medium with higher K2HPO4 and lower NaCl content proved more effective as A medium in case of bacterial cell number also. The exact effects (NaCl, K2HPO4) can be determined by further studies. Linear relationship was observed between the protein concentration and bacterial cell number. Based on this results is detectable the keratin activity and therefore could determine the degree of keratin degradation.
ACKNOWLEDGEMENTS
We thank Prof. Kornél L. Kovács, Dr. Zoltán Bagi (University of Szeged, Department of Biotechnology), Györgyi Biró, Sándorné Kincses, Bence Mátyás (University of Debrecen, MÉK) for professional support and for assistance in the research. This research was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP- 4.2.4.A/ 2-11/1-2012-0001 ‘National Excellence Program’.
REFERENCES
1. ÁDÁM I. (2001) - A toll. A baromfitoll és feldolgozása, Scriptor Bt., Budapest. 11-30. pp. 98-114.
2. BAGI Z. (2006) – Personal consultation. SZTE, TTIK, Biotechnológiai Tanszék.
3. BÁLINT, B., BAGI, Z., TÓTH A., RÁKHELY G., PEREI K., KOVÁCS, K. L. (2005) - Utilization of keratin-containing biowaste to produce biohydrogen. Applied Microbiology and Biotechnol. 69. 4. pp. 404-410.
4. BÍRÓ T., MÉZES L., PETIS M., KOVÁCS L.K., BAGI Z., HUNYADI G.
(2008) - A baromfi toll, mint biogáz alapanyag. Kiss T., Somogyvári M.
(szerk.). Via Futuri 2007. A biomassza alapú energiatermelés. BIOKOM Kft. Pécs. pp. 156-163.
5. BRADFORD, M. M. (1976) - A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72. pp. 248-254.
6. COHLBERG, J. A. (1993) - The strukture of L-keratin. Trends Biochem.
Sci. 18. pp, 360-362.
7. ELŐDI P. (1980) - Biokémia. Akadémia Kiadó. Budapest. pp. 72-128., 543-621.
8. FAKHFAKH N., GARAGOURI M., DAHMENL I., SELLAMI- KAMOUN A., EL FEKI A., NASRI M. (2012) - Improvement of antioxidant potential in rats consuming feathers protein hydrolysate obtained by fermentation of the keratinolytic bacterium, Bacillus pumilus A1. African Journal of Biotechnolgy. 01.11. pp. 938-949.
9. HARRAP, B. S., WOODS, E. F. (1964) - Soluble derivatives of feather keratin. Biochem. J. 92. pp. 19-26.
10. HEGEDŰS M., SCHMINDT J., RAFAI P. (1998) - Állati eredetű melléktermékek hasznosítása. Mezőgazda Kiadó. Budapest. 15-29., 65-93.
11. GUNDERSON, A. R., FORSYTH, M. H., SWADDLE, J. P. (2009) - Evidence that plumage bacteria influence feather coloration and body condition of eastern bluebirds, Sialia sialis. Journal of Avian Biology. 40.
pp. 440-447.
12. KALUZEWSKA M., WAWRZKIEWICZ K., LOBARZEWSKI J. (1991) - Microscopic examination of keratin substrates subjected to the action of the enzymes of Streptomyces fradiae. Int. Biodeterior. 27. pp. 11-26.
13. KAO, M. M., LAI, H. Y. (1995): The study of the selection of feather- degrading microorganismus. J. Chin. Inst. Environmental Eng. 5. pp. 37-43.
14. KOVÁCS, K. L., BAGI, Z., PEREI, R. K., CSANÁDI, GY., FODOR, B., KOVÁCS, Á. T., MARÓTI, G., MAGONY, M., BÁLINT, B., VALASTYÁN, P., RÁKHELY, G. (2002) - Biohydrogen, biogas, bioremediation. Proc. "Power of Microbes in Industry and Environment"
Conf., Opatija, Croatia, 7-9 June, 2002. 17.
15. KUNERT J. (1973) - Keratin decomposition by dermatophytes: evidence of sulphitolysis of the protein. Experientia. 33. pp. 489-498.
16. LETOURNEAU F., SOUSSOTTE V., BRESSOLLIER P., BRANLAND P., VERNEUIL B. (1998) - Keratinolytic activity of Streptomyces sp.
S.K.1-02: a new isolated strain. Lett. Appl. Microbiol. 26. pp. 77-80.
17. LIN, X., DELEMEN, D. W., MILLER, E. S., SHIH, J. C. (1995) - DNA nucleotide sequence of keratinase gene of Bacillus licheniformis. Appl.
Environmental Microbiol. 61. 4. pp. 1469-1474.
18. LUCAS, F. S., BROENNIMANN, O., FEBBRARO, I., HEEB, P. (2003) - High diversity among feather degrading bacteria from a dry meadow soil.
Microbial Ecology. 45. pp. 282–290.
19. LUCAS, F. S., MOUREAU, B., JOURDIE, V., HEEB, P. (2005) - Brood size modifications affect plumage bacterial assemblages of European starlings. Molecular Ecology. 14. pp. 639–646.
20. MILLER J. H. (1972) - Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
21. OKOROMA E. A., GARELICK H., ABIOLA O. O., PURCHASE D.
(2012) - Identification and characterisation of a Bacillus licheniformis strain with profound keratinase activity for degradation of melanised feather. International Biodeterioration & Biodegradation. 74. pp. 54–60.
22. ONIFADE, A. A., AL-SANE, N. A., AL-MUSSALLAM, A. A., AL- ZARBAM, S. (1998) - A review: Potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Biores. Technol. 66. pp. 1-11.
23. PAPADOPOULOS, M. C. (1985) - Processed chicken feathers as feedstuff for poultry and swine. A review. Agric. Wastes. 14. pp. 275–290.
24. PEREI K., BAGI Z., BÁLINT B., CSANÁDI GY., HOFNER P., HORVÁTH L, KARDOS GY., MAGONY M., RÁKHELY G., ROMÁN GY., TÓTH A., ZSÍROS SZ., KOVÁCS L. K. (2004) - Mikrobák környezetvédelmi biotechnológiai hasznosításra. In: Székács A. (szerk.).
Biokémia. A Magyar Biokémiai Egyesület tájékoztatója. 28. 3. pp. 54–58.
25. PESTI M. (2001) - Általános mikrobiológia. Dialóg Campus Kiadó.
Budapest–Pécs.
26. SHIH, J. C .H. (1993) - Recent development in poultry waste digestion and feather utilization – a review. Poultry Sci. 72. pp. 1617–1620.
27. STEINERT, P. M. (1993) - Structure, function, and dynamics of keratin intermediate filaments. J. Invest. Dermatol. 100. pp. 729-734.
28. WALKER J. M. (2002) - The Protein Protocols Handbook. Humana Press Inc. Totowa. NJ. pp. 1-16.
29. WANG, J. J., SHIH, J. C. H. (1999) - Fermentation production of keratinase from Bacillus licheniformis PWD-1 and a recombinant B.
subtilis FDB-29. Journal of Industrial Microbiology and Biotechnology, June 1999. 22. 6. pp. 608-616.
30. WILLIAMS, C. M., RICHESTER, C. S., MACKENZI, J. M., SHIH, J. C.
H. (1990) - Isolation, identification and characterization of a feather- degrading bacterium. Appl. Environ. Microbiol. 56. pp. 1509-1515.
31. WILLIAMS C.M., LEE CG., GARLICH J.D., SHIH J.C.H. (1991) - Evaluation of a bacterial feather fermentation product, feather-lysate as a feed protein. Poultry Sci. 70. pp. 85-94.
32. 1576/2007/EK decree (modified 1774/2002/EK decree)
33. MSZ 318-3:1979 standard for dry matter content and loss on ignition CONTACT
1. Mézes Lili, University of Debrecen, Faculty of Agricultural and Food Sciences and Environmental Management, Institute of Water and Environmental Management, 4032 Böszörményi str. 138., Debrecen, Hungary, E-mail: mezes@agr.undieb.hu